Abstract
Background
Individuals with schizophrenia have difficulty organizing words semantically to facilitate encoding. This is commonly attributed to organizational rather than semantic processing limitations. By requiring participants to classify and encode words on either a shallow (e.g., uppercase/lowercase) or deep level (e.g., concrete/abstract), the levels-of-processing paradigm eliminates the need to generate organizational strategies.
Methods
This paradigm was administered to 30 patients with schizophrenia and 30 healthy comparison subjects to test whether providing a strategy would improve patient performance.
Results
Word classification during shallow and deep encoding was slower and less accurate in patients. Patients also responded slowly during recognition testing and maintained a more conservative response bias following deep encoding; however, both groups showed a robust levels-of-processing effect on recognition accuracy, with unimpaired patient performance following both shallow and deep encoding.
Conclusions
This normal levels-of-processing effect in the patient sample suggests that semantic processing is sufficiently intact for patients to benefit from organizational cues. Memory remediation efforts may therefore be most successful if they focus on teaching patients to form organizational strategies during initial encoding.
Keywords: Schizophrenia, levels-of-processing, episodic memory, semantic processing, encoding strategy, neuropsychology
Introduction
The central role of semantic organization in verbal episodic memory (Tulving 1983) has been increasingly appreciated. Early studies showed that as information encoding moves from shallow perceptual processing to more elaborate semantic-associative encoding, the strength of the memory trace increases (Craik and Lockhart 1972; Kintsch 1968). The benefit of semantic organization is reflected by better recall of semantically related word lists that can be categorized. It has also been well established that patients with schizophrenia tend not to spontaneously use semantic information to categorize related word lists to benefit encoding and retrieval (Gold et al 1992; Iddon et al 1998; Koh et al 1973; Koh and Peterson 1978; Paulsen et al 1995). Failure to utilize semantic information has contributed to putative differential impairment of episodic memory in schizophrenia (Gold et al 1992; McKenna et al 1990; Rund 1989; Saykin et al 1991; Saykin et al 1994; see Blanchard and Neale 1994 for exception). The mechanism accounting for this impaired use of semantic information is less clear.
A leading explanation for why patients do not use semantic information to facilitate memory is that they have difficulty self-generating organizational strategies (Brébion et al 1997; Iddon et al 1998; Stone et al 1998). These strategic memory explanations conclude that executive problems and related frontostriatal dysfunction interfere with patients’ ability to adopt higher-level organizational strategies. A number of attempts have been made to remediate these strategic impairments. McClain (1983) examined free recall of 18 words following either random or blocked presentation (i.e., grouping words belonging to three different semantic categories together), with or without retrieval cues. Patient performance improved with blocking and equaled control performance only if blocked presentation and retrieval cues were provided. Gold et al (1992) contrasted three encoding presentations: a random list of 20 unrelated nouns; an unblocked list containing five exemplars, each from four randomly presented semantic categories; and a blocked list. Patients benefited from the blocked list but showed no difference between random and unblocked presentation. In contrast, controls benefited from both unblocked and blocked list presentation. Brébion et al (1997) compared recall of two types of word lists, random and unblocked, and obtained similar results. A study comparing blocked versus unblocked recall established that the beneficial effect of blocking was the same for acutely ill and chronically ill patients (Chan et al 2000). Finally, Iddon et al (1998) examined verbal list learning as part of a larger test battery before, during, and after training sessions where patients were instructed in semantic organizational strategies. Patients did not benefit from training and continued to organize the words based on serial order rather than semantic content.
Findings that patients benefit from blocked word presentation provide some support for the strategic memory explanation and suggest that semantic memory (i.e., knowledge) is grossly intact; however, results of below average blocked word performance (Gold et al 1992) and nonresponse to training (Iddon et al 1998) imply that patients have deficits in semantic or other information processing during word encoding apart from higher-level organizational strategies. Evidence of semantic processing deficits in schizophrenia comes primarily from verbal fluency paradigms in which participants generate words belonging to a specific category (e.g., animals). These studies show a consistent performance impairment (Allen and Frith 1983; Goldberg et al 1998) that is among the strongest neuropsychological discriminators of patients from healthy controls (Arango et al 1999). In a recent study (Moelter et al 2001), we found that impaired animal fluency was related to both aberrant automatic semantic-associative network activation and to impaired controlled processes such as search, access, and selection. If these semantic processing impairments are prominent, they may interfere with patients’ ability to engage in deep semantic processing and thereby result in a reduced levels-of-processing effect.
The role of semantic and organizational processes will be investigated in the current study through use of a levels-of-processing (Craik and Lockhart 1972) word encoding and recognition paradigm previously used in functional magnetic resonance imaging (fMRI) research (Buckner et al 1998a, 1998b; Demb et al 1995; Rugg 1998; Wagner et al 1998). In this paradigm, participants are explicitly instructed to process words on either a shallow level (i.e., perceptual features) or deep level (i.e., semantic content). This results in a levels-of-processing effect in which recognition is better for words that undergo deep encoding. By providing explicit instruction, strategic memory demands are reduced. Assuming that patients are able to switch between shallow and deep encoding strategies, this should normalize patient performance if semantic processing is intact; however, we hypothesize that patients have difficulties in both strategic memory and semantic processing and will, therefore, demonstrate a reduced levels-of-processing effect.
Methods and Materials
Participants
Participants were 30 patients (19 male subjects) and 30 healthy comparison subjects (17 male subjects) from the Schizophrenia Research Center at the University of Pennsylvania. The sample did not include data from two patients who were acutely psychotic and unable to attend or respond to the task. Patients and comparison subjects were matched on handedness (four nondextral in each group), age, and parental education. As expected, patients had fewer years of education. Estimated premorbid intelligence quotient (IQ) (National Adult Reading Test [NART]) (Nelson 1982) was also lower in the patient sample, although both groups had similar levels of word knowledge (Pyramids and Palm Trees) (Howard and Patterson 1992). Demographics are summarized in Table 1. Patients had a DSM-IV (American Psychiatric Association 1994) diagnosis of schizophrenia established by medical, neurologic, and psychiatric evaluations (Spitzer et al 1996a; Gur et al 1991). Comparison subjects also underwent standard medical, neurologic, and psychiatric evaluations (Spitzer et al 1996b; Shtasel et al 1991). Participants had no history of substance abuse or other medical, psychiatric, or neurologic disorder that might affect brain function.
Table 1.
Sample Characteristics for Comparison Subjects and Patients with Schizophrenia
| Variable | CS (n = 30) | SC (n = 30) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 30.79 | 10.00 | 33.92 | 8.86 |
| Educationa | 15.70 | 2.17 | 13.63 | 2.51 |
| Mother Education | 13.70 | 3.08 | 13.73 | 3.18 |
| Father Education | 13.67 | 3.64 | 14.50 | 3.47 |
| NART IQa | 107.27 | 7.62 | 98.20 | 13.23 |
| Pyramids and Palm Treesb | 3.47 | 2.50 | 4.68 | 3.01 |
CS, comparison subjects; SC, patients with schizophrenia; NART IQ, National Adult Reading Test Intelligence Quotient estimate
p < .05
number of errors
All patients were tested as clinically stable outpatients and rated as mildly to moderately ill on the following measures: Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham 1980), mean = 30.93, SD = 8.24, range = 18–46; Scale for Assessment of Negative Symptoms (SANS) (Andreasen 1984a), affective flattening = 1.44, SD = 1.50; alogia = 1.37, SD = 1.62; avolition = 1.48, SD = 1.50; anhedonia = 2.48, SD = 1.45; attention = .67, SD = 1.18; and Scale for Assessment of Positive Symptoms (SAPS) (Andreasen 1984b), hallucinations = 2.00, SD = 1.77; delusions = 2.15, SD = 1.51; bizarre behavior = 0; thought disorder = .38, SD = 1.16. On the Hamilton Depression Inventory (Hamilton 1960), only two patients scored in the high depression range (i.e., total rating >18) (Kohler et al 1998), and ratings for the full patient sample were below the cutoff for significant signs of depression (mean = 8.0, SD = 6.8, range = 0–23). Patients were 22.92 years old (SD = 6.34) at illness onset and had been ill an average of 10.24 years (SD = 7.85). All patients were receiving a stable dose of medication at time of testing: 11 typical, 18 atypical, and 1 both typical and atypical. Following the intake evaluation, a complete description of the study was provided and written informed consent was obtained before participation. The study was approved by the Institutional Review Board at the University of Pennsylvania.
Task Development and Administration
The Shallow/Deep Word Encoding and Recognition Task was developed using stimuli and procedures described in previous fMRI studies (Buckner et al 1998a, 1998b; Demb et al 1995). Test stimuli were chosen from a set of 240 abstract and 240 concrete 3- to 8-letter words (Buckner et al 1998a, 1998b). For encoding, four word lists containing 20 words each were constructed, with the constraint that 10 words were in uppercase letters and 10 words in lowercase letters, with half of the words in each case classified as abstract and half classified as concrete. Words in each set appeared in a pseudorandom order, and the four word lists were divided between 8 blocks with 10 words each. Words were presented at a rate of 2 seconds each, with a 1-second interstimulus interval (ISI), resulting in 30-second blocks. During shallow encoding, subjects were instructed to make a button press if the word was in uppercase letters. During deep encoding, a button press was made if the word was concrete. Shallow and deep conditions were alternated using an A-A-B-B design to reduce the number of transitions, since patients often have difficulty alternating response strategies. For recognition, 40 target words from encoding were randomly chosen (20 shallow, 20 deep) and mixed with 20 distractors (10 uppercase, 10 lowercase) that were matched on word length, frequency, and concreteness. Words were presented for 3 seconds each with a variable (or “jittered”) interstimulus interval (ISI) ranging from 5 to 13 seconds. A jittered ISI was included to permit future event-related fMRI studies. Subjects were instructed to press a left button if the word was from the encoding list and to press a right button if it was not from the encoding list. Response instructions (e.g., “press if concrete”) remained visible during encoding and recognition to improve comprehension and eliminate the need to maintain task instructions in working memory. Subjects were required to successfully complete practice trials and permitted to ask questions before the start of the experiment.
The task was developed and administered on Macintosh computers using the PowerLaboratory platform (MacLaboratory, Inc., Devon, Pennsylvania) (Chute and Westall 1996). The Shallow/Deep Word Encoding and Recognition Task, the National Adult Reading Test (Nelson 1982), and the Pyramids and Palm Trees test (Howard and Patterson 1992) were administered as part of a larger battery designed to assess memory and semantic processing (Moelter et al, unpublished data). The Pyramids and Palm Trees test requires participants to access higher order semantic information by deciding which of two written concepts (e.g., palm tree and pine tree) is most closely related to a target concept (e.g., pyramid). Graduate level students administered tasks under faculty supervision.
Data Analysis
Individual responses were recorded for shallow and deep encoding and recognition conditions. Mean percent correct (%COR) and median reaction time (RT) (in milliseconds) scores were calculated as indices of classification accuracy and speed during shallow and deep encoding. The sampling distribution of a proportion does not satisfy the normality assumption required for common parametric analysis, such as analysis of variance. The arcsine (a.k.a. angular) transformation, which normalizes the distribution, thus facilitating the use of methods requiring the normality assumption, was applied to the encoding percent correct scores. The arcsine transformation has the form: %COR_T = Sin−1(Total Correct/Total Responses)(1/2) (Cohen 1988). Participants’ responses during the recognition task were recorded as true positive, false positive, true negative, or false negative. Three primary recognition indices were examined. Discriminability (Pr) and response bias (Br) were calculated following the Two-High Threshold Theory (Snodgrass and Corwin 1988) that generates statistically independent signal detection indices. Discriminability reflects the proportion of time that an individual is either certain that an item is a target or certain that it is a foil and is an index of recognition accuracy. Response bias is an index of response bias with values greater than .5 indicating a “liberal” bias (i.e., a tendency to say “yes” when unsure) and values below .5 revealing a “conservative” bias (i.e., a tendency to say “no” when unsure). Median reaction time (RT) was calculated for true positive responses as an index of psychomotor speed. Discriminability, Br, and RT indices were calculated separately for words that had undergone deep versus shallow encoding.
Group differences during word encoding and recognition were examined by entering respective performance indices into separate 2 (patient, comparison subject) by 2 (shallow, deep) multivariate analyses of variance (MANOVA) (Proc GLM; SAS Institute 1999) with repeated-measures for the second factor. Post hoc analysis of variance (ANOVA) was used to decompose any significant interactions.
Results
Word Classification Accuracy
Patients and comparison participants performed well on shallow and deep classification tasks during word encoding. Word classification accuracy was close to ceiling for healthy participants (%COR = 99.67, SD = .16) and patients (%COR = 95.25, SD = 1.30) during shallow encoding and somewhat lower in both groups during deep encoding (comparison subjects, %COR = 92.41, SD = 1.15; patients, %COR = 83.75, SD = 2.12). Multivariate analysis confirmed that accuracy was lower for deep encoding [F(1,58) = 119.51, p < .0001] and for patients across conditions [F(1,58) = 20.88, p < .0001], with no interaction [F(1,58) = .01, p = .99]. Examination of reaction time data found that classification decisions were made within about a second in both groups (comparison subjects: shallow = 709 milliseconds, SD = 26, deep = 1004 milliseconds, SD = 27; patients: shallow = 1011 milliseconds, SD = 47, deep = 1282 milliseconds, SD = 47). Multivariate analysis revealed that reaction times were slower for the deep encoding task [F(1,58) = 101.90, p < .0001] and for patients [F(1,58) = 38.97, p < .0001], with no interaction [F(1,58) = .19, p = .67]. Thus, patients showed normal level-of-processing effects on word classification speed and accuracy but remained slower and less accurate than the comparison sample across conditions.
Word Recognition
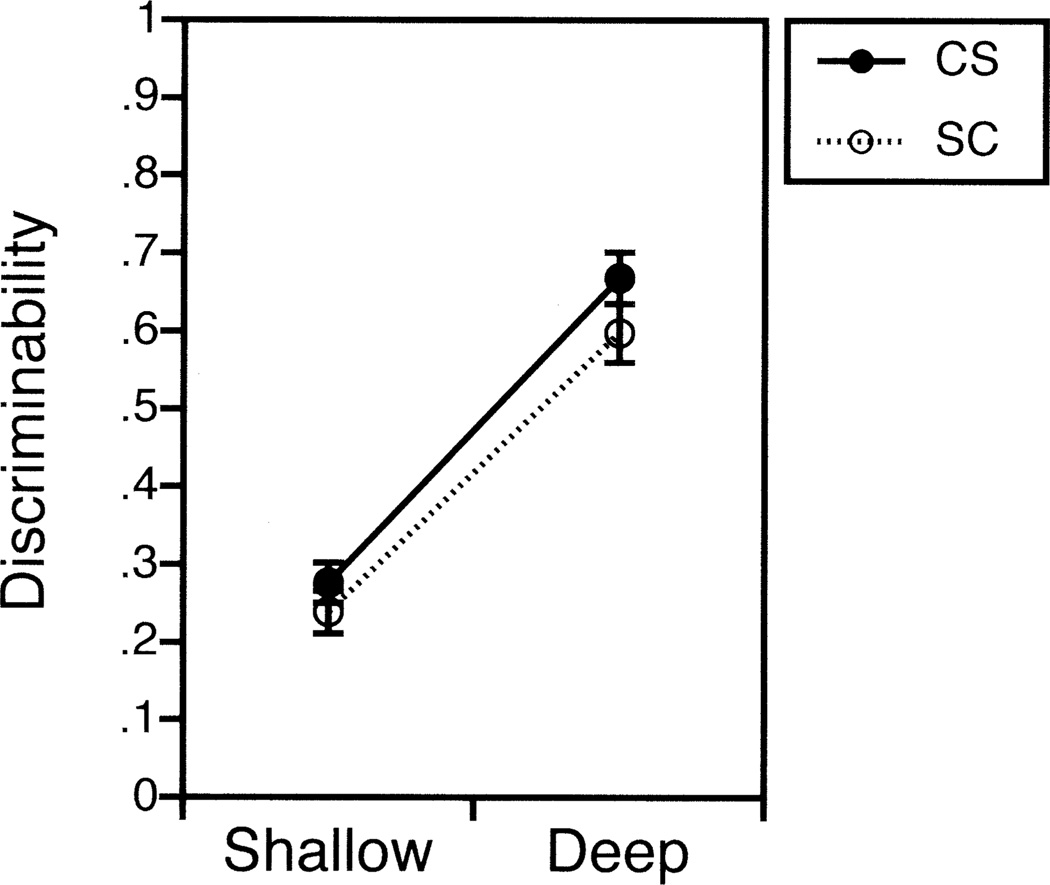
As can be seen in Figure 1, both groups had better recognition discriminability following deep encoding [F(1,58) = 289.58, p < .0001], with no effect of diagnosis [F(1,58) = 2.00, p = .16] or any diagnosis by level of processing interaction [F(1,58) = .57, p = .45]. This levels-of-processing effect was quite robust, with discriminability improving by over twofold in both groups (comparison subjects = 174 + 22% increase; patients = 102 + 82% increase). Thus, patients showed the same benefit of deep processing on recognition accuracy as the comparison group. To investigate the lack of group differences in recognition discriminability, an observed effect size (d) (Cohen 1988) was calculated. This index provides an estimate of the test score overlap between groups, with values of .2 representing a “small” effect size, .5 a “medium” effect size, and .8 a “large” effect size. Relatively small effect sizes were found for both shallow (d = .27) and deep recognition discriminability (d = .39), suggesting that it is unlikely that group differences in recognition accuracy would have been revealed with larger samples.
Figure 1.
Mean (±SEM) recognition discriminability for 30 healthy comparison subjects (solid line) and 30 patients with schizophrenia (dotted line).
When response bias was examined (Figure 2), there were effects of diagnosis [F(1,58) = 19.59, p < .0001] and level-of-processing [F(1,58) = 116.94, p < .0001] and a diagnosis by level-of-processing interaction [F(1,58) = 17.90, p < .0001]. As illustrated in Figure 2, patients had a slightly more conservative response bias for words that had undergone shallow encoding [F(1,58) = 5.73, p = .02]. Following deep encoding, comparison subjects had a liberal response bias, whereas patients maintained a more conservative bias [F(1,58) = 25.68, p < .0001]. Whereas healthy participants moved from a conservative to liberal response bias when words were more deeply encoded, patients maintained a tendency to reject words when unsure (a conservative bias) following both shallow and deep encoding.
Figure 2.
Mean (±SEM) recognition response bias for 30 healthy comparison subjects (solid line) and 30 patients with schizophrenia (dotted line).
Finally, when reaction time was examined (Figure 3), speed was found to be faster for words that had undergone deep encoding [F(1,58) = 31.63, p < .0001], and patients were slower across conditions [F(1,58) = 17.22, p < .0001], with no interaction [F(1,58) = 1.26, p = .27].
Figure 3.
Mean (±SEM) reaction time (in milliseconds) for 30 healthy comparison subjects (solid line) and 30 patients with schizophrenia (dotted line).
Additional Analyses
Several possible alternative explanations of patients’ recognition performance were examined in a series of post hoc analyses.
EFFECT OF REDUCED WORD CLASSIFICATION SPEED AND ACCURACY
Groups were not equal on word classification accuracy or speed during initial encoding, which may have influenced subsequent recognition performance. This was investigated by performing analyses of covariance (ANCOVA)1 to test for main effects of diagnosis while accounting for group differences in classification accuracy and speed. When encoding accuracy was covaried, results for recognition discriminability and response bias remained unchanged. For recognition discriminability there were still no group differences for either shallow [F(1,57) = .10, p = .75] or deep conditions [F(1,57) = .10, p = .76]. The ANCOVA for response bias showed main effects of diagnosis for both shallow [F(1,57) = 4.14, p = .046] and deep encoding [F(1,57) = 22.5, p < .0001]. Reaction time results changed slightly. There was no longer a main effect of diagnosis on reaction time for words that had undergone shallow encoding [F(1,57) = 3.55, p = .06]; however, the main effect of diagnosis remained for reaction time during the deep condition [F(1,57) = 5.42, p < .05].
When ANCOVAs were performed using encoding reaction time as a covariate, there was again no change in the pattern of results for recognition discriminability and response bias. As before, there was no group difference in recognition discriminability [shallow: F(1,57) = .90, p = .35; deep: F(1,57) = .09, p = .77], and the previously described group differences in response bias remained unchanged [shallow: F(1,57) = 4.47, p < .05; deep: F(1,57) = 16.68, p < .0001]; however, for the reaction time index, there was no longer evidence of patient slowing during either shallow [F(1,57) = 2.33, p = .13] or deep conditions [F(1,57) = 2.56, p = .11] once initial slowing in word classification accuracy was accounted for. This reflects how pervasive psychomotor slowing is in schizophrenia (Sovani and Thatte 1998) and indicates that it was not unique to the recognition condition. Although reaction time slowing did not appear to account for the interaction in response bias in the current study, processing speed deserves close attention in future studies, as it may serve as an index of available resources for higher cognitive processing. In sum, the original pattern of similarities in recognition discriminability and differences in response bias did not appear due to group differences in word encoding classification speed and accuracy. Reaction time slowing in patients during recognition testing, however, appeared secondary to a generalized slowing that was also present during initial word classification.
EFFECT OF PREMORBID INTELLECTUAL DIFFERENCES
The second analysis assessed the possible impact of group differences in estimated premorbid verbal intelligence. Although groups were matched on parental education and word knowledge, patients had worse NART (Nelson 1982) performance, raising the possibility that differences in verbal intelligence may have influenced encoding and recognition task performance. Therefore, the original multivariate analyses were repeated on a subsample of 19 patients with schizophrenia (11 male patients) and 19 healthy comparison subjects (12 male subjects) who were matched on NART performance.
Analyses of word classification accuracy no longer revealed a difference between patients and comparison participants [F(1,36) = 2.64, p = .11]. Classification remained less accurate for the deep condition [F(1,36) = 73.84, p < .0001], and there was still no interaction between group and level-of-processing [F(1,36) = .10, p = .76]. It therefore appears that patients’ relative difficulty classifying words as uppercase/lowercase or concrete/abstract was related to their lower estimated premorbid verbal intellectual abilities. When word classification speed was examined, reaction times remained slower for the deep condition [F(1,36) = 85.9, p < .001] and for patients [F(1,36) = 23.6, p < .0001], with no interaction [F(1,36) = .36, p = .55]. When recognition performance was examined, results were relatively unchanged. Discriminability remained lower for words that had undergone shallow encoding [F(1,36) = 226.26, p < .0001], and there was still no main effect of group [F(1,36) = .02, p = .89] or any group by condition interaction [F(1,36) = .05, p = .82]. Response bias results were also similar, with main effects of condition [F(1,36) = 78.52, p < .0001], group [F(1,36) = 8.09, p < .05], and a group by condition interaction [F(1,36) = 7.59, p < .05]. When the interaction was examined, there was no longer a group difference for the shallow condition [F(1,36) = 2.74, p = .11], but patients remained more conservative following deep encoding [F(1,36) = 10.61, p < .05]. Finally, reaction times remained faster for words that had been deeply encoded [F(1,36) = 22.18, p < .0001], and patients remained slower across conditions [F(1,36) = 8.79, p < .05], with no group by condition interaction [F(1,36) = 2.01, p = .16]. In sum, group differences in estimated premorbid verbal intellectual ability appeared to contribute to less accurate word classification during initial encoding; however, these premorbid differences did not appear to influence recognition performance.
RELATIONSHIP BETWEEN RESPONSE BIAS AND CLINICAL SYMPTOMS
This final analysis investigated whether clinical factors may have played a role in the patients’ more conservative response bias. There are some reports that conservative response biases more likely accompany depression or negative symptoms, whereas a liberal response bias is more likely to occur in mania or be related to positive symptoms (Corwin et al 1990; Brébion et al 1999, 2000). Given the absence of clinically significant depression in our patient sample, correlations with Hamilton Depression Inventory ratings were not examined; however, a series of regression analyses were performed to determine if response bias could be predicted by clinical measures of negative (Affective Flattening, Alogia, Avolition, Anhedonia, and Attention subscales of the SANS) or positive symptoms (Hallucinations, Delusions, and Thought Disorder subscales on the SAPS). The Bizarre Behavior subscale on the SAPS was not examined, as all but three subjects had values of zero.
Because there is not a simple linear interpretation of response bias, both linear and quadratic regression models were investigated. There were no significant quadratic effects, so linear results are reported. Delusion and Thought Disorder subscales on the SAPS were found to be positive predictors of response bias. Regression results for SAPS subscales were in line with previous findings (Brébion et al 1999, 2000) in that more severe residual positive symptoms following treatment predicted less conservative response biases. The Thought Disorder subscale predicted response bias during both shallow [F(1,24) = 11.12, p = .002] and deep conditions [F(1,24) = 11.87, p = .002], accounting for 32% and 33% of the respective variance. The Delusion subscale was predictive for the deep condition only [F(1,24) = 6.90, p = .01], with 22% of the variance accounted for. None of the subscales on the SANS had predictive value.
Discussion
The levels-of-processing effect (Craik and Lockhart 1972) is a robust phenomenon in which episodic memory is better for information that undergoes deep (i.e., conceptual) versus shallow (i.e., perceptual) processing. Contrary to our initial hypothesis, we found that patients showed a normal levels-of-processing effect on recognition discriminability and reaction time. Both groups experienced more than a twofold increase in recognition discriminability. This is even greater than the 25% effect reported in a recent imaging study of word stem completion that linked deep processing with right posterior hippocampal activation (Heckers et al 2002). The current levels-of-processing effect was found despite patients having lower estimated premorbid verbal intellectual abilities and slower and less accurate word classification during initial encoding. When these factors were controlled statistically and through analysis of matched subgroups, recognition discriminability and response bias results remained unchanged. This normal levels-of-processing effect suggests that patients’ semantic processing is sufficiently intact for them to fully benefit from being provided with organizational strategies during initial encoding when tested in a recognition format.
In support of earlier claims of recognition memory being intact in schizophrenia (Calev 1984; Goldberg et al 1989), we found that patients’ recognition discriminability was unimpaired for words that had undergone both shallow and deep encoding; however, unimpaired recognition performance does not necessarily imply that patients’ episodic memory is unaffected. As revealed in subsequent studies that found mild impairments in recognition discriminability (Aleman et al 1999; Danion et al 1999; Weiss et al 2002), nonrelational recognition tasks can be successfully performed by relying on feelings of familiarity (noetic knowledge) rather than actual reliving of the encoding event (autonoetic knowledge). When autonoetic knowledge is tested through source memory probes and similar procedures, patient performance is once again impaired. The implication of this for the current study is that a normal levels-of-processing effect on recognition discriminability may not translate to intact patient performance on free recall or source memory tasks. This may help explain why McClain (1983) had to use both blocked word presentation and retrieval cues to normalize patient performance in her free recall study and suggests that providing organizational cues alone will not be sufficient to fully remediate memory performance in schizophrenia.
Although recognition discriminability was intact, there were group differences in response bias, with patients maintaining a more conservative bias (i.e., tendency to say “no” when unsure) following deep encoding. This effect could not be explained by group differences in initial word classification or in estimated verbal intellectual abilities. Conservative response biases have been reported in several recent studies of recognition discriminability (Danion et al 1999; Weiss et al 2002), with the suggestion that investigators examine correlations with clinical features. When clinical variables were examined, no relationship was found with negative symptoms on the SANS; however, on the SAPS, response bias was found to become less conservative as thought disorder and delusion ratings became more severe. This result is consistent with previous findings (Brébion et al 1999, 2000) and has been interpreted as reflecting problems in source-monitoring (Johnson et al 1993) or reality-monitoring (Frith 1992) that may accompany positive symptoms in schizophrenia.
A limitation of the current study is that it did not employ free recall or source memory probes to more thoroughly test the strength of the memory trace. Addition of free recall and source memory probes to a levels-of-processing paradigm would permit a more rigorous test of retrieval processes and help determine whether providing organizational cues during encoding is sufficient to normalize all aspects of verbal episodic memory. The range of clinical symptoms was also restricted, with most patients falling in the mild range and all patients receiving a stable dose of medication. This restricts the generalizability of the current findings to stable medicated patients. Nevertheless, this pattern of results should be seen as encouraging for those patients who are clinically stable and amenable to cognitive remediation efforts. The finding of a normal levels-of-processing effect suggests that further research should be directed toward investigating memory remediation programs that focus on teaching encoding strategies. Teaching patients to adopt deeper encoding strategies when presented with new information is likely to improve subsequent recognition memory performance and thereby contribute to improved functional outcome.
Acknowledgments
This research was supported by the EJLB Foundation and by National Institutes of Health Grants MH62103, MH-19112, MH-00586, MH-48539, MH 43880, MH64405, and M01RR0040.
We thank Mariana Mendez-Tadel, M.D., for assistance with data collection and Bruce Turetsky, M.D., and Terry Goldberg, Ph.D., for comments on earlier versions of the study.
Footnotes
Covariance analysis was performed with ANOVA rather than the initial MANOVA because separate covariate terms existed for shallow and deep encoding conditions.
Presented at the Annual Meeting of the Academy of Clinical Neuropsychopharmacology, 2001, Waikaloa, Hawaii.
References
- Aleman A, Hijman R, de Haan EHF, Kahn RS. Memory impairment in schizophrenia: A meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Allen HA, Frith CD. Selective retrieval and free emission of category exemplars in schizophrenia. Br J Psychology. 1983;74:481–490. doi: 10.1111/j.2044-8295.1983.tb01881.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, Iowa: The University of Iowa; 1984a. [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, Iowa: The University of Iowa; 1984b. [Google Scholar]
- Arango C, Bartko JJ, Gold JM, Buchanan R. Prediction of neuropsychological performance by neurological signs in schizophrenia. Am J Psychiatry. 1999;156:1349–1357. doi: 10.1176/ajp.156.9.1349. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Neale JM. The neuropsychological signature of schizophrenia: Generalized or differential deficit? Am J Psychiatry. 1994;151:40–48. doi: 10.1176/ajp.151.1.40. [DOI] [PubMed] [Google Scholar]
- Brébion G, Amador X, David A, Malaspina D, Sharif Z, Gorman JM. Positive symptomatology and source-monitoring failure in schizophrenia—an analysis of symptom-specific effects. Psychiatry Res. 2000;95:119–131. doi: 10.1016/s0165-1781(00)00174-8. [DOI] [PubMed] [Google Scholar]
- Brébion G, Amador X, Smith MJ, Gorman JM. Mechanisms underlying memory impairment in schizophrenia. Psychol Med. 1997;27:383–393. doi: 10.1017/s0033291796004448. [DOI] [PubMed] [Google Scholar]
- Brébion G, Amador X, Smith MJ, Malaspina D, Sharif Z, Gorman JM. Opposite links of positive and negative symptomatology with memory errors in schizophrenia. Psychiatry Res. 1999;88:15–24. doi: 10.1016/s0165-1781(99)00076-1. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Dale AM, Rotte M, Rosen BR. Functional-anatomic study of episodic retrieval using fMRI II. Selective averaging of event-related fMRI trials to test the retrieval success hypothesis. Neuroimage. 1998a;7:163–175. doi: 10.1006/nimg.1998.0328. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Wagner AD, Rosen BF. Functional-anatomic study of episodic retrieval using fMRI I. Retrieval effort versus retrieval success. Neuroimage. 1998b;7:151–162. doi: 10.1006/nimg.1998.0327. [DOI] [PubMed] [Google Scholar]
- Calev A. Recall and recognition in chronic non-demented schizophrenics: Use of matched tasks. J Abnorm Psychol. 1984;93:172–177. doi: 10.1037//0021-843x.93.2.172. [DOI] [PubMed] [Google Scholar]
- Chan AS, Kwok IC, Chiu H, Lam L, Pang A, Chow L. Memory and organizational strategies in chronic and acute schizophrenic patients. Schizophr Res. 2000;41:431–445. doi: 10.1016/s0920-9964(99)00078-x. [DOI] [PubMed] [Google Scholar]
- Chute DL, Westall RF. PowerLaboratory for MacOS. Devon, PA: BrooksCole Publishing/MacLaboratory, Inc.; 1996. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Corwin J, Peselow E, Feenan K, Rotrosen J, Fieve R. Disorders of decision in affective disease: An effect of b-adrenergic dysfunction? Biol Psychiatry. 1990;27:813–833. doi: 10.1016/0006-3223(90)90463-c. [DOI] [PubMed] [Google Scholar]
- Craik F, Lockhart R. Levels of processing: A framework for memory research. J Verbal Learn Verbal Behav. 1972;11:671–684. [Google Scholar]
- Danion J-M, Rizzo L, Bruant A. Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Arch Gen Psychiatry. 1999;56:639–644. doi: 10.1001/archpsyc.56.7.639. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. The Cognitive Neuropsychology of Schizophrenia. Hove, UK: Lawrence Erlbaum; 1992. [Google Scholar]
- Gold JM, Randolf C, Carpenter CJ, Goldberg TE, Weinberger DR. Forms of memory failure in schizophrenia. J Abnorm Psychol. 1992;101:487–494. doi: 10.1037//0021-843x.101.3.487. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Aloia MS, Gourovitch ML, Missar D, Pickar D, Weinberger DR. Cognitive substrates of thought disorder. I: The Semantic System. Am J Psychiatry. 1998;155:1671–1676. doi: 10.1176/ajp.155.12.1671. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR, Pliskin NH, Berman KF, Podd MH. Recall memory deficit in schizophrenia: A possible manifestation of prefrontal dysfunction. Schizophr Res. 1989;2:251–257. doi: 10.1016/0920-9964(89)90001-7. [DOI] [PubMed] [Google Scholar]
- Gur RE, Mozley D, Resnick SM, Levick S, Erwin R, Saykin AJ, et al. Relations among clinical scales in schizophrenia: Overlap and subtypes. Am J Psychiatry. 1991;148:472–478. doi: 10.1176/ajp.148.4.472. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Weiss AP, Alpert NM, Schacter DL. Hippocampal and brain stem activation during word retrieval after repeated and semantic encoding. Cereb Cortex. 2002;12:900–907. doi: 10.1093/cercor/12.9.900. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. The Pyramids and Palm Trees Test: A Test of Semantic Access from Words and Pictures. Bury St. Edmunds, UK: Thames Valley Test Company; 1992. [Google Scholar]
- Iddon JL, McKenna PJ, Sahakian BJ, Robbins TW. Impaired generation and use of strategy in schizophrenia: Evidence from visuospatial and verbal tasks. Psychol Med. 1998;28:1049–1062. doi: 10.1017/s0033291798006758. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Kintsch W. Recognition and free recall of organized lists. J Exp Psychol Gen. 1968;78:481–487. [Google Scholar]
- Koh S, Kayton L, Berry R. Mnemonic organization in young nonpsychotic schizophrenics. J Abnorm Psychol. 1973;81:299–310. doi: 10.1037/h0034525. [DOI] [PubMed] [Google Scholar]
- Koh S, Peterson R. Encoding orientation and the remembering of schizophrenic young adults. J Abnorm Psychol. 1978;87:303–313. [PubMed] [Google Scholar]
- Kohler C, Gur RC, Swanson CL, Petty R, Gur RE. Depression in schizophrenia: I. Association with neuropsychological deficits. Biol Psychiatry. 1998;43:165–172. doi: 10.1016/S0006-3223(97)00033-4. [DOI] [PubMed] [Google Scholar]
- McClain L. Encoding and retrieval in schizophrenic free recall. J Nerv Ment Dis. 1983;171:471–479. doi: 10.1097/00005053-198308000-00004. [DOI] [PubMed] [Google Scholar]
- McKenna PJ, Tamlyn D, Lund CE, Mortimer AM, Hammond S, Baddeley AD. Amnesic syndrome in schizophrenia. Psychol Med. 1990;20:967–972. doi: 10.1017/s0033291700036667. [DOI] [PubMed] [Google Scholar]
- Moelter ST, Hill SK, Ragland JD, Lunardelli A, Gur RC, Gur RE, et al. Controlled and automatic processing during animal word list generation in schizophrenia. Neuropsychology. 2001;15:492–501. [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART) Windsor, Berkshire, UK: NFER-Nelson; 1982. [Google Scholar]
- Overall JR, Gorham D. The brief psychiatric rating scale. J Oper Psychiatry. 1980;ll:48–64. [Google Scholar]
- Paulsen JS, Heaton RK, Sadek JR, Perry W, Delis DC, Braff D, et al. The nature of learning and memory impairments in schizophrenia. J Int Neuropsychol Soc. 1995;1:88–99. doi: 10.1017/s135561770000014x. [DOI] [PubMed] [Google Scholar]
- Rugg MD. Memories are made of this. Science. 1998;281:1151–1152. doi: 10.1126/science.281.5380.1151. [DOI] [PubMed] [Google Scholar]
- Rund BR. Distractibility and recall capability in schizophrenics: A 4 year longitudinal study of stability in cognitive performance. Schizophr Res. 1989;2:265–275. doi: 10.1016/0920-9964(89)90003-0. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT Software, Version 8. Cary, NC: SAS Institute Inc.; 1999. [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley D, Mozley LH, Resnick SM, et al. Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester B, Mozley LH, Stafiniak P, et al. Neuropsychological deficits in neuroleptic naïve patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Shtasel DL, Gur RE, Mozley PD, Richards J, Taleff MM, Heimberg C, et al. Volunteers for biomedical research: Recruitment and screening of normal controls. Arch Gen Psychiatry. 1991;48:1022–1025. doi: 10.1001/archpsyc.1991.01810350062010. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Sovani A, Thatte S. Effects of psychosocial interventions on motor speed and accuracy of chronic positive, negative, and mixed schizophrenic symptoms. Int J Soc Psychiatry. 1998;44:117–126. doi: 10.1177/002076409804400204. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M. Instruction Manual for the Structured Clinical Interview for DSM-IV (SCID-P) New York: New York State Psychiatric Institute; 1996a. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M. Structured Clinical Interview for DSM-IV: Non-Patient Version (SCID-NP) New York: New York State Psychiatric Institute; 1996b. [Google Scholar]
- Stone M, Gabrieli JDE, Stebbins GT, Sullivan EV. Working and strategic memory deficits in schizophrenia. Neuropsychology. 1998;12:278–288. doi: 10.1037//0894-4105.12.2.278. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of Episodic Memory. Oxford, UK: Oxford University Press; 1983. [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstall W, Maril A, Dale AM, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Dodson CS, Goff DC, Schacter DL, Heckers S. Intact suppression of increased false recognition in schizophrenia. Am J Psychiatry. 2002;159:1506–1513. doi: 10.1176/appi.ajp.159.9.1506. [DOI] [PubMed] [Google Scholar]