Abstract
Objective
The purpose of this study was to examine the incidence of nuisance bleeding after AMI and its impact on QOL.
Background
Prolonged dual antiplatelet therapy (DAPT) is recommended after acute myocardial infarction (AMI) to reduce ischemic events, but it is associated with increased rates of major and minor bleeding. The incidence of even lesser degrees of post-discharge “nuisance” bleeding with DAPT and its impact on quality of life (QOL) are unknown.
Methods
Data from the 24-center TRIUMPH (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status) study of 3,560 patients, who were interviewed at 1, 6, and 12 months after AMI, were used to investigate the incidence of nuisance bleeding (defined as Bleeding Academic Research Consortium type 1). Baseline characteristics associated with “nuisance” bleeding and its association with QOL, as measured by the EuroQol 5 Dimension visual analog scale, and subsequent re-hospitalization were examined.
Results
Nuisance (Bleeding Academic Research Consortium type 1) bleeding occurred in 1,335 patients (37.5%) over the 12 months after AMI. After adjusting for baseline bleeding and mortality risk, ongoing DAPT was the strongest predictor of nuisance bleeding (rate ratio [RR]: 1.44, 95% confidence interval [CI]: 1.17 to 1.76 at 1 month; RR: 1.89, 95% CI: 1.35 to 2.65 at 6 months; and RR: 1.39, 95% CI: 1.08 to 1.79 at 12 months; p < 0.01 for all comparisons). Nuisance bleeding at 1 month was independently associated with a decrement in QOL at 1 month (−2.81 points on EuroQol 5 Dimension visual analog scale; 95% CI: 1.09 to 5.64) and non-significantly toward higher re-hospitalization (hazard ratio: 1.20; 95% CI: 0.95 to 1.52).
Conclusions
Nuisance bleeding is common in the year after AMI, associated with ongoing use of DAPT, and independently associated with worse QOL. Improved selection of patients for prolonged DAPT may help minimize the incidence and adverse consequences of nuisance bleeding.
INTRODUCTION
The treatment of acute myocardial infarction (AMI) and chronic ischemic heart disease entails a careful balance between maximizing antiplatelet therapy to minimize ischemic events (1) while avoiding bleeding (2–5). The incidence and prognostic importance of major and minor bleeding after AMI or percutaneous coronary intervention (PCI) are well recognized (6–10). Clinical predictors of in-hospital bleeding have been defined, and its risk can be estimated using validated risk scores, such as the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines) bleeding model (11,12). In contrast, little is known about post-discharge bleeding, including “nuisance” bleeding, which can be defined as easy bleeding, bruising, and nose or gum bleeds. The importance of this more minor degree of bleeding was recently underscored by the Bleeding Academic Research Consortium (BARC) definition of bleeding (13), which defined BARC type 1 bleeding as “bleeding that does not cause the patient to seek medical care by a healthcare professional or hospitalization, and is not actionable” but may still be significant from patients’ perspectives. The prevalence, predictors, and impact of nuisance bleeds on outcomes after AMI are currently unknown.
What few reports (14,15) do exist on the long-term incidence and consequences of nuisance bleeding are limited to single-center experiences of lower-risk patients after PCI, where it has been suggested that post-discharge nuisance bleeding is common and potentially associated with adverse cardiac events. No report has ever examined the association of nuisance bleeding with patients’ quality of life (QOL). To better address the existing gap in knowledge in the prevalence, predictors, and consequences of nuisance bleeding in the setting of AMI, we examined data from the prospective multicenter, TRIUMPH (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status) study (16), which included detailed follow-up of patients’ self-reported bleeding events and QOL. We sought to clarify the magnitude and significance of nuisance bleeding after AMI. We investigated the incidence of nuisance bleeding after AMI in TRIUMPH, its predictors including its association with ongoing dual antiplatelet therapy (DAPT) use during follow-up, and its impact on health-related QOL and subsequent re-hospitalization events.
METHODS
TRIUMPH Population
The TRIUMPH study is a prospective, multicenter cohort study of 4,340 patients with AMI (both non–ST-segment and ST-segment elevation myocardial infarction) enrolled at 24 US centers between April 11, 2005, and December 31, 2008 (16). Patients were eligible for inclusion in the TRIUMPH study if they were aged ≥18 years and had an AMI, supported by elevated biomarkers and either electrocardiographic changes or symptoms consistent with the diagnosis (16). Patients were not eligible for enrollment if they were transferred to the participating hospital from another facility >24 h after their original AMI presentation, refused, were incarcerated, or could not provide informed consent. TRIUMPH was approved by the institutional review boards at each participating site, and written informed consent was obtained from all participants.
Study Population
For the purposes of this study, we included patients who had at least one follow-up visit after the index AMI hospitalization at 1, 6, or 12 months from which information on patient-reported bleeding events and DAPT use was recorded.
Predictors and Outcomes
In TRIUMPH, nuisance bleeding was assessed via the following four questions at 1-, 6- and 12-month interviews: “Since leaving the hospital after your heart attack, have you had: 1) Easy or significant bleeding? 2) Significant bruising? 3) Gum bleeds or nose bleed? 4) Serious bleeding?” We applied the newly developed BARC bleeding definition (13) as a framework to define the primary predictor variable of interest, nuisance bleeding (BARC type 1), as the occurrence of any of the above four bruising/bleeding events, which did not lead to: 1) hospitalization (BARC type 2 or higher bleeding, n=13), 2) blood transfusion (BARC type 3 or higher bleeding, n=27), or 3) change of medications by a physician (BARC type 2 or higher bleeding, n=32). The final analytic cohort included 3,560 patients.
Health-related QOL was the main outcome of interest. The EuroQOL-5D (EQ-5D) instrument was used to assess QOL in the TRIUMPH study. The EQ-5D is a generic measure of health status developed by the EuroQoL group (17) which measures health status in five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension is rated on the following levels: 1) no problems, 2) some problems, 3) extreme problems. The patients also estimate their total health status on a 20-centimeter visual analog scale (VAS) with the end-points being “Best imaginable health state” = a score of 100, and “Worst imaginable health state” = a score of 0 (17). The EQ-5D VAS is thus a quantitative measure of patients’ perceived health – a patient-centered health outcome. EQ-5D has been validated for patients with acute coronary syndrome (18,19). Rehospitalization at 1 year was a secondary outcome of interest. Trained data collectors tracked rehospitalization events, which were then centrally adjudicated, independently of the site investigator(s).
Statistical analyses
Baseline clinical and demographic characteristics of patients who reported nuisance bleeding versus those who did not were compared using the chi-square or Fisher exact test for categoric variables, as appropriate. Independent t tests or Mann–Whitney Wilcoxon U tests were used to compare continuous variables. Independent correlates of nuisance bleeding were determined using a multivariable, repeated measures, modified Poisson regression model with robust variance estimation (20). A repeated-measures approach was chosen because repeated measurements within a cluster (e.g., a person’s likelihood to bleed over time intervals) are more correlated than measurements in different clusters. A repeated-measures design offers the advantage of controlling for within-subject variance (the tendency to bleed) and is a more conservative analytic approach. We modeled nuisance bleeding at 1, 6, and 12 months as the dependent variable (repeated measures). A key predictor variable of interest was DAPT therapy at 1, 6, or 12 months. For patients discharged with DAPT therapy and continuing to report DAPT use at 1, 6, or 12 months, we assumed continued use of DAPT between assessments. Other covariates included confounders of bleeding, such as age, female gender, insurance status, GRACE (Global Registry of Acute Coronary Events) 6-month mortality risk score (21), CRUSADE bleeding risk score (12), history of atrial fibrillation, occurrence of in-hospital bleeding, baseline hemoglobin and creatinine, development of atrial fibrillation or warfarin use during the initial AMI hospitalization, or coronary artery bypass grafting (CABG) at the time of the initial AMI. We explored interactions of every covariate with the 3 time points and included the following 3 significant time interactions: CABG during the initial AMI hospitalization, atrial fibrillation or warfarin use during the initial AMI hospitalization, and baseline creatinine. In this repeated-measures modeling approach, exposure to clopidogrel during the 3 time points (1, 6, and 12 months), was matched with bleeding during the corresponding time intervals, and it preserved the temporal association between the exposure and the outcome during the corresponding time intervals.
The association of self-reported bleeding at 1 month with 1-month health status outcomes was evaluated using hierarchical (adjusting for site as a random effect) multivariable linear regression model for the EQ-5D health status outcome. This model adjusted for the baseline health status EQ-5D VAS score, age (per 10 years), female gender, insurance status, in-hospital PCI, in-hospital CABG, GRACE 6-month mortality risk score, CRUSADE bleeding risk score, warfarin use, history of atrial fibrillation, occurrence of in-hospital bleeding, baseline hemoglobin, and baseline creatinine.
As a secondary analysis, the association of nuisance bleeding at 1 month with subsequent (beyond 1 month) re-hospitalization during the 12-month follow-up period was assessed by using Kaplan-Meier survival analysis and compared using the log-rank test. Multivariable Cox regression, stratified by site and adjusting for the listed confounders associated with bleeding, mortality, and re-hospitalization, was performed to identify the independent association of nuisance bleeding at 1 month with subsequent rehospitalization. We also examined the association of early nuisance bleeding at 1 month after discharge with future more serious Thrombolysis In Myocardial Infarction (TIMI) major or minor bleeding, as well as nuisance bleeding (at 6 or 12 months).
RESULTS
Of the 3,560 included patients with AMI, 1,335 (37.5%) reported a nuisance (BARC type I) bleeding episode during the 12-month follow-up period. Most nuisance bleeding occurred early, at 1 month (n = 864; 64.7% of all reports of bleeding); nevertheless, nuisance bleeding continued to occur throughout the 12 months of follow-up (n = 338, 25.3% at 6 months; n = 476, 35.7% at 12 months). The most common type of nuisance bleeding reported was “bruising and bleeding” (n = 714, 53.5% at 1 month; n = 272, 20.4% at 6 months; n = 412, 30.9% at 12 months).
In unadjusted analyses, patients with nuisance bleeding were more likely to be older, to be female, to have experienced severe bleeding at the time of their initial hospitalization, and to have had a higher CRUSADE bleeding risk score. Patients with nuisance bleeding also were more likely to have a history of or develop atrial fibrilation during hospitalization or follow-up and more likely to be initiated on warfarin at discharge or during follow-up (Table 1).
Table 1.
Baseline clinical and demographic characteristics by nuisance bleeding at any time point.
| Nuisance bleeding at any time point | P-Value | ||
|---|---|---|---|
| Yes n = 1335 |
No n = 2225 |
||
| Socio-demographics | |||
| Age | 59.7 ± 12.2 | 59.0 ± 11.9 | 0.102 |
| Sex Male Female |
832 (62.3%) 503 (37.7%) |
1552 (69.8%) 673 (30.2%) |
< 0.001 |
| Race White/Caucasian Black/African-American Other |
1035 (77.7%) 203 (15.2%) 94 (7.1%) |
1444 (65.1%) 636 (28.7%) 138 (6.2%) |
< 0.001 |
| Education > High School | 712 (53.5%) | 1084 (49.0%) | 0.009 |
| No insurance / Self-Pay | 246 (18.8%) | 508 (23.2%) | 0.002 |
| BMI | 29.4 ± 6.4 | 29.8 ± 6.5 | 0.109 |
| Risk factors | |||
| Diabetes | 366 (27.4%) | 689 (31.0%) | 0.025 |
| Dyslipidemia | 656 (49.1%) | 1110 (49.9%) | 0.665 |
| Hypertension | 869 (65.1%) | 1481 (66.6%) | 0.371 |
| Chronic Heart Failure | 85 (6.4%) | 176 (7.9%) | 0.087 |
| Peripheral Vascular Disease | 64 (4.8%) | 107 (4.8%) | 0.984 |
| Prior CAD (MI/PCI/CABG) | 407 (30.5%) | 699 (31.4%) | 0.562 |
| Prior CVA | 68 (5.1%) | 98 (4.4%) | 0.345 |
| Chronic Kidney Disease | 84 (6.3%) | 147 (6.6%) | 0.712 |
| Atrial Fibrillation | 75 (5.6%) | 86 (3.9%) | 0.015 |
| Disease severity and therapies | |||
| CRUSADE bleeding risk score | 25.9 ± 15.2 | 24.8 ± 15.0 | 0.048 |
| GRACE 6m Mortality Risk Score | 99.4 ± 29.7 | 100.2 ± 28.8 | 0.395 |
| Final MI Diagnosis STEMI NSTEMI |
628 (47.0%) 707 (53.0%) |
950 (42.7%) 1275 (57.3%) |
0.012 |
| In-Hospital PCI | 986 (73.9%) | 1399 (62.9%) | < 0.001 |
| Drug Eluting Stent | 554 (41.5%) | 847 (38.1%) | 0.043 |
| In-Hospital CABG | 87 (6.5%) | 255 (11.5%) | < 0.001 |
| In-Hospital Bleeding | 155 (11.6%) | 207 (9.3%) | 0.027 |
| In-Hospital Bleeding Class TIMI major TIMI minor TIMI minimal |
22 (14.3%) 57 (37.0%) 75 (48.7%) |
52 (25.1%) 70 (33.8%) 85 (41.1%) |
0.039 |
| Warfarin at discharge | 180 (13.5%) | 181 (8.1%) | < 0.001 |
| Warfarin at 1m | 154 (11.5%) | 122 (5.5%) | < 0.001 |
| Warfarin at 6m | 94 (7.0%) | 104 (4.7%) | 0.003 |
| Warfarin at 1y | 77 (5.8%) | 77 (3.5%) | 0.001 |
| Warfarin at DC or in-hospital Atrial Fib | 224 (16.8%) | 270 (12.1%) | < 0.001 |
| Thienopyridine at DC | 1133 (84.9%) | 1595 (71.7%) | < 0.001 |
| Thienopyridine at 1m | 869 (82.1%) | 985 (66.4%) | < 0.001 |
| Thienopyridine at 6m | 731 (77.3%) | 878 (62.8%) | < 0.001 |
| Thienopyridine at 1y | 675 (71.6%) | 705 (56.5%) | < 0.001 |
Association of DAPT with nuisance bleeding
In unadjusted analysis, DAPT use at discharge was more common among patients who developed nuisance bleeding than in those who did not (85.0% vs. 72.4%, p < 0.001). DAPT use at 1, 6, and 12 months was strongly associated with nuisance bleeding (Table 1). In multivariable analyses, ongoing DAPT was strongly and independently associated with an approximately 2-fold higher risk of nuisance bleeding. This association was present even after adjustment for the CRUSADE bleeding risk score, the GRACE mortality risk score, atrial fibrillation, warfarin use, and other covariates (Fig. 1). Other variables independently associated with nuisance bleeding were female gender (rate ratio [RR]: 1.29, 95% confidence interval [CI]: 1.19 to 1.40, p < 0.0001) and history of atrial fibrillation (RR: 1.35, 95% CI: 1.15 to 1.57, p = 0.0002).
Figure 1. Independent association of self-reported nuisance bleeding with DAPT at discharge or 1 month, 6 months or 1 year.
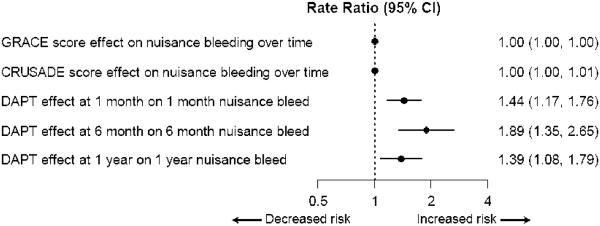
This is a multivariable, repeated-measures, modified Poisson regression model with robust variance estimation. Nuisance bleeding at 1, 6, and 12 months is the dependent variable (repeated measures), and the independent variables were those considered to be predictors of bleeding, including DES use, DAPT at discharge, 1 month, 6 months, or 12 months (main exposure variable), age, female gender, insurance status, GRACE 6-month mortality risk score, CRUSADE bleeding risk score, history of atrial fibrillation at baseline, occurrence of in-hospital bleeding, baseline hemoglobin and baseline creatinine, development of atrial fibrillation or warfarin use during hospitalization, or coronary artery bypass graft (CABG) surgery in-hospital and all significant interactions of above covariates with the three time-points. Variables other than CRUSADE and GRACE risk scores not shown. Other statistically significant variables associated independently with nuisance bleeding were female gender (RR 1.29, 95% CI 1.19 to 1.40, P-value <0.0001) and history of atrial fibrillation (RR 1.35, 95% CI 1.15 to 1.57, P-value = 0.0002) [Data not shown in the figure]. X-axis graphs the rate ratio (RR). Numbers alongside the floating bars denote the corresponding rate ratios and 95% CI.
Association of nuisance bleeding with quality of life
Patients who developed nuisance bleeding had more pain/discomfort and anxiety/depression, and an overall lower EQ-5D visual analog score at 1, 6, or 12 months (Table 2).
Table 2.
Health status outcomes measured via the EQ5D questionnaire by categories of nuisance bleeding.
| Nuisance bleeding at any time-point | P-value | ||
|---|---|---|---|
| Yes (N =1335) | No (N = 2225) | ||
| EQ-5D: Pain/discomfort (baseline) I have no pain or discomfort I have moderate pain or discomfort I am extreme pain or discomfort |
795 (60.1%) 466 (35.2%) 62 (4.7%) |
1405 (63.7%) 711 (32.2%) 91 (4.1%) |
0.103 |
| EQ-5D: Anxiety/depression (baseline) I am not anxious or depressed I am moderately anxious or depressed I am extremely anxious or depressed |
820 (62.1%) 424 (32.1%) 77 (5.8%) |
1481 (67.2%) 624 (28.3%) 98 (4.4%) |
0.005 |
| EQ-5D: Visual analog scale (baseline) | 64.4 ± 21.4 | 65.4 ± 21.5 | 0.148 |
| EQ-5D: Pain/discomfort (1 month) I have no pain or discomfort I have moderate pain or discomfort I am extreme pain or discomfort |
698 (58.8%) 444 (37.4%) 46 (3.9%) |
1057 (63.7%) 546 (32.9%) 56 (3.4%) |
0.027 |
| EQ-5D: Anxiety/depression (1 month) I am not anxious or depressed I am moderately anxious or depressed I am extremely anxious or depressed |
777 (65.3%) 362 (30.4%) 50 (4.2%) |
1164 (70.3%) 451 (27.2%) 41 (2.5%) |
0.003 |
| EQ-5D: Visual analog scale (1 month) | 72.0 ± 19.9 | 73.5 ± 19.5 | 0.046 |
| EQ-5D: Pain/discomfort (6 months) I have no pain or discomfort I have moderate pain or discomfort I am extreme pain or discomfort |
595 (56.6%) 389 (37.0%) 67 (6.4%) |
978 (61.9%) 525 (33.2%) 76 (4.8%) |
0.015 |
| EQ-5D: Anxiety/depression (6 months) I am not anxious or depressed I am moderately anxious or depressed I am extremely anxious or depressed |
668 (63.9%) 325 (31.1%) 52 (5.0%) |
1086 (69.3%) 422 (26.9%) 59 (3.8%) |
0.013 |
| EQ-5D: Visual analog scale (6 months) | 73.7 ± 21.0 | 75.2 ± 19.7 | 0.068 |
| EQ-5D: Pain/discomfort (12 months) I have no pain or discomfort I have moderate pain or discomfort I am extreme pain or discomfort |
568 (52.9%) 434 (40.4%) 71 (6.6%) |
940 (59.8%) 542 (34.5%) 90 (5.7%) |
0.002 |
| EQ-5D: Anxiety/depression (12 months) I am not anxious or depressed I am moderately anxious or depressed I am extremely anxious or depressed |
677 (63.0%) 347 (32.3%) 50 (4.7%) |
1115 (70.9%) 404 (25.7%) 54 (3.4%) |
< 0.001 |
| EQ-5D: Visual analog scale (12 months) | 74.3 ± 20.6 | 76.0 ± 20.1 | 0.043 |
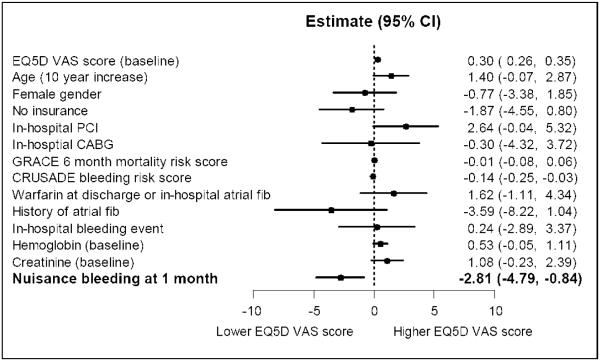
Adjusted, multivariable analysis showed that nuisance bleeding by 1 month was associated with a significant decline of 2.81 EQ-5D VAS score points (95% CI: 0.84 to 4.79 points) at 1 month that was independent of baseline QOL and other covariates (Fig. 2).
Figure 2. Independent association of nuisance bleeding with quality of life (EQ5D VAS).
Estimates (with 95% CI) indicate absolute points on the EQ5D VAS score. Negative sign indicates a decrement in QOL. This is a multivariable, hierarchical linear regression model with EQ5D VAS score at 1 month as the dependent variable and nuisance bleeding at 1 month as the predictor variable of interest. The model adjusts for other confounding variables including age, female gender, insurance status, GRACE 6-month mortality risk score, CRUSADE bleeding risk score, history of atrial fibrillation at baseline, baseline hemoglobin and baseline creatinine, development of atrial fibrillation or warfarin use during hospitalization, or coronary artery bypass graft (CABG) surgery in-hospital. X-axis graphs the change in QOL scores assessed via the EQ5D VAS. Numbers alongside the floating bars denote the corresponding estimate with its 95% CI.
Association of nuisance bleeding with subsequent rehospitalization
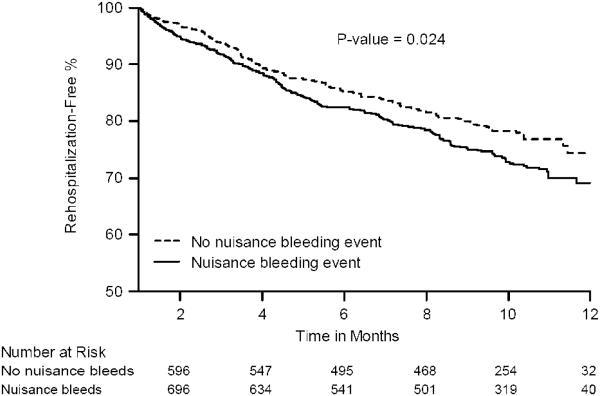
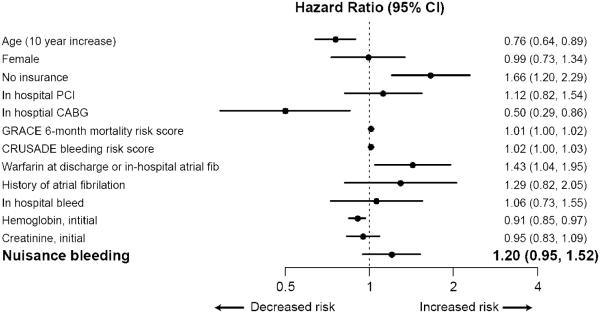
In unadjusted Kaplan-Meier analyses, patients with nuisance bleeding at 1 month were more likely to experience re-hospitalization by 12 months (Fig. 3A). However, after multivariable adjustment in Cox proportional hazards regression analysis, this association was attenuated and no longer significant (hazard ratio: 1.20, 95% CI: 0.95 to 1.52, p = 0.13) (Fig. 3B).
Figure 3. Association of nuisance bleeding with re-hospitalization.
Panel A: Kaplan-Meier survival estimates of rehospitalization-free event rate
X-axis represents time in months. Y-axis represents the probability of being rehospitalization-free. P-value is for the log-rank test.
Panel B: Cox multivariable regression model.
X-axis graphs the hazard ratio and the floating bars denote the corresponding estimate with its 95% CI.
Association of nuisance bleeding with overt bleeding
We found no association of nuisance bleeding within the first 30 days of discharge with more severe TIMI major or minor bleeding during subsequent follow-up (0.2% in the no nuisance bleeding group vs. 1.35% in the nuisance bleeding group, log-rank test p = 0.057, adjusted hazard ratio: 3.12, 95% CI: 0.33 to 29.34, p = 0.32). However, we found an association of 1-month nuisance bleeding with subsequent nuisance bleeding; subjects with nuisance bleeding at 1 month were twice as more likely to develop subsequent nuisance bleeding at 6 or 12 months (62.5%) than those who did not have nuisance bleeding at 1 month (38.25%) (adjusted RR: 1.61, 95% CI: 1.39 to 1.86, p < 0.0001). In TRIUMPH, 202 patients (5.7%) had an in-hospital TIMI major or minor bleeding event during the index AMI admission before hospital discharge. After excluding this group of patients, the incidence of nuisance bleeding during follow-up remained unchanged, from 1,335 (37.5%) in the full cohort to 1,255 (37.4%).
DISCUSSION
Among investigations examining the frequency and consequences of bleeding for patients after hospitalization for AMI, this is the first study to report the rates of less severe nuisance bleeding quantified within the framework of the recent BARC bleeding classification (13) as BARC type 1 bleeding. By analyzing data from the TRIUMPH study, a multicenter registry of patients with AMI that collected extensive baseline and follow-up data, we observed that approximately 40% of patients reported nuisance bleeding in the year after their AMI. Even after adjusting for numerous patient characteristics, including bleeding risk and other anticoagulants, there was a strong, independent association between post-discharge nuiansance bleeding and DAPT use. Moreover, such nuisance bleeding was associated with a significantly worse QOL and nonsignificantly higher rates of re-hospitalization. The clinical significant of our observed 3-point difference in those with and without nuisance bleeding is comparable to the difference in osteoporotic patients with and without vertebral fractures (22). Our findings suggest that these adverse patient experiences, which are largely ignored in clinical trials, may be important issues in clinical practice and warrant consideration at the time of AMI treatment.
Our study extends previous observations from the ranomized trials of DAPT use and has potentially important clinical implications. Previous clinical trials have reported an excess incidence of major and minor bleeding (BARC 2 or greater classification), but not nuisance bleeding (BARC 1). For example, both the CREDO (Clopidogrel for the Reduction of Events During Observation) trial (4) and the CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) study (23) observed an absolute increase in 1% to 2% for major bleeding in the year after the addition of clopidogrel to aspirin. More recently, the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance) (2) and the PRAGUE-8 (Clopidogrel Loading Dose for ad-Hoc Percutaneous Coronary Intervention Immediately Following Elective Coronary Angiography: Randomized Multicenter Trial Comparing Pre-Treatment >6 Hours Before Every Angiography vs. Cath-Lab Administration After Angiography [Just Before Intervention]) trials (5) reported an excess of bleeding with long-term clopidogrel and aspirin therapy. The present study provides novel information by describing the prevalence of BARC 1 bleeding events in routine clinical care and elucidating its association with patients’ QOL. In light of these data, discussing the downstream risks of DAPT with patients after AMI or drug-eluting stent (DES) implantation can better inform them of what to expect and heighten awareness. Other variables independently associated with nuisance bleeding were female gender and history of atrial fibrillation, and these patients, in particular, may be interested in considering therapies that could minimize their bleeding risks.
Our results also indicate that early nuisance bleeding events early after discharge after AMI predict future bleeding events during later follow-up. Thus, the recognition of early nuisance bleeding after AMI could alert the clinician to prompt closer monitoring of these patients for future bleeding events. However, we observed no difference in the nuisance bleeding rate after excluding those who had significant in-hospital TIMI major or minor bleeding events, suggesting in-hospital overt bleeding does not predict future nuisance bleeding.
These findings also have important implications for the design of future studies and comparative effectiveness trials of newer, more potent antiplatelet agents (24,25). Whereas traditional studies have only emphasized clinically large bleeding events in their outcomes assessments, we were able to categorize more mild degrees of bleeding using patient-reported interviews and to demonstrate a worsening of outcomes in those who reported such events. The association of BARC 1 bleeding with demonstrable decrements in QOL and a trend toward increased risks of readmission support the hierarchically graded, consensus classification for bleeding advocated by the BARC investigators (13). Identifying these risks of DAPT may be even more relevant with the more potent P2Y12 inhibitors. For example, the PLATO (PLATelet inhibition and patient Outcomes) trial, which did not use BARC bleeding definition, found bleeding rates of ticagrelor versus clopidogrel were similar to PLATO major bleeding (11.6% vs. 11.2%; p = 0.43), TIMI major bleeding (7.9% vs. 7.7%, p = 0.56), and the GUSTO (Global Utilization Of Streptokinase And Tpa For Occluded arteries) trial severe bleeding (2.9% vs. 3.1%, p = 0.22) (26). However, these comparisons with clopidogrel fail to quantify the potential impact of therapy on more minor bleeding events, which we found to be common and clinically relevant. Future studies may be improved by incorporating follow-up questions to classify milder degrees of nuisance bleeding (BARC 1) to provide a more complete assessment of important patient-centered outcomes. Such assessments also should be conducted in revascularization trials, when DES use may mandate prolonged DAPT. As second-generation DES platforms continue to evolve, emerging evidence suggests that prolonged DAPT therapy for contemporary stent designs exceeding 6 months may have lesser benefit, while increasing rates of bleeding (27,28). Incorporating patient-reported assessments of BARC bleeding in DES and antiplatelet therapy trials may therefore provide a more balanced perspective and add value in translating this evidence to clinical practice.
Our findings have important implications for adherence to DAPT (29). It has been reported in qualitative studies that an important reason for DAPT discontinuation was patients’ perception of unpleasant side effects from DAPT (rash, bleeding, bruising) and that the majority of DAPT continuers recalled having received education specifically on clopidogrel adherence (30,31). Our study, which found nuisance bleeding occurs commonly, underscores the importance of educating patients to anticipate nuisance bleeding and to not use that as a reason to discontinue DAPT without talking first with their physician.
Study limitations
First, we evaluated nuisance bleeding in the context of the BARC bleeding definitions based on patient self-report. Although the observed association with other adverse clinical outcomes supports the clinical significance of these reports, further validation of how best to ascertain BARC 1 bleeding events is likely warranted. Second, DAPT adherence rates were also self-reported, and we did not have access to pharmacy data to quantify the exact duration of DAPT use. It is possible that some degree of recall bias may confound these results, because patients taking DAPT regularly may be more likely to recall nuisance bleeding. Likewise, those not compliant with DAPT may be less likely to report nuisance bleeding. Third, despite multivariable adjustment of important confounders in all of the models, there remains the possibility of unmeasured confounding. In addition, the possibility of selection bias due to the exclusion of 16% patients with missing follow-up information may limit the precision and generalizability of our estimates. Finally, despite observing a statistically significant association between nuisance bleeding and a decrement in QOL, whether this is a causal relationship is unclear. It is possible that reduced QOL is multifactorial and due to comorbidities other than nuisance bleeding.
Conclusions
We found that patients commonly experience nuisance bleeding after AMI. Nuisance bleeding is reported by one of every two to three patients and is associated with worse QOL. Prolonged DAPT use was associated with a 1.5- to 2-fold higher risk of nuisance bleeding in the year after AMI. These findings collectively suggest even nuisance bleeds are significant to patients and deserve greater attention and consideration both in clinical recommendations for treatment and in future clinical trials of prolonged anti-thrombotic therapies.
Acknowledgments
Sources of funding: TRIUMPH was funded by P50 HL077113 from the NHLBI and support for these analyses were provided by an award from the American Heart Association Pharmaceutical Round Table and David and Stevie Spina.
ABBREVIATIONS
- PCI
Percutaneous coronary intervention
- DAPT
Dual anti-platelet therapy
- TIMI
Thrombolysis in myocardial infarction
- BARC
Bleeding Academic Research Consortium
- NB
Nuisance bleeding
- CAD
Coronary artery disease
- MI
Myocardial infarction
- AMI
Acute myocardial infarction
- DES
Drug eluting stents
- BMS
Bare metal stents
- QOL
Quality of life
- EQ-5D
Euro QOL – 5 Dimension
- VAS
Visual analog scale
Footnotes
Role of any Sponsor: No sponsor participated in the design and conduct of the study, collection, analysis, or interpretation of the data, nor in the preparation, review, nor approval of the manuscript.
Data Access and Responsibility: Drs. Amin, Spertus, and Ms. Reid had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- (1).Kushner FG, Hand M, Smith SC, Jr., et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- (2).Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. 20. [DOI] [PubMed] [Google Scholar]
- (3).Squizzato A, Keller T, Romualdi E, Middeldorp S. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD005158.pub3. %19;CD005158. [DOI] [PubMed] [Google Scholar]
- (4).Steinhubl SR, Berger PB, Mann JT, III, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. %20. [DOI] [PubMed] [Google Scholar]
- (5).Widimsky P, Motovska Z, Simek S, et al. Clopidogrel pre-treatment in stable angina: for all patients > 6 h before elective coronary angiography or only for angiographically selected patients a few minutes before PCI? A randomized multicentre trial PRAGUE-8. Eur Heart J. 2008;29:1495–1503. doi: 10.1093/eurheartj/ehn169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Steg PG, Huber K, Andreotti F, et al. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J. 2011;32:1854–1864. doi: 10.1093/eurheartj/ehr204. [DOI] [PubMed] [Google Scholar]
- (7).Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM, Bassand JP. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1193–1204. doi: 10.1093/eurheartj/ehm019. [DOI] [PubMed] [Google Scholar]
- (8).Mehran R, Pocock S, Nikolsky E, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4:654–664. doi: 10.1016/j.jcin.2011.02.011. [DOI] [PubMed] [Google Scholar]
- (9).Manoukian SV. The relationship between bleeding and adverse outcomes in ACS and PCI: pharmacologic and nonpharmacologic modification of risk. J Invasive Cardiol. 2010;22:132–141. [PubMed] [Google Scholar]
- (10).Manoukian SV. Predictors and impact of bleeding complications in percutaneous coronary intervention, acute coronary syndromes, and ST-segment elevation myocardial infarction. Am J Cardiol. 2009;104:9C–15C. doi: 10.1016/j.amjcard.2009.06.020. [DOI] [PubMed] [Google Scholar]
- (11).bu-Assi E, Gracia-Acuna JM, Ferreira-Gonzalez I, Pena-Gil C, Gayoso-Diz P, Gonzalez-Juanatey JR. Evaluating the Performance of the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines (CRUSADE) bleeding score in a contemporary Spanish cohort of patients with non-ST-segment elevation acute myocardial infarction. Circulation. 2010;121:2419–2426. doi: 10.1161/CIRCULATIONAHA.109.925594. [DOI] [PubMed] [Google Scholar]
- (12).Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:1873–1882. doi: 10.1161/CIRCULATIONAHA.108.828541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- (14).Ben-Dor I, Torguson R, Scheinowitz M, et al. Incidence, correlates, and clinical impact of nuisance bleeding after antiplatelet therapy for patients with drug-eluting stents. Am Heart J. 2010;159:871–875. doi: 10.1016/j.ahj.2010.01.016. [DOI] [PubMed] [Google Scholar]
- (15).Roy P, Bonello L, Torguson R, et al. Impact of "nuisance" bleeding on clopidogrel compliance in patients undergoing intracoronary drug-eluting stent implantation. Am J Cardiol. 2008;102:1614–1617. doi: 10.1016/j.amjcard.2008.07.063. [DOI] [PubMed] [Google Scholar]
- (16).Arnold SV, Chan PS, Jones PG, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Rabin R, de CF. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- (18).Schweikert B, Hahmann H, Leidl R. Validation of the EuroQol questionnaire in cardiac rehabilitation. Heart. 2006;92:62–67. doi: 10.1136/hrt.2004.052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ellis JJ, Eagle KA, Kline-Rogers EM, Erickson SR. Validation of the EQ-5D in patients with a history of acute coronary syndrome. Curr Med Res Opin. 2005;21:1209–1216. doi: 10.1185/030079905X56349. [DOI] [PubMed] [Google Scholar]
- (20).Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- (21).Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- (22).Dhillon V, Hurst N, Hannan J, Nuki G. Association of low general health status, measured prospectively by Euroqol EQ5D, with osteoporosis, independent of a history of prior fracture. Osteoporos Int. 2005;16:483–489. doi: 10.1007/s00198-004-1705-3. [DOI] [PubMed] [Google Scholar]
- (23).Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- (24).Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- (25).Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- (26).Becker RC, Bassand JP, Budaj A, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2011;32:2933–2944. doi: 10.1093/eurheartj/ehr422. [DOI] [PubMed] [Google Scholar]
- (27).Valgimigli M, Campo G, Monti M, et al. Short-versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–2026. doi: 10.1161/CIRCULATIONAHA.111.071589. [DOI] [PubMed] [Google Scholar]
- (28).Kedhi E, Stone GW, Kereiakes D, Serruys P, Parise H, Fahy M, Simonton C, Sudhir K, Sood P, Smits P. Stent thrombosis: Insights on outcomes, predictors and impact of dual antiplatelet therapy interruption from the SPIRIT II, SPIRIT III, SPIRIT IV and COMPARE trials. J Am Coll Cardiol. 59 doi: 10.4244/EIJV8I5A92. [13s1], E321. 2012. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- (29).Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113:2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. %20. [DOI] [PubMed] [Google Scholar]
- (30).Decker C, Garavalia L, Garavalia B, Spertus JA. Clopidogrel-taking behavior by drug-eluting stent patients: Discontinuers versus continuers. Patient Prefer Adherence. 2008;2:167–75. doi: 10.2147/ppa.s3443. 167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Garavalia L, Ho PM, Garavalia B, et al. Clinician-patient discord: exploring differences in perspectives for discontinuing clopidogrel. Eur J Cardiovasc Nurs. 2011;10:50–55. doi: 10.1016/j.ejcnurse.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]