Abstract
Functional magnetic resonance imaging (fMRI) has played a prominent role in the quest to identify the brain systems responsible for cognitive dysfunction in schizophrenia. This chapter describes the evolution of these research efforts, which have alternated between efforts to localize specific cognitive impairments to work trying to understand broader network dysfunction. After a concise summary of localization efforts, the remainder of the chapter describes how different groups of scientists have developed and tested broader network theories. This includes a description of both task-activation and resting state studies, and involves a wide array of analytic techniques. The chapter closes with an understanding of how current default-mode and task-positive network theories grew out of these earlier resting-state and task-activation approaches, and provides some recommendations about future directions.
Keywords: Functional neuroimaging, Human brain mapping, Brain physiology, Psychosis, Cognitive neuroscience
1 Introduction
From the beginning of functional imaging research on cognition in schizophrenia, scientists have oscillated between functional localization approaches designed to map the pathophysiology of schizophrenia to discrete brain regions and cognitive functions, and functional integration approaches attempting to demonstrate how a broader breakdown in communication between multiple brain regions may explain disordered cognition in schizophrenia. Fortunately, these two worlds often collide— providing us with a more nuanced understanding of how information processing goes awry in the brains of individuals diagnosed with schizophrenia. The goal of this chapter is to describe how studies examining group differences in broader network activity (e.g., functional connectivity) grew out of initial studies designed to functionally segregate distinct areas of task-related activation. In so doing, we will briefly touch upon what has been learned from focal activation studies, including references to recent quantitative meta-analyses. However, these focal activation studies have been extensively reviewed (Ragland et al. 2007; Brown and Thompson 2010; Gur and Gur 2010), and the primary focus of this chapter will be on functional integration approaches—beginning with functional connectivity analyses using regions of interest (ROI) during active task conditions, moving to multivariate approaches aimed at examining resting default mode network (DMN) and activated task positive network (TPN) states, and ending with a discussion of more recent approaches employing multi-modal functional and anatomical imaging techniques.
2 Functional Localization
Functional imaging of individuals with schizophrenia has traced an interesting circle, from resting state, to “activation” studies employing task paradigms, and back again to resting studies. The first functional imaging study to localize pathology in schizophrenia was performed by Ingvar and Franzen (1974) who used 133Xenon blood flow (rCBF) during resting state to demonstrate that, compared to healthy participants, individuals with schizophrenia had relatively lower flows in the frontal cortex (i.e., “hypofrontality”), and higher flows in occipito-temporal regions. However, this resting hypofrontality was not always replicated (Gur et al. 1987a, b), leading to a refinement of the model, stressing that, “hypofrontality appears to be dependent on the behavioral state of the patients during the brain imaging experiment” (Weinberger et al. 1991, p. 276). This refinement emphasized the importance of using the subtraction technique to contrast activity during a task state with activity during a baseline condition designed to constrain what subjects were thinking about, and isolate brain regions responsible for the specific function that the task was designed to measure. The power of this approach had previously been demonstrated by Peterson et al., in a word fluency study (Posner et al. 1988), and was widely embraced by the schizophrenia research community.
Since this transition from initial resting state studies, activation paradigms have measured the impact of schizophrenia on regional brain function during a wide array of cognitive, sensory, and emotional processing tasks, leading to over 800 references in a recent PubMed search. These studies initially struggled with group performance differences that confounded interpretation of physiological differences. This difficulty was largely overcome with the development of event-related designs (Zarahn et al. 1997), which allowed one to follow recommendations (Carter et al. 2008) to restrict analysis to correct only trials. The difficulty of reviewing such a vast literature was somewhat simplified with the development of a quantitative meta-analytic technique termed Activation Likelihood Estimation (Turkeltaub et al. 2002; Laird et al. 2005). ALE measures the probability that spatially smoothed activation foci from individual studies occur across multiple studies, and tests the null hypothesis that the location of these activated foci is equal at every voxel, against an alternative hypothesis that activated foci are spatially distributed. To date, we are aware of six studies that have used ALE to perform meta-analyses of functional imaging studies of schizophrenia.
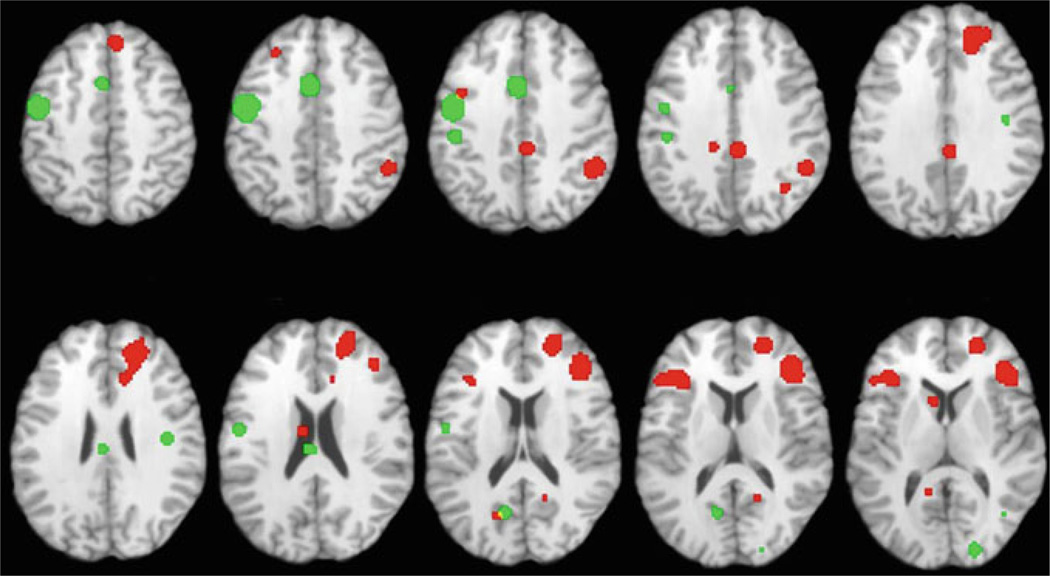
The first three ALE analyses examined working and episodic memory studies, respectively. In the first analysis, Glahn et al. (2005) examined working memory studies employing the N-Back task, and found predicted reductions in dorsolateral prefrontal cortex (DLPFC) activity in patients during task performance. However, patient reductions were not restricted to the DLPFC, extending to more rostral and ventrolateral/insular portions of the prefrontal cortex (PFC). Moreover, there was evidence of patient “overactivation” in the frontal pole, dorsomedial prefrontal cortex, and anterior cingulate gyrus, leading the authors to conclude that working memory dysfunction in schizophrenia cannot be classified as a simple DLPFC deficit, but should be considered in the context of the larger network of activity engaged by the task. Subsequent analyses of episodic memory encoding and retrieval studies also showed areas of both under- and over-activation in patients across multiple brain regions (see Fig. 1). Reduced activation in patients was observed in multiple areas of the PFC including dorsolateral and ventrolateral prefrontal cortex (VLPFC) (Achim and Lepage 2005; Ragland et al. 2009), and in non-prefrontal regions including the medial temporal lobe (Achim and Lepage 2005), thalamus (Ragland et al. 2009) and posterior cingulate gyrus (Achim and Lepage 2005; Ragland et al. 2009). Both analyses found that patients abnormally increased parahippocampal activation during episodic retrieval, and the study by Ragland et al. (2009) found that group differences in VLPFC activity were not present in studies that provided subjects with encoding instructions—thereby reducing strategic memory demands.
Fig. 1.
Illustration of group differences in fMRI activation during memory encoding in healthy participants and patients with schizophrenia based upon an activation likelihood estimation (ALE) meta-analysis of seven previously published studies. Areas of greater activation in healthy participants versus patients are indicated in red, and areas of greater activation in patients versus healthy participants are indicated in green. Image is adapted from original figure published in Ragland et al. (2009)
The three remaining ALE studies also found a distributed pattern of group differences. In an examination of a variety of executive function tasks Minzenberg et al. (2009) and Li et al. (2010) found patient reductions in the DLPFC, posterior cingulate gyrus, rostral and dorsal anterior cingulate gyrus and thalamus, and patient increases in pre-supplementary motor area and inferior parietal cortex. A study of facial emotion processing (Li et al. 2010) found patient over-activation in the insula, and patient underactivation in the amygdala, parahippocampal gyrus, fusiform gyrus, superior frontal gyrus, and lentiform nucleus. Finally, analysis of three studies of time perception (Ortuño et al. 2011), found that patients had reduced activation in middle and superior frontal gyrus, precentral gyrus, lentiform nucleus, thalamus, precuneus, and anterior cingulate, and did not show any areas of atypically increased activation.
As can be appreciated from this review of ALE studies, functional imaging research of schizophrenia using task paradigms and univariate statistical approaches has failed to reveal a “smoking gun” in which dysfunction of a single brain region can fully explain group differences in task performance. This should probably not come as a surprise as the behaviors most commonly impacted by and studied in schizophrenia (e.g., attention, memory, executive control) are multi-factorial—requiring integration of multiple brain regions. Moreover, even if a functional deficit is secondary to impairment of a single brain region, such a focal lesion would likely produce downstream effects on other brain areas, making it difficult to isolate group differences to a single location. There have been two primary responses to the challenges of functional localization. One has been to try to improve localization procedures by translating cognitive neuroscience paradigms designed to identify specific neural mechanisms into clinical studies that can more precisely map brain-behavior relationships. This approach holds great promise and is being developed for clinical trials research to discover new cognitive-enhancing agents, and is being facilitated by the NIH sponsored CNTRICS initiative begun in 2007 (Carter and Barch 2007). The second approach, and the focus of the remainder of this chapter, has been to employ multivariate statistical approaches designed to characterize the impact of schizophrenia on broader network activity.
3 Early Network Theories
One year after Ingvar and Franzen (1974) posited their “hypofrontality” hypothesis, they speculated that their results could be explained by disrupted transmission in a mediothalamic-frontocortical projection system (Franzén and Ingvar 1975) rather than disruption in a single brain region. The notion of disrupted integration of brain activity in schizophrenia was not formally tested until almost 10 years later when Clark et al. (1984) calculated Pearson correlations in positron emission tomography (PET) glucose metabolism between specified ROIs during electrical stimulation (mild shock) versus resting baseline conditions. In a descriptive comparison of patient and control groups, they found that the strong frontal coupling observed in healthy volunteers appeared reduced in the patient sample. Patients also appeared to show reduced anterior–posterior coupling in the left hemisphere and reduced bilateral coupling within anterior brain regions. Group differences in PET regional metabolic correlations were more rigorously tested several years later by Volkow et al. (1988) who employed resting and smooth pursuit eye movement conditions, and tested whether the overall pattern of regional correlations differed between patients and controls using the Kullback procedure. This analysis revealed that patients had a global decrease in the number of significant correlations, which was descriptively characterized as reduced anterior–posterior and cortico-thalamic correlations.
These initial correlational results, combined with clinical speculations that hallucinations and delusions might result from abnormal integration between different brain regions, led Friston and Frith (1995) to propose that schizophrenia may best be characterized as a disconnection syndrome. In this word production study, investigators adapted methodology developed in electrophysiological research to create a measure of functional connectivity defined as, “the temporal correlation between spatially remote neurophysiological events.” (Friston and Frith 1995). By using a stepwise regression-like procedure to identify patterns of functional connectivity between all of the voxels in the brain (i.e., eigenimages) the investigators found that the majority of the covariance in the time-series was captured by a negative interaction between prefrontal and superior temporal cortex in healthy controls during the word production task. In contrast, patients showed a positive correlation in fronto-temporal connectivity, leading the authors to conclude that schizophrenia is characterized by a reversal in typical prefrontal and temporal lobe integration. In a subsequent paper, Andreasen et al. (1996) reviewed a number of their previous PET activation studies to argue that this disconnection syndrome is not limited to the frontal and temporal lobe, and extends to a broader failure of integration between prefrontal–thalamic–cerebellar circuitry (termed “cognitive dysmetria”). The disconnection syndrome and cognitive dysmetria hypotheses provided a theoretical foundation for subsequent functional connectivity studies.
4 Task-Related Functional Connectivity
Prior to 2006 when researchers began to investigate resting-state networks, functional connectivity studies of schizophrenia examined group differences in network activity while participants performed various task activation paradigms. Several of these studies used working memory tasks, including the first study by Meyer-Lindenberg et al. (2001) who had healthy participants and patients with schizophrenia perform an N-Back working memory task and a sensorimotor baseline during PET rCBF imaging. As in the previous Friston and Frith (1995) study eigenimages were generated to characterize the variance–covariance matrix of the time-series. These eigenimages were created for a prespecified set of prefrontal, temporal, and parietal ROIs based on task activation results. The pattern that captured the most variance was negative relationships among the temporal cortex, hippocampus, and cerebellum in patients, and a positive relationship between the DLPFC and cingulate gyrus in healthy controls. This group dissociation in functional connectivity remained regardless of task condition, leading the authors to conclude that disruption of fronto-temporal interactions appears to be a trait marker of schizophrenia.
In the late 1990s the field transitioned from PET rCBF to blood oxygen level-dependent (BOLD) fMRI imaging procedures, which provided investigators with better temporal resolution, allowing implementation of new methods to examine functional connectivity. These subsequent fMRI studies examined functional connectivity with a variety of task paradigms. As a test of the disconnection syndrome hypothesis Lawrie et al. (2002) examined correlations between DLPFC and superior temporal gyrus (STG) ROIs in patients and controls while subjects performed a sentence completion task during fMRI. In support of the hypothesis of fronto-temporal disconnection, they found that left fronto-temporal correlations were reduced in schizophrenia, and showed greater reductions for patients with more severe hallucinations. The next study used a somewhat different analytical approach termed psychophysiological interaction analysis (PPI), which examined how correlations between BOLD signal activity in a “seed” region in the right anterior cingulate gyrus (ACC) and activity in the rest of the brain were modulated by task demands (Boksman et al. 2005). This was done for fMRI data collected while participants were performing a word generation task, and revealed that the right ACC showed localized interaction with the left temporal lobe associated with increased verbal fluency in healthy volunteers, that was absent in patients. Using a similar approach with fMRI data obtained during a continuous performance attention task, Honey et al. (2005) found evidence of both increased and decreased ACC connectivity in patients with schizophrenia during a degraded stimulus version of an attentional continuous performance task. When functional connectivity was examined in relation to degraded versus non-degraded stimulus conditions, patient decreases were seen in ACC connectivity to the cerebellum and, patient increases were seen in ACC connectivity to the prefrontal cortex. These disruptions in connectivity with cerebellar and motor areas were viewed in support of the cognitive dysmetria hypothesis. Finally, this timeseries correlational approach was also used to examine connectivity between STG and parahippocampal gyrus seed regions in the temporal cortex with voxels in the rest of the brain during a word encoding task that manipulated levels-of-processing (Wolf et al. 2007). This analysis of fMRI data revealed that, for deep versus shallow encoding conditions, patients had reduced temporal lobe connectivity to the DLPFC, but increased connectivity to the VLPFC, suggesting that the ventrolateral portion of the PFC may play a compensatory role for patients during episodic encoding.
One of the limitations of previously described functional connectivity analyses is that they do not provide information about the directionality of functional interactions. Therefore, an additional study of the N-Back paradigm (Schlösser et al. 2003) used structural equation modeling to assess “effective connectivity” in patients and controls. Effective connectivity is defined as, “the influence one neural system exerts over another” (Friston et al. 1994), and is examined by constructing anatomical models (i.e., path models) of the directional connections between pre-specified ROIs. Goodness-of-fit statistics are used to select the path models with the smallest residual error. Based upon previous anatomical and functional studies, Schlösser et al. (2003) proposed a unidirectional pathway from parietal cortex to the DLPFC and VLPFC, reciprocal pathways between DLPFC and VLPFC, and a cortico-cerebellar feedback loop involving bi-directional connections among prefrontal cortex, thalamus, and cerebellum. Between-group comparisons of these models revealed that patients receiving typical antipsychotics had reduced prefrontal-cerebellar and cerebellar-thalamic connectivity, and enhanced thalamo-cortical path coefficients. There was also evidence of enhanced interhemispheric connectivity in a group of patients receiving second-generation antipsychotic medications, suggesting that investigators should consider medication effects when performing these studies.
In sum, these functional connectivity studies employing activation tasks and ROI or seed-based correlational approaches have fairly consistently demonstrated that connectivity of the prefrontal cortex and the anterior cingulate gyrus with the temporal cortex appears to be reduced in patients with schizophrenia, consistent with the dissconection syndrome hypothesis. However, these results are not invariant—sometimes showing increased connectivity in patients, and sometimes differing depending upon clinical state, medication status, and the regions that are selected as seeds or as ROIs. As these studies progressed, concerns were also raised about the use of task activation paradigms to examine functional connectivity. When interpreting functional connectivity results, investigators were interested in drawing conclusions about anatomical connections (i.e., areas that fire together, wire together), and one concern about use of activation tasks was that they could create task-related correlations in the time-series that may obscure trait-like differences in anatomical connectivity (Biswal et al. 1995; Lowe et al. 1998; Raichle and Gusnard 2005). A related concern was that group differences in how subjects perform these activation tasks could contribute to group differences in functional connectivity apart from true differences in brain physiology. These concerns contributed to a directional shift back toward resting-state studies, which eliminate performance demands, and might, ostensibly, provide better trait-like measures of brain physiology.
5 Default Mode Network and Task-Positive Network
Resting-state functional connectivity analysis capitalizes on the finding that the brain appears to be intrinsically organized into functionally correlated networks that are independent of task (Fox et al. 2005; Fransson 2005; Buckner et al. 2008). Early observations in PET imaging found a consistent set of brain areas that showed higher metabolism during resting versus task conditions, particularly in posterior parietal and medial frontal areas (Gusnard and Raichle 2001; Raichle et al. 2001). Around the same time, fMRI studies revealed that BOLD signal in a similar set of brain regions fluctuated spontaneously and coherently at low frequencies (<0.1 Hz) when subjects were resting in the scanner, or simply “doing nothing” (Biswal et al. 1995; Greicius et al. 2003; Fransson 2005). This network comprises medial prefrontal, posterior parietal, and medial temporal regions—all brain areas that have been implicated as dysfunctional in schizophrenia. Moreover, signal fluctuations in this network are anti-correlated with BOLD signal in regions typically engaged by tasks, such as the lateral prefrontal cortex, suggesting a physiological tradeoff between the engagement of “task-negative” and “task-positive” brain states (Fox et al. 2005). This “task-negative” network is thought to represent a baseline brain state from which metabolic resources are diverted during cognitive demand, and has been termed the “default-mode” network (DMN; (Greicius et al. 2003)). The DMN has also been conceptualized as supporting internally directed thought, whereas task-positive networks are thought to support externally oriented cognitive processes (Buckner et al. 2008). Given previously discussed communication breakdowns between brain regions needed for cognitive tasks in schizophrenia, investigators hypothesized that group differences in functional connectivity during activation tasks could be co-occurring with, or driven by abnormal functional connectivity in the DMN. This has led to several fundamental questions about the nature of intrinsic brain networks in schizophrenia.
One question of primary interest has been whether functional connectivity differences between patients with schizophrenia and controls are detectable at rest. Liang et al. (2006) were the first to address this question, using an automated anatomical parcellation procedure to divide the brain into nine regions, and then comparing pairwise regional correlations in resting BOLD fluctuations between patients and controls. They found that resting-state correlations were reduced in patients across brain regions, with the most prominent differences noted in the insula, temporal lobe, striatum, and prefrontal cortex. Additionally, correlations between the cerebellum and all other regions were increased in patients during rest. The results from Liang et al. (2006) established that functional connectivity is abnormal in patients during rest, and that abnormalities occur across the entire brain, and are not limited to a few regions.
Rather than parcellating the whole brain into anatomical regions, Bluhm et al. (2007) focused their analysis of resting-state functional connectivity in schizophrenia on candidate regions of the DMN. To accomplish this, they began with a seed in the posterior parietal cortex (PPC), a region consistently identified as part of the DMN, and identified correlated voxels across the brain. Then, for each voxel in this network, they compared the degree of connectivity with the PCC between the healthy and patient groups. Functional connectivity between PCC and medial prefrontal cortex, parietal cortex, and cerebellum was reduced in patients. These combined studies suggested widespread functional disintegration within the DMN in schizophrenia patients even at rest. However, it remained unclear whether DMN connectivity was also abnormal in patients during active cognitive tasks, and whether DMN abnormalities were related to dysfunction in brain areas recruited by these tasks.
Thus, a second question was whether group network differences observed during rest were also apparent during task. To address this question, investigators employed a technique called independent component analysis (ICA), which is a data-driven approach to pinpointing temporally consistent, spatially independent brain networks in fMRI data, that occur regardless of task conditions (Calhoun et al. 2009). ICA can be applied equally in resting-state and task-related fMRI, and is particularly useful for identifying the task-negative DMN even during cognitively demanding tasks. The first study to detect DMN abnormalities in schizophrenia patients during task conditions (Garrity et al. 2007) used ICA on fMRI data acquired while subjects performed an auditory oddball detection paradigm. Researchers identified the independent component that most overlapped with DMN brain regions, and then compared the temporal and spatial characteristics of this component between patients and controls. Across both groups, the DMN component showed the greatest activation decrease during presentation of the auditory target stimuli, consistent with the notion that the DMN engagement decreases with greater cognitive demand. Patients had reduced activation compared to controls in the posterior cingulate and precuneus, but increased activation in a different part of the posterior cingulate, the anterior cingulate, and in the superior medial frontal gyrus. It is important to note that these group differences represented differences in regional BOLD signal intensity in those regions, rather than differences in functional connectivity, and reflected topographical differences in the DMN between patients and controls. Similarly, in the temporal domain, patients had relatively less power in low-frequency DMN oscillations, but relatively greater power in higher frequency DMN oscillations, suggesting that DMN abnormalities in patients extended beyond regional differences in network localization. A subsequent study from the same group (Calhoun et al. 2008) established that spatial and temporal DMN differences in patients found during the oddball task were also atypical during rest, suggesting that DMN findings during rest generalize to tasks, and vice versa. However, the spatial layout of both the DMN component and a component consistent with the task-positive network (TPN) were slightly different between activated and resting scans, suggesting that DMN networks were somewhat modulated by task, and that this modulation was not consistent across diagnostic groups.
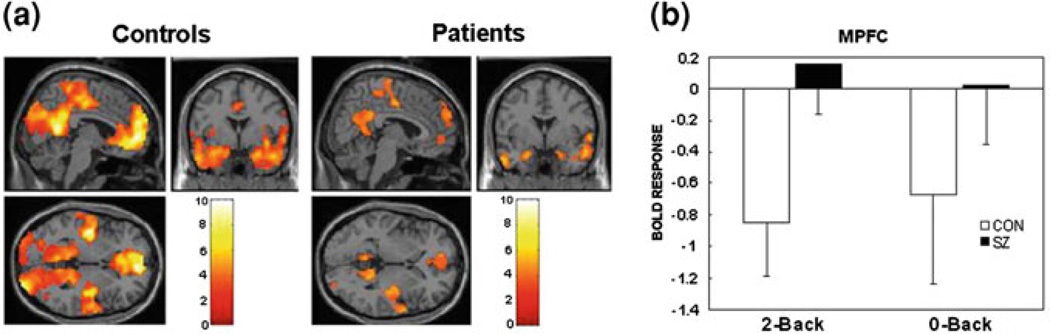
Several studies have attempted to unravel the relationship between the DMN and the TPN in patients with schizophrenia, with conflicting results. Comparing resting-state networks associated with signal in PCC and DLPFC seed regions, Zhou et al. (2007) found that anti-correlations between the DMN and TPN were greater in patients than in controls. Contrary to earlier findings of reduced connectivity, the study revealed greater within-network functional connectivity in patients. However, this analysis examined only anti-correlated brain regions, and may have missed other important between-group differences. A resting-state study by the Calhoun group (Jafri et al. 2008) compared correlations between seven key independent components and found that, in patients, the DMN independent component was more temporally similar to three of the other non-DMN components including the lateral frontal cortex. This finding suggested that schizophrenia patients might not adequately suppress the DMN when task-related regions come online. Whitefield-Gabrieli et al. (2009) tested this hypothesis by examining interactions between DMN and TPN activity across periods of rest and periods of performance on a working memory task in patients and controls. They found that DMN activity decreased during task, that better task-related DMN suppression was related to better working memory performance in patients, and that this task-related DMN suppression was greater in controls than in patients (see Fig. 2). Additionally, in patients, areas of the DMN were relatively more functionally connected during task, and less anti-correlated with TPN regions during both rest and task conditions. Based on these results, investigators posited a DMN hyperconnectivity/hyperactivity hypothesis, proposing that, in patients, a state of DMN over-synchronization results in failure to divert physiological resources away from the DMN during cognitively demanding tasks.
Fig. 2.
Task-related suppression of default network regions is lower in patients. a Greater activation during rest than task (2-back working memory paradigm) in DMN regions for controls (CON) and patients (SZ). b Task-related suppression of MPFC (a DMN region) during 2-back and 0-back conditions. Error bars are 95% confidence intervals. Image is adapted from original Figure published in Whitfield-Gabrieli et al. (2009)
Whitfield-Gabrieli et al. (2009) also speculated that this hyper-DMN state, in which regions putatively responsible for internally directed thinking are constantly more engaged, may be related to thought disturbance in schizophrenia. They showed that reduced task-related DMN suppression was related to higher scores on a psychopathology index in both the schizophrenia patient group and in healthy individuals. In the Bluhm et al. (2007) study, greater functional connectivity within the DMN during rest was positively associated with positive and negative symptoms in patients. Because of its relationship to both cognitive and clinical variables, it is possible that abnormally high engagement of the DMN is a state-level feature of schizophrenia that can explain both psychopathology and cognitive deficits. However, in these previous studies, patients were receiving antipsychotic medications, which may have contributed to group differences in DMN activity. Olanzapine, an atypical antipsychotic, has been shown to ameliorate clinical symptoms and potentially improve working memory performance. In a double-blind, counterbalanced study examining the effects olanzapine treatment, Sambataro et al. (2010) compared DMN functional connectivity during a working memory task when patients were on and off medication. Olanzapine dose was associated with increased functional connectivity in medial frontal areas of the DMN, but did not affect posterior regions. Although this study revealed that antipsychotic medication can mediate DMN connectivity, and may contribute to variability in functional connectivity differences across schizophrenia studies, DMN connectivity remained aberrant in the un-medicated patient group, suggesting that network-level differences in schizophrenia patients reflect underlying neuropathology regardless of medication effects.
While network connectivity analysis adds another level of complexity to our understanding of brain disruption in schizophrenia, questions remain about exactly what biological mechanisms drive the coherence of these functional networks. We now know that coherent signal fluctuations in intrinsic brain networks, particularly the DMN, are highly robust over time and across subjects in healthy populations (Biswal et al. 2010; Van Dijk et al. 2010), suggesting trait-like properties. Moreover, combinations of resting-state fMRI and either anatomical tracer studies in animals (Vincent et al. 2007) or white matter tractography in healthy humans (Greicius et al. 2009) show that intrinsic functional connectivity maps well—but not perfectly—with anatomical connections in the brain. That is, areas that are connected directly or indirectly by white matter tracts show correlated BOLD signal fluctuation during resting-state fMRI. If this functional– anatomical relationship holds in patients, one possible explanation for differences in intrinsic functional connectivity in schizophrenia is that the framework of anatomical connectivity that underlies a functional network is similarly atypical. Zhou et al. (2008) examined both functional and structural connectivity of the anterior hippocampus, a region important for episodic memory often implicated in schizophrenia, and found that both were reduced in patients, suggesting a relationship between functional disintegration and degraded white matter tracts. However, this functional–anatomical relationship may be less tightly coupled in patients than in controls. Skudlarski et al. (2010) directly compared diffusion tensor-based tractography and resting-state functional connectivity, and found that, while certain brain regions showed either increased or decreased intrinsic functional connectivity in patients versus controls, anatomical connectivity was generally degraded regardless of brain region in the patient sample. Furthermore, while functional networks reflected anatomical connectivity moderately well in controls, functional connectivity between any two brain regions was less predictive of the anatomical connections of those regions in patients. A key assumption of functional connectivity analysis is that network coherence is an indirect measure of anatomical connections. These most recent results challenge whether this assumption can be applied to schizophrenia populations.
6 Conclusions
Has the addition of functional connectivity analysis improved our understanding of pathophysiology in patients with schizophrenia? This research has clearly established that task-related and intrinsic functional connectivity are dysfunctional in patients, but studies do not agree on the exact nature of these disruptions. Task-based and default-mode networks are inversely related in healthy subjects, and this relation appears disrupted by schizophrenia. Patients generally fail to disengage DMN during task conditions, but it is not clear whether this impairment is driven by failure of task-related regions such as the lateral prefrontal cortex to deactivate DMN activity, or by hyperconnectivity within the DMN that makes it more resistant to task-related inhibition (Whitfield-Gabrieli et al. 2009). An added challenge of resting-state versus task activation studies is that resting-state studies do not employ experimental manipulations to test specific hypotheses, thereby reducing the interpretative leverage of the resting-state approach. Regardless of the answer to this question, this current state of affairs suggests caution in interpreting group differences in activation studies that use resting baselines as reference conditions, since these differences can be driven either by the task or by the reference condition. This work has clearly demonstrated that we can no longer think of schizophrenia as an impairment of a single brain region, and that we need to continue to work toward an understanding of group differences at a network level. This progress will likely depend upon the convergence of multiple approaches, including use of carefully controlled cognitive neuroscience tasks to more precisely map brain and behavior interactions, and use of multi-modal imaging techniques (structural and functional) to provide a convergent understanding of the DMN in schizophrenia.
Acknowledgments
This work was supported by NIMH grant R01MH084895.
References
- Achim A, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. Br J Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Théberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksman K, Théberge J, Williamson P, Drost DJ, Malla A, Densmore M, Takhar J, Pavlosky W, Menon RS, Neufeld RW. A 4.0-T fMRI study of brain connectivity during word fluency in first-episode schizophrenia. Schizophr Res. 2005;75:247–263. doi: 10.1016/j.schres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Brown GG, Thompson WK. Functional brain imaging in schizophrenia: selected results and methods. Curr Top Behav Neurosci. 2010;4:181–214. doi: 10.1007/7854_2010_54. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: a review. Front Hum Neurosci. 2009;3:17. doi: 10.3389/neuro.09.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Heckers S, Nichols T, Pine DS, Strother S. Optimizing the design and analysis of clinical functional magnetic resonance imaging research studies. Biol Psychiat. 2008;64:842–849. doi: 10.1016/j.biopsych.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Kessler R, Buchsbaum MS, Margolin RA, Holcomb HH. Correlational methods for determining regional coupling of cerebral glucose metabolism: a pilot study. Biol Psychiatry. 1984;19:663–678. [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzén G, Ingvar DH. Absence of activation in frontal structures during psychological testing of chronic schizophrenics. J Neurol Neurosurg Psychiatry. 1975;38:1027–1032. doi: 10.1136/jnnp.38.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Friston KJ, Tononi G, Reeke GN, Sporns O, Edelman GM. Value-dependent selection in the brain: simulation in a synthetic neural model. Neuroscience. 1994;59:229–243. doi: 10.1016/0306-4522(94)90592-4. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Gur RC. Functional magnetic resonance imaging in schizophrenia. Dialogues Clin Neurosci. 2010;12:333–343. doi: 10.31887/DCNS.2010.12.3/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Resnick SM, Gur RC, Alavi A, Caroff S, Kushner M, Reivich M. Regional brain function in schizophrenia. II. Repeated evaluation with positron emission tomography. Arch Gen Psychiatry. 1987a;44:126–129. doi: 10.1001/archpsyc.1987.01800140028004. [DOI] [PubMed] [Google Scholar]
- Gur RE, Resnick SM, Alavi A, Gur RC, Caroff S, Dann R, Silver FL, Saykin AJ, Chawluk JB, Kushner M. Regional brain function in schizophreniaI, A positron emission tomography study. Arch Gen Psychiatry. 1987b;44:119–125. doi: 10.1001/archpsyc.1987.01800140021003. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar DH, Franzén G. Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta Psychiatr Scand. 1974;50:425–462. doi: 10.1111/j.1600-0447.1974.tb09707.x. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Li H, Chan RC, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. 2010;36:1029–1039. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortuño F, Guillén-Grima F, López-García P, Gómez J, Pla J. Functional neural networks of time perception: challenge and opportunity for schizophrenia research. Schizophr Res. 2011;125:129–135. doi: 10.1016/j.schres.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Ragland J, Yoon J, Minzenberg M, Carter C. Neuroimaging of cognitive disability in schizophrenia: search for a pathophysiological mechanism. Int Rev Psychiatry. 2007;19:417–427. doi: 10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166:863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA. Intrinsic brain activity sets the stage for expression of motivated behavior. J Comp Neurol. 2005;493:167–176. doi: 10.1002/cne.20752. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Blasi G, Fazio L, Caforio G, Taurisano P, Romano R, Di Giorgio A, Gelao B, Lo Bianco L, Papazacharias A, Popolizio T, Nardini M, Bertolino A. Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology. 2010;35:904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, Stoeter P. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wolf AP, Brodie JD, Cancro R, Overall JE, Rhoades H, Van Gelder P. Brain interactions in chronic schizophrenics under resting and activation conditions. Schizophr Res. 1988;1:47–53. doi: 10.1016/0920-9964(88)90039-4. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Daniel DG. Prefrontal cortex dysfunction in schizophrenia. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal lobe function and dysfunction. New York: Oxford University Press; 1991. pp. 275–287. [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Gur RC, Valdez JN, Loughead J, Elliott MA, Gur RE, Ragland JD. Alterations of fronto-temporal connectivity during word encoding in schizophrenia. Psychiatry Res. 2007;154:221–232. doi: 10.1016/j.pscychresns.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D’Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, Yu C, Liu Z, Jiang T. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 2008;100:120–132. doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]