Abstract
Neuropsychological performance in 151 patients with schizophrenia was examined using cluster analysis to identify neurocognitive subtypes. Hierarchical and iterative partitioning methods identified four clusters using an extended neuropsychological battery. Consistent with previous findings two extreme clusters were characterized by near normative performance and profound global dysfunction, respectively. The two remaining neurocognitive clusters displayed moderate-severe dysfunction and were differentiated by unique patterns of abstraction and flexibility, attention, spatial memory, and sensory-perception. Analysis of variance revealed an interaction between global memory and executive function for clusters III and IV. Although limited cluster differences were found relative to clinical and historical data, the distribution of previously defined clinical subtypes was uneven among neurocognitive clusters. Paranoid patients were significantly more likely to be classified into cluster II and disproportionately absent from clusters I and IV. Patients with negative and disorganized clinical subtypes comprised a disproportionate component of clusters I and IV but were less likely to be classified in cluster II. This suggests greater correspondence than previously postulated between systems responsible for clinical symptomatology and those moderating neurocognitive dysfunction.
Heterogeneity of schizophrenia has long been an area of theoretical and empirical inquiry. Numerous attempts have been made to classify the disorder into meaningful subtypes. Several classification systems based on clinical symptomatology have been proposed with the goal of identifying distinct etiology. Despite advances in subtyping and understanding clinical and cognitive symptomatology, heterogeneity in schizophrenia remains troublesome for investigators. Kraepelin (1925) and Bleuler (1952) initially suggested four subtypes including simple, catatonic, hebephrenic, and paranoid. Subsequent clinical classification systems focused on paranoid/nonparanoid, deficit/nondeficit, and positive/negative distinctions. Classification systems involving multivariate dimensions have also been proposed (Bilder, Mukherjee, Rieder, & Pandurangi, 1985; Carpenter, Bartko, Carpenter, & Strauss, 1976; Liddle, 1987) and a recent meta-analysis found three subtypes to be the most stable (positive, negative, and disorganized; Grube, Bilder, & Goldman, 1998). However, classification systems based on clinical phenomenology have been criticized on both methodological and theoretical grounds (Andreasen, Flaum, Schultz, Duyurek, & Miller, 1997; Goldberg & Weinberger, 1995). Specific criticisms included instability of clinical classification over time, reliance on self-report measures, and use of rating scales that require subjective interpretation. Moreover, growing understanding of schizophrenia as a neurocognitive disorder has focused attention on neural system involvement. One difficulty with subtypes based on clinical phenomenology is the difficulty relating symptoms directly to neural mechanisms.
Studies of the relationship between clinical subtypes and neuropsychological function have produced mixed results. Deficit/nondeficit and paranoid/nonparanoid classifications have received the most attention in the literature. Deficit patients have shown greater dysfunction in a wide range of areas including executive function, memory and learning, and visual-spatial skills (Buchanan et al., 1994; Hill, Ragland, Gur, & Gur, 2001; Mattson, Berk, & Lucas, 1997). Paranoid patients have shown relatively less impairment on tests of executive functions, attention, memory, and motor skills (Hill et al., 2001; Zalewski, Johnson-Selfridge, Ohriner, Zarrella, & Seltzer, 1998).
Given the established reliability of many neuropsychological measures over time, Heinrichs and Awad (1993) argued that a neurocognitive approach to subtyping schizophrenia would offer greater stability of classification. Furthermore, neuropsychological measures were developed based on their sensitivity to cerebral dysfunction. Thus, neuropsychological impairment can be directly linked to underlying neural function. To capitalize on the sensitivity of neuropsychological measures to neural systems, Heinrichs and Awad (1993) performed cluster analysis with selected neuropsychological tests to identify distinct neurocognitive clusters. A five-cluster solution based on aspects of executive function, memory, motor skills, and intelligence was revealed. Two extreme clusters were characterized by global impairment and normative performance, respectively. The remaining three intermediate clusters demonstrated executive dysfunction, fine motor dysfunction, and combined executive and fine motor dysfunction, respectively. More recent studies of neurocognitive clustering have used specific batteries of abstract reasoning and problem solving (Goldstein, Beers, & Shemansky, 1996) and intelligence scales (Goldstein, Allen, & Seaton, 1998; Seaton, Allen, Goldstein, Kelley, & van Kammen, 1999). Findings consistently identified four clusters including two extreme clusters with one displaying near normal function and the other characterized by severe and pervasive impairment. The remaining clusters were characterized by moderate impairments and differentiated primarily by psychomotor skills.
Previous investigations using empirical clustering methods to identify neurocognitive clusters in schizophrenia have focused on selective batteries of abstraction, intelligence, and selected aspects of memory and motor skills. Therefore, the impact of attention, memory, and other areas of known dysfunction in schizophrenia are not fully understood. Previous investigations have demonstrated relatively greater memory impairment in schizophrenia as well as nontrivial contributions of poor organizational and other executive skills to memory impairment in schizophrenia (Brebion, Amador, Smith, & Gorman, 1997; Censits, Ragland, Gur, & Gur, 1997; Chan et al., 2000; Gold, Randolph, Carpenter, Goldberg, & Weinberger, 1992; Ragland et al., 2001; Saykin et al., 1991, 1994). When investigating cognitive heterogeneity in schizophrenia, these findings demonstrate the need to consider broad neurocognitive domains simultaneously, specifically executive and memory functions. The present study was designed to identify empirical clusters of schizophrenia based on an extended neuropsychological battery assessing abstraction and flexibility, attention, verbal and visual memory, spatial abilities, language, sensory-perception, and motor skills. To assess the relationship between neurocognitive clusters and clinical symptomatology, comparisons among neurocognitive clusters were conducted using demographic, historical, and clinical data. It was hypothesized that clusters with less severe neuropsychological dysfunction would exhibit higher ratings of positive symptoms. On the other hand, clusters demonstrating greater neuropsychological dysfunction were postulated to demonstrate greater levels of negative symptoms. Finally, the relationship between neurocognitive and clinical subtypes was compared by assessing the proportional distribution of clinical subtypes within each derived cluster.
METHOD
Participants
The sample consisted of 151 patients who had previously been recruited to participate in neurobiological studies at the Schizophrenia Center of the University of Pennsylvania. Participants were diagnosed with schizophrenia according to DSM III–R/DSM IV (American Psychiatric Association [APA], 1987, 1994) criteria based on medical, neurologic, and psychiatric evaluation. Participants were carefully screened to eliminate possible confounds related to substance abuse, other psychiatric disorders, head injury or concussions, other medical disorders that may effect brain function, and medication effects. Screening was based on a structured clinical interview, laboratory tests, review of available medical records as well as self-report and informant report (Gur et al., 1991; Shtasel et al., 1992). Exclusion criteria were as follows: past or present Axis I disorder, other than schizophrenia; past or present personality disorder; history of neurologic disease such as cerebral ischemia, vascular headache, carotid artery disease, cerebral palsy, epilepsy, brain tumor, dementia, chronic meningitis, multiple sclerosis, pernicious anemia, normal-pressure hydrocephalus, Parkinson's disease, and Huntington's disease; and medical diseases that may affect brain function including hypertension, cardiac disease, diabetes mellitus, endocrine disorders, renal disease, and chronic obstructive pulmonary disease. Tardive dyskinesia is a movement disorder secondary to neuroleptic treatment, which may compound existing cognitive deficits in schizophrenia (Paulsen, Heaton, & Jeste, 1994). According to ratings on the Abnormal Involuntary Movement Scale (AIMS; National Institute of Mental Health, 1976) less than 6% of the sample had abnormal movements consistent with tardive dyskinesia. The distribution of these patients was not disproportionate relative to either clinical or neurocognitive grouping, thus it is unlikely that cognitive effects associated with tardive dyskinesia had a significant impact on the present data.
Participants were also excluded for documented or reported history of head trauma with loss of consciousness. To avoid confounds related to medication use or substance abuse, participants were excluded for use of medication with known cognitive effects (e.g., Topamax). Participants were excluded if a lifetime history of substance abuse or treatment was endorsed by the participant or informant. Although confirmation of a negative history of head injury or substance abuse was not possible because comprehensive medical records were not available, all participants displayed negative toxicology screens.
Procedure and Measures
Participants completed a standard neuropsychological battery assessing intelligence, abstraction and flexibility, verbal memory, visual memory, attention, language, visual-spatial abilities, sensory, and motor skills (Saykin et al., 1991). The test battery is listed in Table 1, including administration and scoring references. Not all participants received the same tests as the battery was updated with changes in the field. Earlier participants (56.7%) were administered subtests from the Wechsler Memory Scale (WMS) while more recent participants (43.3%) received updated subtests from Wechsler Memory Scale – Revised (WMS–R). Rather than using separate norm-referenced scores, percent correct scores were calculated for these measures. Battery administration was conducted by examiners with advanced training in standardized testing under faculty supervision. Test data were independently re-scored to eliminate arithmetic and data entry errors.
Table 1.
Neuropsychological Battery with Tests Grouped by Neuropsychological Function.
| Neuropsychological function | Component test variables |
|---|---|
| Abstraction and flexibility | WCST: Categories WCST: Perserverative Errors |
| Attention | Continuous Performance Test: Number correct1
Trail Making Test: Parts A & B speed2 WAIS–R: Digit Span & Digit Symbol Seashore Rhythm Test2 |
| Verbal memory | Logical Memorya I & II (WMS/WMS-R) CVLT: Learning trials 1–5 |
| Visual memory | Visual Reproduction I & II (WMS/WMS–R) |
| Spatial abilities | Judgment of Line Orientation WAIS–R: Block Design |
| Language | Controlled Oral Word Association Raw score3
Animal Naming Raw score (BDAE) Token Test3, 12 item version Boston Naming Test Mattis Aphasia Exam Visual Naming3 |
| Motor skills | Finger Tapping2
Thumb-Finger Sequential Touch4 |
| Sensory skills | Stereognosis Time (sec.), LNNB4
Stereognosis Errors, LNNB4 |
Note.
Gordon Diagnostic Systems, Inc. (Gordon, 1986).
Halstead–Reitan Neuropsychological Battery (Reitan & Wolfson, 1993).
Multilingual Aphasia Examination (MAE; Benton & Hamsher, 1976).
Luria–Nebraska Neuropsychological Battery (Golden, Purisch, & Hammeke, 1981); WCST, Wisconsin Card Sorting Test – card version (Heaton, 1981); WAIS–R, Wechsler Adult Intelligence Scale – Revised (Wechsler, 1981); WMS–R, Wechsler Memory Scale – Revised (Wechsler, 1987); CVLT, California Verbal Learning Test (Delis, Kramer, Kaplan, & Ober, 1983); BDAE, Boston Diagnostic Aphasia Examination (Goodglass & Kaplan, 1983).
Logical Memory percent recall used earlier subjects tested using the original Wechsler Memory Scale (WMS).
Data Analysis
To provide a standard metric for comparisons across subtests, test scores were standardized (z score) to performance of healthy controls in our database. Scores for each neuropsychological function were obtained by computing the mean of tests comprising each function (Censits et al., 1997; Saykin et al., 1991, 1994). Summary measures were calculated for abstraction and flexibility, attention, verbal memory, spatial memory, language abilities, spatial abilities, sensory-perception, and motor skills. Examination of raw neuropsychological function scores revealed extreme scores (5–14 SDs below the mean) that were truncated to z = −5.0. No verbal memory, spatial abilities, and motor skill scores were truncated. In contrast, 6.6% of abstraction and flexibility, 9.9% of attention, 4% of spatial memory, 0.6% of language, and 2% of sensory-perception scores were truncated.
Cluster analysis was based on truncated neuropsychological function scores and executed in two stages. In the first stage a hierarchical agglomerative clustering method was used to identify the optimal number of clusters. Initially each case was defined as its own cluster, then the most similar clusters were combined through successive steps until only one cluster remained. Clusters are combined only if the Euclidean distance between two clusters is lower than the distance between any other cluster pair. However, hierarchical agglomerative methods have been criticized because only one pass is made through the data and poor initial clustering cannot be corrected later (Blashfield & Aldenderfer, 1988). Also, hierarchical agglomerative methods may produce disparate results if cases are reordered. Thus, the second stage of cluster analysis subjected data to an iterative clustering method in which the number of clusters was specified and cases were free to vary among clusters until a minimum overall sum of squares was achieved. In this manner optimal assignment of cases to clusters was achieved. Both clustering procedures utilized Ward's linkage method (Ward, 1963).
Chi-square and one-way ANOVA were used to assess possible demographic differences among neurocognitive clusters (e.g., age, education, medication status, etc.). Bonferroni corrections were used to correct for multiple comparisons and control for inflation of Type I error. Possible differences in clinical symptomatology were assessed through mean comparison. Dependent measures included individual items from the BPRS, SANS, and SAPS as well as composite scores based on factor analysis of these measures (Gur et al., 1991). Finally, based on clinical data, patients were previously classified into clinical subtypes (negative, paranoid, disorganized, Schneiderian, mild; Gur et al., 1994). Patients in the negative subtype were characterized by symptoms such as avolition, blunted affect, and alogia. Paranoid patients displayed prominent delusions and hallucinations. Those classified as disorganized had symptoms consistent with concept disorganization, thought disorder, tangentiality, and incoherence. The Schneiderian subtype displayed a wide range of hallucinations and delusions involving thought broadcasting, thought insertion, and conversing or commenting voices. Finally, the mild subtype displayed less prominent and mixed clinical symptoms. To assess the relationship between clinical and neurocognitive clusters chi-square analysis was used to compare the expected and observed placement of clinical subtypes in each cluster classification.
RESULTS
Hierarchical cluster analysis resulted in four distinct neurocognitive profiles as determined by examination of agglomeration coefficients and visual inspection of the dendrogram. Iterative clustering methods confirmed cluster number and determined final arrangement of cases. Neuropsychological profiles for the four neurocognitive clusters are shown in Figure 1. Cluster I was characterized by severe (z = −2.5 to −3.5) impairment in sensory and motor skills, language, and spatial abilities as well as profound (z > −3.5) deficits on measures of memory and executive skills. This pattern is consistent with severe and diffuse cerebral impairment and may represent a floor effect for most measures. Thus, statements regarding differential neuropsychological impairment within this cluster may be misleading.
Fig. 1.
Mean neuropsychological profiles (± SEM) of the four neurocognitive clusters. Neuropsychological functions are abstraction (ABF), attention (ATT), verbal memory (VMEM), visual-spatial memory (SMEM), language (LAN), spatial abilities (SPA), sensory-perception (SEN), and motor skills (MOT). Cluster I: Severe and Profound Global Dysfunction; Cluster II: Near Normative Performance with Mild Dysfunction in Verbal Memory; Cluster III: Moderate-severe with more Prominent Executive than Memory Dysfunction; Cluster IV: Moderate-severe with more Prominent Memory than Executive Dysfunction.
Nearly one-half of all participants were classified into the second cluster. Level of performance varied from average to mildly impaired ranges with verbal memory being the area of greatest weakness. Previous studies have noted that 25–27% of the patients were classified in the normal range (Palmer et al., 1997; Weickert et al., 2000) as opposed to the nearly 50% of the present sample. However, comparatively normal distributions for IQ and each neurocognitive domain were noted upon examination of histograms. Thus, not all participants in cluster II displayed an overall level of performance in the normal range. Rather, cluster analysis did not “separate out” normal and mildly impaired groups because this statistical technique is more sensitive to profile similarities than level of performance. Participants classified into cluster II were similar with respect to a profile pattern of a relative weakness in verbal memory and, compared to the other clusters, had an otherwise flat profile.
Although the final two clusters displayed similar levels of performance, in the moderate-severely impaired range, the profile pattern differed substantially and was characterized by an interaction between memory and attention/abstraction functions. Cluster III was notable for greater impairments in attention and abstraction relative to memory, particularly visual-spatial memory. In contrast, more prominent memory dysfunction relative to executive skills were characteristic of cluster IV. It is important to note that the severity of the visual-spatial memory performance in cluster IV was greater than would be predicted based on spatial abilities alone. This suggests that spatial memory dysfunction is not solely due to deficits in spatial processing.
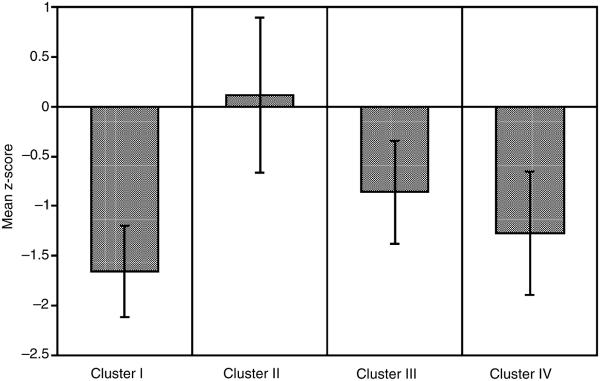
Intelligence quotient, as estimated by a composite of WAIS–R Vocabulary and Block Design, was used as a marker variable. Results of a one-way ANOVA comparing IQ among neurocognitive clusters were significant, F(3, 146) = 47.57, p < .0001), and are presented in Figure 2. Post hoc comparisons revealed that the least impaired group (cluster II) had significantly higher estimated IQ scores than all the other clusters. The profoundly impaired cluster had significantly lower IQ scores than the two moderate-severely impaired clusters. There was no difference between the moderate-severely impaired groups (clusters III & IV) on IQ scores. To better understand the relationship between intelligence and neurocognitive performance zero-order correlations between IQ and each of the eight neurocognitive domains are presented in Table 2. All groups displayed a significant relationship between intelligence and language. Despite limited power in some clusters, the magnitude of the relationships between intelligence and verbal memory and spatial abilities were somewhat consistent across groups. For cluster II, intelligence was significantly related to five of eight neurocognitive domains, perhaps suggesting a stronger cognitive reserve or underlying capabilities. On the other hand, the significant correlations between intelligence and several neuropsychological domains for cluster II may be a statistical artifact of a larger sample with increased power, range, and variation. Relative independence of intelligence from abstraction and, to a lesser extent, attention is evident by the preponderance of non-significant correlations among these domains.
Fig. 2.
Cluster means (± SEM) for estimated intellectual skills (combined WAIS–R Vocabulary and Block Design). Cluster II (near normative performance) displayed significantly higher intelligence scores than any other cluster. On the other hand, cluster I (diffuse and profound dysfunction) had lower intelligence scores than clusters II or III.
Table 2.
Correlation Between Intelligence and Each Neuropsychological Function for Each Neurocognitive Cluster.
| Neuropsychological function |
||||||||
|---|---|---|---|---|---|---|---|---|
| ABF | ATT | VMEM | SMEM | LAN | SPA | SEN | MOT | |
| Cluster I (n = 15) | 0.14 | 0.30 | 0.38 | −0.26 | 0.61* | 0.33 | 0.22 | −0.01 |
| Cluster II (n = 76) | 0.13 | 0.47** | 0.33** | 0.46** | 0.61** | 0.65** | 0.05 | 0.07 |
| Cluster III (n = 41) | −0.04 | 0.14 | 0.37* | −0.06 | 0.54** | 0.49** | −0.03 | 0.34* |
| Cluster IV (n = 19) | 0.29 | −0.02 | 0.15 | 0.16 | 0.59** | 0.46* | 0.53* | 0.20 |
Note. Cluster I: Severe and Profound Global Dysfunction.
Cluster II: Near Normative Performance with Mild Dysfunction in Verbal Memory.
Cluster III: Moderate-severe with more Prominent Executive than Memory Dysfunction.
Cluster IV: Moderate-severe with more Prominent Memory than Executive Dysfunction.
Demographic and clinical data for each of the four neurocognitive clusters are displayed in Table 3. Analysis of demographic and clinical data using a series of one-way ANOVA with four groups revealed significant cluster differences in education, parent education, age of illness onset, SANS total score, and individual SANS/SAPS items assessing alogia, attention, and delusions. Given the strong relationship between estimated IQ and cluster placement, IQ scores were used as covariates when appropriate (i.e., when significant one-way findings were present and when clusters displayed equivalent regression line slopes for the relationship between IQ and the demographic/clinical variable in question). When intelligence was held constant, ANCOVA failed to sustain the significant cluster differences for education, parent education, and SANS total score, as well as alogia and attention items of the SANS. Analysis of co-variance was inappropriate for age of onset and delusions due to nonparallel regression lines. Thus, post hoc comparisons of the one-way ANOVAs indicated that the mildly impaired cluster (II) had a later illness onset compared to cluster IV (stronger memory than abstraction /attention performance), F(1, 86) = 4.93, p = .01. Cluster II was also rated as having significantly more delusions than patients in cluster III with greater executive than visual-spatial memory performance, F(3, 144) = 3.14, p < .05.
Table 3.
Demographic, Historical, and Clinical Data for Each Neurocognitive Cluster of Schizophrenia.
| Neurocognitive cluster |
||||
|---|---|---|---|---|
| Cluster I | Cluster II | Cluster III | Cluster IV | |
|
| ||||
| Variable | n = 15 | n = 76 | n = 41 | n = 19 |
| Sex, M/F | 9/6 | 44/32 | 23/18 | 12/7 |
| Age | 37.00±12.58 | 31.39±9.69 | 31.44±10.48 | 30.86±9.96 |
| Education | 11.20±1.21 | 13.97±2.10 | 12.56±1.70 | 12.00±2.16 |
| Parental education | 10.50±1.52 | 13.15±2.93 | 12.58±2.57 | 12.17±2.72 |
| Age of onseta | 23.75±5.51 | 24.60±6.74 | 23.67±6.94 | 19.50±4.97 |
| Illness duration | 8.04±4.33 | 6.43±5.93 | 7.94±9.12 | 10.23±7.88 |
| No. of hospitalizations | 4.27±6.40 | 2.50±5.83 | 1.65±2.55 | 3.50±4.82 |
| BPRS total | 44.80±10.35 | 44.00±9.46 | 42.78±12.15 | 44.89±12.19 |
| SANS total | 52.93±26.85 | 35.77±20.99 | 45.29±27.81 | 46.11±27.46 |
| SAPS total | 43.80±24.87 | 37.67±19.99 | 32.27±19.43 | 39.58±28.61 |
| Negative – Affect | 2.27±1.10 | 1.96±1.22 | 2.34±1.41 | 2.37±1.61 |
| Negative – Alogia | 2.73±1.33 | 1.21±1.36 | 2.05±1.45 | 2.42±1.46 |
| Negative – Anhedonia | 3.13±1.46 | 2.79±1.35 | 2.66±1.37 | 2.63±1.57 |
| Negative – Avolition | 3.07±1.33 | 2.03±1.40 | 2.10±1.56 | 2.21±1.32 |
| Negative – Attention | 2.20±1.57 | 0.95±1.20 | 1.83±1.30 | 1.74±1.63 |
| Positive – Hallucination | 2.80±1.66 | 2.21±1.52 | 1.88±1.60 | 2.47±1.74 |
| Positive – Delusionb | 3.07±.96 | 3.19±1.14 | 2.41±1.20 | 2.58±1.35 |
| Positive – Bizarre | 1.40±1.30 | 0.89±1.18 | 1.15±1.28 | 1.68±1.29 |
| Positive – Thought Dis. | 2.27±1.83 | 1.41±1.32 | 2.00±1.38 | 1.68±1.38 |
| Negative composite | 2.24±1.13 | 1.67±.94 | 2.00±1.22 | 2.06±1.23 |
| Dis./Thgt composite | 1.60±1.16 | 1.12±.77 | 1.42±.79 | 1.43±.99 |
| Par-Host composite | 2.70±1.28 | 2.97±1.18 | 2.52±1.33 | 2.22±1.08 |
| Schneiderian composite | 1.74±1.10 | 1.46±1.12 | 1.05±.91 | 1.68±1.28 |
Note. Cluster I: Severe and Profound Global Dysfunction.
Cluster II: Near Normative Performance with Mild Dysfunction in Verbal Memory.
Cluster III: Moderate-severe with more Prominent Executive than Memory Dysfunction.
Cluster IV: Moderate-severe with more Prominent Memory than Executive Dysfunction.
II > IV.
II > III.
Deficits in abstraction, problem solving, and attention as well as verbal and visual memory are well established in the literature and have been viewed as hallmarks of schizophrenia (Brebion et al., 1997; Censits et al., 1997; Chan et al., 2000; Gold et al., 1992; Ragland et al., 2001; Saykin et al., 1991, 1994). Given the importance of memory and executive functions noted in previous research as well as the present findings of a unique and defining relationship between memory and attention/abstraction in the two moderate clusters, these constructs were examined in greater detail. For these analyses, a global memory score was computed as the mean of verbal and visual-spatial memory. Similarly, a global executive function was computed for the mean of attention and abstraction scores. Repeated measures analysis of variance (ANOVA) was used to examine between-group main effects of neurocognitive cluster (4 groups), within-groups main effects for global neuropsychological functions (memory and executive), and their interaction. Significant interactions were to be decomposed using one-way ANOVA for each neurocognitive cluster. Results indicated a significant main effect of neurocognitive cluster, F(3, 147) = 171.25, p < .001, and an interaction between cluster and global function, F(3, 147) = 28.20, p < .001, but no main effect of global function, F(1, 147) = 1.66, p = .20. Examination of the significant interaction revealed no differences between global memory and executive function for cluster I, F(14) = 0.86, p = .37. Significantly stronger executive than memory performance was noted for cluster II, F(75)= 15.52, p < .001, and cluster IV, F(18)= 45.43, p < .001, and stronger memory than executive performance was found in cluster III, F(40) = 33.41, p < .001.
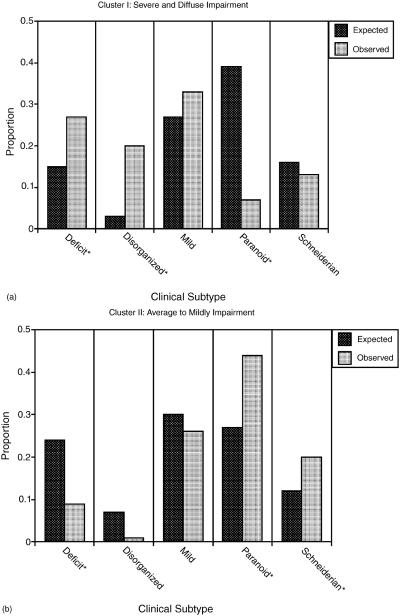
Chi-square analyses were performed to identify possible cluster differences on categorical variables. The sex distribution of all patients was approximately 58% male and was consistent with lifetime prevalence of schizophrenia (Kohn, Dohrenwend, & Mirotznik, 1998). The ratio of males to females within each cluster did not vary significantly from the overall sex distribution. To compare neurocognitive clusters identified in the present study with clinical subtypes previously developed (Gur et al., 1994), the expected distribution of clinical subtypes was calculated independently for each cluster. Comparison of expected and observed classifications was performed using omnibus chi-square analyses for each cluster. The results revealed a nonuniform distribution of clinical subtypes within neurocognitive cluster I (χ2 = 13.03, p < .01), cluster II (χ2 = 21.96, p < .001), and cluster IV (χ2 = 20.12, p < .01) but not cluster III (see Figs. 3a-d). To clarify the significant overall findings and assess which clinical subtypes varied as a function of neurocognitive profile, additional chi-square comparisons were conducted for each cluster. There were significantly more deficit (χ2 = 9.6, p < .05) and disorganized patients (χ2 = 96.3, p < .05) patients, but fewer paranoid patients (χ2 = 26.26, p < .05) in the profoundly impaired cluster I. Neurocognitive cluster II, characterized by low average to mildly impaired performance, was comprised of significantly larger proportions of paranoid (χ2 = 10.1, p < .05) and Schneiderian (χ2 = 5.3, p < .05) clinical subtypes and fewer deficit patients (χ2 = 9.38, p < .05). Those in cluster IV, with a stronger executive than memory neurocognitive profile, had greater proportions of deficit (χ2 = 35.6, p < .05) and disorganized (χ2 = 40.3, p < .05) clinical sub-types, with fewer than expected paranoid patients (χ2 = 15.16, p < .05).
Fig. 3(a)–(d).
Comparison between observed and expected distribution of clinical subtypes by neurocognitive cluster. *χ2 significant (p < .05).
DISCUSSION
The present investigation confirms previous findings of neurocognitive clusters underlying cognitive heterogeneity in schizophrenia using measures of abstraction and flexibility, motor performance, verbal abilities, and visual spatial skills (Goldstein, 1990; Goldstein et al., 1998; Goldstein & Shemansky, 1996; Heinrichs & Awad, 1993). Our results extend these findings to a comprehensive neuropsychological battery, which included multiple measures of memory, attention, language, and sensory performance. These findings also support the notion that cognitive heterogeneity is a robust phenomenon affecting a wide range of abilities in schizophrenia (Goldstein et al., 1998). As with previous studies, cluster analysis identified four neurocognitive clusters including two extreme clusters, characterized by near normative performance compared to severe and diffuse dysfunction, respectively, and two clusters with moderate-severe dysfunction (Goldstein, 1990; Goldstein et al., 1998; Goldstein & Shemansky, 1996; Heinrichs & Awad, 1993). Unlike previous studies the moderate-severe clusters were differentiated according to profile patterns that highlight an interaction between executive function (attention and abstraction), spatial memory, and sensory-perception. Specifically, there was an interaction in which cluster III displayed more prominent executive dysfunction relative to less impaired memory and other functions. This pattern was reversed for cluster IV patients who displayed severe memory dysfunction compared to moderate executive dysfunction.
There are several possible explanations for the double dissociation of memory and executive abilities for clusters III and IV. One hypothesis pertains to differential dysfunction of acquisition, storage, and retrieval systems. Although schizophrenia entails compromise to all three components of memory (Gold et al., 1992), the present data suggest a more intricate pattern. For example, cluster IV patients may have rapid forgetting with limited benefit from recognition prompts. In contrast, cluster III patients may have acquisition deficits relative to less dysfunctional storage and retrieval. These patients may display a pattern of inefficient learning but limited forgetting, and improvement with repetition and recognition prompts. Additional research is necessary to clarify the unique involvement of distinct memory components for each neurocognitive cluster.
Previous studies have classified schizophrenia patients according to normal, cortical, and sub-cortical memory patterns (Paulsen et al., 1995). Results indicated that schizophrenia patients with a normal memory profile were older, had a later age of onset, and were on a lower dose of neuroleptic medication than those with dysfunctional memory patterns. Furthermore, a greater number of negative symptoms were noted for those with a subcortical pattern. This is consistent with the present findings of disproportionate negative and disorganized clinical subtypes in the neurocognitive clusters with the greatest memory deficits (clusters I & IV). However, a more refined classification system, incorporating memory and executive involvement, is suggested by the identification of four neurocognitive clusters.
One issue underlying subtyping in schizophrenia is whether heterogeneity represents a continuum of severity or a limited number of distinct and qualitatively meaningful subtypes. The present findings provide evidence for both a severity continuum and qualitatively distinct clusters. Support for the severity continuum comes from findings of cluster differences in level of performance and similar profile patterns involving verbal memory, spatial-abilities, language, and motor skills (see Fig. 1). Additionally, intellectual abilities were highest in cluster II and lowest for those in cluster I who demonstrated global and profound neuropsychological impairment. On the other hand, unique patterns of neuropsychological function were evident for abstraction, attention, spatial memory, and sensory perception. Profile patterns were most distinct for the two clusters with moderate to severe dysfunction. One explanation for these data may be that severity of illness moderates or overlays qualitatively distinct clusters. These findings are consistent with Goldstein et al. (1998), who suggested that heterogeneity in schizophrenia is the result of differing clusters and variation in severity.
Aside from theoretical underpinnings, the cluster profiles of neuropsychological performance may have implications for functional outcome in the community. Recent research has related verbal memory to a variety of community outcome measures, executive skills with global community function and occupational performance, and vigilance with social problem solving and social skill acquisition (see Green, 1996; Lysaker, Bell, & Beam-Goulet, 1995; Velligan, Bow-Thomas, Mahurin, Miller, & Halgunseth, 2000). Cluster I patients who displayed profound and global dysfunction, would likely require the greatest amount of community support and would benefit least from social/community training. Despite near normative neuropsychological profile, patients in cluster II displayed the most difficulty with verbal memory and may display only mild deficits in overall community function. Social and community training should focus on other areas of relatively intact performance to mediate relative weaknesses in verbal memory. The impact of distinct neuropsychological profiles may be most salient for the two moderate-severely dysfunctional clusters. Based on the interaction between memory and executive function, patients with profile patterns characterized by stronger attention and abstraction than memory (cluster IV) may display relatively stronger occupational performance and social competence. Training with these patients should minimize memory demands and emphasize functional strengths associated with executive, spatial, and motor skills. In contrast, cluster III patients, with greater deficits in executive skills than memory, may demonstrate more difficulty in social and occupational situations and may derive greater benefit from visual-spatial memory aids such as flow charts, graphs, and the use of pictures/symbols.
A recent investigation by Weickert et al. (2000) found evidence for distinct patterns of cognitive dysfunction relative to intellectual change compared to estimated premorbid levels. Specifically, a decline of 10 or more points was associated with executive function, memory, and attention deficits. Those with preserved intelligence displayed executive function and attention impairments and an otherwise near normal profile. Patients with low premorbid and current intelligence displayed more areas of dysfunction including executive function, memory, attention, language, and visual-perception. Although premorbid intelligence was not assessed in the present study, the relationship between intelligence and neurocognitive cluster was addressed. The results indicated that level of intellectual performance corresponded to overall level of neuropsychological performance. Indeed, average range intellectual skills were found in the near normative cluster II compared to low average intellectual skills for the moderate-severely impaired clusters and significantly lower and border-line intellectual function for the most impaired classification (cluster I).
When the role of clinical symptomatology was assessed, few differences among neurocognitive clusters were found. Although contrary to our hypotheses, these data were consistent with previous studies reporting no differences among neurocognitive clusters in terms of symptom profile, symptom severity, and DSM subtypes (Seaton et al., 1999). This would suggest a lack of sensitivity of current clinical classifications, symptom assessments, and demographic variables to brain systems corresponding to both clinical and neurocognitive expression in schizophrenia. Development of future clinical scales using empirical criterion keying may be beneficial in identifying correspondence between clinical and neurocognitive features of schizophrenia.
Initially the lack of clinically related neurocognitive cluster differences would support the proposition by Palmer et al. (1997) that two separate systems may be responsible for clinical and neuropsychological symptoms in schizophrenia. However, despite a lack of robust cluster differences involving clinical data, rating scales, and composite clinical scores, the distribution of clinical subtypes (Gur et al., 1994; Hill et al., 2001) to neurocognitive clusters was uneven. These data are not fully consistent with the proposition that distinct systems are associated with clinical and neurocognitive symptomatology. Rather, the present data provide evidence of some degree of overlap between clinical and neurocognitive presentation suggesting greater overlap among systems than previously speculated (Palmer et al., 1997). This was supported by omnibus and specific chi-square comparisons indicating that paranoid patients, and to a lesser extent Schneiderian patients, were over-represented in the healthiest neurocognitive clusters and under-represented among clusters with the greatest dysfunction. The reverse was noted for negative and disorganized patients who were disproportionately classified into low functioning clusters and relatively absent within the higher functioning cluster II. An alternative explanation that incorporates the present findings with the postulation of Palmer et al. (1997) is that the distinct systems for clinical and neurocognitive symptomatology exist, but are mutually sensitive to a third underlying system. Regardless, these findings are consistent with previous literature indicating that paranoid patients have fewer cognitive impairments, respond better to treatment, and display better overall prognosis. On the other hand, negative or deficit patients display more cognitive impairments, are less responsive to treatment, and have a worse overall prognosis (Fenton & McGlashan, 1994; Hill et al., 2001; Kaplan & Sadock, 1994; Kirkpatrick, 1997; Zalewski et al., 1998).
Before definitive conclusions can be made, cross-validation and replication of both the neurocognitive clusters and their relation to clinical symptoms or subtypes is necessary. Future studies should also focus on possible differences in community outcome for patients with distinct neuropsychological profiles. There were several limitations to the current study. The eight neuropsychological functions were comprised of an unequal number of tests. Neuropsychological functions with fewer items may be less stable. For example, abstraction was comprised of two scores from the Wisconsin Card Sorting Test and had larger standard errors (see Fig. 1). Experiment-wise error may be a concern given the high number of comparisons involving clinical and historical data. However, when covaried for intellectual abilities only two differences were found and post hoc clarification used Bonferonni corrections for possibly inflated alpha error. Moreover, these findings were consistent with previous studies indicating higher ratings of positive symptoms among less neurocognitively impaired patients and earlier age of onset for more chronic and debilitating forms of schizophrenia. Although issues of Type I error were addressed to the extent possible, the unequal size of clusters may have differentially influenced power, and the probability of a Type II error is unclear.
ACKNOWLEDGMENT
This research was supported by National Institute of Health Training Grant MH 19112.
REFERENCES
- American Psychiatric Association . Diagnostic and statistical manual. 3rd. APA; Washington, DC: 1987/1994. revised/4th ed. [Google Scholar]
- Andreasen NC, Flaum M, Schultz S, Duyurek S, Miller D. Diagnosis, methodology and subtypes of schizophrenia. Neuropsychobiology. 1997;35:61–63. doi: 10.1159/000119390. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Multilingual aphasia examination. University of Iowa; Iowa City, IA: 1976. [Google Scholar]
- Bilder RM, Mukherjee S, Rieder RO, Pandurangi AK. Symptomatic and neuropsychological components of defect states. Schizophrenia Bulletin. 1985;11:409–419. doi: 10.1093/schbul/11.3.409. [DOI] [PubMed] [Google Scholar]
- Blashfield RK, Aldenderfer MS. The methods and problems of cluster analysis. In: Nesselroade JR, Cattell RB, editors. Handbook of multivariate experimental psychology. Plenum Press; New York: 1988. pp. 447–473. [Google Scholar]
- Bleuler E. Dementia praecox or the group of schizophrenia. International Universities Press; New York: 1952. [Google Scholar]
- Brebion G, Amador X, Smith MJ, Gorman JM. Mechanisms underlying memory impairment in schizophrenia. Psychological Medicine. 1997;27:383–393. doi: 10.1017/s0033291796004448. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Strauss ME, Kirkpatrick B, Holstein C, Breier A, Carpenter WT. Neuropsychological impairments in deficit vs. non-deficit forms of schizophrenia. Archives of General Psychiatry. 1994;51:804–811. doi: 10.1001/archpsyc.1994.03950100052005. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Bartko JJ, Carpenter CL, Strauss JP. Another view of schizophrenia subtypes: A report from the International Pilot Study of Schizophrenia. Archives of General Psychiatry. 1976;33:508–516. doi: 10.1001/archpsyc.1976.01770040068012. [DOI] [PubMed] [Google Scholar]
- Censits DM, Ragland JD, Gur RC, Gur RE. Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: A longitudinal study. Schizophrenia Research. 1997;24:289–298. doi: 10.1016/s0920-9964(96)00091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AS, Kwok IC, Chiu H, Lam L, Pang A, Chow LY. Memory and organizational strategies in chronic and acute schizophrenia patients. Schizophrenia Research. 2000;41:431–445. doi: 10.1016/s0920-9964(99)00078-x. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test (CVLT) manual. The Psychological Corporation; New York, NY: 1983. [Google Scholar]
- Fenton WS, McGlashan TH. Antecedents, symptom progression and long-term outcome of the deficit syndrome of schizophrenia. American Journal of Psychiatry. 1994;151:351–356. doi: 10.1176/ajp.151.3.351. [DOI] [PubMed] [Google Scholar]
- Gold JM, Randolf C, Carpenter CJ, Goldberg TE, Weinberger DR. Forms of memory failure in schizophrenia. Journal of Abnormal Psychology. 1992;101:487–494. doi: 10.1037//0021-843x.101.3.487. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. A case against subtyping in schizophrenia. Schizophrenia Research. 1995;17:147–152. doi: 10.1016/0920-9964(95)00060-y. [DOI] [PubMed] [Google Scholar]
- Golden CJ, Purisch AD, Hammeke TA. Luria-Nebraska Neuropsychological Battery: Forms I and II. Western Psychological Services; Los Angeles, CA: 1981. [Google Scholar]
- Goldstein G. Neuropsychological heterogeneity in schizophrenia: A consideration of abstraction and problem-solving abilities. Archives of Clinical Neuropsychology. 1990;5:251–264. [PubMed] [Google Scholar]
- Goldstein G, Allen DN, Seaton BE. A comparison of clustering solutions for cognitive heterogeneity in schizophrenia. Journal of the International Neuropsychological Society. 1998;4:353–362. [PubMed] [Google Scholar]
- Goldstein G, Beers SR, Shemansky WJ. Neuropsychological differences between schizophrenic patients with heterogeneous Wisconsin Card Sorting Test Performance. Schizophrenia Research. 1996;21:13–18. doi: 10.1016/0920-9964(96)00019-9. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Shemansky WJ. Neuropsychological differences between schizophrenic patients with heterogeneous Wisconsin Card Sorting Test performance. Schizophrenia Research. 1996;21:13–18. doi: 10.1016/0920-9964(96)00019-9. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. 2nd Lea and Febiger; Philadelphia, PA: 1983. [Google Scholar]
- Gordon M. Manual, Gordon Diagnostic System. Gordon Diagnostic Systems; New York, NY: 1986. [Google Scholar]
- Grube BS, Bilder RM, Goldman RS. Meta-analysis of symptom factors in schizophrenia. Schizophrenia Research. 1998;31:113–120. doi: 10.1016/s0920-9964(98)00011-5. [DOI] [PubMed] [Google Scholar]
- Gur RE, Mozley PD, Resnick SM, Levick S, Erwin R, Saykin AJ, Gur RC. Relations among clinical scales in schizophrenia. American Journal of Psychiatry. 1991;148:472–478. doi: 10.1176/ajp.148.4.472. [DOI] [PubMed] [Google Scholar]
- Gur RE, Mozley PD, Shtasel DL, Cannon TD, Gallacher F, Turetsky B, Grossman R, Gur RC. Clinical subtypes of schizophrenia: Differences in brain and CSF volume. American Journal of Psychiatry. 1994;151:343–350. doi: 10.1176/ajp.151.3.343. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test Manual. Psychological Assessment Resources; Odessa: 1981. [Google Scholar]
- Heinrichs RW, Awad AW. Neurocognitive subtypes of chronic schizophrenia. Schizophrenia Research. 1993;9:49–58. doi: 10.1016/0920-9964(93)90009-8. [DOI] [PubMed] [Google Scholar]
- Hill SK, Ragland JD, Gur RC, Gur RE. Neuropsychological differences among empirically derived clinical subtypes of schizophrenia. Neuropsychology. 2001;15:492–501. [PubMed] [Google Scholar]
- Kaplan HI, Sadock BJ. Synopsis of psychiatry: Behavioral sciences, clinical psychiatry. 7th Williams & Wilkins Company; Baltimore, MD: 1994. [Google Scholar]
- Kirkpatrick B. Affiliation and neuropsychiatric disorders: The deficit syndrome of schizophrenia. Annals New York Academy of Sciences. 1997;807:455–468. doi: 10.1111/j.1749-6632.1997.tb51939.x. [DOI] [PubMed] [Google Scholar]
- Kohn R, Dohrenwend BP, Mirotznik J. Epidemiological findings on selected psychiatric disorders in the general population. In: Dohrenwend BP, editor. Adversity, stress, and psychopathology. Oxford University Press; New York, NY: 1998. pp. 235–278. [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia. Livingston; Edinburgh: 1925. [Google Scholar]
- Liddle PF. Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychological Medicine. 1987;17:49–57. doi: 10.1017/s0033291700012976. [DOI] [PubMed] [Google Scholar]
- Lysaker P, Bell M, Beam-Goulet J. Wisconsin Card Sorting Test and work performance in schizophrenia. Schizophrenia Research. 1995;56:45–51. doi: 10.1016/0165-1781(94)02641-u. [DOI] [PubMed] [Google Scholar]
- Mattson DT, Berk M, Lucas MD. A neuropsychological study of prefrontal lobe function in the positive and negative subtypes of schizophrenia. The Journal of Genetic Psychology. 1997;158:487–494. doi: 10.1080/00221329709596685. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health . Abnormal Involuntary Movement Scale. In: Guy W, editor. ECDEU assessment manual for psychopharmacology. Rev. Department of Health, Education, and Welfare; Rockville, MD: 1976. pp. 534–537. [Google Scholar]
- Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, Zisook S, Jeste DV. Is it possible to be schizophrenic and neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Heaton RK, Jeste DV. Neuropsychological impairment in tardive dyskinesia. Neuropsychology. 1994;8:227–241. [Google Scholar]
- Paulsen JS, Heaton RK, Sadek JR, Perry W, Delis DC, Braff D, Kuck J, Zisook S, Jeste DV. The nature of learning and memory impairments in schizophrenia. Journal of the International Neuropsychological Society. 1995;1:88–99. doi: 10.1017/s135561770000014x. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: A PET cerebral blood flow study. American Journal of Psychiatry. 2001;158:1114–1125. doi: 10.1176/appi.ajp.158.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. 2nd Neuropsychology Press; Tucson, AZ: 1993. [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester B, Stafiniak P. Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Archives of General Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Archives of General Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Seaton BE, Allen DN, Goldstein G, Kelley ME, van Kammen DP. Relations between cognitive and symptom profile heterogeneity in schizophrenia. Journal of Nervous and Mental Disease. 1999;187:414–419. doi: 10.1097/00005053-199907000-00004. [DOI] [PubMed] [Google Scholar]
- Shtasel DL, Gur RE, Gallacher F, Heimberg C, Cannon T, Gur RC. Phenomenology and functioning in first episode schizophrenia. Schizophrenia Bulletin. 1992;18:449–462. doi: 10.1093/schbul/18.3.449. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Bow-Thomas CC, Mahurin RK, Miller AL, Halgunseth BA. Do specific neurocognitive deficits predict specific domains of community function in schizophrenia? Journal of Nervous and Mental Disease. 2000;188:518–524. doi: 10.1097/00005053-200008000-00007. [DOI] [PubMed] [Google Scholar]
- Ward J. Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association. 1963;58:236–244. [Google Scholar]
- Wechsler DA. Wechsler Adult Intelligence Scale Revised (WAIS–R), Manual. The Psychological Corporation; Cleveland, OH: 1981. [Google Scholar]
- Wechsler DA. Wechsler Memory Scale Revised (WMS-R) The Psychological Corporation; Cleveland, OH: 1987. [Google Scholar]
- Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger MD. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Archives of General Psychiatry. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- Zalewski C, Johnson-Selfridge MT, Ohriner S, Zarrella K, Seltzer JC. A review of neuropsychological differences between paranoid and non-paranoid schizophrenia patients. Schizophrenia Bulletin. 1998;24:127–145. doi: 10.1093/oxfordjournals.schbul.a033305. [DOI] [PubMed] [Google Scholar]