Abstract
Poly(A) RNA binding proteins (Pabs) bind with high affinity and specificity to polyadenosine RNA. Textbook models show a nuclear Pab, PABPN1, and a cytoplasmic Pab, PABPC, where the nuclear PABPN1 modulates poly(A) tail length and the cytoplasmic PABPC stabilizes poly(A) RNA in the cytoplasm and also enhances translation. While these conventional roles are critically important, the Pab family has expanded recently both in number and in function. A number of novel roles have emerged for both PAPBPN1 and PABPC that contribute to the fine-tuning of gene expression. Furthermore, as the characterization of the nucleic acid binding properties of RNA binding proteins advances, additional proteins that show high affinity and specificity for polyadenosine RNA are being discovered. With this expansion of the Pab family, comes a concomitant increase in the potential for Pabs to modulate gene expression. Further complication comes from an expansion of the potential binding sites for Pab proteins revealed by an analysis of templated polyadenosine stretches present within the transcriptome. Thus, Pabs could influence mRNA fate and function not only by binding to non-templated poly(A) tail but also to internal stretches of adenosine. Understanding the diverse functions of Pab proteins is not only critical to understand how gene expression is regulated but also to understand the molecular basis for tissue-specific diseases that occur when Pab proteins are altered. Here we describe both conventional and recently emerged functions for PABPN1 and PABPC and then introduce and discuss three new Pab family members, ZC3H14, hnRNP-Q1, and LARP4.
Introduction
Messenger RNA (mRNA) is the intermediate molecule in the highly regulated flow of genetic information from DNA to protein. mRNA processing events, which are required throughout the life of the mRNA, are referred to as post-transcriptional processing and include the capping, splicing, cleavage and 3’end processing, export from the nucleus, localization, translation, and ultimate turnover of the mRNA1. Each of these events is tightly regulated and is mediated by a myriad of RNA binding proteins that coat the mRNA from its birth in the nucleus until its eventual degradation in the cytoplasm1, 2. The importance of these RNA binding proteins and the post-transcriptional processing events that they mediate is highlighted by a number of human diseases that are associated with dysregulation of their function3, 4.
One key post-transcriptional event is the 3’end processing of an mRNA transcript, which occurs via a two-step mechanism. In the first step, the mRNA undergoes an endonucleolytic cleavage event at a designated position within the transcript. Upon cleavage, a non-templated polyadenosine “tail”, or poly(A) tail, is added to the upstream cleavage product5, 6. The vast majority of eukaryotic mRNAs are cleaved and polyadenylated in this manner. The resulting poly(A) tails are bound by a specific family of RNA binding proteins termed poly(A) RNA binding proteins or Pabs7. The most conventional roles of the poly(A) tail are regulation of mRNA stability and translation8, 9. Two well-studied, or canonical, Pabs, poly(A) binding nuclear protein 1 (PABPN1) and cytoplasmic poly(A) binding protein (PABPC), bind to the poly(A) tail and regulate these processes7. PABPN1 stimulates polyadenylation and regulates poly(A) tail length in the nucleus while PABPC facilitates translation and modulates mRNA stability in the cytoplasm10, 11. Although these functions are critically important, Pabs perform additional functions that influence gene expression.
In recent years, a number of novel functions for the canonical Pabs have come to light7 and will be discussed in this review. Furthermore, recent studies have also led to the identification of additional members of the Pab family, three of which are discussed here: 1) Zinc finger CCCH-type containing protein #14 (ZC3H14); 2) Heterogeneous ribonucleoprotein Q1 (hnRNP-Q1); and 3) La-related protein 4 (LARP4). These proteins expand the Pab family not only by their unique roles in regulating various aspects of post-transcriptional processing, but also by the diversity in how they recognize and bind to polyadenosine RNA. Finally, the presence of internal, or templated, polyadenosine stretches within many mRNA transcripts suggests an additional point of entry for regulation of gene expression by Pabs. Not all transcripts contain internal polyadenosine stretches and therefore may provide a clue into the specificity of Pabs for certain classes or types of RNA transcripts, thus expanding our focus on polyadenosine RNA beyond non-templated poly(A) tails to include templated internal stretches of adenosine.
In this review, we briefly summarize the well-studied, or canonical, functions of PABPN1 and PABPC and then describe the newly defined roles for these proteins that have emerged in recent years. We then describe the three novel Pab family members and discuss their roles in modulating gene expression. Finally, we present an analysis that determines the number of templated polyadenosine stretches within the human transcriptome, suggesting the potential for regulation by such a motif. By integrating the wealth of information on Pabs and polyadenosine sequences described in recent years, we gain insight into the role of this protein family in regulating gene expression and build an updated, more comprehensive model for the function of these proteins and their target sequences.
Summary of the Canonical Pabs
Conventional Pab proteins are typically defined by the presence of an RNA recognition motif (RRM) (Figure 1) combined with biochemical evidence to demonstrate specific, high affinity binding to polyadenosine RNA. A single nuclear Pab, termed PABPN1, and multiple cytoplasmic Pabs, termed PAPBC1, 3–5 and L1, have been identified in multiple organisms (Table 1). PABPC1 is ubiquitously expressed while the remaining PABPC homologs differ in their spatial and temporal expression pattern (Table 1). PABPN1 and PABPC1 are the best studied Pab proteins and are hence referred to here as the canonical Pabs. The terms PABPN1 and PABPC will be used throughout the review to refer to any ortholog of PABPN1 or PABPC1 (Table 2).
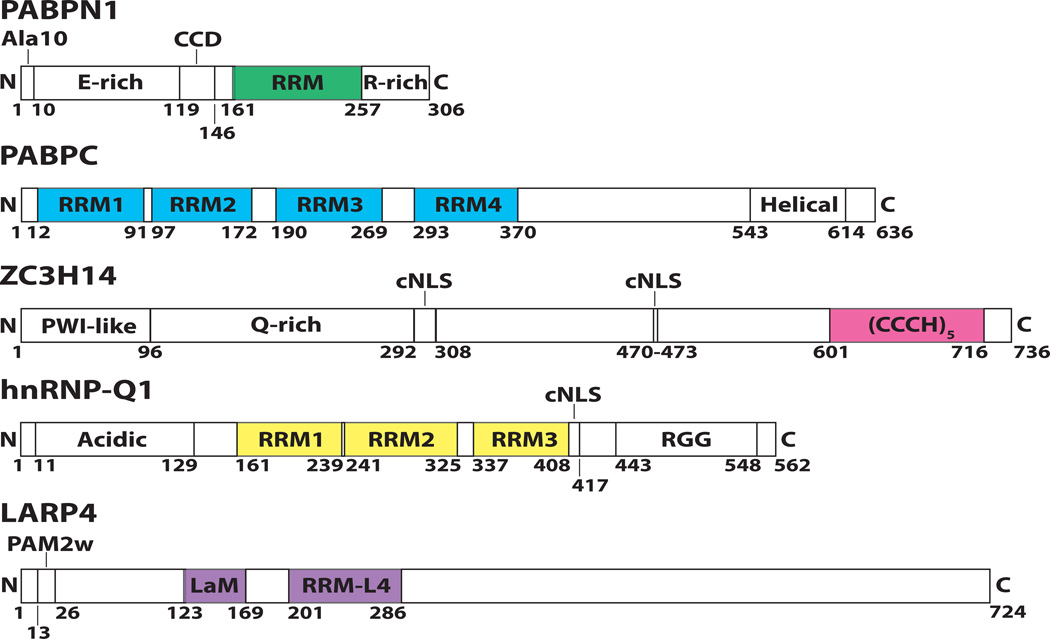
Figure 1. Pab protein domain structures and family members.
The domain structures for the five Pab family members discussed in this review (PABPN1, PABPC, ZC3H14, hnRNP-Q1, and LARP4) are schematized here. RNA binding modules are highlighted in colors that correspond with each of these proteins in subsequent figures. PABPN1 is a nuclear Pab that contains a stretch of 10 alanines (Ala10) that are expanded in OPMD, a glutamic acid-rich domain (E-rich), a coiled-coil domain (CCD), an RNA recognition motif (RRM) that is responsible for high affinity polyadenosine RNA binding, as well as an arginine-rich (R-rich) domain at the C-terminus. PABPC is a cytoplasmic Pab that interacts with polyadenosine RNA via RRMs (RRM1-4) and contains a C-terminal Helical domain. ZC3H14 is a novel nuclear Pab that interacts with polyadenosine RNA via tandem CysCysCysHis (CCCH) zinc fingers and also contains an N-terminal Proline Tryptophan Isoleucine-like (PWI-like) fold that mediates interactions with the nuclear pore, a glutamine-rich (Q-rich) domain of unknown function, and two putative classical nuclear localization signals (cNLS). hnRNP-Q1 is a novel cytoplasmic Pab that is presumed to bind polyadenosine RNA via RRMs (RRM1-3) and contains an Acidic N-terminal domain as well as an Arginine Glycine Glycine (RGG) domain, both of which mediate protein-protein interactions. A putative weak cNLS is also present in hnRNP-Q1. LARP4 is a novel cytoplasmic Pab that interacts with polyadenosine RNA via a La Motif (LaM) in conjunction with an RRM-like 4 domain (RRM-L4). LARP4 interacts with PABPC via a poly(A) binding protein interacting motif (PAM2) domain that contains an atypical tryptophan in the consensus sequence (PAM2w).
Table 1. PABPC Family Members.
PABPC homologs present in H. sapiens are listed. The mRNA expression pattern of each homolog, as described by the UCSC Genome Browser, is listed. Known protein functions and percent protein similarity to PABPC1 are indicated.
| Gene Name | Expression | Known Functions | Similarity |
|---|---|---|---|
| PABPC1164 | Ubiquitous | mRNA Translation, mRNA Decay, miRNA-mediated Repression, NMD, L1 Retrotransposition, mRNA Localization, Local Translation | -------- |
| PABPC3165 | Testes-specific | Spermatid mRNA Translation | 64% |
| PABPC4165 | T Cells & Other Tissues | Erythroid Differentiation, Upregulated upon T Cell Activation, PABPC1 Compensation | 67% |
| PABPC5166 | Fetal Brain & Other Adult Tissues | PABPC1 Compensation | 7% |
| PABPC1L167 | Ovaries, Testes & Other Adult Tissues | Oocyte mRNA Translation | 59% |
Table 2. Poly(A) Binding Protein Family Members.
The protein names of Pab family members discussed are shown. The names of the corresponding proteins in a number of common model organisms are provided. Proteins in parentheses have not been extensively studied in the organisms indicated. A dashed line indicates no known/obvious/described ortholog present in that organism.
| Poly(A) Binding Proteins | ||||||
|---|---|---|---|---|---|---|
| Species | H. sapiens | PABPN1 | PABPC1 | ZC3H14 | hnRNP-Q1 | LARP4 |
| M. musculus | Pabpn1 | Pabpc1 | Zc3h14 | Syncrip | (Larp4a) | |
| X. laevis | pabpn1 | pabpc1a/b | (zc3h14) | (syncrip) | (larp4a/b) | |
| D. rerio | (pabpn1) | pabpc1a/b | (zc3h14) | (syncrip) | (larp4a/b) | |
| D. melanogaster | Pabp2 | PABP | dNab2 | Syncrip | (Larp4) | |
| C. elegans | PABP-2 | PAB-2 | SUT-2 | HRP-2 | (LARP-5) | |
| S. pombe | pab2 | pabp | nab2 | ----- | ----- | |
| S. cerevisiae | ----- | Pab1 | Nab2 | ----- | ----- | |
Canonical PABPN1 Functions
PABPN1 is the primary nuclear Pab and is highly conserved among eukaryotes12. PABPN1 is expressed ubiquitously in metazoans and PABPN1 orthologs have been analyzed in D. melanogaster and C. elegans, as well as S. pombe. However, to date there is no comparable PAPBN1 counterpart in S. cerevisiae10 (Table 2). The PABPN1 protein consists of a number of functional domains, which are diagramed in Figure 1. The best characterized domain of PABPN1 is the RNA RRM found toward the C-terminus of the protein, which mediates high affinity polyadenosine RNA binding13. PABPN1 recognizes 10 to 11 nucleotides14, 15 and detailed binding studies suggest that most of these adenosines, if not all, are bound by PABPN1 in a base-specific manner15. The arginine-rich region located C-terminal to the RRM also contributes to adenosine RNA recognition16. This domain is asymmetrically methylated; however, the methylation has no apparent effect on the affinity, specificity, or cooperativity of RNA binding13, 16, and instead modulates interactions with the PABPN1 nuclear import receptor, transportin17, 18. The helical domain N-terminal to the RRM is required for stimulation of poly(A) polymerase (PAP). Of note, the stretch of ten alanines located immediately adjacent to the initiating methionine is expanded to anywhere from 12–17 alanines in the late-onset human disease, oculopharyngeal muscular dystrophy (OPMD), which is characterized by a progressive weakening of a specific group of muscles in the head as well as limb muscles in some instances of the disease12. How this modest alanine expansion leads to this tissue-specific disease is unknown.
The role of PABPN1 in polyadenylation has been extensively studied largely through elegant biochemical studies19–21. These conventional functions of PABPN1 are schematized in Figure 2. As stated in the Introduction, 3’-end processing of pre-mRNA transcripts occurs via a two-part process consisting of cleavage and subsequent polyadenylation. These processing events are mediated by a large, heterooligomeric protein complex that is comprised in part of PAP and the cleavage and polyadenylation specificity factor (CPSF)6. Upon cleavage of the pre-mRNA by CPSF-73, PAP begins to add adenosine residues to the upstream cleavage product in a slow, or distributive, manner. Binding of PABPN1 to the first 11 adenosines stimulates PAP activity and triggers processive polyadenylation19, 20. Evidence that PABPN1 increases the affinity of PAP for RNA, thus leading to processive polyadenylation by a tethering mechanism is provided through analysis of RNA binding mutants of PABPN1 that are unable to stimulate processive polyadenylation21. In addition, amino acid changes in the helical domain of PABPN1, which do not affect the affinity of PABPN1 for RNA, but do abrogate binding to PAP, also eliminate the ability PABPN1-mediated stimulation of processive polyadenylation20. In the “molecular ruler” model, PABPN1 binds the initial 11–14 adenosines, and then coats the growing poly(A) tail to form 21 nm spherical particles that enable the RNA to fold back and maintain an interaction between CPSF and PAP21, 22. When a length of 200–300 adenosines is reached, this complex is disrupted and the phase of processive polyadenylation is complete (Figure 2)21. This model is supported by experiments using electron and scanning force microscopy with oligo(A) RNA and purified PABPN1 protein22. These studies using primarily purified proteins have provided the field with significant insight into the role of PABPN1 in regulating polyadenylation; however, further studies to assess the role of PABPN1 in the context of other RNA binding proteins as well as other functions that have emerged for this protein are required to build a complete model of polyadenylation.
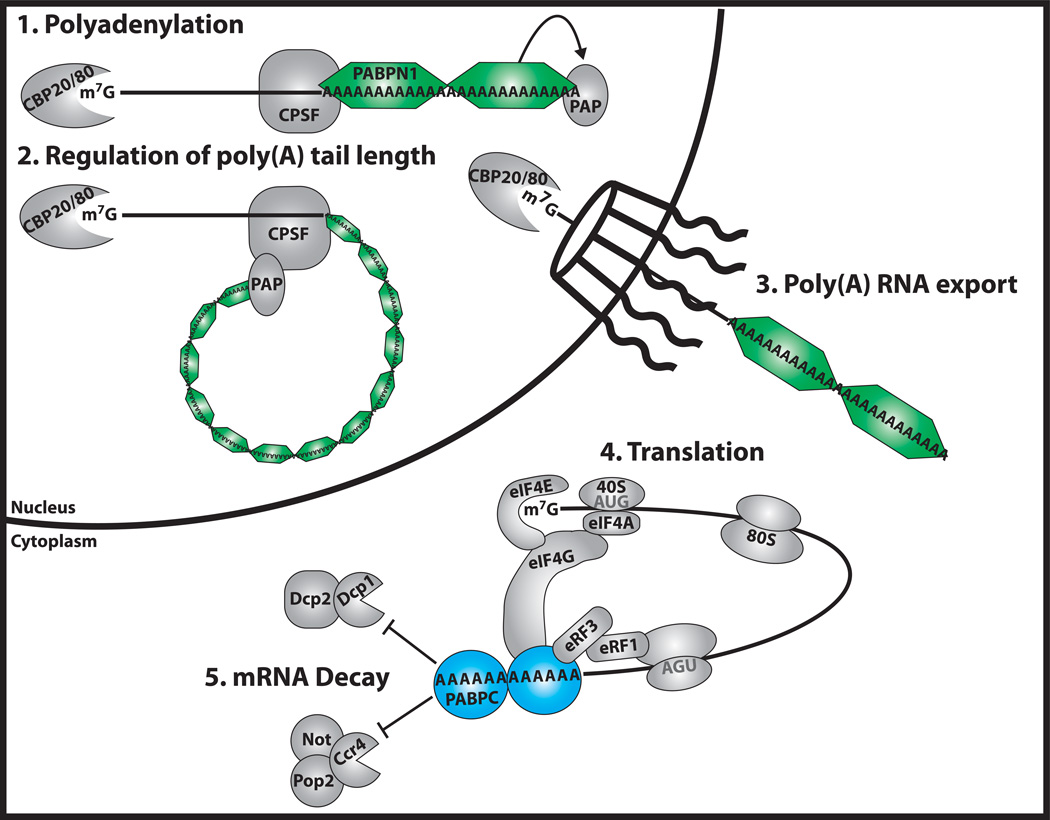
Figure 2. Model for the Canonical Functions of PABPN1 and PABPC.
The role of PABPN1 (green hexagon) in modulating 3’end processing of mRNA transcripts is well established and consists of the following molecular functions: 1. Polyadenylation: PABPN1 interacts with poly(A) polymerase to stimulate processive polyadenylation; 2. Regulation of poly(A) tail length: PABPN1 interacts with the cleavage and polyadenylation specificity factor (CPSF) to modulate and ensure proper poly(A) tail length; and 3. Poly(A) RNA export: Although whether the role is direct or indirect is unknown, defects in PABPN1 function can lead to nuclear accumulation of poly(A) RNA. This observation together with the fact that PABPN1 shuttles between the nucleus and the cytoplasm have led to the suggestion that PABPN1 function is required for efficient poly(A) RNA export from the nucleus. PABPC (blue circle) plays a well-defined role in modulating gene expression including: 4. Translation: PABPC binds to eukaryotic translation initiation factor 4G (eIF4G), which bridges interactions between the 5’- and 3’-ends of the mRNA and facilitates efficient translation initiation and 5. mRNA decay: PABPC binds to eukaryotic release factor 3 (eRF3), which facilitates ribosome recycling and thus inhibits mRNA decay by protecting the poly(A) tail from decapping enzymes (Dcp1 and Dcp2) as well as exonucleases such as the Ccr4-Pop2-Not complex. The following factors are also incorporated into the model shown: Cap binding proteins 20 and 80 (CBP20/80); 7 methylguanosine cap (m7G); eukaryotic translation initiation factor 4E (eIF4E); eukaryotic small ribosome (40S); eukaryotic translation initiation factor 4A (eIF4A); eukaryotic ribosome (80S); and eukaryotic release factor 1 (eRF1).
Proper 3’end processing and polyadenylation of transcripts is required for efficient mRNA export from the nucleus to the cytoplasm6. The finding that PABPN1 can shuttle between the nucleus and the cytoplasm has led to the suggestion that PABPN1 could contribute to poly(A) RNA export from the nucleus23 (Figure 2). Consistent with this suggestion, nuclear accumulation of poly(A) RNA is observed in cells expressing the viral NS1A protein, which binds to and inhibits the nuclear-cytoplasmic shuttling of PABPN124. Furthermore, primary muscle cells depleted of PABPN1 also display nuclear accumulation of bulk poly(A) RNA25. Altered steps in the polyadenylation process could cause this nuclear poly(A) RNA retention, as proper polyadenylation is required for efficient mRNA export from the nucleus6, 26, 27. However, two independent studies using templated poly(A) tracts present at the 3’-ends of reporter transcripts show that these artificial substrates are exported,28, 29 suggesting that the presence of a tail alone may be sufficient for RNA export and therefore raising the question of how the steps of polyadenylation are coordinated with the generation of an export-competent mRNP complex. These studies suggest that PABPN1 could regulate mRNA export from the nucleus, but further analyses are required to understand whether PABPN1 plays a direct role in modulating this critical step in gene expression or whether effects on upstream events such as polyadenylation impair subsequent mRNA export.
Altogether, these studies underscore the critically important role of PABPN1 in stimulating and regulating the polyadenylation of nascent mRNA transcripts. Whether PABPN1 plays a direct role in modulating mRNA export to the nucleus remains unclear but the question highlights the complexity of understanding the direct molecular functions of proteins involved in the highly interconnected steps of post-transcriptional processing.
Canonical PABPC Functions
The major cytoplasmic poly(A) binding protein, PABPC, is ubiquitously expressed and found in all eukaryotes (Table 2). As shown in Figure 1, PABPC contains four tandem RNA recognition motifs (RRMs), an unstructured linker region and a C-terminal helical domain with five α-helices10, 11. PABPC contacts polyadenosine RNA through the RRMs and requires a minimum of 12 nucleotides for high affinity binding30. Together RRMs 1 and 2 mediate oligo(A) binding while other pairwise combinations or single RRMs are not sufficient for binding31. However, binding of all four RRMs is required to account for the ~25 nucleotide footprint of PABPC30, 32. In addition, the N-terminal RRMs and the C-terminal α-helical domain serve as protein-protein interaction domains to mediate the various functions of PABPC. The RRMs facilitate binding to eukaryotic initiation factor 4G (eIF4G)33, the α-helices facilitate binding to eukaryotic release factor 3 (eRF3)34 and promote PABPC dimerization35 and both of these domains are involved in binding Poly(A) binding protein Interacting Proteins (PAIPs)36, 37 and GW182, an RNA induced silencing complex (RISC) protein38. Thus, PABPC serves as a platform to coordinate multiple players that modulate gene expression.
The role of PABPC in regulating mRNA translation has been extensively studied10, 11 (Figure 2). Studies with S. cerevisiae reveal that cytoplasmic mRNAs form a closed loop structure to promote 40S ribosomal subunit recruitment39. PABPC is required to assume this structure, which facilitates efficient translation33, 40, 41. The cap-binding complex, eIF4F, enables formation of the closed loop structure with the eIF4G subunit linking the 5’ cap-bound eIF4E subunit to poly(A) tail-bound PABPC41, 42. Further evidence suggests that PABPC could play a role in 60S ribosomal subunit joining8, 43, 44 and promote ribosome recycling by interacting with eukaryotic release factor 3 (eRF3)45. In summary, PABPC mediates a number of interactions between the translationally poised mRNA and ribosome that underlie the role in ensuring efficient translation.
Similar to the role in translation, the function of PABPC in modulating mRNA decay is well established10, 11 (Figure 2). mRNA decay is a multi-step process that is typically initiated by poly(A) tail shortening to a length of 10–15 nucleotides46. Deadenylation is followed by decapping with the Dcp1–Dcp2 complex and digestion by the 5’- to 3’-exoribonuclease Xrn146. PABPC inhibits the deadenylase, which blocks the initial step of mRNA decay47, 48. PABPC also interacts with other RNA binding proteins, including the novel Pab hnRNP-Q1 (discussed below), to inhibit the decay of specific mRNA transcripts by blocking deadenylase or endonuclease activity49–51. Therefore, PABPC affects both global and transcript-specific mRNA decay.
The well-studied roles of the canonical Pabs, PABPN1 and PABPC, in modulating polyadenylation, translation, and mRNA decay demonstrate the importance of these proteins in post-transcriptional processing. However, recent studies reveal diverse, novel functions of these canonical Pabs7, expanding our understanding of how these key proteins regulate gene expression and pointing toward a new model that integrates the novel and canonical functions of PABPN1 and PABPC.
Novel Functions of the Canonical Pabs
As discussed in the previous section, PABPN1 and PABPC play well-defined roles in polyadenylation, poly(A) tail length regulation, mRNA stability, and translation10, 11. However, in recent years a number of studies have unveiled multiple novel functions7 (Figure 3) for these two proteins in the post-transcriptional regulation of mRNAs which, can influence both the temporal and spatial control of gene expression.
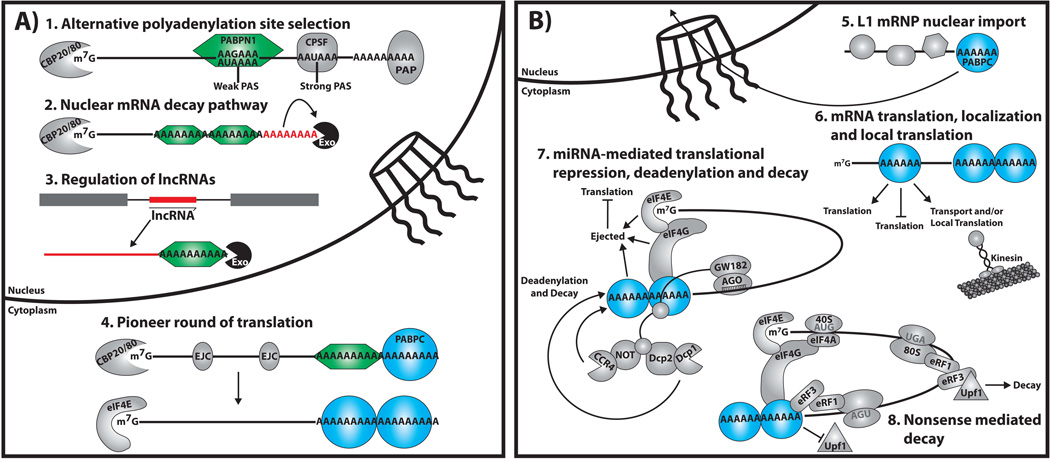
Figure 3. Model for Novel Functions of PABPN1 and PABPC.
A) Recent studies reveal that PABPN1 (green hexagon) plays diverse roles in modulating gene expression that extend beyond those shown in Figure 2 including impacting: 1. Alternative polyadenylation site selection: Loss of PABPN1 function leads to preferential use of proximal PolyAdenylation Sites (PAS), which are often weak sites relative to the commonly used distal sites; 2. Nuclear mRNA decay pathway: PABPN1 coordinates with other RNA processing factors (not shown) to generate hyperadenylated mRNAs (indicated by red As) that target the transcript for degradation via a novel nuclear mRNA decay pathway; 3. Regulation of lncRNA: PABPN1 also promotes the turnover, via the RNA exosome (Exo), of a specific subset of long noncoding RNAs (lncRNAs) in an oligoadenylation-dependent manner; and 4. Pioneer round of translation: PABPN1 remains bound to mRNAs as they exit the nucleus and enter the pioneer round of translation. PABPN1 likely cooperates with the exon junction complex (EJC) and PABPC and is replaced by PABPC following the pioneer round of translation. B) Recent work reveals that PABPC (blue circle) is required for a number of functions beyond control of translation and regulation of mRNA decay illustrated in Figure 2. These functions include: 5. L1 mRNP nuclear import: Although the mechanism is unknown, PABPC is required for the nuclear accumulation of the LINE1 Ribonucleoprotein (L1 RNP); 6. mRNA translation, localization, and local translation: PABPC can modulate the translation and may modulate the localization and local translation of specific mRNA transcripts by binding to internal A-rich sequences; 7. miRNA-mediated translational repression, deadenylation, and decay: PABPC promotes RNA-induced silencing complex (RISC) recruitment by binding to the RISC protein, GW182, which leads to the removal of PABPC, disruption of the closed loop structure and deadenylation by recruited deadenylase complexes; and 8. Nonsense mediated decay: PABPC inhibits recruitment of the nonsense mediated decay protein, Upf1, by interacting with eukaryotic release factor 3 (eRF3). The following factors are also incorporated into the model shown: Cap binding proteins 20 and 80 (CBP20/80); 7 methylguanosine cap (m7G); cleavage and polyadenylation specificity factor (CPSF); Poly(A) polymerase (PAP); eukaryotic translation initiation factor 4E (eIF4E); eukaryotic small ribosome (40S); eukaryotic translation initiation factor 4A (eIF4A); eukaryotic ribosome (80S); and eukaryotic release factor 1 (eRF1).
Novel PABPN1 Functions
Alternative polyadenylation
Recent work has uncovered a role for PABPN1 in influencing alternative cleavage and polyadenylation site selection (Figure 3A). This role for PABPN1 was initially uncovered in an unbiased genome-wide screen for modulators of alternative polyadenylation (APA) using an APA reporter52. This study found that siRNA-mediated depletion of PABPN1 results in 3’UTR shortening and preferential usage of proximal poly(A) sites (PASs). An alternative approach also uncovered a role for PABPN1 in APA in muscle, the tissue affected in OPMD patients12. This analysis employed 3’-end RNA-Seq analysis on transgenic mouse tissues that express mutant alanine-expanded PAPBPN1 (Ala17) to interrogate the impact of mutant PABPN1 on RNA53. Consistent with the study directed at defining modulators of APA52, this analysis revealed a major shift toward proximal PAS utilization in tissues expressing mutant PABPN1. Thus, two distinct approaches reveal a critical role for PABPN1 in modulating PAS site selection. The consequences of this shift to proximal cleavage sites include a decrease in repression by 3’UTR binding factors such as miRNAs. Additional experiments in both of these studies led both groups to invoke a model in which alanine-expanded PABPN1 sequesters endogenous PABPN1 in nuclear aggregates, which are commonly observed in OPMD patient cells12, resulting in a decrease of functional PABPN1 in the nucleus54. A decrease in functional PABPN1 in OPMD patient muscles could lead to shifts in APA that could contribute to patient phenotypes. These independent approaches strongly support a role for PABPN1 in APA; however, the mechanism by which PABPN1 modulates APA is unclear, as evidenced by distinct mechanistic models for how PABPN1 modulates APA selection proposed in the two studies. These differences demonstrate that further biochemical analyses are required to elucidate the precise mechanism by which PABPN1 influences APA.
lncRNAs
A role for PABPN1 in the regulation of long noncoding RNAs (lncRNAs), which are defined as non-coding RNAs that are greater than 200 nucleotides in length55, has recently been reported56 (Figure 3A). This function for PABPN1 emerged from an RNA-Seq analysis of the transcriptome of HeLa cells depleted of PABPN1 by siRNA. Interestingly, the majority of transcripts from protein-coding genes were unaffected by knockdown of PABPN1, which was somewhat surprising given the well-defined biochemical role of PABPN1 in 3’end processing and polyadenylation12. However, a subset of lncRNAs showed an increase in steady-state levels upon knockdown of PABPN1. A model lncRNA derived from a snoRNA host gene was used to demonstrate that PABPN1 promotes lncRNA turnover through an oligoadenylation-dependent mechanism. Further analysis revealed that this class of PABPN1-sensitive lncRNAs is targeted by specific subunits of the human Trf4-Air2-Mtr4 Polyadenylation (TRAMP) complex, an exosome co-factor57, in cooperation with the exosome, suggesting that PABPN1 regulates lncRNA expression, in a manner similar to that described for PABPN1 in polyadenylation-dependent RNA decay in fission yeast58.
Nuclear RNA Quality Control
A role for PABPN1 in promoting the decay of mRNAs subject to nuclear RNA quality control as well as polyadenylated nuclear noncoding RNAs (ncRNAs) has also recently been reported59 (Figure 3A). A nuclear RNA decay pathway that includes PABPN1, two isoforms of PAP (PAPα and PAPγ), and the catalytically active exonuclease subunits of the RNA exosome (RRP6 and DIS3) was defined in this work. In this pathway, PABPN1 and the PAP enzymes produce hyperadenylated RNA products that are subsequently targeted and degraded by the exosome. Experiments using cordycepin, which causes premature termination of polyadenylation60, and PAP-stimulating mutants of PABPN1 demonstrate that the extension of the poly(A) tail is necessary for decay as cordycepin treatment led to the accumulation of more stable transcripts with shorter poly(A) tails. Transcripts that undergo efficient splicing and export are not impacted by this pathway, invoking a model in which PABPN1, together with PAPα and PAPγ, plays a role in nuclear RNA quality control and promotes exosome-mediated decay of nuclear polyadenylated transcripts that are delayed in processing.
Pioneer round of translation
The pioneer round of translation of an mRNA is a key quality control checkpoint that selects for correctly processed and translation-competent mRNAs61. Pioneer translation initiation complexes differ from steady-state translation initiation complexes by the complement and stoichiometry of RNA-binding proteins and associated complexes61. However, how the process of translation facilitates the remodeling of the pioneer translation initiation complexes into steady-state translation initiation complexes is not yet known. PABPN1, which localizes to the nucleus at steady-state, can shuttle to the cytoplasm and associate with nascent mRNA transcripts that have undergone the pioneer round of translation62. However, in polysome profile analyses, PABPN1 is associated with fewer ribosomes than PABPC63, suggesting that PABPN1 may be replaced by PABPC on translating mRNAs. Immunoprecipitation of PABPN1 and PABPC from HeLa cell lysates expressing iron-responsive mRNA reporters revealed a significant decrease in the amount of reporter mRNA that was enriched upon PABPN1 pulldown following the stimulation of translation64. Together, these data support a model in which the process of translation either stimulates the removal of PABPN1 and/or enhances binding of PABPC, ultimately resulting in the replacement of PABPN1 by PABPC on translating mRNAs (Figure 3A).
These recent studies greatly expand our understanding of the function of PABPN1, extending beyond the regulation of polyadenylation in the nucleus to diverse roles in mRNA processing events. These newly discovered roles for PABPN1 as a key factor in influencing poly(A) site selection, regulating lncRNA expression, and coordinating a novel nuclear RNA decay pathway paint a new picture of PABPN1 as a multifunctional player in the highly regulated post-transcriptional control of gene expression.
Novel PABPC Functions
miRNA-mediated translational repression, deadenylation and decay
In an extension of the canonical role for PABPC in regulating mRNA translation and stability, PABPC and the poly(A) tail have been implicated in facilitating miRNA-mediated translational repression, deadenylation and decay7, 38 (Figure 3B). Recent work supports a model where PABPC promotes the association of the RNA induced silencing complex (RISC), specifically Argonaute 1 (AGO1), with target mRNAs65 and the interaction of the RISC component GW182 with both PABPC and deadenylase complexes is required for efficient silencing66. PABPC is released from the target mRNA prior to deadenylation and the recruitment of the deadenylase complexes to the miRNA target by GW182 is required for PABPC release to occur67. Subsequently, eIF4E and eIF4G are released from the mRNA in a decapping- and deadenylation-dependent manner, respectively67, and GW182 is released from the mRNA after deadenylation suggesting that it is only required to initiate silencing68. Taken together, these data suggest that binding of the RNA induced silencing complex (RISC), which includes AGO1 and GW182, leads to translational repression, deadenylation and decay of the miRNA target due to removal of PABPC and consequently the disruption of the closed loop structure which inhibits translation and allows deadenylase access to the poly(A) tail (Figure 3B). However, PABPC does not appear to play a role in miRNA-mediated repression in D. melanogaster S2 cells or D. rerio embryos, which suggests that this mechanism may not be conserved in all organisms and/or experimental systems69, 70.
Nonsense mediated decay
PABPC plays a role in identifying premature termination codons in the nonsense mediated decay (NMD) pathway7 (Figure 3B). NMD is an mRNA surveillance pathway that leads to the degradation of mRNAs that contain premature termination/stop codons (PTCs)71. This process involves an interaction between the central NMD protein Upf1, an ATPase and RNA helicase, and the eukaryotic release factors eRF1 and eRF3, which bind to ribosomes that are positioned over stop codons71. Only recently have studies begun to unravel how cells distinguish PTCs from normal stop codons. The conventional model for how the NMD machinery distinguishes between these two centers around the position of the exon junction complex (EJC), which marks exon-exon junctions that result from splicing71. This model posits that stop codons upstream of splice junctions and hence, deposited EJCs, are recognized as premature71. However, this mechanism does not explain NMD of transcripts not subject to splicing or with a PTC located after the terminal splice site.
Recent results suggest that PABPC may also contribute to the identification of PTCs and consequently NMD7 (Figure 3B). PTCs located in close proximity to the 3’UTR evade NMD more efficiently than those more distal72. Studies implicating PABPC in PTC recognition show that PABPC inhibits NMD at a distal PTC when it is tethered immediately downstream73. Thus, PABPC marks the end of the mRNA transcript and that the translational machinery distinguishes a normal stop codon from a PTC by the presence or absence of PABPC, respectively. However, PTCs located proximal to the AUG start codon can also evade NMD, which may be due to the continued binding of PABPC to the 43S ribosomal complex throughout the AUG scanning process74. NMD evasion by tethered PABPC is dependent on the C-terminus of PABPC74, which contains the binding site for eRF310, 11. PABPC and Upf1 compete for binding to eRF375, which leads to a model in which a PABPC-eRF3 interaction suppresses NMD and a Upf1-eRF3 interaction facilitates NMD (Figure 3B). However, this mechanism remains controversial76 and how PABPC and the EJC coordinate NMD remains to be elucidated.
Long interspersed nuclear elements (LINE) retrotransposition
Long interspersed nuclear elements (L1 or LINEs) are 500–8,000 base pair, non-long terminal repeat (non-LTR) retrotransposons that are very abundant in humans, accounting for ~17% of the genome77. Shortening the poly(A) tail of the L1 RNA or depletion of PABPC significantly reduced L1 retrotransposition78. Furthermore, PABPC is a component of the L1 ribonucleoprotein (RNP)78, which is an RNA-protein complex that is required for retrotransposition79. Depletion of PABPC leads to reduced accumulation of the L1 RNP protein, Orf1, in the nucleus78. These results suggest that PABPC affects retrotransposition by facilitating nuclear accumulation of the L1 RNP (Figure 3B). PABPC also interacts with templated A-rich sequences in short interspersed nuclear elements (SINEs)80, which are 100–300 base pair, non-LTR retrotransposons that account for ~11% of the human genome77, but evidence supporting a role for PABPC in regulating the retrotransposition of SINEs is lacking81–83. The mechanisms underlying these contributions of PABPC to regulation of transposition have not yet been defined.
mRNA localization
mRNAs are localized in many cell types as a mechanism to spatially regulate gene expression and this localization is particularly key in developmental processes84. A role for PABPC in mRNA localization has been proposed based on studies in D. melanogaster embryos (Figure 3B), where PABPC co-immunoprecipitates, in an RNA-dependent manner85, with the Egl/Bic-D transport machinery, which functions as an adaptor to link mRNA cargoes to dynein motors84. Of interest, PABPC also co-localizes with osk mRNA and the localization of osk mRNA to the posterior compartment of the embryo is disrupted in PABPC mutants85. PABPC mutants also show reduced osk mRNA levels suggesting that the localization defect detected may be due to altered osk mRNA stability and/or localization85. Furthermore, the osk mRNA sequences required for this regulation have been mapped to an A-rich region in the 3’UTR, which may be bound by PABPC85. PABPC is also part of a complex of proteins that associates with RNA fragments containing the minimal localization signal of the endogenous, localized mRNA bicoid, further supporting the idea that PABPC could influence mRNA localization through the formation of a multiprotein complex86 (Figure 3B). However, additional work is needed to dissect whether PABPBC plays direct or indirect roles in mRNA localization.
Translation and local translation
PABPC regulates the translation of some specific mRNAs through mechanisms that appear to be independent of the poly(A) tail (Figure 3B). For example, PABPC binds to A-rich sequences in the 5’-UTR of the PABPC mRNA transcript and consequently represses its own translation in vitro and in vivo87–89. PABPC also binds to an ~50 nucleotide A-rich sequence in the 3’-UTR of Y-box binding protein 1 (YB-1) mRNA to relieve translational inhibition by bound YB-1 protein90, which normally functions as a transcription factor91. These studies demonstrate that PABPC can regulate translation by binding to templated adenosines within the mRNA transcript. In addition, PABPC binds to BC200 and BC180, noncoding RNAs in H. sapiens and M. musculus92, 93, respectively, which inhibit translation by blocking the helicase activity of eIF4A81–83. PABPC blocks BC200 and BC1-mediated translation inhibition both in vitro and in vivo94. These noncoding RNAs contain over 40 nucleotides of adenosine-rich sequences, and nine consecutive adenosine residues were shown to be the minimal sequence necessary for binding by PABPC RRM domains95. In addition, BC200 and BC1 are localized to the dendrites of neurons96, 97 where they were reported to interact with FMRP98, a local translation regulator, and hnRNP-Q199. These results suggest that PABPC may also play a role in regulating translation locally in neuronal processes. In support of this hypothesis, PABPC binds to Vasopressin mRNA through sequences in the end of the coding region and/or the 3’UTR100. These regions of the mRNA are also required for localization of the mRNA to dendrites where Vasopressin mRNA is locally translated100, 101. In summary, PABPC regulates translation through a variety of mechanisms and has also been implicated in mRNA transport, which suggests a role for PABPC in regulating translation locally within specific subcellular compartments (Figure 3B).
These recent findings demonstrate that PABPC plays important roles in virtually all cytoplasmic post-transcriptional regulatory events, including mRNA transport, translation and decay (Figure 3B). PABPC regulates multiple aspects of these processes, either directly or indirectly, indicating that PABPC plays a key role in coordinating the post-transcriptional control of gene expression. Future studies of interest include analyzing how the multiple functions of PABPN1 and PABPC are coordinated to influence gene expression, as well as how novel Pabs such as ZC3H14, hnRNP-Q1, and LARP4 fit into the picture of post-transcriptional processing via binding to polyadenosine RNA.
New Pab Family Members
The ongoing discovery and characterization of RNA binding proteins has led to the expansion of many classes of RNA binding proteins, including the Pab protein family. Here we present three novel members of the Pab family: ZC3H14, hnRNP-Q1 and LARP4 (Figure 1). These proteins are defined as Pabs based on in vitro binding studies that demonstrate specific, high affinity binding to polyadenosine RNA. ZC3H14, hnRNP-Q1, and LARP4 play diverse roles in the post-transcriptional control of gene expression, which are schematized in Figure 4.
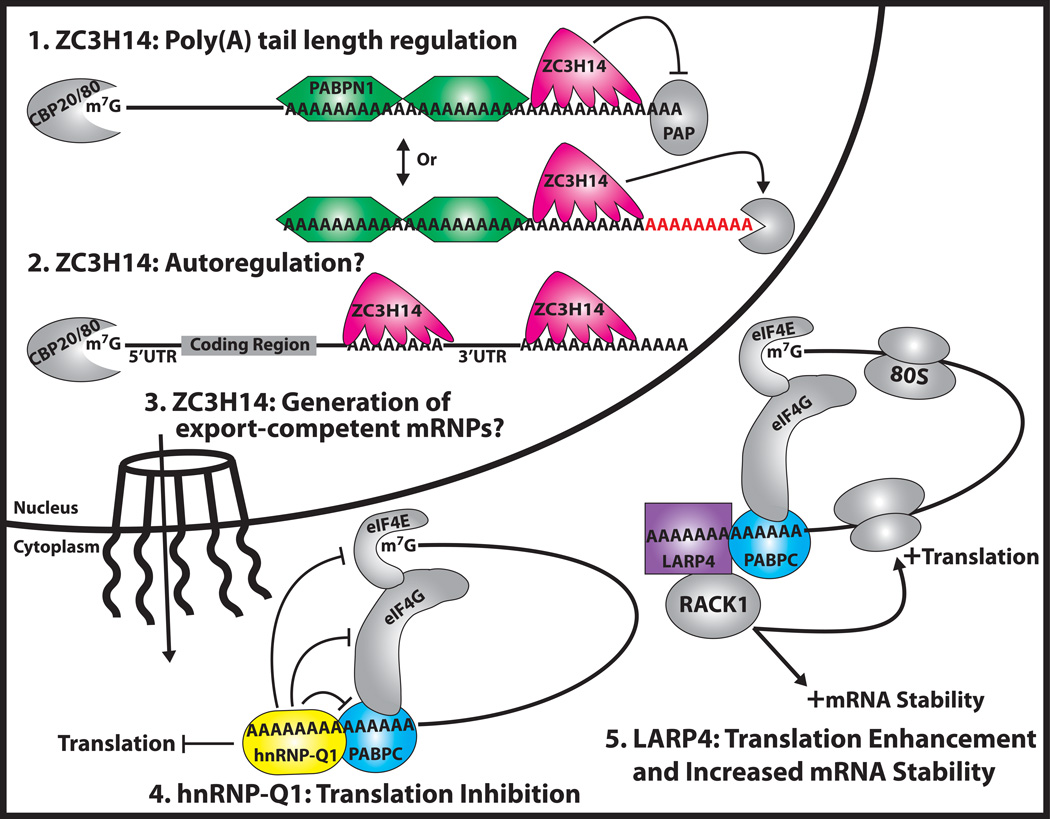
Figure 4. Model for the Function of Novel Pab Family Members.
The functions proposed for the new members of the Pab family described here (ZC3H14, hnRNP-Q1, and LARP4) are illustrated. The novel nuclear zinc finger Pab, ZC3H14 (pink five-fingered shape) plays a role in 1. poly(A) tail length regulation: ZC3H14 could limit poly(A) tail length either by inhibiting Poly(A) Polymerase (PAP) or by recruiting a 3’–5’ exonuclease (grey Pac-man); 2. Autoregulation: Like its S. cerevisiae counterpart, Nab2, ZC3H14 may bind to and autoregulate its own mRNA transcript via an A15 stretch present in the 3’UTR; and 3. Generation of export-competent mRNPs: ZC3H14 could play a direct role in the generation of properly packaged mRNPs that are poised for export but most data to support this function comes from studies of S. cerevisiae Nab2. Alternatively, proper polyadenylation could be required to assemble export-competent mRNPs and the role for Nab2/ZC3H14 could be indirect. The novel cytoplasmic Pab, hnRNP-Q1 (yellow ellipse), plays a role in 4. Translation inhibition: hnRNP-Q1 competes with PABPC for binding to poly(A) tails and consequently preventing the formation of the translation initiation complex. The other novel cytoplasmic Pab, LARP4 (purple rectangle), is implicated in 5. Translation enhancement and increased mRNA stability: LARP4 interacts with PABPC as well as the ribosome-associated protein, RACK1, to positively modulate mRNA translation and decay. The following factors are also incorporated into the model shown: Cap binding proteins 20 and 80 (CBP20/80); 7 methylguanosine cap (m7G); eukaryotic translation initiation factor 4E (eIF4E); eukaryotic translation initiation factor 4G (eIF4G); and eukaryotic ribosome (80S).
ZC3H14
Zinc finger CCCH-type containing protein #14 (ZC3H14) is an evolutionarily conserved nuclear Pab that binds to polyadenosine RNA via tandem CCCH zinc fingers102. Although most Pab proteins bind polyadenosine RNA via RRM motifs, biochemical studies provide evidence that the zinc fingers in ZC3H14 confer specific binding to polyadenosine RNA102. Interest in this class of zinc finger Pabs has been sparked by recent studies showing that a mutation of ZC3H14 that results in loss of this ubiquitously expressed protein leads to an autosomal recessive form of nonsyndromic intellectual disability103. The brain-specific phenotype in these patients suggests that the function of ZC3H14 is particularly critical in the highly specialized cells found in the brain.
Putative orthologs of ZC3H14, which share a common domain structure (Figure 1), have been studied in a variety of model organisms (Table 2), including S. cerevisiae104 (Nab2), D. melanogaster103 (dNab2), C. elegans105 (SUT-2), and M. musculus106 (ZC3H14/MSUT2). The N-terminus of ZC3H14 adopts a proline tryptophan isoleucine (PWI)-like fold, which mediates interactions with nuclear pore-associated proteins and facilitates proper mRNA export from the nucleus107. A classical nuclear localization signal (cNLS) is thought to target ZC3H14 to the nucleus108. The tandem CCCH zinc fingers located at the C-terminus of the protein are responsible for high affinity polyadenosine RNA recognition102, 104. Extensive binding studies demonstrate that in S. cerevisiae, only three out of the seven total zinc fingers are necessary for high affinity polyadenosine RNA recognition. The additional zinc fingers may bind other nucleic acid sequences or even other proteins102, 109.
Recent structural analyses of both S. cerevisiae and C. thermophilum ZC3H14 reveal that three zinc fingers interact to form an RNA binding module110, 111. The crystal structure of zinc fingers 3–5 of C. thermophilum Nab2 in complex with an A8 RNA oligo revealed that these zinc fingers form the binding unit that interacts with polyadenosine RNA with base-specific interactions on multiple adenosine residues111. These three zinc fingers share the greatest homology with hZC3H14 zinc fingers 1–3 and S. cerevisiae Nab2 zinc fingers 5–7, which is consistent with previous studies implicating the final three zinc fingers of Nab2 in high affinity polyadenosine RNA binding102 as well as poly(A) tail length control112, 113. Further analysis of the crystal structure revealed that although multiple adenosine-specific interactions are present, the spacing of the individual zinc fingers within the binding unit could result in Nab2 also binding to degenerate, or A-rich sequences instead of solely to poly(A) tracts. This information opens the possibility that this family of proteins could bind to poly(A) tails as well as A-rich sequences, and presents a model in which ZC3H14 could interact with a diverse spectrum of RNA transcripts.
Although little is known about the molecular function of human ZC3H14, studies in model organisms provide insight into potential roles in post-transcriptional processing. The majority of these studies have been carried out in S. cerevisiae analyzing Nab2. The Nab2 protein localizes to the nucleus at steady-state, but shuttles between the nucleus and cytoplasm114. The ZC3H14 protein also localizes to the nucleus at steady-state, more specifically to nuclear speckles, which are sites of RNA processing108, 115. Nab2 associates with poly(A) mRNA transcripts prior to export, likely modulating polyadenylation and facilitating the generation of export-competent mRNPs116. S. cerevisiae cells expressing N-terminal deletion mutants of Nab2 display nuclear accumulation of poly(A) RNA as well as extended poly(A) tails114, 117. However, the existence of Nab2 RNA binding mutants that confer defects in poly(A) tail length with no nuclear accumulation of poly(A) RNA highlights the critical role of the zinc finger domain in poly(A) tail length regulation118. Additional insight into a potential function for ZC3H14 has been obtained from a D. melanogaster loss-of-function model of ZC3H14, dNab2 null flies103. Studies in this model demonstrate that dNab2 has specificity for polyadenosine RNA in vitro and that RNA isolated from dNab2 null flies displays extended poly(A) tails. However, no defect in bulk poly(A) mRNA export has been detected103, lending support to a primary role for ZC3H14 in control of poly(A) tail length. Consistent with this model, siRNA-mediated depletion of ZC3H14 from cultured mammalian cells causes extended poly(A) tails with no apparent impact on poly(A) RNA localization119.
With respect to the role of zinc finger Pabs in the brain suggested by the phenotype of ZC3H14 patients, behavioral defects observed in dNab2 null flies can be rescued by transgenic expression of either Drosophila Nab2 or human ZC3H14 specifically in neurons119. This result demonstrates the critical role of Nab2/ZC3H14 in ensuring proper neuronal function and also provides experimental evidence for the functional conservation of this protein family through evolution. Rescue of neuronal phenotypes is accompanied by rescue of the molecular phenotype of extended poly(A) tails also observed in dNab2 mutant flies103, 119. Together, these data suggest that the role of ZC3H14 in poly(A) tail length control may underlie its function in development and disease. Two simple models for how ZC3H14 could limit poly(A) tail length include: 1) a limiting model where ZC3H14 limits the activity of poly(A) polymerase; or 2) a model in which ZC3H14 recruits or triggers a ribonuclease to trim the poly(A) tail118 (Figure 4).
An independent line of experimentation provides a further link between ZC3H14 and neuronal function. A screen for suppressors of the neurotoxicity of aggregated tau protein in C. elegans identified mutations in the Suppressor of Tauopathy 2 (SUT-2) gene, which encodes the nematode ortholog of ZC3H14105. Loss of function mutations in SUT-2 suppressed the uncoordinated phenotype, tau aggregation, and the observed neurodegenerative changes in this worm model, suggesting that SUT-2, when present, enhances tau-induced neurotoxicity105. Further analysis of the human protein ZC3H14/mammalian SUT-2 (MSUT2) demonstrate reduced MSUT2 staining in the hippocampus, a region of the brain affected by tau pathology120, in patients with Alzheimer’s disease106. The authors suggest that decreased MSUT2 staining in patient neurons may reflect a population of neurons that are spared from tau pathology subsequent neurodegeneration106.
The existence of multiple loss-of-function models of ZC3H14 with neuronal phenotypes strongly suggests a critical role for ZC3H14 in neurons. However, ZC3H14 is ubiquitously expressed, so the protein likely cooperates with PABPN1 to achieve precise control of polyadenylation. Whether ZC3H14, like the canonical Pabs, has additional, perhaps neuron-specific functions is not yet known.
hnRNPQ1
Heterogeneous ribonucleoprotein Q1 (hnRNP-Q1; also termed Nsap1,121 Syncrip122 and hnRNP-Q2123) is the cytoplasmic isoform of the mRNA binding protein hnRNP-Q124 (Mus musculus: NP_062770.1; Homo Sapiens: NP_001153149.1). Alternative splicing of the gene also leads to two nuclear isoforms of hnRNP-Q, termed hnRNP-Q2 and hnRNP-Q3124, but the function of these proteins is less well-characterized as compared to hnRNP-Q1. hnRNP-Q1 is ubiquitously expressed122, 125, 126 and evolutionarily conserved (Table 2). The domain structure of hnRNP-Q1 consists of an N-terminal acidic domain, three RRMs, a putative nuclear localization sequence and a C-terminal RGG box124. hnRNP-Q2 and hnRNP-Q3 share the same domains but have an additional NLS and a different C-terminus that results from alternative splicing124. Both the acidic domain and the RGG box are involved in protein-protein interactions124, 127 and the RRM domains are presumed to be required for binding RNA. However, experimental studies to define specific domains required for RNA binding have not yet been performed.
hnRNP-Q1 has been implicated in a variety of post-transcriptional regulatory processes including mRNA splicing124, 128–130, editing127, 131, 132, transport133–137, translation99, 126, 138–145, and degradation51, 140, 146–149. In addition, hnRNP-Q1 was recently identified as a novel polyadenosine RNA binding protein123, consistent with previous studies that revealed binding to oligo(A) RNA122. Data demonstrating that hnRNP-Q1 binds specifically to polyadenosine RNA with high affinity include in vitro binding assays coupled with competition experiments. The in vitro binding studies demonstrate that neither poly(C) nor poly(G) RNA can compete with poly(A) RNA for binding to hnRNP-Q1122, 123.
Recent work suggests that hnRNP-Q1 competes with PABPC for binding to poly(A) tails and inhibits translation123 (Figure 4). In this model, hnRNP-Q1 represses translation by inhibiting eIF4F complex recruitment to the mRNA and consequently 48S and 80S initiation complex formation and ultimately cap-dependent translation123. This mechanism is consistent with previous studies that have identified hnRNP-Q1 as a translational repressor126, 144. Furthermore, hnRNP-Q1-mediated translational repression depends on both the poly(A) tail and PABPC. The fold change of hnRNP-Q1-mediated translational inhibition increases with the length of the poly(A) tail123. Additionally, when PABPC is sequestered by the repressor poly(A) binding protein interacting protein 2 (Paip2), hnRNP-Q1 does not affect translation123. Depletion of hnRNP-Q via shRNA sequences targeting all isoforms of hnRNPQ leads to increased global translation as detected by [35S]methionine/cysteine incorporation123. However, previous microarray data identified only ~2,250 mRNAs enriched in hnRNP-Q1 immunoprecipitation pellets which corresponds to ~10% of the mRNAs interrogated. These results suggest that hnRNP-Q1 does not bind to all mRNA targets and that there is some specificity involved in this mechanism134. In fact, two consensus sequences for hnRNP-Q1 binding were identified from the microarray analysis: AYAAYY and UAUYRR (Y = C/U and R = A/G)134. Furthermore, hnRNP-Q1 binds to the 3’-UTR of RhoA mRNA, which does not contain A-rich sequences126. Therefore, hnRNP-Q1 may bind to both A-rich and non-A-rich sequences within an mRNA transcript and specifically repress the translation of a subset of mRNAs unlike the other Pab family members.
These results demonstrate that hnRNP-Q1 represses translation by competing with PABPC for binding to poly(A) tails (Figure 4). However, the mechanism that defines the interplay between hnRNP-Q1 and PABPC and consequently whether translation is enhanced or repressed has yet to be identified. Similarly, whether hnRNP-Q1 affects the translation of all mRNAs is unknown as is the possible contribution of the nuclear isoforms of hnRNP-Q, which contain the same RNA binding domains as hnRNP-Q1.
LARP4
La-related protein 4, or LARP4, is one member of the superfamily of La-related RNA binding proteins150 that are conserved across eukaryotic evolution151, 152 (Table 2). The La family of proteins use a La motif (LaM) as well as an RRM to recognize terminal UUU-3’OHs on small, nascent RNA transcripts to protect the bound RNAs from 3’ exonucleases, such as the RNA exosome153. LARPs also contain the LaM-RRM or LaM-RRM-like domain arrangement and have varying specificities for different classes of RNAs150. Although the overall domain structure of LARP4 (Figure 1) is similar to other family members, LARP4 harbors critical divergence from La as well as other LARP family members in its LaM. This change is in two or three key residues involved in UUU-3’OH recognition, suggesting that LARP4 may have unique RNA targets and functions. These critical amino acid changes in LARP4 suggest that it is the most diverged member of the La family152.
A recent study demonstrates that LARP4 is a bona fide cytoplasmic poly(A) RNA binding protein that also binds to PABPC using a variant PABP-interacting Motif (PAM2) to influence global translation as well as the stability of target mRNA transcripts154. The N-terminal domain of LARP4, which contains both the LaM and the RRM, has high affinity for a 20-mer of adenosine RNA, but has low affinity for U20 and essentially no affinity for C20 or G20. Although La binds U10 with high affinity, LARP4 does not bind to A10 with high affinity, but requires a longer stretch of adenosine. Thus, LARP4 binds with high affinity to polyadenosine RNA with a length requirement of more than 10 adenosines154. Polysome profile analysis revealed that LARP4 associates with 40S ribosomes as well as translating polyribosomes154. Subsequent studies uncovered an interaction between LARP4 and the 40S ribosomal-associated protein, RACK1, which is a scaffold protein that recruits activated protein kinase C to the ribosome to stimulate translation154, 155. This interaction may partially explain the association between LARP4 and 40S ribosomal subunits.
In addition to interactions with the 40S ribosome, LARP4 may also directly interact with PABPC. LARP4 contains a conserved N-terminal domain with a PAM2 motif. The PAM2 motif in LARP4 harbors a tryptophan in place of a typically invariant phenylalanine and is consequently designated a PAM2w motif. Indeed, LARP4 and PABPC can be co-immunoprecipitated and the interaction is RNase-resistant, suggesting a protein-protein interaction. Analysis of a series of LARP4 deletion mutants reveals that the PAM2w domain as well as a region C-terminal to the RRM is required for PABPC interaction. Analysis of these LARP4 deletion mutants by polysome profiling reveals a diminished association with polysomes, suggesting that the interaction with PABPC is necessary for LARP4 to maintain association with polysomes. Further studies also implicate the RNA binding motifs in mediating association with 40S components and polysomes. LARP4 RNA binding mutants that are not associated with 40S components also show decreased interaction with PABPC, suggesting that RNA binding by LARP4 stabilizes the LARP4-PABPC interaction. The affinity of LARP4 for polyadenosine RNA suggests that LARP4 and PABPC may associate on the poly(A) tails of mRNAs. In fact, further analyses provide evidence that PABPC and LARP4 interact with one another only in the presence of polyadenosine RNA154.
The primary function ascribed to La proteins is modulation of RNA stability150. Stability assays reveal that LARP4 stabilizes a luciferase reporter as well as several endogenous mRNAs analyzed. However, the stability of some mRNA transcripts, including the non-polyadenylated histone H2A mRNA156 and GAPDH mRNA154, was not affected by LARP4 overexpression. Thus, LARP4 appears to stabilize some, but not all, mRNAs. Results of this study suggest that LARP4 associates with translating polyadenylated mRNAs and that LARP4, via interaction with PABPC, may be one member of a network of proteins that compete for interaction with PABPC. Finally, because overexpression of LARP4 stabilizes some mRNAs, LARP4 may also compete with degradation machinery such as the Pan2/Pan3 and/or the Ccr4-Not-Caf1-Tob2 deadenylation complexes157 to modulate transcript stability. LARP4 clearly plays an important role in influencing gene expression and further analysis is required to not only identify the precise function of LARP4 in modulating translation and stability, but also to understand how LARP4 fits in with the other Pab family members.
These studies not only highlight the diverse functions of the novel Pabs, ZC3H14, hnRNP-Q1 and LARP4, but they also underscore the importance of Pabs in influencing gene expression. Further comprehensive study of Pab family members should reveal whether they interact with or coordinate with other Pab family members to regulate post-transcriptional processes. The ongoing discovery and characterization of novel RNA binding proteins suggests there could be additional Pab family members that we have yet to discover, which would then need to be integrated into an overall picture of Pab protein function.
Novel Polyadenosine Stretches
As discussed extensively in this review, the canonical function of Pab proteins is to bind the poly(A) tails of mRNA transcripts and carry out diverse functions that are essential for proper gene expression. However, these non-templated polyadenosine tracts are not the only stretches of polyadenosine found in the transcriptome. For instance, a handful of studies have described polyadenosine stretches ranging from 9–26 adenosines within mRNA transcripts158–160, but little work has been done to determine the function of these sequences. Given the length of these internal stretches and the typical Pab footprint of ~11–12 A's15, 30, Pabs may bind to these internal sequences as well as to poly(A) tails. In fact, there is precedence for this mode of regulation. As discussed earlier, human PABPC binds to A-rich sequences in the 5’UTR of PABPC mRNA and the 3’UTR of YB-1 mRNA to modulate the translation of these transcripts87–90. Furthermore, binding of S. cerevisiae Nab2 to a stretch of 26 adenosines in the 3’UTR of NAB2 mRNA mediates a negative feedback response in which recruitment of the exosome drives subsequent degradation of the NAB2 transcript161. The human ortholog of Nab2, ZC3H14, also contains a polyadenosine stretch (A15) within the 3’ UTR and preliminary studies suggest that ZC3H14 can bind to its own transcript (unpublished data). Together these data suggest that internal polyadenosine sequences could play an important role in regulating gene expression.
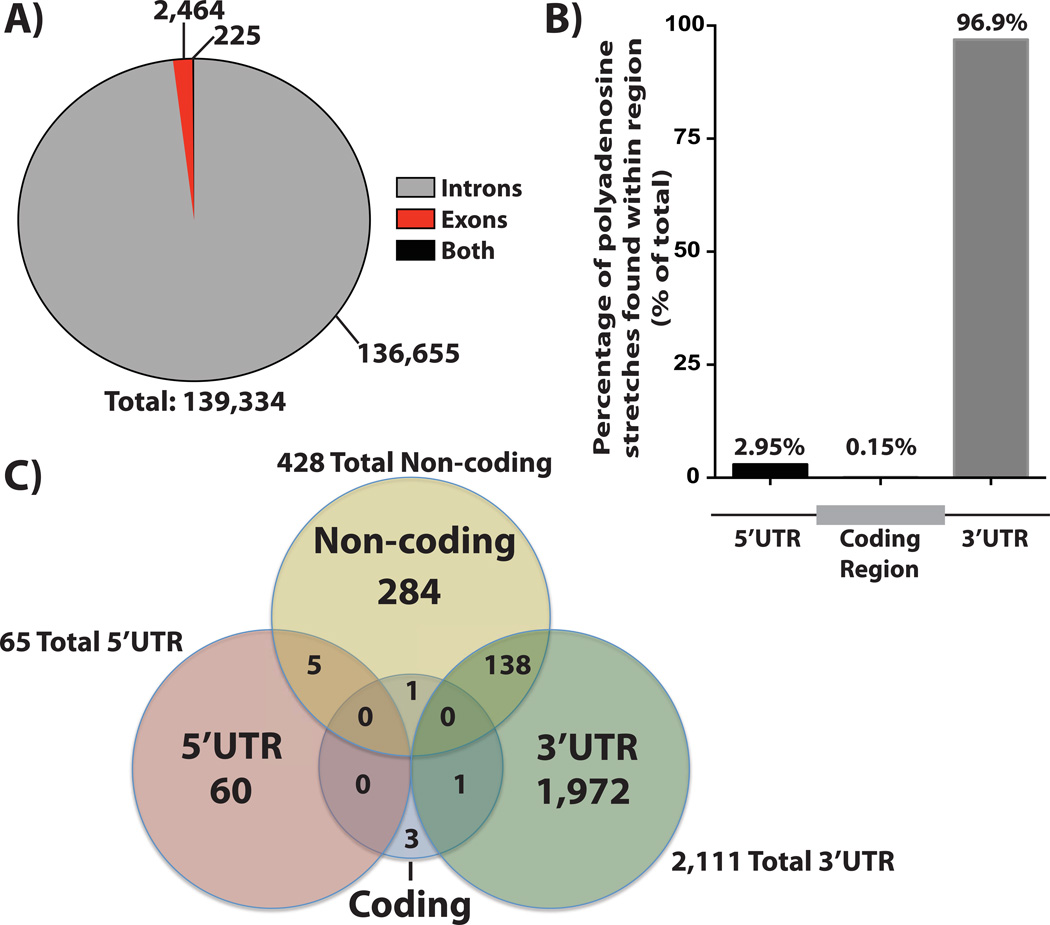
To gain insight into the prevalence of internal polyadenosine stretches within the human transcriptome, we analyzed all annotated human transcripts from the UCSC HG19 genome annotation for the presence of polyadenosine stretches. PABPN1 and PABPC require a stretch of 11–12 adenosines for high affinity binding15, 30, so we extracted stretches containing at least 12 consecutive adenosines, filtered the data for overlapping regions, and mapped the resulting stretches to their locations within transcripts. As a proof of principle, we verified the presence of mRNA transcripts that are known to contain ≥12 nucleotide (nt) adenosine stretches (Dystrophin160 and Vitamin D Receptor158) within our final dataset. Our results indicate that there are 139,334 instances of ≥12 nt adenosine stretches in the human transcriptome, many of which occur in multiple differentially spliced isoforms of the same gene (Figure 5A). As expected, the overwhelming majority of adenosine stretches occur in introns (136,655 instances). Interestingly, there are only 2,464 adenosine stretches that occur exclusively in exons (i.e. in the mature transcript)--1.8% of total instances. Of those, roughly 82% occur exclusively in the UTRs of the gene in which it resides (the majority of which occur in the 3’ UTR) (Figure 5B), suggesting that the aforementioned examples of regulatory mechanisms that depend upon templated polyadenosine stretches in UTRs could be more prevalent than previously appreciated. Remarkably, less than 0.02% of templated polyadenosine instances occur in the coding region of mRNA transcripts, with the remainder found in non-coding RNAs (Figure 5C). These preliminary results reveal that many mRNAs contain internal polyadenosine stretches and therefore have the potential to be bound and modulated by poly(A) binding proteins not only via their non-templated poly(A) tails, but also through these templated internal sequences. The vast enrichment of these stretches in the 3’UTRs of mature transcripts supports the well-establish function of the 3’UTR in post-transcriptional processing162 and suggests a potential additional point of regulation via 3’UTRs. In the future, it will be interesting to test the function of these internal polyadenosine sequences as well as investigate whether these sequences are enriched in certain types or classes of RNA transcripts.
Figure 5. Prevalence and Location of Templated, Internal Polyadenosine Stretches Within the Human Transcriptome.
A) Analysis of the human transcriptome for the frequency and enrichment of internal polyadenosine stretches containing at least 12 consecutive adenosines reveals that the vast majority (136,655 out of 139,334) of these sequences are located in the introns of mRNAs whereas a much smaller fraction are located in exonic regions (2,464 out of 139,334), which includes 5’ and 3’UTR regions. A much smaller number of these internal adenosine stretches are found in sequences that can be either introns or exons as a result of alternative splice variants. B) Internal polyadenosine stretches found in exonic sequences are almost exclusively located in untranslated regions. Further analysis of the ≥12nt polyadenosine sequences that occur in mature mRNA transcripts reveals that almost all (99.9%) of these instances are located in the UTRs, with an extremely large percentage present in the 3’UTRs (96.9%) of mRNA transcripts. C) Non-coding RNAs also include templated stretches of polyadenosine. A number (428) of noncoding RNAs (yellow circle) contain templated polyadenosine sequences, suggesting that Pabs could modulate non-coding RNAs through this templated binding site. Consistent with a model of 3’ UTR regulation, a large number of the internal polyadenosine stretches identified in ncRNAs (138) are transcribed from regions that correspond to the 3’UTRs of other/host genes (yellow and green overlap).
Future Directions and Remaining Questions
As described here, our understanding of the functions and members of the Pab protein family has increased in recent years. The studies presented in this review highlight the diversity found within the Pab family, as represented not only by the diverse roles of Pabs in modulating gene expression, but also by novel Pab family members that bind to RNA via unconventional polyadenosine RNA binding modules. The function of Pab proteins likely depends on the cellular context, specific regulatory elements, and the complement of RNA binding proteins present. Highlighting the importance of context for Pab protein function are the tissue-specific diseases that result from mutation of PABPN1 or ZC3H14. Both PABPN1 and ZC3H14 play important roles in RNA processing, which are likely critical in numerous cell types, so why defects in PABPN1 cause muscle-specific pathology and loss of ZC3H14 causes defects specifically in brain function is not at all obvious. Clearly to understand these tissue-specific diseases, studies will not only need to assess contributions of these proteins within the biological context of the affected tissues but also to define the complete molecular functions of the Pabs that underlie these contributions. Indeed, there are likely additional roles that have yet to be discovered for members of the Pab protein family raising several key questions for contemplation.
The first question is how the diverse functions of the multiple Pab proteins are coordinated throughout the intimately linked steps of post-transcriptional processing. Given that all Pab proteins bind to polyadenosine, they should all technically compete for the same target sequences. Presumably there is remodeling and/or replacement of the complement of Pabs associated with a poly(A) RNA as that RNA moves through processing within the nucleus to ultimately fulfill its cytoplasmic destiny. The question of whether all polyadenylated RNAs are bound at some point in their life by the full complement of Pabs or whether Pabs show preferential binding to specific transcripts or subsets of transcripts will also need to be addressed in order to understand how overall Pab family function is coordinated. Very little is known about how Pab proteins could be exchanged for one another at different stages of poly(A) RNA biogenesis and/or function. One possible mode of Pab regulation is association with protein binding partners. For example, PABPC is regulated by binding to a number of PAb-Interacting Proteins (PAIPs), including LARP4. Given this precedent, there are likely additional partners that interact with and regulate other Pab family members. Such interactions could contribute to selectivity for certain transcripts in cases where these Pab binding partners themselves are sequence-specific RNA binding proteins. Clearly, further studies are needed to define molecular interactions with both target RNAs and protein partners to fully understand how members of the Pab family of proteins cooperate to regulate gene expression.
A second important and related question is whether all adenosine sequences are created equal. For instance, the non-templated poly(A) tails present at the 3’ end of mRNAs represent just one type of polyadenosine found in the human transcriptome. Our analysis of internal, templated polyadenosine stretches in RNAs reveals clear enrichment of these sequences within the introns and 3’UTRs of mRNAs. These sequences could be bound by Pabs to confer regulation. Only in a few cases have the function of such templated A stretches been analyzed but those studies do confirm the potential for their functional importance. With regard to the question of whether all polyadenosine stretches are equivalent, recent work revealed that methylation of adenosine residues (m6A) on circadian clock transcripts contributes to their processing efficiency and plays a role in maintaining proper circadian rhythm163. The observation that post-transcriptional modification of adenosine residues modulates mRNA processing presents the possibility that adenosine residues on poly(A) tails or within internal polyadenosine stretches could be methylated to create distinct motifs that could be differentially recognized by Pab proteins. Future analyses should address the function of internal polyadenosine sequences and also explore whether modification of adenine within Pab target sequences influences Pab recognition and/or regulation.
In summary, the family of Pab proteins is expanding in both number and diversity of function. In fact, there are likely additional Pab family members that we have yet to define as such or to discover. While insight into the molecular roles of the individual Pab proteins is growing, developing a comprehensive model for how the functions of this protein family are coordinated to modulate gene expression remains the goal for the future.
Acknowledgements
We are grateful to members of the Corbett and Bassell laboratories for helpful discussions and to Dr. G. Matera for his support of this work. This work was supported by R01 grants from the NIH to AHC (GM058728) and GJB (MH085617) and F31 grants from the NIH to CPW (CA168321) and KRW (MH095266).
References
- 1.Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 2.Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582:1977–1986. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukong KE, Chang KW, Khandjian EW, Richard S. RNA-binding proteins in human genetic disease. Trends Genet. 2008;24:416–425. doi: 10.1016/j.tig.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Castello A, Fischer B, Hentze MW, Preiss T. RNA-binding proteins in Mendelian disease. Trends Genet. 2013;29:318–327. doi: 10.1016/j.tig.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Wahle E, Ruegsegger U. 3'-End processing of pre-mRNA in eukaryotes. FEMS Microbiol Rev. 1999;23:277–295. doi: 10.1111/j.1574-6976.1999.tb00400.x. [DOI] [PubMed] [Google Scholar]
- 6.Chan S, Choi EA, Shi Y. Pre-mRNA 3'-end processing complex assembly and function. Wiley Interdiscip Rev RNA. 2011;2:321–335. doi: 10.1002/wrna.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goss DJ, Kleiman FE. Poly(A) binding proteins: are they all created equal? Wiley Interdiscip Rev RNA. 2013;4:167–179. doi: 10.1002/wrna.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munroe D, Jacobson A. mRNA poly(A) tail, a 3' enhancer of translational initiation. Mol Cell Biol. 1990;10:3441–3455. doi: 10.1128/mcb.10.7.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saini KS, Summerhayes IC, Thomas P. Molecular events regulating messenger RNA stability in eukaryotes. Mol Cell Biochem. 1990;96:15–23. doi: 10.1007/BF00228449. [DOI] [PubMed] [Google Scholar]
- 10.Mangus DA, Evans MC, Jacobson A. Poly(A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol. 2003;4:223. doi: 10.1186/gb-2003-4-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn U, Wahle E. Structure and function of poly(A) binding proteins. Biochim Biophys Acta. 2004;1678:67–84. doi: 10.1016/j.bbaexp.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee A, Apponi LH, Pavlath GK, Corbett AH. PABPN1: molecular function and muscle disease. FEBS J. 2013;280:4230–4250. doi: 10.1111/febs.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn U, Nemeth A, Meyer S, Wahle E. The RNA binding domains of the nuclear poly(A)-binding protein. J Biol Chem. 2003;278:16916–16925. doi: 10.1074/jbc.M209886200. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth A, Krause S, Blank D, Jenny A, Jeno P, Lustig A, Wahle E. Isolation of genomic and cDNA clones encoding bovine poly(A) binding protein II. Nucleic Acids Res. 1995;23:4034–4041. doi: 10.1093/nar/23.20.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer S, Urbanke C, Wahle E. Equilibrium studies on the association of the nuclear poly(A) binding protein with poly(A) of different lengths. Biochemistry. 2002;41:6082–6089. doi: 10.1021/bi0160866. [DOI] [PubMed] [Google Scholar]
- 16.Smith JJ, Rucknagel KP, Schierhorn A, Tang J, Nemeth A, Linder M, Herschman HR, Wahle E. Unusual sites of arginine methylation in Poly(A)-binding protein II and in vitro methylation by protein arginine methyltransferases PRMT1 and PRMT3. J Biol Chem. 1999;274:13229–13234. doi: 10.1074/jbc.274.19.13229. [DOI] [PubMed] [Google Scholar]
- 17.Fronz K, Guttinger S, Burkert K, Kuhn U, Stohr N, Schierhorn A, Wahle E. Arginine methylation of the nuclear poly(a) binding protein weakens the interaction with its nuclear import receptor, transportin. J Biol Chem. 2011;286:32986–32994. doi: 10.1074/jbc.M111.273912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahle E, Moritz B. Methylation of the nuclear poly(A)-binding protein by type I protein arginine methyltransferases - how and why. Biol Chem. 2013;394:1029–1043. doi: 10.1515/hsz-2013-0121. [DOI] [PubMed] [Google Scholar]
- 19.Wahle E. A novel poly(A)-binding protein acts as a specificity factor in the second phase of messenger RNA polyadenylation. Cell. 1991;66:759–768. doi: 10.1016/0092-8674(91)90119-j. [DOI] [PubMed] [Google Scholar]
- 20.Kerwitz Y, Kuhn U, Lilie H, Knoth A, Scheuermann T, Friedrich H, Schwarz E, Wahle E. Stimulation of poly(A) polymerase through a direct interaction with the nuclear poly(A) binding protein allosterically regulated by RNA. EMBO J. 2003;22:3705–3714. doi: 10.1093/emboj/cdg347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhn U, Gundel M, Knoth A, Kerwitz Y, Rudel S, Wahle E. Poly(A) tail length is controlled by the nuclear poly(A)-binding protein regulating the interaction between poly(A) polymerase and the cleavage and polyadenylation specificity factor. J Biol Chem. 2009;284:22803–22814. doi: 10.1074/jbc.M109.018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller RW, Kuhn U, Aragon M, Bornikova L, Wahle E, Bear DG. The nuclear poly(A) binding protein, PABP2, forms an oligomeric particle covering the length of the poly(A) tail. J Mol Biol. 2000;297:569–583. doi: 10.1006/jmbi.2000.3572. [DOI] [PubMed] [Google Scholar]
- 23.Calado A, Tome FM, Brais B, Rouleau GA, Kuhn U, Wahle E, Carmo-Fonseca M. Nuclear inclusions in oculopharyngeal muscular dystrophy consist of poly(A) binding protein 2 aggregates which sequester poly(A) RNA. Hum Mol Genet. 2000;9:2321–2328. doi: 10.1093/oxfordjournals.hmg.a018924. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Li Y, Krug RM. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3'-end processing machinery. EMBO J. 1999;18:2273–2283. doi: 10.1093/emboj/18.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apponi LH, Leung SW, Williams KR, Valentini SR, Corbett AH, Pavlath GK. Loss of nuclear poly(A)-binding protein 1 causes defects in myogenesis and mRNA biogenesis. Hum Mol Genet. 2010;19:1058–1065. doi: 10.1093/hmg/ddp569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckner R, Ellmeier W, Birnstiel ML. Mature mRNA 3' end formation stimulates RNA export from the nucleus. EMBO J. 1991;10:3513–3522. doi: 10.1002/j.1460-2075.1991.tb04915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, Carmichael GG. Role of polyadenylation in nucleocytoplasmic transport of mRNA. Mol Cell Biol. 1996;16:1534–1542. doi: 10.1128/mcb.16.4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poon LL, Fodor E, Brownlee GG. Polyuridylated mRNA synthesized by a recombinant influenza virus is defective in nuclear export. J Virol. 2000;74:418–427. doi: 10.1128/jvi.74.1.418-427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dower K, Kuperwasser N, Merrikh H, Rosbash M. A synthetic A tail rescues yeast nuclear accumulation of a ribozyme-terminated transcript. RNA. 2004;10:1888–1899. doi: 10.1261/rna.7166704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachs AB, Davis RW, Kornberg RD. A single domain of yeast poly(A)-binding protein is necessary and sufficient for RNA binding and cell viability. Mol Cell Biol. 1987;7:3268–3276. doi: 10.1128/mcb.7.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn U, Pieler T. Xenopus poly(A) binding protein: functional domains in RNA binding and protein-protein interaction. J Mol Biol. 1996;256:20–30. doi: 10.1006/jmbi.1996.0065. [DOI] [PubMed] [Google Scholar]
- 32.Baer BW, Kornberg RD. The protein responsible for the repeating structure of cytoplasmic poly(A)-ribonucleoprotein. J Cell Biol. 1983;96:717–721. doi: 10.1083/jcb.96.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarun SZ, Jr, Wells SE, Deardorff JA, Sachs AB. Translation initiation factor eIF4G mediates in vitro poly(A) tail-dependent translation. Proc Natl Acad Sci U S A. 1997;94:9046–9051. doi: 10.1073/pnas.94.17.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoshino S, Imai M, Kobayashi T, Uchida N, Katada T. The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3'-Poly(A) tail of mRNA. Direct association of erf3/GSPT with polyadenylate-binding protein. J Biol Chem. 1999;274:16677–16680. doi: 10.1074/jbc.274.24.16677. [DOI] [PubMed] [Google Scholar]
- 35.Lin J, Fabian M, Sonenberg N, Meller A. Nanopore detachment kinetics of poly(A) binding proteins from RNA molecules reveals the critical role of C-terminus interactions. Biophys J. 2012;102:1427–1434. doi: 10.1016/j.bpj.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray NK, Coller JM, Dickson KS, Wickens M. Multiple portions of poly(A)-binding protein stimulate translation in vivo. EMBO J. 2000;19:4723–4733. doi: 10.1093/emboj/19.17.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khaleghpour K, Kahvejian A, De Crescenzo G, Roy G, Svitkin YV, Imataka H, O'Connor-McCourt M, Sonenberg N. Dual interactions of the translational repressor Paip2 with poly(A) binding protein. Mol Cell Biol. 2001;21:5200–5213. doi: 10.1128/MCB.21.15.5200-5213.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun JE, Huntzinger E, Izaurralde E. The role of GW182 proteins in miRNA-mediated gene silencing. Adv Exp Med Biol. 2013;768:147–163. doi: 10.1007/978-1-4614-5107-5_9. [DOI] [PubMed] [Google Scholar]
- 39.Kahvejian A, Roy G, Sonenberg N. The mRNA closed-loop model: the function of PABP and PABP-interacting proteins in mRNA translation. Cold Spring Harb Symp Quant Biol. 2001;66:293–300. doi: 10.1101/sqb.2001.66.293. [DOI] [PubMed] [Google Scholar]
- 40.Tarun SZ, Jr, Sachs AB. A common function for mRNA 5' and 3' ends in translation initiation in yeast. Genes Dev. 1995;9:2997–3007. doi: 10.1101/gad.9.23.2997. [DOI] [PubMed] [Google Scholar]
- 41.Tarun SZ, Jr, Sachs AB. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- 42.Wells SE, Hillner PE, Vale RD, Sachs AB. Circularization of mRNA by eukaryotic translation initiation factors. Mol Cell. 1998;2:135–140. doi: 10.1016/s1097-2765(00)80122-7. [DOI] [PubMed] [Google Scholar]
- 43.Kahvejian A, Svitkin YV, Sukarieh R, M'Boutchou MN, Sonenberg N. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev. 2005;19:104–113. doi: 10.1101/gad.1262905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Searfoss A, Dever TE, Wickner R. Linking the 3' poly(A) tail to the subunit joining step of translation initiation: relations of Pab1p, eukaryotic translation initiation factor 5b (Fun12p), and Ski2p-Slh1p. Mol Cell Biol. 2001;21:4900–4908. doi: 10.1128/MCB.21.15.4900-4908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida N, Hoshino S, Imataka H, Sonenberg N, Katada T. A novel role of the mammalian GSPT/eRF3 associating with poly(A)-binding protein in Cap/Poly(A)-dependent translation. J Biol Chem. 2002;277:50286–50292. doi: 10.1074/jbc.M203029200. [DOI] [PubMed] [Google Scholar]
- 46.Parker R, Song H. The enzymes and control of eukaryotic mRNA turnover. Nat Struct Mol Biol. 2004;11:121–127. doi: 10.1038/nsmb724. [DOI] [PubMed] [Google Scholar]
- 47.Korner CG, Wahle E. Poly(A) tail shortening by a mammalian poly(A)-specific 3'-exoribonuclease. J Biol Chem. 1997;272:10448–10456. doi: 10.1074/jbc.272.16.10448. [DOI] [PubMed] [Google Scholar]
- 48.Tucker M, Staples RR, Valencia-Sanchez MA, Muhlrad D, Parker R. Ccr4p is the catalytic subunit of a Ccr4p/Pop2p/Notp mRNA deadenylase complex in Saccharomyces cerevisiae. EMBO J. 2002;21:1427–1436. doi: 10.1093/emboj/21.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Day N, Trifillis P, Kiledjian M. An mRNA stability complex functions with poly(A)-binding protein to stabilize mRNA in vitro. Mol Cell Biol. 1999;19:4552–4560. doi: 10.1128/mcb.19.7.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Kiledjian M. The poly(A)-binding protein and an mRNA stability protein jointly regulate an endoribonuclease activity. Mol Cell Biol. 2000;20:6334–6341. doi: 10.1128/mcb.20.17.6334-6341.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grosset C, Chen CY, Xu N, Sonenberg N, Jacquemin-Sablon H, Shyu AB. A mechanism for translationally coupled mRNA turnover: interaction between the poly(A) tail and a c-fos RNA coding determinant via a protein complex. Cell. 2000;103:29–40. doi: 10.1016/s0092-8674(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 52.Jenal M, Elkon R, Loayza-Puch F, van Haaften G, Kuhn U, Menzies FM, Oude Vrielink JA, Bos AJ, Drost J, Rooijers K, et al. The poly(A)-binding protein nuclear 1 suppresses alternative cleavage and polyadenylation sites. Cell. 2012;149:538–553. doi: 10.1016/j.cell.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 53.de Klerk E, Venema A, Anvar SY, Goeman JJ, Hu O, Trollet C, Dickson G, den Dunnen JT, van der Maarel SM, Raz V, et al. Poly(A) binding protein nuclear 1 levels affect alternative polyadenylation. Nucleic Acids Res. 2012;40:9089–9101. doi: 10.1093/nar/gks655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raz V, Routledge S, Venema A, Buijze H, van der Wal E, Anvar S, Straasheijm KR, Klooster R, Antoniou M, van der Maarel SM. Modeling oculopharyngeal muscular dystrophy in myotube cultures reveals reduced accumulation of soluble mutant PABPN1 protein. Am J Pathol. 2011;179:1988–2000. doi: 10.1016/j.ajpath.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beaulieu YB, Kleinman CL, Landry-Voyer AM, Majewski J, Bachand F. Polyadenylation-dependent control of long noncoding RNA expression by the poly(A)-binding protein nuclear 1. PLoS Genet. 2012;8:e1003078. doi: 10.1371/journal.pgen.1003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt K, Butler JS. Nuclear RNA surveillance: role of TRAMP in controlling exosome specificity. Wiley Interdiscip Rev RNA. 2013;4:217–231. doi: 10.1002/wrna.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemieux C, Marguerat S, Lafontaine J, Barbezier N, Bahler J, Bachand F. A Pre-mRNA degradation pathway that selectively targets intron-containing genes requires the nuclear poly(A)-binding protein. Mol Cell. 2011;44:108–119. doi: 10.1016/j.molcel.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 59.Bresson SM, Conrad NK. The human nuclear poly(a)-binding protein promotes RNA hyperadenylation and decay. PLoS Genet. 2013;9:e1003893. doi: 10.1371/journal.pgen.1003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holbein S, Wengi A, Decourty L, Freimoser FM, Jacquier A, Dichtl B. Cordycepin interferes with 3' end formation in yeast independently of its potential to terminate RNA chain elongation. RNA. 2009;15:837–849. doi: 10.1261/rna.1458909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maquat LE, Hwang J, Sato H, Tang Y. CBP80-promoted mRNP rearrangements during the pioneer round of translation, nonsense-mediated mRNA decay, and thereafter. Cold Spring Harb Symp Quant Biol. 2010;75:127–134. doi: 10.1101/sqb.2010.75.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hosoda N, Lejeune F, Maquat LE. Evidence that poly(A) binding protein C1 binds nuclear pre-mRNA poly(A) tails. Mol Cell Biol. 2006;26:3085–3097. doi: 10.1128/MCB.26.8.3085-3097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiu SY, Lejeune F, Ranganathan AC, Maquat LE. The pioneer translation initiation complex is functionally distinct from but structurally overlaps with the steady-state translation initiation complex. Genes Dev. 2004;18:745–754. doi: 10.1101/gad.1170204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato H, Maquat LE. Remodeling of the pioneer translation initiation complex involves translation and the karyopherin importin beta. Genes Dev. 2009;23:2537–2550. doi: 10.1101/gad.1817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moretti F, Kaiser C, Zdanowicz-Specht A, Hentze MW. PABP and the poly(A) tail augment microRNA repression by facilitated miRISC binding. Nat Struct Mol Biol. 2012;19:603–608. doi: 10.1038/nsmb.2309. [DOI] [PubMed] [Google Scholar]
- 66.Huntzinger E, Kuzuoglu-Ozturk D, Braun JE, Eulalio A, Wohlbold L, Izaurralde E. The interactions of GW182 proteins with PABP and deadenylases are required for both translational repression and degradation of miRNA targets. Nucleic Acids Res. 2013;41:978–994. doi: 10.1093/nar/gks1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zekri L, Kuzuoglu-Ozturk D, Izaurralde E. GW182 proteins cause PABP dissociation from silenced miRNA targets in the absence of deadenylation. EMBO J. 2013;32:1052–1065. doi: 10.1038/emboj.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zekri L, Huntzinger E, Heimstadt S, Izaurralde E. The silencing domain of GW182 interacts with PABPC1 to promote translational repression and degradation of microRNA targets and is required for target release. Mol Cell Biol. 2009;29:6220–6231. doi: 10.1128/MCB.01081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fukaya T, Tomari Y. PABP is not essential for microRNA-mediated translational repression and deadenylation in vitro. EMBO J. 2011;30:4998–5009. doi: 10.1038/emboj.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mishima Y, Fukao A, Kishimoto T, Sakamoto H, Fujiwara T, Inoue K. Translational inhibition by deadenylation-independent mechanisms is central to microRNA-mediated silencing in zebrafish. Proc Natl Acad Sci U S A. 2012;109:1104–1109. doi: 10.1073/pnas.1113350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schoenberg DR, Maquat LE. Regulation of cytoplasmic mRNA decay. Nat Rev Genet. 2012;13:246–259. doi: 10.1038/nrg3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Behm-Ansmant I, Kashima I, Rehwinkel J, Sauliere J, Wittkopp N, Izaurralde E. mRNA quality control: an ancient machinery recognizes and degrades mRNAs with nonsense codons. FEBS Lett. 2007;581:2845–2853. doi: 10.1016/j.febslet.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 73.Amrani N, Ganesan R, Kervestin S, Mangus DA, Ghosh S, Jacobson A. A faux 3'-UTR promotes aberrant termination and triggers nonsense-mediated mRNA decay. Nature. 2004;432:112–118. doi: 10.1038/nature03060. [DOI] [PubMed] [Google Scholar]
- 74.Silva AL, Ribeiro P, Inacio A, Liebhaber SA, Romao L. Proximity of the poly(A)-binding protein to a premature termination codon inhibits mammalian nonsense-mediated mRNA decay. RNA. 2008;14:563–576. doi: 10.1261/rna.815108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kervestin S, Li C, Buckingham R, Jacobson A. Testing the faux-UTR model for NMD: analysis of Upf1p and Pab1p competition for binding to eRF3/Sup35p. Biochimie. 2012;94:1560–1571. doi: 10.1016/j.biochi.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meaux S, van Hoof A, Baker KE. Nonsense-mediated mRNA decay in yeast does not require PAB1 or a poly(A) tail. Mol Cell. 2008;29:134–140. doi: 10.1016/j.molcel.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cordaux R, Batzer MA. The impact of retrotransposons on human genome evolution. Nat Rev Genet. 2009;10:691–703. doi: 10.1038/nrg2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dai L, Taylor MS, O'Donnell KA, Boeke JD. Poly(A) binding protein C1 is essential for efficient L1 retrotransposition and affects L1 RNP formation. Mol Cell Biol. 2012;32:4323–4336. doi: 10.1128/MCB.06785-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roy-Engel AM. LINEs, SINEs and other retroelements: do birds of a feather flock together? Front Biosci (Landmark Ed) 2012;17:1345–1361. doi: 10.2741/3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muddashetty R, Khanam T, Kondrashov A, Bundman M, Iacoangeli A, Kremerskothen J, Duning K, Barnekow A, Huttenhofer A, Tiedge H, et al. Poly(A)-binding protein is associated with neuronal BC1 and BC200 ribonucleoprotein particles. J Mol Biol. 2002;321:433–445. doi: 10.1016/s0022-2836(02)00655-1. [DOI] [PubMed] [Google Scholar]
- 81.Lin D, Pestova TV, Hellen CU, Tiedge H. Translational control by a small RNA: dendritic BC1 RNA targets the eukaryotic initiation factor 4A helicase mechanism. Mol Cell Biol. 2008;28:3008–3019. doi: 10.1128/MCB.01800-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H, Iacoangeli A, Lin D, Williams K, Denman RB, Hellen CU, Tiedge H. Dendritic BC1 RNA in translational control mechanisms. J Cell Biol. 2005;171:811–821. doi: 10.1083/jcb.200506006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H, Iacoangeli A, Popp S, Muslimov IA, Imataka H, Sonenberg N, Lomakin IB, Tiedge H. Dendritic BC1 RNA: functional role in regulation of translation initiation. J Neurosci. 2002;22:10232–10241. doi: 10.1523/JNEUROSCI.22-23-10232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136:719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vazquez-Pianzola P, Urlaub H, Suter B. Pabp binds to the osk 3'UTR and specifically contributes to osk mRNA stability and oocyte accumulation. Dev Biol. 2011;357:404–418. doi: 10.1016/j.ydbio.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 86.Arn EA, Cha BJ, Theurkauf WE, Macdonald PM. Recognition of a bicoid mRNA localization signal by a protein complex containing Swallow, Nod, and RNA binding proteins. Dev Cell. 2003;4:41–51. doi: 10.1016/s1534-5807(02)00397-0. [DOI] [PubMed] [Google Scholar]
- 87.Bag J, Wu J. Translational control of poly(A)-binding protein expression. Eur J Biochem. 1996;237:143–152. doi: 10.1111/j.1432-1033.1996.0143n.x. [DOI] [PubMed] [Google Scholar]
- 88.de Melo Neto OP, Standart N, Martins de Sa C. Autoregulation of poly(A)-binding protein synthesis in vitro. Nucleic Acids Res. 1995;23:2198–2205. doi: 10.1093/nar/23.12.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hornstein E, Harel H, Levy G, Meyuhas O. Overexpression of poly(A)-binding protein down-regulates the translation or the abundance of its own mRNA. FEBS Lett. 1999;457:209–213. doi: 10.1016/s0014-5793(99)01039-x. [DOI] [PubMed] [Google Scholar]
- 90.Skabkina OV, Skabkin MA, Popova NV, Lyabin DN, Penalva LO, Ovchinnikov LP. Poly(A)-binding protein positively affects YB-1 mRNA translation through specific interaction with YB-1 mRNA. J Biol Chem. 2003;278:18191–18198. doi: 10.1074/jbc.M209073200. [DOI] [PubMed] [Google Scholar]
- 91.Skabkina OV, Lyabin DN, Skabkin MA, Ovchinnikov LP. YB-1 autoregulates translation of its own mRNA at or prior to the step of 40S ribosomal subunit joining. Mol Cell Biol. 2005;25:3317–3323. doi: 10.1128/MCB.25.8.3317-3323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martignetti JA, Brosius J. BC200 RNA: a neural RNA polymerase III product encoded by a monomeric Alu element. Proc Natl Acad Sci U S A. 1993;90:11563–11567. doi: 10.1073/pnas.90.24.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martignetti JA, Brosius J. Neural BC1 RNA as an evolutionary marker: guinea pig remains a rodent. Proc Natl Acad Sci U S A. 1993;90:9698–9702. doi: 10.1073/pnas.90.20.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kondrashov AV, Kiefmann M, Ebnet K, Khanam T, Muddashetty RS, Brosius J. Inhibitory effect of naked neural BC1 RNA or BC200 RNA on eukaryotic in vitro translation systems is reversed by poly(A)-binding protein (PABP) J Mol Biol. 2005;353:88–103. doi: 10.1016/j.jmb.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 95.Khanam T, Muddashetty RS, Kahvejian A, Sonenberg N, Brosius J. Poly(A)-binding protein binds to A-rich sequences via RNA-binding domains 1+2 and 3+4. RNA Biol. 2006;3:170–177. doi: 10.4161/rna.3.4.4075. [DOI] [PubMed] [Google Scholar]
- 96.Tiedge H, Chen W, Brosius J. Primary structure, neural-specific expression, and dendritic location of human BC200 RNA. J Neurosci. 1993;13:2382–2390. doi: 10.1523/JNEUROSCI.13-06-02382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tiedge H, Fremeau RT, Jr, Weinstock PH, Arancio O, Brosius J. Dendritic location of neural BC1 RNA. Proc Natl Acad Sci U S A. 1991;88:2093–2097. doi: 10.1073/pnas.88.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zalfa F, Adinolfi S, Napoli I, Kuhn-Holsken E, Urlaub H, Achsel T, Pastore A, Bagni C. Fragile X mental retardation protein (FMRP) binds specifically to the brain cytoplasmic RNAs BC1/BC200 via a novel RNA-binding motif. J Biol Chem. 2005;280:33403–33410. doi: 10.1074/jbc.M504286200. [DOI] [PubMed] [Google Scholar]
- 99.Duning K, Buck F, Barnekow A, Kremerskothen J. SYNCRIP, a component of dendritically localized mRNPs, binds to the translation regulator BC200 RNA. J Neurochem. 2008;105:351–359. doi: 10.1111/j.1471-4159.2007.05138.x. [DOI] [PubMed] [Google Scholar]
- 100.Mohr E, Prakash N, Vieluf K, Fuhrmann C, Buck F, Richter D. Vasopressin mRNA localization in nerve cells: characterization of cis-acting elements and trans-acting factors. Proc Natl Acad Sci U S A. 2001;98:7072–7079. doi: 10.1073/pnas.111146598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohr E, Richter D. Local synthesis of the rat Vasopressin precursor in dendrites of in vitro cultured nerve cells. Brain Res Mol Brain Res. 2003;114:115–122. doi: 10.1016/s0169-328x(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 102.Kelly SM, Pabit SA, Kitchen CM, Guo P, Marfatia KA, Murphy TJ, Corbett AH, Berland KM. Recognition of polyadenosine RNA by zinc finger proteins. Proc Natl Acad Sci U S A. 2007;104:12306–12311. doi: 10.1073/pnas.0701244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pak C, Garshasbi M, Kahrizi K, Gross C, Apponi LH, Noto JJ, Kelly SM, Leung SW, Tzschach A, Behjati F, et al. Mutation of the conserved polyadenosine RNA binding protein, ZC3H14/dNab2, impairs neural function in Drosophila and humans. Proc Natl Acad Sci U S A. 2011;108:12390–12395. doi: 10.1073/pnas.1107103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anderson JT, Wilson SM, Datar KV, Swanson MS. NAB2: a yeast nuclear polyadenylated RNA-binding protein essential for cell viability. Mol Cell Biol. 1993;13:2730–2741. doi: 10.1128/mcb.13.5.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guthrie CR, Schellenberg GD, Kraemer BC. SUT-2 potentiates tau-induced neurotoxicity in Caenorhabditis elegans. Hum Mol Genet. 2009;18:1825–1838. doi: 10.1093/hmg/ddp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guthrie CR, Greenup L, Leverenz JB, Kraemer BC. MSUT2 is a determinant of susceptibility to tau neurotoxicity. Hum Mol Genet. 2011;20:1989–1999. doi: 10.1093/hmg/ddr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fasken MB, Stewart M, Corbett AH. Functional significance of the interaction between the mRNA-binding protein, Nab2, and the nuclear pore-associated protein, Mlp1, in mRNA export. J Biol Chem. 2008;283:27130–27143. doi: 10.1074/jbc.M803649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leung SW, Apponi LH, Cornejo OE, Kitchen CM, Valentini SR, Pavlath GK, Dunham CM, Corbett AH. Splice variants of the human ZC3H14 gene generate multiple isoforms of a zinc finger polyadenosine RNA binding protein. Gene. 2009;439:71–78. doi: 10.1016/j.gene.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Apponi LH, Kelly SM, Harreman MT, Lehner AN, Corbett AH, Valentini SR. An interaction between two RNA binding proteins, Nab2 and Pub1, links mRNA processing/export and mRNA stability. Mol Cell Biol. 2007;27:6569–6579. doi: 10.1128/MCB.00881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brockmann C, Soucek S, Kuhlmann SI, Mills-Lujan K, Kelly SM, Yang JC, Iglesias N, Stutz F, Corbett AH, Neuhaus D, et al. Structural basis for polyadenosine-RNA binding by Nab2 Zn fingers and its function in mRNA nuclear export. Structure. 2012;20:1007–1018. doi: 10.1016/j.str.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuhlmann SI, Valkov E, Stewart M. Structural basis for the molecular recognition of polyadenosine RNA by Nab2 Zn fingers. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Viphakone N, Voisinet-Hakil F, Minvielle-Sebastia L. Molecular dissection of mRNA poly(A) tail length control in yeast. Nucleic Acids Res. 2008;36:2418–2433. doi: 10.1093/nar/gkn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kelly SM, Leung SW, Apponi LH, Bramley AM, Tran EJ, Chekanova JA, Wente SR, Corbett AH. Recognition of polyadenosine RNA by the zinc finger domain of nuclear poly(A) RNA-binding protein 2 (Nab2) is required for correct mRNA 3'-end formation. J Biol Chem. 2010;285:26022–26032. doi: 10.1074/jbc.M110.141127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Green DM, Marfatia KA, Crafton EB, Zhang X, Cheng X, Corbett AH. Nab2p is required for poly(A) RNA export in Saccharomyces cerevisiae and is regulated by arginine methylation via Hmt1p. J Biol Chem. 2002;277:7752–7760. doi: 10.1074/jbc.M110053200. [DOI] [PubMed] [Google Scholar]
- 115.Lamond AI, Spector DL. Nuclear speckles: a model for nuclear organelles. Nat Rev Mol Cell Biol. 2003;4:605–612. doi: 10.1038/nrm1172. [DOI] [PubMed] [Google Scholar]
- 116.Tuck AC, Tollervey D. A transcriptome-wide atlas of RNP composition reveals diverse classes of mRNAs and lncRNAs. Cell. 2013;154:996–1009. doi: 10.1016/j.cell.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marfatia KA, Crafton EB, Green DM, Corbett AH. Domain analysis of the Saccharomyces cerevisiae heterogeneous nuclear ribonucleoprotein, Nab2p. Dissecting the requirements for Nab2p-facilitated poly(A) RNA export. J Biol Chem. 2003;278:6731–6740. doi: 10.1074/jbc.M207571200. [DOI] [PubMed] [Google Scholar]
- 118.Soucek S, Corbett AH, Fasken MB. The long and the short of it: the role of the zinc finger polyadenosine RNA binding protein, Nab2, in control of poly(A) tail length. Biochim Biophys Acta. 2012;1819:546–554. doi: 10.1016/j.bbagrm.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kelly SML SW, Pak C, Banerjee A, Moberg KH, Corbett AH. A Conserved Role for the Zinc Finger Polyadenosine RNA Binding Protein, ZC3H14, in Control of Poly(A) Tail Length. RNA. 2014 doi: 10.1261/rna.043984.113. In Submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rankin CA, Gamblin TC. Assessing the toxicity of tau aggregation. J Alzheimers Dis. 2008;14:411–416. doi: 10.3233/jad-2008-14408. [DOI] [PubMed] [Google Scholar]
- 121.Harris CE, Boden RA, Astell CR. A novel heterogeneous nuclear ribonucleoprotein-like protein interacts with NS1 of the minute virus of mice. J Virol. 1999;73:72–80. doi: 10.1128/jvi.73.1.72-80.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mizutani A, Fukuda M, Ibata K, Shiraishi Y, Mikoshiba K. SYNCRIP, a cytoplasmic counterpart of heterogeneous nuclear ribonucleoprotein R, interacts with ubiquitous synaptotagmin isoforms. J Biol Chem. 2000;275:9823–9831. doi: 10.1074/jbc.275.13.9823. [DOI] [PubMed] [Google Scholar]
- 123.Svitkin YV, Yanagiya A, Karetnikov AE, Alain T, Fabian MR, Khoutorsky A, Perreault S, Topisirovic I, Sonenberg N. Control of translation and miRNA-dependent repression by a novel poly(A) binding protein, hnRNP-Q. PLoS Biol. 2013;11:e1001564. doi: 10.1371/journal.pbio.1001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mourelatos Z, Abel L, Yong J, Kataoka N, Dreyfuss G. SMN interacts with a novel family of hnRNP and spliceosomal proteins. EMBO J. 2001;20:5443–5452. doi: 10.1093/emboj/20.19.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rossoll W, Kroning AK, Ohndorf UM, Steegborn C, Jablonka S, Sendtner M. Specific interaction of Smn, the spinal muscular atrophy determining gene product, with hnRNP-R and gry-rbp/hnRNP-Q: a role for Smn in RNA processing in motor axons? Hum Mol Genet. 2002;11:93–105. doi: 10.1093/hmg/11.1.93. [DOI] [PubMed] [Google Scholar]
- 126.Xing L, Yao X, Williams KR, Bassell GJ. Negative regulation of RhoA translation and signaling by hnRNP-Q1 affects cellular morphogenesis. Mol Biol Cell. 2012;23:1500–1509. doi: 10.1091/mbc.E11-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Quaresma AJ, Oyama S, Jr, Barbosa JA, Kobarg J. The acidic domain of hnRNPQ (NSAP1) has structural similarity to Barstar and binds to Apobec1. Biochem Biophys Res Commun. 2006;350:288–297. doi: 10.1016/j.bbrc.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 128.Chen HH, Chang JG, Lu RM, Peng TY, Tarn WY. The RNA binding protein hnRNP Q modulates the utilization of exon 7 in the survival motor neuron 2 (SMN2) gene. Mol Cell Biol. 2008;28:6929–6938. doi: 10.1128/MCB.01332-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kabat JL, Barberan-Soler S, Zahler AM. HRP-2, the Caenorhabditis elegans homolog of mammalian heterogeneous nuclear ribonucleoproteins Q and R, is an alternative splicing factor that binds to UCUAUC splicing regulatory elements. J Biol Chem. 2009;284:28490–28497. doi: 10.1074/jbc.M109.023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Neubauer G, King A, Rappsilber J, Calvio C, Watson M, Ajuh P, Sleeman J, Lamond A, Mann M. Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat Genet. 1998;20:46–50. doi: 10.1038/1700. [DOI] [PubMed] [Google Scholar]
- 131.Blanc V, Navaratnam N, Henderson JO, Anant S, Kennedy S, Jarmuz A, Scott J, Davidson NO. Identification of GRY-RBP as an apolipoprotein B RNA-binding protein that interacts with both apobec-1 and apobec-1 complementation factor to modulate C to U editing. J Biol Chem. 2001;276:10272–10283. doi: 10.1074/jbc.M006435200. [DOI] [PubMed] [Google Scholar]
- 132.Lau PP, Chang BH, Chan L. Two-hybrid cloning identifies an RNA-binding protein, GRY-RBP, as a component of apobec-1 editosome. Biochem Biophys Res Commun. 2001;282:977–983. doi: 10.1006/bbrc.2001.4679. [DOI] [PubMed] [Google Scholar]
- 133.Bannai H, Fukatsu K, Mizutani A, Natsume T, Iemura S, Ikegami T, Inoue T, Mikoshiba K. An RNA-interacting protein, SYNCRIP (heterogeneous nuclear ribonuclear protein Q1/NSAP1) is a component of mRNA granule transported with inositol 1,4,5-trisphosphate receptor type 1 mRNA in neuronal dendrites. J Biol Chem. 2004;279:53427–53434. doi: 10.1074/jbc.M409732200. [DOI] [PubMed] [Google Scholar]
- 134.Chen HH, Yu HI, Chiang WC, Lin YD, Shia BC, Tarn WY. hnRNP Q regulates Cdc42-mediated neuronal morphogenesis. Mol Cell Biol. 2012;32:2224–2238. doi: 10.1128/MCB.06550-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Elvira G, Wasiak S, Blandford V, Tong XK, Serrano A, Fan X, del Rayo Sanchez-Carbente M, Servant F, Bell AW, Boismenu D, et al. Characterization of an RNA granule from developing brain. Mol Cell Proteomics. 2006;5:635–651. doi: 10.1074/mcp.M500255-MCP200. [DOI] [PubMed] [Google Scholar]
- 136.Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 137.McDermott SM, Meignin C, Rappsilber J, Davis I. Drosophila Syncrip binds the gurken mRNA localisation signal and regulates localised transcripts during axis specification. Biol Open. 2012;1:488–497. doi: 10.1242/bio.2012885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cho S, Park SM, Kim TD, Kim JH, Kim KT, Jang SK. BiP internal ribosomal entry site activity is controlled by heat-induced interaction of NSAP1. Mol Cell Biol. 2007;27:368–383. doi: 10.1128/MCB.00814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim DY, Kim W, Lee KH, Kim SH, Lee HR, Kim HJ, Jung Y, Choi JH, Kim KT. hnRNP Q regulates translation of p53 in normal and stress conditions. Cell Death Differ. 2013;20:226–234. doi: 10.1038/cdd.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim DY, Kwak E, Kim SH, Lee KH, Woo KC, Kim KT. hnRNP Q mediates a phase-dependent translation-coupled mRNA decay of mouse Period3. Nucleic Acids Res. 2011;39:8901–8914. doi: 10.1093/nar/gkr605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim DY, Woo KC, Lee KH, Kim TD, Kim KT. hnRNP Q and PTB modulate the circadian oscillation of mouse Rev-erb alpha via IRES-mediated translation. Nucleic Acids Res. 2010;38:7068–7078. doi: 10.1093/nar/gkq569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim JH, Paek KY, Ha SH, Cho S, Choi K, Kim CS, Ryu SH, Jang SK. A cellular RNA-binding protein enhances internal ribosomal entry site-dependent translation through an interaction downstream of the hepatitis C virus polyprotein initiation codon. Mol Cell Biol. 2004;24:7878–7890. doi: 10.1128/MCB.24.18.7878-7890.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee KH, Woo KC, Kim DY, Kim TD, Shin J, Park SM, Jang SK, Kim KT. Rhythmic interaction between Period1 mRNA and hnRNP Q leads to circadian time-dependent translation. Mol Cell Biol. 2012;32:717–728. doi: 10.1128/MCB.06177-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lyabin DN, Nigmatullina LF, Doronin AN, Eliseeva IA, Ovchinnikov LP. Identification of proteins specifically interacting with YB-1 mRNA 3' UTR and the effect of hnRNP Q on YB-1 mRNA translation. Biochemistry (Mosc) 2013;78:651–659. doi: 10.1134/S0006297913060102. [DOI] [PubMed] [Google Scholar]
- 145.Vincendeau M, Nagel D, Brenke JK, Brack-Werner R, Hadian K. Heterogenous nuclear ribonucleoprotein Q increases protein expression from HIV-1 Rev-dependent transcripts. Virol J. 2013;10:151. doi: 10.1186/1743-422X-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen CY, Shyu AB. Rapid deadenylation triggered by a nonsense codon precedes decay of the RNA body in a mammalian cytoplasmic nonsense-mediated decay pathway. Mol Cell Biol. 2003;23:4805–4813. doi: 10.1128/MCB.23.14.4805-4813.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim TD, Kim JS, Kim JH, Myung J, Chae HD, Woo KC, Jang SK, Koh DS, Kim KT. Rhythmic serotonin N-acetyltransferase mRNA degradation is essential for the maintenance of its circadian oscillation. Mol Cell Biol. 2005;25:3232–3246. doi: 10.1128/MCB.25.8.3232-3246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moser JJ, Eystathioy T, Chan EK, Fritzler MJ. Markers of mRNA stabilization and degradation, and RNAi within astrocytoma GW bodies. J Neurosci Res. 2007;85:3619–3631. doi: 10.1002/jnr.21439. [DOI] [PubMed] [Google Scholar]
- 149.Weidensdorfer D, Stohr N, Baude A, Lederer M, Kohn M, Schierhorn A, Buchmeier S, Wahle E, Huttelmaier S. Control of c-myc mRNA stability by IGF2BP1-associated cytoplasmic RNPs. RNA. 2009;15:104–115. doi: 10.1261/rna.1175909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bayfield MA, Yang R, Maraia RJ. Conserved and divergent features of the structure and function of La and La-related proteins (LARPs) Biochim Biophys Acta. 2010;1799:365–378. doi: 10.1016/j.bbagrm.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bousquet-Antonelli C, Deragon JM. A comprehensive analysis of the La-motif protein superfamily. RNA. 2009;15:750–764. doi: 10.1261/rna.1478709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Merret R, Martino L, Bousquet-Antonelli C, Fneich S, Descombin J, Billey E, Conte MR, Deragon JM. The association of a La module with the PABP-interacting motif PAM2 is a recurrent evolutionary process that led to the neofunctionalization of La-related proteins. RNA. 2013;19:36–50. doi: 10.1261/rna.035469.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chlebowski A, Lubas M, Jensen TH, Dziembowski A. RNA decay machines: the exosome. Biochim Biophys Acta. 2013;1829:552–560. doi: 10.1016/j.bbagrm.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 154.Yang R, Gaidamakov SA, Xie J, Lee J, Martino L, Kozlov G, Crawford AK, Russo AN, Conte MR, Gehring K, et al. La-related protein 4 binds poly(A), interacts with the poly(A)-binding protein MLLE domain via a variant PAM2w motif, and can promote mRNA stability. Mol Cell Biol. 2011;31:542–556. doi: 10.1128/MCB.01162-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nilsson J, Sengupta J, Frank J, Nissen P. Regulation of eukaryotic translation by the RACK1 protein: a platform for signalling molecules on the ribosome. EMBO Rep. 2004;5:1137–1141. doi: 10.1038/sj.embor.7400291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marzluff WF, Wagner EJ, Duronio RJ. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nat Rev Genet. 2008;9:843–854. doi: 10.1038/nrg2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Funakoshi Y, Doi Y, Hosoda N, Uchida N, Osawa M, Shimada I, Tsujimoto M, Suzuki T, Katada T, Hoshino S. Mechanism of mRNA deadenylation: evidence for a molecular interplay between translation termination factor eRF3 and mRNA deadenylases. Genes Dev. 2007;21:3135–3148. doi: 10.1101/gad.1597707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grundberg E, Brandstrom H, Ribom EL, Ljunggren O, Kindmark A, Mallmin H. A poly adenosine repeat in the human vitamin D receptor gene is associated with bone mineral density in young Swedish women. Calcif Tissue Int. 2003;73:455–462. doi: 10.1007/s00223-002-0032-y. [DOI] [PubMed] [Google Scholar]
- 159.Leeb T, Kriegesmann B, Baumgartner BG, Klett C, Yerle M, Hameister H, Brenig B. Molecular cloning of the porcine beta-1,2-N-acetylglucosaminyltransferase II gene and assignment to chromosome 1q23-q27. Biochim Biophys Acta. 1997;1336:361–366. doi: 10.1016/s0304-4165(97)00072-x. [DOI] [PubMed] [Google Scholar]
- 160.Tuffery S, Moine P, Demaille J, Claustres M. Identification of variable length polyadenosine tract at the dystrophin locus. Hum Genet. 1995;95:590–592. doi: 10.1007/BF00223878. [DOI] [PubMed] [Google Scholar]
- 161.Roth KM, Wolf MK, Rossi M, Butler JS. The nuclear exosome contributes to autogenous control of NAB2 mRNA levels. Mol Cell Biol. 2005;25:1577–1585. doi: 10.1128/MCB.25.5.1577-1585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Matoulkova E, Michalova E, Vojtesek B, Hrstka R. The role of the 3' untranslated region in post-transcriptional regulation of protein expression in mammalian cells. RNA Biol. 2012;9:563–576. doi: 10.4161/rna.20231. [DOI] [PubMed] [Google Scholar]
- 163.Fustin JM, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, Isagawa T, Morioka MS, Kakeya H, Manabe I, et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 164.Grange T, de Sa CM, Oddos J, Pictet R. Human mRNA polyadenylate binding protein: evolutionary conservation of a nucleic acid binding motif. Nucleic Acids Res. 1987;15:4771–4787. doi: 10.1093/nar/15.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Feral C, Mattei MG, Pawlak A, Guellaen G. Chromosomal localization of three human poly(A)-binding protein genes and four related pseudogenes. Hum Genet. 1999;105:347–353. doi: 10.1007/s004399900148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Blanco P, Sargent CA, Boucher CA, Howell G, Ross M, Affara NA. A novel poly(A)-binding protein gene (PABPC5) maps to an X-specific subinterval in the Xq21.3/Yp11.2 homology block of the human sex chromosomes. Genomics. 2001;74:1–11. doi: 10.1006/geno.2001.6530. [DOI] [PubMed] [Google Scholar]
- 167.Guzeloglu-Kayisli O, Pauli S, Demir H, Lalioti MD, Sakkas D, Seli E. Identification and characterization of human embryonic poly(A) binding protein (EPAB) Mol Hum Reprod. 2008;14:581–588. doi: 10.1093/molehr/gan047. [DOI] [PubMed] [Google Scholar]