Abstract
Population-based data have documented a continued increase in NTM prevalence since 2000. Annual prevalence in North American and Australia ranges from 3.2–9.8 per 100,000, and is generally higher than in Europe. Studies of NTM PD from South Korea, Japan, and Taiwan, also suggest increasing prevalence. In Africa and the Middle East, prevalence of NTM ranges from 4% –15% among suspected TB cases and 18%–20% among suspected multi-drug resistant TB (MDR TB) cases. M. avium complex (MAC) is predominant in North America and East Asia, whereas M. kansasii, M. xenopi, and M. malmoense are more common in Europe. Host factors important to the current epidemiology of NTM PD include thoracic skeletal abnormalities, rheumatoid arthritis, and use of immunomodulatory drugs. Clustering of disease within families suggests a heritable genetic predisposition to disease susceptibility. Warm, humid environments with high atmospheric vapor pressure contribute to population risk.
Keywords: epidemiology, nontuberculous mycobacteria, pulmonary disease, global
Introduction
In this chapter we review approaches to studying the epidemiology of NTM pulmonary disease (NTM PD), and update advances in the field since the last review published in 2002 (1).
Methodologic challenges
Studying the epidemiology of NTM PD presents several methodologic challenges that impact the measures obtained. First, with a few exceptions (2), in most countries, NTM PD is not a reportable condition, such that describing the burden, trends, and associated risk factors for this condition depend on special studies, surveys, and sentinel surveillance efforts described later in this chapter. Secondly, isolation from an uncontaminated clinical specimen is insufficient to document disease, because respiratory secretions from patients with underlying lung disease may be colonized with these organisms without overt untoward manifestations For this reason, clinical information is required in concert with microbiologic data to be certain whether disease is present in an individual, although reliance on solely microbiologic information has recently been shown to provide a reasonable approximation (3–5). The diagnosis comprises three components, including microbiologic, radiographic, and clinical criteria (6). These criteria require that a patient with symptoms seek health care, that the health care provider suspects a diagnosis, that appropriate samples are obtained (sputum or lung biopsy), and that radiographic imaging is performed. Finally, the disease is often indolent (slowly progressive) and chronic, and patients may not be routinely evaluated with appropriate samples throughout their disease course. Often patients may have difficulty producing sputum samples, which limits the ability to obtain appropriate samples. In one study of NTM, 40–70% of patients were cultured in only a single year during a study period spanning 5–10+ years (3).
Measures of disease burden
For a chronic disease such as NTM PD, prevalence is the best measure of disease burden in a population. Incidence is a measure of the frequency of new infections and is the best measure for identifying risk factors for disease. Defining incidence, the frequency of new cases occurring in a defined population over a specified time period, requires definition of a time period when patients were disease-free. Because the disease is often indolent and samples for microbiologic analysis may not be taken, this disease-free risk window may not be clear.
Prevalence is a measure of the frequency of all cases existent in a defined population, newly or previously diagnosed. Two measures of prevalence have been used to define the NTM PD burden in a population: average annual prevalence and period prevalence (3, 4, 7, 8). The average annual prevalence is defined by averaging the annual number of cases over a defined period and dividing by the average annual population over that time period. The period prevalence is defined as the total number of cases existent over a defined period divided by the average population in that time period. For a longer time period, for example over 5–10 years, the period prevalence estimates will always be greater than an average annual population estimate because for the former, cases are summed over multiple years, so that cases that are identified only once during a period of interest are included, whereas for an annual prevalence, the same case occurring over multiple years would be counted only in years in which multiple isolates are obtained, despite ongoing chronic disease.
Although mortality data are suboptimal given the uncertain validity of death certificate coding for NTM PD as a cause of death, nonetheless, such data have been used in two instances to provide a national picture of the epidemiology of NTM. In the United States, mortality patterns mirrored prevalence data with respect to time, place, and person (9). In Japan, mortality data were useful for providing insight into patterns by age, sex, and region, and for to estimating prevalence (10).
Risk factors: approaches
To more fully understand the population patterns and trends for NTM PD, both host and environmental risk factors must be considered, as both contribute to disease patterns. Because these organisms are widespread in soil and water in most countries, and yet disease is rare, host susceptibility likely plays a key role. The role of inherited genetic factors can be studied by both traditional and modern genetic methods. Traditional methods include pedigree analysis to indicate clustering of NTM PD and related traits within families (11, 12). Future studies can include approaches such as whole exome sequencing or candidate gene approaches to identify variants associated with disease or severe disease, similar to the work that has been done for CF to identify genetic modifiers of disease (13). For studies involving genetic sequencing, as with any control group, the comparison population should be as similar as possible to the case group except with respect to the factor under study. Characterization of HLA types has also been used to identify genetic variants associated with NTM disease (14, 15).
An area of great concern has been the role of specific environmental exposures to soil and water, and particularly to water aerosols from showers and baths (16). One approach to studying this has been to design case-control studies with detailed ascertainment of these exposures. However, the measurement of individual behaviors is limited by recall bias as well as the unknown incubation period (time between exposure and disease onset). In addition, these studies are limited to some degree by the lack of knowledge regarding infectious dose for NTM PD, which would help guide the detail needed for exposure ascertainment. An alternative and more recent approach has been to study these factors using spatial analysis which allows detection of disease clusters and analysis of the association of these clusters with atmospheric and other environmental conditions (2, 17). Because NTM are environmental organisms, an analytic approach that seeks to relate population patterns to environmental conditions may be a better approach, as these factors act beyond the individual (i.e. influencing mycobacterial grown in and near bodies of water), in a truly ecologic fashion.
Host and environmental risk factors: findings
Host and environmental risk factors identified to date are summarized in Table 1. Case-control studies which have attempted to identify environmental and host factors associated with disease have had somewhat inconsistent results, likely due to both methodologic and population differences. One study in the United States which measured exposure to both soil and water aerosol as well as host factors found a number of host factors with significant associations with disease, whereas few to no environmental exposures were identified as risk factors (18). In the latter study, the only important protective factors identified were any swimming pool use (indoor or outdoor) and washing dishes by hand. However, these effects were felt to be likely due to a bias whereby healthier patients are more likely to engage in these activities (18). In another case-control study of the risk of environmental exposures for NTM among persons with CF, indoor swimming pool use at least monthly was significantly associated with NTM infection (19) (Table 1). One study in Japan found that exposure to soil more than twice weekly was significantly associated with disease (20).
Table 1.
Risk factors for NTM infection and disease
| Risk Factor | Relative risk, Odds ratio, or Relative Prevalence |
Disease or Infection |
|---|---|---|
| Environmental- individual exposures | ||
| Soil exposure | 5.9 (20) | Disease |
| Indoor swimming pool use (in the past four months) | 5.9 (1.3, 26.1) (19) | Infection |
| Swimming pool use at least once per month (indoor or outdoor, over the past five years) | 0.15 (0.04–0.67)(18) | Disease |
| Host factors | ||
| Lung cancer (neoplasms of larynx, trachea, and bronchus) | 3.4 (7) | Disease |
| COPD | 2–10 (7) (18) (27) | Disease |
| Bronchiectasis | 44–187.5 (7) (22) | Disease (coding) (7) Disease (validated microbiologic surrogate) (20) |
| Thoracic skeletal abnormalities | 5.4 (18) | Disease |
| Low body weight | 9.09* (18) | Disease |
| Rheumatoid arthritis | 1.5 (7),1.9# (25) – undefined (26) | Disease |
| Immunomodulatory drugs/anti-TNF agents | 1.3- Undefined (OR=infinity) (18) anti-TNF agents 2.2 (79) Others 1.6–2.9 (79) |
Disease |
| Steroid use | 8 (18) 1.6 (79) |
Disease |
| Gastro-esophageal reflux disease | 5.3# (27) 1.5* (25) |
Disease |
| Environment- climatic and population factors | ||
| Proportion of area as surface water | 4.6 (17) | Disease |
| Mean daily potential evapotranspiration | 4.0 (17) | Disease |
| Copper soil levels, per 1 ppm increase | 1.2 (1.0,1.2) (17) | Disease |
| Sodium soil levels, per 0.1 ppm increase | 1.9 (1.2,2.9) (17) | Disease |
| Manganese soil levels, per 100 ppm increase | 0.7 (0.4, 1.0) (17) | Disease |
| Increased average topsoil depth | 0.87 (M. intracellulare) (2) | Disease |
| Soil bulk density | 1.8 (M. kansasii) (2) | Disease |
Estimated from data in paper
Hazard ratio, fully adjusted for age, sex, income, rurality, and comorbidities for NTM (HIV, COPD, asthma, and GERD)
Comparison of patients with NTM/TB with uninfected anti-TNF users
Medical host factors are also detailed in Table 1. When measures of association are available, these are presented; our focus was on comparisons to non NTM (or TB) infected populations. Historically NTM was associated with neoplasms as well as structural lung diseases such as COPD (1), and these underlying diseases remain important predisposing factors for NTM (Table 1). The population affected by COPD has tended to be predominantly male, which reflected to a large degree patterns of smoking in the population. In countries where smoking remains prevalent, COPD remains an important predisposing risk factor, and will be detailed later in this chapter in the regional sections. Persons with cystic fibrosis are disproportionately affected by NTM, with a national prevalence estimated at 13% (21), and this topic will be covered in a separate chapter. In a nationally representative sample of older adults in the US, bronchiectasis was also strongly associated with NTM PD, although this condition was more prevalent among women with NTM PD than men with NTM PD (7). Bronchiectasis was also found to be strongly associated with NTM lung disease in a nation-wide study in Denmark, with an odds ratio of 187.5 (22).
In recent decades, coincident with a major increase in NTM, a predominance of women without known risk factors has been observed (23). In this population, the “idiopathic” NTM PD, women with a tall, thin morphotype predominate (24); recently identified risk factors include thoracic skeletal abnormalities and low BMI (18). Use of immunomodulatory drugs or steroids are also associated with NTM PD (18), as is rheumatoid arthritis per se, with risk increasing further in the presence of more potent immunosuppressive therapy (25, 26). (Table 1). No consistent association with diabetes and NTM PD has been found (7, 18, 27).
Although individual factors predispose for disease, an interaction with environmental conditions clearly exists (28). In the United States, both M. intracellulare skin-test sensitization (29) and NTM PD are more prevalent in the warmer more humid areas of the Southeast and Southwest. In Japan, NTM PD mortality, a surrogate measure for disease prevalence and distribution, has found a higher prevalence in the warmer and more humid coastal areas and in an area with a large lake. Recent studies have used geospatial approaches to identify climatic factors which influence the population risk of NTM PD. Two studies in the United States have identified atmospheric factors predictive of disease prevalence or disease clustering in a geographic area (Table 1). Adjemian et al found that evapotranspiration (the potential of the atmosphere to absorb water), and the proportion of the area as surface water was predictive of disease clustering in an area, and both studies found that vapor pressure (a measure of the water in the atmosphere at a given temperature) was predictive of disease prevalence among CF patients (19, 28). These population findings are consistent with an earlier environmental microbiology study which found that warmer temperature, low pH, low dissolved oxygen, high soluble zinc, high humic acid, and high fulvic acid are related to the environmental prevalence of NTM (30). In Queensland, Australia, in one of the few epidemiologic studies to evaluate environmental factors by species, increased average topsoil depth was protective against M. intracellulare, while increased soil bulk density was positively associated with M. kansasii disease (Table 1) (31).
Prevalence and incidence
In areas where NTM is reportable such as the province of Queensland, Australia (2), data based on isolates linked to clinical information provide a representative pattern of disease by time, place, and person. Sentinel surveillance comprises a single site or sample of sites which voluntarily report data on isolates, with a standard set of clinical information collected for patients. If the surveillance network is nationally representative, this system can provide good prevalence estimates. One example is the mycobacterial study group in France, which reported data on NTM isolates and associated clinical information during a three year period (32).
Several other types of prevalence studies have been published. These include those limited to microbiologic isolation, those with microbiologic and limited clinical information, and those with complete clinical information (fully defined). For example, the NTM-Network of the European Trials group (NET) compiles species information from isolates throughout participating laboratories in Europe and elsewhere. A strength of this collaboration is the number of isolates and participating countries, and the data provide a snapshot of the species distribution across regions and countries (33). This measure would likely overestimate disease prevalence, as not all persons with one or more isolates will meet ATS disease criteria. Similarly, regional laboratories with complete capture of a defined geographic region and population, such as provincial laboratories in Canada (8), can also provide a representative picture of isolates.
When surveillance or regionally representative data are not available, other approaches can provide a representative picture. An ideal study type is one which has electronic laboratory data linked to clinical data and other diagnostic information in a defined population, such as that available for integrated health care delivery systems in the United States (3), or integrated health care systems in other countries. When laboratory data are not available, ICD9 codes provide a surrogate measure of diagnosis and are useful to estimating the prevalence of this rare condition from large administrative datasets such as Medicare (7). Estimates for the sensitivity of ICD9 codes vary from 27%–50% (3, 27). Although these codes underestimate the true prevalence of NTM, this bias is not likely to be systematic, and therefore these codes provide an important tool for estimating prevalence by demographic features and comorbid conditions. Within these various approaches to estimating prevalence, study populations may be regionally representative (33), nationally representative (7), or representative of an area or state within a country (3, 34).
Epidemiology of NTM Isolation and Disease by Global Region
In our review of epidemiological data of pulmonary NTM, we strive to include the highest quality data published since 2000. Ideal studies comprise population-based investigations including both clinical and microbiologic data, and encompassing an adequate temporal period to best capture the burden of infection in the population. We present reviews of the best available studies by global region.
North America
The study of pulmonary NTM epidemiology in North America has advanced substantially in recent years. Numerous studies that are either population-based or from epidemiologically relevant population sub-groups have been published, and several studies have provided estimates of period prevalence in addition to annual prevalence. We excluded studies focusing exclusively on hospitalized patients, or wherein case identification criteria were deemed inadequate. We included studies presenting data from a single institution for the assessment of relative species frequency and for information regarding population patterns including age, sex and pre-existing lung disease. Table 2 presents results regarding the frequency of NTM PD, while Table 3 presents results regarding relative frequency of different species among populations with NTM PD.
Table 2.
Studies of Rates of Pulmonary NTM Isolation and Disease by Region
| North America | |||||
| Location (dates) | Disease Definition |
Annual Rates*
(species frequency) |
Period Prevalence of Disease | ||
| Isolation | Disease | Period Duration | Prevalence | ||
| Oregon, U.S.A. (2005–6) (34)** | Microbiological | 12.7 | 5.6 | 2 years | 11.1 |
| Portland, Oregon, U.S.A. (2005–6)(4)** | Microbiological and clinical | NR | NR | 2 years | 8.6 (general population)*** 20.4 (age ≥50 years)*** MAC 87.5% M. abscessus / chelonae 6% |
| Beneficiaries of four integrated health care delivery systems in California, Colorado, Pennsylvania and Washington, U.S.A. (2004–6) (3)** | Microbiological results with ICD-9 coding with subset clinical review for validation | 11.8 (all sites combined) | 5.5 (all sites combined) | 3 years | <60 yr ~ 4 60–69 yr ~ 40 70–79 yr ~ 100 ≥80 yr ~ 220 |
| Ontario, Canada (1997–2007)(36)** | NA | 19.2 | NR | NA | NR |
| U.S.A.(nation-wide) Medicare beneficiaries (≥65 years of age) (1997–2007)(7) |
ICD-9 coding | NA | ~47 | ≤11 years | ≥65 yrs = 112 |
| Ontario, Canada (1998–2010)(8)** | Microbiological | 22.2 | 9.8 | 5 years | 41.3 (2006–2010) |
| Central and South America | |||||
| Location (dates) | Disease Definition |
Annual Rates (species frequency) |
Period Prevalence of Disease†† | ||
| Isolation† | Disease† | Period Duration | Prevalence† | ||
| Baixada Santista region, Sao Paulo, Brazil (2000–2005)(39) | Microbiological | Total 1.31 HIV negative 0.77 HIV positive 0.55 |
Total 0.25 | 6 years | 1.5 |
| Sao Jose do Rio Preto, Brazil (1996–2005) (40) | Microbiological | 5.3 | 1.0 | 10 years | 10 |
| Europe | |||||
| Location (dates) | Disease Definition | Annual Rates (species frequency) | Period Prevalence of Disease†† | ||
| Isolation | Disease |
Period Duration |
Prevalence | ||
| Southwest Ireland (1987–2000) (44) | Microbiological and clinical | 1.9 | 0.2 | NA | NR |
| Borders Region, Scotland (1992–2010) (50) | Microbiological; subset clinical | NR | 0.86 (1992–2004) 3.1 (2005–2010) |
NR | NR |
| Leeds, UK (1995–1999) (46) | Microbiological and clinical | 2.9 | 1.7 | 5 years | 8.5 |
| Denmark, nation-wide (1997–2008) (5) | Microbiological; subset clinical | 2.5 | 1.1 | 12 | 5–8 |
| Nijmegen-Arnhem region, Netherlands (1999–2005) (48) | Microbiological and clinical | 6.3 | 1.4 | 7 years | 9.8 |
| France, multi-site (2000–2002) (32) ‡ | Microbiological and clinical | MAC 0.4 | MAC 0.2 | 3 years | 0.6 |
| Crete, Greece (2000–2009) (49) | Microbiological; subset clinical | NR | 0.6 | NA | NR |
| France, multi-site (2001–2003)(45) ‡ | Microbiological and clinical | NR | 0.73 | 3 years | 2.2 |
| Central Greece (2004–2006) (47) | Microbiological and clinical | 7.0 | 0.7 | 3 years | 2.1 |
| Croatia, nation-wide (2006–2010) (51) | Microbiologic | 5.3 | 0.75 | ||
| Australia | |||||
| Location (dates) | Disease Definition |
Annual Rates*
(species frequency) |
Period Prevalence of Disease†† | ||
| Isolation | Disease | Period Duration | Prevalence | ||
| Australia, national (2000) (77) | Microbiological and clinical | 5.9 | 0.56 | NA | NR |
| Queensland, Australia, state-wide (1999–2005) (2) | Microbiological and clinical | NR | 3.2 | NA | NR |
| New Zealand, national (2004) (78) | Microbiological and clinical | 3.77 | 0.56 | NA | NR |
Rates expressed as annual rates per 100,000 population, averaged over study period unless otherwise specified
Studies which excluded M. gordonae
Prevalence figures represent underestimates - 30% of records unavailable to determine disease status
Based on assumption of population-based study
Estimated from data in reports
Sentinel site methodology used to extrapolate multi-site prevalence estimates over entire nation
ICD-9 – International diagnostic classification of diseases – ninth revision
RGM – rapidly growing mycobacteria
NR – Not Reported
Table 3.
NTM lung disease by species and region
| North America | ||||||
| Location (dates) | N | Most Common Species | ||||
| New York City, U.S.A. single institution(2000–2004) (80) | 81 | MAC (80%) | M. abscessus /chelonae (13%) | M. fortuitum (8%) | M. xenopi (6%) | M. kansasii (5%) |
| Oregon, U.S.A., state-wide population-based (2005–2006) (34) | 407 | MAC (85%) | M. abscessus /chelonae (4%) | M. xenopi (1%) | M. fortuitum (<1%) | M. goodi (<1%) |
| Toronto, Canada, single institution (2002–2003) (37) | 255 | MAC (69%) | M. xenopi (21%) | RGM (7%) | ||
| Four integrated health care delivery systems, U.S.A., in California, Colorado, Pennsylvania and Washington (1994–2006) (3) | 1,865 | MAC (80%) | M. abscessus /chelonae (12%) | M. fortuitum (6%) | M. kansasii (6%) | M. simiae (3%) |
| Charlottesville, Virginia, U.S.A., single institution (2001–2009) (38) | 83 | MAC (69%) | M. kansasii (5%) | M. xenopi (5%) | M. abscessus (4%) | M. fortuitum (4%) |
| Ontario, Canada (2010) (8) | 1,294 | MAC (64%) | M. xenopi (23%) | M. abscessus (3%) | M. fortuitum (3%) | |
| Central and South America | ||||||
| Location (dates) | N | Most Common Species | ||||
| Baixada Santista region, Sao Paulo, Brazil (2000–2005) (39) | 125 | M. kansasii (20.8%) | MAC (20%) | M. fortuitum (16%) | ||
| Sao Jose do Rio Preto, Brazil (1996–2005) (40) | 184 | MAC (62.9%) | M. fortuitum (11.4%) | M. gordonae (8.5%) | M. chelonae (5.7%) |
M. kansasii M. abscessus (each 2.9%) |
| Buenos Aires Province, Argentina (2004–2010) (41) | 54 | MAC (48%) M. avium (20.4%) M. intracellu lare (27.8%) |
M. kansasii (13%) | M. gordonae (13%) | M. fortuitum (3.7%) | M. scrofulaceum (3.7%) |
| Rio de Janeiro, Brazil (1993–2011) (42) | 127 | MAC (35.4%) | M. kansasii (33.1%) | M. abscessus (18.9%) | M. fortuitum (8.7%) | |
| Para State, Brazil (2010–2011) (43) | 29 | M. massiliense (44.8%) | MAC (20.7%) M. avium (10.3%) M. intracellulare (10.3%) |
M. abscessus (6.9%) |
M. bolletii, M. celatum, M. fortuitum. M. kansasii, M. mariokaense (each 3.4%) |
|
| Europe | ||||||
| Location (dates) | N | Most Common Species | ||||
| Southwest Ireland (1987–2000) (44) | 17 | MAC (82%) | M. malmoense (12%) | M. abscessus (6%) | ||
| France, multi-site (2001–2003) (45) | 263 | MAC (48%) | M. xenopi (25%) | M. kansasii (13%) | M. abscessus (9%) | |
| Leeds, UK (1995–1999) (46) | 49 | MAC (45%) | M. malmoense (37%) | M. xenopi (6%) | M. kansasii (6%) | |
| Nijmegen-Arnhem region, Netherlands (1999–2005) (48) | 53 | MAC (49%) M. avium (45%) M. intracellu lare (3.8%) |
M. kansasii (23%) | M. szulgai (7.5%) | M. xenopi (5.6%) | |
| Denmark, nation-wide (1997–2008) (5) | 335 | MAC (57%) | M. malmoense (8.1%) | M. xenopi (7.8%) | M. abscessus (6.9%) | M. gordonae (5.4%) |
| Central Greece (2004–2006) (47) | 16 | M. fortuitum (62.5%) | MAC (25%) | M. malmoense (12.5%) | ||
| Lisbon, Portugal (2008–2009) (81) | 58 | MAC (22.4%) M. avium (6.9%) M. intracellu lare (15.5%) |
M. fortuitum (13.8%) | M. gordonae (12.1%) | M. kansasii (10.3%) | M. chelonae (8.6%) |
| Naples, Italy (2006–2009) (82) | 16 | M. intracellulare (43.8%) | M. kansasii (31.3%) | M. xenopi (12.5%) | M. fortuitum (6.3%) | |
| Crete, Greece (2000–2009) (49) | 38 | MAC (40%) | M. kansasii (26%) | M. abscessus (8%) |
M. fortuitum M. chelonae M. gordonae (each 5%) |
|
| London, England, single institution (2000–2007, slowly growing NTM) (83) | 57 | M. kansasii (70%) | MAC (40%) | M. malmoense (9%) | M. xenopi (7%) | |
| Borders Region, Scotland (1992–2010) (50) | 32 | MAC (43.8%) | M. malmoense (12.5%) | M. chelonae (9.4%) | M. gordonae (9.4%) |
M. nonchromog enicum M. terrae M. xenopi (each 6.3%) |
| Croatia, nation-wide (2006–2010) (51) | 167 | M. xenopi (28.1%) | M. gordonae (20.4%) |
MAC (19.8%) M. avium (14.4%) M. intracellula re (5.4%) |
M. fortuitum (11.4%) | |
| Middle East and South Asia | ||||||
| Location (dates) | N | Most Common Species | ||||
| Turkey, single institution (2004–2009) (60) | 31 | MAC 42% M. intracellulare 32% M. avium 10% |
M. abscessus 16% | M. kansasii 16% | ||
| Israel, single institution (2004–2010) (61) | 45 | M. xenopi 29% | M. kansasii 20% | MAC 18% | M. fortuitum 9% | |
| Saudi Arabia, sampling of several institutions (2009–2010) (62) | 49 | M. abscessus 30.6% | M. fortuitum 28.6% | MAC 16.3% M. intracellulare 10.2% M. avium 6.1% |
M. kansasii 8.2% | |
| Oman, sample of NTM lung disease cases from central laboratory (2006–2007) (63) | 7 | MAC 71.4% M. intracellulare 28.6% M. chimaera 28.6% |
M. colombiense 14.3% | M. simiae 14.3% | ||
| India, single institution (2005–2008) (64) | 67 | MAC 34% M. intracellulare 22% M. avium 4.5% |
M. abscessus 22% | M. simiae 22% | M. fortuitum 12% | |
| East Asia | ||||||
| Location (dates) | N | Most Common Species | ||||
| Seoul, South Korea, single institution (2002–2003) (65) | 195 | MAC (48%) M. intracellulare (29%) M. avium (19%) |
M. abscessus (33%) | M. fortuitum (11%) | M. kansasii (4%) | |
| Seoul, South Korea, single institution (2002–2008) (84) | 651 | MAC 62.9% | M. abscessus (26.7%) | M. abscessus (26.7%) | ||
| Seoul, South Korea, single institution (2006–2010) (85) | 345 | MAC (76.2%) M. avium (40.9%) M. intracellulare (35.4%) |
M. abscessus (18.2%) | M. fortuitum (2.3%) | M. kansasii (2.0%) | |
| Kaohsiung City, Southern Taiwan, single institution (2004–2005) (72) | 67 | M. abscessus (44.8%) | M. fortuitum (23.9%) | MAC (14.9%) | M. kansasii (13.4%) | |
| Taipei City, Northern Taiwan, single institution (2007–2009) (74)* | 252 | MAC 39.7% | M. chelonae-abscessus 30.2% | M. kansasii 11.1% | M. fortuitum 9.5% | |
| Australia | ||||||
| Location (dates) | N | Most Common Species | ||||
| Australia, national (2000) (77) | 107 | MAC (67.3%) | M. kansasii (19.6%) | M. abscessus (6.5%) | ||
| Queensland, Australia, state-wide (1999–2005) (2) | 130 | MAC (73.8%) M. intracellulare (60%) M. avium (13.8%) |
M. kansasii (7.7%) | M. abscessus (5.4%) | ||
| New Zealand, national (2004) (78) | 47 | MAC (83%) | M. abscessus (9%) | |||
MAC – Mycobacterium avium complex
RGM – rapidly growing nontuberculous mycobacterial species
Included only patients whose radiographic disease pattern could be classified as cavitary, bronochiectatic, or consolidative
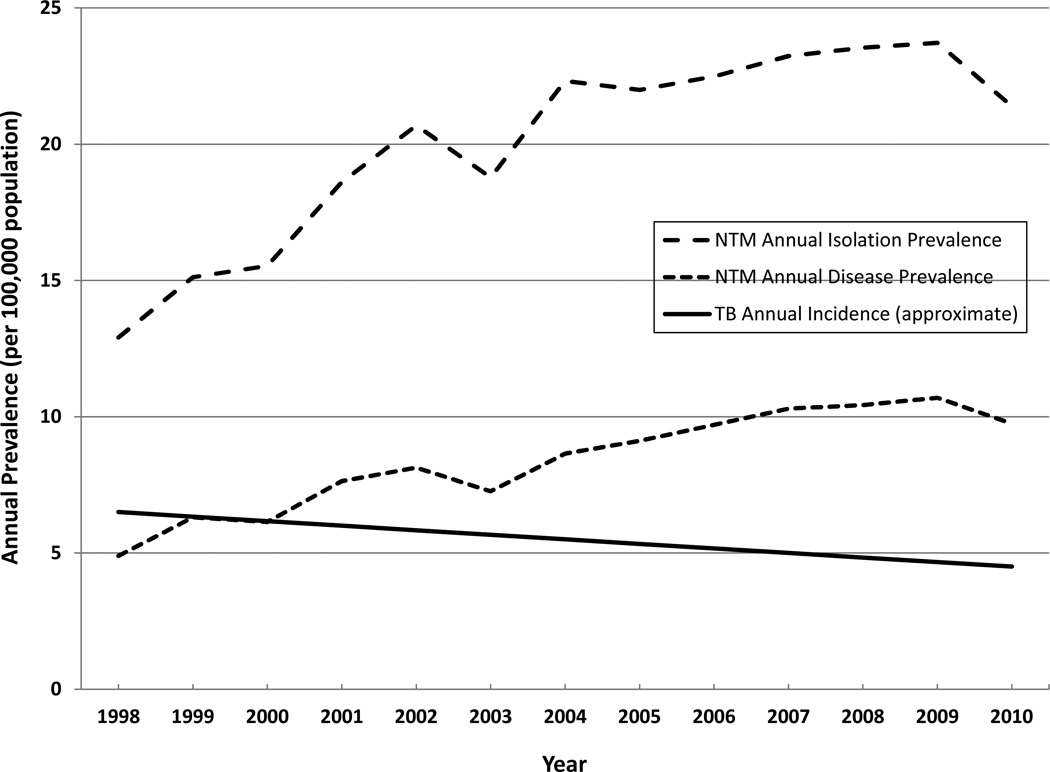
Comprehensive population-based studies in Ontario, Canada have demonstrated annual rates of isolation prevalence of 14.1–22.2/100,000 population (8, 35, 36). Because these studies were limited by a lack of clinical information, a surrogate microbiologic definition of disease was employed (≥2 positive sputum samples or ≥1 positive bronchoscopic or biopsy sample). In one of these studies, annual disease prevalence was estimated at 9.8/100,000 in 2010, while period prevalence of disease was estimated at 41.3/100,000 over the five-year period 2006–2010 (8). All of the above studies excluded M. gordonae. In Ontario, M. avium complex (MAC) was most commonly isolated and identified as a cause of disease, followed by M. xenopi, and the rapidly growing mycobacteria (RGM) (Table 2). These data offer accurate estimates of isolation prevalence in a population of approximately 13 million people, but estimates of disease were limited by the lack of clinical information.
Following implementation of statewide NTM isolation reporting for 2005–2006, and using standardized protocols for NTM case investigation, investigators in Oregon, U.S.A. described NTM epidemiology in a comprehensive state-wide microbiologic based study, with complete clinical record reviews among a subset of subjects in the Portland area (4, 34). Excluding M. gordonae, annualized isolation prevalence of pulmonary NTM was 12.7/100,000 (34) and the two-year period prevalence of disease, defined by full microbiological and clinical criteria, was estimated at 8.6/100,000 (4). Records were available for review in approximately 70% of the Portland area subjects, so disease rates were underestimated. The Oregon data offer robust population-based estimates of isolation prevalence in 3.7 million people, but due to the incomplete availability of clinical records, substantially underestimated disease prevalence.
The epidemiology of NTM has been studied in several closed system managed care organizations in the U.S.A. A multi-center study was performed in four integrated health care delivery systems (IHCDS), including 4.1 million beneficiaries in California, Colorado, Pennsylvania, and Washington (3). The investigators extracted microbiologic and ICD-9 coded records data, and validated a subset of patients for radiographic evidence of disease. The annual site-specific prevalence of NTM PD (excluding M. gordoane) was found to be 1.4–6.6/100,000. Strengths of these data rest on the diversity of the studied geographic regions and the validation of cases.
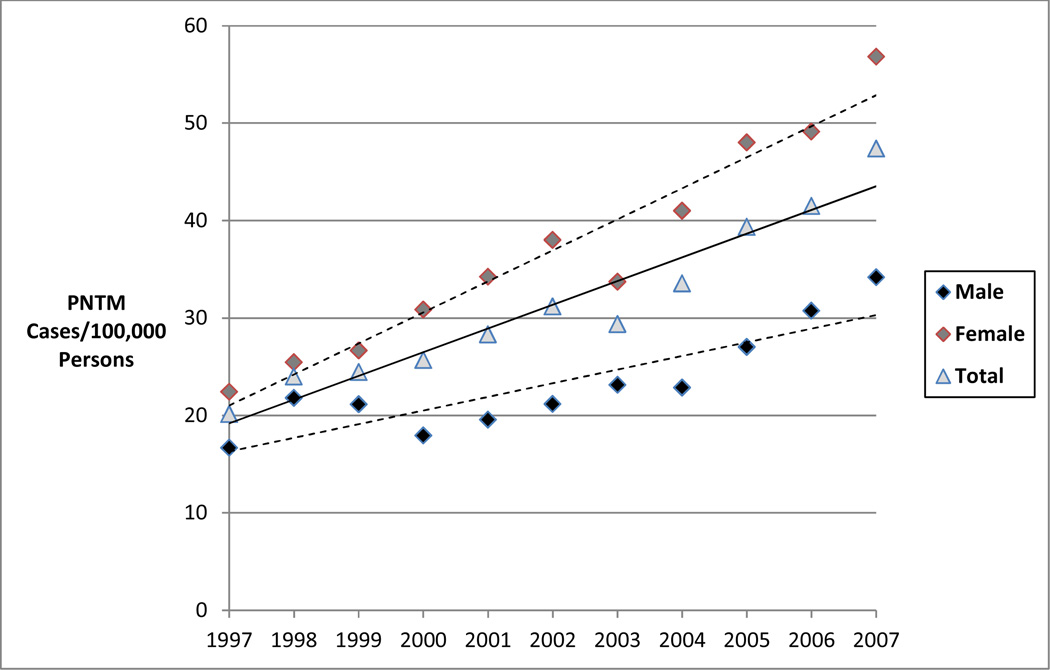
A nation-wide U.S.A. study of Medicare beneficiaries (≥ 65 years of age), from 1997 through 2007 (7) provides valuable insight regarding disease frequency in older adults, who comprise the vast majority of NTM patients. The investigators used ICD-9 coding to identify cases of NTM disease and observed an annual prevalence of approximately 47/100,000 in 2007. Intriguing geographic heterogeneity was identified. Regional period prevalence was highest in the West (149/100,000), driven largely by California (191/100,000) and Hawaii (396/100,000), and second highest in the Southeast (131/100,000). In this older adult population, an average annual increase of NTM disease of 8.5% was observed over 1997–2007 (7) (Figure 1). In U.S.A. managed care organizations, increases of nearly 3% per year were observed over 1994–2006 (3). In Ontario, Canada, the average increase in annual disease prevalence was 6.3% per year over 1998–2010 (8) (Figure 2) while the five-year period prevalence increased from 29.3/100,000 (1998–2002) to 41.3/100,000 (2006–2010) (8).
Figure 1.
Annual prevalence of PNTM cases among a sample of U.S. Medicare Part B enrollees by sex from 1997 to 2007.
Figure 2.
Prevalence of NTM isolation and disease, and TB incidence, Canada, 1998–2010
Information from studies presenting data on the proportion of NTM PD caused by different species is presented in Table 3. In all studies, MAC was the most common species complex (64–85% of cases), followed in most studies by M. abscessus/chelonae (3–13%), M. xenopi (1–23%), M. fortuitum (<1–8%), and M. kansasii (<1–6%). Recently, data from various North American centers were compiled as part of a global NTM collaboration (33).
In North America, the majority of patients tended to be women (range of 55–66%), and were more likely to be older adults (mean age 68.2 years, range 59–70 years, Table 4). Most recent North American studies did not report oin whether patients had pre-existing lung disease, but the frequency of COPD was described in four studies (3, 4, 37, 38) wherein 28%–66% of patients had COPD (Table 4). With respect to prevalence by racial\ethnic groups, of interest is the finding that in the USA, among persons aged ≥65 years, persons classified as “Asian” had relative prevalence rates approximately twofold higher than persons categorized as “white”.
Table 4.
Age and Sex distribution NTM lung disease by Region
| North America | |||||
| Location (dates) | N |
Mean age (years) |
Female | COPD |
Pre-existing pulmonary disease |
| New York City, U.S.A. single institution(2000–2004) (80) | 81 | 70 | Overall 60% | NR | 44% |
| Oregon, U.S.A., state-wide population-based (2005–2006) (34) | 407 | 68 (median) | 60.5% | NR | NR |
| Portland, Oregon, U.S.A. (2005–6) (4) | 134 | (subset of state-wide study) | (subset of state-wide study) | 28% | NR |
| Ontario, Canada, population based (2008; MAC only) (86) | 880 | 69.1 (median) | 56% | NR | NR |
| Toronto, Canada, single institution (2002–2003) (37) | 255 | 68 | 55% | 39% | 56% |
| Charlottesville, Virginia, U.S.A., single institution (2001–2009) (38) | 83 | 59 | 66% | 23% | NR |
| Europe | |||||
| Location (dates) | N |
Mean age (years) |
Female | COPD |
Pre-existing pulmonary disease |
| Croatia, nation-wide (2006–2010) (51) | 167 | 66 (median) | 44.9% | NR | NR |
| France, multi-site (2001–2003) (45) | 263 | Overall 64.5 MAC 70 M. xenopi 60 M. kansasii 54 RGM 62 |
Overall 46.9% MAC 60.2% M. xenopi 22.7% M. kansasii 20.6% RGM 68.8% |
Overall 54.8% MAC 71.2% M. xenopi 42.4% M. kansasii 20.6% RGM 50% |
|
| Netherlands, nation-wide (1999–2005, M. xenopi) (87) | 25 | M. xenopi 60 | M. xenopi 24% | M. xenopi 68% | M. xenopi 84% |
| Croatia, nation-wide (1980–2005, M. xenopi) (52) | 24 | M. xenopi 61.7 | M. xenopi 10% | M. xenopi 90% | |
| Central Greece (2004–2006) (47) | 16 | 66.1 | 31.3% | 68.8% | |
| Nijmegen-Arnhem region, Netherlands (1999–2005) (48) | 53 | 57 | 30% | 43% | 70% |
| Netherlands, nation-wide (1999–2004, M. chelonae-abscessus) (88) | 16† | Overall* 60.2 M. abscessus† 60.8 M. chelonae 59.6 |
Overall* 25% M. abscessus†25% M. chelonae 25% |
Overall 37.5% M. abscessus† 37.5% M. chelonae 37.5% |
Overall 62.5% M. abscessus† 62.5% M. chelonae 62.5% |
| Denmark, nation-wide (1997–2008) (5) | 335 | 61.2 | 41.2% | 37.3% | 46% |
| London, England, single institution (2000–2007, slowly growing NTM) (83) | 57 | 61 | 32% | 37% | |
| Netherlands, nation-wide (2002–2005, M. malmoense)(89) | 32 | M. malmoense 56 | M. malmoense 34.4% | M. malmoense 66% | M. malmoense 81% |
| Naples, Italy (2006–2009) (82) | 16 | 67.6 | 31.3% | 31.3% | |
| Lisbon, Portugal (2008–2009) (81) | 58 | Overall 54.7 (med) MAC 54.2 (med) |
Overall 53.4% MAC 53.8% |
NR | NR |
MAC – Mycobacterium avium complex
RGM – rapidly growing nontuberculous mycobacteria
Patients with known cystic fibrosis excluded from above (calculated from data presented)
Includes M. abscessus, M. massiliense, M. bolletii
In summary, data from recent North American studies generally suggest annual rates of NTM isolation of 6–22/100,000, and annual rates of disease of 5–10/100,000. Period prevalence estimates, undoubtedly a superior measure of the burden of disease, varied widely based on the period duration and age group studied. Period prevalence in studies including all ages ranged from 9–41/100,000, and was far higher when focusing on older sub-populations. Most studies demonstrated temporal increases in the frequency of NTM PD over a 3 to 11 year period. Limitations included heterogeneous case ascertainment and disease definitions, and incomplete age-group or geographic coverage. In the USA, nationally representative estimates of the <65 population are not available. In Canada, large geographic areas outside the province of Ontario have not been systematically studied, and there has been limited assessment employing clinical record review within Ontario. NTM PD in North America is very strongly associated with increasing age (especially common in the elderly) and COPD, and is reported more commonly in females. The prevalence of NTM PD varies both with the presence of COPD and gender, whereby men seem to more commonly have “secondary” NTM, with predisposing COPD, while women most commonly appear to have ”idiopathic” NTM (37).
Central / South America
A number of recent studies, generally from single institutions, have added to the understanding of the epidemiology of NTM PD in Central and South America. The limitation common to all of these studies is the lack of a reliable denominator in a defined population, making estimates regarding NTM frequency uncertain. In addition, data regarding species distribution may also be affected by referral bias to the institutions from which data were reported. The latter may be relevant if NTM species varies according to clinical features, like COPD, or if clinicians’ referral may depend on the species isolated and the perception of its pathogenicity. Table 2 presents results regarding NTM frequency, while Table 3 presents results regarding relative frequency of different NTM species among populations with NTM disease.
In the Baixada Santista region of Sao Paolo, Brazil, investigators reported on a series of patients who, as patients with suspected or presumed TB, had sputum collected in regional healthcare facilities, and sent to their institution for culture over 2000–2005 (39). Based on contemporary population data, their report suggests an annual prevalence of 1.3/100,000 for NTM isolation, and 0.25/100,000 for NTM disease, as well as a six-year period prevalence of disease of 1.5/100,000. Limitations include sampling that was not comprehensive (underestimating true rates), and disease definition based on microbiologic criteria alone, perhaps overestimating the proportion with disease. Over 1996–2005, in Sao Jose do Rio Preto, Brazil, investigators reported on pulmonary NTM isolation in 184 patients whose specimens had been referred to a regional TB laboratory for suspected or presumed TB (40). Based on contemporary population data, their report suggests annual prevalence rates of 5.3/100,000 for NTM isolation, and 1.0/100,000 for NTM disease, as well as ten-year period prevalence of 10/100,000 for NTM disease. This report lacked information regarding the underlying sampling. If the survey was not comprehensive, the calculated rates are underestimates.
Species distribution was presented in some recent studies. In Argentina, among 54 patients with NTM PD over 2004–2010, whose specimens were submitted to a regional specialty hospital, the species distribution was MAC first (48%), followed by M. kansasii (13%) and M. gordonae (13%)(41). The species distribution may not apply to the entire region, depending on possible referral biases, which were not addressed. From a national mycobacterial referral center in Rio de Janeiro, Brazil, 174 patients with pulmonary NTM during 1993–2011, were described (42). Because TB is generally diagnosed based on smear microscopy rather than culture, 79% of patients had been treated empirically for up to six months for TB before the diagnosis of NTM was made. NTM disease, defined by ATS criteria, was diagnosed in 73% (127/174). Among patients with NTM disease, species distribution was MAC first (35.4%), followed closely by M. kansasii (33.1%), then M. abscessus (18.9%), and M. fortuitum (8.7%). It is uncertain whether the species distribution is regionally representative, because the report came from a highly specialized referral center, where one would suspect greater disease severity or complexity.
The finding that a large proportion of patients were initially receiving empiric, sometimes prolonged, TB therapy, is consistent with the notion that NTM is likely under-recognized in regions with substantial TB rates, particularly in much of the world where laboratory capacity is not sufficient to peform cultures for diagnosis of all TB suspects. In the Para state of Brazil, a report regarding patients with pulmonary NTM isolated over 2010–2011 was presented (43). Species distribution among the 29 patients who fulfilled ATS disease criteria was M. massiliense first (44.8%), followed by MAC (20.7%), then M. abscessus (6.9%). Of all positive mycobacterial cultures, 13.5% were NTM, suggestive that there under-recognition of NTM among TB suspects when there is exclusive reliance on smear microscopy.
Regarding population patterns, two recent South American studies presented data on age and sex distribution in NTM PD. Among 173 patients with NTM PD at a national mycobacterial referral centre in Rio de Janeiro, Brazil, during 1993–2011, 62% were male and the median age was 55 years (42). Among 29 patients with NTM PD in the Para state of Brazil with NTM PD during 2010–2011 72% were female and the mean age was 52.3 years (43). The presence of pre-existing lung disease was addressed to some extent. In the study from Rio de Janeiro, bronchiectasis and COPD were present in 22% and 21% of patients respectively, suggesting strong associations with NTM PD (42). In the study from the state of Para, 76% (22/29) of cases were thought to have prior TB (43). On the one hand, it is plausible that bronchiectasis or other structural lung disease after TB was a major risk factor in these patients. On the other hand, the designation of prior TB was likely made without mycobacterial culture, so it is possible that at least some of the patients had NTM lung disease from the outset.
In summary, relatively little can be concluded regarding the population frequency of pulmonary NTM in Central and South America. Two studies provide data that could be used for such estimates, but the lack of a reliable population denominator makes the estimates questionable. Regional variation in the species distribution was evident among studies of NTM disease. MAC was generally most common, and M. kansasii was also frequently reported. Due to the possibility of bias in case ascertainment, it is difficult to make conclusions regarding age and sex distributions from these two studies.
Europe
In the European region numerous recent studies exist using variable methodologies to study NTM epidemiology - some population-based while others using sentinel-site methods to extrapolate disease frequency over a larger region. Table 2 presents results of studies with data on NTM frequency, while Table 3 presents results regarding relative frequency of different NTM specie among populations with NTM disease. In Southwest Ireland, a population-based study of pulmonary NTM reported average annual rates of 1.9/100,000 (isolation) and 0.2/100,000 (disease) over 1987–2000 (44). Temporal frequency data were presented, but not by anatomic site, so inferences regarding temporal trends of pulmonary NTM could not be made.
Studies from France have used a sentinel-site methodology, collecting data over 2000–2003, from 32 sites widely distributed across the country (32, 45). Average annual rates of lung disease in patients without HIV infection were estimated for all NTM at 0.73/100,000 (45) and for MAC at 0.2/100,000 (32). Estimates from these sentinel-site studies depended on several assumptions, introducing some uncertainty. The network of sentinel sites was established to provide surveillance of TB drug resistance, and prevalence estimates for NTM depend on the assumption that the population serviced by the network is identical for TB and NTM, with identical referral patterns into the network for TB and NTM. If referral into the network is not comprehensive, perhaps affected by regional providers’ perception of network sites’ interest or expertise, then results from sentinel sites may not be appropriately generalized to the entire population.
Data from Leeds, United Kingdom, over 1995–1999, indicated an annual rate of NTM isolation of approximately 2.9/100,000 and disease of 1.7/100,000 (46). In Central Greece, over 2004–2006, annual rates of pulmonary NTM were 7.0/100,000 (isolation) and 0.7/100,000 (disease) (47). The Nijmegen-Arnhem region of the Netherlands has been extensively studied. Investigators reported, over 1999–2005, annual isolation of approximately 6.3/100,000 and disease of approximately 1.4/100,000 (48). Detailed analysis of NTM epidemiology throughout Denmark over 1997–2008 revealed rates of average annual isolation of 2.5/100,000 and disease of 1.1/100,000 (5). Period prevalence was not presented, but a crude approximation, based on the reported median survival of approximately 5 years, is 5–8/100,000. A population-based study from Crete, Greece over 2000–2009 reported an annual rate of disease of 0.6/100,000 (49). In the Borders region of Scotland, over 1992–2010, the average annual rate of NTM lung disease was observed to be 1.6/100,000 (50). In Croatia, over 2006–2010, the annual rates of NTM isolation and disease (based on ATS microbiologic criteria) were observed to be 5.3/100,000 and 0.75/100,000 respectively (51).
Temporal trends in pulmonary NTM were presented, to some extent, in four studies. There was a suggestion of a temporal increase in NTM PD in Leeds, United Kingdom, over 1995–1999, although the observed absolute prevalence rates were low (46). In Croatia, the annual identification of M. xenopi pulmonary disease increased steadily from 0.05/100,000 from the early 1980s to 0.18/100,000 in the early 2000s (52). Data from the national reference laboratory for mycobacteria in the In the Netherlands (receiving 85% of clinically isolated NTM) over 2000–2006, demonstrated an approximate four-fold increase in the number of patients with M. avium isolates referred in (53). Although changes in referral rates could explain some of the increase, there was likely a substantial increase in pulmonary M. avium. In the Scottish Borders region, over 1992–2010, there was a substantial increase in NTM lung disease, from 0.86/100,000 in 1992–2004, to 3.1/100,000 in 2005–2010 (50).
A number of European studies have presented useful data regarding age, sex, and pre-existing lung disease in NTM PD (Table 4). The following weighted mean calculations were performed among studies presented in Table 4 with non-overlapping populations. There was an average age of approximately 62.5 years, apparently somewhat younger than the mean age of NTM PD patients in North American studies. All but one study reported that patients were mostly men - mean 57%, in contrast to findings in North America. The presence of COPD, when reported, was described in 39% of NTM PD patients.
In summary, recent literature from Europe continues to identify substantially lower rates of pulmonary NTM isolation and disease than in North America, with several studies demonstrating recent temporal increases. In contrast to observations in North America, NTM PD patients in European studies tend be mostly men, and slightly younger. Some of the differences likely relate to differences in smoking patterns. Recent data suggest that in Europe, 23% of adults are smokers (with a 16% reduction during the prior decade), while in the U.S.A. and Canada, approximately 15% of adults are smokers (with a 16% reduction during the prior decade). Based on differences in recent smoking data, one might assume that a higher proportion of NTM PD in Europe would be COPD/smoking-related,(54) and perhaps due to a historical excess of men smoking, that men would comprise a higher proportion of NTM PD patients.
Africa
Numerous recent African studies have investigated the frequency of pulmonary NTM in two main types of populations. First, several investigations searched for NTM among patients suspected of having pulmonary TB. In 10 hospitals in Western Kenya, over 2007–2009, 361/872 (45.1%) patients suspected of having pulmonary TB tested positive for mycobacteria. Of patients with positive cultures, 95.8% (346/361) had TB, while 4.2% (15/361) had NTM (55). In two TB centers in Nigeria, during 2010 and 2011, consecutive TB suspects were assessed with mycobacterial cultures in addition to AFB smear (56). Of 1,603 TB suspects, 444 (28%) had pulmonary mycobacteria identified, of whom 375 (85%) had M. tuberculosis complex species and 69 (15%) had NTM (56). Finally, in a surveillance study of children and adolescents in rural Eastern Uganda, including 2,200 individuals identified as pulmonary TB suspects, only eight patients (0.36%) had M. tuberculosis complex species isolated, while 95 patients (4.3%) had NTM isolated (57). Although the above studies did not provide adequate details regarding the proportion of patients who fulfilled criteria for NTM disease, the results suggest that a potentially significant proportion of African TB suspects may have NTM disease, but may not be detected in routine care.
The second relevant population type that has been studied comprises patients with “chronic” pulmonary TB. In Burkina Faso, over 2007–2008, patients classified as having “chronic” TB (positive acid-fast sputum smear after five months of standard therapy), were investigated microbiologically to assess the common assumption, that “chronic” TB accurately identifies multi-drug resistant (MDR) TB (58). Of 63 cases investigated, 38 patients (60.3%) had M. tuberculosis complex species isolated, while 13 (20.6%) had NTM. In a similar study from a TB referral center in Mali, 61 “chronic” TB patients were investigated, of whom 11 (18%) were found to have only NTM in their sputum (59). These findings suggest that a substantial proportion of patients in Africa with suspected MDR TB may actually have NTM PD.
Middle East and South Asia
No recent population-based studies of pulmonary NTM were identified from this region. A number of studies, generally performed in single institutions, provide information regarding species distribution among patients with NTM PD in this region. At a single center in Turkey, during 2004–2009, among 53 patients with pulmonary NTM isolation, 60% (31/53) were judged to have disease (60). The mean age of patients with disease was 54 years and 87.1% (27/31) were males (60). In a single institution in Israel, among 215 patients identified with pulmonary NTM isolation over 2004–2010, 21% (45/215) were judged to have definite or probable disease, but clinical information was not detailed (61). From various laboratories in Saudi Arabia, a sample of 95 patients with NTM isolation was identified during 2009–2010, including 73 patients with pulmonary isolation, 67% of whom (49/73) were judged to have pulmonary disease but clinical information was not detailed (62). In Oman, over 2006–2007, a random sample of 13 NTM isolates from the Central Public Health Laboratory in Muscat, was selected for case review (63). Eleven isolates were pulmonary, and ATS disease criteria were fulfilled in 63.6% (7/11). Among patients with NTM PD, the mean age was 46.3 years and 57.1% (4/7) were males. At a single institution in Mumbai, India, during 2005–2008, 103 patients had pulmonary NTM isolates, with 65.0% (67/103) judged to have either “definite” or “probable” disease, but clinical information was not detailed (64). Table 3 presents the species distributions among patients with NTM PD from the above studies.
East Asia
No population-based studies of pulmonary NTM epidemiology in East Asia were identified. A number of studies have been done from single institutions and are described below. We generally excluded studies that presented exclusively isolate-level data (lacking patient-level data) and studies which focused on specific patient groups (critical care unit patients, cancer patients, etc.). A number of South Korean studies offer insight into NTM PD species distribution and patient characteristics, and suggest that NTM PD is becoming increasingly common. At one institution in Seoul, over 2002–2003, 794 patients with pulmonary NTM isolates were classified according to disease status (65), using the 1997 American Thoracic Society criteria (66) and the 2000 British Thoracic Society criteria (67) ascertained by full review of medical records (65). Definite disease was defined by fulfilling both ATS and BTS criteria, probable disease cases fulfilled only the BTS (less stringent) criteria, and unlikely disease comprised the remainder. Of 195 patients judged to have either definite (n= 131) or possible (n=64) disease, the mean age was 63 years, and 55% were women. The species distribution is shown in Table 3.
Recent Japanese studies provide insight into the prevalence of NTM PD, species distribution, and patient features. A recent study used the mortality rate, among patients with NTM PD, as a way to derive estimates of prevalence of NTM PD (10). The authors estimated a national prevalence of NTM PD in Japan in 2005 at 33–65/100,000, and report that most cases are caused by MAC (10). A single-institution study in Tokyo, describing 273 newly-diagnosed patients with MAC lung disease during 1996–2002, reported an average age of 64 years, and a predominance of females (70%) (68). The authors divided the disease into radiographic type according to the presence of cavitation, and observed that 30% of females had cavitation, compared with 65–70% of males. Two additional studies of MAC PD patients from Japan provide interesting insights (69–71). Among 634 HIV-negative patients diagnosed with MAC PD over 1995–2005 in Saitama, the mean age at cohort entry was 68.9 years, 58.5% were women, and 38.3% had pre-existing respiratory disease (including 5.8% with emphysema) (69). Another group presented data on a cohort of 164 patients identified with MAC PD over 1999–2005 in Kyoto, wherein the mean age was 66 years, 56.7% were women, and 61.6% had COPD (70). These studies support the notion that MAC PD patients in Japan are generally older and mostly women, although the presence of COPD was variable. However, referral bias may affect cohort characteristics in studies from single institutions.
A number of studies from single institutions in Taiwan, provide insights into species distribution and suggest that NTM PD is increasingly common. At an institution in Kaohsiung City, Southern Taiwan, it was observed during 2004–2005, that among the 67 patients with NTM PD identified, the average age was 67 years, 70% were males, and 88.1% had pre-existing lung disease, including 61.2% with COPD (72). At a hospital in Taipei City, Northern Taiwan, the annual prevalence of NTM PD increased from 1.28/100,000 in 2000, to 7.94/100,000 in 2008 (73). From the same institution, 252 NTM PD patients with classifiable radiographic patterns (cavitary, bronchiectatic, or consolidative) were identified over 2007–2009 (74). The mean age was 63.7 years and 50.8% were men (74). The studies from Taiwan identified NTM PD patients as being in the same age range as most other studies. However, in contrast to work from Japan and Korea, Taiwanese studies described a slight male predominance. Referral biases may be relevant in these single institution studies. Species distribution data from these studies are presented in Table 3.
Two recent studies from China reported on the frequency of NTM isolation among patients with mycobacterial isolates. From a single institution in Shanghai over 2005–2008, 5.1% of positive mycobacterial cultures were NTM, increasing from 4.3% in 2005 to 6.4% in 2008 (75). In several counties in rural Shandong Province, of 2,949 specimens that were culture-positive for mycobacteria, 64 were NTM (1.6%) (76). More detailed epidemiologic data from China are not yet readily available.
In summary, recent NTM PD studies from East Asia describe patients who are usually in their seventh decade (mean/median age 63–69 years). South Korean and Japanese investigators identified a majority of females, while Taiwanese work reported a slight male predominance.
Australia and New Zealand
Recent literature from Australia and New Zealand includes three excellent population-based studies describing the epidemiology of NTM PD. Table 2 presents data regarding disease frequency, while Table 3 presents data regarding NTM species distribution. A nation-wide Australian survey during 2000, focusing on incidence (patients without previously identified pulmonary NTM), reported an annual isolation incidence of 5.9/100,000 and disease incidence of 0.56/100,000 (77). In the state of Queensland, Australia, data were presented from 1999–2005. In the most recent year of study, the annual prevalence of NTM PD was 3.2/100,000 (2). In New Zealand, in 2004, it was observed that the prevalence of NTM PD was 1.17/100,000 (78). Temporal trends in NTM PD were presented in two Australian studies. At the nationwide level, comparing 2000 with 1988, there appeared to be a minimal increase in annual incidence, from 0.51/100,000 to 0.56/100,000 (77). In the state of Queensland, from 1999 to 2005, there was an increase from 2.2/100,000 to 3.2/100,000 (2). In the latter study, note was made of a predominance of fibronodular (nodular bronchiectatic) disease in the recent year of the study, compared with a previously reported predominance of cavitary disease.
Regarding age and sex distributions, in Queensland, Australia, appreciable rates of NTM PD occurred among patients who were at least 45 years old, and increased dramatically with age, with a tripling in rates between the 45–59 year age group and the 60–74 year age group (2). In addition, women outnumbered men overall and in all age strata from 30 years and older. In New Zealand, 79% of people with NTM PD were female, but age data were not presented (78). In New Zealand, 82% of NTM PD patients were described as having predisposing lung conditions, including 18% COPD, and 56% with bronchiectasis, where cause versus effect may be difficult to discern (78).
Summary and Research Gaps
Population-based data for prevalence and trends estimation are available primarily from North America, Europe, as well as for New Zealand and Australia, where NTM PD is a reportable condition; these data have documented a continued increase in NTM prevalence since 2000. Annual prevalence in North American and Australia, ranging from 3.2–9.8, is generally higher than in Europe, where available estimates are all below 2 per 100,000. Tertiary care-based studies of NTM PD from South Korea, Japan, and Taiwan, also suggest increasing prevalence of NTM PD. In South America, Africa, and the Middle East, studies among patients with suspected TB and MDR have documented a previously unappreciated prevalence of NTM in this population, ranging from 4–15% for suspected TB and 18–20% for suspected multi-drug resistant TB (MDR TB).
Clinical data available from several regions have allowed better characterization of the affected populations. Across regions, the majority of cases occur among persons aged >50 years, with a mean age of 54–70 years. In North America and East Asia, most NTM PD cases are among women whereas in Europe men predominate among NTM PD cases, with varying proportions of preexisting lung disease across regions. The species distribution also varies by region, with MAC predominant in North America and East Asia, whereas in Europe MAC is less predominant and M. kansasii, M. xenopi, and M. malmoense are found with greater frequency. In addition, species vary by patient sex and the presence of preexisting lung disease.
The available data highlight data needs and research gaps. To more fully characterize prevalence and trends for NTM PD, population-based data on NTM isolates with species information linked to clinical data and patient characteristics (age, sex, geographic location) are needed from more geographic areas of the world. These data could be obtained by linking NTM surveillance to existing TB surveillance, with reporting of all AFB isolates linked to the above clinical and demographic information. In areas where such data are not feasible to obtain, sentinel surveillance could be established at tertiary care centers serving a large population. In particular, to advance the understanding of the influence of species and subspecies type on disease type and progression, as well as the influence of environmental reservoirs on disease presentation, more detailed information related to both patient and demographic factors are needed. Standard microbiologic and clinical data collection will allow assessment of trends. The detection of important proportions of patients with NTM isolates among patients screened for TB and MDR TB highlights the need for enhanced mycobacterial laboratory capacity to discriminate between cases of TB and NTM, ensuring correct diagnosis and management, as well as identifying the burden of NTM in these settings.
With respect to risk factors for NTM, host and environmental factors interact to influence disease risk. Beyond previously described structural abnormalities and immunosuppressive conditions, host factors important to the current epidemiology of NTM PD include thoracic skeletal abnormalities, rheumatoid arthritis, immunomodulatory drugs such as TNF-alpha inhibitors (particularly in the RA population), steroid use, and GERD. Clustering of disease within families suggests a heritable genetic predisposition to disease susceptibility, and additional research is needed to identify these genetic factors. With respect to environmental exposures to water and soil aerosols, very few individual exposures have been identified, but new analytic approaches have highlighted the important of climatic factors such as warm, humid environments with high atmospheric vapor pressure as contributing to population risk. Additional research is needed to more specifically identify additional climatic and soil factors, and their contribution to microbial growth. Further, the interaction of human populations with these environments will be key to understanding the interaction of host and environmental factors.
Key Points.
Population-based data from North America, Europe, and Australia, show that NTM PD prevalence continues to increase, and that prevalence is lower in Europe than in North America and Australia.
Large tertiary-care facility based studies in East Asia (Japan, South Korea, Taiwan) also suggest high and increasing prevalence of NTM PD.
In selected African countries, NTM were identified in 4.2% to 15% of suspected TB cases and 18–20% of persons with “chronic” suspected MDR TB.
Improved surveillance for NTM is needed, including population based surveillance and sentinel studies
Host and environmental factors interact in disease risk; host factors include neoplasms, preexisting lung disease and more recently identified factors such as thoracic skeletal abnormalities, rheumatoid arthritis and immunomodulatory drugs. Environmental risk factors for disease clustering include high vapor pressure such as is found in warm, humid environments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- 1.Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clinics in Chest Medicine. 2002;23:553–567. doi: 10.1016/s0272-5231(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 2.Thomson RM NTM working group at Queensland TB Control Centre and Queensland Mycobacterial Reference Laboratory. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerging Infectious Diseases. 2010;16:1576–1583. doi: 10.3201/eid1610.091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977–982. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 5.Andrejak C, Thomsen VO, Johansen IS, et al. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med. 2010;181:514–521. doi: 10.1164/rccm.200905-0778OC. [DOI] [PubMed] [Google Scholar]
- 6.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 7.Adjemian J, Olivier KN, Seitz AE, et al. Prevalence of nontuberculous mycobacterial lung disease in U.S. medicare beneficiaries. Am J Respir Crit Care Med. 2012;185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marras TK, Mendelson D, Marchand-Austin A, et al. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998–2010. Emerging Infectious Diseases. 2013;19:1889–1891. doi: 10.3201/eid1911.130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirsaeidi M, Machado RF, Garcia JG, et al. Nontuberculous mycobacterial disease mortality in the United States, 1999–2010: a population-based comparative study. PLoS One. 2014;9:e91879. doi: 10.1371/journal.pone.0091879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto K, Iwai K, Uchimura K, et al. A Steady Increase in Nontuberculous Mycobacteriosis Mortality and Estimated Prevalence in Japan. Annals of the American Thoracic Society. 2014;11:1–8. doi: 10.1513/AnnalsATS.201303-067OC. [DOI] [PubMed] [Google Scholar]
- 11.Colombo RE, Hill SC, Claypool RJ, et al. Familial clustering of pulmonary nontuberculous mycobacterial disease. Chest. 2010;137:629–634. doi: 10.1378/chest.09-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung JM, Fowler C, Smith C, et al. A familial syndrome of pulmonary nontuberculous mycobacteria infections. Am J Respir Crit Care Med. 2013;188:1373–1376. doi: 10.1164/rccm.201306-1059LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutting GR. Modifier genes in mendelian disorders: the example of cystic fibrosis. Annals of the New York Academy of Sciences. 2010;1214:57–69. doi: 10.1111/j.1749-6632.2010.05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo K, Yamazaki Y, Hanaoka M, et al. Analysis of HLA antigens in mycobacterium avium-intracellulare pulmonary infection. Am J Respir Crit Care Med. 2000;161:1368–1371. doi: 10.1164/ajrccm.161.4.9906094. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi M, Ishizaka A, Nakamura H, et al. Specific HLA in pulmonary MAC infection in a Japanese population. Am J Respir Crit Care Med. 2000;162:316–318. doi: 10.1164/ajrccm.162.1.9908071. [DOI] [PubMed] [Google Scholar]
- 16.Feazel LM, Baumgartner LK, Peterson KL, et al. Opportunistic pathogens enriched in showerhead biofilms. Proceedings of the National Academy of Sciences USA. 2009;106:16393–16399. doi: 10.1073/pnas.0908446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adjemian J, Olivier KN, Seitz AE, et al. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med. 2012;186:553–558. doi: 10.1164/rccm.201205-0913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dirac MA, Horan KL, Doody DR, et al. Environment or host?: A case-control study of risk factors for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;186:684–691. doi: 10.1164/rccm.201205-0825OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prevots DR, Adjemian J, Fernandez AG, et al. Environmental risks for nontuberculous mycobacteria: individual exposures and climatic factors in the cystic fibrosis population. Annals of the American Thoracic Society. 2014 doi: 10.1513/AnnalsATS.201404-184OC. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maekawa K, Ito Y, Hirai T, et al. Environmental risk factors for pulmonary mycobacterium avium-intracellulare complex disease. Chest. 2011;140:723–729. doi: 10.1378/chest.10-2315. [DOI] [PubMed] [Google Scholar]
- 21.Olivier KN, Weber DJ, Wallace RJ, Jr, et al. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167:828–834. doi: 10.1164/rccm.200207-678OC. [DOI] [PubMed] [Google Scholar]
- 22.Andrejak C, Nielsen R, Thomsen VO, et al. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68:256–262. doi: 10.1136/thoraxjnl-2012-201772. [DOI] [PubMed] [Google Scholar]
- 23.Prince DS, Peterson DD, Steiner RM, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321:863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 24.Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brode SK, Jamieson FB, Ng R, et al. Risk of mycobacterial infections associated with rheumatoid arthritis in Ontario, Canada. Chest. 2014 doi: 10.1378/chest.13-2058. [DOI] [PubMed] [Google Scholar]
- 26.Winthrop KL. Infections and biologic therapy in rheumatoid arthritis: our changing understanding of risk and prevention. Rheumatic Diseases Clinics of North America. 2012;38:727–745. doi: 10.1016/j.rdc.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Winthrop KL, Baxter R, Liu L, et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Annals of the Rheumatic Diseases. 2013;72:37–42. doi: 10.1136/annrheumdis-2011-200690. [DOI] [PubMed] [Google Scholar]
- 28.Adjemian JOK, Prevots DR. Nontuberculous mycobacteria among cystic fibrosis patients in the United States: screen practices and environmental risk. Am J Respir Crit Care Med. 2014 doi: 10.1164/rccm.201405-0884OC. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards LB, Acquaviva FA, Livesay VT, et al. Clinical and laboratory studies of tuberculosis and respiratory disease: The Navy Recruit Program. The American Review of Respiratory Disease. 1969;99:1–132. [PubMed] [Google Scholar]
- 30.Kirschner RA, Jr, Parker BC, Falkinham JO., III Epidemiology of infection by nontuberculous mycobacteria. Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum in acid, brown-water swamps of the southeastern United States and their association with environmental variables. The American Review of Respiratory Disease. 1992;145:271–275. doi: 10.1164/ajrccm/145.2_Pt_1.271. [DOI] [PubMed] [Google Scholar]
- 31.Chou MP, Clements AC, Thomson RM. A spatial epidemiological analysis of nontuberculous mycobacterial infections in Queensland, Australia. BMC Infect Dis. 2014;14:279. doi: 10.1186/1471-2334-14-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maugein J, Dailloux M, Carbonelle B, et al. Sentinel site surveillance of Mycobacterium avium complex pulmonary disease. European Respiratory Journal. 2005;26:1092–1096. doi: 10.1183/09031936.05.00148604. [DOI] [PubMed] [Google Scholar]
- 33.Hoefsloot W, van Ingen J, Andrejak C, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. European Respiratory Journal. 2013;42:1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 34.Cassidy PM, Hedberg K, Saulson A, et al. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clinical Infectious Diseases. 2009;49:e124–e129. doi: 10.1086/648443. [DOI] [PubMed] [Google Scholar]
- 35.Marras TK, Chedore P, Ying AM, et al. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario 1997–2003. Thorax. 2007;62:661–666. doi: 10.1136/thx.2006.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Houqani M, Jamieson F, Chedore P, et al. Isolation prevalence of pulmonary nontuberculous mycobacteria in Ontario in 2007. Canadian Respiratory Journal. 2011;18:19–24. doi: 10.1155/2011/865831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marras TK, Mehta M, Chedore P, et al. Nontuberculous mycobacterial lung infections in Ontario, Canada: clinical and microbiological characteristics. Lung. 2010;188:289–299. doi: 10.1007/s00408-010-9241-8. [DOI] [PubMed] [Google Scholar]
- 38.Satyanarayana G, Heysell SK, Scully KW, et al. Mycobacterial infections in a large Virginia hospital, 2001–2009. BMC Infectious Diseases. 2011;11 doi: 10.1186/1471-2334-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamarioli LA, Coelho AGV, Pereira CM, et al. Descriptive study of the frequency of nontuberculous mycobacteria in the Baixada Santista region of the state of Sao Paulo, Brazil. Jornal Brasileiro de Pneumologia. 2008;34 doi: 10.1590/s1806-37132008000800008. [DOI] [PubMed] [Google Scholar]
- 40.Pedro HSP, Pereira MIF, Goloni MRA, et al. Nontuberculous mycobacteria isolated in Sao Jose do Rio Preto, Brazil between 1996 and 2005. Jornal Brasileiro de Pneumologia. 2008;34 doi: 10.1590/s1806-37132008001100010. [DOI] [PubMed] [Google Scholar]
- 41.Imperiale B, Zumarraga M, Gioffre A, et al. Disease caused by non-tuberculous mycobacteria: diagnostic procedures and treatment evaluation in the north of Buenos Aires Province. Revista Argentina de Microbiologia. 2012;44:3–9. doi: 10.1590/S0325-75412012000100002. [DOI] [PubMed] [Google Scholar]
- 42.de Mello KG, Mello FC, Borga L, et al. Clinical and therapeutic features of pulmonary nontuberculous mycobacterial disease, Brazil, 1993–2011. Emerging Infectious Diseases. 2013;19:393–399. doi: 10.3201/eid1903.120735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fusco da Costa AR, Falkinham JO, III, Lopes ML, et al. Occurrence of nontuberculous mycobacterial pulmonary infection in an endemic area of tuberculosis. PLoS Neglected Tropical Diseases [electronic resource] 2013;7:e2340. doi: 10.1371/journal.pntd.0002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy MP, O'Connor TM, Ryan C, et al. Nontuberculous mycobacteria: incidence in Southwest Ireland from 1987 to 2000. Respiratory Medicine. 2003;97:257–263. doi: 10.1053/rmed.2003.1431. [DOI] [PubMed] [Google Scholar]
- 45.Dailloux M, Abalain ML, Laurain C, et al. Respiratory infections associated with nontuberculous mycobacteria in non-HIV patients. European Respiratory Journal. 2006;28:1211–1215. doi: 10.1183/09031936.00063806. [DOI] [PubMed] [Google Scholar]
- 46.Henry MT, Inamdar L, O'Riordain D, et al. Nontuberculous mycobacteria in non-HIV patients: epidemiology, treatment and response. European Respiratory Journal. 2004;23:741–746. doi: 10.1183/09031936.04.00114004. [DOI] [PubMed] [Google Scholar]
- 47.Gerogianni I, Papala M, Kostikas K, et al. Epidemiology and clinical significance of mycobacterial respiratory infections in Central Greece. International Journal of Tuberculosis and Lung Disease. 2008;12:807–812. [PubMed] [Google Scholar]
- 48.Van Ingen J, Bendien SA, de Lange WCM, et al. Clinical relevance of nontuberculous mycobacteria isolated in Nijmegen-Arnhem region, The Netherlands. Thorax. 2009;64:502–506. doi: 10.1136/thx.2008.110957. [DOI] [PubMed] [Google Scholar]
- 49.Gitti Z, Mantadakis E, Maraki S, et al. Clinical significance and antibiotic susceptibilities of nontuberculous mycobacteria from patients in Crete, Greece. Future Microbiology. 2011;6:1099–1109. doi: 10.2217/fmb.11.91. [DOI] [PubMed] [Google Scholar]
- 50.McCallum AD, Watkin SW, Faccenda JF. Non-tuberculous mycobacterial infections in the Scottish Borders: identification, management and treatment outcomes--a retrospective review. Journal of the Royal College of Physicians of Edinburgh. 2011;41:294–303. doi: 10.4997/JRCPE.2011.403. [DOI] [PubMed] [Google Scholar]
- 51.Jankovic M, Samarzija M, Sabol I, et al. Geographical distribution and clinical relevance of nontuberculous mycobacteria in Croatia. International Journal of Tuberculosis & Lung Disease. 2013;17:836–841. doi: 10.5588/ijtld.12.0843. [DOI] [PubMed] [Google Scholar]
- 52.Marusic A, Katalinic-Jankovic V, Popovic-Grle s, et al. Mycobacterium xenopi pulmonary disease - epidemiology and clinical features in non-immunocompromised patients. Journal of Infection. 2009;58:108–112. doi: 10.1016/j.jinf.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 53.van Ingen J, Hoefsloot W, Dekhuijzen PN, et al. The changing pattern of clinical Mycobacterium avium isolation in the Netherlands. International Journal of Tuberculosis & Lung Disease. 2010;14:1176–1180. [PubMed] [Google Scholar]
- 54.OECD. OECD factbook 2014: economic, environmental and social statistics. OECD Publishing. 2014 [Google Scholar]
- 55.Nyamogoba HD, Mbuthia G, Mining S, et al. HIV co-infection with tuberculous and non-tuberculous mycobacteria in western Kenya: challenges in the diagnosis and management. African Health Sciences. 2012;12:305–311. doi: 10.4314/ahs.v12i3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aliyu G, El-Kamary SS, Abimiku A, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS ONE [Electronic Resource] 2013;8:e63170. doi: 10.1371/journal.pone.0063170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asiimwe BB, Bagyenzi GB, Ssengooba W, et al. Species and genotypic diversity of non-tuberculous mycobacteria isolated from children investigated for pulmonary tuberculosis in rural Uganda. BMC Infectious Diseases. 2013;13:88. doi: 10.1186/1471-2334-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Badoum G, Saleri N, Dembele MS, et al. Failing a re-treatment regimen does not predict MDR/XDR tuberculosis: is "blind" treatment dangerous? European Respiratory Journal. 2011;37:1283–1285. doi: 10.1183/09031936.00144710. [DOI] [PubMed] [Google Scholar]
- 59.Maiga M, Siddiqui S, Diallo S, et al. Failure to recognize nontuberculous mycobacteria leads to misdiagnosis of chronic pulmonary tuberculosis. PLoS ONE [Electronic Resource] 2012;7:e36902. doi: 10.1371/journal.pone.0036902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bicmen C, Coskun M, Gunduz AT, et al. Nontuberculous mycobacteria isolated from pulmonary specimens between 2004 and 2009: causative agent or not? New Microbiologica. 2010;33:399–403. [PubMed] [Google Scholar]
- 61.Braun E, Sprecher H, Davidson S, et al. Epidemiology and clinical significance of non-tuberculous mycobacteria isolated from pulmonary specimens. International Journal of Tuberculosis & Lung Disease. 2012;17:96–99. doi: 10.5588/ijtld.12.0237. [DOI] [PubMed] [Google Scholar]
- 62.Varghese B, Memish Z, Abuljadayel N, et al. Emergence of clinically relevant non-tuberculous mycobacterial infections in Saudi Arabia. PLoS Neglected Tropical Diseases [electronic resource] 2013;7:e2234. doi: 10.1371/journal.pntd.0002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Mahruqi SH, van-Ingen J, Al-Busaidy S, et al. Clinical relevance of nontuberculous Mycobacteria, Oman. Emerging Infectious Diseases. 2009;15:292–294. doi: 10.3201/eid1502.080977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shenai S, Rodrigues C, Mehta A. Time to identify and define non-tuberculous mycobacteria in a tuberculosis-endemic region. International Journal of Tuberculosis & Lung Disease. 2010;14:1001–1008. [PubMed] [Google Scholar]
- 65.Koh WJ, Kwon OJ, Jeon K, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006;129:341–348. doi: 10.1378/chest.129.2.341. [DOI] [PubMed] [Google Scholar]
- 66.American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997;156:S1–S25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- 67.British Thoracic Society. Management of opportunist mycobacterial infections: Joint tuberculosis committee guidelines 1999. Thorax. 2000;55:210–218. doi: 10.1136/thorax.55.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Okumura M, Iwai K, Ogata H, et al. Clinical factors on cavitary and nodular bronchiectatic types in pulmonary Mycobacterium avium complex disease. Internal Medicine. 2008;47:1465–1472. doi: 10.2169/internalmedicine.47.1114. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi M, Takayanagi N, Kanauchi T, et al. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;185:575–583. doi: 10.1164/rccm.201107-1203OC. [DOI] [PubMed] [Google Scholar]
- 70.Ito Y, Hirai T, Maekawa K, et al. Predictors of 5-year mortality in pulmonary mycobacterium avium-intracellulare complex disease. International Journal of Tuberculosis & Lung Disease. 2012;16:408–414. doi: 10.5588/ijtld.11.0148. [DOI] [PubMed] [Google Scholar]
- 71.Kitada S, Uenami T, Yoshimura K, et al. Long-term radiographic outcome of nodular bronchiectatic Mycobacterium avium complex pulmonary disease. International Journal of Tuberculosis & Lung Disease. 2012;16:660–664. doi: 10.5588/ijtld.11.0534. [DOI] [PubMed] [Google Scholar]
- 72.Wang CC, Lin MC, Liu JW, et al. Nontuberculous mycobacterial lung disease in southern Taiwan. Chang Gung Medical Journal. 2009;32:499–508. [PubMed] [Google Scholar]
- 73.Lai CC, Tan CK, Chou CH, et al. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000–2008. Emerging Infectious Diseases. 2010;16:294–296. doi: 10.3201/eid1602.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shu CC, Lee CH, Hsu CL, et al. Clinical characteristics and prognosis of nontuberculous mycobacterial lung disease with different radiographic patterns. Lung. 2011;189:467–474. doi: 10.1007/s00408-011-9321-4. [DOI] [PubMed] [Google Scholar]
- 75.Wang HX, Yue J, Han M, et al. Nontuberculous mycobacteria: susceptibility pattern and prevalence rate in Shanghai from 2005 to 2008. Chinese Medical Journal. 2010;123:184–187. [PubMed] [Google Scholar]
- 76.Jing H, Wang H, Wang Y, et al. Prevalence of nontuberculous mycobacteria infection, China, 2004–2009. Emerging Infectious Diseases. 2012;18:527–528. doi: 10.3201/eid1803.110175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haverkort F. National atypical mycobacteria survey, 2000. Communicable Diseases Intelligence. 2003;27:180–189. doi: 10.33321/cdi.2003.27.42. [DOI] [PubMed] [Google Scholar]
- 78.Freeman J, Morris A, Blackmore T, et al. Incidence of nontuberculous mycobacterial disease in New Zealand, 2004. New Zealand Medical Journal. 2007;120 [PubMed] [Google Scholar]
- 79.Brode SK, Jamieson FB, Ng R, et al. Increased risk of mycobacterial infections associated with anti-rheumatic medications. doi: 10.1136/thoraxjnl-2014-206470. In Press. [DOI] [PubMed] [Google Scholar]
- 80.Bodle EE, Cunnigham JA, Della-Latta P, et al. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerging Infectious Diseases. 2008;14:390–396. doi: 10.3201/eid1403.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amorim A, Macedo R, Lopes A, et al. Non-tuberculous mycobacteria in HIV-negative patients with pulmonary disease in Lisbon, Portugal. Scandinavian Journal of Infectious Diseases. 2010;42:626–628. doi: 10.3109/00365541003754485. [DOI] [PubMed] [Google Scholar]
- 82.Del Giudice G, Iadevaia C, Santoro G, et al. Nontuberculous mycobacterial lung disease in patients without HIV infection: a retrospective analysis over 3 years. The clinical respiratory journal. 2011;5:203–210. doi: 10.1111/j.1752-699X.2010.00220.x. [DOI] [PubMed] [Google Scholar]
- 83.Davies BS, Roberts CH, Kaul S, et al. Non-tuberculous slow-growing mycobacterial pulmonary infections in non-HIV-infected patients in south London. Scandinavian Journal of Infectious Diseases. 2012;44:815–819. doi: 10.3109/00365548.2012.694469. [DOI] [PubMed] [Google Scholar]
- 84.Park YS, Lee CH, Lee SM, et al. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. International Journal of Tuberculosis and Lung Disease. 2010;14:1069–1071. [PubMed] [Google Scholar]
- 85.Lee SK, Lee EJ, Kim SK, et al. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scandinavian Journal of Infectious Diseases. 2012;44:733–738. doi: 10.3109/00365548.2012.681695. [DOI] [PubMed] [Google Scholar]
- 86.Al-Houqani M, Jamieson F, Mehta M, et al. Aging, COPD and other risk factors do not explain the increased prevalence of pulmonary Mycobacterium avium complex in Ontario. Chest. 2012;141:190–197. doi: 10.1378/chest.11-0089. [DOI] [PubMed] [Google Scholar]
- 87.van Ingen J, Boeree MJ, de Lange WC, et al. Mycobacterium xenopi clinical relevance and determinants, the Netherlands. Emerging Infectious Diseases. 2008;14:385–389. doi: 10.3201/eid1403.061393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Ingen J, de Zwaan R, Dekhuijzen RP, et al. Clinical relevance of Mycobacterium chelonae-abscessus group isolation in 95 patients. Journal of Infection. 2009;59:324–331. doi: 10.1016/j.jinf.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 89.Hoefsloot W, van Ingen J, de Lange WC, et al. Clinical relevance of Mycobacterium malmoense isolation in The Netherlands. European Respiratory Journal. 2009;34:926–931. doi: 10.1183/09031936.00039009. [DOI] [PubMed] [Google Scholar]