Abstract
Stress sensitivity may be one process that can explain why some genetically at-risk individuals are more susceptible to some types of stress-reactive psychopathologies. Dysregulation of the Limbic Hypothalamic Pituitary Adrenal (LHPA) axis, including cortisol reactivity to challenge, represents a key aspect of stress sensitivity. However, the degree of stability over time among youth, especially differential stability as a function of particular genetic variants, has not been investigated. A general community sample of children and adolescents (mean age = 11.4; 56% girls) provided a DNA sample and completed two separate laboratory stress challenges, across an 18-month follow-up (N =224 at Time 1; N = 194 at Time 2), with repeated measures of salivary cortisol. Results showed that test-retest stability for several indices of cortisol reactivity across the laboratory challenge visits were significant and of moderate magnitude for the whole sample. Moreover, gene variants of several biologically plausible systems relevant for stress sensitivity (especially 5-HTTLPR and CRHR1) demonstrated differential stability of cortisol reactivity over 18-months, such that carriers of genotypes conferring enhanced environmental susceptibility exhibited greater stability of cortisol levels over time for some LHPA axis indices. Findings suggest that LHPA axis dysregulation may exhibit some trait-like aspects underlying stress sensitivity in youth, especially for those who carry genes related to greater genetic susceptibility to environmental stress.
It is well accepted and demonstrated that stress contributes to the development of many forms of commonly occurring psychopathological symptoms and disorders (e.g., Grant, Compas, Stuhlmacher, Thurm, & McMahon, 2003; Hostinar & Gunnar, 2013; Mazure, 1998; Monroe, 2008). However, at the same time, decades of research have demonstrated that not everyone succumbs to stress exposure with increases in psychopathology. This has led to theoretical and empirical work on individual differences in stress sensitivity (e.g., Belsky, Pluess, & Widamen, 2013; Meehl, 1962; Monroe & Simons, 1991; Zubin & Spring, 1977). Although the literature does not have a clearly, precisely, and agreed upon definition of the construct of stress sensitivity, an implicit consensus across most approaches converges on the idea that there are individual differences in stress response which affect the likelihood of exhibiting increases in psychopathology after stress exposure. In other words, individuals who are stress sensitive require less stress relative to others to elicit symptoms of certain forms of some psychopathologies (e.g., Monroe & Harkness, 2005; Post, 1992).
At the same time, many approaches for capturing and understanding these individual differences in moderating the effect of stress on the prediction of later psychopathology have been proposed and examined, including genetic (Lemery & Doelger, 2005; Rende & Waldman, 2006), cognitive (Hankin, Snyder, & Gulley, in press; Alloy & Riskind, 2006), interpersonal (Van Orden et al., 2005), temperament (Rothbart & Posner, 2006; Nigg, 2006), and neurobiological (Gunnar & Vazquez, 2006; Walker et al., 2004) factors. This vulnerability (or diathesis)-stress approach to the development of psychopathology has generally proven successful (Hankin & Abela, 2005; Ingram & Price, 2010). Yet identifying which individual difference factors moderate the effect of stress exposure on certain psychopathological symptoms does not reveal the underlying processes by which stress sensitivity provokes elevations in some symptoms and eventual onset of stress-related disorders. Many researchers have focused on the Limbic Hypothalamic Pituitary Adrenal (LHPA) axis as one core intermediate process and biological mechanism that may undergird stress sensitivity.
In this paper, we investigated the degree of stability of a key aspect of LHPA axis activity, specifically cortisol reactivity to stress, among children and adolescents. In particular, we examined the novel and intriguing hypothesis that stability of cortisol reactivity to stress over time may vary by selected genotypes related to stress sensitivity. As we will introduce, prior work has found that particular genetic variants are associated with cortisol response and that, in general, cortisol reactivity exhibits moderate stability over time. By synthesizing these findings, we hypothesize that particular genetic variants related to environmental susceptibility may contribute to stress sensitivity via enhanced stability over time in cortisol reactivity to stress. The findings from this study support this hypothesis and show that: 1) various aspects of cortisol reactivity to stress, albeit not all, are moderately stable in youth over a relatively long test-retest interval (18-months), 2) several, but not all, biologically relevant, a priori selected candidate genes for heightened stress sensitivity are significantly associated with certain components of cortisol reactivity to stress across the laboratory visit, and 3) there are meaningful differential stabilities over time in cortisol reactivity to stress that systematically vary across these key genetic variants for stress sensitivity.
LHPA axis dysregulation and psychopathology: Cortisol reactivity to stress
LHPA axis dysregulation represents a promising candidate biological mechanism that may relate to stress sensitivity. Cortisol is the glucocorticoid end product of the LHPA system. An essential aspect of the body’s interconnected set of physiological systems for responding to challenges, the LHPA axis is especially sensitive to stressful situations, especially those involving novelty, uncontrollability, or social threat (Dickerson & Kemeny, 2004).
Abnormal cortisol function and reactivity to stress are established markers of many forms of stress-related psychopathologies, including some anxiety disorders, depression, and substance use problems in adults and youth (Burke, Davis, Otte, & Mohr, 2005; Gunnar & Quevedo, 2007; Gunnar & Vazquez, 2006; Lopez-Duran, Kovacs, & George, 2009; Schepis, Rao, Yadav, & Adinoff, 2011; Schiefelbein & Susman, 2006; Vreeburg et al., 2010; Walker et al., 2001; Walker, Sabuwalla, & Huot, 2004). However, the relations between cortisol levels and various forms of stress-reactive psychopathologies are complex. Most research with adults tends to show that depression is associated with elevated cortisol levels and reduced feedback inhibition, whereas cortisol levels are often low in PTSD and some other anxiety disorders, relative to healthy controls in stress reactivity paradigms. Importantly, research suggests that cortisol reactivity predates prospective symptom elevations and onset of some stress-related disorders (e.g., Badanes, Watamura, & Hankin, 2011; Schiefelbein & Susman, 2010; Shirtcliff et al., 2012; Walker et al., 2001) and may contribute causally in the development of some stress-reactive psychiatric disorders, such as depression (Azar, Paquette, Zoccolillo, Baltzer, & Tremblay, 2007; Feldman et al., 2009; Hasler, Drevets, Manji, & Charney, 2004; Oswald et al., 2006). In sum, these studies are consistent in showing that cortisol levels are associated with particular forms of stress-related psychopathologies, even though the precise nature of this relationship can be complex and vary across different disorders.
Cortisol reactivity to stress, stability, and associations with genetic susceptibility
Many common forms of stress-reactive psychopathologies, such as depression and several anxiety disorders, exhibit significant, albeit moderate, heritability (Lemery & Doelger, 2005; Rende & Waldman, 2006). Beyond main effects of latent heritability and observed genetic variants, research has also extensively examined and demonstrated gene-environment interplay, including gene-environment interactions (GxE) and correlations (rGE) (e.g., Jaffee & Price, 2007; Belsky et al., 2013; Caspi et al., 2010; Moffitt, Caspi, & Rutter, 2005). In light of these advances, it has been argued that there is a need for better understanding potential causal pathways that link genetic risk to eventual onset of symptoms for stress-reactive forms of psychiatric disorder. Some empirical and theoretical work has suggested that LHPA axis dysregulation may connect together part of the association between latent genetic risk and later psychopathologies (e.g., Clarke et al., 2008, Federenko, Nagamine, Hellhammer, Wadwha, & Wust, 2004; Mehta & Binder, 2012, Oldehinkel & Bouma, 2011). However, little empirical research has explicitly examined whether particular, biologically relevant, a priori selected candidate genes relevant for heightened stress sensitivity are associated with cortisol reactivity to stress, and especially the degree of stability in LHPA axis reactivity over time. Establishing that stability of cortisol reactivity to stress is related to selected susceptibility to stress alleles would thus add new knowledge to the literature. Investigating this question is the primary aim of the current study.
Cortisol reactivity and genetic susceptibility
First, it is important to demonstrate associations between biologically plausible genetic variants, known to be associated with stress sensitivity, and link these genotypes with cortisol reactivity. In this study, we selected particular biologically relevant genetic variants, including 5-HTTLPR, DRD2, COMT, and CRHR1, because these genotypes have been linked with common stress-related psychopathologies, general stress sensitivity, and LHPA axis activity specifically. Prior evidence has established significant relations between these genetic variants and LHPA axis dysregulation, including cortisol reactivity to laboratory stress.
5-HTTLPR
Probably the most investigated association between genetic susceptibility and genotype-dependent effects in stress sensitivity is the link between the Transporter-Linked Polymorphic Region (5-HTTLPR) and cortisol reactivity to laboratory stress. A recent meta-analysis (Miller, Wankerl, Stalder, Kirschbaum, & Alexander, 2013) demonstrated a significant association between 5-HTTLPR genotype and LHPA axis reactivity to stress. There are potential mechanisms by which 5-HTTLPR could yield enhanced cortisol stability over time. One possibility is that 5-HTTLPR is involved in the neural circuitry, including via greater amygdala activity, involved with processing of negatively valenced emotional stimuli (e.g., Hariri et al., 2005; Heinz et al., 2004) and biased processing of emotional stimuli (e.g., Beevers et al., 2009).
COMT
Similarly, the Val108/158 SNP in the catechol-O-methyltransferase (COMT) gene, which is involved with coding for the gene that is responsible for metabolizing dopamine, has been hypothesized to affect stress sensitivity (Zubieta et al., 2003). One possible mechanism by which COMT could lead to greater cortisol stability involves different neural substrates that are linked with greater executive functioning problems (Val allele carriers) (Mier, Kirsch, & Meyer-Lindenberg, 2010). Alternatively, another possibility is that Val carriers exhibit abnormal reward processing as found in a neuroimaging study (Camara et al., 2010). COMT is significantly associated with various psychiatric disorders (e.g., Hosak, 2007) as well as levels of cortisol response (e.g., cortisol stress reactivity in Armburster et al., 2012, Jabbi et al., 2007; total morning cortisol output in Walder et al., 2010).
DRD2
Next, various studies have demonstrated GxE effects with the gene coding for the D2 subtype of the Dopamine Receptor (DRD2) in various psychopathologies (Belsky & Pluess, 2009; Dunn et al., 2011; Koenen et al., 2009), and there are significant associations between dopamine release in the brain and cortisol levels (increased cortisol production after amphetamine challenge; Oswald et al., 2005). Although less research has investigated mechanisms relating DRD2 to stress sensitivity, one possibility, based on animal research, is that repeated stress (e.g., social defeat) can reduce D2 mRNA expression in key brain reward centers (i.e., dorsal striatum and nucleus accumbens) (Dietz, Dietz, Moore, Ouimet, & Kabbaj, 2008).
CRHR1
Finally, corticotropin-releasing hormone receptors (CRHR) are critical proteins for regulating LHPA axis activity. There are two receptors for CRHR, designated as type 1 and 2, and these are encoded genetically by CRHR1 and CRHR2, respectively. Various studies have demonstrated associations between cortisol reactivity to stressful challenge and specific SNPs in CRHR1 (e.g., Cicchetti, Rogosch, & Oshri, 2011; Mahon et al., 2013; Pagliaccio et al., 2014; Sumner et al., 2014). Based on this research, we selected CRHR1 for investigation in this study. One potential mechanism by which CRHR1 may lead to greater stress sensitivity derives from animal research, in which a lowered stress threshold was related to increased alcohol seeking, and this effect occurred via CRHR1 genetic activity (Hansson et al., 2006). Similar interactions between CRHR1 and stress have been found in human adolescents who transitioned into higher rates of alcohol consumption and drinking progression (Schmid et al., 2010).
In summary, these studies provide evidence that some aspects of levels of cortisol response exhibit significant molecular genetic associations. These data supplement behavioral genetic findings (e.g., Federenko et al., 2004) showing that there is a heritable component to cortisol reactivity.
LHPA axis stability over time
Second, to establish that stability of cortisol reactivity to stress is related to selected susceptibility to stress alleles, research is needed to ascertain that this index of LHPA axis dysregulation exhibits trait-like characteristics of stability over time. Some studies (e.g., Goldberg et al., 2003; Liening, Stanton, & Schultheiss, 2010; Platje et al., 2013; Pruessner et al., 1997; Shirtcliff et al., 2012), but not all (e.g., Ross et al., 2014), show moderate test-retest consistency in cortisol response as indexed by levels of diurnal cortisol or cortisol awakening response (CAR). Similarly, longitudinal studies investigating the degree of stability for levels of cortisol reactivity to stressful challenges demonstrate moderate test-retest consistency over time for cortisol reactivity to stress (e.g., Federenko et al., 2004; Kirschbaum, Prussner, Stone, Federenko, Gaab, Lintz, Schommer, & Hellhammer, 1995; Schommer, Hellhammer, & Kirschbaum, 2003; Susman, Dorn, Inoff-German, Nottelmann, & Chrousos, 1997).
Still, there are gaps in the extant cortisol stability literature that the present research can address. Most of the prior studies included adult male samples (Federenko et al., 2004; Kirschbaum et al., 1995, but see Susman et al., 1997, for exception with adolescents). Also, the test-retest interval between cortisol reactivity laboratory challenges was relatively brief—daily across 5 days (Kirschbaum et al., 1995), three times across 1-week intervals (Federenko et al., 2004), and three times across 4-week intervals (Schommer et al., 2003). These adult-sample studies with relatively short time intervals between cortisol reactivity assessments demonstrated moderate stabilities (r’s ranging from .38-.62) using Area Under the Curve (AUC) as an index of cortisol reactivity. Thus, the extant literature suggests moderate levels of test-retest stability for cortisol reactivity to laboratory stressor, consistent with the notion of a relatively stable, trait-like component that may underlie LHPA activity to stressful challenges.
The present study adds to this corpus of research by reporting on stability in levels of cortisol response with multiple samplings across the full stressful laboratory challenge at two time points using a longer, 18-month longitudinal follow-up and with a sample of youth. Moreover, several of the prior studies used AUC (Kirschbaum et al., 1995; Federenko et al., 2004), or simple change score (Susman et al., 1997), as the index of cortisol reactivity, although more complex and nuanced approaches of LHPA axis stress reactivity can be considered (e.g., peak level, activation and recovery intensity; Lopez-Duran, Mayer, & Abelson, 2014). As such, in this study, we investigate the degree of stability using both AUC and different components of the cortisol stress reactivity profile to evaluate stability in more nuanced ways.
Differential stability of cortisol reactivity by genetic susceptibility to stress
Finally, by synthesizing these two main findings—that particular genetic variants are associated with cortisol response, and cortisol reactivity exhibits stability over time—a unique hypothesis can be postulated in that stability of cortisol reactivity to stress over time may vary by genotype. In other words, individuals carrying susceptibility genotypes that are associated with higher reactivity to stressful environmental contexts would likely exhibit greater stability in cortisol reactivity to challenge over time. The genetic variants selected for investigation in this study have demonstrated enhanced reactivity to various relevant contextual environments, such as stressful life events (e.g., Belsky & Pluess, 2009), that are associated with risk to particular stress-related psychopathologies. In sum, it is reasonable to expect greater continuity of dysregulated cortisol reactivity in individuals who are at enhanced genetic susceptibility to environmental events. We test this hypothesis by evaluating whether particular susceptibility to environment genes moderate the degree of stability in various indices of cortisol reactivity to stress.
The present investigation
In summary, we examined the stability of cortisol reactivity to stress, as one index of LHPA axis dysregulation, and whether there is differential stability that is influenced by selected susceptibility to stress candidate genes. Studying a sample of children and adolescents recruited from the general community who were longitudinally followed over an 18-month period with two administrations of stressful laboratory challenges and repeated salivary cortisol measurements across each laboratory visit, we addressed the following hypotheses. First, cortisol reactivity to stress would exhibit moderate stability over the 18-month follow-up for the sample of youth as a whole. Second, biologically plausible genetic variants associated with stress sensitivity and susceptibility to environment would moderate the degree of stability in cortisol levels over time. Last, these genetic variants would relate to higher cortisol reactivity for key components of stress reactivity across the laboratory visit.
Method
Participants
Children and adolescents were recruited by letters sent home to families with a child in 3rd, 6th, or 9th grades of public schools. Interested parents called the laboratory and responded to a brief phone screen that established that both the parent and child were fluent in English, and the child did not carry an autism spectrum or psychotic disorder and had an IQ > 70. Participants were 224 youth ranging in age from 9-15 (M = 11.4, SD = 2.27) at Time 1; 194 youth (86.6%) returned 18-months later at Time 2. The sample was approximately evenly divided by sex (boys: 44%, girls: 56%) and grade (32% 3rd grade, 32% 6th grade, 36% 9th grade). Ethnicity was as follows: Caucasian: 64%, African American: 8%, Latino: 7%, Asian/Pacific Islander: 5%, Other/Mixed Race: 16%.
The primary caretaker and the child participant visited the laboratory for the assessments. This consisted of collection of youth cortisol via saliva at both time points and collection of DNA via saliva at Time 1. Parents provided informed written consent for their own and their child’s participation; youth provided written assent.
Laboratory stress paradigms at Times 1 and 2 and cortisol assessment
The stress paradigm at Time 1 (see Hankin, Badanes, Abela, & Watamura, 2010) included two components. First was a 5-10 minute problem-solving task, which has been used previously in stress reactivity research (Granger, Weisz, & Kauneckis, 1994). The caretaker and child discuss a conflict and talk about this recent argument (Robin & Foster, 1989). Second, youth auditioned for a “reality TV show” by giving a speech directly into a video camera while their parent watched; youth were instructed that judges would evaluate their performance. The combination of both components yielded a stressful laboratory challenge lasting approximately 15 minutes.
At Time 2, the youth completed a different stress paradigm that was modeled after other reliable, validated distress-inducing laboratory challenges (Nock & Mendes, 2008; Ruggero & Johnson, 2006; Stroud, Salovey, & Epel, 2002) and also involved two components. The combination of both elements lasted approximately 15 minutes. First, youth completed a modified Wisconsin Card Sort Task (Nock & Mendes, 2008). The experimenter told the child that s/he had correctly matched the four key cards after the first three trials (to involve the child) and then told the child the next seven responses were incorrect (to induce distress); thereafter, the child was informed that the 11th trial was correct while the next nine were incorrect. The second half of the challenge included a stressful math performance task, modified from a validated math achievement stress task (Stroud et al., 2002) to be developmentally appropriate for youth. The participants had to complete a series of difficult math problems (on average 2 grade levels above the child’s grade) on a white board, while time pressure was imposed, and the children had to explain their reasoning and answers to a panel (2-3 trained individuals) of impassive judges.
There is a lack of consensus on stress paradigms that elicit cortisol reactivity across different ages (Gunnar, Talge, & Herrera, 2009). These laboratory challenge tasks were used because they were expected to be developmentally appropriate across the ages of the participants, they have been used previously in other stress-inducing laboratory challenge paradigms in reliable and valid ways, and they involved the essential elements (e.g., threat of social rejection and social evaluation, anticipatory and processive stress) known to activate the LHPA axis in children and adolescents (Lupien, McEwen, Gunnar, & Heim, 2009). Prior research using these stress elicitation paradigms demonstrates their validity for relevant outcomes.
The number of saliva samples assessed across the full laboratory challenge differed slightly between Times 1 and 2. For Time 1, we collected five total saliva samples: the first was upon immediate entry to the laboratory, the second was an hour later (baseline), the third was after the challenge (reactivity), and the fourth and fifth (recoveries) were at 15-minute intervals after the challenge. For Time 2, we collected seven total samples: the first two were taken upon immediate entry to the laboratory with a 10-minute separation, the third was an hour later (baseline), the fourth was after the challenge (reactivity), and the remaining three (recoveries) were at 15-minute intervals after the challenge. These procedures were used because we expected a high cortisol response with initial entry to the laboratory, which involves stress anticipation given that arriving at a university research laboratory represents an uncertain and novel context, in addition to elevated cortisol in reaction to the laboratory stressful challenge, involving social evaluation and rejection. Importantly, youth were informed not to eat, especially dairy products, within an hour before coming to the laboratory, and youth were asked about consumption of dairy and last food and drink consumed, as well as other known confounds, such as steroid/inhaler use and asthma medications, allergies, recent illness, nicotine use, and oral contraceptives. Then, to allow for a decrease in any cortisol reactivity and return to an individual’s normal baseline that was expected to occur in response to the uncertainty and arriving at the research laboratory, the youth participated in the stress paradigm after an hour of acclimation. At both Times 1 and 2, on average, initial cortisol samples were collected at 17:00 (range 16:00-18:30). Overall, we followed standard procedures that we have used previously (Badanes et al., 2011; Hankin et al., 2010; Hayden et al., in press).
Saliva samples were obtained via synthetic salivette collection devices (Sarstedt, Nuembrecht, Germany). Saliva was extracted by centrifuging for 4 minutes at 2500 RPM. Vials and salivettes were frozen at −20° C until data collection was complete. Samples were then defrosted and batched for assay in groups of 36 and were assigned to batches; all samples from the same child were analyzed in the same batch. Samples were sent to the Biochemical Laboratory, Psychobiology, University of Trier, Germany to be assayed. Cortisol levels were determined by employing a competitive solid phase time-resolved fluorescence immunoassay with fluorometric end point detection (DELFIA; Hoferl, Krist, & Buchbauer, 2005). For samples retained in the analyses described next, the mean interassay coefficients of variation (CV) for controls were 6.6% to 8.5%. For duplicates of samples in this study, the intraassay CV was 5%.
Cortisol distributions were positively skewed as is frequently observed (Gunnar & Talge, 2005). Consistent with the standard approach, a log10 transformation of the raw cortisol values was applied and produced unskewed cortisol values; these were used in analyses. We used different indices of cortisol stress reactivity in our analyses for test-retest stability. We calculated the area under the curve (AUC) with respect to ground (AUCg) and with respect to increment (AUGi) (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003) for all of the available cortisol measures for each laboratory visit (i.e., 5 for Time 1; 7 for Time 2). AUCg is the total area under the curve of all measurements with reference from ground, or zero, whereas AUCi is the area under the curve with reference to the first value and emphasizes change over time (Pruessner et al., 2003). Finally, we also examined associations between the different key components of cortisol values across the laboratory visit and challenge, including initial entry into laboratory, baseline, challenge, and recovery 1 and 2, so that a more nuanced analysis of these indices could be provided.
Genotyping
Saliva samples were obtained from all study participants with Oragene™ (DNA Genotek, Ontario, Canada) collection kits, and DNA was extracted using standard salting-out and solvent precipitation methods. The method for 5-HTTLPR and SNP rs25531 is detailed in Whisman, Richardson, and Smolen (2011). Genotyping of TaqIA rs1800497 in DRD2 and Val158Met rs4680 in COMT are outlined in Haberstick and Smolen (2004). The successful call-rate was 97.5% for 5-HTTLPR, 97% for DRD2, and 96.3% for COMT. The SNPs (rs242924, rs878886) in CRHR1 were done on an Illumina BeadXpress® GoldenGate platform. SNP assays were ordered by rs number, and genotyping was performed according to company-supplied protocols (http://www.illumina.com/technology/goldengate_genotyping_assay.ilmn). The successful call rate for these SNPs was 85%. All of the genotypes were in Hardy-Weinberg Equilibrium, except for DRD2 (p = .02)
Pubertal Development
All youth were administered the Pubertal Development Scale (PDS; Petersen et al., 1988), which includes five questions about physical development, scored from 1 (no) to 4 (development complete). Reliability and validity of the PDS is high (Petersen et al., 1988; Shirtcliff et al., 2008), as PDS scores relate significantly with physical examination for pubertal development (Shirtcliff et al., 2008). We followed standard PDS scoring to create prepubertal and postpubertal groups separately for girls and boys.
RESULTS
Validity of laboratory challenge: Elicitation of cortisol reactivity pattern at Time 1 and 2
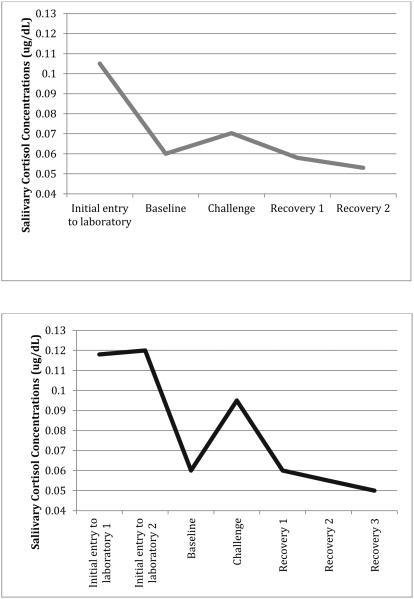
Prior to conducting the analyses to test main study hypotheses, it was important to demonstrate that the laboratory challenge tasks we employed at both time points indeed induced the anticipated cortisol reactivity pattern that has been consistently demonstrated in the literature. As seen in Figure 1, both laboratory stress challenge paradigms produced the expected cortisol reactivity pattern with a rise from baseline to reactivity and then decline in cortisol values during recovery. Repeated measures ANOVA analyses of the cortisol values from baseline to final recovery (i.e., 4 assessments for Time 1; 5 measurements for Time 2 eighteen months later) confirm that the overall stress response was quadratic. For Time 1, the quadratic component was significant, F(1, 223) = 12.11, p = .001; a similar significant quadratic component was seen for Time 2: F(1, 193) = 59.01, p < .001. The difference between cortisol values at baseline and reactivity was significant, for Time 1, t (1, 223) = 2.30, p < .05, and for Time 2, t (1, 193) = 9.59, p < .001. In sum, these analyses provide validity that each laboratory challenge elicited the cortisol reactivity patterns as expected with use of different laboratory challenge paradigms at Times 1 and 2.
Figure 1.
Mean cortisol response to psychosocial challenge among youth at Time 1 (top panel) and Time 2 (bottom panel).
Overall test-retest stability: Whole sample
Given the different ages of the youth in this study and demonstrations of significant pubertal effects in cortisol reactivity (e.g., Gunnar et al, 2009; Hankin et al., 2010; Stroud et al., 2009), we used partial correlations to examine test-retest correlations after partialling out the effect of pubertal development. Results, using the whole sample of children and adolescents available across both time points (N = 192), showed mild to moderate test-retest stability in cortisol levels assessed in the separate laboratory challenges at the two time points across the 18-month follow-up, depending on the index: AUCg (r = .41, p < .001), AUCi (r = .26, p < .001), and significant correlations between different constituent components of stress reactivity, including initial entry into lab (r = .32, p < .001), baseline (r = .30, p < .001), reactivity to challenge (r = .39, p < .001), recovery 1 (r = .38, p < .001), and recovery 2 (r = .30, p < .001).
Test-retest of cortisol reactivity to stress: Differential stability by susceptibility genotype
We examined whether the genotypes—5-HTTLPR, DRD2, COMT, and CRHR1—demonstrated differential levels of test-retest stability in AUCg, AUCi, and the various individual components of cortisol reactivity across the laboratory visit, over the 18-month follow-up. Given that there would be numerous statistical tests with the five genetic markers and the multiple LHPA indices, we used multiple regression to ascertain the main hypothesis that genetic susceptibility would moderate stability from Time 1 to Time 2 for LHPA indices. Specifically, we created regressions in which all five genetic markers (main effects and then the interaction of the genetic marker X Time 1 LHPA axis index) were included in a single analysis to predict change from Time 2 (i.e., 18-month later) HPA and Time 1 HPA axis index. We controlled for puberty and race/ethnicity (to manage population stratification; Tang et al., 2005). This analytic approach controls for any overlap among genetic markers and groups the analysis within families of test, so this provides a conservative test of hypotheses by controlling for shared variance among genotypes while providing an appropriate balance for examining multiple effects simultaneously. Step 4 of the regression represents the interaction between genetic marker and Time 1 LHPA axis activity (e.g., 5-HTTLPR x initial entry into the laboratory) predicting to Time 2 LHPA axis activity for that component and provides the critical analysis of our main hypothesis that genotype significantly affects the stability over time for a particular aspect of LHPA axis activity.
Results of these regressions (see Table 1) show that particular genetic markers significantly moderated stability over time (see Step 4) for AUCg, initial entry into the lab, and reactivity to laboratory challenge. There was no omnibus significant genotype interaction at Step 4 in these regressions for AUCi (ΔF for Step 4 = 2.26), baseline (ΔF for Step 4 = 0.49), Recovery 1 (ΔF for Step 4 = 0.81), or Recovery 2 (ΔF for Step 4 = 0.70). The test-retest correlations between baseline and 18-months visits are shown for AUCg and AUCi in Table 2; stability of constituent components of cortisol reactivity across the laboratory visit are reported in Table 3.
Table 1.
Regression analyses examining which genotypes for environmental susceptibility moderated degree of stability across 18-month follow-up for different LHPA indices.
| (AUCg) | |||||||
| Overall Model | Change Statistics | ||||||
| df | R2 | F | df | ΔR2 | ΔF | β | |
| Step 1 | 1, 187 | .066 | 8.90** | 1 | .066 | 8.90*** | |
| Baseline cortisol | .41** | ||||||
| Step 2 | 3, 185 | .069 | 3.06** | 2 | .033 | .20 | |
| Race | .04 | ||||||
| Puberty | .04 | ||||||
| Step 3 | 8, 180 | .091 | 1.49 | 5 | .022 | .57 | |
| 5-HTTLPR | .02 | ||||||
| DRD2 | .03 | ||||||
| COMT | .09 | ||||||
| CRHR1 rs242924 | .03 | ||||||
| CRHR1 rs 87886 | .08 | ||||||
| Step 4 | 13, 175 | .234 | 2.68** | 5 | .143 | 4.26 | |
| 5-HTTLPR × baseline | .57* | ||||||
| DRD2 × baseline | .33 | ||||||
| COMT × baseline | .11 | ||||||
| CRHR1 rs242924 × baseline | .61* | ||||||
| CRHR1 rs87886 × baseline | .74** | ||||||
| (Initial Entry to Laboratory) | |||||||
| Overall Model | Change Statistics | ||||||
| df | R2 | F | df | ΔR2 | ΔF | β | |
| Step 1 | 1, 187 | .134 | 19.62** | 1 | .134 | 19.62*** | |
| Baseline cortisol | .32*** | ||||||
| Step 2 | 3, 185 | .149 | 7.28*** | 2 | .015 | 1.09 | |
| Race | .03 | ||||||
| Puberty | .13 | ||||||
| Step 3 | 8, 180 | .157 | 2.79** | 5 | .008 | .24 | |
| 5-HTTLPR | .06 | ||||||
| DRD2 | .002 | ||||||
| COMT | .02 | ||||||
| CRHR1 rs242924 | .05 | ||||||
| CRHR1 rs 87886 | .06 | ||||||
| Step 4 | 13, 175 | .380 | 5.43*** | 5 | .223 | 8.29*** | |
| 5-HTTLPR × baseline | .296* | ||||||
| DRD2 × baseline | .12 | ||||||
| COMT × baseline | .35** | ||||||
| CRHR1 rs242924 × baseline | .21 | ||||||
| CRHR1 rs87886 × baseline | .68*** | ||||||
| (Reactivity to Laboratory Challenge) | |||||||
| Overall Model | Change Statistics | ||||||
| df | R2 | F | df | ΔR2 | ΔF | β | |
| Step 1 | 1, 187 | .139 | 20.28*** | 1 | .139 | 20.28*** | |
| Baseline cortisol | .39*** | ||||||
| Step 2 | 3, 185 | .141 | 6.76*** | 2 | .002 | .14 | |
| Race | .04 | ||||||
| Puberty | .01 | ||||||
| Step 3 | 8, 180 | .171 | 3.08** | 5 | .031 | .89 | |
| 5-HTTLPR | .05 | ||||||
| DRD2 | .04 | ||||||
| COMT | .06 | ||||||
| CRHR1 rs242924 | .13 | ||||||
| CRHR1 rs 87886 | .14 | ||||||
| Step 4 | 13, 175 | .418 | 6.29*** | 5 | .246 | 9.64*** | |
| 5-HTTLPR × baseline | .42* | ||||||
| DRD2 × baseline | .25 | ||||||
| COMT × baseline | .16 | ||||||
| CRHR1 rs242924 × baseline | .63*** | ||||||
| CRHR1 rs87886 × baseline | .77*** | ||||||
Table 2.
Stability estimates in AUCg and AUCi as indices of sensitivity differences in cortisol levels, across 18-month follow-up as a function of youth genetic susceptibility.
| AUCg | |||
| Test-retest stability coefficients over 18-months | |||
| Genotype | N | High susceptibility variant | Low susceptibility variant |
|
| |||
| 5-HTTLPR | 188 | (SS/SL; r = .50***) | (LL, r = .07) |
| DRD2 | 190 | (A2A2; r = .47***) | (A1A1/A1A2; r = .22) |
| COMT | 188 | (Val/Val;Val/Met; r = .47***) | (Met; r = .09) |
|
CRHR1
rs242924 |
189 | (AA; r = .41***) | (AC/ CC; r = .18) |
|
CRHR1
rs878886 |
188 | (CC/CG; r = .50***) | (GG; r = .13) |
| AUCi | |||
| Test-retest stability coefficients over 18-months | |||
| Genotype | N | High susceptibility variant | Low susceptibility variant |
|
| |||
| 5-HTTLPR | 188 | (SS/SL; r = .42***) | (LL, r = .19*) |
| DRD2 | 190 | (A2A2; r = .29*) | (A1A1/A1A2; r = .25*) |
| COMT | 188 | (Val/Val;Val/Met; r = .35**) | (Met; r = .12) |
|
CRHR1
rs242924 |
189 | (AA; r = .29*) | (AC/ CC; r = .27*) |
|
CRHR1
rs878886 |
188 | (CC/CG; r = .37**) | (GG; r = .13) |
Table 3.
Stability estimates in key components of stress reactivity across 18-month follow-up as a function of youth genetic susceptibility.
| Test-retest correlations for each stress reactivity component | |||||
| Genotype | Lab entry | Baseline | Challenge | Recovery 1 | Recovery 2 |
|
| |||||
| 5-HTTLPR | |||||
| LL | .15 | .06 | .02 | .27* | .13 |
| SL/SS | .43*** | .18* | .60*** | .49*** | .21* |
| DRD2 | |||||
| A1A1/A1A2 | .31* | .16 | .25* | .41*** | .22* |
| A2A2 | .37*** | .17* | .47*** | .47*** | .17* |
| COMT | |||||
| Met | .05 | .00 | .05 | .17 | .38** |
| V/V;V/M | .45*** | .20* | .56*** | .49*** | .47*** |
| CRHR1 rs242924 | |||||
| AC/CC | .29*** | .14 | .22** | .17* | .14 |
| AA | .25 | .33* | .72*** | .15 | .13 |
| CRHR1 rs878886 | |||||
| GG | .14 | .14 | .36*** | .06 | .06 |
| CC/CG | .65*** | .30* | .28* | .44*** | .40** |
These findings show that several, but not all, of the a priori identified high susceptibility variant of certain genotypes demonstrated significantly higher levels of test-retest stability over time compared to the low susceptibility variants’ stability coefficients. The strength of test-retest stabilities and the degree of magnitude in stability coefficients between genotype groups also depended on which index of cortisol reactivity was evaluated. More specifically and as seen in Table 1, high susceptibility genetic variants, including 5-HTTLPR and CRHR1 (both rs242924 and rs87886), showed significantly greater stability for AUCg. A more nuanced dissection of LHPA stress reactivity, including the cortisol levels across the laboratory visit, revealed a similar pattern in the genotype group differences regarding which aspects of LHPA axis stress reactivity exhibited differential test-retest stabilities. It is notable that there was no significant genotype difference for stability of the baseline component or the recovery components for any of the susceptibility alleles examined in this study. In contrast, there were significant genetic variant differences in stability over time for two of the presumably more stressful components of the laboratory visit. For initial entry into the laboratory, significant differential test-retest stability was obtained for 5-HTTLPR, COMT and CRHR1 (rs878886). For the stressful laboratory challenge, 5-HTTLPR and CRHR1 (both rs242924 and rs878886) exhibited significant differential stability over time. The omnibus Step 4 was not significant for AUCi, baseline, or Recovery 1 or 2, so we did not interpret any individual genotype x LHPA axis effects.
Susceptibility genotype and cortisol reactivity to laboratory challenge
Finally, we examined whether the genotypes ascertained in this study, especially those that revealed more consistent differential stabilities in LHPA activity over time, were associated with cortisol response levels across the laboratory visit. We evaluated this question via two sets of analyses. First was a repeated measures ANOVA in which susceptibility genotype group was the independent variable and repeated assessments of cortisol across each lab visit was the dependent variable to examine whether there was a main effect of genotype, or a genotype X time influence, on cortisol level across the laboratory visit. Second was a Multivariate Analysis of Variance (MANOVA) to examine more precisely whether genotype affected cortisol reactivity to the more stressful components of the laboratory visit. Given the preceding set of results with differential stabilities, especially for 5-HTTLPR, COMT, and CRHR1, we focused our analyses on these genotypes.
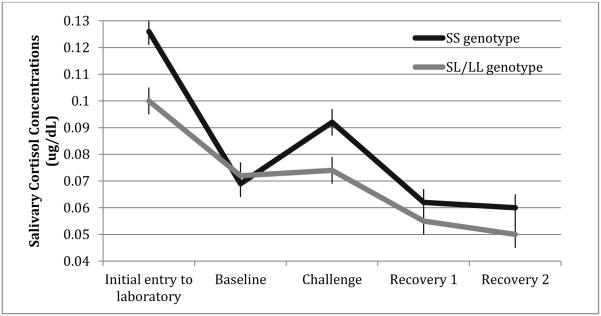
Results of the repeated measures ANOVA showed a significant main effect on cortisol levels for 5-HTTLPR at Time 1: F(1, 223) = 4.92, p < .05, and Time 2, F(1, 193) = 5.75, p < .05. These results show that S-homozygotes for 5-HTTLPR exhibited higher cortisol levels across the entire laboratory visit at both time points. However, these main effects were qualified by a significant genotype x time interaction for 5-HTTLPR at Time 1, F(2.37, 638.02) = 3.13, p < .05, and this was driven by a significant cubic effect (F = 5.59, p < .05). Figure 2 illustrates this effect and shows that S-homozygotes exhibited greater reactivity at both initial entry into the lab and during the laboratory challenge compared to L-carriers. Results from repeated measures ANOVAs for the other genotypes were all non-significant for main effects of genotype and for genotype x time interactions.
Figure 2.
Mean cortisol response to psychosocial challenge among youth at Time 1 as a function of 5-HTTLPR genotype. SS=Short/Short; SL=Short/Long, LL=Long/Long.
Findings for the MANOVAs for a more nuanced analysis of potential genotype effects on specific constituent components of LHPA stress reactivity across the laboratory visit showed a significant effect for 5-HTTLPR (F(11, 183) = 2.01, p = .03, Wilk's Λ = 0.89, partial η2 = .11), CRHR1 rs242924 (F(11, 183) = 3.09, p = .001, Wilk's Λ = 0.78, partial η2 = .20), and CRHR1 rs878886 (F(11, 183) = 1.86, p = .05, Wilk's Λ = 0.87, partial η2 = .13). For COMT, the omnibus effect was not significant (F(11, 183) = 1.28, p = .24, Wilk's Λ = 0.92, partial η2 = .07). Planned follow-up analyses showed that 5-HTTLPR S-homozygotes exhibited significantly higher cortisol levels at challenge reactivity at both Time 1 (F = 4.65, p = .03, partial η2 = .024) and Time 2 (F = 7.59, p < .01, partial η2 = .039) along with a marginally significant effect at initial entry to the laboratory at Time 1 (F = 3.27, p = .07, partial η2 = .017). For CRHR1 rs242924, there were significant differences at Time 1 initial lab entry (F = 8.66, p = .004, partial η2 = .057) and challenge reactivity for both Time 1 (F = 7.59, p = .007, partial η2 = .05) and Time 2 (F = 8.61, p = .004, partial η2 = .057). Last, there were significant differences for CRHR1 rs878886 at Time 1 initial lab entry (F = 7.91, p = .006, partial η2 = .052) and challenge reactivity at Time 1 (F = 4.95, p = .03, partial η2 = .033).
DISCUSSION
Stress sensitivity has been proposed as at least one intermediate process that can explain why some vulnerable individuals are more susceptible to particular forms of stress-related psychopathologies. Dysregulation of the LHPA axis, including cortisol reactivity to stress, represents a key biological aspect of stress sensitivity. Findings from this longitudinal study with repeated measures of salivary cortisol taken among a general community sample of children and adolescents, who completed two separate laboratory stress challenges across an 18-month interval, provide suggestive new data supporting the perspective that some components of cortisol reactivity to stress likely may function as at least one relatively stable, trait-like intermediate processes that could connect some aspects of genetic risk to certain forms of stress-related psychopathologies. Examination of particular genotypes of biologically plausible systems (5-HTTLPR, DRD2, COMT, and CRHR1) that are relevant for stress sensitivity showed that some of these heightened sensitivity genetic variants, especially 5-HTTLPR and CRHR1, were associated with greater stability of cortisol reactivity to stress over time compared to low susceptibility alleles.
The primary novel contribution of this study is the demonstration that several indices of stability of cortisol reactivity to stress across time varied by some, but not all, of the stress susceptibility genotypes. We reasoned that if dysregulated biological stress response functions as one potential mechanism that confers elevated risk to stress-related psychopathologies, then we would expect to find greater stability of LHPA axis activity in stressful contexts, such as the uncertainty of visiting a novel research laboratory and a laboratory-induced stressful challenge, and we would observe greater continuity of this LHPA axis dysregulation among individuals who are at enhanced genetic susceptibility to stressful environments.
Our findings supported this novel proposition and extension of the stress sensitivity hypothesis. The high susceptibility variants of 5-HTTLPR and CRHR1 showed significantly higher stability for cortisol reactivity, as indexed by AUCg. Moreover, when more nuanced analyses of the constituent components of cortisol reactivity were examined—initial entry into the laboratory and the stressful laboratory challenge—5-HTTLPR and CRHR1 revealed significantly different stability estimates, such that youth who carried high susceptibility variants of these genes exhibited significantly greater test-retest stabilities for these components in cortisol reactivity. COMT also exhibited significant differential stability for the stressful uncertainty of initial entry to the laboratory. These specific molecular genetic data add to the prior behavioral genetic literature research demonstrating significant, moderate heritability estimates for stability of cortisol reactivity to stress (Federenko et al., 2004), and other aspects of LHPA axis dysregulation, including CAR and diurnal cortisol levels (Bartels, de Geus, Kirschbaum, Sluyter, & Boosma 2003; Fries, Dettenorn, & Kirschbaum, 2009).
Considerable prior research, although not always consistent, shows that several of the susceptibility genotypes examined in this study demonstrate significant interactions with stressful environments (i.e., GxE) in association with various stress-related psychopathologies (Caspi et al., 2005; Karg et al., 2011; Dunn et al., 2011; Caspi et al., 2010; Belsky & Pluess, 2009; Belsky et al., 2013; Koenen et al., 2009). However, relatively little research has examined potential mechanisms that may underlie demonstrations of GxE in relation to such psychopathologies. Findings from this study suggest the possibility that significant GxE, at least with some of the genes examined in this study, may occur because genetically susceptible individuals are more likely to react to stressful life events in a moderately trait-like, stable manner via dysregulated biological stress response—LHPA axis activity as indexed by levels of cortisol reactivity to stress. We found that certain susceptibility genotypes, especially 5-HTTLPR and CRHR1, were associated with cortisol reactivity. There was a significant effect for 5-HTTLPR to exhibit higher average cortisol levels across the laboratory visit at both time points, although this general main effect trend was qualified by a significant interaction over time at Time 1. Specifically, 5-HTTLPR S-homozygotes were found to have higher cortisol levels for the more stressful components of the first laboratory visit, including initial entry into the lab, which includes stress anticipation of a novel and uncertain context, as well as the stressful laboratory challenge component, which involves social evaluation, processive stress, and threat of social rejection. CRHR1 also showed some effects for higher cortisol levels for specific components across the laboratory visit, including initial entry into the lab at Time 1 and the laboratory challenge component (at both time points for rs242924, and Time 1 for rs878886). In general, these findings are consistent with prior research demonstrating that some susceptibility genotypes, including 5-HTTLPR and CRHR1, are associated with cortisol reactivity (e.g., Gotlib et al., 2008; Mahon et al., 2013; Miller et al., 2013; Pagliaccio et al., 2014; Sumner et al., 2014). Other research investigating genetic sensitivity to stress shows that particular susceptibility genotypes (e.g., 5-HTTLPR) enhance individuals’ own unique reactions to stressful environmental contexts, as demonstrated by GxE effects with idiographically defined reactivity to stressful environments (i.e., deviations in individuals’ own stress levels over time, Hankin, Jenness et al., 2011; fluctuations in maternal depressive symptoms over time as conferring a negative environment to the child, Oppenheimer et al., 2013) as well as differential susceptibility effects to low levels of positive parenting (Hankin, Nederhof et al., 2011).
More generally, the overall cortisol reactivity stability data from this study replicate and extend prior research to suggest that there is a relatively stable, trait-like component driving stability of cortisol reactivity patterns to a laboratory stressor, because the pattern and magnitude of test-retest correlations was similar across studies despite differences in sample ages and timing of test-retest intervals. The previous studies (e.g., Federenko et al., 2004; Kirschbaum et al., 1995; Schommer et al., 2003; Susman et al., 1997) that specifically examined cortisol reactivity to laboratory challenges, conducted predominantly with adults and with relatively short time-frames for test-retest intervals, showed stability coefficients that ranged from .38-.62, with AUCg as the index. We obtained an overall general stability estimate of .41 with AUCg over a substantially longer follow-up (18-months) in this study with youth. It is interesting and notable that the relative strength of the test-retest correlations is moderate, and in a relatively similar effect size range, across the available studies, despite different aged-samples and varying test-retest time intervals. Other aspects of LHPA axis dysregulation, including CAR and diurnal cortisol levels, have also been shown to exhibit similarly moderate levels of stability over time in several, but not all (cf., Ross et al., 2014), studies. For example, test-retest stability for CAR ranged from .39-.67 over very short 2-3 day follow-up intervals (Pruessner et al).
Taken as a whole, the pattern of results, from this study and when considered alongside prior work, are consistent with the suggestion that cortisol reactivity to stress, as one index of LHPA axis activity, may operate as an endophenotype for some types of stress-related psychopathologies. Other empirical and theoretical works have similarly suggested that LHPA axis dysregulation may constitute an endophentype between latent genetic risk and later psychopathologies (e.g., Clarke et al., 2008, Federenko et al., 2004, Hasler et al., 2004, Mehta & Binder, 2012, Oldehinkel & Bouma, 2011). Overall, the data show that some of the conditions are consistent with those postulated according to the definitional criteria for an endophenotype. As proposed by Gottesman and Shields (1972) and further expanded by Gottesman and Gould (2003), the following criteria need to be met for a construct or process to qualify as an endophenotype: (1) is associated with the illness; (2) is heritable; (3) is primarily state independent, although a challenge may be required to make it manifest; (4) co-segregates with the illness within families; and (5) is found in affected family members at a greater proportion than seen in the general population. More recently, Chan and Gottesman (2008) added an additional criterion, namely that an endophenotype possess qualities of a trait that can be measured reliably. While our evidence shows that the particular genetic variants of 5-HTTLPR and CRHR1 were associated with differential stability in cortisol reactivity over time among youth, no study to date has tested all of the criteria needed to demonstrate that cortisol reactivity to stress operates, in fact, as an endophenotype. For example, the criteria of state independence, co-segregation with psychopathology within families, and being observed more frequently in affected family members than expected in general population were not investigated in our study or most prior work. Future research aimed at simultaneously examining all criteria needed to demonstrate that LHPA axis dysregulation functions as an endophenotype will be important because an endophenotype is not simply a biomarker of psychopathology, but rather represents an expression of a core propensity to the illness (Lenzenweger, 2013). Moreover, a particular advantage of unearthing endophenotypes is the likelihood that when the biology of an endophenotype is better understood, candidate genes can be more easily and systematically identified and linked to the endophenotype (Lenzenweger, 2013).
The mechanistic reasons why allelic variation in these susceptibility genotypes showed some evidence of differential stability over time for some indices of LHPA axis are not clear. Elucidating why carriers of high susceptibility genotypes manifest higher test-retest consistency in cortisol reactivity to stress remains an open question that needs additional investigation. For example, a history of childhood maltreatment and adversity, as well as prenatal stress, predicts infant and child cortisol reactivity (e.g., Davis, Glynn, Waffarn, & Sandman, 2011; Ehlert, 2013), and early adversity moderates the effect of susceptibility genes on cortisol reactivity (e.g., Sumner et al., 2014). One plausible mechanism may be the interaction of susceptibility genes with adverse childhood experiences contributing to a more stably dysregulated LHPA axis. This and other possible processes require future research.
Not all of our hypotheses were supported in this study, and some effects were subtle and depended on which index of cortisol reactivity was investigated. No significant differential stability effects were found for DRD2. We initially selected DRD2 as a susceptibility gene and hypothesized that it may exhibit differential stability based on evidence for GxE effects in some psychopathologies (e.g., Dunn et al., 2011; Koenen et al., 2009) and evidence pointing to correlations between dopamine release in the brain and peripheral cortisol levels (Oswald et al., 2005). However, DRD2 did not significantly moderate stability of cortisol reactivity over time. This suggests that other mechanisms, instead of cortisol reactivity to stress, may confer risk to stress-related psychopathologies for those individuals carrying the high susceptibility variant of DRD2. Finally, no genotype moderated stability of AUCi as the index of cortisol reactivity. Overall and for the sample as a whole, test-retest for AUCi was significant, albeit lower relatively compared to AUCg and several of the components of stress reactivity (e.g., reactivity to the lab challenge). It may be that the underlying basal level, as reflected in AUCg, is what is most stable. This highlights the complexity of the LHPA axis in stress reactivity and the need for investigating multiple indices of activity to provide a more comprehensive, nuanced perspective as opposed to a single, overly simplistic index of LHPA functioning.
Findings from this study need to be considered in light of its strengths and weaknesses. Strengths include a moderately large sample of youth who completed two separate stressful laboratory challenge paradigms across a relatively long prospective follow-up to evaluate test-retest stability. Also and importantly, validity analyses showed that both of these developmentally appropriate laboratory challenges elicited the expected spike in cortisol levels after the stressful challenge from baseline and then return to baseline levels during recovery. Last, we examined the novel hypothesis that particular, a priori identified, biologically plausible genotypes, known to be associated with broad stress sensitivity, the LHPA axis, and differential susceptibility, would moderate the degree of stability in cortisol reactivity over time.
At the same time, limitations of the study provide avenues for future research. First, only a few selected genotypes were examined, so future research is needed to expand the scope of inquiry into other stress-sensitive genes (e.g., FKBP5). Second, stability of levels of cortisol reactivity were examined with a two-time point design, although more sophisticated stability analyses can be evaluated with multi-time point designs (Hankin, 2008; Fraley & Roberts, 2005). Future research can address this issue by investigating mean-level and rank-order stabilities in these various indices of LHPA axis dysregulation via a repeated measures design with three or more time points of cortisol reactivity. Third, our use of different laboratory challenges at Times 1 and 2, although intended to minimize habituation and/or learning and practice effects, may have attenuated stability estimates in cortisol reactivity across time. Prior studies examining test-retest stability used the same laboratory stressor at both time points. Fourth, we used a general community sample of youth, in which the expected symptom levels and prevalence rates of common psychopathologies are observed (e.g., see Badanes et al., 2001; Hankin, Badanes et al., 2010; Hayden et al., in press), and no explicit information on early life stress or trauma exposure was assessed. Findings on differential stability by susceptibility genotype may differ when examined among a psychiatric clinical sample or when early adversity is included as a moderator.
In summary, the selected genes investigated in this study have consistently demonstrated significant associations with multiple forms of stress-related psychopathology, have been found to interact with stressful environments (GxE) to predict some types of psychopathology, and are related to biological stress sensitivity as instantiated via cortisol reactivity to stressful laboratory challenges. LHPA axis dysregulation, especially cortisol reactivity, has been postulated as a plausible biological mechanism related to stress sensitivity that could explain, at least in part, why genetically susceptible individuals are more likely to develop some forms of stress-reactive psychopathology, especially under stressful conditions. Specific results from this study showed that cortisol levels during a stressful laboratory challenge exhibited moderate stability over 18-months for the whole sample of youth, and those youth carrying some genes related to heightened susceptibility to the environment showed significantly higher cortisol reactivity to stress for some indices of stability over time. Findings from this longitudinal study with children and adolescents expand evidence that cortisol reactivity to stressful challenges, an important index of LHPA axis activity, may operate as at least one intermediate process that connects some aspects of genetic risk to particular forms of some stress-related psychopathology.
Contributor Information
Benjamin L. Hankin, University of Denver
Lisa S. Badanes, Metropolitan State University of Denver
Andrew Smolen, University of Colorado.
Jami F. Young, Rutgers University
References
- Alloy LB, Riskind JH, editors. Cognitive vulnerability to emotional disorders. Routledge; 2006. [Google Scholar]
- Armbruster D, Mueller A, Strobel A, Lesch KP, Brocke B, Kirschbaum C. Children under stress–COMT genotype and stressful life events predict cortisol increase in an acute social stress paradigm. The International Journal of Neuropsychopharmacology. 2012;15(09):1229–1239. doi: 10.1017/S1461145711001763. [DOI] [PubMed] [Google Scholar]
- Azar R, Paquette D, Zoccolillo M, Baltzer F, Tremblay RE. The association of major depression, conduct disorder, and maternal overcontrol with a failure to show a cortisol buffered response in four-month-old infants of teenage mothers. Biological Psychiatry. 2007;62:573–579. doi: 10.1016/j.biopsych.2006.11.009. doi:10.1016/j.biopsych.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Development and Psychopathology. 2011;23:881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, de Geus EJ, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol levels in children. Behavior Genetics. 2003;33(4):421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. Journal of Abnormal Psychology. 2009;118(3):670. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M, Widaman KF. Confirmatory and competitive evaluation of alternative gene-environment interaction hypotheses. Journal of Child Psychology and Psychiatry. 2013;54:1135–1143. doi: 10.1111/jcpp.12075. doi:http://dx.doi.org/10.1111/jcpp.12075. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychosocial stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Camara E, Krämer UM, Cunillera T, Marco-Pallarés J, Cucurell D, Nager W. The effects of COMT (Val108/158Met) and DRD4 (SNP- 521) dopamine genotypes on brain activations related to valence and magnitude of rewards. Cerebral Cortex. 2010;20(8):1985–1996. doi: 10.1093/cercor/bhp263. others. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biological psychiatry. 2005;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Chan RC, Gottesman II. Neurological soft signs as candidate endophenotypes for schizophrenia: a shooting star or a Northern star? Neuroscience & Biobehavioral Reviews. 2008;32(5):957–971. doi: 10.1016/j.neubiorev.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Oshri A. Interactive effects of corticotropin releasing hormone receptor 1, serotonin transporter linked polymorphic region, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Development and Psychopathology. 2011;23(4):1125–1138. doi: 10.1017/S0954579411000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Treutlein J, Zimmermann US, Kiefer F, Skowronek MH, Rietschel M, Schumann G. Review: HPA-axis activity in alcoholism: examples for a gene-environment interaction. Addiction Biology. 2008;13(1):1–14. doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry. 2011;52(2):119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Dietz KC, Moore S, Ouimet CC, Kabbaj M. Repeated social defeat stress-induced sensitization to the locomotor activating effects of d-amphetamine: role of individual differences. Psychopharmacology. 2008;198(1):51–62. doi: 10.1007/s00213-008-1078-y. [DOI] [PubMed] [Google Scholar]
- Dunn EC, Uddin M, Subramanian SV, Smoller JW, Galea S, Koenen KC. Research Review: Gene–environment interaction research in youth depression–a systematic review with recommendations for future research. Journal of Child Psychology and Psychiatry. 2011;52(12):1223–1238. doi: 10.1111/j.1469-7610.2011.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert U. Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology. 2013;38(9):1850–1857. doi: 10.1016/j.psyneuen.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Federenko IS, Nagamine M, Hellhammer DH, Wadhwa PD, Wüst S. The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. The Journal of Clinical Endocrinology & Metabolism. 2004;89(12):6244–6250. doi: 10.1210/jc.2004-0981. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente MA, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. doi:10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Foster SL, Robin AL. Parent-adolescent conflict. In: Mash EJ, Barkley RA, editors. Treatment of Childhood Disorders. Guilford Press; NY: 1989. pp. 493–528. [Google Scholar]
- Fraley RC, Roberts BW. Patterns of continuity: a dynamic model for conceptualizing the stability of individual differences in psychological constructs across the life course. Psychological review. 2005;112(1):60. doi: 10.1037/0033-295X.112.1.60. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Goldberg S, Levitan R, Leung E, Masellis M, Basile VS, Nemeroff CB, Atkinson L. Cortisol concentrations in 12-to 18-month-old infants: Stability over time, location, and stressor. Biological Psychiatry. 2003;54(7):719–726. doi: 10.1016/s0006-3223(03)00010-6. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Schizophrenia and genetics: A twin study vantage point. Academic Press; NY: 1972. [Google Scholar]
- Granger DA, Weisz JR, Kauneckis D. Neuroendocrine reactivity, internalizing behavior problems, and control-related cognitions in clinic-referred children and adolescents. Journal of Abnormal Psychology. 1994;103:267–276. doi: 10.1037//0021-843x.103.2.267. [DOI] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Stuhlmacher AF, Thurm AE, McMahon SD, Halpert JA. Stressors and child and adolescent psychopathology: moving from markers to mechanisms of risk. Psychological Bulletin. 2003;129(3):447. doi: 10.1037/0033-2909.129.3.447. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. Developmental Psychopathology. 2006;2:533–547. [Google Scholar]
- Gunnar MR, Talge NM. Neuroendocrine measures in developmental research. In: Schmidt LA, Segalowitz SJ, editors. Developmental Psychophysiology. Cambridge University Press; New York, NY: 2005. pp. 343–356. [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu. Rev. Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Developmental Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick BC, Smolen A. Genotyping of three single nucleotide polymorphisms following whole genome preamplification of DNA collected from buccal cells. Behavioral Genetics. 2004;34:541–547. doi: 10.1023/B:BEGE.0000038492.50446.25. [DOI] [PubMed] [Google Scholar]
- Hankin BL. Stability of cognitive vulnerabilities to depression: A short-term prospective multi-wave study. Journal of Abnormal Psychology. 2008;117:324–333. doi: 10.1037/0021-843X.117.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abela JRZ. Depression from childhood through adolescence and adulthood: A developmental vulnerability-stress perspective. In: Hankin BL, Abela JRZ, editors. Development of Psychopathology: A Vulnerability-Stress Perspective. Sage Publications; Thousand Oaks, CA: 2005. pp. 245–288. [Google Scholar]
- Hankin BL, Badanes LS, Abela JRZ, Watamura SE. Hypothalamic pituitary adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychological stress from preschool through middle adolescence. Biological Psychiatry. 2010;68:484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Jenness J, Abela JRZ, Smolen A. Interaction of 5HTTLPR and idiographic stressors predicts prospective depressive symptoms specifically among youth in a multi-wave study. Journal of Child and Adolescent Clinical Psychology. 2011;40:572–585. doi: 10.1080/15374416.2011.581613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness JL, Young JF, Abela JRZ, Smolen A, Ormel J, Oldehinkel AJ. Differential susceptibility in youth: Evidence that 5-HTTLPR x positive parenting is associated with positive affect “for better and worse.”. Translational Psychiatry. 2011;1:1–7. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Snyder HR, Gulley LD. Cognitive risks in developmental psychopathology. In: Cicchetti D, editor. Developmental Psychopathology. Wiley; in press. [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Björk K, Soverchia L, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proceedings of the National Academy of Sciences. 2006;103(41):15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62(2):146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10) doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Hankin BL, Mackrell SVM, Sheikh HI, Jordan PL, Dozois DJA, Singh SM, Badanes LS. Youths’ cognitive vulnerability and cortisol: Moderating influence of parental depression history. Development and Psychopathology. doi: 10.1017/S0954579414001138. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neuroscience. 2004;8(1):20–21. doi: 10.1038/nn1366. others. [DOI] [PubMed] [Google Scholar]
- Höferl M, Krist S, Buchbauer G. Adaptation of DELFIATM Cortisol Kit for Determination of Salivary Cortisol Concentration. Archiv der Pharmazie. 2005;338(10):493–497. doi: 10.1002/ardp.200500116. [DOI] [PubMed] [Google Scholar]
- Hosák L. Role of the COMT gene Val158Met polymorphism in mental disorders: a review. European Psychiatry. 2007;22(5):276–281. doi: 10.1016/j.eurpsy.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Gunnar MR. The developmental effects of early life stress: An overview of current theoretical frameworks. Current Directions in Psychological Science. 2013;22:400–406. doi: 10.1177/0963721413488889. doi:http://dx.doi.org/10.1177/0963721413488889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RE, Price JM, editors. Vulnerability to psychopathology: Risk across the lifespan. Guilford Press; 2010. [Google Scholar]
- Jabbi M, Korf J, Kema IP, Hartman C, Van der Pompe G, Minderaa RB, Den Boer JA. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Molecular Psychiatry. 2007;12(5):483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene–environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12(5):432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression: meta-analysis revisited. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Amstadter AB, Nugent NR. Gene-environment interaction in posttraumatic stress disorder: An update. Journal of Traumatic Stress. 2009;22(5):416–426. doi: 10.1002/jts.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemery KS, Doelger LISA. Genetic vulnerabilities to the development of psychopathology. In: Hankin BL, Abela JRZ, editors. Development of psychopathology: A vulnerability–stress perspective. Sage Publications; Thousand Oaks, CA: 2005. pp. 161–198. [Google Scholar]
- Lenzenweger MF. Thinking clearly about the endophenotype–intermediate phenotype–biomarker distinctions in developmental psychopathology research. Development and psychopathology. 2013;25:1347–1357. doi: 10.1017/S0954579413000655. 4pt2. [DOI] [PubMed] [Google Scholar]
- Liening SH, Stanton SJ, Saini EK, Schultheiss OC. Salivary testosterone, cortisol, and progesterone: two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiology & Behavior. 2010;99(1):8–16. doi: 10.1016/j.physbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George C. Hypothalamic pituitary adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. doi:10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran NL, Mayer SE, Abelson JL. Modeling neuroendocrine stress reactivity in salivary cortisol: Adjusting for peak latency variability. Stress. 2014;17:285–295. doi: 10.3109/10253890.2014.915517. doi:10.3109/10253890.2014.915517. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews: Neuroscience. 2009;10:434–345. doi: 10.1038/nrn2639. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology. 2013;227(2):231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM. Life stressors as risk factors in depression. Clinical Psychology: Science and Practice. 1998;5:291–313. [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Meehl PE. Schizotaxia, schizotypy, schizophrenia. American Psychologist. 1962;17:827–838. doi:http://dx.doi.org/10.1037/h0041029. [Google Scholar]
- Mehta D, Binder EB. Gene× environment vulnerability factors for PTSD: The HPA-axis. Neuropharmacology. 2012;62(2):654–662. doi: 10.1016/j.neuropharm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Molecular psychiatry. 2009;15(9):918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Miller R, Wankerl M, Stalder T, Kirschbaum C, Alexander N. The serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cortisol stress reactivity: a meta-analysis. Molecular Psychiatry. 2013;18(9):1018–1024. doi: 10.1038/mp.2012.124. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of general psychiatry. 2005;62(5):473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Monroe SM. Modern approaches to conceptualizing and measuring life stress. Annual Review of Clinical Psychology. 2008;4:33–52. doi: 10.1146/annurev.clinpsy.4.022007.141207. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Recurrence in major depression: A conceptual analysis. Psychological Review. 2011;118:655–74. doi: 10.1037/a0025190. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis stress in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry. 2006;47(3-4):395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Nock MK, Mendes WB. Physiological arousal, distress tolerance, and social problem-solving deficits among adolescent self-injurers. Journal of Consulting and Clinical Psychology. 2008;76(1):28. doi: 10.1037/0022-006X.76.1.28. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Bouma E. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: a review of gender differences. Neuroscience & Biobehavioral Reviews. 2011;35(8):1757–1770. doi: 10.1016/j.neubiorev.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Oppenheimer CW, Hankin BL, Young JF, Abela JRZ, Smolen A. Youth Genetic Vulnerability to Maternal Depressive Symptoms: 5-HTTLPR as Moderator of Intergenerational Transmission Effects in a Multiwave Prospective Study. Depression and Anxiety. 2013;30:190–196. doi: 10.1002/da.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Wand GS. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30(4):821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Zandi P, Nestadt G, Potash JB, Kalaydjian AE, Wand GS. Relationship between cortisol responses to stress and personality. Neuropsychopharmacology. 2006;31:1583–1591. doi: 10.1038/sj.npp.1301012. doi:10.1038/sj.npp.1301012. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, Botteron KN, Harms MP, Barch DM. Stress-System Genes and Life Stress Predict Cortisol Levels and Amygdala and Hippocampal Volumes in Children. Neuropsychopharmacology. 2014;39:1245–1253. doi: 10.1038/npp.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett LJ, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of Psychosocial Stress Into the Neurobiology. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, Von Auer K, Jobst S, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61(26):2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Rende R, Waldman I. Behavioral and molecular genetics and developmental psychopathology. Developmental Psychopathology. 2006;1:427–64. [Google Scholar]
- Rothbart MK, Posner MI. Temperament, attention, and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology, 2nd edition, Developmental Neuroscience. Wiley; NY: 2006. [Google Scholar]
- Ruggero CJ, Johnson SL. Reactivity to a laboratory stressor among individuals with bipolar I disorder in full or partial remission. Journal of Abnormal Psychology. 2006;115(3):539–544. doi: 10.1037/0021-843X.115.3.539. [DOI] [PubMed] [Google Scholar]
- Schepis TS, Rao U, Yadav H, Adinoff B. The limbic–hypothalamic–pituitary–adrenal axis and the development of alcohol use disorders in youth. Alcoholism: Clinical and Experimental Research. 2011;35(4):595–605. doi: 10.1111/j.1530-0277.2010.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein VL, Susman EJ. Cortisol levels and longitudinal cortisol change as predictors of anxiety in adolescents. The Journal of Early Adolescence. 2006;26(4):397–413. [Google Scholar]
- Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, Laucht M. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. The International Journal of Neuropsychopharmacology. 2010;13(06):703–714. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Developmental Psychobiology. 2012;54(5):493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Development. 2008;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biological Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Sumner JA, McLaughlin KA, Walsh K, Sheridan MA, Koenen KC. CRHR1 Genotype and History of Maltreatment Predict Cortisol Reactivity to Stress in Adolescents. Psychoneuroendocrinology. 2014;43:71–80. doi: 10.1016/j.psyneuen.2014.02.002. DOI: 10.1016/j.psyneuen.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Granger DA, Blades KT, Randazzo W, Heaton JA, Dorn LD. Cortisol and alpha amylase reactivity and timing of puberty: Vulnerabilities for antisocial behaviour in young adolescents. Psychoneuroendocrinology. 2010;35(4):557–569. doi: 10.1016/j.psyneuen.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Inoff-Germain G, Nottelmann ED, Chrousos GP. Cortisol reactivity, distress behavior, and behavioral and psychological problems in young adolescents: A longitudinal perspective. Journal of Research on Adolescence. 1997;7(1):81–105. [Google Scholar]
- Tang H, Quertermous T, Rodriguez B, Kardia SL, Zhu X, Brown A, Rishch NJ. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. American Journal of Human Genetics. 2005;76:268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Orden K, Wingate LR, Gordon KH, Joiner TE. Interpersonal factors as vulnerability to psychopathology over the life course. Development of Psychopathology: A Vulnerability-Stress Perspective. 2005:136–160. [Google Scholar]
- Vreeburg SA, Zitman FG, van Pelt J, DeRijk RH, Verhagen JC, van Dyck R, Penninx BW. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosomatic Medicine. 2010;72(4):340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- Walder DJ, Trotman HD, Cubells JF, Brasfield J, Tang Y, Walker EF. Catechol-O-Methyltransferase (COMT) modulation of cortisol secretion in psychiatrically at-risk and healthy adolescents. Psychiatric Genetics. 2010;20(4):166. doi: 10.1097/YPG.0b013e32833a1ff3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Sabuwalla Z, Huot R. Pubertal neuromaturation, stress sensitivity, and psychopathology. Development and Psychopathology. 2004;16(4):807–824. doi: 10.1017/s0954579404040027. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Development and Psychopathology. 2001;13(3):721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Richardson ED, Smolen A. Behavioral inhibition and triallelic genotyping of the serotonin transporter promoter (5-HTTLPR) polymorphism. Journal of Research in Personality. 2011;45:706–709. [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- Zubin J, Spring B. Vulnerability: A new view of schizophrenia. Journal of Abnormal Psychology. 1977;86:103–126. doi: 10.1037//0021-843x.86.2.103. doi:http://dx.doi.org/10.1037/0021-843X.86.2.103. [DOI] [PubMed] [Google Scholar]