Abstract
The substantial health burden associated with Major Depressive Disorder is a product of both its high prevalence and the significant risk of relapse, recurrence and chronicity. Establishing recurrence vulnerability factors (VFs) could improve the long-term management of MDD by identifying the need for further intervention in seemingly recovered patients. We present a model of sensitization in depression vulnerability, with an emphasis on the integration of behavioral and neural systems accounts. Evidence suggests that VFs fall into two categories: dysphoric attention and dysphoric elaboration. Dysphoric attention is driven by fixation on negative life events, and is characterized behaviorally by reduced executive control, and neurally by elevated activity in the brain’s salience network. Dysphoric elaboration is driven by rumination that promotes over-general self and contextual appraisals, and is characterized behaviorally by dysfunctional attitudes, and neurally by elevated connectivity within normally-distinct prefrontal brain networks. While, at present, few prospective VF studies exist from which to catalogue a definitive neurobehavioral account, extant data support the value of the proposed two-factor model. Measuring the continued presence of these two VFs during recovery may more accurately identify remitted patients who would benefit from targeted prophylactic intervention.
Keywords: Depression, Relapse, Recurrence Vulnerability, Sensitization, Reactivity
With empirical evidence supporting pharmacologic (Rush et al., 2006) and psychotherapeutic (Hollon et al., 2005) treatment of the acute phase of Major Depressive Disorder (MDD), patients and clinicians increasingly face the challenge of averting future episodes. Consistent with the tenets of prevention science (Munoz, Cuijpers, Smit, Barrera, & Leykin, 2010), a potentially productive strategy is to identify the stress sensitivity factors that predispose individuals to relapse and recurrence. In particular, the identification of malleable vulnerability factors (VFs) may allow for personalized risk assessment following symptom remission and the provision of prophylactic, individualized interventions. While VFs remain understudied in the research literature, emerging cognitive-behavioral and neuroimaging studies have begun to provide parallel accounts of MDD vulnerability, revealing potential avenues for effective risk assessment and intervention. In this article, we outline the rationale for the integration of these two literatures into a two-factor model of depression vulnerability.
Depression vulnerability has been defined in terms of increased risk for re-emergence of symptoms (relapse) or a new episode (recurrence) following a period of recovery (APA, 2000; Frank et al., 1991). As recently articulated (Monroe & Harkness, 2011), the dominant focus on relapse/recurrence ignores the large percentage of patients who experience only a single lifetime episode. This disjunction suggests a sensitization process that distinguishes single episode vulnerability from risk for a more chronic course of illness. In characterizing this sensitization, the mutually-reinforcing relationship between negative life events and depression vulnerability across the lifespan has been a fruitful area of investigation (Liu & Alloy, 2010). Such vulnerability is ultimately gauged by the return of depressive episodes, either in terms of relapse following the incomplete remission of depressive symptoms, or in the form of recurrence following successful remission. However, the literature distinguishing relapse and recurrence risk is sparse, and vulnerability for relapse and recurrence are likely at least partially determined by a common set of VFs. For this reason, we posit our model as potentially indicative of both relapse and recurrence vulnerability, at least until further research provides greater evidence for distinct risk profiles underlying the two forms of vulnerability.
To date, most established VFs, such as personality (Klein, Kotov, & Bufferd, 2011), genetics (Talati, Weissman, & Hamilton, 2013), and clinical history (Judd et al., 1998), reflect largely fixed patient factors. To add to these conceptualizations, we propose two VFs representing different aspects of cognitive dysregulation: dysphoric attention and dysphoric elaboration. Positioned between environmental stress and the expression of depressive symptoms, it is our hope that these constructs will help to characterize sensitization effects as the dynamic product of sequenced dysregulations. If measurement of these dysregulations can be refined, it could help to reveal enduring vulnerability following MDD remission/recovery.
The Two-Factor Sensitization Model
The two-factor model is intended to be integrative, describing dynamic mechanisms of initial stress sensitivity and subsequent sensitization in MDD. Stress is proposed to be the product of detecting threats and appraising them as unmanageable, in keeping with appraisal theories of emotion (Lazarus & Folkman, 1984). For example, one might experience adverse life events such as losing one’s job or suffering a personal injury. While such events are relatively universal examples of adversity, the stress generated by these events varies between people on a continuum ranging from resilience to vulnerability. The central claim of the model is that one’s stress in the face of adverse life events depends on how specific features of these events are integrated into a person’s interpretive context, a process biased both by what kinds of features capture attention, and how one subsequently elaborates on attended features. In the example of losing one’s job, one may fixate on the mortifying feelings of being fired or on the visceral sense of relief to be leaving an acerbic work environment. Subsequently, one might appraise oneself dysphorically as being a failure, or euphorically as being a bit wiser for having gone through the experience. Such appraisals then reinforce attentional habits to fixate on mood-congruent features of events, forming an interpretive cycle between attention and elaboration. The central prediction of the two-factor model is that sensitization occurs because of increased coupling between dysphoric attention and elaboration. This coupling serves to elevate relapse/recurrence risk with each subsequent episode, as even relatively minor signs of adversity gain the power to activate powerful dysphoric elaborations.
The proposal of dysphoric attention and elaboration as VFs acknowledges that vulnerability stems from both extrinsic and intrinsic sources. There is substantial evidence that while major stressors promote the onset of initial depressive episodes, minor stressors become increasingly predictive of relapse/recurrence with successive episodes, indicating a potential sensitization process (Monroe & Harkness, 2005). With each episode, depression vulnerability and the arising of life stressors become increasingly correlated, suggesting a lowered threshold for stress appraisals as a consequence of depressive life history (Liu & Alloy, 2010). In effect, each episode engenders stress sensitization, leaving apparently recovered individuals at elevated risk for future depressive episodes, although the mechanisms by which such sensitization occurs are largely unknown.
The two-factor model proposes a process for how such stress sensitization occurs (Figure 1). Sensitization occurs as a consequence of fixation and rumination: fixation favors sustained representation of negative over positive features of events, leading to weak associations between simultaneously represented negative features. Rumination then integrates concrete negative feature representations into abstract dysphoric schema about one’s self, future, and role in the world, e.g., “I’m a failure”. The presence of dysphoric schemas shift a person’s attention towards mood congruent content and increases the associative potential of features from novel negative events, enhancing dysphoric fixation and reinforcing dysphoric attitudes.
Figure 1.
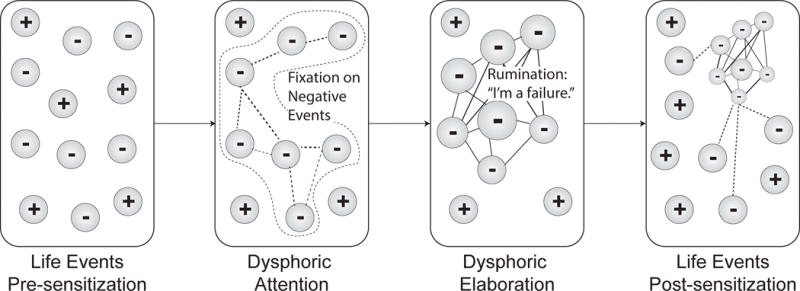
Introducing the two-factor model of depression vulnerability. Subjective experience is presented in each rounded rectangle, reflecting the dynamic process of sensitization over time. Experience is constituted by both positive (+) and negative (−) events, which are dynamically modified by cognition. Weak associations are illustrated as dotted lines and strong associations by solid lines.
Traditionally, cognitive models of sensitization implicate rumination as a monolithic cognitive process in which vulnerable individuals focus attention on experienced stressors and their implications (Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). Our model suggests that this definition of rumination might conflate 2 VFs, attention and elaboration, which have distinct roles in the sensitization process. Dysphoric attention to stressors constitutes an early VF, increasing the relative salience of negative life events (Armstrong & Olatunji, 2012; Peckham, McHugh, & Otto, 2010; Sanchez, Vazquez, Marker, LeMoult, & Joormann, 2013). This salience promotes a temporally-extended attentional capture by negative events that we will refer to as fixation. We propose that dysphoric attention is common to both first episode and recurrent forms of depression. However, fixation on negative life events also promotes dysphoric elaboration, skewing the integration of events to form dysphoric schema about the self, the future and the world. Such schema provide a conceptual anchor that unify and normalize the interpretation of further adverse experiences.
Sensitization is hypothesized to occur when dysphoric elaboration binds fixated negative events with negative, self-referential implications – it is this more elaborative rather than attentional process that we will refer to as rumination. Rumination creates strong associations between negative events and a centralized self-concept (Lyubomirsky & Nolen-Hoeksema, 1995), leading to generalized dysphoric attitudes towards the self, one’s future, and one’s place in the world, in keeping with cognitive models of depression (Beck, 1970). These attitudes are relatively independent of changing environmental contexts as they refer to stable, global, and internal properties (Seligman, Abramson, Semmel, & Von Baeyer, 1979) As such, dysphoric attitudes are themselves depressogenic, serving as semantic attractors for novel negative life events, magnifying the perceived implication of minor life events as confirming pre-existing dysphoric attitudes. These stress-emergent negative schemas manifest in the form of cognitive reactivity, a pattern of dysphoric elaboration that acts as a second VF (Teasdale, 1999).
As an example, let us consider James, an accountant in his mid 30’s whose mother passes away after an unexpected illness. While his siblings grieve and adapt to the loss, James fixates upon his mother’s passing, how she won’t be there to see his kids grow up or celebrate his promotion within the firm. He notices that his energy at work starts to flag and he finds himself making mistakes on his clients’ files. To James, each day seems to pass with little to look forward to or enjoy. Throughout this time, James ruminates on why he alone seems to have these negative thoughts and feelings, and wonders whether he lacks the ability to thrive in the world without his mother’s support and guidance. As time passes, James starts to return to his old self, but now sees the world differently. Seemingly minor upsets such as spilling his coffee one morning immediately bring to mind thoughts of inadequacy and worthlessness, serving as one more confirmatory example of his low view of self. Left unchecked, such seemingly innocuous triggers put James at a heightened risk for the re-emergence of depression compared to before his bereavement. Critically, it is the combination of fixation on negative life events combined with ruminative self-elaboration that characterize the sensitization process rather than these factors operating in isolation.
The idea that multiple cognitive factors are involved in depression is not novel, having been described in recent reviews (Gotlib & Joormann, 2010; Jacobs, Reinecke, Gollan, & Kane, 2008). However, while such reviews cast depression vulnerability in terms of a failure for explicit reflective processes to inhibit pre-existing dysphoric associations (Beevers, 2005), the two-factor model seeks to describe how such failures allow enduring conceptual associations to form insidiously, progressing from maladaptive fixation on negative experiences to entrenched global attitudes. While this is a complex and cyclical process, the model can be broken down into four major assumptions that lead to a prediction of stress sensitization in depression:
Vulnerability operates at both concrete and implicational levels of processing. The central assumption of the model is drawn from construal theory, which proposes that human cognition operates at both concrete and implicational levels of construal to determine subjective experience (Barnard & Teasdale, 1991; Trope & Liberman, 2010). Experience is therefore subject to two distinct sources of bias, attention biases towards negative events, and schematic biases about how such events inform our understanding of ourselves in the world. The ‘starting values’ for such biases are not arbitrary, but are likely influenced by a host of genetic, developmental, and environmental factors.
Dysphoric processing biases are malleable. It is likely that people develop resilience or sensitivity to stress over time, to their benefit in the presence of social and clinical support, or to their detriment following loss, isolation or trauma. Sensitization in this model is defined as the dysphoric tuning of affective integration biases.
Attention and schematic biases have independent tuning mechanisms. Attention bias towards life events is tuned through fixation on negative events, repeated failure to disengage from negative information despite normal thresholds for the detection of such events (Gotlib & Joormann, 2010). Schematic bias is tuned through rumination, a recursive analysis of one’s self, future and environment. To the extent that an analytic focus weakens positive attitudes and reinforces negative attitudes, it promotes dysphoric elaboration, engendering global dysphoric attitudes (Rimes & Watkins, 2005).
While fixation and rumination and analytic focus are distinct cognitive operations, they are inter-related through their effects. Fixation on negative events provides rich opportunities for dysphoric elaboration (Koster, De Lissnyder, Derakshan, & De Raedt, 2011). Repeated conceptual analysis of the causes and consequences of negative events then consolidates negative associations in memory as dysphoric schemas. Conversely, once such schemas supersede the optimistic elaborative biases observed in healthy individuals, dysphoric elaboration supports attentional capture by schema-congruent depressive events, reducing the plasticity of the representational system (Pittenger & Duman, 2007).
Integrating many of the points above, the major prediction of the model is that dysphoric elaboration is a consequence of sustained dysphoric attention, representing a sensitization mechanism for risk of relapse/recurrence. Most individuals begin life with resilience against depression, in the form of positively-skewed, self-serving interpretive biases that are largely absent in depressed samples (Mezulis, Abramson, Hyde, & Hankin, 2004). Even before exposure to major stressors, the tuning of attention and interpretive biases likely varies between individuals as a function of genetic, developmental, social, and strategic factors. Early vulnerability to depression likely plays out through fixation on negative life events, creating dissonance between such negative events and positive implicational biases about the self, world, and future. While it may be possible that fixation alone could lead to an initial depressive episode, it seems more likely that initial vulnerability involves the intersection of fixation tendencies with major life stressors. In cases where major life stressors are present, pervasive exposure to negative events dominates subjective experience, leading to the onset of the first depressive episode. During this time, factors such as personality (Shea & Yen, 2005), regulatory strategy (Silk, Steinberg, & Morris, 2003), explanatory style (Nolen-Hoeksema, Girgus, & Seligman, 1986), all of which are affected by both genetics and environment throughout development, serve to determine the tendency to fixate and ruminate on these major life stressors.
To the extent that one’s regulatory habits promote fixation and rumination, sensitization begins through the gradual deterioration of positive implications as they are updated to reflect negative life experience. Rumination populates subjective experience with negative implications, lowering the threshold needed for negative life events to create a net dysphoric experience conducive to MDD recurrence. With each depressive episode, fixation on negative events provides additional opportunities for rumination, disrupting positive attitudes and entrenching global negative attitudes about the self, future, and world. Conversely, increased dysphoric elaboration feeds back onto attention processes, imbuing negative tone to ambiguous situations and lowering the threshold for stress perception. In this way, progressively tighter coupling between dysphoric attention and elaboration serves to explain risk elevation with subsequent depressive relapses/recurrences, a theory that is supported by contemporary clinical and neuroimaging investigations of depression vulnerability.
Clinical Research Supporting the Two-Factor Model
The two factor model makes a series of empirical claims about the relationship between attention, elaboration, and relapse/recurrence vulnerability. For each VF, numerous operationalizations are possible. For each of dysphoric attention and dysphoric elaboration, some of the central operational paradigms and their intended vulnerability markers are summarized in Table 1. Through research using these paradigms, each of the model’s claims has found some measure of support in the empirical literature; these claims, examples of the research supporting them, and future research directions are summarized in Table 2. Below, we review these findings and address the need for further research in greater depth.
Table 1.
Candidate methods for assessing dysphoric attention and dysphoric elaboration.
| Task Name | Description | Vulnerability Marker |
|---|---|---|
| Dysphoric attention | ||
| Dot Probe | Present emotional and neutral word simultaneously for > 1 sec, then replace with target ‘dot’ behind one of the words. | Sensitivity as reaction time to negative vs. neutral words (Fritzsche et al., 2010); bias only visible at longer presentation time might indicate combined attention / elaboration components. |
| Emotional Stroop | Record time for reading emotional vs. neutral words printed in different colors. | Sensitivity as reaction time to negative vs. neutral words (Williams, Mathews, & MacLeod, 1996) |
| Spatial Cueing | Cue 1 of 2 spatial locations with either a neutral or negative word or image; cue either rightly or wrongly predicts target location. | Sensitivity as greater effect of cue validity for negative vs. neutral words (Leyman et al., 2007). |
| Eye Tracking | Naturalistic viewing of neutral and dysphoric photographs. | Sensitivity as greater fixation time to dysphoric images (Caseras et al., 2007). |
| Neuroimaging | fMRI analysis of negative vs. neutral stimulus presentation. | Sensitivity as greater amygdala and attenuated DLPFC response to negative images (Ramel et al., 2007). |
| Dysphoric elaboration | ||
| Negative Self-Ideation | Compare endorsement of dysphoric self-descriptors before and after negative mood induction. | Reactivity as elevation of dysphoric self-descriptors (Segal, Gemar, & Williams, 1999); in situations where elevation is not apparent, negative mood elevation or high baseline self-ideation may also serve as risk predictors (van Rijsbergen et al., 2013). |
| Scrambled Sentence Completion | Create either positively or negatively valenced sentences from scrambled words. | Negative completion trend predicts MDD symptoms (Rude, Wenzlaff, Gibbs, Vane, & Whitney, 2002) and future diagnosis (Rude, Durham-Fowler, Baum, Rooney, & Maestas, 2010). |
| Dysfunctional Attitudes | Compare endorsement of dysphoric attitudes before and after negative mood induction. | Extended Attributional Style Questionnaire (Peterson & Villanova, 1988) assesses global cause attribution, and the Cognitive Style Questionnaire (Abramson & Metalsky, 1986) assesses attitudes about future and the self. Dysfunctional Attitudes Scale assesses negative thinking patterns (Segal et al., 2006). |
| Avoidance of Negative Affect | Assess self-reported acceptance of negative emotion and ability to nonjudgmentally observe thoughts. | Reactivity as low levels of self-endorsed acceptance or decentering (Bieling et al., 2012). |
| Neuroimaging | Compare neural reactivity between neutral and dysphoric film clips. | Reactivity as elevated medial prefrontal and reduced sensory cortex activation during sad film viewing (Farb et al., 2011). |
Note: see De Raedt & Koster (2010) for a different review of attentional paradigms in depression.
Table 2.
A summary of the claims and prediction of the two-factor model of sensitization in depression vulnerability.
| Claim / Prediction | Key Evidence | Future Questions |
|---|---|---|
| 1) Vulnerability operates at concrete and implicational levels of processing |
|
|
| 2) Processing biases are malleable |
|
|
| 3) Attention and schematic biases have independent tuning mechanisms |
|
|
| 4) Fixation and rumination have mutually reinforcing effects |
|
|
| Prediction: MDD sensitization involves fixation causing rumination |
|
|
Claim #1: Vulnerability operates at concrete and implicational levels of processing
We posit that dysphoric attention is a VF that reflects sensitivity to negative life events, while dysphoric elaboration is a VF that reflects more general dysphoric attitudes. The separation of attentional and elaborative VFs has historical precedent in the depression research literature. For example, the tendency to rehearse dysphoric attitudes about the self has been linked to depressive symptoms, an association independent from the effects of negative life events (Smith, Ingram, & Roth, 1985). This finding led researchers to distinguish between concrete thoughts tied to features of negative events themselves, and more abstract thoughts about the implications of these features in supporting narratives of global, chronic difficulties (Avison & Turner, 1988). In this way, the seeds of the attention/elaboration distinction were formed.
Subsequently, dysphoric attention has been theorized to underlie sensitivity to negative life events, predicated upon an associative bias for the processing of negative information and a deficit of reflective attentional control (Beevers, 2005; Carver, Johnson, & Joormann, 2008). Consequently, depression vulnerability has been linked to exaggerated, temporally-extended attentional capture by negatively-valenced stimuli and subsequent behavioral inhibition (Caseras, Garner, Bradley, & Mogg, 2007; Fritzsche et al., 2010; Leyman, De Raedt, Schacht, & Koster, 2007). Paradigms for tracking dysphoric attention include the dot-probe task, emotional stroop, spatial cueing, and eye tracking tasks (Table 1). Unlike most attentional capture paradigms, in which attention is directed quickly to a salient target, in depression such capture occurs slowly; relative to healthy controls and patients with anxiety, depressed participants linger longer on negative stimuli (Mogg, Bradley, & Williams, 1995), maintaining negative representations that are susceptible to further cognitive elaboration. Dysphoric attention therefore appears to be a deficit in the voluntary control of attention rather than some form of subliminal attentional capture; indeed, depressed participants show little distinction from controls when stimuli are presented at onsets less than 1 sec in duration (Bradley, Mogg, & Lee, 1997).
While deficits in attention control are apparent in response to negative external stimuli, vulnerability also extends to preoccupation with negative ideation (Koster et al., 2011). Dysphoric elaboration is most commonly measured through mood-evoked cognitive reactivity. In such paradigms, negative mood inductions increase the endorsement of global, dysfunctional attitudes in people with a history of depression, but not in people who have never been depressed (Ingram, Atchley, & Segal, 2011; Jeanne, Gross, Persons, & Hahn, 1998; Teasdale & Cox, 2001). Importantly for dysphoric elaboration’s status as a VF, cognitive reactivity predicts failure to recover from depression (Williams, Healy, Teasdale, White, & Paykel, 1990), and is predictive of depressive relapse (Segal et al., 2006). In addition to cognitive reactivity paradigms, dysphoric elaboration is also measured through scrambled sentence completion tasks, self-reported dysfunctional attitudes, and avoidance of negative affect (Table 1).
Claim #2: Dysphoric processing biases are malleable
Substantial recent research has focused on the modification of the two VFs, creating two robust but often separate research literatures. Since attention bias can be manipulated rapidly, it is perhaps easier to demonstrate its malleability. Indeed, negative attention bias appears to be reliably induced by repeated trials of focusing a person’s attention on negative stimuli following simultaneous presentation of negative and neutral stimuli (MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002). Conversely, it also appears possible to use these attention-training paradigms to reduce negative attention bias both in moderately-depressed college students (Wells & Beevers, 2010), and in select cases of acutely depressed individuals (Papageorgiou & Wells, 2000; Wells et al., 2009).
Dysphoric elaboration consists of entrenched negative attitudes, whose malleability is perhaps more difficult to establish empirically. However, cognitive bias modification interventions have been growing in popularity, and a recent meta-analysis of such interventions found moderate effects on elaborative biases in addition to attentional biases (Hallion & Ruscio, 2011). We may also infer malleability of dysphoric elaboration through clinical studies in which depressive thinking styles change following interventions designed to reduce rates of relapse/recurrence (Hollon 2005; Teasdale 2000).
For example, Mindfulness Based Cognitive Therapy (MBCT) is designed to reduce relapse/recurrence vulnerability by limiting dysphoric elaboration (Teasdale et al., 2000). Indeed, mindfulness training has been linked to reduced rumination scores in community participants undergoing mindfulness-based stress reduction (MBSR; Deyo, Wilson, Ong, & Koopman, 2009), and in patients with recurrent depression (Ramel, Goldin, Carmona, & McQuaid, 2004). In an active control study, MBCT reduced rumination significantly more than a relaxation control in a depressed college sample (Jain, Shapiro, Swanick, Roesch, Mills, et al., 2007). In another study, depressed patients were asked to recall a list of mixed-valence words before and after a social stress induction. While control participants reported longer trains of negative words during recall following the stressor, MBCT group members showed the opposite pattern, actually reducing their tendency to recall successive negative words (Van Vugt, Hitchcok, Shahar, & Britton, 2012). Taken together, this evidence is suggestive of the potential for therapeutic interventions to reduce dysphoric elaboration, a testament to the malleability of this second VF.
Claim #3: Attention and schematic biases have independent tuning mechanisms
Specifically, we suggest that dysphoric attention is tuned through fixation, while dysphoric elaboration is tuned through rumination. Furthermore, we argue that fixation and rumination are distinct, albeit interrelated, constructs. One challenge in using current research to distinguish between fixation and rumination is that many efficacious interventions attempt to address both of these biases concurrently, making it difficult to track construct-specific changes. For example, both mindfulness-based interventions (Kabat-Zinn, 1990; Segal, Williams, & Teasdale, 2002) and metacognitive therapy (Papageorgiou & Wells, 2000) employ a combination of attention training practices and training on the importance of limiting dysphoric rumination. Nevertheless, paradigms that focus more purely on fixation and rumination do exist. Training in fixation on negative stimuli leads to greater depressive symptoms following a stressor than training in fixation on neutral stimuli (MacLeod et al., 2002). Conversely, training in fixation on positive stimuli leads to reduced attention towards dysphoric stimuli (Wadlinger & Isaacowitz, 2008), and training-reduced negative attention bias mediates reductions in depressive symptoms in moderately-depressed college students (Wells & Beevers, 2010), and in case studies of acutely depressed individuals ( Wells et al., 2009). Indeed, such data are behind recent claims that affect-biased attention constitutes a form of emotion regulation or dysregulation in its own right (Todd, Cunningham, Anderson, & Thompson, 2012; Wadlinger & Isaacowitz, 2011).
Comparably, studies of rumination’s impact on emotional health have found that rumination promotes overgeneral memory in depression (Watkins & Teasdale, 2001), compromises working memory capacity in both depressed adults (Watkins & Brown, 2002) and dysphoric young adults (Philippot & Brutoux, 2008), reduces sleep quality (Guastella & Moulds, 2007), and impairs interpersonal problem solving (Yoon & Joormann, 2012). In a recent examination of rumination and mindfulness inductions, rumination led to greater negative affect and physiological arousal whereas mindfulness increased positive affect and parasympathetic activity (Gilbert & Gruber, 2014), suggesting that elaborative tuning can occur bi-directionally to promote either wellness or psychopathology. Additionally, a study of MBCT with formerly depressed participants found that rumination scores were reduced following MBCT, with residual levels of rumination post-treatment predicting depressive relapse over a 1 year follow up (Michalak, Holz, & Teismann, 2011). To the extent that rumination can be reduced, such tuning may be protective in the struggle against reemergence of depressive symptoms following episode.
Given evidence of both fixation and rumination tuning, we may question how independent these mechanisms truly are. Although fixation and rumination may seem similar in their preoccupation with negative experience, experimental evidence supports a distinction between these cognitive processes. In empirical investigations, rumination is related to, but not identical to fixation (Donaldson, Lam, & Mathews, 2007; Joormann, 2006; Joormann, Dkane, & Gotlib, 2006; Joormann & Gotlib, 2008), with rumination scores accounting for 10–20% of the variance in attention bias scores after controlling for depressive symptom severity.
One reason for this modest overlap is that fixation appears to be an abnormality of selection rather than elaboration. In other words, fixation involves the inhibition of attentional capture by positive information as well as exaggerated capture by negative information. Unlike the operationalization of attention capture in the broader cognitive literature, fixation appears to occur over more protracted time courses of at least a second following stimulus onset, unlike the early selection biases towards threat stimuli that is found in an anxiety disorders (Bradley et al., 1997). However, this 1 second time course is still far different from a chronic pattern of conceptual evaluation. For example, a meta-analysis of eye-tracking studies in affective disorders observed that relative to anxiety disorders, depression is uniquely characterized by both reduced orienting towards positive stimuli and maintenance of gaze towards these stimuli, in addition to exaggerated maintenance towards negative stimuli (Armstrong & Olatunji, 2012). By contrast, brooding, the dysphoric aspect of rumination, is characterized primarily by items expressing some dissatisfaction with the self, rather than a pre-occupation with the inciting negative events themselves (Armey et al., 2009; Joormann et al., 2006). Consistent with this account, induction of a ruminative focus in patients with depression increased self-ratings of worthlessness and incompetence (Rimes & Watkins, 2005). Thus from a content analysis, the constructs of fixation and rumination appear to have separate targets.
Granting that there is some independence between fixation and rumination, research has begun to assess how rumination and fixation separately mediate vulnerability. For example, the magnitude of dysphoric fixation in patients with MDD has recently been associated with slower recovery from sadness in subsequent mood induction (Clasen, Wells, Ellis, & Beevers, 2013; Sanchez et al., 2013). In a study of adolescents with a history of MDD, rumination was predicted by past depressive episodes, but also predicted episode return and duration (Abela & Hankin, 2011). Studies of this kind provide naturalistic evidence that fixation and rumination can be tuned over time to modify levels of risk.
The presence of distinct contributing mechanisms may help to explain why both MDD remission (Guidi, Fava, Fava, & Papakostas, 2011) and prevention of relapse/recurrence (Segal et al., 2010) are better achieved through combinations of therapeutic interventions compared to monotherapy, as different treatments may target different vulnerability factors. By this logic, identification of dominant VFs may be important for selecting the highest priority clinical intervention. For example, patients whose VFs primarily suggest dysphoric attention may benefit most from attention bias modification (Browning, Holmes, Charles, Cowen, & Harmer, 2012) or maintenance antidepressant medication (ADM), whereas those demonstrating mixed VFs may benefit additionally from cognitive therapy (CT) or mindfulness-based cognitive therapy, treatments that focus on building metacognitive awareness (Teasdale et al., 2002). Further research will be needed to validate algorithms for matching clinical interventions to particular VF patterns.
Claim #4: Fixation and rumination have mutually reinforcing effects
Fixation and rumination may be mutually reinforcing in provoking depression vulnerability, with a potential causal pathway moving from fixation to rumination to increased vulnerability. This causal hypothesis is based on findings from a pilot study (n=4) of therapeutic attention training in recurrent depression that led to reduced rumination scores over a 12 month follow up (Papageorgiou & Wells, 2000), while Donaldson et al. (2007) found no immediate effect of a rumination induction on levels of dysphoric fixation. Nevertheless, when rumination and fixation are measured concurrently, they do appear to be moderately related, with 10–20% shared variance in depressed samples, and a more moderate 6–10% shared variance in subclinical samples (Everaert et al., 2014; Everaert, Tierens, Uzieblo, & Koster, 2013). However, even within such samples, rumination mediates the relationship between fixation and subsequent memory biases, supporting the notion of a causal pathway from fixation to rumination. Longitudinal research is needed to investigate the possibility of a tightening correspondence between attention and elaboration with increasing episode vulnerability. In particular such studies could evaluate whether there is a temporal precedence to changes in dysphoric attention and elaboration that would imply a causal flow.
The connection between fixation and rumination does help to explain the idiosyncrasies of attentional biases in depression. While remitted depressed patients demonstrate a compromised ability to disengage attention from sad faces in dot-probe tasks (Joormann & Gotlib, 2007), these biases are only apparent following long (> 1000 msec) exposure periods to dysphoric words (Fritzsche et al., 2010). This prolonged attentional requirement and neural response suggests a slower, elaborative mechanism of vulnerability that is distinct from stimulus-driven, dysphoric attentional capture that is observed in anxiety disorders. Instead, the ‘lingering gaze’ in remission may reflect an attentional bias reinforced by cognitive elaboration.
Furthermore, dysphoric elaboration appears to influence attention to external events. For example, remitted depressed patients tend to rate neutral faces as possessing negative affective tone more often than controls (Bhagwagar, Cowen, Goodwin, & Harmer, 2004; Leppanen, Milders, Bell, Terriere, & Hietanen, 2004). In this way, dysphoric elaboration may shape behavior to increase the likelihood of stressful life experiences (Liu & Alloy, 2010), and also impose powerful retrospective biases that act as a ‘late selector’ of negative attributes from complex social events (Hammen, 2005).
Prediction: Sensitization occurs because dysphoric attention becomes conflated with elaboration
While there is strong evidence to support a connection between dysphoric attention and elaboration, there is relatively little evidence to support a causal flow between the two. However, given the evidence presented thus far, a reasonable prediction would be that chronic dysphoric attention facilitates dysphoric elaboration, which leads to tighter coupling between the two VFs. This coupling constitutes a sensitization to negative experience that increases the risk of depressive relapse/recurrence. Consistent with this view, a year-long study of 6–14 year olds found that those possessing low self-esteem and high dysfunctional attitudes demonstrated the greatest elevation in depressive symptoms following elevations in daily hassles compared to others in the same cohort (Abela & Skitch, 2007). It is this insidious coupling between dysphoric attention and elaboration of even minor events that elevates relapse/recurrence vulnerability. Within this framework, dysphoric elaboration may constitute a process supporting an existing theory that cognitive vulnerabilities contribute to the appraisal of ambiguous or seemingly minor stressors as highly stressful events, which in turn compromise a person’s sense of well-being (Liu & Alloy, 2010). This iterative dynamic interplay between daily stressors, fixation on these stressors, and their amplification via cognitive reactivity may well be responsible for increased vulnerability to episode return with successive depressive episodes.
A number of basic science paradigms have been used to examine the potential sensitization relationship between dysphoric attention and elaboration. A central sensitization mechanism is the withdrawal of engagement with positive life events in the face of stress. For example, emotional stressors appear to reduce reward responsiveness (Bogdan & Pizzagalli, 2006; Foti & Hajcak, 2010). Thus, exposure to salient stressors through dysphoric attention may initially reduce processing of positive stimuli, a sign of early relapse/recurrence vulnerability. Blunting of the neural response to reward predicts subsequent depression vulnerability in female adolescents (Bress, Foti, Kotov, Klein, & Hajcak, 2013), an effect particularly evident in recurrent compared to early episode depression (Hall, Milne, & Macqueen, 2013). Such blunting may represent the entrenchment of attentional biases to exclude positive features of life events.
As stress-related disengagement from positive features of events intensifies, dissonance is created between a person’s positive self-construal and the lack of such positivity in daily life. A proclivity for rumination may then serve to erode these positive attitudes, such as the personalization of negative experiences, rather than seeing them as the consequence of external circumstances (Ciesla, Felton, & Roberts, 2011; Smith & Alloy, 2009). Accordingly, the self-schema model of depression (Dance & Kuiper, 1987), is predicated on research demonstrating that conventional, positive self-schema roles are disrupted both in MDD, but also in people vulnerable to MDD (MacDonald, Kuiper, & Olinger, 1985). In the Macdonald et al. study, participants with both mild and moderate depressive symptoms showed less consistency than control participants in their self-ratings of neutral and dysphoric trait adjectives, suggesting a destabilization of self-referential attitudes. Similarly, greater anhedonic symptoms in depression have been associated with reductions in positive, self-enhancing bias (Dunn, Stefanovitch, Buchan, Lawrence, & Dalgleish, 2009).
We propose that sensitization occurs as rumination erodes positive attitudes and strengthens dysfunctional attitudes. In keeping with this idea, multiple clinical studies suggest that dysphoric elaboration is the dominant predictor of relapse/recurrence in patients with multiple previous MDD episodes (Halvorsen, Wang, Eisemann, & Waterloo, 2010), even when compared to patients with a single prior episode (Yamamoto, Yamano, Shimada, Ichikawa, & Nakaya, 2014). As self-schemas are repeatedly disrupted, dysphoric elaboration may become habitual, reducing awareness of such associations and thereby reducing opportunities to engage in self-regulation. For example, healthy controls exposed to mood challenge demonstrate increased negative implicit attitudes, but remitted participants maintain a pre-existing negative bias that was found prior to induction (Meites, Deveney, Steele, Holmes, & Pizzagalli, 2008). This finding suggests an ‘always-on’ negative self association that persists following remission.
The consequence of powerful dysphoric elaboration is that nominally benign stressors become triggers for the rehearsal of dysphoric attitudes (Segal, Williams, Teasdale, & Gemar, 1996). Supporting this idea, the number of non-severe adverse life events appears to predict new episodes of recurrent depression (Monroe et al., 2006), suggesting that the severity of adversity is less important than one’s interpretations of any adversity. Instead, it is possible that the importance of negative features of even minor stressors is exaggerated through dysphoric elaboration. Indeed, catastrophising is a unique predictor of depressive symptoms in chronic pain patients, even when controlling for other forms of responding such as distraction, dissociation, or re-appraisal (Sullivan & D’Eon, 1990). Conversely, MBCT, an intervention which appears to function through the reduction in dysphoric elaboration (as elaborated in claim 2 above), has its greatest prophylactic efficacy in patients with 3 or more past episodes of depression (Ma & Teasdale, 2004), consistent with elaboration driving vulnerability in later but not earlier episodes of recurrent depression.
The claims and predictions discussed above suggest a number of fruitful research questions. The overarching need in evaluating the two-factor model will be the simultaneous measurement of both dysphoric attention and elaboration within a single experimental paradigm, which, if conducted over multiple time points would allow for the explicit modeling of the relationship between adverse life events, dysphoric attention and elaboration, and their relationship to future symptom or episode status.
A Neural Systems Account of the Two-Factor Model
The findings reviewed above provide intriguing evidence that dysphoric elaboration may arise as a progression from dysphoric attention, elevating relapse/recurrence risk as the two VF processes become more densely interdependent with each episode. While more behavioral research is needed to substantiate this claim, neuroimaging research on depression vulnerability also speaks to the idea of conflated cognitive processes in depression vulnerability.
Neural Mechanisms of Dysphoric Attention
Maladaptive cognitive processes observed in acute MDD may persist in the form of dysphoric attention, a susceptibility to attentional capture by negative events. Neurally, this model is characterized by elevated activity in the amygdala, anterior insula and anterior cingulate cortex, and attenuated activity in the dorsolateral prefrontal cortex (DLPFC) (Drevets, Savitz, & Trimble, 2008; Hamilton et al., 2012; Siegle, Thompson, Carter, Steinhauer, & Thase, 2007). Heightened amygdala activity is the most prototypical VF of dysphoric attention, having been detected both metabolically through PET imaging and functionally through fMRI-derived responses to negative stimuli. Indeed, amygdala hyperarousal in depressed individuals persists even after negative emotional stimuli are no longer present (Siegle et al., 2007).
One of the amygdala’s primary functions is to enable the rapid orienting of attention to motivationally-salient stimuli (Vuilleumier, 2005), a capacity which is biased towards negative information during acute episodes of depression (Gotlib, Krasnoperova, Yue, & Joormann, 2004). Habitual dysphoric attention creates a negatively-skewed context for appraisals of subjective well-being, and accordingly metabolic rate in the right amygdala predicts negative mood in depressed patients (Abercrombie et al., 1998). Conversely, one proposed mechanism of ADMs is that they promote a positive bias in attention to counter dysphoric attention (Harmer & Cowen, 2013). While amygdala reactivity to negative stimuli often normalizes following successful ADM (Arnone et al., 2012; Sheline et al., 2001) or CT (Fu et al., 2008), elevated amygdala reactivity is still observed in remitted patients following negative mood challenge and is associated with dysphoric attention and memory biases (Ramel et al., 2007).
The amygdala does not operate in isolation. In healthy individuals, the amygdala response to negative stimuli is regulated by dorsal and ventral aspects of the prefrontal cortex (PFC). For example, the act of labeling emotional facial expression relative to other characteristics such as gender (Lieberman et al., 2007) promotes ventral PFC activation and a commensurate reduction in amygdala activity. In individuals who suffer from MDD, the ability to regulate negative information is compromised, as evidenced by disrupted connectivity between the PFC and amygdala (Heller et al., 2009; Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007). Relative to healthy controls, remitted MDD patients demonstrate reduced rostral anterior cingulate and dorsomedial PFC activation in response to task feedback on a response inhibition (Go/No-Go) paradigm, commensurate with reduced executive recruitment even in the face of performance errors (Nixon, Liddle, Worwood, Liotti, & Nixon, 2013). Reduced DLPFC reactivity to dysphoric stimuli is also associated with high levels of hopelessness (Zhong et al., 2011), potentially reflecting habituation to repeated failures to redirect attention. Consistent with its characterization as a residual symptom marker, DLPFC normalization is associated with depressive symptom resolution following both ADM (Schaefer, Putnam, Benca, & Davidson, 2006) and CT (Brody et al., 2001). Sustained DLFPC hypoactivity in remission is therefore an additional neural indicator of dysphoric attention.
Neural Mechanisms of Dysphoric Elaboration
Neurally, dysphoric elaboration can be framed in terms of habitual engagement in an analytic or evaluative mode of processing, particularly in response to emotional stress. As such, the neural predictions for dysphoric elaboration are contrary to that of dysphoric attention: greater PFC activity is expected when such self-referential elaboration is triggered, compared to reduced activation commonly observed during dysphoric attention to external stimuli. Which of these patterns is evident may depend on experimental paradigms, with unconstrained responses to mood challenge promoting analytic self-focus (and hence PFC activation) compared to PFC engagement failures during externally-directed experimental tasks. Consistent with this account, rumination about the self may be characterized by elevated rather than reduced PFC stress reactivity. For example, people high in rumination show elevated PFC activity when directing attention away from dysphoric stimuli (Chuen Yee Lo, Lau, Cheung, & Allen, 2012; Diener, Kuehner, Brusniak, Struve, & Flor, 2009), potentially reflecting the difficulty of disengaging from habitual elaborative processes.
Emerging findings have begun to implicate the medial PFC as the primary neural correlate of dysphoric elaboration. Elevated medial PFC activity has been observed during self-referential processing in depression (Lemogne et al., 2009) and in response to mood challenge in both subclinical (Farb et al., 2010) and remitted patients (Farb, Anderson, Bloch, & Segal, 2011). While decreased DLPFC activity may be a marker for MDD that normalizes with symptom remission (Brody et al., 2001; Siegle et al., 2007; Zhong et al., 2011), the medial PFC continues to display elevated stress reactivity (Lemogne et al., 2010) and resting-state connectivity (Li et al., 2012). Similarly, ADM reduces medial PFC reactivity to negative self-descriptors in individuals at high risk for MDD (Di Simplicio, Norbury, & Harmer, 2012), but medial PFC reactivity to mood challenge persists in medicated, remitted patients, with reactivity in this region predicting future relapse/recurrence (Farb et al., 2011).
Vulnerability factors as interacting neural systems
On a neural level, sensitization occurs because of increased coupling between brain networks that support dysphoric attention and elaboration, which together elevate relapse/recurrence risk with each episode experienced. This elevated risk may be the product of interacting brain networks, sets of intrinsically correlated brain regions that are each normally associated with a different form of information processing (Figure 2). In accordance with the coupling-as-vulnerability prediction of the two-factor model, a central finding in network studies of MDD is the conflation of multiple mental processes into a common ruminative cycle. Normally, the prefrontal lobe hosts three distinct networks, including the ‘task-independent’ default mode network (DMN), the ‘task-positive’ executive network (EXN), and the ‘task-switching’ network sensitive to affective salience (SLN) (Menon & Uddin, 2010; Seeley et al., 2007). In MDD these networks are simultaneously activated in the dorsal MPFC (Sheline, Price, Yan, & Mintun, 2010), interfering with the resolution of dysphoric cognition. The consequence of this conflation is that automatic appraisals of affective salience and subsequent conceptual elaboration interfere with efforts to adaptively direct attention and behavior.
Figure 2.
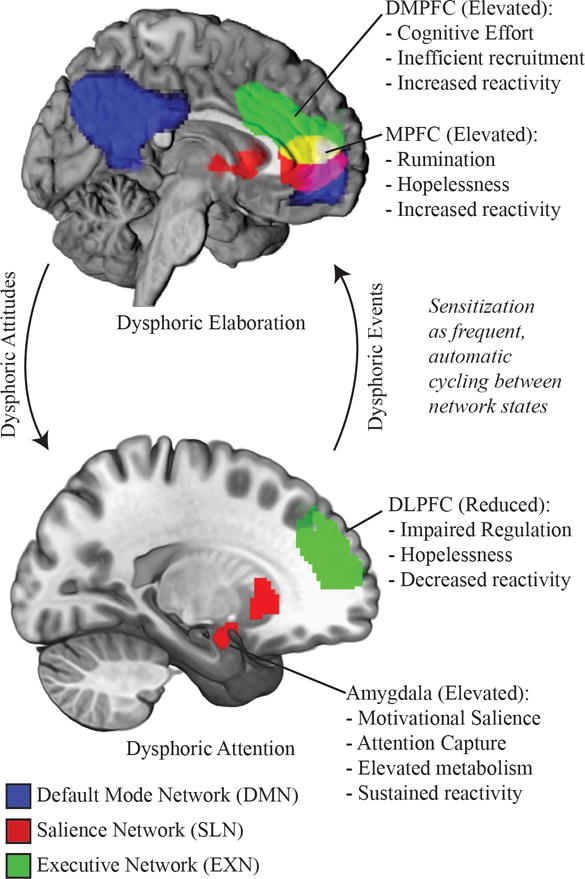
A schematic of the 3 intrinsically-connected networks associated with depression vulnerability. Dysphoric attention is characterized by increased salience network (SLN) and reduced executive network (EXN) activity. Prolonged attention to dysphoric content may promote dysphoric elaboration, co-opting EXN function into habitual self-evaluation and elaboration characteristic of the default mode network (DMN). Dysphoric elaboration may in turn create dysphoric expectations, biasing selection and maintaining dysphoric attention.
Of the three networks, the DMN has been most consistently linked to MDD; its activation during task-irrelevant, internally directed thought (Mason et al., 2007) is consistent with a theory of habitual elaboration disrupting regulatory efforts. Evidence for DMN hyperactivity is apparent in remission from MDD, both in terms of elevated functional connectivity and hypogyrification of the DMNs central hub in the precuneus and posterior cingulate of the brain (Nixon, Liddle, Nixon, et al., 2013). Consistent with its hypothesized relationship to dysphoric elaboration, the prefrontal hub of the DMN has been associated with elaborative self-referential processing in depression (Lemogne, Delaveau, Freton, Guionnet, & Fossati, 2012). Furthermore, the prefrontal DMN has shown exaggerated connection strength and diffuseness in MDD (Sheline et al., 2009), failing to deactivate during cognitively demanding tasks, which may indicate interfering rumination (Lemogne et al., 2012).
In parallel, progressive EXN dysfunction associated with dysphoric attention may help to explain why DMN processing is able to dominate dorsal nexus activity. The DLPFC is one of the principal nodes of the EXN, and is associated with control over visceral impulses (Baumgartner, Knoch, Hotz, Eisenegger, & Fehr, 2011) and adaptive emotion regulation (Goldin, McRae, Ramel, & Gross, 2008), empowering goal-directed behavior (Ballard et al., 2011). However, DLPFC regulatory capacity may be compromised by acute stress (Qin, Hermans, van Marle, Luo, & Fernandez, 2009). Rather than lying dormant, DLPFC activity appears to become co-opted into ruminative processes during both MDD episode and remission (Cooney, Joormann, Eugene, Dennis, & Gotlib, 2010; Farb et al., 2011), contributing to findings of diffuse DMN activity in depression (Sheline et al., 2009). Accordingly, higher DLPFC activity during self-referential processing has been associated with lower rates of remission in MDD (Lemogne et al., 2010). Similarly, higher DLPFC activity during emotional tasks has been linked to increased depression severity and feelings of hopelessness (Grimm et al., 2009).
Our discussion of neural networks has focused primarily on the presence of dysphoric elaboration and the absence of cognitive control in dysphoric attention. However, dysphoric attention manifests as an attentional bias driven both by elevated DLPFC reactivity (Kerestes et al., 2012), and recruitment of the third dorsal nexus network, the SLN, which includes the amygdala, a structure prominent in dysphoric attention. The SLN is involved in the redirection of attention towards emotionally salient stimuli (Seeley et al., 2007), which is characterized by anterior right insula activation (Menon & Uddin, 2010). In MDD, anterior insula activation occurs most often following high levels of EXN activity, indicating a tendency to switch to DMN rumination, whereas in healthy controls, insula activation follows high levels of DMN activity, indicating a habit of disengaging from rumination and getting back to the task at hand (Hamilton et al., 2011).
The ability to flexibly allocate attention via the SLN may be important for sustained well-being. When attention is constructively engaged, as in a guided breathing exercise, trait non-reactivity is associated with reduced insula switch-signals to emotional stimuli and better task performance (Paul, Stanton, Greeson, Smoski, & Wang, 2013). Conversely, following emotional challenge, trait rumination is linked to increased insula switch-signals, consistent with the prioritization, and consequently attention, to negative or unexpected events. Such attention may then facilitate further dysphoric elaboration. Thus, the co-activation of these three networks is associated with rumination, compromised cognitive control, and redirection of attention to dysphoric information that further reinforces ruminative patterns (Figure 2). Such co-activation resonates with the two-factor model’s sensitization prediction of increased coupling between dysphoric attention and dysphoric elaboration. Within this reactive cycle, vulnerability may be characterized by event interpretation that is prejudiced by habitual expectations: in a study of remitted patients, prefrontal hyperactivity has been observed during reward anticipation, relative to SLN hypoactivation observed during reward outcomes (Dichter, Kozink, McClernon, & Smoski, 2012). Thus, habitual expectations may begin to dominate perception, whereas actual outcomes are met with an attenuated prefrontal response.
It would be reasonable to infer from this research that DMN activity is inherently maladaptive, representing a withdrawal from the external world and goal-directed cognition (e.g., Marchetti, Koster, Sonuga-Barke, & De Raedt, 2012). However, it is important to recall that dysphoric elaboration is defined as engagement of an analytic process with dysphoric content. From this perspective, DMN activity represents only the analytic process, which is not in itself adaptive or maladaptive. Indeed, a comparison of EXN and DMN activity over time suggests no differences in the ratio of network dominance between MDD and healthy controls (Hamilton et al., 2011). However, in people with a history of depression, DMN dominance correlates with higher levels of depressive rumination (Berman et al., 2011; Hamilton et al., 2011). Prospective clinical research corroborates this process/content interaction: rumination predicts depressive symptoms, but only following the dysphoric content introduced by negative life events (Abela, Hankin, Sheshko, Fishman, & Stolow, 2012). Similarly, negative cognitive style, the tendency to generate global negative thoughts, interacts with rumination to predict the incidence, number and duration of future depressive episodes, while rumination alone is not predictive when the interaction term is included in the model (Robinson & Alloy, 2003).
Thus, DMN hyperconnectivity may indicate relapse/recurrence sensitization insofar as it reflects conflation between dysphoric attention (SLN/EXN) and elaboration (DMN) processes. In such situations, reducing DMN activity may have prophylactic benefits. Adults with early life stress but without psychiatric illness have demonstrated reduced DMN connectivity compared to healthy controls (Philip et al., 2013), suggesting that avoiding the process of self-referential rumination may be a stress coping mechanism. One mechanism by which Ketamine, an NMDA antagonist with antidepressant properties, may exert its effects is by decoupling DMN connectivity with the dorsal nexus (Scheidegger et al., 2012). Cognitive therapy may increase subgenual cingulate and MPFC reactivity to positive stimuli while attenuating reactivity to negative stimuli, a neural activity change that accounts for reductions in depressive symptoms (Yoshimura et al., 2013). Thus, reductions in vulnerability may be assessed by the extent to which networks for attention, executive control, and elaboration are rendered more distinct, commensurate with decoupling of dysphoric attitudes and elaboration.
Concluding Remarks
Existing research supports a two-factor model of sensitization in recurrent depression, predicated on dysregulation in the cognitive domains of attention and elaboration. Based on the established contribution of both dysphoric attention and elaboration to depressive affect, the model predicts that fixation on negative features of life events and subsequent dysphoric rumination serve as a mutually reinforcing cognitive pattern, a pattern strengthened through repeated association during depressive episodes. The product of such rehearsal is sensitization to adverse life events, as even relatively minor features of adverse experience are fixated upon and allowed to trigger powerful dysphoric elaborations about the self, future, and world.
The idea that the coupling of dysphoric attention and elaboration promotes a more chronic illness course is a largely untested but empirically tractable claim, as the impact of these two VFs has been established independently but rarely measured in tandem. If the two factor account can be validated, the interaction between dysphoric attention and elaboration would offer a plausible account for sensitization effects in unipolar mood disorder. If future validation studies produce positive findings, the two-factor model may aid in the identification of remitted patients who would be best matched to targeted therapies, thereby increasing effective MDD prevention.
Footnotes
With regards to the current manuscript, authors NASF, JAI, and AKA have no economic or commercial conflicts of interest to disclose. ZVF discloses commercial interest in Mindfulness-Based Cogntiive Therapy (MBCT), which is mentioned as a therapeutic intervention in the text. ZVF receives commercial support in the form of royalties for the MBCT clinical manual, as well as for leading MBCT facilitator training courses.
References
- Abela JR, Hankin BL. Rumination as a vulnerability factor to depression during the transition from early to middle adolescence: a multiwave longitudinal study. Journal of Abnormal Psychology. 2011;120(2):259–271. doi: 10.1037/a0022796. [DOI] [PubMed] [Google Scholar]
- Abela JR, Hankin BL, Sheshko DM, Fishman MB, Stolow D. Multi-wave prospective examination of the stress-reactivity extension of response styles theory of depression in high-risk children and early adolescents. Journal of Abnormal Child Psychology. 2012;40(2):277–287. doi: 10.1007/s10802-011-9563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abela JR, Skitch SA. Dysfunctional attitudes, self-esteem, and hassles: Cognitive vulnerability to depression in children of affectively ill parents. Behaviour Research and Therapy. 2007;45(6):1127–1140. doi: 10.1016/j.brat.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Abercrombie HC, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, Davidson RJ. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9(14):3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Abramson L, Metalsky G. The Cognitive Style Questionnaire: Measurement of negative cognitive styles about self and consequences. 1986. Unpublished Manuscript. [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. American Psychiatric Press; Washington: 2000. [Google Scholar]
- Armey MF, Fresco DM, Moore MT, Mennin DS, Turk CL, Heimberg RG, Alloy LB. Brooding and pondering: Isolating the active ingredients of depressive rumination with exploratory factor analysis and structural equation modeling. Assessment. 2009;16(4):315–327. doi: 10.1177/1073191109340388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: a meta-analytic review and synthesis. Clinical Psychology Review. 2012;32(8):704–723. doi: 10.1016/j.cpr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, McKie S, Elliott R, Thomas EJ, Downey D, Juhasz G, Anderson IM. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. American Journal of Psychiatry. 2012;169(8):841–850. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- Avison WR, Turner RJ. Stressful life events and depressive symptoms: Disaggregating the effects of acute stressors and chronic strains. Journal of Health and Social Behavior. 1988:253–264. [PubMed] [Google Scholar]
- Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. Journal of Neuroscience. 2011;31(28):10340–10346. doi: 10.1523/JNEUROSCI.0895-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard PJ, Teasdale JD. Interacting Cognitive Subsystems: A systemic apporach to cognitive-affective interaction and chagne. Cognition & Emotion. 1991;5:1–39. [Google Scholar]
- Baumgartner T, Knoch D, Hotz P, Eisenegger C, Fehr E. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nature Neuroscience. 2011;14:1468–1474. doi: 10.1038/nn.2933. [DOI] [PubMed] [Google Scholar]
- Beck AT. The core problem in depression: The cognitive triad. Depression: Theories and Therapies. 1970:47–55. [Google Scholar]
- Beevers CG. Cognitive vulnerability to depression: a dual process model. Clinical Psychology Review. 2005;25(7):975–1002. doi: 10.1016/j.cpr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social Cognitive & Affective Neuroscience. 2011;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z, Cowen PJ, Goodwin GM, Harmer CJ. Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. American Journal of Psychiatry. 2004;161(1):166–168. doi: 10.1176/appi.ajp.161.1.166. [DOI] [PubMed] [Google Scholar]
- Bieling PJ, Hawley LL, Bloch RT, Corcoran KM, Levitan RD, Young LT, Segal ZV. Treatment-specific changes in decentering following mindfulness-based cognitive therapy versus antidepressant medication or placebo for prevention of depressive relapse. Journal of Consulting & Clinical Psychology. 2012;80(3):365–372. doi: 10.1037/a0027483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biological Psychiatry. 2006;60(10):1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occurring dysphoria. Behaviour Research and Therapy. 1997;35(10):911–927. doi: 10.1016/s0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50(1):74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Baxter LR., Jr Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Archives of General Psychiatry. 2001;58(7):631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Charles M, Cowen PJ, Harmer CJ. Using attentional bias modification as a cognitive vaccine against depression. Biological Psychiatry. 2012;72(7):572–579. doi: 10.1016/j.biopsych.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychological Bulletin. 2008;134(6):912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X, Garner M, Bradley BP, Mogg K. Biases in visual orienting to negative and positive scenes in dysphoria: An eye movement study. Journal of Abnormal Psychology. 2007;116(3):491–497. doi: 10.1037/0021-843X.116.3.491. [DOI] [PubMed] [Google Scholar]
- Chuen Yee Lo B, Lau S, Cheung SH, Allen NB. The impact of rumination on internal attention switching. Cognition & Emotion. 2012;26(2):209–223. doi: 10.1080/02699931.2011.574997. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, Felton JW, Roberts JE. Testing the cognitive catalyst model of depression: Does rumination amplify the impact of cognitive diatheses in response to stress? Cognition & Emotion. 2011;25(8):1349–1357. doi: 10.1080/02699931.2010.543330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen PC, Wells TT, Ellis AJ, Beevers CG. Attentional biases and the persistence of sad mood in major depressive disorder. Journal of Abnormal Psychology. 2013;122(1):74–85. doi: 10.1037/a0029211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cognitive Affective & Behavioral Neuroscience. 2010;10(4):470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dance KA, Kuiper NA. Self-schemata, social roles, and a self-worth contingency model of depression. Motivation & Emotion. 1987;11(3):251–268. [Google Scholar]
- De Raedt R, Koster EH. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive Affective & Behavioral Neuroscience. 2010;10(1):50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- Deyo M, Wilson KA, Ong J, Koopman C. Mindfulness and rumination: does mindfulness training lead to reductions in the ruminative thinking associated with depression? EXPLORE: The Journal of Science and Healing. 2009;5(5):265–271. doi: 10.1016/j.explore.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Norbury R, Harmer CJ. Short-term antidepressant administration reduces negative self-referential processing in the medial prefrontal cortex in subjects at risk for depression. Molecular Psychiatry. 2012;17(5):503–510. doi: 10.1038/mp.2011.16. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. Journal of Affective Disorders. 2012;136(3):1126–1134. doi: 10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener C, Kuehner C, Brusniak W, Struve M, Flor H. Effects of stressor controllability on psychophysiological, cognitive and behavioural responses in patients with major depression and dysthymia. Psychological Medicine. 2009;39(1):77–86. doi: 10.1017/S0033291708003437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson C, Lam D, Mathews A. Rumination and attention in major depression. Behaviour Research and Therapy. 2007;45(11):2664–2678. doi: 10.1016/j.brat.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Stefanovitch I, Buchan K, Lawrence AD, Dalgleish T. A reduction in positive self-judgment bias is uniquely related to the anhedonic symptoms of depression. Behaviour Research and Therapy. 2009;47(5):374–381. doi: 10.1016/j.brat.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaert J, Duyck W, Koster EH. Attention, interpretation, and memory biases in subclinical depression: A proof-of-principle test of the combined cognitive biases hypothesis. Emotion. 2014;14(2):331–340. doi: 10.1037/a0035250. [DOI] [PubMed] [Google Scholar]
- Everaert J, Tierens M, Uzieblo K, Koster EH. The indirect effect of attention bias on memory via interpretation bias: Evidence for the combined cognitive bias hypothesis in subclinical depression. Cognition & Emotion. 2013;27(8):1450–1459. doi: 10.1080/02699931.2013.787972. [DOI] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Bloch RT, Segal ZV. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biological Psychiatry. 2011;70(4):366–372. doi: 10.1016/j.biopsych.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one’s emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10(1):25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G. State sadness reduces neural sensitivity to nonrewards versus rewards. Neuroreport. 2010;21(2):143–147. doi: 10.1097/WNR.0b013e3283356448. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48(9):851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Fritzsche A, Dahme B, Gotlib IH, Joormann J, Magnussen H, Watz H, von Leupoldt A. Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychological Medicine. 2010;40(5):815–826. doi: 10.1017/S0033291709990948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, Murray RM. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biological Psychiatry. 2008;64(6):505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Gilbert K, Gruber J. Emotion Regulation of Goals in Bipolar Disorder and Major Depression: A Comparison of Rumination and Mindfulness. Cognitive Therapy & Research. 2014:1–14. [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113(1):121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34(4):932–943. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Moulds ML. The impact of rumination on sleep quality following a stressful life event. Personality and Individual Differences. 2007;42(6):1151–1162. [Google Scholar]
- Guidi J, Fava GA, Fava M, Papakostas GI. Efficacy of the sequential integration of psychotherapy and pharmacotherapy in major depressive disorder: a preliminary meta-analysis. Psychological Medicine. 2011;41(2):321–331. doi: 10.1017/S0033291710000826. [DOI] [PubMed] [Google Scholar]
- Hall GB, Milne AM, Macqueen GM. An fMRI study of reward circuitry in patients with minimal or extensive history of major depression. European Archives of Psychiatry & Clinical Neuroscience. 2014;264:187–198. doi: 10.1007/s00406-013-0437-9. [DOI] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011;137(6):940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Halvorsen M, Wang CE, Eisemann M, Waterloo K. Dysfunctional attitudes and early maladaptive schemas as predictors of depression: a 9-year follow-up study. Cognitive Therapy and Research. 2010;34(4):368–379. doi: 10.1016/j.jbtep.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Cowen PJ. ’It’s the way that you look at it’–a cognitive neuropsychological account of SSRI action in depression. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2013;368(1615):20120407. doi: 10.1098/rstb.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 2009;106(52):22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, Gallop R. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Archives of General Psychiatry. 2005;62(4):417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- Ingram RE, Atchley RA, Segal ZV. Vulnerability to depression: From cognitive neuroscience to prevention and treatment. Guilford Press; 2011. [Google Scholar]
- Jacobs RH, Reinecke MA, Gollan JK, Kane P. Empirical evidence of cognitive vulnerability for depression among children and adolescents: A cognitive science and developmental perspective. Clinical Psychology Review. 2008;28(5):759–782. doi: 10.1016/j.cpr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Shapiro SL, Swanick S, Roesch SC, Mills PJ, Bell I, Schwartz GE. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Annals of Behavioral Medicine. 2007;33(1):11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- Jeanne M, Gross JJ, Persons JB, Hahn J. Mood matters: Negative mood induction activates dysfunctional attitudes in women vulnerable to depression. Cognitive Therapy and Research. 1998;22(4):363–376. [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J. Differential effects of rumination and dysphoria on the inhibition of irrelevant emotional material: Evidence from a negative priming task. Cognitive Therapy and Research. 2006;30(2):149–160. [Google Scholar]
- Joormann J, Dkane M, Gotlib IH. Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behavior Therapy. 2006;37(3):269–280. doi: 10.1016/j.beth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology. 2007;116(1):80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117(1):182–192. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Keller MB. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. Journal of Affective Disorders. 1998;50(2–3):97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain and Illness. New York: Delacorte; 1990. [Google Scholar]
- Kerestes R, Ladouceur CD, Meda S, Nathan PJ, Blumberg HP, Maloney K, Phillips ML. Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychological Medicine. 2012;42(1):29–40. doi: 10.1017/S0033291711001097. [DOI] [PubMed] [Google Scholar]
- Klein DN, Kotov R, Bufferd SJ. Personality and depression: explanatory models and review of the evidence. Annual review of clinical psychology. 2011;7:269–295. doi: 10.1146/annurev-clinpsy-032210-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EH, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: the impaired disengagement hypothesis. Clinical Psychology Review. 2011;31(1):138–145. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Lazarus R, Folkman S. Cognitive appraisal processes. Stress, appraisal and coping. 1984:22–54. [Google Scholar]
- Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. Journal of Affective Disorders. 2012;136(1–2):e1–e11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, Volle E, Bergouignan L, Lehericy S, Fossati P. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social Cognitive & Affective Neuroscience. 2009;4(3):305–312. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Mayberg H, Bergouignan L, Volle E, Delaveau P, Lehericy S, Fossati P. Self-referential processing and the prefrontal cortex over the course of depression: a pilot study. Journal of Affective Disorders. 2010;124(1–2):196–201. doi: 10.1016/j.jad.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Leppanen JM, Milders M, Bell JS, Terriere E, Hietanen JK. Depression biases the recognition of emotionally neutral faces. Psychiatry Research. 2004;128(2):123–133. doi: 10.1016/j.psychres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Leyman L, De Raedt R, Schacht R, Koster EH. Attentional biases for angry faces in unipolar depression. Psychological Medicine. 2007;37(3):393–402. doi: 10.1017/S003329170600910X. [DOI] [PubMed] [Google Scholar]
- Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng LL, Hu D. A Treatment-Resistant Default Mode Subnetwork in Major Depression. Biological Psychiatry. 2012;74(1):48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Liu RT, Alloy LB. Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clinical Psychology Review. 2010;30(5):582–593. doi: 10.1016/j.cpr.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S, Nolen-Hoeksema S. Effects of self-focused rumination on negative thinking and interpersonal problem solving. Journal of Personality and Social Psychology. 1995;69(1):176–190. doi: 10.1037//0022-3514.69.1.176. [DOI] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. Journal of Consulting & Clinical Psychology. 2004;72(1):31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- MacDonald MR, Kuiper NA, Olinger LJ. Vulnerability to depression, mild depression, and degree of self-schema consolidation. Motivation and emotion. 1985;9(4):369–379. [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111(1):107–123. [PubMed] [Google Scholar]
- Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychology Review. 2012;22(3):229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites TM, Deveney CM, Steele KT, Holmes AJ, Pizzagalli DA. Implicit depression and hopelessness in remitted depressed individuals. Behaviour Research and Therapy. 2008;46(9):1078–1084. doi: 10.1016/j.brat.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezulis AH, Abramson LY, Hyde JS, Hankin BL. Is there a universal positivity bias in attributions? A meta-analytic review of individual, developmental, and cultural differences in the self-serving attributional bias. Psychological Bulletin. 2004;130(5):711–747. doi: 10.1037/0033-2909.130.5.711. [DOI] [PubMed] [Google Scholar]
- Michalak J, Hölz A, Teismann T. Rumination as a predictor of relapse in mindfulness-based cognitive therapy for depression. Psychology and Psychotherapy: Theory, Research and Practice. 2011;84(2):230–236. doi: 10.1348/147608310X520166. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: The role of awareness. British Journal of Clinical Psychology. 1995;34(1):17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychological Review. 2005;112(2):417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Recurrence in major depression: A conceptual analysis. Psychological Review. 2011;118(4):655–674. doi: 10.1037/a0025190. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Roberts JE, Kupfer DJ, Frank E. Life stress and treatment course of recurrent depression: II. Postrecovery associations with attrition, symptom course, and recurrence over 3 years. Journal of Abnormal Psychology. 1996;105(3):313–328. doi: 10.1037//0021-843x.105.3.313. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Torres LD, Guillaumot J, Harkness KL, Roberts JE, Frank E, Kupfer D. Life stress and the long-term treatment course of recurrent depression: III. Nonsevere life events predict recurrence for medicated patients over 3 years. Journal of Consulting and Clinical Psychology. 2006;74(1):112–120. doi: 10.1037/0022-006X.74.1.112. [DOI] [PubMed] [Google Scholar]
- Munoz RF, Cuijpers P, Smit F, Barrera AZ, Leykin Y. Prevention of major depression. Annual Review of Clinical Psychology. 2010;6:181–212. doi: 10.1146/annurev-clinpsy-033109-132040. [DOI] [PubMed] [Google Scholar]
- Nixon NL, Liddle PF, Nixon E, Worwood G, Liotti M, Palaniyappan L. Biological vulnerability to depression: linked structural and functional brain network findings. British Journal of Psychiatry. 2014;204:283–289. doi: 10.1192/bjp.bp.113.129965. [DOI] [PubMed] [Google Scholar]
- Nixon NL, Liddle PF, Worwood G, Liotti M, Nixon E. Prefrontal cortex function in remitted major depressive disorder. Psychological Medicine. 2013;43(6):1219–1230. doi: 10.1017/S0033291712002164. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS, Seligman ME. Learned helplessness in children: a longitudinal study of depression, achievement, and explanatory style. Journal of Personality and Social Psychology. 1986;51(2):435–442. doi: 10.1037//0022-3514.51.2.435. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspectives on Psychological Science. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Papageorgiou C, Wells A. Treatment of recurrent major depression with attention training. Cognitive and Behavioral Practice. 2000;7(4):407–413. [Google Scholar]
- Paul NA, Stanton SJ, Greeson JM, Smoski MJ, Wang L. Psychological and neural mechanisms of trait mindfulness in reducing depression vulnerability. Social Cognitive & Affective Neuroscience. 2013;8(1):56–64. doi: 10.1093/scan/nss070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depression and anxiety. 2010;27(12):1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- Peterson C, Villanova P. An Expanded Attributional Style Questionnaire. Journal of Abnormal Psychology. 1988;97(1):87–89. doi: 10.1037//0021-843x.97.1.87. [DOI] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Price LH, Bloom RF, Carpenter LL. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. European Journal of Neuropsychopharmacology. 2013;23(1):24–32. doi: 10.1016/j.euroneuro.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot P, Brutoux F. Induced rumination dampens executive processes in dysphoric young adults. Journal of Behavior Therapy and ExperimentalPsychiatry. 2008;39(3):219–227. doi: 10.1016/j.jbtep.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2007;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology. American Journal of Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biological Psychiatry. 2009;66(1):25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Carmona PE, McQuaid JR. The effects of mindfulness meditation on cognitive processes and affect in patients with past depression. Cognitive Therapy and Research. 2004;28(4):433–455. [Google Scholar]
- Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biological Psychiatry. 2007;61(2):231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Rimes KA, Watkins E. The effects of self-focused rumination on global negative self-judgements in depression. Behaviour Research and Therapy. 2005;43(12):1673–1681. doi: 10.1016/j.brat.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Robinson MS, Alloy LB. Negative Cognitive Styles and Stress-Reactive Rumination Interact to Predict Depression: A Prospective Study. Cognitive Therapy & Research. 2003;27(3):275–292. [Google Scholar]
- Rude SS, Durham-Fowler JA, Baum ES, Rooney SB, Maestas KL. Self-report and cognitive processing measures of depressive thinking predict subsequent major depressive disorder. Cognitive Therapy and Research. 2010;34(2):107–115. [Google Scholar]
- Rude SS, Wenzlaff RM, Gibbs B, Vane J, Whitney T. Negative processing biases predict subsequent depressive symptoms. Cognition & Emotion. 2002;16(3):423–440. [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Vazquez C, Marker C, LeMoult J, Joormann J. Attentional disengagement predicts stress recovery in depression: An eye-tracking study. Journal of Abnormal Psychology. 2013;122(2):303–313. doi: 10.1037/a0031529. [DOI] [PubMed] [Google Scholar]
- Schaefer HS, Putnam KM, Benca RM, Davidson RJ. Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biological Psychiatry. 2006;60(9):974–986. doi: 10.1016/j.biopsych.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Seifritz E. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7(9):e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L, Levitan RD. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Archives of General Psychiatry. 2010;67(12):1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal ZV, Gemar M, Williams S. Differential cognitive response to a mood challenge following successful cognitive therapy or pharmacotherapy for unipolar depression. Journal of Abnormal Psychology. 1999;108(1):3–10. doi: 10.1037//0021-843x.108.1.3. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Archives of General Psychiatry. 2006;63(7):749–755. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams J, Teasdale J, Gemar M. A cognitive science perspective on kindling and episode sensitization in recurrent affective disorder. Psychological Medicine. 1996;26(2):371–380. doi: 10.1017/s0033291700034760. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York: Guilford Press; 2002. [Google Scholar]
- Seligman ME, Abramson LY, Semmel A, Von Baeyer C. Depressive attributional style. Journal of Abnormal Psychology. 1979;88(3):242–247. doi: 10.1037//0021-843x.88.3.242. [DOI] [PubMed] [Google Scholar]
- Shea MT, Yen S. Personality traits/disorders and depression: A summary of conceptual and empirical findings. In: Rosenbluth M, Kennedy SH, Bagby RM, editors. Depression and Personality: Conceptual and Clinical Challenges. Arlington, VA, US: American Psychiatric Publishing, Inc; 2005. pp. 43–64. [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74(6):1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Smith JM, Alloy LB. A roadmap to rumination: A review of the definition, assessment, and conceptualization of this multifaceted construct. Clinical Psychology Review. 2009;29(2):116–128. doi: 10.1016/j.cpr.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TW, Ingram RE, Roth DL. Self-focused attention and depression: Self-evaluation, affect, and life stress. Motivation and emotion. 1985;9(4):381–389. [Google Scholar]
- Sullivan MJ, D’Eon JL. Relation between catastrophizing and depression in chronic pain patients. Journal of Abnormal Psychology. 1990;99(3):260–63. doi: 10.1037//0021-843x.99.3.260. [DOI] [PubMed] [Google Scholar]
- Talati A, Weissman MM, Hamilton SP. Using the high-risk family design to identify biomarkers for major depression. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2013;368(1615):20120129, 1–11. doi: 10.1098/rstb.2012.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD. Emotional processing, three modes of mind and the prevention of relapse in depression. Behaviour Research and Therapy. 1999;37:S53–S77. doi: 10.1016/s0005-7967(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Cox SG. Dysphoria: self-devaluative and affective components in recovered depressed patients and never depressed controls. Psychological Medicine. 2001;31(07):1311–1316. doi: 10.1017/s003329170100424x. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, Segal ZV. Metacognitive awareness and prevention of relapse in depression: empirical evidence. Journal of Consulting and Clinical Psychology. 2002;70(2):275–287. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Todd RM, Cunningham WA, Anderson AK, Thompson E. Affect-biased attention as emotion regulation. Trends in cognitive sciences. 2012;16(7):365–372. doi: 10.1016/j.tics.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Trope Y, Liberman N. Construal-level theory of psychological distance. Psychological Review. 2010;117(2):440–463. doi: 10.1037/a0018963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijsbergen G, Bockting C, Burger H, Spinhoven P, Koeter M, Ruhé H, Schene A. Mood Reactivity Rather Than Cognitive Reactivity Is Predictive of Depressive Relapse: A Randomized Study With 5.5-Year Follow-Up. Journal of Consulting & Clinical Psychology. 2013;81:508–517. doi: 10.1037/a0032223. [DOI] [PubMed] [Google Scholar]
- Van Vugt MK, Hitchcock P, Shahar B, Britton W. The effects of mindfulness-based cognitive therapy on affective memory recall dynamics in depression: a mechanistic model of rumination. Frontiers in human neuroscience. 2012;6:257, 1–13. doi: 10.3389/fnhum.2012.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Wadlinger HA, Isaacowitz DM. Looking happy: the experimental manipulation of a positive visual attention bias. Emotion. 2008;8(1):121–126. doi: 10.1037/1528-3542.8.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, Isaacowitz DM. Fixing our focus: training attention to regulate emotion. Pers Soc Psychol Rev. 2011;15(1):75–102. doi: 10.1177/1088868310365565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E, Brown R. Rumination and executive function in depression: An experimental study. Journal of Neurology, Neurosurgery & Psychiatry. 2002;72(3):400–402. doi: 10.1136/jnnp.72.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E, Teasdale JD. Rumination and overgeneral memory in depression: effects of self-focus and analytic thinking. Journal of Abnormal Psychology. 2001;110(2):353–357. doi: 10.1037/0021-843x.110.2.333. [DOI] [PubMed] [Google Scholar]
- Wells A, Fisher P, Myers S, Wheatley J, Patel T, Brewin CR. Metacognitive therapy in recurrent and persistent depression: A multiple-baseline study of a new treatment. Cognitive Therapy and Research. 2009;33(3):291–300. [Google Scholar]
- Wells TT, Beevers CG. Biased attention and dysphoria: Manipulating selective attention reduces subsequent depressive symptoms. Cognition & Emotion. 2010;24(4):719–728. [Google Scholar]
- Williams JM, Healy D, Teasdale JD, White W, Paykel ES. Dysfunctional attitudes and vulnerability to persistent depression. Psychol Med. 1990;20(2):375–381. doi: 10.1017/s0033291700017694. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120(1):3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yamano M, Shimada H, Ichikawa K, Nakaya M. The specificity of cognitive reactivity in recurrent major depressive episodes. The Japanese Journal of Psychology. 2014;85(1):29–39. doi: 10.4992/jjpsy.85.29. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Joormann J. Is timing everything? Sequential effects of rumination and distraction on interpersonal problem solving. Cognitive Therapy and Research. 2012;36(3):165–172. [Google Scholar]
- Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Okada G, Kunisato Y, Yamawaki S. Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Social Cognitive & Affective Neuroscience. 2014;9(4):487–493. doi: 10.1093/scan/nst009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, Yao S. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biological Psychology. 2011;88(2–3):233–242. doi: 10.1016/j.biopsycho.2011.08.007. [DOI] [PubMed] [Google Scholar]


