Abstract
Most smokers attempting to quit will relapse, even when using evidence-based cessation treatment. This illustrates the need for better understanding of the relapse process to thereby improve cessation treatments. While the impact of stress sensitivity on relapse is clear, little research has precisely examined stress reactivity in addicted individuals. Further, most research on relapse focuses on affect surrounding self-administration, and doesn't address potentially important pre-consumption processes such as anticipation of use. We examined the effects of anticipation and actual smoking on stress reactivity in 34 deprived smokers withdrawn for 24 hours and 37 non-deprived smokers, with 37 non-smoker controls. Using a cued shock stressor task, we measured stress reactivity via startle potentiation and self-reported anxiety. After completing the task once, smokers anticipated smoking a cigarette resting in front of them while they completed the task a second time. Smokers then smoked before completing the task a third and final time. Non-smokers anticipated and drank water as a control. Anticipation of smoking significantly attenuated both startle potentiation and self-reported anxiety to shock cues for deprived smokers relative to non-deprived smokers. Smokers' stress reactivity was not reduced by smoking beyond the prior effect of anticipation. These results suggest that anticipation, rather than actual drug consumption, may drive the primary reinforcing effect of reduced stress reactivity in smoking. Future research is needed to understand this effect of anticipation on drug use and determine whether anticipation would make an effective intervention target for addiction and other psychopathology which exhibits increased stress sensitivity.
Keywords: Stress reactivity, Stress sensitivity, Addiction, Anticipation of drug use, Smoking, Startle potentiation
It has been 50 years since the 1964 U.S. Surgeon General's report on the negative health consequences of smoking. Still, tobacco-related illnesses continue to cause the death of more than 480,000 annually in the United States of America alone (National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health, 2014). While the majority of smokers want to quit smoking and more than half try to quit (Centers for Disease Control and Prevention, 2011), relapse remains the most common outcome, even for smokers who used evidence-based cessation treatment (Fiore et al., 2008). In order to develop more effective cessation treatments, we need a better understanding of the cascade of cognitive and affective processes that both precede and ultimately result in relapse. Such understanding could provide unique insight into the relapse process which could be used to improve smoking cessation treatment as well as treatments for other drug addictions.
Many addiction models suggest that stress plays an important role in smoking and other drug use (Kassel, Stroud, & Paronis, 2003). For instance, human-animal translational research has indicated that chronic drug use leads to adaptations in affect-related brain structures resulting in increased stress sensitivity which may manifest as exaggerated negative affect in response to stressors when in withdrawal (Hefner, Moberg, Hachiya, & Curtin, 2013; Koob & Volkow, 2010). Consistent with translational research, negative reinforcement models of addiction posit that alleviation of negative affect is a primary motivator for drug use (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). Individual experiences also support the importance of stress sensitivity in maintaining addiction as alleviation of the negative affective component of stress is commonly reported as a primary motive for smoking or other drug use (Novak, Burgess, Clark, Zvolensky, & Brown, 2003; Parrott, 1999). Furthermore, both animal and human data have led to models of “stress-induced relapse” which posit that the presentation of discrete, acute stressors motivates individuals in abstinence to again use drugs perhaps due to the sensitivity these individuals have to stressors (Bossert, Marchant, Calu, & Shaham, 2013; Koob & Volkow, 2010; Sinha, 2001).
Despite clear demonstration of increased sensitivity to stressors, stress-induced relapse and smokers' beliefs that smoking will reduce their negative affect, direct effects of smoking on stress responses have been difficult to demonstrate in the laboratory (Kassel et al., 2003). Our study was designed to explore two potential reasons for this inconsistency. First, there is often a lack of nuance in the experimental measurement of stress reactivity in addiction as many studies have relied on complicated stressors and/or measures of stress which do not take advantage of recent findings on neural mechanisms afforded by animal-human translational stress/addiction research (Curtin & Lang, 2007; Kassel et al., 2003; Perkins, Karelitz, Conklin, Sayette, & Giedgowd, 2010). Second, the experimental study of stress response in addiction is usually limited to the impact of stressors on actual consumption and vice versa, despite the potential importance of stress reactivity in relation to other important drug use processes, such as anticipation of drug use.
Our research takes advantage of translational methods used to study the central nervous system (CNS) component of the stress response by using potentiation of the defensive startle reflex to cued threat of electric shock (Bradford, Shapiro, & Curtin, 2013; Davis, 2006; Hefner et al., 2013; Hogle, Kaye, & Curtin, 2010). The primary startle circuit is directly modulated through projections from some of the same brain structures implicated in neuroadaptation addiction models (e.g., the extended amygdala; Davis, 2006; Koob & Volkow, 2010). Startle potentiation is a direct measure of defensive reactivity to stressors (i.e., stress reactivity) which is congruent with negative affect and not simply arousal (Davis, 2006; Vaidyanathan, Patrick, & Cuthbert, 2009). The startle response is resistant to volitional control and less susceptible to responder bias than self-report measures of stress reactivity in addiction (Kassel et al., 2003). Our research also uses threat of shock which is a robust, discrete, and reliable stressor used to elicit negative affect across species thus providing a translational bridge for stress-addiction research (Engelmann, Radke, & Gewirtz, 2009; Hogle et al., 2010; Koob & Volkow, 2010).A few other studies have used startle potentiation to measure negative affect in responses to stressors (i.e., threat of electric shock)in smokers (Grillon, Avenevoli, Daurignac, & Merikangas, 2007; Hogle & Curtin, 2006; Hogle et al., 2010) but these have focused on the impact of drug deprivation and consumption on stress reactivity.
Stress-addiction research has traditionally focused on connections between stress and consumption of the drug thus bypassing any processes that lead up to that consumption. Relapse is a process with identified components (e.g., initial cessation, lapse, relapse; Shiffman et al., 2006). However it is possible to further parse a single lapse event into sub-components. For example, recent work using drug “cue-availability” paradigms suggests that the period before imminent drug use may involve a host of cognitive, affective, and attentional changes (Carter & Tiffany, 2001; Robinson et al., 2014). We refer to this phenomenon immediately prior to drug use as “anticipation”. Anticipation of smoking could provide an ideal target for intervention because the smoker has not yet lapsed (i.e., succumbed to that first cigarette post-quit) – almost certainly the death knell in a cessation attempt (Kenford et al., 1994).
The goals of this study were to examine the effects of anticipation and subsequent smoking on stress reactivity in smokers. Specifically, we tested: 1) The effect of anticipation of smoking on stress reactivity among deprived smokers relative to non-deprived smokers; and 2) The effect of cigarette consumption on stress reactivity beyond anticipation of smoking among deprived smokers relative to non-deprived smokers. To examine these effects, smokers completed a stressor task at baseline, in anticipation of smoking and then after smoking. To control for extraneous variables such as anticipation of consumption broadly defined or other smoker characteristics, we ran an additional sample of non-smokers who simply anticipated then drank water. We measured participants' stress reactivity by measuring the potentiation of their defensive startle reflex during threat of shock using a modified version of the No-shock, Predictable-shock, Unpredictable-shock (NPU) cued threat task (Schmitz & Grillon, 2012).The NPU task allowed us to assess startle potentiation to threat generally as well as unpredictable and predictable subtypes since selective sensitivity to unpredictable threat is an important component of some models of drug addiction (Hefner et al., 2013; Hogle et al., 2010; Koob & Volkow, 2010). We also assessed self-reported anxiety to index participants' subjective stress reactivity to the threat cues (Bradford et al., 2013).
Method
Recruitment and screening
We recruited 84 daily smokers and 45 non-smokers from the greater Madison, WI community via newspaper, web, and TV advertisements. Following a phone screen, eligible potential participants attended an in-person session to further assess eligibility, learn about the study, provide written informed consent, and complete self-report measures of demographics and smoking history. Smokers also completed the Fagerström Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), a well-validated, 6-item dependence measure.
Eligible participants were: aged ≥ 18 years, able to read and write in English, not currently using psychiatric medication, and reported no current physical or psychological condition that would contraindicate participation in the threat of shock task (e.g., recent heart problems, anxiety disorders). Eligible smokers also had to report smoking ≥10 cigarettes/day for at least 1 year, no current participation in any smoking cessation program or treatment, as well as provide a screening session exhaled carbon monoxide (CO) level ≥ 10 ppm, (Hogle et al., 2010; Piper & Curtin, 2006). Nonsmokers (NS) had to report smoking <100 cigarettes in their lifetime, no current or past daily cigarette use, and provide a CO level < 10 ppm.
Smokers were stratified by gender and randomly assigned to either the deprived or the non-deprived smoker group. Deprived smokers abstained from all nicotine-containing products for 24 hours prior to their laboratory session while non-deprived smokers maintained their typical cigarette usage pattern prior to their laboratory session.
Experimental session
At the beginning of the experimental session, we measured CO level again to biochemically confirm self-reported abstinence (≤ 50% of screening CO level) among the deprived smokers. Deprived smokers who did not meet this criterion were rescheduled. If non-deprived smokers reported last smoking more than 30 min before the experimental session, we asked them to smoke a cigarette immediately prior to beginning the session to minimize withdrawal symptoms during the task. Smoking withdrawal symptoms were assessed prior to task start (Wisconsin Smoking Withdrawal Scale, WSWS; Welsch et al., 1999).
Participants' general startle reactivity to acoustic startle probes was assessed while they viewed a series of nine colored squares (threat cues) presented on a computer screen for 5 s each with a variable inter-trial interval (ITI, 14–20 s, mean=17 s). Next we measured participants' subjective shock tolerance per standardized procedures from our laboratory (e.g., Curtin, Patrick, Lang, Cacioppo, & Birbaumer, 2001; Hogle et al., 2010; Moberg & Curtin, 2009). Participants reported their response to a series of increasing intensity, 200 ms duration, electric shocks (7 mA maximum) administered across the distal phalanges of the index and ring fingers of participants' left hand. We stopped the procedure once participants reached the maximum level of shock they could tolerate. We set shock intensity during the main task to each participant's subjective maximum tolerance threshold to minimize individual differences in shock sensitivity.
Participants completed the cued threat task three times (see Cued threat task for description). After the first run of the task, smokers were asked to take out one of their cigarettes and hold it while the experimenter set up some things in another room. Non-smokers were given a bottle of water to hold. After three minutes, the experimenter reentered the room, told the participant they would be able to smoke (or drink) after the next run of the task and placed the cigarette (or water) directly below the computer screen where it remained during completion of the second run of the task. Next, participants were escorted outside to either smoke as much as they wanted (or drink water). We measured amount of cigarettes smoked to the nearest ¼ of the original tobacco rod of each cigarette (see Table 1). Approximately 15 minutes later, participants completed the task for the final time. Immediately after each run of the task, participants rated how anxious they were when they saw the threat and non-threat cues on a 7 point rating scale (1=Not at all anxious; 7=Extremely anxious). We reimbursed all participants $20/hour for a total of approximately 4 hours in the laboratory and deprived participants an additional $50 for adherence to the tobacco deprivation criterion.
Table 1. Descriptive statistics and manipulation checks for the sample.
| Deprived Smokers | Non-deprived Smokers | Non-Smokers | |
|---|---|---|---|
| Demographics | |||
| Total N | 34 | 37 | 37 |
| Female (%) | 47 | 51 | 51 |
| White (%) | 50 | 70 | 73 |
| High school or equivalent degree (%)S | 44 | 57 | 84 |
| Age | 43.2 (11.2) Range=23-68 |
42.1 (11.8) Range=21-65 |
38.9 (15.5) Range=19-67 |
|
| |||
| Screening session measures | |||
| Cigarettes per day | 17.1 (5.3) Range=10-30 |
18.1 (6.6) Range=10-40 |
- |
| Age of first cigarette | 15.3 (3.7) Range=9-23 |
14.1 (3.0) Range=9-23 |
- |
| Years smoking daily | 25.6 (10.6) Range=6-48 |
26.2 (11.1) Range=6-47 |
- |
| FTND | 5.50 (1.6) Range=2-9 |
5.43 (2.2) Range=0-9 |
- |
| Screening session CO (ppm)S | 19.0 (6.2) Range=10-39 |
20.0 (12.2) Range=10-62 |
2.1 (1.4) Range=0-7 |
|
| |||
| Experimental session measures | |||
| General startle reactivity (uV) | 54.6 (38.0) Range=7-143 |
52.6 (44.8) Range=5-193 |
66.3 (43.5) Range=5-183 |
| Experimental session CO (ppm)D | 3.6 (1.7) Range=1-8 |
18.4 (7.1) Range=10-40 |
- |
| WSWSD | 2.3 (0.5) Range=1-3 |
1.9 (.6) Range=1-3 |
- |
| Cigarettes during consumption | 0.9 (0.4) Range=0.25-2 |
0.9 (0.3) Range=0.25-2 |
- |
Note.
Descriptive statistics (mean and standard deviation unless otherwise noted) for manipulation checks for the entire final sample (N=108). However, data recording errors led to missing “cigarettes smoked during consumption” data and “Age of first cigarette” for 3 and 1 non-deprived smokers, respectively. Chi-squared tests were used for qualitative variables (e.g., gender). T tests were used for quantitative variables (e.g., CO level).
Dependent measures that displayed significant effects (p< .05) of deprivation (deprived vs. non-deprived smokers) are indicated with the superscript ‘D’.
Dependent measures that displayed significant effects (p < .05) of smoker status (non-deprived smoker vs. non-smoker) are indicated by the superscript ‘S’.
Cued threat task
Participants viewed a series of three colored square cues presented on a computer screen for 5 s each separated by a variable ITI (14–20 s, mean=17 s). These cues were presented in one of three block types: unpredictable shock blocks (U), predictable shock blocks (P), and no-shock blocks (N). A pre-block message on the monitor informed participants of the next block type. In unpredictable shock blocks, participants were instructed that electric shocks could be administered at any point during the block, both during the cues and in the ITI. A total of six shocks were administered across two unpredictable shock blocks (2 shocks at either 2 or 4.8 s post-cue onset and 4 shocks during the ITI at 3, 6, or 9 s post-cue offset). In the predictable shock blocks, participants were instructed that electric shocks would be administered only during the cues and that no shocks would ever be administered during the ITI. A total of six shocks were administered at 4.5 s post-cue onset across two predictable shock blocks (i.e., during every cue; 3 shocks in each block). Three no-shock blocks were included as a nonaversive control condition from which to calculate startle potentiation during cues in predictable and unpredictable shock blocks. In no-shock blocks, participants were instructed that no shocks would be administered either during the cues or the ITIs. There were two block orders counterbalanced across participants: UNPNPNU and PNUNUNP. Startle potentiation was calculated as the increase in startle response to probes during cues in the shock blocks relative to cues in the no-shock blocks. Self-reported anxiety was analogously calculated as the increase in anxiety to the shock cues relative to the no-shock cues.
Startle response measurement and data reduction
We used a bioamplifier (James Long Company) to sample electromyographic activity in the orbicularis oculi muscle at 1,000 Hz from electrodes placed under the right eye according to published guidelines (Blumenthal et al., 2005; van Boxtel, Boelhouwer, & Bos, 1998). We measured eyeblink startle response to 50 ms white noise probes at 102 dB with near instantaneous rise time. During the general startle reactivity task there were 6 noise probes during a subset of the visual cues at 3.5 or 4.5 s post cue onset. There were 14 noise probes during a subset of the cues in the threat of shock (8 probes; 4 for each threat type) and no-shock (6 probes) blocks at 3.5 or 4.5 s, post cue onset, with equal probable timing. We presented additional noise probes during the inter-trial intervals in the general startle reactivity (3 probes) and cued threat tasks (7 probes) to decrease probe predictability. A minimum of 13.5 s separated each probe from any previous startle eliciting event (i.e., another probe or electric shock). We matched serial position of the probes across block types within participants during the cued threat task.
Data reduction and processing followed published guidelines (Blumenthal et al., 2005). Specifically, offline processing included high pass filtering (4th order 28 Hz Butterworth filter), signal epoching (-50 – 250 ms period surrounding noise probe), rectification, and smoothing (4th order 30 Hz Butterworth low pass filter). Trials with greater than 40 μV deflections in the 50 ms pre-probe baseline were rejected as artifact (i.e., unstable baseline). We excluded 13 participants with <5 μV general startle reactivity (non-responders) during data processing. These participants did not differ from startle responders on any of the variables listed in Table 1 (ps > 0.05). We scored peak eyeblink response between 20 and 100 ms post-probe onset relative to mean 50 ms pre-probe baseline.
Analysis plan
Data were analyzed using R (R Development Core Team, 2014). We analyzed startle potentiation and self-reported anxiety during cue presentations in separate general linear models (GLM) each with a between subjects factor for smoking group (deprived smokers, non-deprived smokers, non-smokers) and repeated measures for task time (baseline, anticipation, consumption) and threat type (unpredictable, predictable). We followed up omnibus effects with planned contrasts using Fisher's LSD approach to protect against inflation of family-wise error (Kirk, 1995). We analyzed the smoking group factor using planned between subject contrasts to examine the effects of Deprivation (deprived smokers vs non-deprived smokers) and Smoker Status (non-deprived smokers vs non-smokers). We analyzed the task time factor using planned within-subject contrasts to examine the effects of Anticipation (anticipation of smoking vs. baseline) and Consumption (post-consumption vs anticipation of smoking) consistent with our research goals. We included an interactive between-subject regressor for general startle reactivity (mean centered) to control for individual differences in startle potentiation (Bradford, Kaye, & Curtin, 2014; Hogle et al., 2010; Schmitz & Grillon, 2012)1. We report raw GLM coefficients (b) and partial eta squared (ηp2) to document effect sizes.
Results
The final sample consisted of 34 deprived smokers, 37 non-deprived smokers and 37 non-smokers2. The three groups were comparable with respect to demographics and smoking variables, although nonsmokers were more educated than smokers (see Table 1). At the experimental session, deprived smokers reported significantly more withdrawal symptoms and provided significantly lower CO readings than non-deprived smokers (see Table 1).
The NPU task was successful in inducing stress as indicated by significant (non-zero) startle potentiation (b = 13.5, t(102) = 11.77, p < .001, ηp2 = .57) and significantly increased self-reported anxiety to threat cues (b = 2.3, t(102) = 11.96, p < .001, ηp2 = .58) across smoking groups, task times, and threat types (see Table 2).
Table 2. Startle potentiation by threat type, task time, and smoking group.
| Baseline | Anticipation | After Consumption | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Overall | U | P | Overall | U | P | Overall | U | P | |
| Deprived Smokers | 20.0 (3.2) | 19.9 (3.5) | 20.2 (3.9) | 9.1* (2.4) | 8.2 (2.8) | 10.0 (2.9) | 6.1 (2.2) | 7.2 (2.6) | 5.0 (2.7) |
| Non-deprived Smokers | 16.0 (3.1) | 14.7 (3.3) | 17.4 (3.7) | 15.5 (2.3) | 13.2 (2.7) | 17.7 (2.8) | 15.0 (2.1) | 11.4 (2.5) | 18.5 (2.6) |
| Non-Smokers | 14.9 (3.1) | 14.7 (3.4) | 15.2 (3.8) | 14.9 (2.4) | 14.8 (2.7) | 14.9 (2.8) | 9.6 (2.2) | 8.5 (2.5) | 10.7 (2.6) |
Note.
Means and standard errors for startle potentiation by threat type and task time adjusted for the interactive between-subject regressor for general startle reactivity (mean centered) for the entire final sample (N=108). U = Unpredictable shock; P = Predictable shock; Overall = (unpredictable shock + predictable shock)/2.
Denotes the significant effect (p < .05) of the planned contrast for Anticipation (anticipation of smoking vs. baseline).
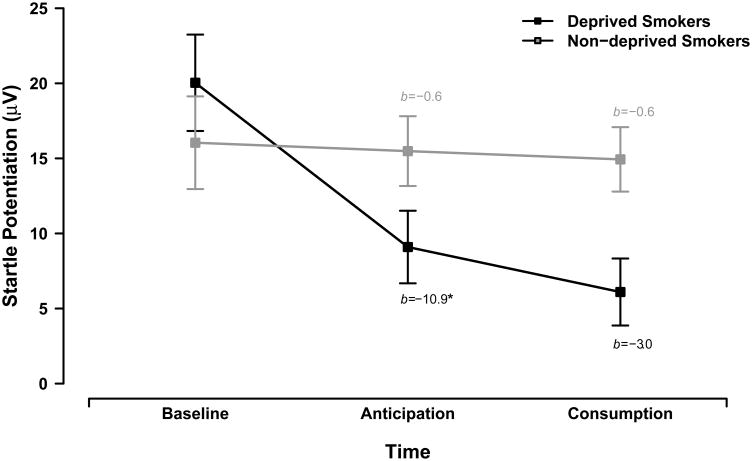
Analysis of startle potentiation revealed a significant smoking group × task time interaction, F(4,204) = 3.35, p = .011, ηp2 = .06. Follow up planned interaction contrasts revealed that anticipation of smoking had a greater dampening effect on startle potentiation for the deprived smokers than for the non-deprived smokers across threat types, b = -10.4, t(102) = 2.43, p = .017, ηp2 = .06. Follow-up tests revealed that anticipation significantly attenuated startle potentiation for deprived smokers, b = -10.9, t(102) = 3.55, p = .001, ηp2 = .11 but not non-deprived smokers, b = -0.6, t(102) = 0.19, p = .851, ηp2 < .01 across threat types, see Figure 1.
Figure 1. Startle potentiation by smoking group.
Lines display the effects of anticipation and consumption on startle potentiation for deprived smokers (black) and non-deprived smokers (gray). Error bars indicate +/- one standard error for point estimates of overall startle potentiation from the GLM. We report GLM coefficients for the simple effects of anticipation and consumption for each smoking group (* p < .001).
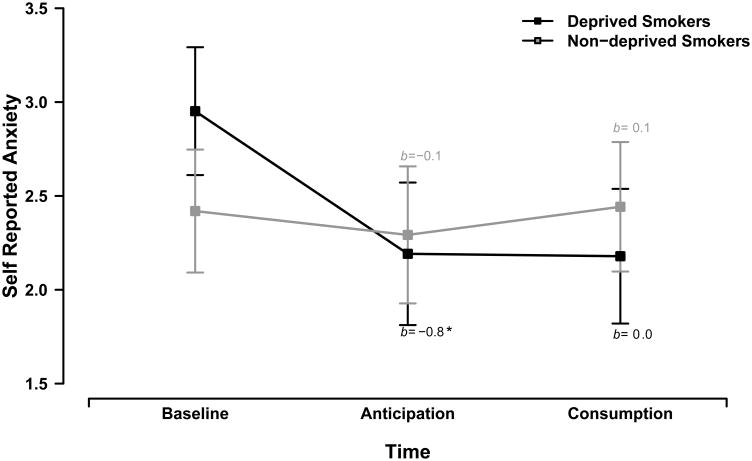
Analysis of self-reported anxiety also revealed a significant smoking group × task time interaction, F(4,204) = 2.44, p = .048, ηp2 = .05. Anticipation of smoking also had a greater dampening effect on self-reported anxiety for deprived smokers than for non-deprived smokers across threat types, b = -0.6, t(102) = 2.01, p = .047, ηp2 = .04. Follow-up tests revealed that anticipation significantly attenuated self-reported anxiety for deprived smokers, b = -0.8, t(102) = 3.34, p = .001, ηp2 = .10, but not non-deprived smokers across threat types, b = -0.1, t(102) = 0.58, p = .563, ηp2 < .01, see Figure 2.
Figure 2. Self-reported anxiety by smoking group.
Lines display the effects of anticipation and consumption on self-reported anxiety for deprived smokers (black) and non-deprived smokers (gray). Error bars indicate +/- one standard error for point estimates of mean self-reported anxiety from the GLM. We report GLM coefficients for the simple effects of anticipation and consumption for each smoking group (* p < .001).
As expected, no significant effects of smoker status (non-deprived smokers vs. non-smokers) were observed for startle potentiation or self-reported anxiety (ps > .05). In addition, there were no significant effects of the consumption contrast (consumption vs. anticipation) or threat type (uncertain vs. certain) on startle potentiation or self-reported anxiety (ps > .05).
Discussion
This experiment provided clear evidence that anticipation of smoking is sufficient to reduce stress reactivity to discrete stressors for smokers in withdrawal. This finding emerged from a task that used potent, discrete stressors (i.e., threat of electric shock) to elicit stress reactivity that was assessed objectively by a well-validated measure of the CNS modulated negative affective component of stress reactivity (i.e., startle potentiation). We assessed stress reactivity during three components of smoking as they would occur in the real world (withdrawal, anticipation, smoking), which adds ecological validity to the current results. In addition, the effects of anticipation on startle potentiation were mirrored in supplemental analyses of participants' self-reported anxiety to the cues, which further validates these findings and rules out many methodological/measurement explanations for this effect. The lack of anticipation or consumption effects in both non-deprived and non-smokers suggest the 24 hour deprivation manipulation drove these effects. Finally, anticipation of smoking dampened stress reactivity whether the threat of shock was predictable or unpredictable, which suggests that this effect is robust across various types of stressors that smokers may encounter in the real world. While some research shows selective effects of drug consumption or withdrawal on response to unpredictable threat, the broad effects of anticipation seen here may implicate distinct neurologically and/or psychological mechanisms from previous translational work which did not measure anticipation (e.g., Hefner et al., 2013).
The effects of anticipation of drug use on stress reactivity can be compared and contrasted to cue reactivity research which most often focuses on the emergence of cravings, changes in attention and increased motivation to smoke in the presence of smoking cues (Carter & Tiffany, 2001). Much of the cue reactivity work uses smoking cues which are not directly predictive of cigarette consumption (i.e., pictures of other people smoking) and thus may not activate the cognitive and affective processes which occur in preparation for and anticipation of smoking (Juliano & Brandon, 2002; Levin, Rose, Behm, & Caskey, 1991; Perkins et al., 2008). Our paradigm is more comparable to “cue-availability” paradigms in which smokers are informed that they will be able to smoke immediately after completing a task, however these tasks also often utilize pictures in lieu of more powerful manipulations (Droungas, Ehrman, Childress, & O'Brien, 1995; McBride, Barrett, Kelly, Aw, & Dagher, 2006; but see Carter & Tiffany, 2001). In our study, we provided a powerful manipulation of smoking anticipation by having participants' own cigarettes remain in sight during the anticipation run of the task. Furthermore, most availability studies have not measured response to discrete stressors while participants are simultaneously anticipating drug use as was done in this study.
The current findings suggest the need for more research in dissecting the cognitive-attentional and affective processes that lead up to a relapse (i.e., anticipation) and how such processes themselves may be rewarding by allowing a smoker to experience reduced stress and thus further perpetuated addiction. For instance, sensorimotor factors such as the sight, flavor or inhalation of the smoke become secondary reinforces for smoking due to their repeated pairings with smoking itself (Caggiula et al., 2001; Perkins et al., 2001; Perkins et al., 2008; Rose, Behm, Westman, & Johnson, 2000). Given that anticipation of smoking is frequently paired with smoking in a similar matter as sensorimotor cues, the rapid reduction in stress response triggered by anticipation suggests that anticipation of smoking may serve as another secondary reinforcer of smoking.
Distraction by the participant's cigarettes may also have contributed to our results. Recent research using cognitive tasks in availability paradigms have provided evidence that nicotine- deprived participants are more distracted by smoking-related cues (Juliano & Brandon, 1998; Robinson et al., 2014; but see Wertz & Sayette, 2001).Deprived smokers in our study may have been distracted by the cognitive processes involved in viewing their cigarettes or in thinking about smoking. Future research using non-cigarette distractors as well as cigarette cues that do not signal imminent smoking in our paradigm may clarify the contribution of distraction to the stress dampening effects of anticipation seen in our study.
Smokers' stress reactivity was not reduced by smoking beyond the prior effect of anticipation. This result is somewhat in contrast to smokers' report that consumption of cigarettes reduces their stress (Parrott, 1999). However, this research may illustrate the inability of self-report to distinguish the processes involved in daily smoking. In other words, it may be that smokers associate the anticipation and planning with self-administration as a single event and are unable to differentiate the effects of the components (Baker, Japuntich, Hogle, McCarthy, & Curtin, 2006). Research with Ecological Momentary Assessment during anticipation of smoking may be able to disentangle these processes in real world smoking (McCarthy, Piasecki, Fiore, & Baker, 2006; Piper et al., 2011). These findings are consistent with smoking cue reactivity studies that suggest that smoking cues alone have effects on smokers' mood when they believe they have consumed nicotine (Juliano & Brandon, 2002; Levin et al., 1991).
The present study narrowly focuses on the CNS component of the stress response. However, the stress response includes a complex interaction of CNS, peripheral nervous system (PNS) and hypothalamic-pituitary-adrenal (HPA) activations which produce changes in affect, arousal, and attention (McEwen, Eiland, Hunter, & Miller, 2012; McEwen, 2001; Sapolsky, 2002, 2003; Segerstrom & Miller, 2004). Recent translational models of addiction heavily emphasize changes in components of the CNS (e.g., the extended amygdala) after chronic drug use (Koob & Volkow, 2010). These changes are believed to manifest as an increase in sensitivity to stressors which may be reflected in humans by increases in negative affective response. In fact, work from our laboratory using similar methods as those used here has demonstrated increased sensitivity to various types of stressors in deprived smokers compared to non-deprived smokers (Hogle & Curtin, 2006; Hogle et al., 2010).It should be noted that we did not observe evidence of between subject differences in baseline consistent with putative stress neuroadaptations in this study. However, this may not be too surprising given that this study was not powered to detect such effects in only one run of the task (i.e., baseline). Nevertheless, our findings suggest that anticipation of smoking may have the potential to dampen the increased stress sensitivity previously seen in deprived smokers.
The affective and cognitive processes involved in drug use anticipation may represent an opportunity for intervention, prior to drug use. For example, if anticipation of smoking holds equal or greater reinforcing value than smoking itself due to smokers' expectancies or implicit conditioning processes, education about such effects may help smokers to better understand their addiction. Or, exposure therapy designed to weaken or break potential conditioned bonds between the cues, behaviors and cognitions leading up to smoking and the pharmacological effects thereof may be an effective intervention. Future research is needed to better understand mechanisms responsible for any role anticipation has in maintaining addiction and to develop interventions that target this potential relapse process (e.g., McKee, Weinberger, Shi, Tetrault, & Coppola, 2012).
Smokers in the present study were allowed to smoke as much as they wanted during the consumption manipulation yet they only smoked one cigarette, on average. This may raise concerns about whether there was sufficient dosing to reduce stress reactivity (Hogle & Curtin, 2006; Mueller, Mucha, & Pauli, 1998). While the current research did not allow us to examine dose-specific effects, the ad lib smoking manipulation likely reflected the amount that smokers would smoke in reaction to some stressors in the real world. Future research could clarify dose effects by directly manipulating amount smoked or time allowed to smoke while comparing the effects of anticipation and actual smoking.
Future research could also assess what effect standard pharmacotherapies such as nicotine replacement (NRT) or varenicline have on stress reactivity in paradigms which measure smoking anticipation using human laboratory models that have been developed for screening interventions (McKee et al., 2012). While NRT and varenicline have been shown to be effective in reducing post-quit negative affect (Bolt, Piper, Theobald, & Baker, 2011; Cinciripini et al., 2013; Piper et al., 2008), it remains to be seen what effect they have on stress reactivity during anticipation of smoking. Of course, no current smoking cessation treatment is effective for all smokers (Fiore et al., 2008). Similarly, the effects seen here may not hold for all smokers. Smokers in this study were moderately dependent. Further work must be done to assess the effects of smoking anticipation on non-dependent smokers (i.e., novice or non-daily smokers) or more dependent smokers as they may exhibit even greater stress sensitivity. It is also important to explore these effects among smokers who may have increased sensitivity for other reasons (e.g., those with comorbid anxiety) who were excluded from this study.
In sum, the current research illustrates the importance of anticipation in reducing stress responses when a smoker is in withdrawal. These findings suggest that anticipation, rather than actual drug consumption, may drive the primary reinforcing effect of reduced stress responses in the context of drug use. The effects of anticipation should be further explored across different samples and using different levels of analysis of stress reactivity. It is possible that the stress dampening effects of anticipation of use may generalize to other drug dependence. For example, alcohol consumption dampens startle potentiation to threat of shock in social drinkers (e.g., (Bradford et al., 2013; Hefner et al., 2013; Moberg & Curtin, 2009), but it remains to be seen whether anticipation of drinking ameliorates stress responses in alcoholics. It is also remains to be seen if the effects reported here manifest in indices of stress reactivity other than startle potentiation and self-report (e.g., direct measurement of HPA activity). Confirmation of this would inform research on the complex interplay between various subcomponents of the stress response system. More broadly, the anticipation effect seen for smoking could generalize to anticipation of other adaptive and maladaptive stress relieving behaviors (e.g., eating, compulsions). If so, cognitive behavioral treatments for various clinical disorders may benefit by incorporating anticipation of behavior as a target of intervention.
Acknowledgments
The research was funded by University of Wisconsin start-up funds and NIH grant 1KL2RR025012 for Megan E. Piper and NIH grant R01 DA033809 for John J. Curtin.
Footnotes
We tested for sex differences in preliminary analyses. However, no interactions involving sex were significant so sex was removed from the final reported GLM.
We removed from analysis an additional 4 deprived, 2 non-deprived and 2 non-smokers that were identified as regression outliers (i.e., studentized residual with Bonferroni corrected p <.05) in preliminary GLM analyses on startle potentiation.
References
- Baker T, Japuntich SJ, Hogle JM, McCarthy DE, Curtin JJ. Pharmacologic and behavioral withdrawal from addictive drugs. Current Directions in Psychological Science. 2006;15(5):232–236. doi: 10.1111/j.1467-8721.2006.00442.x. [DOI] [Google Scholar]
- Baker T, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, Theobald WE, Baker T. Why two smoking cessation agents work better than one: Role of craving suppression. Journal of Consulting and Clinical Psychology. 2011;80(1):54–65. doi: 10.1037/a0026366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229(3):453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford DE, Kaye JT, Curtin JJ. Not just noise: individual differences in general startle reactivity predict startle response to uncertain and certain threat. Psychophysiology. 2014;51(5):407–411. doi: 10.1111/psyp.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford DE, Shapiro BL, Curtin JJ. How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychological Science. 2013;24(12):2541–2549. doi: 10.1177/0956797613499923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacology, Biochemistry, and Behavior. 2001;70(4):515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001a;9(2):183–190. doi: 10.1037/1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental & Clinical Psychopharmacology. 2001b;9(2):183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Quitting smoking among adults--United States, 2001-2010. MMWR Morbidity and Mortality Weekly Report. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, et al. Wetter DW. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry. 2013;70(5):522–533. doi: 10.1001/jamapsychiatry.2013.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JJ, Lang AR. Alcohol and emotion: Insights and directives from affective science. Emotion and Psychopathology: Bridging Affective and Clinical Science. 2007;8:191–213. [Google Scholar]
- Curtin JJ, Patrick CJ, Lang AR, Cacioppo JT, Birbaumer N. Alcohol affects emotion through cognition. Psychological Science. 2001;12(6):527–531. doi: 10.1111/1467-9280.00397. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61(8):741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O'Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addictive Behaviors. 1995;20(5):657–673. doi: 10.1016/0306-4603(95)00029-C. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Radke AK, Gewirtz JC. Potentiated startle as a measure of the negative affective consequences of repeated exposure to nicotine in rats. Psychopharmacology. 2009;207(1):13–25. doi: 10.1007/s00213-009-1632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker T, Bailey WC, Benowitz NL, Curry SJ, et al. Goldstein MG. A clinical practice guideline for treating tobacco use and dependence: 2008 update A U S Public Health Service report. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- Grillon C, Avenevoli S, Daurignac E, Merikangas KR. Fear-potentiated startle to threat, and prepulse inhibition among young adult nonsmokers, abstinent smokers, and nonabstinent smokers. Biological Psychiatry. 2007;62:1155–1161. doi: 10.1016/j.biopsych.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstroem Test for Nicotine Dependence: A revision of the Fagerstroem Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hefner KR, Moberg CA, Hachiya LY, Curtin JJ. Alcohol stress response dampening during imminent versus distal, uncertain threat. Journal of Abnormal Psychology. 2013;122(3):756–769. doi: 10.1037/a0033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43(4):344–356. doi: 10.1111/j.1469-2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Kaye JT, Curtin JJ. Nicotine withdrawal increases threat-induced anxiety but not fear: Neuroadaptation in human addiction. Biological Psychiatry. 2010;68(8):687–688. doi: 10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: evidence with urge and reaction time. Experimental and Clinical Psychopharmacology. 1998;6(1):45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. Journal of Abnormal Psychology. 2002;111(1):88–97. doi: 10.1037//0021-843x.111.1.88. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker T. Predicting Smoking Cessation. JAMA: The Journal of the American Medical Association. 1994;271(8):589–594. doi: 10.1001/jama.1994.03510320029025. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental design: Procedures for the behavioral sciences. Pacific Grove, CA: Brooks/Cole; 1995. Multiple Comparison Tests; pp. 90–133. [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology Reviews. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rose J, Behm F, Caskey NH. The effects of smoking-related sensory cues on psychological stress. Pharmacology, Biochemistry, and Behavior. 1991;39(2):265–268. doi: 10.1016/0091-3057(91)90177-4. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2006;31(12):2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker T. Life Before and After Quitting Smoking: An Electronic Diary Study. Journal of Abnormal Psychology. 2006;115(3):454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- McEwen BS. From molecules to mind. Stress, individual differences, and the social environment. Ann N Y Acad Sci. 2001;935:42–9. [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62(1):3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and Validating a Human Laboratory Model to Screen Medications for Smoking Cessation. Nicotine & Tobacco Research. 2012 doi: 10.1093/ntr/nts090. nts090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable vs. predictable threat. Journal of Abnormal Psychology. 2009;118(2):335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller V, Mucha RF, Pauli P. Dependence on smoking and the acoustic startle response in healthy smokers. Pharmacology Biochemistry and Behavior. 1998;59(4):1031–8. doi: 10.1016/s0091-3057(97)00508-x. [DOI] [PubMed] [Google Scholar]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK179276/ [PubMed] [Google Scholar]
- Novak A, Burgess ES, Clark M, Zvolensky MJ, Brown RA. Anxiety sensitivity, self-reported motives for alcohol and nicotine use, and level of consumption. Journal of Anxiety Disorders. 2003;17(2):165–180. doi: 10.1016/S0887-6185(02)00175-5. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does cigarette smoking cause stress? The American Psychologist. 1999;54(10):817–20. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- Perkins K, Ciccocioppo M, Conklin C, Milanak ME, Grottenthaler A, Sayette MA. Mood influences on acute smoking responses are independent of nicotine intake and dose expectancy. Journal of Abnormal Psychology. 2008;117(1):79–93. doi: 10.1037/0021-843X.117.1.79. [DOI] [PubMed] [Google Scholar]
- Perkins K, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2001;3(2):141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Perkins K, Karelitz JL, Conklin C, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biological Psychiatry. 2010;67(8):707–714. doi: 10.1016/j.biopsych.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Curtin JJ. Tobacco withdrawal and negative affect: An analysis of initial emotional response intensity and voluntary emotion regulation. Journal of Abnormal Psychology. 2006;115(1):96–102. doi: 10.1037/0021-843X.115.1.96. [DOI] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, Baker T. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. Journal of Abnormal Psychology. 2008;117:94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, et al. Baker T. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology. 2011;216(4):569–578. doi: 10.1007/s00213-011-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. Retrieved from http://www.R-project.org. [Google Scholar]
- Robinson JD, Engelmann JM, Cui Y, Versace F, Waters AJ, Gilbert DG, et al. Cinciripini PM. The Effects of Nicotine Dose Expectancy and Motivationally Relevant Distracters on Vigilance. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2014 doi: 10.1037/a0035122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J, Behm F, Westman E, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacology, Biochemistry, and Behavior. 2000;67(1):71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Endocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral endocrinology. 2nd. MIT Press; 2002. pp. 409–450. [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochemical Research. 2003;28(11):1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nature Protocols. 2012;7(3):527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology. 2006;74(2):276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Cuthbert BN. Linking dimensional models of internalizing psychopathology to neurobiological systems: Affect-modulated startle as an indicator of fear and distress disorders and affiliated traits. Psychological Bulletin. 2009;135(6):909–942. doi: 10.1037/a0017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boxtel A, Boelhouwer AJW, Bos AR. Optimal EMG signal bandwidth and interelectrode distance for the recording of acoustic, electrocutaneous and photic blink reflexes. Psychophysiology. 1998;35(6):690–697. [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker T. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychol Addict Behav. 2001;15(3):268–71. [PMC free article] [PubMed] [Google Scholar]