Summary
Small molecule ligands that target to TGR5 and FXR have shown promise in treating various metabolic and inflammation-related human diseases. New insights into the mechanisms underlying the bariatric surgery and bile acid sequestrant treatment suggest that targeting the enterohepatic circulation to modulate gut-liver bile acid signaling, incretin production and microbiota represents a new strategy to treat obesity and type-2 diabetes.
Keywords: diabetes, bile acids, FGF15/19, incretin, microbiota
INTRODUCTION
Bile acids are physiological detergent molecules that facilitate the absorption of dietary lipids and vitamins in the gut (1). Studies in the past decades revealed that bile acids are signaling molecules that regulate lipid, glucose and energy metabolism. This regulatory function of bile acids is predominantly mediated by the bile acid-activated nuclear receptor farnesoid X receptor (FXR) and G protein coupled receptor TGR5, which is reviewed in details elsewhere (2). Emerging evidence suggests that bile acids in the small and large intestine regulate gut microbiota, incretin secretion and fibroblast growth factor 15/19 (FGF15/19) production, which modulate whole body lipid, glucose and energy homeostasis. In addition, recent findings revealed that the rapid improvement of glycemic control after gastric bypass surgery may be attributed to intestine bile acid signaling. This review focuses on the most recent findings that underscore the importance of the enterohepatic bile acid signaling in the regulation of metabolic homeostasis.
Regulation of bile acid synthesis by the gut-liver signaling axis
Bile acids are synthesized from cholesterol in the liver (2). As shown in Figure 1, cholesterol 7αhydroxylase (CYP7A1), a cytochrome P450 enzyme residing in the endoplasmic reticulum, catalyzes the first and rate-limiting step in the classic bile acid synthesis pathway. Newly synthesized bile acids are conjugated to amino acid glycine or taurine and are secreted across the apical membrane of the hepatocytes and stored in the gallbladder. A meal intake stimulates the release of bile acids into the intestinal tract to facilitate the absorption of dietary lipids and vitamins. Bile acids are efficiently reabsorbed in the ileum and transported back to the liver via portal circulation for re-secretion into the bile. The daily fecal loss of bile acids is only about 5%, which is replenished by de novo bile acid synthesis in the liver. The process of bile acid transport between the liver and the intestine is referred to as the enterohepatic circulation of the bile, which not only is important in nutrient absorption, but also plays a key role in regulating bile acid homeostasis and bile acid signaling.
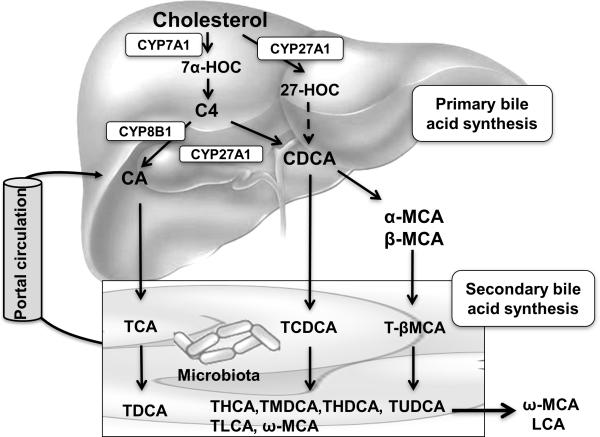
Figure 1. Bile acid biosynthetic pathways.
Two major bile acid biosynthetic pathways are shown. The classic pathway is the major bile acid synthetic pathway in the liver. In this pathway, cholesterol is converted to 7α-hydroxycholesterol (7α-HOC) by the rate-limiting enzyme cholesterol 7α-hydroxylase (CYP7A1), which is located in the endoplasmic reticulum. The sterol 12α-hydroxylase (CYP8B1) converts the intermediate 7α-hydroxy-4 cholesten-3-one (C4) to 7α, 12α-dihydroxy-4- cholesten-3-one, leading to synthesis of cholic acid (CA). Without 12αhydroxylation by CYP8B1, C4 is eventually converted to chenodeoxycholic acid (CDCA). The mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes the steroid side-chain oxidation in both CA and CDCA synthesis. In the alternative pathway, CYP27A1 converts cholesterol to 27hydroxycholesterol (27-HOC), which eventually is converted to CDCA. In mouse liver, most CDCA is converted to α- and β-muricholic acid (MCA). MCA is only found in trace amount in humans. In the large intestine, bacterial 7-dehydroxylase removes a hydroxyl group from C-7 and converts CA to deoxycholic acid (DCA) and converts CDCA to lithocholic acid (LCA). CYP3A1 and epimerases also convert CDCA to the secondary bile acids, including hyocholic acid (HCA), murideoxycholic acid (MDCA), ω-muricholic acid (ω-MCA), hyodeoxycholic acid (HDCA) and ursodeoxycholic acid (UDCA). Most LCA and ω-MCA are excreted into feces.
Under normal conditions, the CYP7A1 gene transcription, thus hepatic bile acid synthesis, is tightly regulated via bile acid-mediated negative feedback mechanisms in order to maintain a relatively constant bile acid pool (Figure 2). It is now clear that FXR plays a central role in mediating the negative feedback regulation of bile acid synthesis (2). In the hepatocytes, the bile acid/FXR/SHP (small heterodimer partner) cascade was first identified to inhibit CYP7A1 gene transcription in response to elevated bile acids in the liver (3,4). In extra-hepatic tissues, FXR is highly expressed in the intestine that is also constantly exposed to high levels of bile acids. In 2005, Inagaki et al. revealed an intestinal FXR/FGF15/liver FGF receptor 4 (FGFR4) (human homolog: FGF19) signaling axis that links the gut bile acid sensing to the regulation of hepatic bile acid synthesis (5). The expression of FGF15 was detected at high levels in the ileum but presented at very low levels in other parts of the small intestine or colon, and was not expressed in mouse hepatocytes. Activation of intestine FXR by bile acids resulted in the transcriptional induction of FGF15. FGF15 then acts as an endocrine hormone to inhibit hepatic CYP7A1 gene transcription via binding to the cell surface FGFR4 on hepatocytes. The FGF15/19-mediated gut to liver signaling has been demonstrated in fgfr4 knockout mice (6,7), tissue-specific fxr knockout mice (8) and in human hepatocytes (9). More recently, Lan et al. showed that mice lacking the intestine basolateral bile acid efflux transporter ostα had significantly reduced bile acid pool size but also lower hepatic CYP7A1 gene expression (10), which was resulted from bile acid retention in the intestine that leads to FGF15 induction. The same group also demonstrated that mice lacking the intestine apical sodium dependent bile acid transporter (ASBT) showed reduced intestine FGF15 expression, higher hepatic CYP7A1 expression and resistance to atherosclerosis development (11). These studies reiterated the importance of gut-liver signaling axis in the regulation of bile acid and lipid homeostasis.
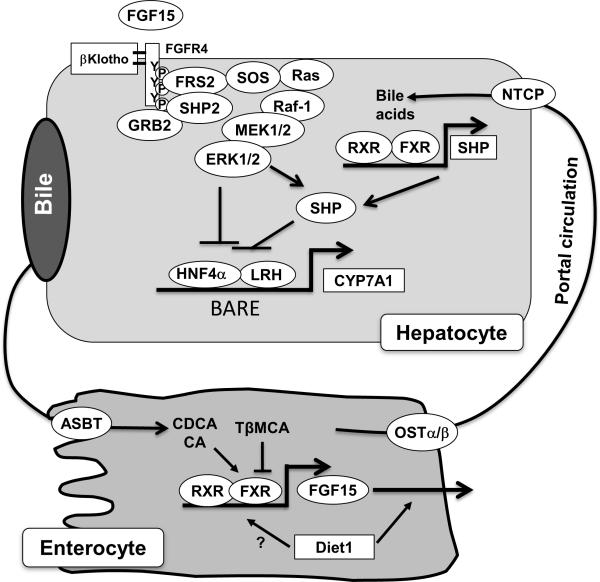
Figure 2. Mechanisms of bile acid feedback inhibition of bile acid synthesis.
Bile acidactivated signaling inhibits CYP7A1 and therefore reduces hepatic bile acid synthesis. Nuclear receptors HNF4α and LRH1 bind to the bile acid response element (BARE) located in the CYP7A1 gene promoter and stimulate CYP7A1 gene transcription. In hepatocytes, bile acids activate FXR, which induces the repressor SHP. SHP interacts with and represses the transactivating action of HNF4α and LRH. In the intestine, bile acid-activated FXR induces the transcription of FGF15 (FGF19 in humans). Diet1 may promote FGF15/19 secretion from the intestine. Diet1 may also regulate FGF15 mRNA levels. FGF15/19 binds and activates FGFR4 on the hepatocytes. FGFR4 activates intracellular signaling pathways such as ERK, which leads to the repression of CYP7A1 gene transcription. SHP2 and FRS2 are identified as key components of the FGFR4 signaling complex that activates ERK1/2 in response to FGF15. The intracellular event downstream of ERK1/2 in CYP7A1 inhibition is less well understood, but may involve the inhibition of HNF4α or LRH. ERK has also been shown to stabilize SHP via direct phosphorylation. Disruption of the bile acid feedback signaling caused increased hepatic CYP7A1 expression and enlarged bile acid pool size.
A positive feedback mechanism links gut microbiota to bile acid synthesis
Recent evidence suggests that the gut microbiota composition directly affects energy metabolism, leading to remarkable alterations of lipid, glucose and energy metabolism (12). The intestinal microbes generate short chain fatty acids from dietary carbohydrates that otherwise cannot be utilized as energy (13-15). The gut microbiota also affected the release of gut hormones such as glucagon like peptide 1 (GLP1) (16-19) and inflammatory mediators (20,21). Both macronutrients and bile acids can reshape the gut microbiota, which in turn regulates the development of obesity and metabolic syndromes (22,23). Alterations of gut microbiota after bariatric surgery were also linked to weight loss (24-27).
It is well known that bile acids inhibit gut microbial growth through their detergent property and also FXR-dependent signaling mechanisms (28). On the other hand, gut bacteria also regulate bile acid biotransformation in the intestine, which alters bile acid composition (29). Germ-free rats and mice had increased bile acid synthesis and enlarged bile acid pool, and were resistant to diet-induced weight gain (30). More recent studies showed that in germ-free mice tauroconjugated bile acids, especially tauro-β-muricholic acid (T-βMCA), became predominant (31).
This is thought to be due to decreased bacterial bile salt hydrolase activity, which de-conjugates T-βMCA before it can be converted to secondary bile acids in the gut (Figure 1). A recent study in germ-free mice showed that despite an overall enlarged bile acid pool, increased muricholic acids acted as FXR antagonists and inhibited intestine FXR activity and FGF15 expression (Figure 2), which explains increased bile acid synthesis seen in germ-free mice (32). Same bile acid phenotypes can also be achieved by preventing cholic acid synthesis via cyp8b1 knockout (33). Another study by Li et al. reported that treating mice with an antioxidant tempol, which has been shown to reduce body weight (34), resulted in two changes in mice (35). First, it caused a shift of the microbial community from Firmicutes towards Bacteroidetes, which is consistent with reduced short chain fatty acid production and weight loss. Second, decreased bile salt hydrolase activity from the Firmicutes resulted in T-βMCA accumulation, leading to enlarged bile acid pool. Importantly, bile acid composition critically determines the hydrophobicity of the bile acid pool, which affects both gut nutrient absorption and gut bile acid signaling. These recent studies underscore the important impact of bile acid composition on bile acid homeostasis and nutrient homeostasis.
SHP-2 is a key signaling component of the hepatic FGF15/19 signaling pathway
The intracellular signaling mechanisms that mediate FGF15/19 inhibition of CYP7A1 gene in the hepatocytes are less well understood. FGFR4 is the predominant FGF receptor expressed in hepatocytes. The FGF15 signaling through FGFR4 requires another transmembrane protein called β-Klotho (36,37). A recent study by Li et al. identified the cytoplasmic tyrosine phosphatase SHP-2 as another key component in the FGF15/19 signaling in the hepatocytes (38). Hepatocyte-specific deletion of SHP2 resulted in increased hepatic expression of CYP7A1 gene, hepatic bile acid accumulation and enlarged bile acid pool. On a chow diet, these mice developed liver injury consistent with bile acid damage to the hepatobiliary system. SHP-2 is a non-receptor tyrosine phosphatase with two Src-homology 2 (SH2) domains that allow its association with cell surface signaling components. SHP2 is required for activation of intracellular Ras/ERK pathway in response to mitogens and cytokines (39,40), which is consistent with ERK-dependent repression of CYP7A1 by FGF19 (9). Other than direct binding to activated receptor tyrosine kinases, SHP2 can also be recruited to the cell surface signaling complex via interacting with adaptor proteins including the FGF receptor substrate 2 (FRS2). Interestingly, a recent report showed that FRS2 also plays a role in relaying FGF15 signaling and ablation of FSR2 in hepatocytes abrogated FGF15/FGFR4 regulation of CYP7A1 (41). It is well known that SHP2 regulates cell proliferation and tumorigenesis (39,42). Consistently, recent studies also suggested a role of FGF15 signaling in promoting liver regeneration in mice (43,44). A better understanding of the FGF15/19/FGFR4 signaling complex may facilitate the development of nontumorigenic variants of FGF19 for the treatment of human diseases (45).
Intestine Diet1 is a novel regulator of hepatic bile acid synthesis
About a decade ago, Mouzeyan et al. first reported that the Diet1 locus confers protection against diet-induced hypercholesterolemia in the C57BL/6J (B6By) mouse sub-strain compared to the nearly genetically identical but atherosclerosis-prone C57BL/6J mouse strain (46). Further characterization revealed that the B6By strain showed elevated hepatic expression of CYP7A1 mRNA and enlarged bile acid pool, which likely conferred resistance to diet-induced hypercholesterolemia (47). In a recent report, the mutant gene that is responsible for increased hepatic bile acid synthesis in the B6By sub-strain was identified by genetic mapping and termed Diet1 (48). This gene, encoding a 236 KDa protein, was shown to be expressed throughout the small intestine with relatively higher mRNA levels in the Ileum. Diet1 mRNA was not detected in the mouse liver, muscle or adipose tissues. It was noticed that the ileal FGF15 mRNA and protein was reduced in Diet1 deficient mice. In a human intestine cell line, Diet1 over-expression showed a modest effect on FGF19 mRNA. In contrast, Diet1 over-expression increased FGF19 protein secretion by ~3-fold. The function of Diet1 was not clear, but it shares sequence similarity to another protein Endotubin, which might be involved in intracellular protein trafficking (49). Interestingly, Diet1 was shown to interact with FGF19 and co-localize with FGF19 in an intracellular compartment distinct from endosomes/lysosomes in the intestine cell line. The identification of Diet1 that may control FGF15/19 secretion added a second layer of regulation of the FGF15/19 signaling axis in the intestine. Because the FGF15 protein has not been detected experimentally in mice so far, the investigation of the role of Diet1 on portal FGF15 levels was therefore limited. Further investigation of Diet1 function in hepatocyte-specific fgfr4 knockout mice and analysis of genetic variations or mutations in DIET1 gene in humans may shed more light on the role of Diet1 in the regulation of bile acid and cholesterol metabolism.
TGR5 mediates the bile acid sequestrant effect on glycemic control
It is well known that disruption of the gut-liver bile acid negative feedback signaling by bile acid sequestrants stimulates hepatic bile acid synthesis, which is effective in treating hypercholesterolemia in humans. Interestingly, adding colesevelam to statin therapy or other anti-diabetic drugs also improved glycemic control in type-2 diabetes mellitus (50-52). Until now the involvement of FXR in this glucose-lowering effect is still not certain given that hepatic and intestine FXR activity are likely either unchanged or decreased after sequestrant treatment (53). On the other hand, increased gut incretin GLP-1 may mediate the glucose-lowering effect of bile acid sequestrants (54). GLP-1, secreted from the ileal and colonic L-cells, enhances insulin secretion from the pancreatic β cells and inhibits glucagon production from the α cells (55). GLP-1 regulation of gastric emptying and satiety may also contribute to its effect on glycemic control (56,57). Macronutrients (dietary carbohydrates and fats) stimulate GLP-1 secretion in the gut. Activation of TGR5 by bile acid or TGR5 agonist treatments also stimulated GLP-1 production in vitro and in vivo (58,59). However, whether endogenous bile acids, especially lithocholic acid, the most potent TGR5 ligand among all bile acid species, are able to activate TGR5 in the gut under normal physiology remains unclear. In this regard, two recent studies showed that bile acid sequestrants increased bile acid concentration in the distal ileum and colon where intestine L cells and TGR5 expression were highly enriched, which led to activation of TGR5 and GLP-1 secretion. Importantly, both studies showed that the bile acid sequestrantinduced GLP-1 secretion and glucose lowering were blunted in mice lacking TGR5 (60,61). An alternative proposed mechanism by which bile acid sequestrants increased GLP-1 production is that bile acid sequestrants interfere with dietary fat solubilization and absorption, which allows a higher concentration of dietary fatty acids to reach the distal ileum to induce GLP-1 secretion (62). In this case, the synergistic effect of dietary nutrients and bile acids on postprandial GLP-1 production will be enhanced by bile acid sequestrants.
Intestine bile acid signaling after bariatric surgery
A number of bariatric surgical procedures including the most common Roux-en-Y gastric bypass (RYGB) and the adjustable gastric banding (AGB) are being used as effective treatment for obesity and type-2 diabetes. Studies have shown that bariatric surgeries reduce food intake and increase satiety, and lead to gradual weight loss (63). However, significant improvement in glycemic control, β cell function and hepatic insulin resistance occurred even before weight loss (64). Extensive investigations into the mechanisms showed that post-surgery was associated with increased postprandial circulating gut hormones (Peptide YY, GLP-1), bile acids and FGF19 that correlated with improved glycemic control, weight loss and diabetic remission. Bariatric surgery promotes bile acids and undigested food to reach the distal intestine, which is thought to enhance incretin secretion. Elevated circulating FGF19 and bile acids can also lower postprandial blood glucose post-surgery, and these mechanisms would be dependent on FXR activation (65). In this regard, Ryan et al. compared the metabolic improvements after vertical sleeve gastrectomy (VSG) in wild type and whole body fxr knockout mice (27). Different from RYGB, VSG reduces gastric volume through ~70% removal of the stomach and does not involve the bypass of the proximal section of small intestine (66). They found that although VSG caused a sustained weight loss and improved glycemic control in wild type mice, FXR deficient mice regained weight at 8 weeks post-surgery and lost improved glycemic control. This is the first study to suggest a key role of FXR in mediating the effects of bariatric surgery. Unfortunately, changes in plasma bile acids and GLP-1 and intestine FGF15 levels post-surgery were not shown in this study, and whether the effect was mediated by intestine or liver FXR is not known. In addition, the result interpretation may be further complicated by the fact that fxr knockout mice already had lower body weight and enlarged bile acid pool size at the time of surgery, which may prevent further weight reduction post-surgery. It would be interesting to test the role of TGR5 in mediating the effects of bariatric surgery in a similar approach.
CONCLUSION
Research in the past two decades has unveiled important roles for bile acids in the regulation of hepatic lipid, glucose and energy metabolism. Small molecule ligands that target to TGR5 and FXR have shown promise in treating various metabolic and inflammation-related human diseases. New findings on the regulatory relationship between bile acid signaling, gut hormones and microbiota and energy balance are intriguing. Particularly, investigations into the mechanisms underlying the bariatric surgery and bile acid binding resin treatment suggest that modulation of the enterohepatic bile acid signaling represents a new strategy to treat obesity and type-2 diabetes. Changes in gut incretin production, bile acid signaling, hepatic CYP7A1 activity and gut microbiota likely contribute to the various metabolic benefits. It should also be kept in mind that the underlying mechanisms of these interactions are complex. Experimental outcomes may highly depend on the conditions and model systems used. Furthermore, the metabolic consequences of modulating enterohepatic bile acid metabolism could be very different in humans than in mice owing to the significant species difference in bile acid composition and metabolism.
Key points.
A positive feedback mechanism links gut microbiota to hepatic bile acid synthesis through intestine FXR antagonism
Diet1 is a novel regulator of intestine FGF19 secretion and controls hepatic bile acid and cholesterol homeostasis.
The protein tyrosine phosphatase SHP2 is identified as a key signaling component in the FGF15/19 signaling regulation of CYP7A1 in the hepatocytes.
Enhanced intestine FXR and TGR5 signaling contributes to the metabolic benefits of bariatric surgery and bile acid sequestrants.
Purpose of review.
This review focuses on the latest understanding of the molecular mechanisms underlying the complex interactions between intestine and liver bile acid signaling, gut microbiota, and their impact on whole body lipid, glucose and energy metabolism.
Recent findings.
Hepatic bile acid synthesis is tightly regulated by the bile acid negative feedback mechanisms. Modulating the enterohepatic bile acid signaling greatly impacts whole body metabolic homeostasis. Recently, a positive feedback mechanism through intestine FXR antagonism has been proposed to link gut microbiota to the regulation of bile acid composition and pool size. Two studies identified intestine Diet1 and hepatic SHP2 as novel regulators of CYP7A1 and bile acid synthesis through the gut-liver FXR/FGF15/19/FGFR4 signaling axis. New evidence suggests that enhancing bile acid signaling in the distal ileum and colon contributes to the metabolic benefits of bile acid sequestrants and bariatric surgery.
Acknowledgement
The authors acknowledge the following funding support: NIH grants DK58379 (J.C.) and DK44442 (J.C.), DK102487-01 (T.L.); the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health (T.L.); the American Diabetes Association 7-12-JF-35 (T.L.).
Footnotes
Conflict of interest
none
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Chiang JY. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr Rev. 2002;23:443–463. doi: 10.1210/er.2000-0035. [DOI] [PubMed] [Google Scholar]
- 2.Li T, Chiang JY. Bile Acid Signaling in Metabolic Disease and Drug Therapy. Pharmacological reviews. 2014;66:948–983. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 5.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Yu C, Wang F, Kan M, Jin C, Jones RB, Weinstein M, Deng CX, McKeehan WL. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. The Journal of biological chemistry. 2000;275:15482–15489. doi: 10.1074/jbc.275.20.15482. [DOI] [PubMed] [Google Scholar]
- 7.Yu C, Wang F, Jin C, Huang X, McKeehan WL. Independent repression of bile acid synthesis and activation of c-Jun N-terminal kinase (JNK) by activated hepatocyte fibroblast growth factor receptor 4 (FGFR4) and bile acids. The Journal of biological chemistry. 2005;280:17707–17714. doi: 10.1074/jbc.M411771200. [DOI] [PubMed] [Google Scholar]
- 8.Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alphahydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan T, Rao A, Haywood J, Kock ND, Dawson PA. Mouse organic solute transporter alpha deficiency alters FGF15 expression and bile acid metabolism. Journal of hepatology. 2012;57:359–365. doi: 10.1016/j.jhep.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan T, Haywood J, Dawson PA. Inhibition of ileal apical but not basolateral bile acid transport reduces atherosclerosis in apoE(−)/(−) mice. Atherosclerosis. 2013;229:374–380. doi: 10.1016/j.atherosclerosis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013;19:338–348. doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 14.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiological reviews. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 16.Reimer RA, McBurney MI. Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology. 1996;137:3948–3956. doi: 10.1210/endo.137.9.8756571. [DOI] [PubMed] [Google Scholar]
- 17.Kok NN, Morgan LM, Williams CM, Roberfroid MB, Thissen JP, Delzenne NM. Insulin, glucagon-like peptide 1, glucose-dependent insulinotropic polypeptide and insulin-like growth factor I as putative mediators of the hypolipidemic effect of oligofructose in rats. The Journal of nutrition. 1998;128:1099–1103. doi: 10.1093/jn/128.7.1099. [DOI] [PubMed] [Google Scholar]
- 18.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. The British journal of nutrition. 2004;92:521–526. doi: 10.1079/bjn20041225. [DOI] [PubMed] [Google Scholar]
- 19.Delzenne NM, Cani PD, Daubioul C, Neyrinck AM. Impact of inulin and oligofructose on gastrointestinal peptides. The British journal of nutrition. 2005;93(Suppl 1):S157–161. doi: 10.1079/bjn20041342. [DOI] [PubMed] [Google Scholar]
- 20.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends in microbiology. 2004;12:562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 22.Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 23.Clarke SF, Murphy EF, Nilaweera K, Ross PR, Shanahan F, O'Toole PW, Cotter PD. The gut microbiota and its relationship to diet and obesity: new insights. Gut microbes. 2012;3:186–202. doi: 10.4161/gmic.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, Bloom SR, Darzi A, Athanasiou T, Marchesi JR, Nicholson JK, Holmes E. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60:1214–1223. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Dore J, Henegar C, Rizkalla S, Clement K. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Perez HE, Sandoval DA, Kohli R, Backhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [This study used FXR deficienct mouse models to investigate the role of FXR signaling in mediating the beneficial effects of bypass surgery in promoting weight loss and improving glucose homeostasis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. Journal of lipid research. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Wostmann BS. Intestinal bile acids and cholesterol absorption in the germfree rat. The Journal of nutrition. 1973;103:982–990. doi: 10.1093/jn/103.7.982. [DOI] [PubMed] [Google Scholar]
- 31.Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proceedings of the National Academy of Sciences of the United States of America 108 Suppl. 2011;1:4523–4530. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [This study revealed a novel mechanism by which gut bacteria modulate the enterohepatic signaling regulation of hepatic bile acid synthesis.] [DOI] [PubMed] [Google Scholar]
- 33**.Hu X, Bonde Y, Eggertsen G, Rudling M. Muricholic bile acids are potent regulators of bile acid synthesis via a positive feedback mechanism. Journal of internal medicine. 2014;275:27–38. doi: 10.1111/joim.12140. [This study demonstrates that importance of bile acid composition in the regulation of hepatic bile acid synthesis in antibiotic-treated mice and in CYP8B1 deficienct mice.] [DOI] [PubMed] [Google Scholar]
- 34.Mitchell JB, Xavier S, DeLuca AM, Sowers AL, Cook JA, Krishna MC, Hahn SM, Russo A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free radical biology & medicine. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 35*.Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nature communications. 2013;4:2384. doi: 10.1038/ncomms3384. [This study reports that alteration of gut microbiome impacts bile acid composition, FXR signaling and metabolic homeostasis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin BC, Wang M, Blackmore C, Desnoyers LR. Liver-specific activities of FGF19 require Klotho beta. The Journal of biological chemistry. 2007;282:27277–27284. doi: 10.1074/jbc.M704244200. [DOI] [PubMed] [Google Scholar]
- 37.Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y, Nabeshima Y. Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. The Journal of clinical investigation. 2005;115:2202–2208. doi: 10.1172/JCI23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Li S, Hsu DD, Li B, Luo X, Alderson N, Qiao L, Ma L, Zhu HH, He Z, Suino-Powell K, Ji K, Li J, Shao J, Xu HE, Li T, Feng GS. Cytoplasmic Tyrosine Phosphatase Shp2 Coordinates Hepatic Regulation of Bile Acid and FGF15/19 Signaling to Repress Bile Acid Synthesis. Cell Metab. 2014;20:320–332. doi: 10.1016/j.cmet.2014.05.020. [This study identified a key signaling component in mediating the FGF15/19 regulation of bile acid synthesis in the hepatocytes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S, Hsu DD, Wang H, Feng GS. Dual faces of SH2-containing protein-tyrosine phosphatase Shp2/PTPN11 in tumorigenesis. Frontiers of medicine. 2012;6:275–279. doi: 10.1007/s11684-012-0216-4. [DOI] [PubMed] [Google Scholar]
- 40.Chan RJ, Feng GS. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007;109:862–867. doi: 10.1182/blood-2006-07-028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Wang C, Yang C, Chang JY, You P, Li Y, Jin C, Luo Y, Li X, McKeehan WL, Wang F. Hepatocyte FRS2alpha is Essential for the Endocrine Fibroblast Growth Factor to Limit the Amplitude of Bile Acid Production Induced by Prandial Activity. Current molecular medicine. 2014;14:703–711. doi: 10.2174/1566524014666140724095112. [This study identified FRS as a novel signaling component in mediating the FGF15/19 regulation of bile acid synthesis in the hepatocytes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bard-Chapeau EA, Li S, Ding J, Zhang SS, Zhu HH, Princen F, Fang DD, Han T, Bailly-Maitre B, Poli V, Varki NM, Wang H, Feng GS. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer cell. 2011;19:629–639. doi: 10.1016/j.ccr.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong B, Huang J, Zhu Y, Li G, Williams J, Shen S, Aleksunes LM, Richardson JR, Apte U, Rudnick DA, Guo GL. Fibroblast growth factor 15 deficiency impairs liver regeneration in mice. American journal of physiology. Gastrointestinal and liver physiology. 2014;306:G893–902. doi: 10.1152/ajpgi.00337.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uriarte I, Fernandez-Barrena MG, Monte MJ, Latasa MU, Chang HC, Carotti S, Vespasiani-Gentilucci U, Morini S, Vicente E, Concepcion AR, Medina JF, Marin JJ, Berasain C, Prieto J, Avila MA. Identification of fibroblast growth factor 15 as a novel mediator of liver regeneration and its application in the prevention of post-resection liver failure in mice. Gut. 2013;62:899–910. doi: 10.1136/gutjnl-2012-302945. [DOI] [PubMed] [Google Scholar]
- 45.Luo J, Ko B, Elliott M, Zhou M, Lindhout DA, Phung V, To C, Learned RM, Tian H, DePaoli AM, Ling L. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Science translational medicine. 2014;6:247ra100. doi: 10.1126/scitranslmed.3009098. [DOI] [PubMed] [Google Scholar]
- 46.Mouzeyan A, Choi J, Allayee H, Wang X, Sinsheimer J, Phan J, Castellani LW, Reue K, Lusis AJ, Davis RC. A locus conferring resistance to dietinduced hypercholesterolemia and atherosclerosis on mouse chromosome 2. Journal of lipid research. 2000;41:573–582. [PubMed] [Google Scholar]
- 47.Phan J, Pesaran T, Davis RC, Reue K. The Diet1 locus confers protection against hypercholesterolemia through enhanced bile acid metabolism. The Journal of biological chemistry. 2002;277:469–477. doi: 10.1074/jbc.M107107200. [DOI] [PubMed] [Google Scholar]
- 48**.Vergnes L, Lee JM, Chin RG, Auwerx J, Reue K. Diet1 functions in the FGF15/19 enterohepatic signaling axis to modulate bile acid and lipid levels. Cell Metab. 2013;17:916–928. doi: 10.1016/j.cmet.2013.04.007. [This study revealed that Diet1 is a novel regulator of entherohepatic FGF15/19 signaling in the regulation of bile acid homeostasis and provided a molecular link between Diet1 gene and atherogenesis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCarter SD, Johnson DL, Kitt KN, Donohue C, Adams A, Wilson JM. Regulation of tight junction assembly and epithelial polarity by a resident protein of apical endosomes. Traffic. 2010;11:856–866. doi: 10.1111/j.1600-0854.2010.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168:1531–1540. doi: 10.1001/archinte.168.14.1531. [DOI] [PubMed] [Google Scholar]
- 51.Staels B, Kuipers F. Bile acid sequestrants and the treatment of type 2 diabetes mellitus. Drugs. 2007;67:1383–1392. doi: 10.2165/00003495-200767100-00001. [DOI] [PubMed] [Google Scholar]
- 52.Garg A, Grundy SM. Cholestyramine therapy for dyslipidemia in noninsulin-dependent diabetes mellitus. A short-term, double-blind, crossover trial. Annals of internal medicine. 1994;121:416–422. doi: 10.7326/0003-4819-121-6-199409150-00004. [DOI] [PubMed] [Google Scholar]
- 53.Brufau G, Stellaard F, Prado K, Bloks VW, Jonkers E, Boverhof R, Kuipers F, Murphy EJ. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52:1455–1464. doi: 10.1002/hep.23831. [DOI] [PubMed] [Google Scholar]
- 54.Sonne DP, Hansen M, Knop FK. Bile acid sequestrants in type 2 diabetes: potential effects on GLP1 secretion. European journal of endocrinology / European Federation of Endocrine Societies. 2014;171:R47–65. doi: 10.1530/EJE-14-0154. [DOI] [PubMed] [Google Scholar]
- 55.Holst JJ. The physiology of glucagon-like peptide 1. Physiological reviews. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 56.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. The American journal of physiology. 1997;273:E981–988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 57.Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide 1(7-36) amide's central inhibition of feeding and peripheral inhibition of drinking are abolished by neonatal monosodium glutamate treatment. Diabetes. 1998;47:530–537. doi: 10.2337/diabetes.47.4.530. [DOI] [PubMed] [Google Scholar]
- 58.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 59.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harach T, Pols TW, Nomura M, Maida A, Watanabe M, Auwerx J, Schoonjans K. TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Scientific reports. 2012;2:430. doi: 10.1038/srep00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potthoff MJ, Potts A, He T, Duarte JA, Taussig R, Mangelsdorf DJ, Kliewer SA, Burgess SC. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. American journal of physiology. Gastrointestinal and liver physiology. 2013;304:G371–380. doi: 10.1152/ajpgi.00400.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hofmann AF. Bile acid sequestrants improve glycemic control in type 2 diabetes: a proposed mechanism implicating glucagon-like peptide 1 release. Hepatology. 2011;53:1784. doi: 10.1002/hep.24100. [DOI] [PubMed] [Google Scholar]
- 63.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Annals of surgery. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Annals of surgery. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miras AD, le Roux CW. Can medical therapy mimic the clinical efficacy or physiological effects of bariatric surgery? International journal of obesity. 2014;38:325–333. doi: 10.1038/ijo.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]