Abstract
The complexity of the human female reproductive tract (FRT) with its multiple levels of hormonally controlled immune protection has only begun to be understood. Dissecting the functions and roles of the immune system in the FRT is complicated by the differential hormonal regulation of its distinct anatomical structures that vary throughout the menstrual cycle. Although many fundamental mechanisms of steroid regulation of reproductive tract immune function have been determined, the effects of exogenous synthetic steroids or endocrine disruptors on immune function and disease susceptibility in the FRT have yet to be evaluated in detail. There is increasing evidence that environmental or synthetic molecules can alter normal immune function. This review provides an overview of the innate and adaptive immune systems, the current status of immune function in the FRT and the potential risks of environmental or pharmacological molecules that may perturb this system.
Keywords: Reproduction, Immunology, Toxicology, Environment, Fertility, Hormone
1. Introduction
Immune systems have been identified across the different kingdoms of life (Animalia, Plantae, Fungi, Protista, Archaea and Bacteria) that provide a formidable and sophisticated defense against pathogens (Marchalonis, et al. 1977; Rolff and Siva-Jothy 2003; Tiffin and Moeller 2006). Because of the implications in human health, however, many of the studies of the immune system have focused on the human. In order for the immune response to function properly, it must act rapidly with a response that is further self-limiting and causes no harm to the individual (Hickey, et al. 2011). The immune system must further retain a “memory” for invading foreign organisms or pathogens in order to facilitate an even more rapid response to subsequent invasions.
1.1. Two Arms of the Immune System: Innate and Adaptive Immune Responses
1.1.1. The Innate Immune System
The innate immune system is evolutionarily ancient compared to the adaptive immune system with elements of it present across all the kingdoms of life. Its major components include: a) protective structural barriers (e.g. mucosal surface of the skin, gastrointestinal, reproductive and respiratory tracts); b) pattern recognition receptors such as Toll-like receptors (TLR), RIG-like receptors (RLR) and NOD-like receptors (NLR) that recognize conserved moieties also known as pathogen-associated molecular patterns (PAMPS) that are uniquely present in viral, bacterial and fungal pathogens; c) cytokines and chemokines that recruit immune cells (macrophages, dendritic cells, T cells) to the site of pathogen exposure; d) endogenous antimicrobials that actively inhibit pathogen survival; and e) innate immune cells (epithelial cells, stromal cells, macrophages, dendritic cells, neutrophils, natural killer cells) that drive this protective response and clear foreign pathogens.
1.1.2. The Adaptive Immune System
The adaptive immune system is composed of specialized cells, which are highly adaptable because of the ability for the acceleration of somatic mutations and irreversible genetic recombinations in the antigen receptor gene regions (Iwasaki 2010). The lymphocyte population can therefore express a vast number of distinct antigen receptors. Furthermore, as this gene rearrangement is irreversible in each cell, the progeny of each of these cells (e.g. memory B and T cells) will inherit the genes encoding the same antigen receptor specificity giving long-lasting specific immunity as well as mount stronger reactions when a pathogen is encountered again. The function of adaptive immune responses is to destroy invading pathogens and any toxic molecules they produce. Although the function of the adaptive immune system is to attack invading pathogens these responses can be destructive. It is therefore crucial that their immune responses are only in reaction to molecules that are foreign to the host and not to the host itself. The ability to distinguish foreign-molecules from self-molecules is a fundamental principal of adaptive immunity. A general comparison of the innate and adaptive immune system is given in Table 1. The innate and adaptive immune systems in the FRT have been described in detail in reviews (Wira and Fahey 2004; Wira, et al. 2005b; Wira, et al. 2011). The variety of immune responses to the plethora of pathogens that can infect the FRT maintains health for the woman and her potential/unborn child.
Table 1.
General Comparison of Innate and Adaptive Immunity in Vertebrates.
| Innate (Non-specific Immunity) | Adaptive (Specific Immunity) |
|---|---|
| First line of defense | Second line of defense |
| Cellular and secreted components: cytokines, chemokines, microbicides | Humoral and Cell Mediated Protection |
| Response is antigen-independent | Response is antigen-dependent |
| Immediate response | Lag time between exposure and maximal response (antibody production) |
| Not antigen-specific | Antigen-specific (Antibody specificity) |
| Exposure results in no immunologic memory, but can be enhanced after exposure to antigen through effects of cytokines | Exposure results in immunologic memory |
1.2. Mucosal vs. Systemic Immunity
For many years, the studies on the immune system emphasized “systemic” immune responses with much emphasis on circulating cells, antibodies and other soluble factors in body fluids. It has, however, become increasingly apparent that the body’s mucosal surfaces, which separate the external from the internal environment, are a critical first line of immune defense. These physical barriers constantly confront environments, which are rich in potential pathogens, and thus they possess mechanisms to protect against invading hostile pathogens while harboring harmless molecules such as food, airborne antigens or commensal bacterial flora. To meet these specialized needs, mucosal surfaces has developed as a complex but sophisticated immune system (innate and adaptive), which is both anatomically and functionally distinct from the systemic immune system (Heremans 1974; Mestecky and McGhee 1987). Characterized by the presence of secretory IgA and IgG, immune protection is also dependent upon T- and B-lymphocytes, monocytes and macrophages, as well as other antigen-presenting cells which recognize and respond to antigenic challenge (Brandtzaeg and Prydz 1984; McDermott and Bienenstock 1979; Ogra, et al. 1981; Underdown and Schiff 1986; Wira, et al. 2003). A summary of the general functions of some of the major proteins involved in mucosal immunity is given in Table 2. These immune factors contribute to immune responses in multiple ways, including acting as antimicrobials against bacterial, fungal and viral pathogens, attracting a diverse immune cell population, activating/differentiating immune cells, stimulating secretion of other cytokines and chemokines, affecting proliferation of immune cells and regulating proteolytic enzymes (Wira, et al. 2005a).
Table 2.
General Functions of Major Immune Proteins involved in Mucosal Immunity and the Female Reproductive Tract.
| Protein (Abbreviation) | Function | References |
|---|---|---|
| Cathelicidin | Produced by neutrophils, epithelial cells and macrophages; regulated by vitamin D | (Liu, et al. 2006) |
| Elafin/Trappin-2 | Neutrophil elastase inhibitor with broad anti-microbial activity | (Wiedow, et al. 1990) |
| Granulocyte macrophage colony stimulating factor (GM-CSF) | Stimulates stem cell production of granulocytes and monocytes | (Caux, et al. 1992) |
| Human β-Defensin 2 (HBD2) | Produced by epithelial cells, has potent anti-microbial activity against gram-negative bacteria. | (Bensch, et al. 1995; Quayle, et al. 1998; Selsted and Ouellette 1995; Wira et al. 2010) |
| Interleukin 6 (IL6) | Both a pro- and anti-inflammatory cytokine; Induces differentiation of leukocytes. | (Heinrich, et al. 1998; Kishimoto, et al. 1995; van der Poll, et al. 1997) |
| Interleukin 8 (IL8) | Mediator of inflammatory responses | (Wolf, et al. 1998) |
| Macrophage Inflammatory Protein (MIP3α) | Recruits lymphocytes and neutrophils; binds and activates CCR6; Interfere with CCR5 and CXCR4 to inhibit HIV infection. | (Bleul, et al. 1996; Cocchi, et al. 1995; Schutyser, et al. 2003; Wira et al. 2010) |
| Monocyte Chemotactic Protein-1 (MCP-1) | Monocyte chemoattractant factor | (Marra, et al. 1999) |
| Transforming Growth Factor β (TGFβ) | Controls proliferation, cellular differentiation; anti-proliferative factor in normal epithelial cells | (Annes, et al. 2003; Shi and Massague 2003) |
| Tumor Necrosis Factor α (TNFα) | Cytokine involved in inflammation and cell apoptosis; Induce differentiation of leukocytes. | (Sedgwick, et al. 2000; Tracey and Cerami 1994) |
| Secretory Leukocyte Protease Inhibitor (SLPI) | Protects mucosa from neutrophil elastase; Anti-viral properties. | (King, et al. 2000; King, et al. 2003; McNeely, et al. 1995) |
1.2.1 Sexually Transmitted Diseases
According to the World Health Organization (WHO), sexually transmitted diseases (STDs) are one of the most serious public health issues with 340 million new cases of potentially curable STDs (Syphilis, Gonorrhoeae, Chlamydia and Trichomoniasis) occurring annually amongst adults aged 15–49 years (WHO 2007). In developing countries STDs and their complications rank in the top five disease categories for which adults seek health care. Infection with STDs can lead to acute symptoms, chronic infection and serious delayed consequences such as infertility, ectopic pregnancy, cervical cancer and the untimely death of infants. Human Immunodeficiency Virus (HIV) has caused approximately 25 million deaths with an additional 33.4 million people infected world-wide (UNAIDS 2007). Women living with HIV make up approximately 60% of the infected patients (UNAIDS 2009). The majority of HIV and STD transmission events occur across the mucosal surface of the FRT. Thus defining and understanding the immune response at this site is essential in preventing the spread of these pathogens.
2. Immunology of the female reproductive tract
While most research has concentrated on the mucosal immunity of the gastro-intestinal or respiratory tracts, emerging studies on the function of the immune system in the FRT have demonstrated the critical role it has in balancing protection against STDs while allowing the survival of foreign sperm and an allogeneic embryo (Fahey, et al. 2011; Kutteh 2005; Wira, et al. 2010; Wira et al. 2005b; Wira et al. 2011). Studies by Wira and colleagues have shown that all aspects of the innate and adaptive immune systems throughout the female reproductive tract are under sex hormone control.
Each of the five anatomical sites of the FRT (Fallopian tubes, endometrium, endocervix, ectocervix and vagina), while functioning separately, provides a collaborative environment to both protect the host from infection while allowing fertilization of the egg and subsequent implantation of the embryo which expresses the sperm’s foreign genes. Each of these anatomical sites is differentially controlled by estradiol and progesterone, which in turn modulate the production and secretion of various immune factors at different times of the menstrual cycle. Extensive studies (Wira, et al. 2005a; Wira et al. 2010; Wira et al. 2005b; Wira, et al. 2005c; Wira, et al. 2002) have defined how these functions are synchronized to optimize the chances for successful fertilization, implantation, and pregnancy.
2.1. Immune cells involved in immune function in the FRT
In order to begin to dissect the immune function of the distinct tissues of the FRT it is important to identify the immune cells, their location in these tissues as well as their potential regulation by steroids. Table 3 summarizes the immune cells that have been identified in the human FRT as well as their general functions and their expression of the sex hormone (estrogen and progesterone) receptors. Collectively these studies show that there is a full set of active immune cells in the FRT and that the differential regulation of these cells in the distinct compartments of the FRT is critical for reproductive success. Of further interest is that the FRT immune cells express steroid hormone receptors suggesting that they are directly responsive to hormonal stimuli thus demonstrating the complex interplay between endocrine, reproductive and immune function (Wira et al. 2005a).
Table 3.
Summary of Immune Cells in the Human FRT including Location and Hormone Receptor Properties.
| Cell Type | Location in FRT (*Indicates high cell numbers) | Major Immune Functions (Innate Immunity, II Adaptive immunity, AI) | ERα | ERβ | PR | References |
|---|---|---|---|---|---|---|
| Macrophage | Vagina, ectocervix endocervix*, uterus* (lymphocyte aggregates), Fallopian tubes | II. Phagocytosis, kill microorganisms and infected cells; Antigen presentation | ++++ | + | ++ | (Fahey et al. 2011; Janeway 2004; Khan, et al. 2005; Murphy, et al. 2009; Wira et al. 2010) |
| Neutrophils | Vagina, ectocervix endocervix*, uterus* | II. Phagocytosis, Antimicrobial α Defensins | +++ | ++ | − | (Fahey et al. 2011; Janeway 2004; Moreno, et al. 2003; Wira et al. 2010) |
| Dendritic Cell | Vagina, ectocervix, endocervix*, uterus* | II. Antigen presenting cells (links adaptive and innate immune systems) | ++ | ++ | + | (Fahey et al. 2011; Janeway 2004; Komi and Lassila 2000; Wira et al. 2010) |
| Uterine NK cell | Vagina*, ectocervix -*, endocervix, uterus | II. Nonspecifically kill virus infected and tumor cells. | − | +++ | ++ | (Arruvito, et al. 2008; Fahey et al. 2011; Henderson, et al. 2003; Janeway 2004; Wira et al. 2010) |
| CD4+ T Cells | Vagina, ectocervix, endocervix*, uterus*, Fallopian tubes * | AI. TH-1 cell mediated responses; IFN secretion – antiviral activity. | +++ | ++ | + | (Fahey et al. 2011; Janeway 2004; Phiel, et al. 2005; Szekeres-Bartho, et al. 1990; Wira et al. 2010) |
| CD8+ (Cytotoxic T lymphocyte) | Vagina, ectocervix, endocervix*, uterus*, Fallopian tubes* | AI. TH-1 cell mediated responses; Apoptosis of infected cells | + | +++ | + | (Fahey et al. 2011; Janeway 2004; Phiel et al. 2005; Szekeres-Bartho et al. 1990; Wira et al. 2010) |
| B cell | Vagina, ectocervix, endocervix, uterus (lymphocyte aggregate core), Fallopian tubes | AI. TH-2 Humoral responses; Maturation into IgA/IgG secreting plasma cells | + | ++++ | − | (Fahey et al. 2011; Janeway 2004; Phiel et al. 2005; Sapino, et al. 2003; Wira et al. 2010) |
2.2. Epithelial and Stromal Cells of the FRT and Their Roles in Immunity
In addition to the full set of immune cells distributed throughout the FRT, epithelial and stromal cells are capable of both mounting an immune response and modulating immune cell function (Wira et al. 2005c). A summary of these properties is given in Table 4. Collectively, these studies show which hormone-regulated reproductive tract cells contain hormone receptors as well as surface receptors involved in recognizing (e.g. TLR) and responding (cytokines, chemokines and endogenous microbicides) to pathogens (Schaefer, et al. 2005). Also shown in Table 4 is how secreted immune factors vary during the menstrual cycle (see column on secreted immune factors and Table footnote). It is evident that cells and their immune response potential, as well as their ability to be directly modulated by sex hormones, vary throughout the FRT.
Table 4.
Properties of Epithelial and Stromal Cells in the Human Female Reproductive Tract involved in Immune Function (ERα, Estrogen Receptor alpha; ERβ, Estrogen Receptor beta; PR, Progesterone Receptor; Hormone receptor levels premenopausal).
Low during the proliferative stage of cycle and peak at time of ovulation;
Levels drop significantly at mid-cycle.
Estradiol stimulates ↑; Estradiol inhibits ↓
2.3. Key Immunoregulatory Modulators of the Innate and Adaptive Immune System in the FRT
As summarized in Table 4, many peptide/protein molecules, including chemokines, cytokines, proteases, protease inhibitors, immunoglobulins, matrix metalloproteases, antimicrobials and growth factors have been identified in the FRT and could potentially modulate immune function (Fahey et al. 2011; Wira et al. 2010; Wira et al. 2011). It has been estimated that there are over 600 proteins in the fluids from cervical lavages (Shaw, et al. 2007). There are likely to be many immune factors that have not been identified. It is apparent that these observations address a critical field of women’s health that requires further study. Of particular interest is a growing body of evidence that commensal bacteria in the lower female reproductive tract as well as at other mucosal surfaces are dependant on estrogen-driven presence of glycogen in epithelial cells and play a central role in providing immune protection (Boskey, et al. 2001). For example, the acidic microenvironment of the vagina is maintained by lactic acid producing commensal bacteria, the most common of which is Lactobacillus found in normal pre-menopausal healthy women (Witkin, et al. 2007). In addition to regulating vaginal pH, specific commensal microdomes protect against HIV infection (Ahmed, et al. 2010). Escherichia coli, Veillonella parvula and Neisseria mucosa suppress HIV-1 infection through TLR-4 activation. In contrast, TLR-2 activation by Lactobacillus acidophilus, Prevotella melaninogenica, Prevotella bivia and Mycobacterium smegmatis enhanced infection (Ahmed et al. 2010). Further research is needed to more fully understand how commensal bacteria alter the vaginal immune protection.
2.3.1. Cytokines, Chemokines and Antimicrobials
Cytokines and chemokines are a structurally and functionally diverse group of proteins (Cannon 2000; Foster, et al. 2004a; Foster, et al. 2004b; Liles and Van Voorhis 1995; Steinke and Borish 2006). These proteins were initially shown to act as mediators and regulators of immune processes but studies have also shown that cytokines are also produced by cells other than immune cells and can also affect non-immune cells. The most common cytokines include: (a) lymphokines (secreted by activated lymphocytes, especially T helper cells); (b) interleukins (mediators between leukocytes); (c) chemokines (small cytokines primarily responsible for leukocyte attraction and migration); and (d) monokines (produced by mononuclear phagocytic cells). Cytokines are produced by cells of both the innate and adaptive immune systems and may act on many cell types (i.e., they are pleiotrophic). In many instances they may have similar actions (i.e., redundancy). Redundancy is due to the usage of the cytokine receptors, which are shared amongst multiple signaling molecules. For example the Type I interferon receptor complex in humans is shared amongst 13 isoforms of IFNα and one isoforms of IFNβ, IFNκ, IFNω and IFNε respectively all of which are believed to generate a protective antiviral response.
Cytokines can induce both damaging and protective responses as well as induce or suppress synthesis of other cytokines. These networks are made even more complex by the receptors that bind these regulatory molecules (Kitamura, et al. 1992; Lopez, et al. 2010; Miyajima, et al. 1992). The response of cytokine/chemokine binding to receptors is associated with various factors including the affinity of binding as well as differential expression and signal transduction pathways.
With respect to the FRT, it has been shown that cytokines and cytokine receptors are expressed by both immune and non-immune cells, and can be regulated by steroid hormones. Cytokines influence a range of uterine functions during the menstrual cycle, as well as implantation, pregnancy and labor Tribe (Orsi and Tribe 2008). There are intricate and dynamic synergistic interactions among individual cytokines, how they are modulated by pregnancy hormones and how perturbations to cytokine signaling can be associated with adverse pregnancy outcomes, such as miscarriage, pre-eclampsia, preterm labor and fetal brain injury.
2.3.2. Immunoglobulin Secretion in the FRT
It has long been known that immunoglobulins (both IgG and secretory IgA) are present in the genital tract of women and that the levels of these proteins vary throughout the reproductive tract and during the menstrual cycle (Kozlowski, et al. 2002; Lu, et al. 1999; Schumacher, et al. 1977; Usala, et al. 1989). While origin of these antibodies is uncertain, it is apparent that both plasma-derived and locally produced antibodies contribute to the immunoglobulin pool (Kutteh 2005). More recent studies have confirmed these early results. Women vaccinated with the human papillomavirus (HPV) had cervical antibody titers that were highest in the proliferative phase but decreased approximately nine-fold around ovulation, and increased 3-fold during the luteal phase (Nardelli-Haefliger, et al. 2003). Whether decreases in antibody titer around ovulation result in lowered protection of women during the peri-ovulatory phase remains to be determined. Findings of cyclic changes in antibody levels during the menstrual cycle indicate that vaccine trials need to include analyses of genital tract secretions for all sexually transmitted vaccines, especially HIV. Further, in view of the large number or reports of adverse reactions to the US FDA Adverse Reaction Reporting Data base, the efficacy versus safety issues need to be further addressed.
2.4. Hormonal regulation of the FRT and the “Window of Vulnerability”
For successful fertilization and embryo survival, the immune system must therefore be modulated during mid-cycle of the menstrual cycle. Many studies have now been carried out to define the components of the immune system present in the FRT and to determine how these protect against pathogens, and how they are controlled by sex hormones (Fahey et al. 2011; Fahey, et al. 2008; Kutteh 2005; Wira et al. 2011).
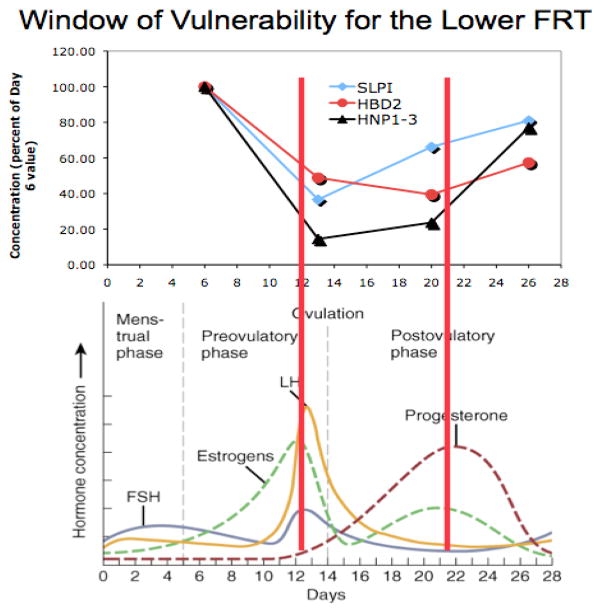
A significant observation was made by Wira and Fahey when they asked the question: “From a viral perspective, what times during the menstrual cycle come closest to being optimal for infection?” By examining multiple immunological parameters the conclusion was made that within the FRT during a normal menstrual cycle, there is a period lasting 7–10 days when important components of innate, humoral, and cell-mediated immunity are suppressed by estradiol and/or progesterone, enhancing the potential for viral infection (Wira and Fahey 2008). As seen in Figure 1, onset of the “window of vulnerability” coincides with an increase in estradiol at about the time of ovulation. It has now been shown that immunological suppression occurs in both the upper and the lower FRT as an integral part of the physiological processes that underlie successful reproduction, and that this suppression coincides with recruitment of potentially infectable cells and upregulation of coreceptors on target cells that are essential for viral uptake (Wira and Fahey 2008). These observations have serious implications for increased susceptibility to STDs during the ovulatory-to-secretory phase of the menstrual cycle. Additionally there are now concerns that a variety of exogenous environmental and pharmaceutical compounds could dramatically alter normal immune function within the FRT. Whether these compounds alter susceptibility to external pathogens has yet to be adequately studied.
Figure 1. Hormonal regulation of the FRT and the “Window of Vulnerability”.
During the window of vulnerability, which begins at ovulation and lasts 7–10 days, there is a marked drop aspects of the innate and adaptive immune systems as well as in several anti-HIV molecules that serve as major sententials in the innate immune system of the FRT. Innate immune antimicrobials that decrease in secretions from the lower FRT include human α-defensin-1-3 (HNP1-3), β-defensins 2 (HBD2), and secretory leukocyte protease inhibitor (SLPI). Adapted from (Keller et al. 2007).
3. Potential of Environmental and Pharmaceutical Compounds to Alter the FRT Immune system
3.1. Endocrine Disruptors
Endocrine disruptors (EDs) are exogenous molecules that affect the normal action of hormones in the body including their synthesis, secretion, metabolism and transport (Brevini, et al. 2005; Cheek and McLachlan 1998; Cheek, et al. 1998; Fox 2004; Kavlock and Ankley 1996; Olea, et al. 1998). EDs include not only synthetic chemicals used in pharmaceutical or agricultural applications (e.g. pesticides, herbicides, plastics, therapeutic hormones) but also naturally occurring compounds present in the environment (e.g. phytoestrogens). There are three types of endocrine disrupting mechanisms independent of ED concentration (Brevini et al. 2005). These include: (a) Binding to and irreversibly locking up the specific hormone receptor (hormone blocking); (b) Mimicking naturally occurring hormones (hormone mimicking); and (c) Acting through hormone-like pathways but initiating abnormal reactions (hormone triggering). While it is beyond the scope of this discussion to give a detailed overview of the many EDs (which will be discussed elsewhere in this special issue) it is important to appreciate their potential for altering the unique immune system within the FRT.
Altering the immune system by EDs could affect the ability to mount well-regulated immune responses to microbial and viral pathogens, vaccine antigens, allergens, as well as self and tumor antigens (Ahmed 2000; Chalubinski and Kowalski 2006; Forawi, et al. 2004). EDs can influence the synthesis of cytokines, immunoglobulins and cell mediators as well as modulating immune cell activation and survival via IL-4 production, Th1/Th2 balance and IgE production thus altering the balance between protection and susceptibility.
3.2. Effect of EDs on Reproduction and Development
The effects of EDs on mammalian reproduction and development are well studied. However, remarkably little is known about their effects on the development of the immune system, especially of the female reproductive tract. There have been numerous studies and reviews demonstrating effects of EDs on embryonic development and germ cell development and these are discussed in detail elsewhere. Table 5 summarizes the major classes of EDs, their mechanism of action and the reproductive and/or immune effects. Most bind to the estrogen receptor to either enhance or inhibit estrogenic effects.
Table 5.
Examples of Different Classes of Endocrine Disruptors (EDs) Known to Alter Functions of Both the Reproductive and Immune Systems.
| Compound/Source | Hormone Activity | Reproductive/Immune Effects | Reference |
|---|---|---|---|
| Zearalenone (ZEN)/fungal micotoxin in cereal crops | Binds estrogen receptors | Decreased fertility; altered Estradiol and Progesterone levels; Decrease in IL-8 synthesis by PMNs | (EFSA 2011; Marin, et al. 2010) |
| Bisphenol A (BPA)/poly-carbonate plastics, polystyrene | Estrogen agonist; androgen/thyroid hormone antagonist | Accelerates puberty onset, altered estrous cyclicity (permanent estrus); Alters IFN-γ, immunoglobulin and interleukin production | (Fernandez, et al. 2009; Sawai, et al. 2003; Yoshino, et al. 2004; Yoshino, et al. 2003) |
| 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), Industrial chemical | Binds aryl hydrocarbon receptor; anti-estrogenic | Inhibits CD4+ T cell differentiation into T helper effector cells; Inhibits uterine epithelial mitogenesis. | (Buchanan, et al. 2002; Marshall and Kerkvliet 2010) |
| DDT, organochlorine/insecticide | Binds estrogen receptor, antiandrogenic | Humoral immune suppression; stimulates the production of NO and proinflammatory cytokines; Reduces Lymphocytes; Adverse fertility effects | (Kim, et al. 2004; Lahvis, et al. 1995; Tiemann 2008) |
| Genistein, phytoestrogen | Binds Estrogen Receptor | Impairs oocyte maturation and embryonic growth; fish sex reversal; Increases in the activities of cytotoxic T cells and NK cells; Suppresses Humoral immunity and alters thymocyte development. |
(Chan 2009; Guo, et al. 2001; Kiparissis, et al. 2003; Scholz and Gutzeit 2000; Vodkova, et al. 2008; Yellayi, et al. 2002) |
Some EDs, particularly the phytoestrogens, have been shown to have some beneficial effects in humans (Cheek and McLachlan 1998; Cheek et al. 1998). Individuals living in regions where traditional diets are high in plant estrogens (e.g. soya meal) are reported to have lower incidences of breast and prostate cancer as well as atherosclerotic cardiovascular disease than people who consume a “Western” diet. More recent studies, however, have demonstrated that Genistein, an isoflavone estrogen, can negatively impact oocyte maturation and subsequent embryonic development (Chan 2009). However, the use of soya meal in infant formula is somewhat controversial and an expert panel concluded that more detailed studies are required to evaluate the long-term effects of phytoestrogen exposure and intake (Rozman, et al. 2006).
3.2 Effect of EDs on Immune Function of the FRT
While mechanisms for the effects of EDs on reproduction and development are not fully understood, even less is known about their effects on the immune system of the FRT. Table 5 summarizes some EDs, which are known to affect both the immune and reproductive systems. It will be important to determine their effects directly on the immune system of the FRT. For example, Heat Shock Proteins (HSPs) are known as “molecular chaperones which are essential for maintaining cell function by the prevention of protein misfolding resulting in protein aggregation (Fink 1999; Hartl and Hayer-Hartl 2002; Tsan and Gao 2009). They have also been proposed for use as biomarkers of environmental perturbation (Bierkens 2000). More recently, numerous HSPs have been implicated to play important roles in immune function including antigen presentation, activation of lymphocytes and macrophages, and activation and maturation of dendritic cells (Li, et al. 2002; Tsan and Gao 2004; Wallin, et al. 2002). With respect to the FRT, Papaconstantinou and colleagues (Papaconstantinou, et al. 2001) have shown that Bis-phenol A (BPA), an ED and a constituent of some plastics, resembles estradiol in its ability to induce increases in uterine heat shock protein levels, mainly hsp90α and glucose-regulated protein. They further demonstrated that both estradiol and BPA increased levels of HSPs at doses lower than those necessary for a significant increase in uterine weight (Papaconstantinou, et al. 2000). Given the role of HSPs in immune function, it can be hypothesized that EDs alter the ability of HSPs to modulate the immune system within the FRT.
Among EDs with deleterious side effects, DDT stands out as a result of its wide spread use to control malaria, a major cause of death in Sub-Saharan Africa. Its endocrine activity has been observed in mice and rat toxicological studies, and available epidemiological evidence indicates that these effects may be occurring in humans as a result of DDT exposure. DDT exposure damages the reproductive system, reduces reproductive success, semen quality, menstruation, gestational length, and duration of lactation (Chen and Rogan 2003; Roberts, et al. 2004; Rogan and Ragan 2003). In addition, exposure to DDT that would be needed in malaria control might cause preterm birth, which is a major contributor to infant mortality (Longnecker, et al. 2001; Rogan and Chen 2005). Given this association, it is imperative that future studies examine the effects of DDT and other chemicals on the FRT immune system. Given the long half-life of this molecule, up to 16 years, it will be important to study girls going into puberty who may have been exposed to these chemicals regularly throughout their lifetime.
As discussed in this review, the healthy immune system of women is important not only for general health but for prevention of STDs (including HIV) and disease progression. Hormone levels markedly affect immune function in the FRT. Thus the presence of EDs could have a substantial impact on normal immune protection. As the majority of women and children in Africa also have insufficient diets, their reproductive immune systems may be even at more risk for endocrine disruptors. In summary, it will be extremely critical in the coming years to more closely monitor the long term effects of these environmental and as well as industrial and pharmaceutical chemicals to determine if the population is being compromised and if there is an increase in susceptibility to STDs.
Research Highlights.
We examine innate and adaptive immunity in the female reproductive tract (FRT).
We define the role of sex hormones in regulating FRT mucosal immunity.
Endocrine disruptors alter disease susceptibility in the FRT.
Reproductive health depends on understanding how endocrine disruptors work.
Information about endocrine disruptors will increase reproductive health.
List of Abbreviations
- FRT
Female Reproductive Tract
- GM-CSF
Granulocyte macrophage colony stimulating factor
- SLIPI
Secretory Leukocyte Protease Inhibitor
- HβD2
Human β Defensin 2
- MIP
Macrophage Inflammatory Protein
- MCP-1
Monocyte Chemotactic protein-1
- IL
Interleukin
- TNF
Tumor Necrosis Factor
- EDs
Estrogen Disruptors
- HSP
Heat Shock Protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed N, Hayashi T, Hasegawa A, Furukawa H, Okamura N, Chida T, Masuda T, Kannagi M. Suppression of human immunodeficiency virus type 1 replication in macrophages by commensal bacteria preferentially stimulating Toll-like receptor 4. J Gen Virol. 2010;91:2804–2813. doi: 10.1099/vir.0.022442-0. [DOI] [PubMed] [Google Scholar]
- Ahmed SA. The immune system as a potential target for environmental estrogens (endocrine disrupters): a new emerging field. Toxicology. 2000;150:191–206. doi: 10.1016/s0300-483x(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Arruvito L, Giulianelli S, Flores AC, Paladino N, Barboza M, Lanari C, Fainboim L. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J Immunol. 2008;180:5746–5753. doi: 10.4049/jimmunol.180.8.5746. [DOI] [PubMed] [Google Scholar]
- Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- Bierkens JG. Applications and pitfalls of stress-proteins in biomonitoring. Toxicology. 2000;153:61–72. doi: 10.1016/s0300-483x(00)00304-8. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16:1809–1813. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984;311:71–73. doi: 10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- Brevini TA, Zanetto SB, Cillo F. Effects of endocrine disruptors on developmental and reproductive functions. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:1–10. doi: 10.2174/1568008053174750. [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Ohsako S, Tohyama C, Cooke PS, Iguchi T. Dioxin inhibition of estrogen-induced mouse uterine epithelial mitogenesis involves changes in cyclin and transforming growth factor-beta expression. Toxicol Sci. 2002;66:62–68. doi: 10.1093/toxsci/66.1.62. [DOI] [PubMed] [Google Scholar]
- Cannon JG. Inflammatory Cytokines in Nonpathological States. News Physiol Sci. 2000;15:298–303. doi: 10.1152/physiologyonline.2000.15.6.298. [DOI] [PubMed] [Google Scholar]
- Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- Chalubinski M, Kowalski ML. Endocrine disrupters--potential modulators of the immune system and allergic response. Allergy. 2006;61:1326–1335. doi: 10.1111/j.1398-9995.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- Chan WH. Impact of genistein on maturation of mouse oocytes, fertilization, and fetal development. Reprod Toxicol. 2009;28:52–58. doi: 10.1016/j.reprotox.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Cheek AO, McLachlan JA. Environmental hormones and the male reproductive system. J Androl. 1998;19:5–10. [PubMed] [Google Scholar]
- Cheek AO, Vonier PM, Oberdorster E, Burow BC, McLachlan JA. Environmental signaling: a biological context for endocrine disruption. Environ Health Perspect. 1998;106(Suppl 1):5–10. doi: 10.1289/ehp.106-1533276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Rogan WJ. Nonmalarial infant deaths and DDT use for malaria control. Emerg Infect Dis. 2003;9:960–964. doi: 10.3201/eid0908.030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Cretoiu S, Cretoiu D, Suciu L, Popescu L. Interstitial Cajal-like cells of human Fallopian tube express estrogen and progesterone receptors. Journal of Molecular Histology. 2009;40:387–394. doi: 10.1007/s10735-009-9252-z. [DOI] [PubMed] [Google Scholar]
- EFSA. EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on the risks for public health related to the presence of zearalenone in food. In. EFSA Journal. 2011:2197. [Google Scholar]
- Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- Fahey JV, Bodwell JE, Hickey DK, Ghosh M, Muia MN, Wira CR. New approaches to making the microenvironment of the female reproductive tract hostile to HIV. Am J Reprod Immunol. 2011;65:334–343. doi: 10.1111/j.1600-0897.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, Wira CR. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol. 2008;1:317–325. doi: 10.1038/mi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MP, Noguerol TN, Lacorte S, Buchanan I, Pina B. Toxicity identification fractionation of environmental estrogens in waste water and sludge using gas and liquid chromatography coupled to mass spectrometry and recombinant yeast assay. Anal Bioanal Chem. 2009;393:957–968. doi: 10.1007/s00216-008-2516-8. [DOI] [PubMed] [Google Scholar]
- Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- Forawi HA, Tchounwou PB, McMurray RW. Xenoestrogen modulation of the immune system: effects of dichlorodiphenyltrichloroethane (DDT) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Rev Environ Health. 2004;19:1–13. doi: 10.1515/reveh.2004.19.1.1. [DOI] [PubMed] [Google Scholar]
- Foster AE, Forrester K, Li YC, Gottlieb DJ. Ex-vivo uses and applications of cytokines for adoptive immunotherapy in cancer. Curr Pharm Des. 2004a;10:1207–1220. doi: 10.2174/1381612043452631. [DOI] [PubMed] [Google Scholar]
- Foster D, Parrish-Novak J, Fox B, Xu W. Cytokine-receptor pairing: accelerating discovery of cytokine function. Nat Rev Drug Discov. 2004b;3:160–170. doi: 10.1038/nrd1305. [DOI] [PubMed] [Google Scholar]
- Fox JE. Chemical communication threatened by endocrine-disrupting chemicals. Environ Health Perspect. 2004;112:648–653. doi: 10.1289/ehp.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Rezapour M, Wu X, Li L, Sjogren C, Ulmsten U. Expression of estrogen receptor-alpha and -beta in anterior vaginal walls of genuine stress incontinent women. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:276–281. doi: 10.1007/s00192-003-1042-7. discussion 281. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Schaefer TM, Fahey JV, Wright JA, Wira CR. Antiviral responses of human Fallopian tube epithelial cells to toll-like receptor 3 agonist poly(I:C) Fertil Steril. 2008;89:1497–1506. doi: 10.1016/j.fertnstert.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/Elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology. 2010;129:207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TL, McCay JA, Zhang LX, Brown RD, You L, Karrow NA, Germolec DR, White KL., Jr Genistein modulates immune responses and increases host resistance to B16F10 tumor in adult female B6C3F1 mice. J Nutr. 2001;131:3251–3258. doi: 10.1093/jn/131.12.3251. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Horn F, Graeve L, Dittrich E, Kerr I, Muller-Newen G, Grotzinger J, Wollmer A. Interleukin-6 and related cytokines: effect on the acute phase reaction. Z Ernahrungswiss. 1998;37(Suppl 1):43–49. [PubMed] [Google Scholar]
- Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab. 2003;88:440–449. doi: 10.1210/jc.2002-021174. [DOI] [PubMed] [Google Scholar]
- Herbst-Kralovetz MM, Quayle AJ, Ficarra M, Greene S, Rose WA, 2nd, Chesson R, Spagnuolo RA, Pyles RB. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol. 2008;59:212–224. doi: 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Heremans JF. The IgA system in connection with local and systemic immunity. Adv Exp Med Biol. 1974;45:3–11. doi: 10.1007/978-1-4613-4550-3_1. [DOI] [PubMed] [Google Scholar]
- Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol. 2011;88:185–194. doi: 10.1016/j.jri.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins MB, Spike RC, Mackie RM, MacLean AB. An immunohistochemical study of androgen, oestrogen and progesterone receptors in the vulva and vagina. Br J Obstet Gynaecol. 1998;105:216–222. doi: 10.1111/j.1471-0528.1998.tb10056.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Travers Paul, Walport Mark, Shlomchik Mark J. Immunobiology. 6. New York: Garland Science; 2004. [Google Scholar]
- Jones RK, Bulmer JN, Searle RF. Immunohistochemical characterization of proliferation, oestrogen receptor and progesterone receptor expression in endometriosis: comparison of eutopic and ectopic endometrium with normal cycling endometrium. Hum Reprod. 1995;10:3272–3279. doi: 10.1093/oxfordjournals.humrep.a135901. [DOI] [PubMed] [Google Scholar]
- Kavlock RJ, Ankley GT. A perspective on the risk assessment process for endocrine-disruptive effects on wildlife and human health. Risk Anal. 1996;16:731–739. doi: 10.1111/j.1539-6924.1996.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Keller MJ, Guzman E, Hazrati E, Kasowitz A, Cheshenko N, Wallenstein S, Cole AL, Cole AM, Profy AT, Wira CR, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21:467–476. doi: 10.1097/QAD.0b013e328013d9b5. 410.1097/QAD.1090b1013e328013d328019b328015. [DOI] [PubMed] [Google Scholar]
- Khan KN, Masuzaki H, Fujishita A, Kitajima M, Kohno T, Sekine I, Matsuyama T, Ishimaru T. Regulation of hepatocyte growth factor by basal and stimulated macrophages in women with endometriosis. Human Reproduction. 2005;20:49–60. doi: 10.1093/humrep/deh525. [DOI] [PubMed] [Google Scholar]
- Kim JY, Choi CY, Lee KJ, Shin DW, Jung KS, Chung YC, Lee SS, Shin JG, Jeong HG. Induction of inducible nitric oxide synthase and proinflammatory cytokines expression by o,p’-DDT in macrophages. Toxicol Lett. 2004;147:261–269. doi: 10.1016/j.toxlet.2003.12.001. [DOI] [PubMed] [Google Scholar]
- King AE, Critchley HO, Kelly RW. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol Hum Reprod. 2000;6:191–196. doi: 10.1093/molehr/6.2.191. [DOI] [PubMed] [Google Scholar]
- King AE, Morgan K, Sallenave JM, Kelly RW. Differential regulation of secretory leukocyte protease inhibitor and elafin by progesterone. Biochem Biophys Res Commun. 2003;310:594–599. doi: 10.1016/j.bbrc.2003.08.151. [DOI] [PubMed] [Google Scholar]
- Kiparissis Y, Balch GC, Metcalfe TL, Metcalfe CD. Effects of the isoflavones genistein and equol on the gonadal development of Japanese medaka Oryzias latipes. Environ Health Perspect. 2003;111:1158–1163. doi: 10.1289/ehp.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- Kitamura M, Maruyama N, Mitarai T, Nagasawa R, Yoshida H, Sakai O. Extracellular matrix contraction by cultured mesangial cells: modulation by transforming growth factor-beta and matrix components. Exp Mol Pathol. 1992;56:132–143. doi: 10.1016/0014-4800(92)90030-f. [DOI] [PubMed] [Google Scholar]
- Komi J, Lassila O. Nonsteroidal anti-estrogens inhibit the functional differentiation of human monocyte-derived dendritic cells. Blood. 2000;95:2875–2882. [PubMed] [Google Scholar]
- Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, Neutra MR. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002;169:566–574. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- Kutteh W, Mestecky J, Wira CR. Mucosal immune system in the human female reproductive tract. In: Mestecky J, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. Burlington: Elselvier Academic Press; 2005. pp. 1631–1646. [Google Scholar]
- Lahvis GP, Wells RS, Kuehl DW, Stewart JL, Rhinehart HL, Via CS. Decreased lymphocyte responses in free-ranging bottlenose dolphins (Tursiops truncatus) are associated with increased concentrations of PCBs and DDT in peripheral blood. Environ Health Perspect. 1995;103(Suppl 4):67–72. doi: 10.1289/ehp.95103s467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecce G, Meduri G, Ancelin M, Bergeron C, Perrot-Applanat M. Presence of estrogen receptor beta in the human endometrium through the cycle: expression in glandular, stromal, and vascular cells. J Clin Endocrinol Metab. 2001;86:1379–1386. doi: 10.1210/jcem.86.3.7322. [DOI] [PubMed] [Google Scholar]
- Li Z, Dai J, Zheng H, Liu B, Caudill M. An integrated view of the roles and mechanisms of heat shock protein gp96-peptide complex in eliciting immune response. Front Biosci. 2002;7:d731–751. doi: 10.2741/a808. [DOI] [PubMed] [Google Scholar]
- Liles WC, Van Voorhis WC. Review: nomenclature and biologic significance of cytokines involved in inflammation and the host immune response. J Infect Dis. 1995;172:1573–1580. doi: 10.1093/infdis/172.6.1573. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Zhou H, Brock JW. Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet. 2001;358:110–114. doi: 10.1016/S0140-6736(01)05329-6. [DOI] [PubMed] [Google Scholar]
- Lopez AF, Hercus TR, Ekert P, Littler DR, Guthridge M, Thomas D, Ramshaw HS, Stomski F, Perugini M, D’Andrea R, et al. Molecular basis of cytokine receptor activation. IUBMB Life. 2010;62:509–518. doi: 10.1002/iub.350. [DOI] [PubMed] [Google Scholar]
- Lu FX, Ma Z, Rourke T, Srinivasan S, McChesney M, Miller CJ. Immunoglobulin concentrations and antigen-specific antibody levels in cervicovaginal lavages of rhesus macaques are influenced by the stage of the menstrual cycle. Infect Immun. 1999;67:6321–6328. doi: 10.1128/iai.67.12.6321-6328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis JJ, Decker JM, DeLuca D, Moseley JM, Smith P, Warr GW. Lymphocyte surface immunoglobulins: evolutionary origins and involvement in activation. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):261–273. doi: 10.1101/sqb.1977.041.01.032. [DOI] [PubMed] [Google Scholar]
- Marin DE, Taranu I, Burlacu R, Tudor DS. Effects of zearalenone and its derivatives on the innate immune response of swine. Toxicon. 2010;56:956–963. doi: 10.1016/j.toxicon.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Marra F, DeFranco R, Grappone C, Parola M, Milani S, Leonarduzzi G, Pastacaldi S, Wenzel UO, Pinzani M, Dianzani MU, et al. Expression of monocyte chemotactic protein-1 precedes monocyte recruitment in a rat model of acute liver injury, and is modulated by vitamin E. J Investig Med. 1999;47:66–75. [PubMed] [Google Scholar]
- Marshall NB, Kerkvliet NI. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann N Y Acad Sci. 2010;1183:25–37. doi: 10.1111/j.1749-6632.2009.05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki S, Fukaya T, Suzuki T, Murakami T, Sasano H, Yajima A. Oestrogen receptor alpha and beta mRNA expression in human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1999;5:559–564. doi: 10.1093/molehr/5.6.559. [DOI] [PubMed] [Google Scholar]
- McDermott MR, Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–1898. [PubMed] [Google Scholar]
- McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Miyajima A, Kitamura T, Harada N, Yokota T, Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- Moreno AN, Jamur MC, Oliver C, Roque-Barreira MC. Mast cell degranulation induced by lectins: effect on neutrophil recruitment. Int Arch Allergy Immunol. 2003;132:221–230. doi: 10.1159/000074303. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Guyre PM, Wira CR, Pioli PA. Estradiol regulates expression of estrogen receptor ERalpha46 in human macrophages. PLoS One. 2009;4:e5539. doi: 10.1371/journal.pone.0005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonas I, Jeschke U, Shabani N, Kuhn C, Balle A, Kriegel S, Kupka MS, Friese K. Immunohistochemical analysis of estrogen receptor alpha, estrogen receptor beta and progesterone receptor in normal human endometrium. Acta Histochem. 2004;106:245–252. doi: 10.1016/j.acthis.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, De Grandi P. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst. 2003;95:1128–1137. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- Ogra PL, Yamanaka T, Losonsky GA. Local immunologic defenses in the genital tract. Prog Clin Biol Res. 1981;70:381–394. [PubMed] [Google Scholar]
- Olea N, Pazos P, Exposito J. Inadvertent exposure to xenoestrogens. Eur J Cancer Prev. 1998;7(Suppl 1):S17–23. doi: 10.1097/00008469-199802001-00005. [DOI] [PubMed] [Google Scholar]
- Orsi NM, Tribe RM. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol. 2008;20:462–469. doi: 10.1111/j.1365-2826.2008.01668.x. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou AD, Fisher BR, Umbreit TH, Goering PL, Lappas NT, Brown KM. Effects of beta-estradiol and bisphenol A on heat shock protein levels and localization in the mouse uterus are antagonized by the antiestrogen ICI 182,780. Toxicol Sci. 2001;63:173–180. doi: 10.1093/toxsci/63.2.173. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou AD, Umbreit TH, Fisher BR, Goering PL, Lappas NT, Brown KM. Bisphenol A-induced increase in uterine weight and alterations in uterine morphology in ovariectomized B6C3F1 mice: role of the estrogen receptor. Toxicol Sci. 2000;56:332–339. doi: 10.1093/toxsci/56.2.332. [DOI] [PubMed] [Google Scholar]
- Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J Clin Endocrinol Metab. 2000;85:4835–4840. doi: 10.1210/jcem.85.12.7029. [DOI] [PubMed] [Google Scholar]
- Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, Mok SC. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- Remoue F, Jacobs N, Miot V, Boniver J, Delvenne P. High intraepithelial expression of estrogen and progesterone receptors in the transformation zone of the uterine cervix. Am J Obstet Gynecol. 2003;189:1660–1665. doi: 10.1016/s0002-9378(03)00852-4. [DOI] [PubMed] [Google Scholar]
- Roberts D, Curtis C, Tren R, Sharp B, Shiff C, Bate R. Malaria control and public health. Emerg Infect Dis. 2004;10:1170–1171. doi: 10.3201/eid1006.030787. author reply 1171–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan WJ, Chen A. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT) Lancet. 2005;366:763–773. doi: 10.1016/S0140-6736(05)67182-6. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, Ragan NB. Evidence of effects of environmental chemicals on the endocrine system in children. Pediatrics. 2003;112:247–252. [PubMed] [Google Scholar]
- Rolff J, Siva-Jothy MT. Invertebrate ecological immunology. Science. 2003;301:472–475. doi: 10.1126/science.1080623. [DOI] [PubMed] [Google Scholar]
- Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, Flaws JA, Hansen DK, Hoyer PB, Jeffery EH, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of soy formula. Birth Defects Res B Dev Reprod Toxicol. 2006;77:280–397. doi: 10.1002/bdrb.20086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapino A, Cassoni P, Ferrero E, Bongiovanni M, Righi L, Fortunati N, Crafa P, Chiarle R, Bussolati G. Estrogen receptor alpha is a novel marker expressed by follicular dendritic cells in lymph nodes and tumor-associated lymphoid infiltrates. Am J Pathol. 2003;163:1313–1320. doi: 10.1016/s0002-9440(10)63490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai C, Anderson K, Walser-Kuntz D. Effect of bisphenol A on murine immune function: modulation of interferon-gamma, IgG2a, and disease symptoms in NZB X NZW F1 mice. Environ Health Perspect. 2003;111:1883–1887. doi: 10.1289/ehp.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- Scholz S, Gutzeit HO. 17-alpha-ethinylestradiol affects reproduction, sexual differentiation and aromatase gene expression of the medaka (Oryzias latipes) Aquat Toxicol. 2000;50:363–373. doi: 10.1016/s0166-445x(00)00090-4. [DOI] [PubMed] [Google Scholar]
- Schumacher GF, Kim MH, Hosseinian AH, Dupon C. Immunoglobulins, proteinase inhibitors, albumin, and lysozyme in human cervical mucus. I. Communication: hormonal profiles and cervical mucus changes--methods and results. Am J Obstet Gynecol. 1977;129:629–636. doi: 10.1016/0002-9378(77)90644-5. [DOI] [PubMed] [Google Scholar]
- Schust DJ, Anderson DJ, Hill JA. Progesterone-induced immunosuppression is not mediated through the progesterone receptor. Hum Reprod. 1996;11:980–985. doi: 10.1093/oxfordjournals.humrep.a019335. [DOI] [PubMed] [Google Scholar]
- Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Riminton DS, Cyster JG, Korner H. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol Today. 2000;21:110–113. doi: 10.1016/s0167-5699(99)01573-x. [DOI] [PubMed] [Google Scholar]
- Selsted ME, Ouellette AJ. Defensins in granules of phagocytic and non-phagocytic cells. Trends Cell Biol. 1995;5:114–119. doi: 10.1016/s0962-8924(00)88961-8. [DOI] [PubMed] [Google Scholar]
- Shaw JL, Smith CR, Diamandis EP. Proteomic analysis of human cervico-vaginal fluid. J Proteome Res. 2007;6:2859–2865. doi: 10.1021/pr0701658. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Steinke JW, Borish L. 3. Cytokines and chemokines. J Allergy Clin Immunol. 2006;117:S441–445. doi: 10.1016/j.jaci.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Philibert D, Chaouat G. Progesterone suppression of pregnancy lymphocytes is not mediated by glucocorticoid effect. Am J Reprod Immunol. 1990;23:42–43. doi: 10.1111/j.1600-0897.1990.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24:145–155. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- Tiemann U. In vivo and in vitro effects of the organochlorine pesticides DDT, TCPM, methoxychlor, and lindane on the female reproductive tract of mammals: a review. Reprod Toxicol. 2008;25:316–326. doi: 10.1016/j.reprotox.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Tiffin P, Moeller DA. Molecular evolution of plant immune system genes. Trends Genet. 2006;22:662–670. doi: 10.1016/j.tig.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Heat shock protein and innate immunity. Cell Mol Immunol. 2004;1:274–279. [PubMed] [Google Scholar]
- Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85:905–910. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- UNAIDS. AIDS epidemic update. 2007. [Google Scholar]
- UNAIDS. AIDS epidemic update. 2009. [Google Scholar]
- Underdown BJ, Schiff JM. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- Usala SJ, Usala FO, Haciski R, Holt JA, Schumacher GF. IgG and IgA content of vaginal fluid during the menstrual cycle. J Reprod Med. 1989;34:292–294. [PubMed] [Google Scholar]
- van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- Vodkova Z, Rajmon R, Petr J, Klabnova P, Jilek F. Effects of genistein and genistin on in vitro maturation of pig oocytes. Czech J Anim Sci. 2008;53:1–8. [Google Scholar]
- Wallin RP, Lundqvist A, More SH, von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–135. doi: 10.1016/s1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- WHO. Sexually transmitted infections. 2007. [Google Scholar]
- Wiedow O, Schroder JM, Gregory H, Young JA, Christophers E. Elafin: an elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence. J Biol Chem. 1990;265:14791–14795. [PubMed] [Google Scholar]
- Wira C, Fahey J, Wallace P, Yeaman G. Effect of the menstrual cycle on immunological parameters in the human female reproductive tract. J Acquir Immune Defic Syndr. 2005a;38(Suppl 1):S34–36. doi: 10.1097/01.qai.0000167040.58181.d5. [DOI] [PubMed] [Google Scholar]
- Wira CR, Fahey JV. The innate immune system: gatekeeper to the female reproductive tract. Immunology. 2004;111:13–15. doi: 10.1111/j.1365-2567.2003.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Abrahams VM, Rossoll RM. Influence of stage of the reproductive cycle and estradiol on thymus cell antigen presentation. J Steroid Biochem Mol Biol. 2003;84:79–87. doi: 10.1016/s0960-0760(03)00002-5. [DOI] [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex Hormone Regulation of Innate Immunity in the Female Reproductive Tract: The Role of Epithelial Cells in Balancing Reproductive Potential with Protection against Sexually Transmitted Pathogens. American Journal of Reproductive Immunology. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005a;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunological Reviews. 2005b;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005c;53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Roche MA, Rossoll RM. Antigen presentation by vaginal cells: role of TGFbeta as a mediator of estradiol inhibition of antigen presentation. Endocrinology. 2002;143:2872–2879. doi: 10.1210/endo.143.8.8938. [DOI] [PubMed] [Google Scholar]
- Witkin SS, Linhares IM, Giraldo P. Bacterial flora of the female genital tract: function and immune regulation. Best Pract Res Clin Obstet Gynaecol. 2007;21:347–354. doi: 10.1016/j.bpobgyn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Wolf M, Delgado MB, Jones SA, Dewald B, Clark-Lewis I, Baggiolini M. Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. Eur J Immunol. 1998;28:164–170. doi: 10.1002/(SICI)1521-4141(199801)28:01<164::AID-IMMU164>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Yeaman GR, Howell AL, Weldon S, Demian DJ, Collins JE, O’Connell DM, Asin SN, Wira CR, Fanger MW. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109:137–146. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellayi S, Naaz A, Szewczykowski MA, Sato T, Woods JA, Chang J, Segre M, Allred CD, Helferich WG, Cooke PS. The phytoestrogen genistein induces thymic and immune changes: a human health concern? Proc Natl Acad Sci U S A. 2002;99:7616–7621. doi: 10.1073/pnas.102650199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S, Yamaki K, Li X, Sai T, Yanagisawa R, Takano H, Taneda S, Hayashi H, Mori Y. Prenatal exposure to bisphenol A up-regulates immune responses, including T helper 1 and T helper 2 responses, in mice. Immunology. 2004;112:489–495. doi: 10.1111/j.1365-2567.2004.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S, Yamaki K, Yanagisawa R, Takano H, Hayashi H, Mori Y. Effects of bisphenol A on antigen-specific antibody production, proliferative responses of lymphoid cells, and TH1 and TH2 immune responses in mice. Br J Pharmacol. 2003;138:1271–1276. doi: 10.1038/sj.bjp.0705166. [DOI] [PMC free article] [PubMed] [Google Scholar]