Abstract
Neuronal nicotinic acetylcholine receptors (nAChRs) are widely distributed in the nervous system and are implicated in many normal and pathological processes. The structural determinants of allostery in nAChRs are not well understood. One class of nAChR allosteric modulators, including the small molecule morantel (Mor), acts from a site that is structurally homologous to the canonical agonist site but exists in the β(+)/α(–) subunit interface. We hypothesized that all nAChR subunits move with respect to each other during channel activation and allosteric modulation. We therefore studied five pairs of residues predicted to span the interfaces of α3β2 receptors, one at the agonist interface and four at the modulator interface. Substituting cysteines in these positions, we used disulfide trapping to perturb receptor function. The pair α3Y168-β2D190, involving the C loop region of the β2 subunit, mediates modulation and agonist activation, because evoked currents were reduced up to 50% following oxidation (H2O2) treatment. The pair α3S125-β2Q39, below the canonical site, is also involved in channel activation, in accord with previous studies of the muscle-type receptor; however, the pair is differentially sensitive to ACh activation and Mor modulation (currents decreased 60% and 80%, respectively). The pairs α3Q37-β2A127 and α3E173-β2R46, both in the non-canonical interface, showed increased currents following oxidation, suggesting that subunit movements are not symmetrical. Together, our results from disulfide trapping and further mutation analysis indicate that subunit interface movement is important for allosteric modulation of nAChRs, but that the two types of interfaces contribute unequally to receptor activation.
Keywords: nicotinic acetylcholine receptors, allosteric regulation, mutagenesis site specific, cysteine-mediating cross-linking, interface, oocyte, Cys-loop receptors
1. INTRODUCTION
Nicotinic acetylcholine receptors (nAChRs) are a diverse family of synaptic proteins belonging to the Cys-loop superfamily of receptors, which also includes GABAA, serotonin, and glycine receptors (1). Numerous subtypes of nAChR are expressed throughout the central and peripheral nervous systems and are implicated in a range of neurological disorders, as well as in synaptic plasticity, learning and memory (2). Several known nAChR ligands are currently in clinical use, although their mechanisms of action are not completely understood (e.g., 3): galanthamine and rivastigmine are used to treat Alzheimer’s and Parkinson’s induced dementia (4, 5) and varenicline is used to treat nicotine addiction (e.g., 6, 7). Interest in nAChR allosteric modulators as therapeutic targets has grown recently, particularly because they may offer greater specificity than agonists and competitive antagonists (8, 9).
nAChRs and other Cys-loop receptors are membrane-bound, ligand-gated ion channels in which five homologous subunits form a central pore (1, 10). The general structure of these receptors, conserved across the superfamily, has been established from a variety of crystal structures of receptors and model-system proteins (e.g., 11–13). Five homologous subunits are arranged around a central pore pseudo-symmetrically. The extracellular domain of each subunit possesses a sidedness such that the (+) and (−) edges contribute to two separate, radial interfaces with neighboring subunits (11). Residues on the a(+) face, including the “C loop,” and some in the (−) face of the neighboring subunit comprise the canonical (orthosteric) agonist binding site. The diversity of nAChR subunits provides a multitude of unique interfaces which are potential ligand binding sites (e.g., 14–16).
Allostery - the interconversion of functional states - is a defining feature of ligand-gated ion channels. Allostery theory applied to nAChRs (e.g., 17) and functional characterization of the phenomenon (e.g., 18) have been quite straightforward compared to identifying the structures that give rise to allostery, let alone the movements that interconvert states. However, results such as the high-resolution structures of bacterial homologues considered to be in open and closed conformations (13, 19) are beginning to elucidate allostery at a structural level. While at present we must rely on indirect methods such as mutagenesis and disulfide trapping to deduce the nature of movement in nicotinic receptors, such techniques are now being used successfully to address questions of movement and conformation changes (e.g., 20, 21).
The pathway by which agonist binding information is transmitted to the channel gate in nAChRs has been elucidated in detail (22, 23); however, considerably less is known about the pathways that must connect allosteric modulator sites to the gate. Some structural determinants of these allosteric pathways have been identified (e.g., 24, 25), but in many cases the binding sites for the modulators are not known (26). Perhaps the most developed case to date is a site (or family of sites) for modulators of α7 receptors in the transmembrane domain just a few Ångstroms from the channel gate; some mutations in this region change both agonist and modulator activity (27, 28). We note that the distance between subunit interfaces (~30 Å) in the extracellular domain is comparable to that between the canonical agonist binding site and the channel gate (~50 Å); thus, determining how interfaces coordinate during conformational changes poses as significant a problem as understanding agonist-to-gate communication.
Our primary goal in this work was to determine the role of interface movement in allosteric modulation of neuronal nAChRs. We hypothesized that the interfaces move during channel gating, and that we could distinguish contributions to agonist activation and modulation processes. Using mutation analysis and disulfide trapping, we found evidence of movement in both the canonical and non-canonical interfaces in both types of activation, and that the interfaces contributed unequally (or asymmetrically) to these processes. Additionally, we uncovered a major role for the β2 C loop that may parallel the function of the α subunit C loop in the canonical binding site.
2. EXPERIMENTAL PROCEDURES
2.1 Reagents
All chemicals, unless otherwise noted, were reagent grade and obtained from Sigma-Aldrich (St. Louis, MO). Morantel is 1,4,5,6-tetrahydro-1-methyl-2-(2-[3-methyl-2-thienyl]ethenyl)pyrimidine, tartrate salt. Oxantel is 1-methyl-2-(3-hydroxyphenylethenyl)-1,4,5,6-tetrahydropyrimidine with 4,4’-methylenebis(3-hydroxy-2-naphthoic acid).
2.2 Nicotinic receptor clones and mutagenesis
Wild type rat α3 and β2 subunits in the plasmid vector pGEMHE were a gift from Dr. Charles Luetje (Miami University, Miami, FL). The clones were originally isolated in the lab of Dr. Jim Patrick (Baylor College of Medicine; 29). We created mutant subunit genes in-house using the QuikChange temperature cycling method (Stratagene, La Jolla, CA) with complementary primers harboring the desired mutation (e.g., 14) or using custom mutagenesis from GenScript (Piscataway, NJ). In either case, mutants were verified by complete sequencing of the entire extracellular domain region using capillary electrophoresis of dye-detected, dideoxy-generated fragments. Unless otherwise noted, all α3 and β2 residue numbering follows that in the structure a3b2.pdb (http://www.ebi.ac.uk/compneur-srv/LGICdb/HTML/a3b2rr.html) (30). These are smaller by two compared with numbering elsewhere in the literature; this discrepancy exists because of length differences with the ACh-binding protein, on which the a3b2.pdb homology model is based. We linearized cDNAs with unique restriction enzymes, and then made them RNase-free by phenol-chloroform extraction. RNAs were synthesized from these cDNAs using the T7 kit from Ambion (Life Technologies; Carlsbad, CA), and were diluted with RNase-free water to 0.5 µg/µL and stored at −20°C.
2.3 Oocyte preparation and injection
We obtained Xenopus laevis oocytes either from Ecocyte Biosciences (Austin, TX) or harvested them from oocyte-positive female frogs (Nasco; Ft. Atkinson, WI) using procedures approved by the Grinnell College Institutional Animal Care and Use Committee, in accord with the National Institutes of Health guidelines. Briefly, stage V and VI oocytes were prepared by collagenase treatment and manual selection. Oocytes were maintained in Barth’s medium (88 mM NaCl, 1.0 mM KCl, 2.5 mM NaHCO3, 0.30 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 15 mM HEPES, and 2.25 mM sodium pyruvate, pH 7.6, supplemented with 100 U/mL penicillin/streptomycin and 50 µg/mL gentamicin). We injected oocytes with 46 nL of a 1:1 (v/v) combination of the desired α and β subunits, prepared from 0.5 µg/µL stock solutions, using a Nanoject microinjector (Drummond, Broomall, PA). Injected oocytes were incubated in Barth’s medium at 16°C for 2–4 days to allow for receptor expression, at which time currents could be recorded for up to 7 subsequent days. On a daily basis, Barth’s medium was changed and dead cells were removed. Expression for most mutants was comparable with that for the wild type α3β2 receptors as judged by currents in the range of 0.2–4 µA evoked with a saturating ACh concentration; some exceptions of poorly expressing mutants are discussed in Results.
2.4 Macroscopic current recordings
We made recordings of macroscopic evoked currents from oocytes using a Gene Clamp 500B amplifier and Digidata 1322A data acquisition system (Molecular Devices; Sunnyvale, CA) using the two-electrode voltage-clamp method, as previously described (14, 31, 32). Voltage was clamped at −60 mV, unless otherwise noted, with leak currents in the 0–200 nA range. Higher leak currents were tolerated in some cases where the baseline was reliably stable. Recording electrodes were filled with 3.0 M KCl and selected for resistances of 0.5–4.0 MΩ. Perfusion and drug administration were controlled with VC-6 solenoid valve systems (Warner Instruments; Hamden, CT). We perfused cells with oocyte Ringer’s medium (OR2; 15 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM HEPES, pH 7.3) until the baseline stabilized prior to recording, typically, 90–120 sec. Drug applications (referred to throughout as “challenges”) were 5 sec unless otherwise noted and oocytes were washed with OR2 for approximately 100 sec between challenges or until currents had returned to a stable baseline. In some cases, additional wash time was used to avoid desensitization. All drugs were prepared in OR2 from concentrated stocks. In titration experiments (e.g., Fig. 3), 2–3 challenges chosen semirandomly in addition to the ACh concentration used for normalizing the set were repeated to ensure stability of responses throughout these 20–30 min experiments.
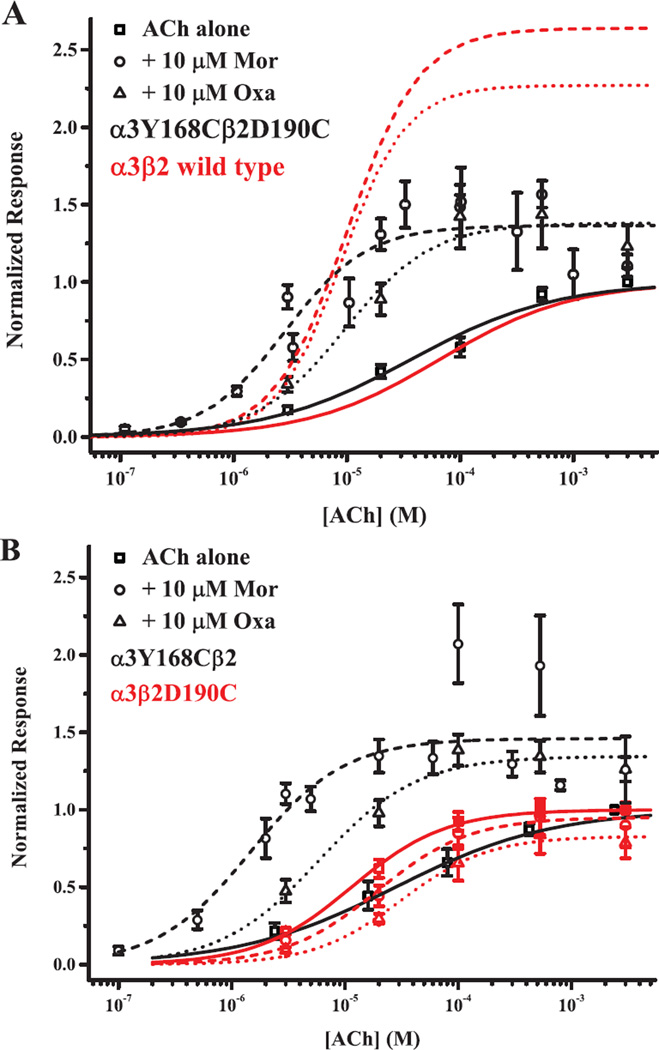
Figure 3.
An interaction involving the C loop β2 is needed for potentiation. Current responses evoked by ACh alone (squares; solid curves), ACh + 10 µM Mor (circles; dashed curves) and ACh + 10 µM Oxa (triangles; dotted curves) for three mutant receptor subtypes are plotted as a function of agonist concentration. A. Activation profiles for the double-cysteine receptor α3Y168Cβ2D190C are shown with black symbols and black fitted curves. Curves shown in red depict the best-fit parameters of the Hill equation for wild type α3β2 (symbols omitted; data from 32). B. Activation profiles for the single mutant α3Y168Cβ2 and α3β2D190C receptors are shown in black and red, respectively; the ACh concentrations for α3Y168Cβ2 are slightly offset for clarity. Symbols represent means (± SEM). Parameters for the Hill equation fits and replicates (n) are given in Table 1.
2.5 Disulfide cross-linking experiments
We performed experiments testing oxidation or reduction of putative disulfide bonds as previously described (31). In brief, currents were elicited with the same challenge (or set of challenges) 2–3 times prior to and following treatments. Responses to these series did not vary by more than 15% and that variation was random; thus responses were judged not to be subject to problems of desensitization or rundown in our experiments. Oxidation treatment was a 5-min perfusion with 4.4 mM H202 solution, either alone, or including 100 µM ACh, 10 µM Mor, or both drugs. To minimize degradation, this solution was usually prepared fresh for every cell, but no more than every third cell, from 30% (w/v) H202 stock kept on ice. Reduction was accomplished with a 5-min perfusion of 40 µM dithiothreitol (DTT) solution, unless otherwise noted, which was prepared fresh for every second or third cell from a concentrated stock. Following treatment with either H202 or DTT, oocytes were washed for 100 sec with OR2 before subsequent drug challenges. Specific drug challenges varied with the experiment and are reported in the figure legends and text. We refer to responses prior to treatment as Icontrol and those after oxidation and reduction treatments as IH2o2 and IDTT, respectively.
2.6 Data and statistical analyses
Our standard characterization of α3β2 mutants includes concentration-response titrations for ACh alone and in the presence of modulator (Mor or Oxa, 10 µM unless otherwise noted), typically over the µM to mM concentration range, but appropriate to cover the response fully. In many cases this consisted of five ACh concentrations with and without modulator, while in the others titrations consisted of 9–10 concentrations to extend the range or to refine the shape of the curve. For comparison of replicate measurements, peak evoked current responses were normalized to that evoked by a saturating concentration of ACh; in experiments testing other agonists, responses were also normalized to saturating ACh. We fit the Hill equation to titration data sets (14, 32):
where EC50 is the concentration evoking half-maximal response, nH is the slope of the curve at EC50 and Emax is the response at saturating agonist. By definition, Emax is 1 for ACh titrations, but Emax > 1 for potentiation and Emax < 1 for inhibition in the presence of modulators; Emax is a measure of relative efficacy in experiments testing other agonists (e.g., Fig. 8). Given the relative errors in the measurements, we consider 2-fold differences in EC50 the limit of significance (31).
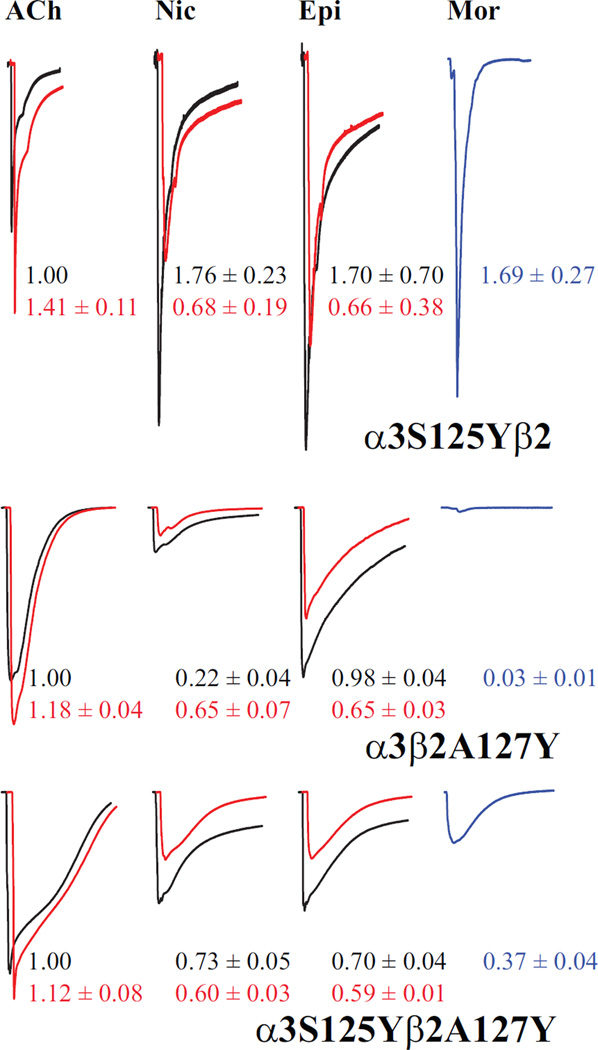
Figure 8.
The agonist interface dominates receptor activation. Representative current traces are shown for the two single mutant α3S125Yβ2 and α3β2A127Y receptors and the co-expressed double mutant α3S125Yβ2A127Y (independent oocytes), voltage-clamped at −60 mV. For each set, responses to agonist alone (black) and agonist + 10 µM Mor (red) are overlaid for ACh, nicotine (Nic) and epibatidine (Epi); currents evoked by 10 µM Mor alone are shown in blue. Overlaid traces are offset for clarity. All responses are normalized to that evoked by ACh alone, the magnitudes of which were 2.1, 1.1 and 1.1 µA for α3S125Yβ2, α3β2A127Y and α3S125Yβ2A127Y, respectively. Saturating agonist concentrations were 300 µM ACh, 300 mM nicotine and 10 µM epibatidine. In all cases responses were evoked by a 5-sec application of drugs; traces are 60 sec in duration, except for the α3S125Yβ2 samples which are 45 sec. The numbers adjacent to the traces are means (± SEM; n = 3–8), color coded to the traces, for replicate measurements in these experiments. For the agonists Nic, Epi and Mor, the values are relative to ACh, whereas for the +Mor condition for ACh, Nic and Epi, the values are relative to agonist alone.
With the premise of studying pairs of residues interacting across interfaces, we evaluated the coupling in these pairs with thermodynamic cycles (33) by calculating
where mut i and j represent single subunit mutations and mut ij represents the co-expressed double mutant. This approach is used to identify nearest-neighbor as well as long-range interactions between amino acid residues (34). In addition, depending on the substitutions used, the type and strength of the interaction can be estimated (e.g., 22). Interaction energies are the theoretical foundation of allosteric behavior at the molecular level (35). A value of Ω = 1 indicates the two mutations are independent, that is, not interacting. Errors propagated in the Ω values in our experiments ranged 23–73% (ave. 46 ± 2%, n = 48); therefore, we consider only values Ω < 0.2 or Ω > 2 meaningful.
By analogy to the thermodynamic cycle, we also calculated the ratio:
where A and A + M represent activation by ACh and ACh +10 µM modulator, respectively, for wild type α3(β2 and a particular mutant (Table 4). These measures illustrate the differential effect of a mutation on modulator and agonist activation properties. This approach is based on the concept that both ligands and amino acid substitutions contribute discrete energy to channel activation, either locally, globally or both (35, 36).
Table 4.
Thermodynamic cycles parse the contributions of interfaces to activation
| Location | Subtype | Ω(ACh) a | Ω(ACh+Mor) a | Rmut-Mor) b | Rmut-Oxa) b |
|---|---|---|---|---|---|
| M1 | α3Y168Cβ2D190C α3Y168Cαβ2 α3β2D190C |
5.7 | 2.2 | 0.52 0.29 10 |
1.9 1.2 19 |
| M1 | α3Y168Fβ2D190Q α3Y168Fαβ2 α3β2D190Q |
18 | 4.9 | 0.57 0.066 7.1 |
1.6 1.5 19 |
| A2 | α3S125Yβ2W149A α3S125Yβ2 α3β2W149A |
0.98 | 2.9 | 7.2 0.0076 7.3 |
|
| A2 | α3S125Cβ2Q39C α3S125Cβ2 α3β2Q39C |
0.16 | 1.8 | 4.5 1.3 0.10 |
|
| M3 | α3Q37Cβ2A127C α3Q37Cβ2 α3β2A127C |
0.64 | 1.15 | 1.7 5.3 3.3 |
|
| M4 | α3E173Cβ2R46C α3E173Cβ2 α3β2R46C |
2.4 | 8.0 | 4.7 8.7 1.7 |
|
| M4 | α3E173Aβ2R46V α3E173Aβ2 α3β2R46V |
15 | 0.57 | 9.0 1.4 4.2 |
|
| A2/M3 | α3S125Yβ2A127Y α3S125Yβ2 α3β2A127Y |
3.2 | 1.4 | 1.2 0.0076 9.4 |
For oxidation and reduction experiments with double cysteine mutants (e.g., Figs. 4, 6 and 7), we used standard statistical analyses, namely, the paired comparison t-test, and for tests of differences among the various oxidation treatments or across mutants, analysis of variance (ANOVA) followed by Tukey’s post hoc test. We consider p < 0.05 significant.
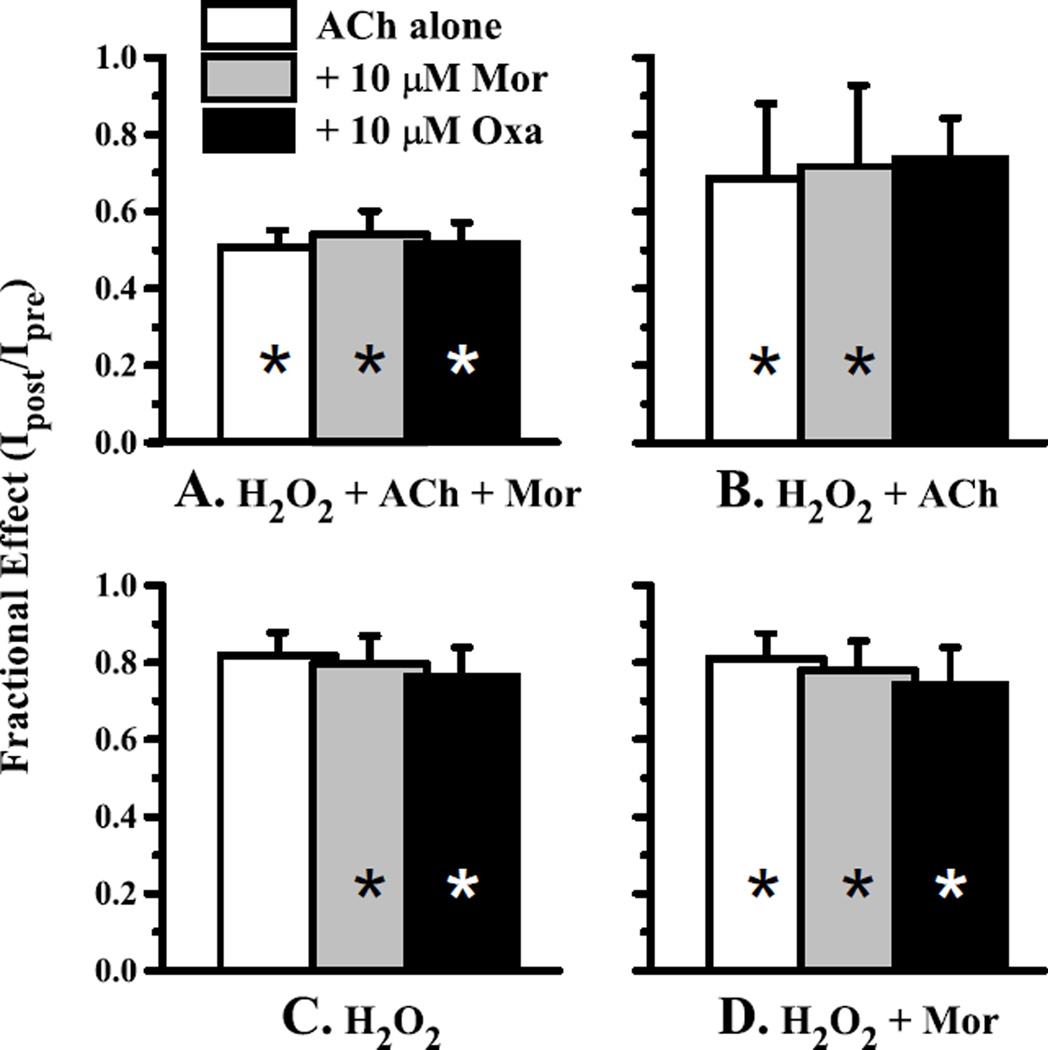
Figure 4.
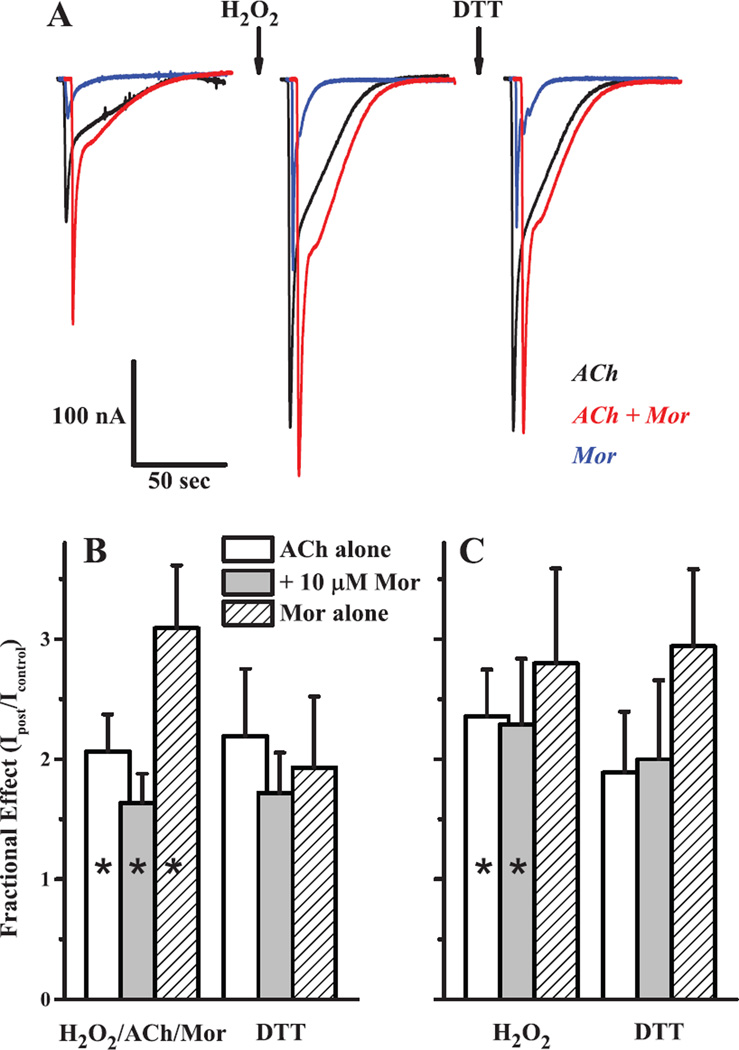
Oxidation of the α3Y168Cβ2D190C receptor decreases activation. Currents evoked by 1 mM ACh alone (open bars) or in combination with 10 µM Mor (gray bars) or 10 µM Oxa (black bars) were measured before and after four oxidation treatments for the double-cysteine mutant α3Y168Cβ2D190C; the fractional current remaining (Ipost/Ipre) is plotted. In each case, the H2O2 concentration was 4.4 mM and the oxidation treatment lasted 5 min; each condition was tested in a separate experiment. The treatments were A. H2O2 co-applied with 100 mM ACh and 10 µM Mor (n = 6); B. H2O2 with 100 mM ACh (n = 5); C. H2O2 alone (n = 8) and D. H2O2 with 10 µM Mor (n = 5). All changes upon treatment were significant at p < 0.05 (range 0.0045–0.047) and are denoted with an asterisk, except C. ACh alone (p = 0.115) and B. ACh + Oxa (p = 0.069). Representative traces for the 4.4 mM H2O2/100 µM ACh/10 µM Mor oxidation condition are shown in Fig. 2.
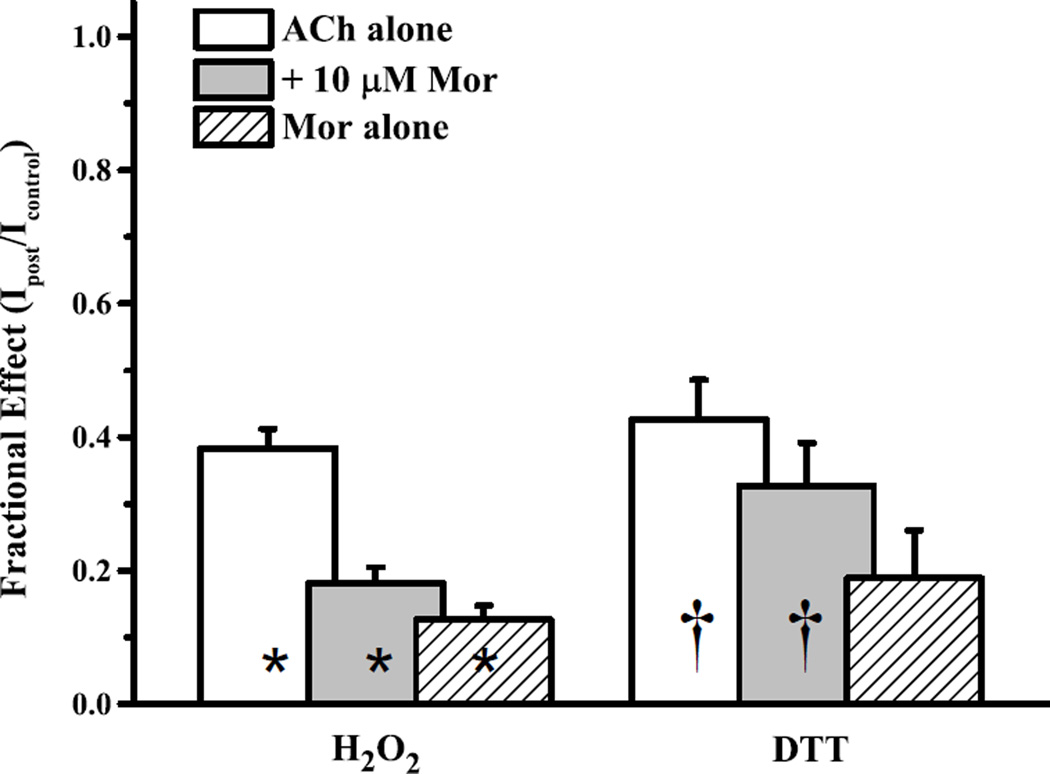
Figure 6.
Oxidation of the α3S125Cβ2Q39C receptor decreases activation. The effects of sequential 4.4 mM H2O2 (alone) and 40–100 µM DTT treatments on α3S125Cβ2Q39C receptors are plotted as currents after treatment normalized to the original (naïve) controls, as evoked by 300 µM ACh alone (open bars), 300 µM ACh + 10 µM Mor (gray bars) or 10 µM Mor alone (hashed bars). Bars represent means (+ SEM). All changes upon oxidation treatment (H2O2; n = 13, all three challenges) were significant at p < 0.05 (range 2.2×10−4-3.3×10−3), and are denoted with an asterisk. The changes following reduction treatment (DTT; n = 7, all three challenges) were significant (denoted with a dagger) for IACh (p = 0.034) and IACh+Mor (p = 0.028), but not for IMor (0.089). Representative traces for the 4.4 mM H2O2 oxidation condition are shown in Fig. 2.
Figure 7.
Oxidation of the α3Q37Cβ2A127C receptor increases activation. The effects of sequential oxidation and reduction treatments on α3S125Cβ2Q39C receptors are shown as representative sample traces (A) and as collated data for currents after treatment normalized to the original (naïve) controls using two oxidation treatments of (B) 4.4 mM H2O2 + 100 µM ACh + 10 µM Mor and (C) 4.4 mM H2O2 alone. In panel A, currents were evoked by 1 mM ACh alone (black), 1 mM ACh + 10 µM Mor (red) or 10 µM Mor alone (blue) with the oxidation treatment (first arrow) as 5 min 4.4 mM H2O2 and reduction (second arrow) with 5 min of 40 µM DTT. For panels B and C, collated data of the type shown in panel A for currents evoked by ACh alone (open bars), ACh + Mor (gray bars) and Mor alone (hashed bars), at the concentrations given above, are plotted as bar graphs; reduction treatment was with 40 µM DTT in both cases. Bars represent means (+ SEM). For experiments of n = 7 (H2O2/ACh/Mor; all three challenges in B), n = 5 (DTT in B) and n = 4 (both treatments; all three challenges in C), all changes upon oxidation were significant at p < 0.05 (range 2.5×10−5−0.035), except C. Mor alone (p = 0.132), and are denoted with an asterisk. DTT did not produce significant changes in any of the three currents for either oxidation treatment (p = 0.096–0.457).
3. RESULTS
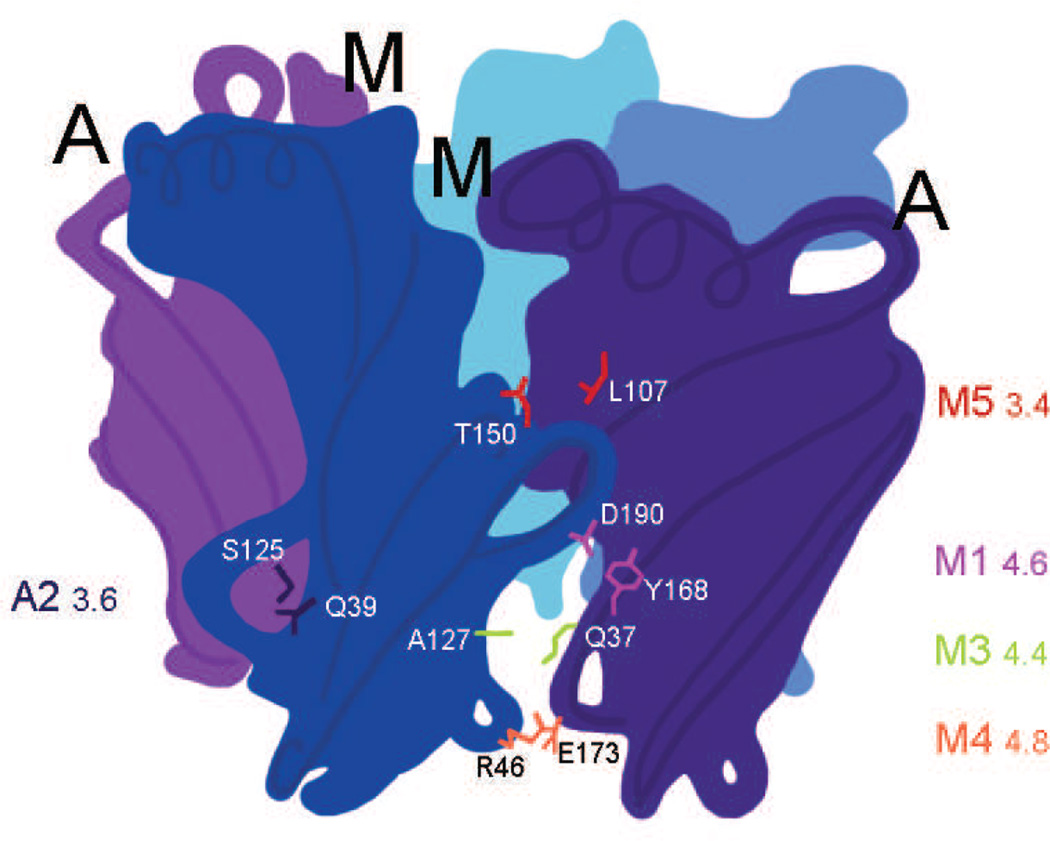
We previously established that a class of anthelmintic compounds allosterically modulates neuronal nicotinic receptors from sites located at non-canonical interfaces (14, 32). Based on others’ work demonstrating that nAChR interface interactions are important in agonist activation (e.g. 20, 23), we asked in this study whether interface movement differs for agonist activation and allosteric modulation. Fig. 1 is a schematic representation of the pentameric extracellular domain of an (α3)2(β2)3 receptor, with α3 subunits in purple and β2 subunits in blue. The relative locations of five pairs of residues we studied are also indicated; four occur in the β2(+)/α3(−) modulator interface (M) and one is in the α3(+)/β2(−) canonical agonist interface (A). We chose these pairs based on the reported importance in agonist activation of homologous pairs from other Cys-loop receptors. We explain these choices below as we describe the results for each location.
Figure 1.
Locations of residue pairs studied. The schematic represents the modulator site (M), at the β2(+)/α(−) interfaces, and the canonical agonist site (A) at the α(+)/β2(−) interfaces, and indicates the relative locations of the pairs of residues examined in this study. The extracellular domain of a pentameric (α3)2(β2)3 receptor is shown, where the α3 subunits are purple and the β2 subunits are blue. The choices of pairs are described in the text. We coded the pair locations as M1: α3Y168/β2D190, A2: α3S125/β2Q39, M3: α3Q37/β2A127, M4: α3E173/β2R46 and M5: α3L107/β2T150 to facilitate referencing these locations and because we studied multiple mutants for some pairs. Numbers next to the pair codes indicate the distance (Å) between nearest heavy atoms for the pairs in the rat homology model a3b2rr.pdb (30).
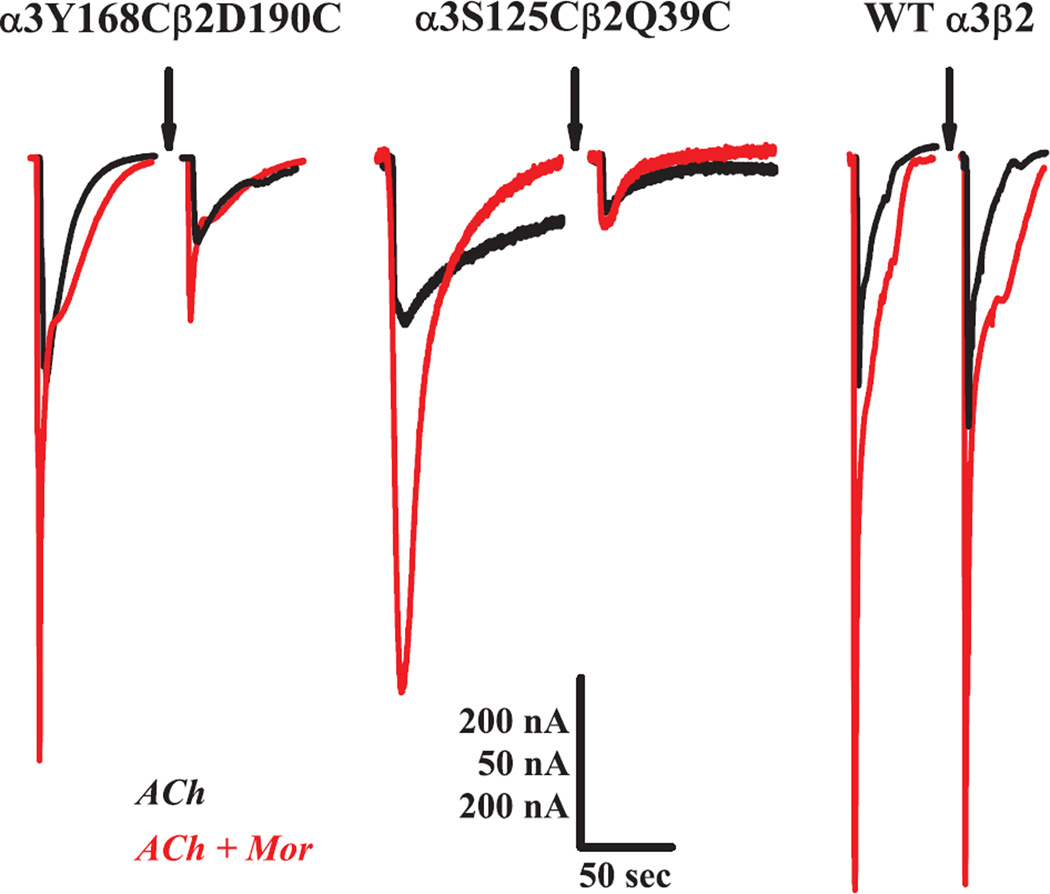
In the α3β2 system, low concentrations (10 µM) of morantel (Mor) potentiate ACh-evoked currents several fold, as shown in each of the left set of traces (in three pairs) in Fig. 2. Under the conditions of these control measurements, potentiation (IACh+Mor/IACh) was greater than 2.5-fold for the modulator interface M1 α3Y168Cβ2D190C mutant, canonical interface A2 α3S125Cβ2Q39C mutant and wild type receptors, respectively. Upon exposure to the oxidizing conditions of H2O2 (arrows), the evoked currents for the mutant receptors were greatly decreased: both currents for α3Y168Cβ2D190C decreased by ~80%, while for the α3S125Cβ2Q39C receptor, IACh decreased by 60% and IACh+Mor decreased 90%. In contrast, the wild type receptor was unaffected by oxidizing treatment, as shown by the third set of traces in Fig. 2, and substantiated by replicate experiments (Supplemental Fig. S1). Together, these findings suggest that the changes in channel function following oxidation of the mutants are due to the cysteine substitutions.
Figure 2.
Oxidation of double-cysteine receptors decreases activation. Representative current traces are shown for two double-cysteine receptors and the wild type α3β2 receptor (independent oocytes), voltage-clamped at −60 mV. For each set, overlaid responses to ACh alone (black) and ACh + 10 µM Mor (red) prior to an oxidizing treatment (arrow) are on the left and responses following the treatment are on the right. Currents were evoked with 1 mM ACh for α3Y168Cβ2D190C and 300 µM for β2Q39C and wild type. The oxidation treatments were 5 min constant perfusion with 4.4 mM H2O2/100 µM ACh/10 µM Mor for α3Y168Cβ2D190C and 4.4 mM H2O2 alone for α3S125Cβ2Q39C and wild type α3β2. Scale bars are 50 sec (horizontal) for all traces; 50 nA (vertical) for α3S125Cβ2Q39C and 200 nA (vertical) for α3Y168Cβ2D190C and wild type α3β2. Collated data for these three experiments are shown in Figs. 4, 6 and Supplemental S1 for α3Y168Cβ2D190C, α3S125Cβ2Q39C and wild type, respectively.
3.1 Movement of the β2 C loop is necessary for potentiation
The C loop of α subunits, which contributes several important residues to the canonical agonist/competitive antagonist binding sites, closes on these ligands during channel activation (reviewed in 10). In addition, pinning the C loop to the adjacent subunit by oxidizing two appropriately substituted cysteines significantly increases channel activity (37). We hypothesize that the homologous C loop structure of the β2 subunit also moves during modulation of α3β2 receptors. To test this, we substituted cysteines for residues α3Y168 and β2D190 (M1; Fig. 1), predicted to be 4.6 Å apart in the homology model (30). Fig. 3A shows the agonist concentration-response behavior of the α3Y168Cβ2D190C receptor for ACh alone, and in the presence of 10 µM Mor or 10 µM oxantel (Oxa), compared to the behavior of the wild type α3β2 receptor. The response of the double-cysteine mutant to ACh was not statistically different from wild type (EC50 = 37 vs. 66 µM, respectively; see Table 1). While the M1 double-cysteine mutant was still potentiated by both Mor and Oxa, the extent of this potentiation was considerably lower than for wild type. For example, Emax = 1.38 for ACh + Mor activation of α3Y168Cβ2D190C, compared to Emax = 2.64 for wild type. However, like wild type, Mor and Oxa modulation for this mutant were quite similar, with the same value of Emax, and EC50 values differing by only 3-fold (Table 1).
Table 1.
Agonist and modulator activation involve the β2 C loop
| Subtype | ACh Activation | ACh + Mor Modulation | ACh + Oxa Modulation | |||||
|---|---|---|---|---|---|---|---|---|
| EC50 (µM) | nH | EC50 (µM) | nH | Emax | EC50 (µM) | nH | Emax | |
| α3β2a | 66 ± 9 | 0.75 ± 0.07 | 10 ± 2 | 1.37 ± 0.80 | 2.64 ± 0.29 | 9 ± 1 | 1.54 ± 0.25 | 2.27 ± 0.06 |
| α3Y168C· β2D190C |
37 ± 6 | 0.68 ± 0.08 [10] |
2.9 ± 0.6 | 1.22 ± 0.33 [4–5] |
1.37 ± 0.06 | 9.5 ± 4.0 | 1.13 ± 0.45 [5] |
1.38 ± 0.11 |
| α3Y168Cβ2 | 36 ± 8 | 0.70 ± 0.11 [11] |
1.6 ± 0.6 | 1.17 ± 0.47 [6] |
1.46 ± 0.08 | 5.9 ± 1.6 | 1.01 ± 0.27 [6] |
1.34 ± 0.06 |
| α3β2D190C | 12 ± 2 | 1.05 ± 0.14 [9] |
19 ± 8 | 1.08 ± 0.42 [4] |
0.95 ± 0.08 | 31 ± 12 | 1.13 ± 0.44 [5] |
0.83 ± 0.08 |
| α3Y168Fβ2 | 14 ± 2 | 0.61 ± 0.04 [12] |
0.14 ± 0.03 | 0.50 ± 0.08 [6] |
1.15 ± 0.03 | 2.9 ± 0.2 | 1.73 ± 0.66 [6] | 1.16 ± 0.02 |
| α3β2D190Q | 12 ± 2 | 1.00 ± 0.15 [8] |
13 ± 6 | 0.73 ± 0.25 [4] |
1.20 ± 0.11 | 31 ± 5 | 1.19 ± 0.21 [4] | 0.97 ± 0.04 |
| α3Y168F β2D190Q |
46 ± 7 | 0.83 ± 0.09 [11] |
4.0 ± 1.2 | 1.17 ± 0.33 [4] |
2.57 ± 0.14 | 10 ± 1 | 1.10 ± 0.09 [4] | 1.06 ± 0.02 |
Fits to the Hill equation for experiments with ACh alone, ACh +10 µM Mor or ACh +10 µM Oxa. In all experiments for the α3Y168Cβ2D190C double mutant, recordings were made on naive oocytes, having had no previous oxidation or reduction treatment [n] indicates the number of replicate oocytes for each measurement.
Data for wild type α3β2 from 32.
The single mutants α3β2D190C and α3Y168Cβ2 displayed important differences in activation properties from both wild type and the double mutant α3Y168Cβ2D190C nAChRs (Fig. 3B). Like the double-cysteine receptor, ACh activation of each single mutant was similar to wild type (Table 1). ACh + Oxa modulation of the α3Y168Cβ2 receptor was comparable to the double-cysteine mutant (EC50 = 5.9 vs. 9.5 µM and Emax = 1.34 vs. 1.38, respectively), as was the ACh + Mor behavior (Table 1). In contrast, Mor and Oxa potentiation were eliminated in the α3β2D190C mutant, and in fact showed signs of inhibition, not unlike the behavior of these compounds on wild type α4β2 receptors (32). The EC50 in the presence of Mor or Oxa was shifted to a higher concentration compared to ACh alone, and for ACh + Oxa, the Emax (0.83 ± 0.08; Table 1) was significantly below one.
We also studied the α3Y168Fβ2, α3β2D190Q, and double α3Y168Fβ2D190Q mutants. Phenylalanine and glutamine occupy the homologous positions in α4 and β4, respectively, and are thus of interest because Mor potentiation in α3β4 and α4β2 receptors is minor and considerably less than that observed α3β2 (32, 38). We predicted that loss of a possible hydrogen-bonding interaction would decrease modulation. Indeed, the Mor and Oxa modulation profiles for the α3Y168Fβ2 and α3β2D190Q mutants paralleled the cysteine mutants, as did ACh activation in the two single mutants and the double mutant (Table 1). However, phenylalanine in position 168 discriminates between Mor and Oxa more strongly than cysteine (21- and 3.7-fold differences in EC50, respectively). Interestingly, Mor potentiation for α3Y168Fβ2D190Q was very similar to wild type α3β2, but Oxa potentiation was substantially decreased. Therefore, with the α3Y168 single mutants behaving more like their respective double mutants (as well as wild type α3β2), α3Y168 appears to compensate for the loss of potentiation in the β2D190 mutants, or α3Y168 dominates in determining the behavior of the double mutants.
Thermodynamic cycle analysis compares the effects of two single mutants to that of the double mutant, and thus measures putative residue interactions in terms of energetic coupling (33). The approach is typically used to evaluate residues predicted from structural data to be nearest neighbors, but it has been applied to long-range interactions in ion channels (34, and references therein). The EC50 from the Hill equation is a composite parameter that includes information about binding and gating (36), but such values have been used to evaluate the coupling parameter Ω when the more rigorous ligand binding constants and gating equilibrium constants are not available (e.g., 39, 40). Hence, in applying this analysis, we assume that the mutations do not radically alter the mechanisms of activation, and we limit our interpretation to differences in activation by agonist alone vs. allosteric modulation.
As indicated in Table 4, with all Ω > 2, the two mutant cycles in location M1 reveal an improvement in channel function; these values are driven by the EC50s for the single mutants being smaller (left-shifted) than wild type, indicating improved potency. The coupling is modest (Ω = 2.1–4.9) for modulation by Mor and Oxa, and surprisingly is less than for ACh activation in the case of the α3Y168F-β2D190Q pair. Extending this approach to the interaction of each mutation with modulator (referenced to ACh activation; two right-hand columns of Table 4) shows that the two residues of the M1 pair mediate modulation, but that the mutations have opposite effects. For wild type α3β2 receptors, the ratio EC50(ACh)/EC50(ACh + Mor) is 6.6 (Table 1); thus, the R(mut-Mor) values of 0.29 and 0.066 for the two α3Y168 single mutations signify a much greater left-shift upon adding Mor than for wild type. In contrast, the β2D190 single mutation values (R = 7.1–19) reflect the effective inhibition discussed above (Fig. 3B, Table 1). Hence, whereas mutations in α3Y168 may “loosen” the receptor, making it more sensitive to Mor and (somewhat) Oxa, mutations in β2D190 appear to disconnect the modulatory mechanism from activation or favor the closed state over the open (relative to wild type).
We next used a disulfide trapping approach to test whether cross-linking the β2 C loop to the α3(−) face would disrupt channel function. We tested four oxidation conditions on α3Y168Cβ2D190C (the absence and presence of agonist and modulators), as we and others have previously done (25, 31), to determine whether disulfide reactivity depended on the activation state of the receptor. Fig. 4 shows that, consistent with the example illustrated in Fig. 2, oxidation decreased currents evoked by ACh alone, ACh + Mor, and ACh + Oxa, all to the same extent (per treatment). Currents decreased 20–30% for oxidation with H2O2 alone, H2O2 + ACh or H2O2 + Mor (panels C, B and D, respectively), but currents decreased by 48% upon oxidation with H2O2 + ACh + Mor (panel A). All but two of these decreases were significant (paired t-test). The larger effect for the H2O2 + ACh + Mor oxidation was significantly different from the H2O2 alone treatment for currents evoked by ACh and ACh + Mor (ANOVA, Tukey’s; F = 4.36, p = 0.016; F = 3.19, p = 0.046, respectively). Similarly, in a separate experiment testing the oxidation onset kinetics (Supplemental Fig. S2A), the final fraction of current remaining at long times was significantly different for oxidation as H2O2 alone, n = 5) vs. H2O2 + ACh + Mor (Ioxidation/Icontrol = 0.61 ± 0.05 and 0.43 ± 0.04, respectively), while the time course was the same for both (t1/2 = 60). Together, these results suggest that movement in the vicinity of the M1 position is necessary for channel activation and that residues α3Y168 and β2D190 are closer and/or are more reactive when the receptor is potentiated compared to the resting state.
We further explored the properties of the putative disulfide bond formed in M1 α3Y168Cβ2D190C receptors with several control experiments. We found 1) that the decrease in currents following oxidation was completely reversible (Supplemental Fig. S2B); 2) that this decrease arose from decreased efficacy of the channels (Supplemental Fig. S2C); and 3) importantly that oxidation treatments for the single mutants α3Y168Cβ2 and α3β2D190C expressed separately failed to changed evoked currents significantly (Supplemental Fig. S3A and S3B).
In sum, our analysis of the M1 position suggests a) a strong interaction between these residues, b) a direct role of the residues in mediating modulation effects, in that for only one of 12 cases is modulation equivalent to wild type, c) a role for these residues in discriminating between Mor and Oxa, and d) relative movement of these residues during channel activation and modulation.
We also attempted to study the α3L107Cβ2T150C double-cysteine mutant (M5 in Fig. 1) because the predicted separation of 3.4 Å for the pair suggests an interaction. We previously showed that β2T150 and α3L107 are involved in allosteric modulation of α3β2 receptors (14, 32). T150, adjacent to W149 of the “aromatic box,” is highly conserved in (−)-interface nAChR subunits, while α3L107 is not, making it one of the determinants of modulator specificity for α3β2. All attempts to express the M5 α3L107Cβ2T150C double-cysteine mutant failed to produce currents large enough for viable study. In total, we tried expressing α3L107Cβ2T150C thirteen times, including several attempts to reduce putative pre-existing disulfide bonds with DTT, without success. This failure must be due to the co-expression of the cysteine pair, because the single cysteine mutants were readily expressed and fully characterized (14, 32).
3.2 α3S125 in the canonical interface is a hotspot for receptor activation
Extensive study has elucidated the transformation of agonist binding energy into channel gating in the muscle-type nAChR system 35, and references therein). For example, Mukhtasimova and Sine (20) demonstrated that the α1Y127-єQ39/δQ41 pairs in canonical interfaces couple agonist binding with rapid and efficient gating. Based on the conservation of a hydroxyl-bearing residue in this α(+) position and the completely invariant glutamine in the (−)-face, neighboring subunit, we studied the role of this location (A2; Fig. 1) in α3β2 modulation.
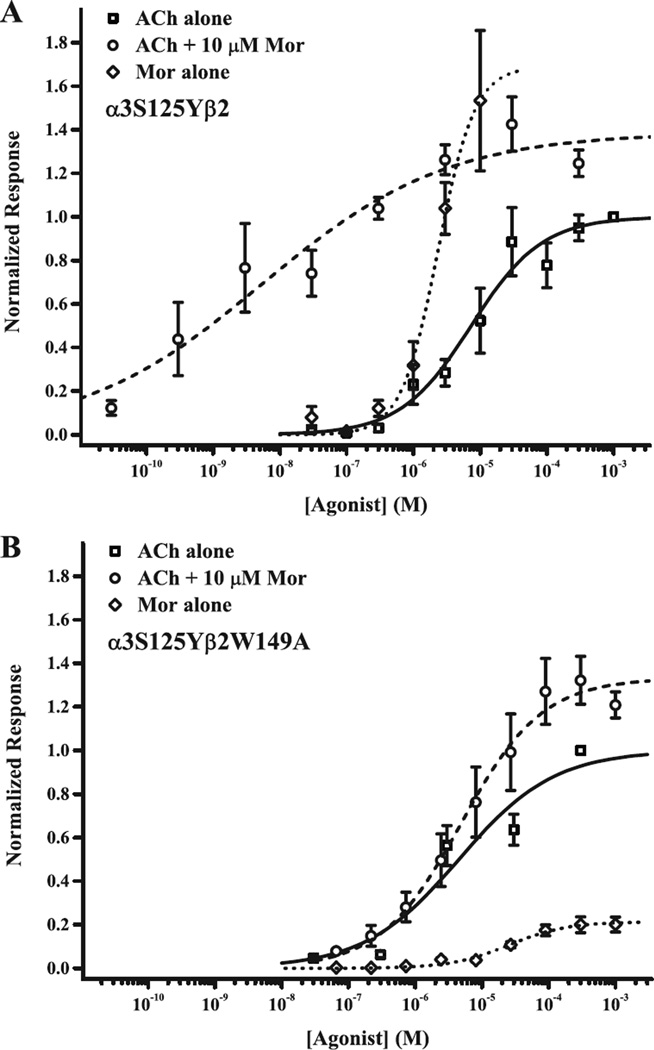
The muscle-type receptor has near-perfect efficacy (e.g., 18) compared to α3β2 (38). We therefore studied the α3S125Yβ2 receptor, predicting increased efficacy for the tyrosine substitution because this amino acid is invariant in α1 sequences. Remarkably, as shown in Fig. 5A, Mor alone was as good an agonist as ACh, with EC50 for activation of 2.3 µM (vs. 7.6 µM for ACh) and Emax of 1.7 relative to saturating ACh (Table 2). In contrast, Mor alone activates wild type α3β2 in the same micromolar range, but with Emax only 0.2 relative to ACh (Table 2; 38). For α3S125Yβ2, we omitted from the fit data collected in the range 30–300 µM Mor (not shown) because these responses were considerably lower than Emax and showed evidence of open channel block (cf. 38). Relative potency for ACh was also increased 8-fold for α3S125Yβ2, consistent with improved efficacy (36). α3S125Yβ2 demonstrated Mor potentiation of ACh-evoked currents, indicating an interaction of the two compounds over a very broad range (EC50 = 9 nM, Table 2); the ACh + Mor curve was shifted to higher potency than for ACh and Mor alone, but the ACh + Mor and Mor alone Emax values were similar. In other words, cooperativity between ACh and Mor persists, because the response was not constant at the level evoked by 10 µM Mor alone, and it increased monotonically with increasing ACh concentration. Exploring the unusual effect of the tyrosine mutation on ACh + Mor modulation further is beyond the scope of this work.
Figure 5.
A putative trigger for activation involves α3S125 and β2Q39. Receptor activation profiles for the two mutant receptors α3S125Yβ2 and α3S125Yβ2W149A are shown in panels A and B, respectively. Currents evoked by ACh alone (squares), ACh with 10 mM Mor (circles) or Mor alone (diamonds), each collected in separate experiments, were normalized to the response evoked by 1 mM ACh (α3S125Yβ2) or 300 mM ACh (α3S125Yβ2W149A). The best fits of the Hill equation to the data are indicated by solid (ACh alone), dashed (ACh + Mor) and dotted curves (Mor alone), respectively; parameters for these fits and replicates (n) are given in Table 2.
Table 2.
Allosteric modulation involves the canonical agonist interface
| Subtype | ACh Activation | ACh + Mor Modulation | Mor Activation | |||||
|---|---|---|---|---|---|---|---|---|
| EC50 (µM) | nH | EC50 (µM) | nH | Eax | EC50 (µM) | nH | Emax | |
| α3β2a | 66 ± 9 | 0.75 ± 0.07 [5–11] | 10 ± 2 | 1.37 ± 0.80 | 2.64 ± 0.29 [5] | 20 ± 2b | 3.1 ± 1.6 | 0.20 ± 0.04 |
| α3S125Yβ2 | 7.6 ± 2.1 | 0.84 ± 0.19 [3–6] | 8.8 ± 4.9nM | 0.31 ± 0.05 | 1.41 ± 0.11 [3–6] | 2.3 ± 0.7 | 1.58 ± 0.42 [3–4] |
1.69 ± 0.27 |
| α3S125Y· β2W149A |
4.5 ± 1.4 | 0.61 ± 0.10 [6] |
4.9 ± 2.0 | 0.72 ± 0.16 [5] |
1.33 ± 0.10 | 25 ± 9 | 1.01 ± 0.29 [5–6] |
0.21 ± 0.02 |
| α3β2W149A a | 40 ± 6 | 0.99 ± 0.12 [5] | 44 ± 6 | 1.38 ± 0.19 | 0.86 ± 0.03 [5] | N.F. | N.F. | 0.015 ± 0.004 [3] |
| α3S125C· β2Q39C |
1.1 ± 0.2 | 0.66 ± 0.09 [3–7] |
0.75 ± 0.40 | 0.62 ± 0.18 [4] |
2.57 ± 0.17 | 0.53 ± 0.21 | 0.92 ± 0.30 [4–8] |
1.00 ± 0.14 |
| α3S125Cβ2 | 3.5 ± 1.3 | 0.64 ± 0.13 [5–7] | 0.71 ± 0.20 | 0.85 ± 0.17 | 1.48 ± 0.07 [5] | 92 ± 33 | 0.54 ± 0.11 [4] |
0.028 ± 0.006 |
| α3β2Q39C | 130 ± 30 | 0.78 ± 0.12 [4] | 2.0 ± 0.4 | 0.91 ± 0.15 | 2.54 ± 0.09 [6] | N.D. | ||
Fits to the Hill equation for experiments with ACh alone, ACh + 10 µM Mor or Mor alone. In all experiments for the (α3S125Cβ2Q39C double mutant, recordings were made on naive oocytes, having had no previous oxidation or reduction treatment [n] indicates the number of replicate oocytes for each measurement. N.F. indicates no fit was possible for data collected in experiment; N.D. indicates no data were collected for experiment.
Data of ACh and ACh + Mor titrations for wild type α3β2, and all data α3β2W149A from 32.
Data of Mor titration for wild type α3β2 from 38.
A possible explanation of the strong agonist behavior for Mor is that the tyrosine substitution unmasks Mor activation from the α(+)/β(−) canonical agonist site, despite the evidence that Mor binds at the β2(+)/β3(−) interface (14, 32). To test this idea, we co-expressed α3S125Y with β2W149A, a mutation in the non-canonical interface that greatly reduces Mor activity (32). These results are shown in Fig. 5B and Table 2. Although ACh activation did not differ from the α3S125Yβ2 receptor (EC50 left-shifted less than 2-fold), Mor activation and Mor potentiation were drastically reduced for α3S125Yβ2W149A compared to the single mutant; these profiles are in fact very similar to wild type α3β2 (Table 2). In other words, the β2W149A substitution selectively decreases Mor activities. Coupling analysis (Table 4) indicates that α3S125 β2W149 do not interact in ACh activation, but do so in Mor modulation, an effect clearly driven by a strong correlation between Mor and the α3S125 position. Thus, we conclude that Mor still acts through the β2(+)/α3(−) site in the α3S125Yβ2 receptor, suggesting that Mor-binding information is fed into the “principal gating pathway” (22) at or near the A2 pair.
With this evidence of the importance of the A2 pair (3.6 Å separation), we substituted α3S125 and β2Q39 with cysteines and tested oxidation and reduction. Fig. 6 (left) shows that treatment with H2O2 alone decreased currents evoked by ACh, ACh + Mor and Mor alone, by 62–89% (also as in Fig. 2). These decreases were all highly significant as measured by paired comparison t-test (p values ranged 0.00022–0.0033), and the effect on IACh was different from IACh+Mor and IMor (ANOVA; F = 29.6, p = 2.5 × 10−8 and Tukey’s post hoc test). The decreases in activation for disulfide trapping of α3S125Cβ2Q39C (Fig. 6) were also greater than for α3Y168Cβ2D190C (Fig. 4; all conditions) (ANOVA; F = 62.3, p = 9.8 × 10−15), indicating that the A2 pair is more solvent-accessible, or that cross-linking in this location decreases efficacy more substantially. We again attribute the changes in currents for the double mutant to formation of a disulfide bond, because H2O2 oxidation treatment led to no changes in evoked currents for the single mutant α3S125Cβ2 (Supplemental Fig. S3C).
The effective resistance of the α3S125C-β2Q39C disulfide bond to reduction by DTT (Fig. 6, right), especially compared to the ready reversibility of the M1 bond, is partial evidence against the A2 pair being easily accessible. In a subset of experiments having first oxidized α3S125Cβ2Q39C, we were able to test reduction (40–100 µM DTT). Although differences (IDTT vs. IH2O2) registered as significant by paired comparison for currents evoked by ACh (p = 0.034) and ACh + Mor (p = 0.028), but not for Mor alone (p = 0.089; n = 7 all three measures), these changes are clearly very far from returning the receptor to original control levels of activation. Though the cysteine substitutions for this A2 location did not exhibit strong coupling (Table 4), the very significant decreases in receptor function upon oxidation suggest an important interaction. Together, the effects of the substitutions in the A2 location implicate these residues not only in agonist activation, but also in allosteric modulation.
3.3 Interface movement is not symmetrical
In order to examine whether the subunit interfaces contribute symmetrically to channel activation, we studied mutations in the β2A127 and α3Q37 positions (M3), which are homologous toα3S125 and β2Q39C, respectively, and predicted to be 4.4 Å apart. We hypothesized that perhaps this pair functions to move Mor-binding information “down” to the channel gate directly, in analogy to agonist-binding information moving through the principal gating pathway of the α1(+) interface (22).
Oxidation of the M3 α3Q37Cβ2A127C double-cysteine mutant had dramatic effects on receptor activation properties. Currents evoked by ACh alone, ACh + Mor and Mor alone, shown as representative traces in Fig. 7A, were all increased by oxidation, whether as H2O2 alone (data collated in Fig. 7C) or in the presence of ACh and Mor (data collated in Fig. 7B). These increases were all significant in paired comparison (p = 2.5×10−5–0.035), except for Mor alone currents in oxidation with H2O2 only (p = 0.132), but the six ratios were not different from one another (ANOVA; F = 2.94, p = 0.130). Given that the currents increased following oxidation for the α3Q37Cβ2A127C receptor, these effects were clearly different from those in the α3Y168Cβ2D190C and α3S125Cβ2Q39C receptors (ANOVA; F = 23.6, p = 1.2 × 10−12). However, like the homologous α3S125C-β2Q39C (A2) pair, the α3Q37C-β2A127C disulfide bond was essentially resistant to reduction by DTT (p = 0.096–0.457 for paired t-test). The effects of the cysteine substitutions in these M3 residues on ACh activation and Mor modulation were modest, with slightly decreased Mor potentiation (Table 3 and Table 4). Nonetheless, given the increased currents upon oxidation of the α3Q37Cβ2A127C receptor, we conclude that these M3 residues participate in channel gating, but that their role is likely different from the canonical A2 position.
Table 3.
Agonist and modulator interface contributions to activation are not symmetrical
| Subtype | ACh Activation | ACh + Mor Modulation | Mor Activation | |||||
|---|---|---|---|---|---|---|---|---|
| EC50 (µM) | nH | EC50 (µM) | nH | Emax | EC50 (µM) | nH | Emax | |
| α3Q37Cβ2 | 45 ± 11 | 1.67 ± 0.44 [4] | 36 ± 11 | 0.84 ± 0.15 | 1.40 ± 0.11 [6] | N.D. | ||
| α3β2A127C | 71 ± 4 | 2.09 ± 0.34 [4] | 35 ± 14 | 0.80 ± 0.17 | 2.83 ± 0.28 [5] | N.D. | ||
| α3Q37C· β2A127C |
31 ± 5 | 0.97 ± 0.13 [6] | 8.1 ± 3.4 | 0.99 ± 0.38 | 1.73 ± 0.15 [6] | N.D. | ||
| α3β2A127Y | 38 ± 4 | 1.46 ± 0.20 [5] | 54 ± 12 | 1.50 ± 0.47 | 1.50 ± 0.09 [3] | 4.8 ± 2.4 | 1.08 ± 0.40 | 0.05 ± 0.01 [5] |
| α3S125Yβ2a | 7.6 ± 2.1 | 0.84 ± 0.19 | 8.8 ± 4.9nM | 0.31 ± 0.05 | 1.41 ± 0.11 | 2.3 ± 0.7 | 1.58 ± 0.42 | 1.69 ± 0.27 |
| α3S125Y· β2A127Y |
14 ± 3 | 1.06 ± 0.19 [3] | 2.6 ± 0.8 | 0.87 ± 0.19 | 1.13 ± 0.06 [3–4] | 1.9 ± 0.2 | 3.18 ± 0.63 | 0.16 ± 0.01 [3] |
| α3E173Cβ2 | 13 ± 1 | 1.16 ± 0.12 [7] | 6.4 ± 1.7 | 0.99 ± 0.22 | 1.02 ± 0.05 [6] | N.D. | ||
| α3β2R46C | 30 ± 6 | 0.88 ± 0.13 [5] | 3.7 ± 1.0 | 1.22 ± 0.28 | 4.33 ± 0.22 [5] | N.D. | ||
| α3E173C· β2R46C |
14 ± 2 | 0.85 ± 0.10 [5–6] | 19 ± 8 | 0.55 ± 0.09 | 1.05 ± 0.08 [5–6] | N.D. | ||
| α3E173Aβ2 | 6.6 ± 0.7 | 1.77 ± 0.26 [3–6] | 1.4 ± 0.5 | 1.15 ± 0.33 | 4.14 ± 0.27 [3] | N.F. | N.F. | 0.16 ± 0.01 [4] |
| α3β2R46V | 5.0 ± 0.7 | 0.92 ± 0.14 [5–7] | 3.2 ± 0.8 | 0.85 ± 0.21 | 1.06 ± 0.06 [5–7] | N.F. | N.F. | 0.03 ± 0.01 [7] |
| α3E173A· β2R46V |
7.3 ± 0.8 | 0.82 ± 0.06 [7–8] | 9.9 ± 2.1 | 1.07 ± 0.23 | 0.95 ± 0.05 [6–7] | N.D. | ||
Fits to the Hill equation for experiments with ACh alone, ACh + 10 µM Mor or Mor alone. In all experiments for the α3Q37Cβ2A127C double mutant, recordings were made on naive oocytes, having had no previous oxidation or reduction treatment [n] indicates the number of replicate oocytes for each measurement. N.F. indicates no fit was possible for data collected in experiment; N.D. indicates no data were collected for experiment.
Data for α3S125Yβ2 repeated from Table 2 for comparison.
To further characterize the contributions the interfaces make to gating, we compared activation and modulation for several agonists. Nicotinic receptor subtypes display characteristic pharmacological profiles, including differences in efficacy for some agonists (38, 41, 42). We aimed to exploit such efficacy differences to determine whether Mor modulation is parallel for all agonists or in some way discriminates among them. Fig. 8 shows traces of currents evoked by ACh ± Mor, nicotine ± Mor, epibatidine ± Mor and Mor alone for α3S125Yβ2, α3β2A127Y, and the double mutant α3S125Yβ2A127Y. These are representative of repeated experiments of this drug profiling, the collated results of which are reported numerically in the figure (means ± SEM; n = 3–8). The concentrations of the three traditional nAChR agonists were saturating, as determined in independent experiments (Table 2 and Table 3, and not shown). For all three subtypes, 10 µM Mor modestly potentiated ACh responses, but inhibited responses evoked by nicotine and epibatidine. This inhibition might make sense in the case of α3S125Yβ2 where the greater efficacy for nicotine and epibatidine (than ACh) might be approaching a ceiling (cf. 32); however, this would not explain the inhibition for the α3β2A127Y and α3S125Yβ2A127Y subtypes. For all three mutants, the responses to Mor alone were very different, ranging from full agonist for α3S125Yβ2 as noted above (Fig. 5A) to the nearly negligible current for α3β2A127Y, which was considerably smaller than for α3β2 (Table 2). Although analyzing them quantitatively is beyond the scope of this study, the kinetics of inactivation were also clearly different for the three subtypes. Interestingly, α3S125Y and β2A127Y appear to couple in ACh activation and for Mor alone activation (Ω = 3.4), and β2A127Y mediates Mor modulation (Table 2, Table 3 and Table 4). In sum, the degree of Mor potentiation or inhibition is effectively independent of the agonist for these three subtypes, despite the agonist activities being different. Accordingly, these substitutions (which are in homologous positions) contribute equally to Mor modulation, or their role is predominantly in agonist activation. However, for Mor acting as an agonist, the interfaces may contribute equally in this location, because the response of the α3S125Yβ2A127Y is intermediate between that of the two single mutants, not dominated by one or the other.
We further explored the possibility of direct channel activation from the β2(+)/α3(−) interface by studying the pair α3E173–β2R46 (M4; Fig. 1). α3E173 and β2R46 might be intimate enough to share an electrostatic attraction (4.8 Å), and the homologous positions are implicated in channel activation processes in other systems (24, 25, 43, 44). In only one of five attempts to express the α3E173Cβ2R46C mutant did we observe currents large enough for study. This allowed us to characterize ACh activation and Mor modulation properties (Table 3) and perform one experiment testing oxidation and reduction. The apparent poor expression of the double-cysteine M4 mutant (Iaverage = 60 ± 10 nA evoked by 2 mM ACh, n = 8, for the one successful expression attempt) was likely due to the cysteines themselves, because we observed robust expression of the M4 double mutant α3E173Aβ2R46V (Iaverage = 990 ± 130 nA evoked by 800 µM ACh, n = 8) and of the single-cysteine mutants.
Like the M3 pair α3Q37C-β2A127C, just 8–10 Å “above,” toward the Mor site, oxidation of α3E173Cβ2R46C with H2O2 alone significantly increased currents (Supplemental Fig. S4). Unlike the M3 pair, reduction of this double mutant with DTT following oxidation did decrease currents, but not completely to the control level (Supplemental Fig. S4). Table 4 indicates that α3E173C and β2R46C are coupled modestly in ACh activation and more substantially in Mor modulation; the pair is dominated by α3E173C, as both mutants have effectively lost Mor potentiation. In contrast, the α3E173A-β2R46V pair, which substitutions we based on the literature (25, 43), do not interact in Mor modulation, but are strongly coupled in ACh activation (Table 4). Interestingly, the double mutant has also lost Mor potentiation, but in this case is dominated by β2R46V (Table 3). Our findings about the M4 position parallel both those of the M1 location, in that the mutations overall seem to “loosen” the receptor for ACh activation and “disconnect” Mor potentiation, and those of the M3 location, in that oxidation of engineered cysteines increases currents. These results suggest the M4 position, and perhaps this region of the receptor more broadly, deserves further study, for example, with charge reversal substitution.
4. DISCUSSION
In this study, we aimed to demonstrate that allosteric modulation of neuronal nicotinic receptors is mediated by movement between subunits. Using mutation analysis and disulfide trapping of cysteine-substituted receptors, we found support for the potentiator Mor enhancing the transmission of agonist binding information, rather than contributing to gating more directly. In addition, interaction of the C loop region of β2 with the α subunit is also integral to modulation. Each type of interface contributes to both agonist activation and allosteric modulation, but the contributions are not equal.
4.1 Allosteric modulation requires movement of all five subunits
Our primary observation supporting the conclusion that interface movement underlies general channel activation is that oxidation of pairs of cysteine residues engineered across these interfaces perturbs evoked currents. For two mutants α3Y168Cβ2D190C (M1) and α3S125Cβ2Q39C (A2), oxidation treatments decreased currents evoked by ACh alone and by the potentiating combination of ACh + Mor/Oxa (Fig. 4 and Fig. 6). For two others, α3Q37Cβ2A127C (M3) and α3E173Cβ2R46C (M4), oxidation increased currents (Fig. 7 and Supplemental Fig. S4).
Our previous work in the α3β2 system (31) and numerous other studies of Cys-loop family receptors (e.g., 20, 45, 46) demonstrate that changes in current caused by oxidation are due to disulfide bond formation between the substituted cysteines. Accordingly in this study, decreases in α3Y168Cβ2D190C currents were completely reversed by subsequent treatment with the reducing agent DTT (Supplemental Fig. S2B), and several single cysteine mutants were unaffected by oxidation (Supplemental Fig. S3). Disulfide bond formation alters channel efficacy, because maximum responses (at saturating agonist concentrations) change while relative potencies do not. Hence, we conclude that movement of the subunits with respect to each other is necessary for optimal channel activation. This interpretation is straightforward for the M1 and A2 pairs for which disulfide bonds impair function. The improved function upon disulfide formation for the M3 and M4 pairs suggests that these bonds favor the open state. This in itself does not mean these pairs must move; however, that the unlinked residues interact comparatively less does suggest a change in orientation or distance at some point during gating. With movement implicated for the non-canonical β2(+)/α3(−) [M1, M3, M4] and canonical α3(+)/β2(−) [A2] interfaces, we infer that all subunits contribute to channel activation, including allosterically modulated states. We did not probe directly the role of homomeric α/α or β/β interfaces that should exist in our system; however, all five subunits contribute to the four interfaces we did examine, and such homomeric interfaces have recently been shown to impact or harbor agonist activities (15, 16).
Although we have no chemical or structural demonstration of disulfide bond formation, we observe large and varied effects of oxidation for cysteine pairs, but no such changes for the wild type or single mutant receptors. Desensitization cannot explain our results because responses were stable before and after treatments, decreases in currents upon oxidation were reversible, and for two pairs oxidation increased currents. Furthermore, we have made no assumptions about channel activation mechanism in regard to the relative occupancy of agonist and modulator sites. Thus, only in the unlikely case that a double mutant greatly perturbed the subunit stoichiometry would comparison of the effects of mutants be open to misinterpretation.
4.2 Subunit interfaces do not contribute symmetrically to channel activation
Previous work has demonstrated that movement in the agonist binding site interface underlies channel activation of Cys-loop receptors (e.g., 20, 47). The Auerbach and Sine groups, through exhaustive mutagenesis and rigorous quantitative modeling, have elucidated the route that agonist binding information follows from the canonical site to the gate, which includes residues in this interface, primarily in the α subunit (22, 23, and references therein). This work has also allowed dissection of the activation process (binding → gating) into discrete components (21, 37, 48). Drawing from these ideas, we postulated that Mor and Oxa, acting from the β2(+)/α3(−) interface, whether as a modulator or partial agonist, could communicate with the gate in a parallel fashion (i.e., “directly down”). The bulk of our evidence in this study, however, weighs against this hypothesis.
Mutations in locations A2 and M1–4 have different effects (from one another) on activation properties, and in some cases discriminate between agonist activation and modulation. Changes in EC50 for ACh activation were generally modest, with the largest increase (2-fold for α3β2Q39C; Table 2) and largest decrease (60-fold for α3S125Cβ2Q39C; Table 2) occurring for A2 mutants. Most changes in Mor modulation (EC50 comparisons) were in a similar range and, as might be expected, occurred in the β2(+)/α3(−) interface: 4-fold increase (α3β2W149A; Table 2) and 70-fold decrease (α3Y168Fβ2; Table 1). However, the largest change in Mor modulation (1000-fold decrease in EC50) was for α3S125Yβ2 (A2) receptors, and improvements in ACh potency (2- to 10-fold) were realized for the six mutants studied in the M4 location (Table 3). Furthermore, the tyrosine substitution of α3S125 (perfectly conserved as tyrosine in all α1 sequences at this position) improved activation by Mor alone, to the point that it was a full agonist, while replacement in β2A127 decreased Mor efficacy relative to wild type (Figs. 5 and Fig. 8; Table 3). Thus, the structural differences between canonical and non-canonical (heteromeric) interfaces in α3β2 dictate unequal contributions to channel activation.
Our disulfide trapping results also indicate differences among the contributions of the probe locations to channel gating, particularly the homologous A2 and M3 sites. Oxidation decreased currents in the M1 and A2 pairs to different degrees (Fig. 4 and Fig. 6). While this may reflect different solvent accessibility of the cysteine pairs, our control experiments indicate that reactions were complete. Thus, the differences are more likely due to different efficacies in the cross-linked receptors, meaning that these locations do not mediate activation the same way. Furthermore, for the α3S125-β2Q39 (A2) pair, the current decreases were greater for IACh + Mor and IMor than for IACh (Fig. 6). More importantly, oxidation has opposite effects at the homologous α3S125-β2Q39 (A2) and α3Q37-β2A127 (M3) locations, possibly suggesting opposite directions of relative movements at these interfaces (cf. 49).
With five unique interfaces, the muscle-type nAChR is inherently asymmetric, and even its two ACh binding sites can be distinguished with various ligands (e.g., 18, 50). Similarly, benzodiazepine-sensitive GABAA receptors have a high degree of asymmetry; interestingly, the benzodiazepine site has a unique position with respect to the two GABA sites (46) vis-a-vis the relationships of modulator and agonist sites in the α3β2 system. In this light, it is perhaps not surprising that our results suggest the various α3β2 interfaces contribute unequally to agonist activation and modulation, but it begs the questions of the number and relative arrangement of agonist and modulator sites necessary for optimal channel activity. In ongoing work we are addressing these questions with subunit concatemers to control the number and relative order of sites.
4.3 The α3 subunit transduces modulator information
If modulator binding information does not travel “down” the β2(+)/α3(−) interface to the channel gate, by what route does it flow in order to enhance receptor activity? The finding that the α3S125Y mutation dramatically enhanced the ability of Mor to activate the receptor on its own was unexpected (Fig. 5A), but it suggests that the modulation pathway includes this location. We have recently demonstrated that residues on the outer face of α3, at the junction of the two sheets constituting the β sandwich structure of the extracellular domain, mediate modulation and channel activation (31). Furthermore, residues in the (−)-face of α3 govern modulator specificity (32), probably in most cases by direct contact, but mutations of several of these residues also substantially alter agonist activation (14, 32). Combining these observations, we propose that modulation consists of Mor binding information routed across or through the α3 subunit and added to the “principal gating pathway” (22) at or near α3S125, which itself is immediately adjacent to one of the cysteines of the Cys-loop.
4.4 The β2 C loop governs modulation
Although we conclude that the α3 subunit contains the primary determinants of the modulation pathway for Mor and Oxa, the β2 C loop also makes an essential contribution. Our data indicate that relative movement in the β2 C loop impacts modulation and that its interaction with the α3 subunit may be involved in discriminating between Mor and Oxa. These findings lead us to an emerging hypothesis about the differences between potentiation and inhibition (positive and negative allosteric modulation) in this system.
Cross-linking the α3 and β2 subunits at location M1 decreased channel function (Fig. 4); hence, movement in this area is necessary for optimal gating, in accord with our central hypothesis, and this disulfide bond favors the closed state. Although the differences are not large, the results suggest that oxidation is more effective in the presence of both ACh and Mor than in the resting state (Fig. 4A), which may indicate that α3Y168 and β2D190 are closer or more reactive in a fully activated state. These observations alone do not enable us to determine a direction for the movement of the β2 C loop relative to the α3(−) face. Restriction of movement of the benzodiazepine interface also decreases potentiation by those modulators of GABAA receptors (46).
The cysteine and phenylalanine substitutions in position 168 of α3 discriminated between Mor and Oxa modulation; when these were co-expressed with their respective β2D190 mutant partners, discrimination was lost for the double-cysteine mutant but maintained for α3Y168Fβ2D190Q (Fig. 3 and Table 1). The (−)-face single mutations α3E113R, α3Q30R and α3E32G, based on the α4 sequence, also differ in selectivity for Mor and Oxa (32, and unpublished observations). Thus, with further delineation of modulator specificity, we may find mutants that differentiate these modulators because specific modulator-receptor interactions have been perturbed directly, or because in binding, the modulator promotes or disrupts discrete interactions between β2 C loop and α3(−) face residues.
In a forthcoming study we have extended the disulfide trapping approach to several cysteine pairs between the β2 C loop and α3(−) face. Cross-linking of at least one pair (α3T115-β2S192) increases currents and including Mor during oxidation alters the changes for another pair (α3Y168-β2S192; unpublished observations). Thus, with judicious choices of pairs, we think it possible to determine the direction of movement of the β2 C loop with respect to α3, as well as pinpoint which residues discriminate between modulators. In turn, such studies may enable us to dissect contributions to gating mechanisms, in particular elucidating the determinants of positive and negative modulation (32), which will facilitate future drug development.
Supplementary Material
HIGHLIGHTS.
Some nAChR allosteric modulators bind to subunit interfaces
Disulfide trapping and mutations affect receptor activation and modulation
Receptor movement during allosteric modulation is distinct from agonist activation
Novel finding of non-canonical interface motion mediating nAChR allosteric modulation
ACKNOWLEDGMENTS
We thank Paul Chrisman for technical support, Steve Sando for the inspiration of the 168–190 pair, Bryan Leland for early pilot studies, and the Danish Institute for Study Abroad for the intangible. We also thank all the reviewers of the manuscript for very helpful critique, and Carrie Sibbald, Nipun Basrur, Annie Campbell and Jim Swartz for further support. This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [grant 1R15 NS070760-01 (to MML)], the Howard Hughes Medical Institute [Undergraduate Science Education grant 52006298 (to Grinnell College)], and by funding from Grinnell College.
ABBREVIATIONS
- nAChR
nicotinic acetylcholine receptor
- Mor
morantel
- Oxa
oxantel
- OR2
oocyte Ringer’s
- DTT
dithiothreitol
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thompson AJ, Lester HA, Lummis SC. The structural basis of function in Cys-loop receptors. Quart. Rev. Biophysics. 2010;43:449–499. doi: 10.1017/S0033583510000168. [DOI] [PubMed] [Google Scholar]
- 2.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 3.Picciotto MR, Kenny PJ. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb. Perspect. Med. 2013;3:1–12. doi: 10.1101/cshperspect.a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database System. Rev. 2006;2006 doi: 10.1002/14651858.CD005593. CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossberg GT, Schmitt FA, Meng X, Tekin S, Olin J. Effects of transdermal rivastigmine on ADAS-cog items in mild-to-moderate Alzheimer’s disease. Am. J. Alzheimers Dis. Other Dementias. 2010;25:627–633. doi: 10.1177/1533317510385808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an α4b2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. J. Amer. Medical Assoc. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 7.Siu EC, Tyndale RF. Non-nicotinic therapies for smoking cessation. Ann. Rev. Pharmacol. Toxicol. 2007;47:541–564. doi: 10.1146/annurev.pharmtox.47.120505.105354. [DOI] [PubMed] [Google Scholar]
- 8.Maelicke A, Albuquerque EX. Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer’s disease. Eur. J. Pharmacol. 2000;393:165–170. doi: 10.1016/s0014-2999(00)00093-5. [DOI] [PubMed] [Google Scholar]
- 9.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem. Pharmacol. 2011;82:915–930. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sine SM. End-plate acetylcholine receptor: structure, mechanism, pharmacology, and disease. Physiol. Rev. 2012;92:1189–1234. doi: 10.1152/physrev.00015.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 12.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J. Mol. Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Hilf RJC, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- 14.Seo S, Henry JT, Lewis AH, Wang N, Levandoski MM. The positive allosteric modulator morantel binds at noncanonical subunit interfaces of neuronal nicotinic acetylcholine receptors. J. Neurosci. 2009;29:8734–8742. doi: 10.1523/JNEUROSCI.1859-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harpsøe K, Ahring PK, Christensen JK, Jensen ML, Peters D, Balle T. Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J. Neurosci. 2011;31:10759–10766. doi: 10.1523/JNEUROSCI.1509-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzaferro S, Benallegue N, Carbone A, Gasparri F, Vijayan R, Biggin PC, Moroni M, Bermudez I. Additional acetylcholine (ACh) binding site at α4/α4 interface of (α4β2)2α4 nicotinic receptor influences agonist sensitivity. J. Biol. Chem. 2011;286:31043–31054. doi: 10.1074/jbc.M111.262014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelstein SJ, Changeux JP. Allosteric transitions of the acetylcholine receptor. Adv. Protein Chem. 1998;51:121–184. doi: 10.1016/s0065-3233(08)60652-x. [DOI] [PubMed] [Google Scholar]
- 18.Jackson MB. Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 1989;86:2199–2203. doi: 10.1073/pnas.86.7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux J-P, Delarue M, Corringer P-J. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 20.Mukhtasimova N, Sine SM. An intersubunit trigger of channel gating in the muscle nicotinic receptor. J. Neurosci. 2007;27:4110–4119. doi: 10.1523/JNEUROSCI.0025-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadey S, Auerbach A. An integrated catch-and-hold mechanism activates nicotinic acetylcholine receptors. J. Gen. Physiol. 2010;140:17–28. doi: 10.1085/jgp.201210801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WY, Sine SM. Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature. 2005;438:243–247. doi: 10.1038/nature04156. [DOI] [PubMed] [Google Scholar]
- 23.Auerbach A. The gating isomerization of neuromuscular acetylcholine receptors. J. Physiol. 2010;588:573–586. doi: 10.1113/jphysiol.2009.182774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyford LK, Sproul AD, Eddins D, McLaughlin JT, Rosenberg RL. Agonist-induced conformational changes in the extracellular domain of α7 nicotinic acetylcholine receptors. Mol. Pharmacol. 2003;64:650–658. doi: 10.1124/mol.64.3.650. [DOI] [PubMed] [Google Scholar]
- 25.Hanson S, Czajkowski C. Structural mechanisms underlying benzodiazepine modulation of the GABAA receptor. J. Neurosci. 2008;28:3490–3499. doi: 10.1523/JNEUROSCI.5727-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arias HR. Positive and negative modulation of nicotinic receptors. Adv. Protein Chem. Struct. Biol. 2010;80:153–203. doi: 10.1016/B978-0-12-381264-3.00005-9. [DOI] [PubMed] [Google Scholar]
- 27.Young GT, Zwart R, Walker AS, Sher E, Millar NS. Potentiation of a7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc. Natl. Acad. Sci. USA. 2008;105:14686–14691. doi: 10.1073/pnas.0804372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill JK, Savolainen M, Young GT, Zwart R, Sher E, Millar NS. Agonist activation of a7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc. Natl. Acad. Sci. USA. 2011;108:5867–5872. doi: 10.1073/pnas.1017975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulter J, Connolly J, Deneris E, Goldman D, Heinemann S, Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc. Natl. Acad. Sci. USA. 1987;84:7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallette J, Bohler S, Benoit P, Soudant M, Pons S, Le Novere N, Changeux J-P, Corringer P-J. An extracellular protein microdomain controls up-regulation of neuronal nicotinic acetylcholine receptors by nicotine. J. Biol. Chem. 2004;279:18767–18775. doi: 10.1074/jbc.M308260200. [DOI] [PubMed] [Google Scholar]
- 31.Chrisman PA, Podair JI, Jobe EM, Levandoski MM. Intra-subunit flexibility underlies activation and allosteric modulation of neuronal nicotinic acetylcholine receptors. Neuropharmacol. 2014;79:420–431. doi: 10.1016/j.neuropharm.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cesa LC, Higgins CA, Sando SR, Kuo DW, Levandoski MM. Specificity determinants of allosteric modulation in the neuronal nicotinic acetylcholine receptor: A fine line between inhibition and potentiation. Mol. Pharmacol. 2012;81:239–249. doi: 10.1124/mol.111.076059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horovitz A, Fersht A. Strategy for analysing the co-operativity of intramolecular interactions in peptides and proteins. J. Mol. Biol. 1990;214:613–617. doi: 10.1016/0022-2836(90)90275-Q. [DOI] [PubMed] [Google Scholar]
- 34.Maksay G. Allostery in pharmacology: Thermodynamics, evolution and design. Prog. Biophys. Mol. Biol. 2011;106:463–473. doi: 10.1016/j.pbiomolbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Auerbach A. The energy and work of a ligand-gated ion channel. J. Mol. Biol. 2013;425:1461–1475. doi: 10.1016/j.jmb.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br. J. Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukhtasimova N, Lee WY, Wang HL, Sine SM. Detection and trapping of intermediate states priming nicotinic receptor channel opening. Nature. 2009;459:451–454. doi: 10.1038/nature07923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu T-Y, Smith CM, Sine SM, Levandoski MM. Morantel allosterically enhances channel gating of neuronal nicotinic acetylcholine a3b2 receptors. Mol. Pharmacol. 2008;74:466–475. doi: 10.1124/mol.107.044388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gleitsman KR, Shanata JAP, Frazier SJ, Lester HA, Dougherty D. Long-range coupling in an allosteric receptor revealed by mutant cycle analysis. Biophysical J. 2009;96:3168–3178. doi: 10.1016/j.bpj.2008.12.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dellisanti CD, Hanson SM, Chen L, Czajkowski C. Packing of the extracellular domain hydrophobic core has evolved to facilitate pentameric ligand-gated ion channel function. J. Biol. Chem. 2011;286:3658–3670. doi: 10.1074/jbc.M110.156851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J. Assembly of human neuronal nicotinic receptor α5 subunits with α3, β2, and β4 subunits. J. Biol. Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- 42.Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4 and hα7 expressed in Xenopus oocytes . J. Pharmacol. Exp. Therap. 1997;280:346–356. [PubMed] [Google Scholar]
- 43.Reeves DC, Jansen M, Bali M, Lemster T, Akabas MH. A role for the β1-β2 loop in the gating of 5-HT3 receptors. J. Neurosci. 2005;25:9358–9366. doi: 10.1523/JNEUROSCI.1045-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee WY, Free CR, Sine SM. Binding to gating transduction in nicotinic receptors: Cys-loop energetically couples to pre-M1 and M2-M3 regions. J. Neurosci. 2009;29:3189–3199. doi: 10.1523/JNEUROSCI.6185-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horenstein J, Wagner DA, Czajkowski C, Akabas MH. Protein mobility and GABA-induced conformational changes in GABAA receptor pore-lining M2 segment. Nature Neurosci. 2001;4:477–485. doi: 10.1038/87425. [DOI] [PubMed] [Google Scholar]
- 46.Hanson SM, Czajkowski C. Disulphide trapping of the GABAA receptor reveals the importance of the coupling interface in the action of benzodiazepines. Br. J. Pharmacol. 2011;162:673–687. doi: 10.1111/j.1476-5381.2010.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purohit P, Auerbach A. Acetylcholine receptor gating: movement in the alpha-subunit extracellular domain. J. Gen. Physiol. 2007;130:569–579. doi: 10.1085/jgp.200709858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lape R, Colquhoun D, Sivilotti LG. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–777. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang YC, Wu W, Zhang JL, Huang Y. Allosteric activation mechanism of the cys-loop receptors. Acta Pharmacol. Sinica. 2009;30:663–672. doi: 10.1038/aps.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prince RJ, Sine SM. Epibatidine binds with unique site and state selectivity to muscle nicotinic acetylcholine receptors. J. Biol. Chem. 1998;273:7843–7849. doi: 10.1074/jbc.273.14.7843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.