Abstract
The incidence of atrial fibrillation (AF) increases with age. Alterations in structure and function of atrial ion channels associated with aging provide the substrate for AF. In this review we provide an overview of current knowledge regarding these age-related changes in atria, focusing on intrinsic ion channel function, impulse initiation and conduction. Studies on the action potentials (APs) of atria have shown that the AP contour is altered with age and the dispersion of AP parameters is increased with age. However, studies using human tissues are not completely consistent with experimental animal studies, since specimens from humans have been obtained from hearts with concomitant cardiovascular diseases and/or that are under the influence of pharmacologic agents. Ionic current studies show that while there are no age-related changes in sodium currents in atrial tissue, the calcium current is reduced and the transient outward and sustained potassium currents are increased in aged cells. While sinoatrial node firing is reduced with age, enhanced impulse initiation may occur in aged atrial cells, for example in the pulmonary veins and coronary sinus. Fibrous tissue is increased in aged atria, which is associated with an increased likelihood of abnormal electrical conduction. Thus, age-related AF involves alterations in the substrate as well as in the passive properties of aged atria.
Keywords: Age, Atria, Ion channels, Calcium, Atrial fibrillation
1 Introduction
The objective of this article is to provide an overview of current knowledge regarding age-related changes in atria, focusing on intrinsic ion channel function, impulse initiation and conduction. It is well established that the incidence and prevalence of atrial fibrillation (AF) increase with age. This observation raises the question: are there changes in intrinsic ion channel function, impulse initiation, and impulse conduction that predispose aged atria to AF?
2 Substrate changes
2.1 Action potential (AP)
In laboratory animals, action potential durations (APDs) in atrial tissue are prolonged with age, thus providing a substrate for reentry. However, clear-cut evidence for age-related changes in action potentials (APs) in normal human atria is lacking, since most studies have been done in patients with significant heart disease, or who are taking various cardiovascular medications. In addition, although some studies have included patients up to 70 years of age, few have included patients over age 70, and none have involved patients 80 years or older. For example, Brorson et al. [1] used four age groups to study right atrial (RA) effective refractory periods (ERP) and monophasic action potentials (MAP) in healthy male humans in vivo. Group one was 25–34 years, group two 35–44 years, group three 45–54 years and group four 55–64 years. They found that group three (45–54 years) showed a significantly longer RA ERP than the others. However, there was no progressive trend in APD with age. Interestingly, the ERP of the oldest group (55–64 years) was comparable to that of the younger age groups. Similarly, the duration of MAP was significantly longer in group three during sinus rhythm and there was no progressive trend with age. Thus, these results do not support the view that APDs are prolonged with age. Spach et al observed the shape of RA APs at different ages (2, 10, 43 and 62 year old subjects) [2]. A similar “spike and hump” AP configuration was demonstrated at all ages (for example see Fig. 1). Although a quantitative analysis of the size of the notch was not performed, the authors suggested that the qualitative similarities in AP shape strongly suggested that there were no significant differences in the basic ionic mechanisms that control the AP shape in atrial pectinate muscle bundles from subjects varying between 1 and 70 years of age [2]. These results imply that passive properties determine propagation and the arrhythmogenic behavior of aged human atria. Notably, left atrial (LA) APs were not studied.
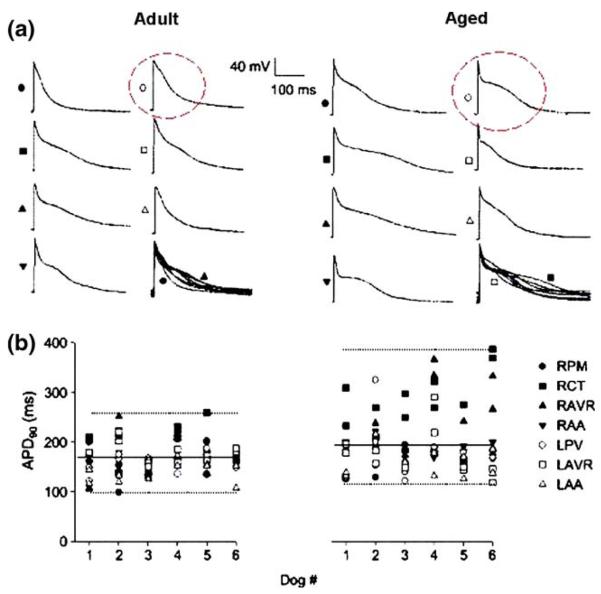
Fig. 1.
Heterogeneity of action potential duration (APD) in adult and aged dogs in normal sinus rhythm. (a) Transmembrane potentials recorded from seven regions of one adult (left) and one aged (right) dog at a cycle length of 1000 ms. In the right lower image at each age, the upstrokes of all action potentials (APs) are superimposed to facilitate comparison of the extent of dispersion. (b) Values of APD at 90% repolarization (APD90) obtained from each impalement in all regions are shown at a cycle length of 1000 ms for each adult and aged dog. Dotted lines are drawn for maximum and minimum values in each group; solid lines represent APD90 averaged over all regions. Two impalements were made in each preparation. Preparations from four regions of the right atrium-pectinate muscles (RPM), crista terminalis (RCT), atrioventricular ring (RAVR) and appendage (RAA)–and three regions of the left atrium–free wall near the pulmonary veins (LPV), atrioventricular ring (LAVR) and appendage (LAA)–were dissected in each dog. Reproduced from reference [7]
Toda [3] reported that APD90 (i.e. APD at 90% repolarization) at day 360 (aged) was significantly longer than that at day 90 (adult) in isolated rabbit RA (Table 1) without concomitant changes in APD10, resting potential, or AP overshoot. No data were reported for animals over 360 days old. Su et al. [4] determined the maximum diastolic potential (MDP), APD50 and APD95 in adult (6–8 months) and aged (26–28 months) rat RA tissues. The MDP was significantly depolarized in atria of aged (−66.8±1.5 mV) versus adult (−76.4±1.8 mV) rats (Table 1). Notably, APD50 and APD95 repolarization did not vary significantly with age. Interestingly, carbachol-induced hyperpolarization was significantly greater in aged compared to adult rats. This age-associated enhancement in carbachol-induced hyperpolarization may be due to a greater increase in IKACh (acetylcholine-induced potassium conductance) in aged atria, since the key mechanism for hyperpolarization of MDP is activation of IKACh through stimulation of muscarinic cholinergic receptors in the atria. More recently, Huang et al. [5] used Langendorff-perfused rat hearts to record MAPs and ERPs of RA and LA epicardium from rats in 3 age groups: adult (7–8 months), middle-aged (13–15 months) and aged (over 2 years). Both MAPD90 and ERPs were prolonged with age in RA (S1S1 = 400 ms) (Table 1), but in LA they were shorter in the aged group than in the other 2 groups. Furthermore, there were no differences in MAPD90 or ERP between the RA and LA in the adult and middle-aged groups, but in the aged group the MAPD90 and ERP of RA were significantly longer than in the LA. Importantly, in the aged group, the MAPD90 of the RA shortened by 38% as the stimulation frequency increased, while LA MAPDs only shortened 11%. Thus with age it appears that the RA MAPD is more sensitive to rate dependent shortening than the LA MAPD.
Table 1.
Comparison of electrophysiological characteristics between adult and age right atria
| Parameters | Adult | Age | Species | References |
|---|---|---|---|---|
| AP | ||||
| MDP(mV) | −76.4±1.8 | −66.8±1.5* | Rat | [4] |
| ~ −77 | ~ −72* | Canine | [7] | |
| APD90(ms) | ~ 130 | ~ 150* | Rabbit | [3] |
| 125±9 | 146±18* | Rat | [5] [7] | |
| ~ 175 | ~ 200* | Canine | ||
| ERP(ms) | 75±6 | 86±4* | Rat | [5] |
| Vmax | ~ 180 | ~ 145* | Canine | [7] |
| APA(mV) | 95±2 | 84±3* | Canine | [7] |
| I Na | ||||
| Density(pA/pF) | 14.3±1.2 | 17.5±1.8 | Canine | [8] |
| I Ca | ||||
| Density(pA/pF) | 19±2.1 | 12±1.5* | Canine | [9] |
| I to | ||||
| Density(pA/pF) at 50 mV | 9.9±0.1 | 12.4±0.1* | Canine | [9] |
| 8.6±0.9(LA) | 11.9±2.1(LA) | Canine | [11] | |
| Decay | τ1(ms): 9.9±0.1 | 12.4±0.1* | Canine | [9] |
| τ2(ms): 47.1±0.5 | 58.8±0.7* | |||
| Steady-state inactivation | V0.5(mV): −34.9±1.8 | −29.8±1.4* | Canine | [9] |
| Recovery from inactivation | τ1(ms): 45.6±5.6 | 76.6±6.8* | Canine | [9] |
| τ2(ms): 313.1±45.6 | 1083.3±162.4* | |||
| I sus | ||||
| Density(pA/pF) at 50 mV | 4.4±0.3 | 5.6±0.5* | Canine | [9] |
| 3.3±0.2(LA) | 2.9±0.3(LA) | Canine | [11] |
AP=action potential; MDP=maximal diastolic potential; APD90=action potential duration to 90% repolarization; ERP=effective refractory period; Vmax= maximum upstroke velocity; INa= sodium current; ICa= calcium current; Ito= transient outward potassium current; Isus= sustained potassium current. LA= left atria.
P<0.05 Age versus Adult.
Anyukhovsky et al. [6] used microelectrode techniques to record APs from the endocardium of the RA free wall of dogs 1–5 years (adult) and >8 years (aged). They found that plateau potential was more negative and that APD90 was longer in the aged versus the adult tissue (Table 1). The differences in APD90 and plateau voltage between the two groups were rate-dependent. APD30 was shorter and rate-dependent in aged atria. The calcium channel blocker nisoldipine markedly lowered the plateau in adults but had no effect on plateau height in aged atria, whereas the ICa,L agonist Bay K 8644 elevated the plateau more prominently in aged tissue. These data suggest that a decrease in the calcium current may be a mechanism for the low plateau voltages in aged RA tissue. Anyukhovsky et al. [7] also recorded APs from four regions of the RA (crista terminalis, appendage, atrioventricular ring and pectinate muscles) and three regions of the LA (appendage, atrioventricular ring and free wall near the pulmonary veins) in dogs of different ages (Fig. 1 (a)). APD90 averaged across all regions was significantly longer in aged compared to adult tissues (Fig. 1 (b)). Notably, the range of APD90 values was wider in the aged group versus adults as a result of an increase in duration occurring mainly in the RA fibers. Statistically, both the standard deviation and the coefficient of variation of APD heterogeneity were higher in aged dogs compared to adults [7]. This increased heterogeneity of atrial repolarization may create a substrate for initiation of AF in aged canines. Interestingly, MDP is significantly more depolarized, Vmax is slower and total AP amplitude is lower in aged than in adult tissues (Table 1)[7] perhaps contributing to slowed conduction.
From the studies described above in different species, we conclude that APD90 and ERP are prolonged and continue to show rate-dependence with age in the RA. In the LA, APs shorten with age and are less rate-dependent, although confirmation by other groups and extension to other species is needed.
2.2 Ion currents
2.2.1 Sodium current (INa)
Our laboratory was the first to study age-related changes in INa in canine atrial cells [8]. We found that INa density in the LA was greater than in the RA in both adult and aged atrial cells (Fig. 2 (a)), and that there were no age-related changes in INa density in either the RA or LA. Thus, the difference in INa density between atrial chambers is unaffected by age (Table 1). INa kinetic differences between aged and adult cells include a significant acceleration into the inactivated state and an enhanced use-dependent decrease in peak-current in aged RA cells. However, in the LA there is no significant difference in the rate of development of inactivation of INa between aged and adult cells. Other kinetic parameters (activation, steady-state inactivation, and recovery from inactivation) of INa are not altered by age in either RA or LA cells [8]. Immunocytochemistry data show that Nav1.5 (the protein underlying the α subunit of the major cardiac sodium channel) staining in RA and LA cells from adult and aged atria is uniform, with staining all along the sarcolemma as well as in the gap junction region [8]. Therefore, there is no structural remodeling of the cardiac Na+ channel protein Nav1.5 in aged canine atrial cells. Currently there are no data in humans examining the effect of age on atrial Na+ channel remodeling.
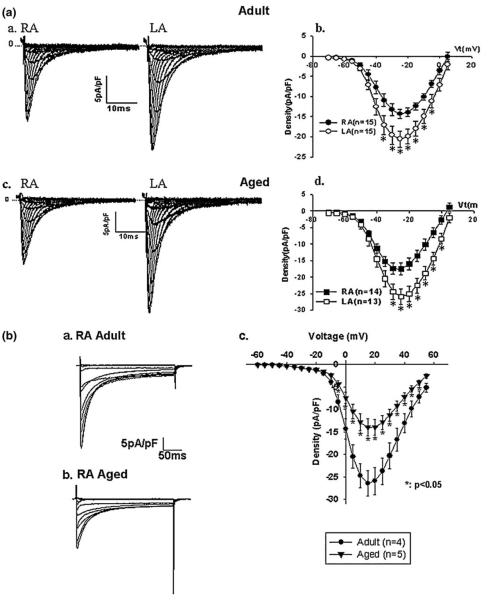
Fig. 2.
(a), Family of tracings of Na+ current (INa) in right (RA) and left (LA) atrial cells from adult (Panel a) and aged (Panel c) dogs. INa density-voltage relationship in RA and LA of adult (Panel b) and aged (Panel d) dogs. Reproduced from reference [8]. 2 (b), Current tracings in an adult (Panel a) and aged (Panel b) right atrial cell from dogs under conditions of this experiment: 3 mM Ca2+/10 mM BAPTAi(internal BAPTA concentration), 2 μM ryanodine, holding voltage of−70 mV to various test voltages. Panel c shows the average peak ICaL density in adult and aged cells. Reproduced from reference [9]
2.3 Calcium currents (ICa)
The effect of age on basal calcium (Ca2+) currents differs. Our laboratory has reported that L-type Ca2+ currents with either Ca2+ (ICaL) or Ba2+ (IBaL) as the charge carrier are significantly reduced by 47% and 43% respectively in aged canine RA cells compared to adults (Fig. 2 (b), Table 1)[9]. The current reduction in aged cells was unaccompanied by a significant change in calcium channel availability or recovery from inactivation. Isoproterenol abolished the differences in IBaL between aged and adult cells, suggesting that adrenergic stimulation can restore Ba2+ currents in aged cells [9]. Using the chelator EGTA with Ca2+ as the charge carrier, Tipparaju et al. [10] also reported that basal ICa and maximal ICa produced by isoproterenol were similar in RA cells from young adult (16±1 year old) and aged patients (66±3 year old). These findings suggest that there may be a pronounced Ca2+-dependent inactivation of ICaL in atrial cells under these recording conditions. There are no published data on the effects of age on LA ICaL.
2.4 Potassium currents
The transient outward (Ito) and sustained potassium current (Isus) densities are significantly increased in aged canine RA cells compared to adult cells (Fig. 3, Table 1)[9]. Kinetic studies of Ito show that Ito decay is slowed and that the steady-state inactivation curve is shifted positively in aged cells. Further, TEA (tetra-ethylammonium)-sensitive current densities of Ito and Isus are greater in aged versus adult cells. However, KN-93, a specific CaMKII inhibitor, produces increases in Ito in both adult and aged RA cells, while dramatically inhibiting Isus in aged compared to adult RA cells. Thus, increased Isus in aged RA cells is most likely due to CaMKII upregulation. Interestingly, upregulated Ito and Isus are not observed in aged LA cells [11]. To our knowledge, no studies have examined the function and molecular basis of other potassium currents (IKr,IKs,IK1, IK(ATP)) from adult and aged atrial cells.
Fig. 3.
K+ currents in cells dispersed from an adult (Panel a) and aged (Panel b) dog right atrial cell. (Panel c) and (d) show average current-voltage relations of Ito and ISUS densities in adult and aged right atrial cells. Reproduced from reference 9
3 Abnormal impulse initiation
Increasing age is associated with abnormal impulse initiation. For example, it is well known that sinoatrial node (SAN) dysfunction increases with age [12]. If the sinus node fails to fire, then there is an increased likelihood that atrial cells with abnormal impulse initiation will become manifest.
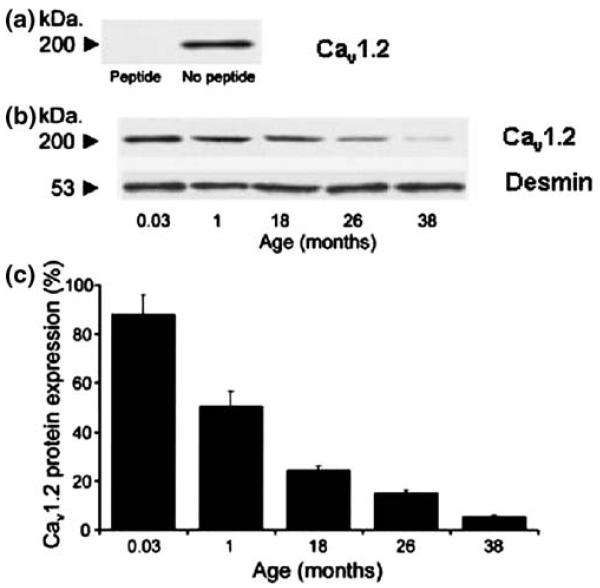
To our knowledge, there are no studies available that illustrate the effects of age on the function of If in the atrium. However, recent studies on guinea pigs suggest that the fundamental reason for a change in SAN function with age is that expression of the Cav1.2 protein (protein underlying the pore-forming unit of the cardiac calcium channel) within the SAN declines during aging (Fig. 4) [13]. This change is accompanied by a reduction in SAN electrical activity. These findings are consistent with observations that the L-type Ca2+ current provides Ca2+ influx into the SAN cell and that Ca2+ release may control SAN pacemaker function [14]. The status of the other cardiac isoform of this protein, Cav1.3, was not assessed in this study. Importantly, areas of atrial tissue staining for a major gap junctional protein (Cx43) also decreased with age in this model [15]. Thus, age-associated sinus brady-cardia may be due to declines in Ca2+ channel protein and Cx43 expression. The mechanisms underlying these changes are unknown.
Fig. 4.
The decline of Cav1.2 protein expression within the sinoatrial (SA) node with increased age in guinea pigs. (a). Specificity of the antibody anti-CNC1 to determine Cav1.2 protein expression was confirmed by the absence of the 200-kDa band in the presence of the supplied control antigen. (b). A typical blot illustrates the expression of desmin (53 kDa) and Cav1.2 (200 kDa) proteins across the examined age range. (c) For every animal from each age group, Cav1.2 protein expression in the SA node region was expressed as a percentage of the paired atrial muscle sample. Data show mean±SEM. Cav1.2 protein expression fell in the SA node region vs age (n=5; ANOVA, P<0.01; y=−1.8363x+67.024; R2=0.81). Reproduced from reference [13]
If the SAN has a reduced rate of firing, then a population of atrial cells that exhibit abnormal pacemaker function may be revealed. Canine atrial fibers, while showing a phenotypic change with age (see section above on substrate changes), apparently show no increased or de novo pacemaker activity with age [16]. On the other hand, several cell regions within the atria show triggered activity both in vivo and in vitro, prompting the question: does the capability for triggered activity increase with age? Over 25 years ago, studies on Purkinje fibers from aged canines demonstrated an increased susceptibility to the Na-K pump inhibitor ouabain [17], manifested by delayed after-depolarizations (DADs) and thus increased potential for triggered activity. Since then it has become clear that abnormal intracellular Ca2+ handling underlies the Ca2+ waves that give rise to DADs [18]. In specialized atriovenous junctions, groups of pulmonary vein (PV) cells, coronary sinus cells or mitral valve cells also show triggered activity [19], and recent data suggest that these types of cells can show enhanced DADs in tissues from aged animals (Fig. 5) [20',21]. Interestingly, in both of these studies, a pharmacological agent was needed to provoke the DADs and thus abnormal impulse initiation (glycolytic inhibition or ouabain). In fact, in one study both early after-depolarizations (EADs) and DADs were induced [21]. From our own work, we have reported that there is a change in AP phenotype that occurs with age in the canine (see Fig. 1(a)) such that APs of left PV cells (within circles) show an increased plateau and APD with age. Given the strict relation between APD and the ability of cells to show DADs [19], it follows that the mechanism of enhanced DADs in PV cells from aged animals may be primarily due to changes in APD that occur with age.
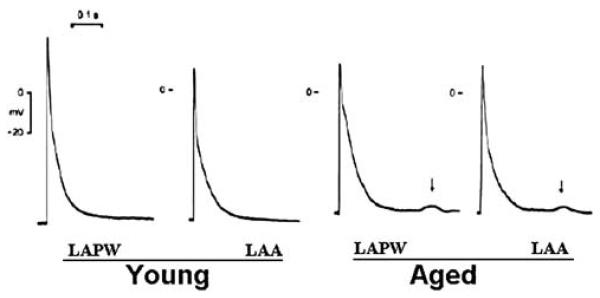
Fig. 5.
Tracings showing examples of action potentials from young and aged rabbit left atrial posterior walls (LAPWs) and left atrial appendages (LAAs). The delayed after-depolarizations (DADs) (↓) were recorded in the aged LAPW and LAA, but not in young LAPWand LAA, with a 2-Hz electrical stimulus. Reproduced from reference [20]
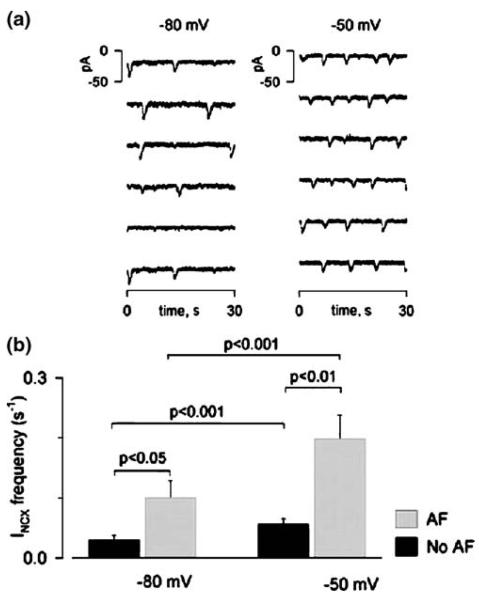
While it is clear that abnormal intracellular Ca2+ dynamics may underlie enhanced DADs in specialized aged atrial cells, as yet there has been no systematic study of intracellular Ca2+ handling in atrial cells from aged human atria. In old rats, one report [21] suggested that the paced Ca2+ transient is delayed in aged animals versus the young, but as noted above, this may be just a reflection of an age-related change in APD. In experimental animal models, there has been no systematic study of the effects of age alone on the components of atrial excitation-contraction (EC) coupling. Perhaps this is a challenge since unlike ventricular cells, normal EC coupling in adult atrial cells involves a coordinated release from two subcellular compartments [18]. However, a recent study in older patients (average 66 years) with AF [22] reported increased Ca2+ spark frequency (RA cells), increased Ca2+ wave frequency, spontaneous inward Na-Ca exchange current (INCX), and DADs (Fig. 6) relative to patients in the same age range who were in sinus rhythm (but with coronary artery bypass surgery, aortic valve replacement, and/or mitral valve replacement). Comparisons between atrial Ca2+ spark characteristics in younger and older patients with sinus rhythm have not been done for atrial cells, but data are available for aged ventricular cells [23].
Fig. 6.
Effect of holding potential on spontaneous INCX frequency in human atrial cells. (a). Switching holding potential from−80 to−50 mV increased the number of spontaneous INCX.(b). Average INCX frequencies at−80 and−50 mV in the two patient groups. Patients with atrial fibrillation (AF) (66.4±11.2 years) had more spontaneous INCX than those without AF (65.5±13.2 years). Reproduced from reference [22]
The mechanism of enhanced Ca2+ wave and spark formation in AF atria from aged patients has been examined, and one study suggests that ryanodine receptor dysfunction (hyperphosphorylation) [24] occurs in human atria from older AF patients (mean 65 years old). However, due to the lack of a young age control group, it is not clear whether the cause of the ryanodine receptor change is due to age, the presence of AF, or another mechanism. The molecular underpinnings for these changes are also unknown. Some have suggested that aging reduces the activity of the sarcoplasmic reticulum calcium (SERCA) pump (a major protein needed to transport Ca2+ back into the sarcoplasmic reticulum) [25]. Recent data using aged human atrial tissues (mean 68 years) suggest that abnormal Ca2+ handling is due to impaired phosphorylation-dependent regulation of many Ca2+ handling proteins, one of which is phospholamban [26]. Again, whether levels of phosphorylation of these proteins change with age alone has not been evaluated.
In summary, data on the effects of age alone on the cell properties of specialized atrial cells suggest that enhanced impulse initiation may occur in such cells, while SAN firing is reduced. However, the specific mechanisms for these changes remain unknown.
4 Abnormal impulse conduction
To understand changes in conduction in the aged atria, it is important to comprehend the nature of both the electrical and structural remodeling that occur with age. In particular, the profound structural remodeling that occurs in aged atria affects the ability of a premature beat that is triggered in the PV cells, for example, to penetrate the substrate and initiate propagation.
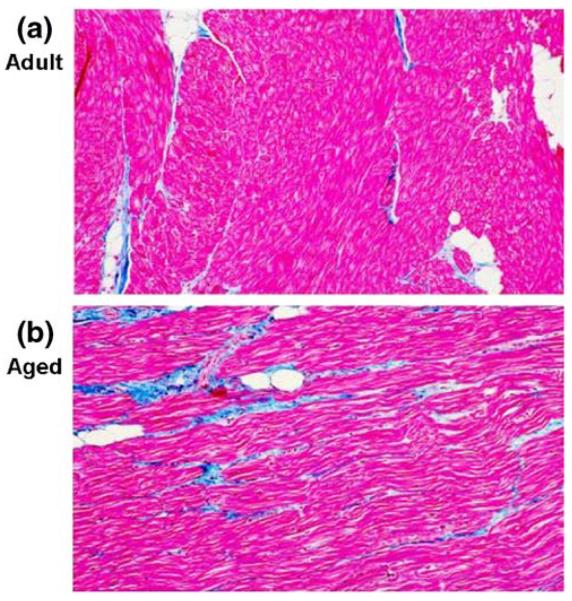
The most significant change in aged atrial bundles is the increased content of fibrous tissue interspersed between the atrial cells (Fig. 7) [6] and characterized by accumulation of collagen in the extracellular space. The impact of remodeling of the atrial microstructure on impulse conduction in atrial bundles isolated from patients with no atrial arrhythmias has been studied by Spach et al. [27-31]. Recent data from this laboratory [31] suggest that arrhythmogenic conduction depends on the atrial microarchitecture, the precise location of the premature beat within the atrial bundle, and its precise timing.
Fig. 7.
Representative sections of right atrial (RA) pectinate muscle bundles from an adult (a) and aged (b) dog showing myocytes in red and collagen in blue. Image analysis of these and additional fields showed the amount of fibrous tissue in adult atrium = 4.5% (a) and the amount of diffuse interstitial fibrous tissue in old atrium = 17.9% (b) of the myocardium. Masson trichrom stain used. Reproduced from reference [6]
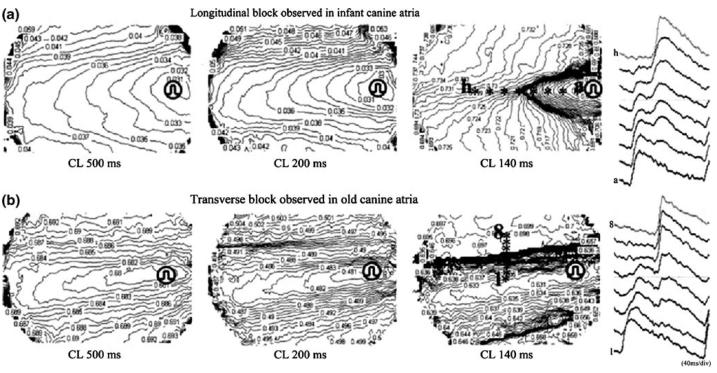
Another important effect of increased fibrosis is a change from typical uniform anisotropic conduction to non-uniform conduction [27]. Studies in human atria (17 to 75 years) show an age-related reduction in wavefront velocities in both atria [32], but only septal refractoriness correlated with age. In another study [33], changes in anisotropic conduction properties in atrial tissue were quantified in infant, young and aged canines (Fig. 8). This study showed that age-related fibrosis preferentially affects transverse conduction (Table 2). Furthermore, as discussed above, this abnormal conduction pattern is not due to a change in intrinsic Na+ channel function.
Fig. 8.
Different patterns of conduction block in infant and old canines. The activation sequences of atrial tissue (optical images) of an infant and an old dog under stimulation at cycle length (CL) 500 ms, 200 ms, and 140 ms are presented by 1-ms isochrones. Conduction block at short (140 ms) CL occurred in the longitudinal (L) direction in the infant dog (a), whereas it was observed in the transverse (T) direction in the old dog (b). Eight action potentials recorded across the block line (a to h in the infant tissue, and 1 to 8 in the old tissue) are shown on the right. Reproduced from reference [33]
Table 2.
Incidence of longitudinal and transverse block in infant, young and old dogs
| Longitudinal Block |
Transverse Block |
Whole Refractorinessa |
|
|---|---|---|---|
| Infant (n=10) | 40%(4/10)b | 20%(2/10)b | 40%(4/10) |
| Young(n=11) | 27%(3/11) | 36%(4/11) | 36%(4/11) |
| Old(n=10) | 0%(0/10) | 50%(5/10) | 50%(5/10) |
Activation ceased simultaneously in all directions because of refractoriness
P<0.05 for infant vs old (X2 test). Reproduced from reference [33]
The implication of these studies is that as collagen is laid down between atrial bundles, muscle cells and bundles become electrically isolated, forcing impulse propagation along nontraditional alternative pathways. In guinea-pig hearts, protein quantification by immunofluorescence and Western blot confirmed a significant loss of Cx43 with age in SAN tissue but not in RA tissue [15]. The expression of other cardiac connexins, Cx40 and Cx45, was unaffected by age in either SAN or RA tissues. However, connexin quantity cannot always be equated with altered function of gap junctions. Thus, regional studies on the effects of age on intercellular connections in both the RA and LA are needed.
5 Summary
Age related changes in both Ca2+ and K+ currents underlie specific AP changes in RA but not LA cells. It is unlikely that conduction changes in aged atrial tissues are due to changes in intrinsic sodium channel function, but rather are due to the complex interplay between age-related fibrosis (whether patchy or diffuse), wavefront velocities, and increased likelihood of block. AF will be initiated if and only if suitable electrophysiologic conditions are present, including a trigger or initiation of an ectopic beat, propagation of the waveform to atrial tissue, and perpetuation of the dysrhythmia through a re-entrant circuit. Age-related changes in atrial tissue increase the likelihood that these conditions will be present in older compared to younger hearts.
Acknowledgments
Supported by grant HL58860 and HL66140 from the National Heart Lung and Blood Institute Bethesda, Maryland
Ionic currents
- IBaL
Barium, L-type
- ICaL
Calcium, L-type
- If
funny current, pacemaker current
- IK1
Potassium, inward rectifier current
- IKACh
Potassium, acetylcholine-induced
- IK(ATP)
Potassium, ATP modulated
- IKr
Potassium, rapid delayed rectifier
- IKs
Potassium, slow delayed rectifier
- INa
Fast Sodium
- INCX
Sodium-calcium exchanger
- Isus
Potassium, sustained
- Ito
Potassium, transient outward
References
- 1.Brorson L, Ollsson SB. Right atrial monophasic action potentials in healthy males, studies during spontaneous sinus rhythm and atrial pacing. Acta Medica Scandinavica. 1976;199(6):433–446. doi: 10.1111/j.0954-6820.1976.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 2.Spach MS, Dolber PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circulation Research. 1988;62(4):811–832. doi: 10.1161/01.res.62.4.811. [DOI] [PubMed] [Google Scholar]
- 3.Toda N. Age related changes in the transmembrane potential of isolated rabbit sino-atrial nodes and atria. Cardiovascular Research. 1980;14(1):58–63. doi: 10.1093/cvr/14.1.58. [DOI] [PubMed] [Google Scholar]
- 4.Su N, Duan J, Moffat MP, Narayanan N. Age related changes in electrophysiological responses to muscarinic receptor stimulation in rat myocardium. Canadian Journal of Physiology and Pharmacology. 1995;73(10):1430–1436. doi: 10.1139/y95-199. [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Ding W, Li L, Zhao D. Differences in the aging associated trends of the monophasic action potential duration and effective refractory period of the right and left atria of the rat. Circulation Journal. 2006;70(3):352–357. doi: 10.1253/circj.70.352. [DOI] [PubMed] [Google Scholar]
- 6.Anyukhovsky EP, Sosunov EA, Plotnikov A, Gainullin RZ, Jhang JS, Marboe CC, et al. Cellular electrophysiologic properties of old canine atria provide a substrate for arrhythmogenesis. Cardiovascular Research. 2002;54(2):462–469. doi: 10.1016/s0008-6363(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 7.Anyukhousky EP, Sosunov EA, Chandra P, Rosen TS, Boyden PA, Danilo P, Jr, et al. Age-associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovascular Research. 2005;66(2):353–363. doi: 10.1016/j.cardiores.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Baba S, Dun W, Hirose M, Boyden PA. Sodium current function in adult and aged canine atrial cells. American Journal of Physiology. Heart and Circulatory Physiology. 2006;291(2):H756–H761. doi: 10.1152/ajpheart.00063.2006. [DOI] [PubMed] [Google Scholar]
- 9.Dun W, Yagi T, Rosen MR, Boyden PA. Calcium and Potassium currents in cells from adult and aged canine right atria. Cardiovascular Research. 2003;58(3):526–534. doi: 10.1016/s0008-6363(03)00288-8. [DOI] [PubMed] [Google Scholar]
- 10.Tipparaju SM, Kumar R, Wang Y, Joyner RW, Wagner MB. Developmental differences in L-type calcium current of human atrial myocytes. American Journal of Physiology. Heart and Circulatory Physiology. 2004;286(5):H1963–H1969. doi: 10.1152/ajpheart.01011.2003. [DOI] [PubMed] [Google Scholar]
- 11.Dun W, Baba S, Boyden PA. Aged-related changes in transient outward and sustained currents in canine right atrial freewall cells are not seen in left atrial cells. Molecular Pathology of Cardiac Arrhythmias; Keystone Symposia: 2003. [Google Scholar]
- 12.Lakatta EG, Sollott SJ. The “Heartbreak” of Older Age. Molecular Interventions. 2002;2(7):431–446. doi: 10.1124/mi.2.7.431. [DOI] [PubMed] [Google Scholar]
- 13.Jones SA, Boyett MR, Lancaster MK. Declining Into Failure: The Age-Dependent Loss of the L-Type Calcium Channel Within the Sinoatrial Node. Circulation. 2007;115(10):1183–1190. doi: 10.1161/CIRCULATIONAHA.106.663070. [DOI] [PubMed] [Google Scholar]
- 14.Lyashkov AE, Juhaszova M, Dobrzynski H, Vinogradova TM, Maltsev VA, Juhasz O, et al. Calcium Cycling Protein Density and Functional Importance to Automaticity of Isolated Sinoatrial Nodal Cells Are Independent of Cell Size. Circulation Research. 2007;100(12):1723–1731. doi: 10.1161/CIRCRESAHA.107.153676. [DOI] [PubMed] [Google Scholar]
- 15.Jones SA, Lancaster MK, Boyett MR. Ageing-related changes in connexins and conduction within the sinoatrial node. The Journal of Physiology. 2004;560(Pt 2):429–437. doi: 10.1113/jphysiol.2004.072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grammer JB, Zeng X, Bosch RF, Kuhlkamp V. Atrial L-type Ca2+-channel, b-adrenoreceptor, and 5-hydroxytrptamine type 4 receptor mRNAs in human atrial fibrillation. Basic Research in Cardiology. 2001;96(1):82–90. doi: 10.1007/s003950170081. [DOI] [PubMed] [Google Scholar]
- 17.Hewett K, Vullimoz Y, Rosen MR. Senescence related changes in the responsiveness to ouabain of canine Purkinje fibers. The Journal of Pharmacology and Experimental Therapeutics. 1982;223(1):153–156. [PubMed] [Google Scholar]
- 18.Ter Keurs HEDJ, Boyden PA. Calcium and Arrhythmogenesis. Physiological Reviews. 2007;87(2):457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cranefield PF, Aronson RS. Cardiac Arrhythmias: The Role of Triggered Activity. 1 ed. Futura Publishing Co. Inc.; Mount Kisco: 1988. [Google Scholar]
- 20.Wongchareon W, Chen YC, Chen YJ, Lin CI, Chen SA. Effects of aging and ouabain on left atrial arrhythmogenicity. Journal of Cardiovascular Electrophysiology. 2007;18(5):526–531. doi: 10.1111/j.1540-8167.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 21.Ono N, Hayashi H, Kawase A, Lin SF, Li H, Weiss JN, et al. Spontaneous atrial fibrillation initiated by triggered activity near the pulmonary veins in aged rats subjected to glycolytic inhibition. American Journal of Physiology. Heart and Circulatory Physiology. 2007;292(1):H639–H648. doi: 10.1152/ajpheart.00445.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Font ER, Aris A, et al. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110(11):1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Altschafl BA, Hajjar RJ, Valdivia HH, Schmidt U. Altered Ca2+ sparks and gating properties of ryanodine receptors in aging cardiomyocytes. Cell Calcium. 2005;37(6):583–591. doi: 10.1016/j.ceca.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Vest JA, Wehrens XH, Reiken S, Lehnart SE, Dobrev D, Chandra P, et al. Defective cardiac ryanodine receptor regualtion during atrial fibrillation. Circulation. 2005;111(16):2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt U, del Monte F, Miyamoto MI, Matsui T, Gwathmey JK, Rosenzweig A, et al. Restoration of Diastolic Function in Senescent Rat Hearts Through Adenoviral Gene Transfer of Sarcoplasmic Reticulum Ca2+-ATPase. Circulation. 2000;101(7):790–796. doi: 10.1161/01.cir.101.7.790. [DOI] [PubMed] [Google Scholar]
- 26.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, et al. Molecular Determinants of Altered Ca2+ Handling in Human Chronic Atrial Fibrillation. Circulation. 2006;114(7):670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 27.Spach MS, Dolber PC. Relating extracellular Potentials and their derivatives to anisotropic Propagation at a Microscopic level in Human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circulation Research. 1986;58(3):356–371. doi: 10.1161/01.res.58.3.356. [DOI] [PubMed] [Google Scholar]
- 28.Spach MS, Miller WT, 3rd, Dolber PC, Kootsey JM, Sommer JR, Mosher CE., Jr. The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circulation Research. 1982;50(2):175–191. doi: 10.1161/01.res.50.2.175. [DOI] [PubMed] [Google Scholar]
- 29.Spach MS, Heidlage JF, Dolber PC, Barr RC. Electrophysiological effects of remodeling cardiac gap junctions and cell size and model studies of normal cardiac growth. Circulation Research. 2000;86(3):302–311. doi: 10.1161/01.res.86.3.302. [DOI] [PubMed] [Google Scholar]
- 30.Spach MS, Heidlage JF, Barr RC, Dolber PC. Cell size and communication: role in structural and electrical development and remodeling of the heart. Heart Rhythm. 2004;1(4):500–515. doi: 10.1016/j.hrthm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Spach MS, Heidlage JF, Dolber PC, Barr RC. Mechanism of origin of conduction disturbances in aging human atrial bundles: Experimental and model study. Heart Rhythm. 2007;4(2):175–185. doi: 10.1016/j.hrthm.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojodjojo P, Kanagaratnam P, Markides V, Davies DW, Peters N. Age related changes in human left and right atrial conduction. Journal of Cardiovascular Electrophysiology. 2006;17(2):120–127. doi: 10.1111/j.1540-8167.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 33.Koura T, Hara M, Takeuchi S, Ota K, Okada Y, Miyoshi S, et al. Anisotropic conduction properties in canine atria analyzed by high-resolution optical mapping: preferential direction of conduction block changes from longitudinal to transverse with increasing age. Circulation. 2002;105(17):2092–2098. doi: 10.1161/01.cir.0000015506.36371.0d. [DOI] [PubMed] [Google Scholar]