Abstract
Firemaster® 550 and Firemaster® BZ-54 are two brominated formulations that are in use as replacements for polybrominated diphenyl ether (PBDE) flame retardants. Two major components of these mixtures are 2,3,4,5-tetrabromo-ethylhexylbenzoate (TBB) and 2,3,4,5-tetrabromo-bis(2-ethylhexyl) phthalate (TBPH). Both have been measured in environmental matrices; however, scant toxicological information exists. The present study aimed to determine if these brominated flame-retardant formulations are bioavailable and adversely affect DNA integrity in fish. Fathead minnows (Pimephales promelas) were orally exposed to either FM 550, FM BZ54, or the nonbrominated form of TBPH, di-(2-ethylhexyl) phthalate (DEHP) for 56 d and depurated (e.g., fed clean food) for 22 d. At several time points, liver and blood cells were collected and assessed for DNA damage. Homogenized fish tissues were extracted and analyzed on day 0 and day 56 to determine the residue of TBB and TBPH and the appearance of any metabolites using gas chromatography-electron-capture negative ion mass spectrometry (GC/ECNI-MS). Significant increases ( p<0.05) in DNA strand breaks from liver cells (but not blood cells) were observed during the exposure period compared with controls, although during depuration these levels returned to control. Both parent compounds, TBB and TBPH, were detected in tissues at approximately 1% of daily dosage along with brominated metabolites. The present study provides evidence for accumulation, metabolism, and genotoxicity of these new formulation flame retardants in fish and highlights the potential adverse effects of TBB- and TBPH-formulated fire retardants to aquatic species.
Keywords: Flame retardant, Tetrabromobenzoate, Phthalate, DNA damage, Metabolism
INTRODUCTION
Flame retardants serve a valuable purpose in decreasing risks associated with fire by extending the time required to transition from a smoldering to flaming state [1]. Polybrominated diphenyl ethers (PBDE) are quite effective as retardants and until recently were some of the most widely used brominated flame retardants. However, due to their environmental persistence and toxicological concerns, many of these commercial PBDE formulations have recently been banned or phased out [2,3]. This has resulted in an industry shift toward the use of alternative types of brominated flame-retardant compounds. Among this new wave of halogenated flame retardants are Firemaster® 550 and Firemaster® BZ-54.
In 2004, Great Lakes Chemical began marketing Firemaster 550 as a less bioaccumulative and toxic alternative to the existing Penta-BDE mixtures [4]. Firemaster 550 consists of a suite of triaryl phosphate isomers, triphenyl phosphate, and a brominated formulation identified as Firemaster BZ-54 [5]. Firemaster BZ-54 is a mixture of 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (TBB) and 2-ethylhexyl 2,3,4,5-tetrabromophthalate ([TBPH]; see Table 1 for structures and chemical details). These chemicals are commonly added to foam cushions, polyvinyl chloride (PVC) plumbing, and other fabrications [6] and have recently been identified in environmental samples. For example, their ubiquitous presence in house dust has been shown with concentrations of TBB and TBPH as high as 15,030 and 10,630 ng/g dry weight reported, respectively [3] and are within the range reported for total BDEs in house dust [7-9]. Additionally, TBB was detected (1.240±0.264μg/g dry wt) in biosolids collected from a San Francisco Bay wastewater treatment plant in levels comparable to that of BDE-209 (1.023±0.179 mg/g dry wt) measured in the same sample (S. Klosterhaus, unpublished data; http://www.sfei.org/rmp/posters/08BFR_Poster_klosterhaus_shrunk.pdf). To date, the only existing toxicological data for TBB and TBPH are the standard acute aqueous toxicity tests (i.e., 96-h median lethal concentration [LC50]) summarized on the Material Safety Data Sheet forms for these Firemaster formulations.
Table 1.
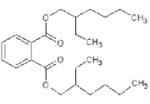
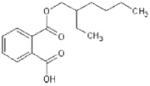
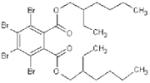
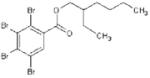
Basic chemical characteristics of di(2-ethylhexyl) phthalate (DEHP), mono(2-ethylhexyl)phthalate (MEHP), 2-ethylhexyl 2,3,4,5-tetrabro-mophthalate (TBPH), and 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (TBB)
| Chemical name | DEHP | MEHP | TBPH | TBB |
|---|---|---|---|---|
| Structure |
|
|
|
|
| Chemical abstracts service number | 117-81-7 | 4376-20-9 | 26040-51-7 | None |
| Molecular weight | 390.57 | 278.35 | 706.15 | 549.93 |
| Solubility (mg/L) | 4.1 × 10-2 [39] | 1.49a | 1.98 × 10-9a | 1.14 × 10-5a |
| Log Kovi | 7.5a | 4.7b | 12.0b | 8.8b |
| Vapor pressure (mm Hg @ 25°C) | 1.33 × 10-8 [39] | 8.15 × 10-7c | 1.71 × 10-11c | 3.43 × 10-8c |
WSKow v 1.41 (U.S. Environmental Protection Agency [U.S. EPA], Washington, DC).
KowWin v 1.67(U.S. EPA).
Mean vapor pressure of antoine and grain methods.
As there is limited information regarding TBB and TBPH bioavailability, uptake, bioaccumulation, metabolism and toxicity (particularly sub-lethal effects), some insights may be gained from studies with the nonbrominated form of TBPH, di(2-ethylhexyl) phthalate (DEHP). The nonbrominated form of TBB is 2-ethylhexyl benzoate (EHB), a chemical for which we could find no toxicological data. Di(2-ethylhexyl) phthalate is rapidly metabolized to mono(2-ethylhexyl)phthalate (MEHP), which has a similar structure to TBB. Toxicological effects have been studied for MEHP exposures and, thus, were considered a chemical potentially representative for TBB. Chemical properties have been well studied for DEHP, but not for MEHP, TBB, or TBPH (see Table 1). These chemicals are fairly insoluble and are highly lipophilic, suggesting that likely exposure scenarios would be by means of food consumption. The flame retardants have four bromines bound to their aromatic ring, which increases its lipophilicity and its molecular size, which may impede movement across cellular membranes. In PBDEs, congeners with a greater number of bromines incorporated in their structure are less bioavailable [10]. However, the largest BDE congener, decabrominated BDE (BDE-209) has a Log KOW similar to TBPH (~10–11) and has been shown to be absorbed in carp [11,12], rats [13], and birds [14], and detected in human tissues [15]. Di(2-ethylhexyl) phthalate has been shown to be readily absorbed and metabolized by rainbow trout and Atlantic salmon [16,17].
A likely effect of TBB and TBPH exposures is the loss of DNA integrity. Genotoxicity has been identified with both PBDEs and DEHP. In the case of PBDEs, BDE-47 exposures affect glutathione levels which may modulate reactive oxygen species generation, apoptosis, and DNA damage [18-20]. The exact mechanism is unclear, but hydroxylated PBDEs themselves appear capable of causing DNA damage in neurological tissues directly [21]. Similar to BDEs, DEHP exposures have also been found to be associated with a depletion of glutathione and an increase in reactive oxygen species in rats [22]. Metabolites of DEHP (monobutyl phthalate, monobenzyl phthalate, and monoethyl phthalate) are associated with loss of DNA integrity in humans, but the mechanism of action is not known [23].
Given the environmental presence of these halogenated flame retardants as well as their structural similarities to chemicals known to be absorbed, metabolized, and associated with DNA damage, concern regarding the potential effects of TBB and TBPH exposures is warranted. The present study was undertaken to investigate the potential for uptake, metabolism, and adverse effects (i.e., DNA damage) in a common toxicity test species in regulatory work, the fathead minnow (Pimephales promelas), exposed to Firemaster 550 and BZ-54 formulations. Specifically, observations were made characterizing growth (i.e., fish mass, length, and lipid content of tissues), lipid normalized tissue accumulation of TBB and TBPH, qualitative evaluation of the formation of metabolites, and the degree of DNA damage in hepatic and blood tissues. Di(2-ethylhexyl) phthalate exposures were also included to compare the extent of DNA damage potential induction between the brominated and nonbrominated structures. To the best of our knowledge, this research is the first to address the uptake, accumulation, metabolism and effects (general and genotoxic) regarding exposures to these brominated compounds.
MATERIALS AND METHODS
Food preparation
Control food was fed to all of the fish during the acclimation and recovery periods as well as to the control treatment for the duration of the exposure period. Firemaster 550 (FM 550) and Firemaster BZ-54 (BZ-54) were procured from Chemtura. Di(2-ethylhexyl) phthalate (98% purity) was purchased from Alfa Aesar. Food amended with FM 550, BZ-54, or DEHP was used for those respective treatments during the 56-d exposure period. All equipment used in food preparation was made of glass or stainless steel and was rinsed with acetone, hexane, and dichloromethane before use. Cod liver oil (CVS®), fish feed (Hunting Creek Fisheries), and fish gel (Aquatic Ecosystems) were used in all food preparation. Fish feed (118 g) and fish gel (51 g) were blended using a glass blender (Crushmaster, Black & Decker) until a fine powder was produced. The BZ-54, FM 550, and DEHP were measured by weight and dissolved into 10 ml cod liver oil. Control food included the cod liver oil without chemical amendment. The oil and the food mixture were combined in a stainless steel mixing bowl. Milli-Q® water (138 ml) was added and the resulting paste was mixed with a stainless steel mixer set at medium for 20 min. The resulting paste was then manually formed into pellets (0.195–0.205 g) and stored at −20°C. Daily aliquots of food were removed and thawed on the day of use.
There is scant toxicological data available to determine at what level effects may be observed. Therefore, an exposure magnitude (1 mg fish/d of F550, BZ-54, or DEHP) roughly two orders of magnitude greater than current measured environmental concentration was chosen [3]. Measured chemical concentrations for FM 550 and BZ-54 treatment food regime are reported in Table 2. Di-(2-ethylhexyl) phthalate is reported only as a nominal concentration as it is readily metabolized and no methods were available to monitor the concentration of DEHP in tissues. Measured concentrations of TBB and TBPH differed from the nominal concentrations as commercial formulations are variable in concentration and some adherence to the carrying vessels may have occurred. The BZ-54 amendment to the feed was expected to be 5,075 μg/g. The sum of the measured concentrations for TBPH and TBB accounted for 59.0% of the combined nominal target. For FM 550, the measured concentration sum for TBPH and TBB was 59.2% of the combined nominal target (4,056μg/g) assuming the formulation had a 1:1 mass ratio between BZ-54 and the other component (tri-aryl and tri-phenyl phosphate) [5]. The resulting daily intake per fish was 150μg TBPH and 330μg TBB in the FM 550 feed, and 180μg TBPH and 420μg TBB in the BZ-54 feed.
Table 2.
Concentrations of 3 contaminant amended diets used in the present study. Measured concentrations of 2-ethylhexyl 2,3,4,5-tetrabro-mophthalate (TBPH), and 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (TBB) are represented as the mean±standard error. Three replicates were extracted and analyzed for each feed type.
| Total (μg/g feed – wet wt) |
|||
|---|---|---|---|
| Feed | % Lipid | TBPH | TBB |
| Control | 6.5±0.3 | <0.26±0.014 | 0.20±0.053 |
| Firemaster® 550a | 7.4±0.7 | 744.7±85.97 | 1658±198.9 |
| Firemaster® BZ-54a | 7.7±1.0 | 907.4±166.3 | 2087±385.0 |
Chemtura.
Organisms were acclimated before exposure
All handling of test organisms was approved by University of Maryland Center for Environmental Sciences’ Institutional Animal Care and Use Committee (F-CBL-08-02). Fathead minnows (Pimephales promelas) are temperate freshwater fish native to North America and used commonly in toxicological testing for regulatory purposes. One-year-old adult males were purchased from Aquatic BioSystems and acclimated to laboratory conditions for eight weeks. During this acclimation period, 10 fish were placed into each of the eight 40-L plastic aquaria. The photoperiod was set at 16:8 h light:dark. Submerged glass heaters kept the temperature at 25°C±1°C. Water quality parameters (i.e., pH, dissolved oxygen) were kept within U.S. Environmental Protection Agency (U.S. EPA) guidelines for moderately hard water [24]. Every other day, waste was removed and half of the water was renewed.
Experimental duration: exposure and recovery periods
A week before test initiation, fish were transferred to fifteen 40-L plastic aquaria at a density of five fish per aquaria. During the entirety of the experiment, 50% water exchanges occurred every other day 6 h after feeding. All food was consumed within 5 min. The experimental duration was 78 d, which consisted of a 56-d period of dietary exposure to the chemical treatments and 22 d recovery where all fish were fed control food. Fish were fed 0.2 g/d (~6% of fish body wt) with food specific to their exposure scenario. Fish in three tanks were administered with control food. Each chemical treatment (FM 550, BZ-54, and DEHP) consisted of four tanks. Fish remaining for the recovery stage remained in their respective tanks and received control pellets regardless of their previous treatment regimen.
Fish were killed by cervical incision during the exposure and recovery periods to measure potential temporal changes in DNA damage. Three to four fish were sampled on days 14, 28, 56, and 78 for the FM 550, BZ-54, and control treatments. Fish exposed to DEHP were sampled on days 28, 56, and 78. Upon killing, hepatic tissue and blood were sub-sampled from each fish. These samples were placed on ice and were immediately processed for the comet assay. Fish length and weight were also recorded. Gonads, liver, and brain were removed and used for other endpoints while the remaining carcass was frozen for later analysis of TBPH and TBB accumulation and metabolite identification on days 0 and 56 only.
Comet assay used to measure DNA damage
The technique performed in the present study was a modification of methods previously reported [25,26]. Liver tissue was minced finely and resuspended in aerated, ice-cold Hanks’ balanced salt solution (HBSS) buffer solution (1 mM Hepes; Ca2+ and Mg2+ free; pH 7.6). Separately, blood was also resuspended in the HBSS buffer solution. Cell density for the liver and blood solutions was 2.6±0.3×105 cells/ml and 4.2±0.5×105 cells/ml, respectively. Both cell suspensions were then placed on ice and subsamples were analyzed for cell viability using trypan blue exclusion. Viability in all samples was greater than 80% and, therefore, all samples were used for the Comet Assay. An aliquot (10μL) of the cell suspension was mixed with 100μL of 37°C, 0.6% low melting point agarose ([LMPA]; dissolved in the buffer solution), layered onto a precoated 1% normal melting point agarose glass slide and solidified on ice. A second LMPA layer was added and again solidified on ice. The slides were then placed in ice cold lysing solution (2.5M NaCl, 100 mM Tris, 1% N-laurylsarcosine, 1% Triton X-100, 10% dimethysulfoxide, pH 10.0) for 1 h at 4°C in the dark. Slides were then drained, rinsed with Milli-Q water, and placed in an electrophoresis chamber with electrophoresis buffer (200mM NaOH, 100mM ethyl-enediaminetetraacetic acid [EDTA], pH<12). The slides were allowed to unwind for 10 min and then electrophoresed (25 V, 300 mA) for 10 min. Slides were removed, drained and rinsed three times with neutralizing buffer (0.4M Tris, pH 7.5). They were then submerged in ice-cold methanol for 5 min, dried in the dark, and stored in a closed container in the dark with desiccant until later re-hydration and analysis.
For DNA damage analysis, slides were rehydrated in a 2μg/g ethidium bromide solution for 10 min. Slides were then observed using an epifluorescence scope (200 × magnification, Olympus BX50) with a Q Imaging Retiga 1300 camera. One hundred cells per slide were selected randomly from the whole slide and quantified for DNA damage using Komet 6.0 (Kinetic Imaging) image analysis software. Percentage of DNA in the tail is the prominent endpoint that was used in the present study as it has been reported as a more accurate and sensitive measure [25,27]. Tail length and olive tail moment are reported for potential comparison to other studies, but are not heavily discussed in the present study.
Chemical analysis
Individual fish samples and three replicates of each food type (0.2 g) were homogenized with clean sodium sulfate to remove residual water using a mortar and pestle. Samples were then loaded into a stainless steel extraction cells, spiked with an internal standard, 4′fluoro-2,3′,4,6-tetrabromodiphenyl ether (F-BDE 69, Chiron), and extracted using pressurized liquid extraction (ASE 300, Dionex). Cells were extracted three times consecutively with a 1:1 solution of hexane and dichloromethane at a temperature of 100°C and at 1500 psi for 10 min. The resulting extract was reduced in volume to roughly 1 ml by use of an automated nitrogen evaporation system (TurboVap II, Zymark). A small subsample of the extract was used to measure lipid content using gravimetric analysis. The remaining extract was then purified by elution through a column containing 4.0 g of alumina (6% deactivated) using a 1:1 ratio of dichloromethane: Hexane. This final extract was then reduced in volume to 0.5 ml and amended with 50 ng of 13C-labeled 2,2′,3,4,5,5′-chlorinated diphenyl ether (CDE 141) to measure the recovery of F-BDE 69. A total of three laboratory blanks and four matrix spikes (spiked with 100 ng of TBB and TBPH) were also incorporated in the extraction process for quality assurance purposes.
Samples were analyzed using gas chromatography mass spectrometry operated in electron capture negative ionization mode (GC/ECNI-MS) in a manner previously reported [3]. A J&W Scientific 0.25 mm (internal diameter)×15 m fused silica capillary column coated with 5% phenyl methylpolysiloxane (0.25μm film thickness) was used for the separation of the brominated compounds. Pressurized temperature vaporization (PTV) was used in the GC inlet which was programmed for the following conditions: an 80°C hold for 0.3 min followed by a 700°C/min ramp to 275°C, which was held throughout the entire sample run. The oven temperature program was set at 40°C and held for 1 min, followed by a temperature ramp of 18°C/min to 250°C, followed by a temperature ramp of 1.5°C/ min to 260°C, and then ramped a final time at 25°C/min to 300°C which was held for an additional 20 min. The transfer line was maintained at 300°C and the ion source at 200°C. 2,3,4,5-Tetrabromo-ethylhexylbenzoate and TBPH were quantified by monitoring molecular ion fragments (m/z 357 and 471 for TBB, and 463 and 515 for TBPH). The mass of each compound was corrected for recovery of F-BDE 69 and normalized to lipid content. Recovery of F-BDE 69 was measured as 72.6±3.3%. 2,3,4,5-Tetrabromo-bis(2-ethylhexyl) phthalate was not detected in the blanks (<1.67±0.17 ng; 3 times the signal of the blanks), whereas TBB was detected in laboratory blanks at 1.65±0.29 ng. The mean and standard error of the mean for TBB and TBPH recovery in the matrix spikes were 113.2±11.4 and 96.3±7.5%, respectively.
Significance determined with analyses of variance and t test
All statistical analyses were run using the statistical package MiniTab (Ver 14). Each tank within a treatment was recognized as a replicate. The data sets were tested for normality and variance homogeneity. One-way analyses of variance (ANOVA) were calculated to determine significance between treatments for a single time point (α = 0.05; p<0.05). If conditions were met for significance by ANOVA, chemical treatments were compared with the controls with a t test to determine which treatments were significantly different from the control treatment (p<0.05).
RESULTS
Fish health and growth
Water quality characteristics were within whole effluent toxicity testing guidance parameters for Pimephales promelas [24]. On day 78, control and BZ-54 treatments had survival of 83 and 88% of their total population. Survival in the other two treatments were 75% for DEHP and 63% for FM 550. The experimental design did not permit sufficient power to determine significance of lethality associated with each feeding regime. Fish weight and length were not significantly different between treatments at any sampling point. At test initiation, average length and weight were 61±1 mm and 2.42±0.21 g, respectively. After 78 d, fish length was 67±1 mm and weight was 3.19±0.21 g. Lipid content in the fish tissue was not significantly different from the controls, except on day 56, when it was greater in the FM 550 treatment (3.00±0.10) in comparison to day 56 controls (1.37±0.32; p<0.01). It is uncertain if this is a statistical anomaly.
Bioaccumulation of TBB/TBPH and metabolite identification
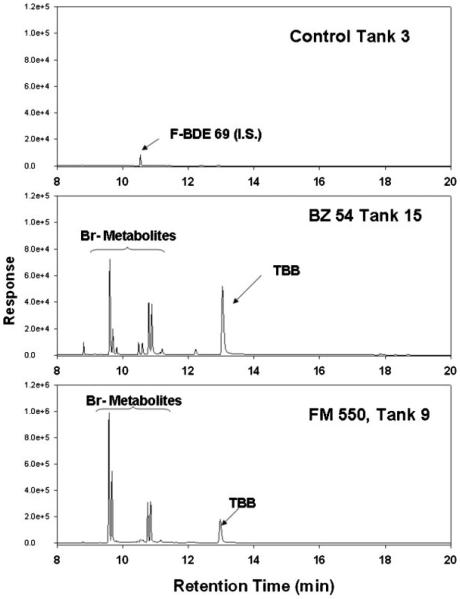
Both TBPH and TBB concentrations in fish on day 56 were significantly greater than those found in day 0 fish or in control fish from day 56 (p<0.05; Fig. 1). Although these are statistically significant observations of the parent compounds presence in the fish, it should be noted that the greatest amount of chemical measured in a single BZ-54-fed fish was 800 ng of TBB and 1,075 ng of TBPH. These numbers represent 0.59 and 0.19% of the daily dosage for TBPH and TBB in the BZ-54-feed, respectively. Total recoverable TBB and TBPH were 70% less in FM 550-fed fish. However, during analysis of the fish tissue samples, several peaks were observed in the GC/MS chromatograms in addition to the parent compounds (Fig. 2). The mass spectrum of these peaks suggested they were brominated metabolites of the parent compounds (i.e., a strong m/z signal for 79 and 81 was observed in exposed fish extracts). The presence of metabolites may explain the low bioaccumulation due to rapid biotransformation and excretion or transformation to a form that is not currently quantifiable.
Fig. 1.
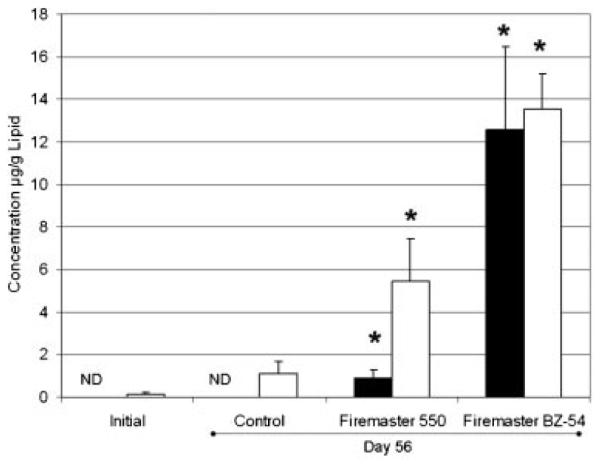
Lipid normalized 2-ethylhexyl 2,3,4,5-tetrabromophthalate (TBPH, black bars) and 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (TBB, white bars) concentrations in fish collected on day 0 (initial) and on day 56. Control, Firemaster 550®, and Firemaster BZ-54® (Chemtura,) treated fish were analyzed. Bars illustrate the mean and the standard error of mean (n=3–4). Asterisk denotes significance (t test; p<0.05). ND=Nondetect (3 times the blank value;<0.152μg/g lipid for Initial and<0.212μg/g lipid for Control).
Fig. 2.
Selected chromatograms from fish samples processed on day 56 from the control, Firemaster BZ-54, and Firemaster 550 treatments (Chemtura). All extracts were measured with gas chromatography-electron-capture negative ion mass spectrometry. The 2,3,4,5-tetrabromo-ethylhexylbenzoate (TBB) and its brominated metabolites are noted. BDE=brominated diphenyl ether; I.S.=internal standard.
DNA damage in liver tissue, not blood
The compounds did not significantly induce DNA damage in blood cells above respective control levels (Table 3; Fig. 3). However, BZ-54 and FM 550 exposures resulted in an increase in DNA damage in liver at more than one sampling point (Table 3; Fig. 4). The BZ-54 exhibited a significant increase in percent tail DNA on days 28 (3.4×greater than controls) and 56 (6.3×). On day 14, the mean was 3.9 times greater than the control mean, but wide variation within the sample population rendered that difference statistically insignificant. Elevated DNA damage levels were not observed after 22 d of recovery. Fish exposed to FM 550 exhibited significant increases in percent tail DNA at all 3 exposure time points. The differences between the treatment and the controls increased from 1.8 times greater at day 14, 3.0 times greater than controls at day 28, and 5.8 times greater than controls at day 56. On day 78, two of the three fish killed for that time point had endpoint levels similar to the controls, while one fish had DNA damage seven times greater than controls. This resulted in a much greater mean, but not one that was significantly greater than the controls due to variability within the data set. Di(2-ethylhexyl) phthalate also exhibited raised levels in percent tail DNA. Although the mean was greater than the controls on day 28, it was only significantly greater on day 56. Means for percent tail DNA were greater for days 28 (2×) and 56 (3.2×). These effects on DNA integrity reduced 22 d after exposure, but were still somewhat raised. Effects did seem to last into the recovery period, with day 78 slightly greater than the controls.
Table 3.
Comet tail length (TL) and tail moment (TM) for fish exposed to all feeds and for all sampling time points. The mean is presented with the standard error and number of fish collected for each sampling and time point. Asterisks (*) note values that were found to be significantly different (analysis of variance, t test, p<0.05).
| Sampling point (Day) |
||||||
|---|---|---|---|---|---|---|
| Treatment | Cell Type | Metric | 14 | 28 | 56 | 78 |
| Control | Blood | TL | 10.49±0.18 (3) | 16.47±4.79 (3) | 9.12±0.73 (3) | 7.53±0.53 (3) |
| TM | 0.282±0.071 (3) | 0.511±0.182 (3) | 0.218±0.042 (3) | 0.196±0.027 (3) | ||
| Liver | TL | 10.77±3.29 (3) | 15.34±2.44 (3) | 5.96±0.24 (3) | 6.33±0.26 (3) | |
| TM | 0.354±0.101 (3) | 0.492±0.087 (3) | 0.114±0.009 (3) | 0.225±0.005 (3) | ||
| Firemaster 550a | Blood | TL | 18.64±1.37 (3)* | 14.22±2.04 (4) | 11.25±3.18 (3) | 11.74±6.36 (3) |
| TM | 0.389±0.112 (3) | 0.569±0.151 (4) | 0.346±0.106 (3) | 0.770±0.529 (3) | ||
| Liver | TL | 22.20±0.87 (3) | 22.32±3.58 (4) | 20.05±1.93 (3)* | 19.58±15.17 (3) | |
| TM | 0.807±0.069 (3) | 1.904±0.501 (4) | 1.298±0.500 (3) | 1.651±1.369 (3) | ||
| Firemaster BZ-54 | Blood | TL | 22.27±2.50 (4)* | 22.10±10.01 (4) | 12.07±3.64 (4) | 4.23±0.08 (3) |
| TM | 0.975±0.482 (4) | 1.822±1.066(4) | 0.590±0.240 (4) | 0.189±0.019 (3) | ||
| Liver | TL | 21.54±6.38 (4) | 34.76±6.36 (4) | 15.79±2.82 (4)* | 4.98±0.42 (3) | |
| TM | 2.365±1.098 (4) | 3.439±1.308 (4) | 1.642±0.635 (4) | 0.255±0.077 (3) | ||
| DEHP | Blood | TL | NM | 12.00±1.95 (3) | 15.33±4.80 (3) | 9.27±2.00 (3) |
| TM | NM | 0.549±0.311 (3) | 0.727±0.339 (3) | 0.430±0.125 (3) | ||
| Liver | TL | NM | 17.06±2.39 (3) | 14.00±1.68 (3)* | 9.21±0.37 (3) | |
| TM | NM | 1.281±0.288 (3) | 0.687±0.127 (3) | 0.399±0.046 (3) | ||
Chemtura.
NM=not measured; DEHP=di(2-ethylhexyl) phthalate.
Fig. 3.
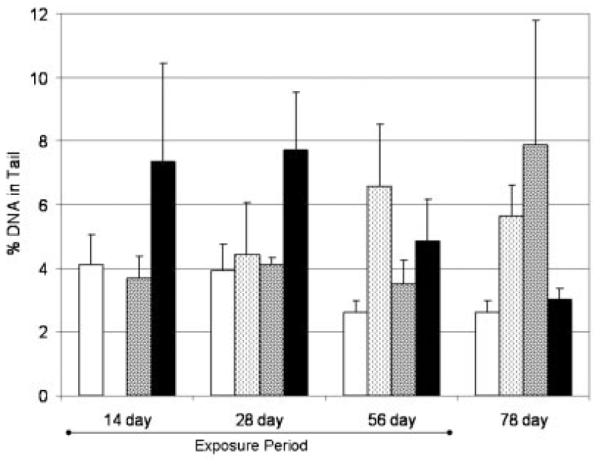
Percentage of DNA in tail from blood samples taken from fish during the experiment. Bars illustrate the mean and the standard error of mean (n=3–4). No treatment was calculated as significantly different from the controls (t test; p<0.05). Control, white bars; DEHP, light hatched bars; F550, dark hatched bars; BZ54, black bars (Firemaster BZ-54, and Firemaster 550 treatments; Chemtura). DEHP=di-(2-ethylhexyl) phthalate.
Fig. 4.
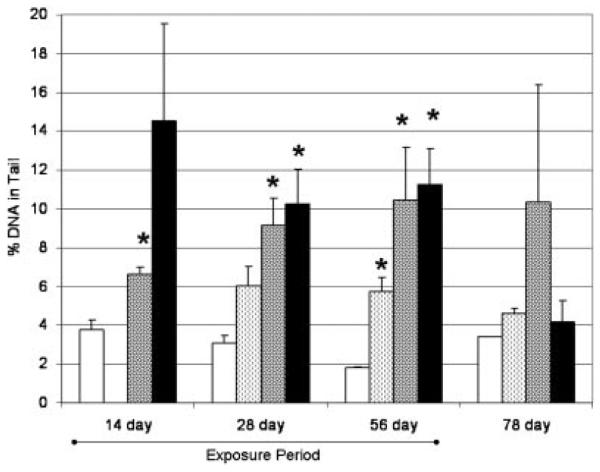
Percentage of DNA in tail from liver samples taken from fish during the experiment. Bars illustrate the mean and the standard error of mean (n=3–4). Treatments significantly different from the controls are identified with an asterisk (t test; p<0.05). Control, white bars; DEHP light hatched bars; F550, dark hatched bars; BZ54, black bars (Firemaster BZ-54, and Firemaster 550 treatments; Chemtura). DEHP=di-(2-ethylhexyl) phthalate.
DISCUSSION
The present study demonstrates that dietary exposures of these chemical formulations at the concentrations and exposure times used in the present study result in uptake and sublethal effects, although they do not adversely affect traditional toxicological endpoints (i.e., lethality and fish growth). However, it is uncertain how representative the exposure scenarios presented in this series of experiments are to environmental conditions. After a 56-d exposure, TBPH and TBB was bio-available and accumulated, although whole fish (sans liver) concentrations after two months of exposure accounted for approximately 1% of the daily oral exposure. It is uncertain to what degree the parent compounds are being absorbed or directly eliminated, but the presence of metabolites indicates that the chemical is being taken up into the fish and is actively metabolized. The locations of some of the peaks in the chromatograms from these fish are similar to chromatograms produced from a study focused on the photodegradation of TBB and TBPH [28]. Photodegradation leads to debromination of both compounds, but only TBB is biologically metabolized in this present study. In a preliminary study, BZ-54 was incubated with active or heat killed common carp (Cyprinus carpio) liver microsomes for 2 h at 25°C (n=3). Similar to our current in vivo study, the concentration of TBB was 73.1±1.3% less in the active microsome samples than in the heat-killed sample while no difference (0.0±4.3%) was detected with respect to the concentration of TBPH. This suggests that TBB is rapidly metabolized in common carp microsomes, but TBPH is not. Although species differences in metabolism may be present between carp and fathead minnows, these preliminary experiments may indicate that there are differences between metabolites produced from photodegradation and biotic processes. Further studies will be performed to elucidate routes of biotransformation and resultant metabolites in fathead minnows.
Although structural analogues, TBPH differs from DEHP as a result of incorporating 4 Br atoms which significantly alter its KOW and size (see Fig. 1). Di(2-ethylhexyl) phthalate readily undergoes catalysis by lipases to produce 2-ethylhexanol and MEHP [29]. Mono(2-ethylhexyl)phthalate can be metabolized further into several products. It can undergo catalysis by means of lipase to phthallic acid and a second 2-ethylhexanol or form oxidative metabolites by means of cytochrome P4504A, alcohol dehydrogenase, or aldehyde dehydrogenase. Mono(2-ethylhexyl) phthalate can also be conjugated by uridine 5′-diphospho-glucuronosyltransferase. In total, at least 13 metabolites consisting of ketones and carboxylic acids are known [30]. In humans, these compounds have a short half-life in the body, because essentially all of the absorbed DEHP and its metabolites were excreted within 24 h. It is these metabolites that are considered to be responsible for the toxic effects of DEHP and MEHP in mammals until they are conjugated by UDPGT enzymes [31]. These processes appear quite similar in fish species. In rainbow trout, microsomal P450s in the liver oxidized MEHP to several metabolites [16]. The parent compounds and the metabolites were rapidly eliminated from the fish tissue. These metabolites were similar to those seen in mammalian species except for an absence of certain diacid metabolites [30,32]. These metabolites have been found in human metabolism and are thought to be the result of the mitochondria having a greater role in biotransformation of MEHP.
Application of the DEHP metabolic route should be tempered when using it to explain TBB and TBPH metabolism. The lack of TBPH biotransformation in fish tissue may be an issue of the large size and hydrophobicity of the compound, which are both the result of the additional four bromine atoms. In BDE metabolism, bromine incorporation in the molecule has been shown to affect reaction rates [33]. These characteristics may inhibit TBPH metabolism, but not TBB. The reduced size and open ring site may permit more enzymatic activity on TBB and enable biotransformation. This could also be true if a different metabolic route is at work.
We found that DNA damage was prevalent in the liver and largely absent in the blood in fish exposed to the Firemaster formulations when compared with control fish. Both brominated chemicals may not absorb as readily as DEHP (~70% absorbance through the gastrointestinal tract) [30] given the influence of halogenation on increasing KOW and size. However, they appear to be actively metabolized in the liver given the localization of DNA damage to the liver and the observation of metabolites in the remaining carcass (Figs. 2, 4). We did not assess the levels of the parent compounds or metabolites in the liver, which may have shown elevated levels. Further studies using DEHP may provide insight on potential metabolic processes and metabolites that are important in determining how TBB and TBPH induce adverse effects on DNA integrity.
Di-(2-ethylhexyl) phthalate has been shown to cause DNA damage in human cells [34]. No study has yet to report DEHP causing DNA damage in hepatocytes. Polybrominated diphenyl ethers have also been found to elicit DNA damage in mussel hepatocytes and mammal neurocytes [35,36]. It is unknown what route these chemicals may cause DNA damage and how this relates to the genotoxicity observed in TBB- and TBPHexposed fish. Potential causes may be the parent compounds or metabolites serve as agonists for peroxisome proliferator-activated receptor, kinases, or aryl hydrocarbon receptor. These pathways have been found to be affected in mammals exposed to DEHP and PBDEs [35,37,38]. An AhR pathway would result in bulky adducts on the DNA while peroxisome proliferator-activated receptor activation and kinase-associated DNA damage is the result of oxidative stress.
CONCLUSION
Dietary exposures of TBB and TBPH induced repairable DNA damage in the hepatic tissue of fathead minnows. Both parent compounds are bioavailable and accumulate in fish tissues but it appears that only TBB is metabolized biologically. The exact metabolic pathways and mechanism of action(s) that result in the observed adverse effects to genetic integrity is largely unknown in the present study. Future studies are under way and are focused on the metabolism of TBB and TBPH, including identification and quantitation of the metabolites observed, elucidation of the enzymatic process(es) involved and how they may result in genotoxicity.
Acknowledgement
Funding for this research was provided by the Drach-Mellody Navigator Research Fellowship, Chesapeake Biological Laboratory, University of Maryland–Baltimore, and the National Institute of Environmental Health Sciences (R01-ES016099). Contribution number 4339 of the University of Maryland Center for Environmental Science.
REFERENCES
- 1.Chao CWH, Wang JH. Transition of smoldering to flaming combustion of horizontally oriented flexible polyurethane foam with natural convection. Combust Flame. 2001;127:2252–2264. [Google Scholar]
- 2.Hites RA. Polybrominated diphenyl ethers in the environment and in people: Meta-analysis of concentrations. Environ Sci Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton HM, Allen JG, Kelly S, Konstantinov A, Klosterhaus S, Watkins D, Mcclean MD, Webster TF. Alternate and new brominated flame retardants detected in US house dust. Environ Sci Tech. 2008;42:6910–6916. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- 4.Chemtura . Firemaster® 550; MSDS00896. Middlebury, CT, USA: 2006. [Google Scholar]
- 5.Chemtura . Firemaster® BZ-54; MSDS 00074. Middlebury, CT, USA: 2006. [Google Scholar]
- 6.Andersson PL, Oberg K, Orn U. Chemical characterization of brominated flame retardants and identification of structurally representative compounds. Environ Toxicol Chem. 2006;25:1275–1282. doi: 10.1897/05-342r.1. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton HM, Dodder NG, Offenberg JH, Schantz MM, Wise SA. Polybrominated diphenyl ethers in house dust and clothes dryer lint. Environ Sci Technol. 2005;39:925–931. doi: 10.1021/es0486824. [DOI] [PubMed] [Google Scholar]
- 8.Allen JG, McClean MD, Stapleton HM, Webster TF. Linking PBDEs in house dust to consumer products using X-ray fluorescence (XRF) Environ Sci Technol. 2008;42:4222–4228. doi: 10.1021/es702964a. [DOI] [PubMed] [Google Scholar]
- 9.Wilford BH, Shoeib M, Harner T, Zhu J, Jones KC. Polybrominated diphenyl ethers in indoor dust in Ottawa, Canada: Implications for sources and exposure. Environ Sci Technol. 2005;39:7027–7035. doi: 10.1021/es050759g. [DOI] [PubMed] [Google Scholar]
- 10.Hardy ML. The toxicology of three commercial Polybrominated diphenyl oxide (ether) flame retardants. Chemosphere. 2002;46:757–777. doi: 10.1016/s0045-6535(01)00240-5. [DOI] [PubMed] [Google Scholar]
- 11.Stapleton HM, Alaee M, Letcher RJ, Baker JE. Debromination of the flame retardant decabromodiphenyl ether by juvenile carp (Cyprinus carpio) following dietary exposure. Environ Sci Technol. 2006;38:112–119. doi: 10.1021/es034746j. [DOI] [PubMed] [Google Scholar]
- 12.Stapleton HM, Letcher RJ, Baker JE. Dietary accumulation and metabolism of Polybrominated diphenyl ethers (PBDEs) by juvenile carp (Cyprinus carpio) Arch Environ Contam Toxicol. 2004;23:1939–1946. doi: 10.1897/03-462. [DOI] [PubMed] [Google Scholar]
- 13.Huwe JK, Smith DJ. Accumulation, whole-body depletion, and debromination of decabromodiphenyl ether (BDE-209) in male Sprague-Dawley rats following dietary exposure. Environ Sci Technol. 2007;41:2371–2377. doi: 10.1021/es061954d. [DOI] [PubMed] [Google Scholar]
- 14.Van den Steen E, Covaci A, Jaspers VLB, Dauwe T, Voorspoels S, Eens M, Pinxten R. Accumulation, tissue specific distribution and debromination of decabromodiphenyl ether (BDE 209) in European starlings (Sturnus vulgaris) Environ Pollut. 2007;148:648–653. doi: 10.1016/j.envpol.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Schector A, Papke O, Harris TR, Tung KC. Partitioning of Polybrominated diphenyl ether (PBDE) congeners in human blood and milk. Toxicol Environ Chem. 2006;88:319–324. [Google Scholar]
- 16.Barron MS, Albro PW, Hayton WL. Biotransformation of di(2-ethylhexyl)phthalate by rainbow trout. Environ Toxicol Chem. 1995;14:873–876. [Google Scholar]
- 17.Norman A, Borjeson H, David F, Tienpont B, Norrgren L. Studies of uptake, elimination, and late effects in Atlantic salmon (Salmo salar) dietary exposed to di-2-ethylhexyl phthalate (DEHP) during early life. Arch Environ Contam Toxicol. 2007;52:235–242. doi: 10.1007/s00244-005-5089-y. [DOI] [PubMed] [Google Scholar]
- 18.Giordano G, Kavanagh TJ, Costa LG. Neurotoxicity of a Polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol Appl Pharmacol. 2008;232:161–168. doi: 10.1016/j.taap.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He P, He WH, Wang AG, Xia T, Xu BY, Zhang M, Chen XM. PBDE-47-induced oxidative stress, DNA damage, and apoptosis in primary cultured rat hippocampal neurons. Neurotoxicology. 2008;29:124–129. doi: 10.1016/j.neuro.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Shao J, White CC, Dabrowski MJ, Kavavagh TJ, Eckert ML, Gallagher EP. The role of mitochondrial and oxidative injury in BDE 47 toxicity to human fetal liver hematopoietic stem cells. Toxicol Sci. 2008;101:81–90. doi: 10.1093/toxsci/kfm256. [DOI] [PubMed] [Google Scholar]
- 21.Song RF, Duarte TL, Almeida GM, Farmer PB, Cooke MS, Zhang WB, Sheng GY, Fu JM, Jones GDD. Cytotoxicity and gene expression profiling of two hydroxylated Polybrominated diphenyl ethers in human H295R adrenocortical carcinoma cells. Toxicol Lett. 2009;185:23–31. doi: 10.1016/j.toxlet.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Kasahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, Tokuda M, Nakano Y, Inoue M. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem J. 2002;365:849–856. doi: 10.1042/BJ20020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): Human metabolism and internal exposure - an update and latest results. Int J Androl. 2006;29:155–165. doi: 10.1111/j.1365-2605.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Environmental Protection Agency . Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms. 4th. Washington, DC: 2002. EPA-821-R-02-013. [Google Scholar]
- 25.Mitchelmore CL, Chipman JK. Detection of DNA strand breaks in Brown Trout (Salmo trutta) hepatocytes and blood cells using the single cell gel electrophoresis (comet) assay. Aquat Toxicol. 1998;41:161–182. [Google Scholar]
- 26.Sullivan C, Mitchelmore CL, Hale RC, Van Veld PA. Induction of CYP1A and DNA damage in the fathead minnow (Pimephales promelas) following exposure to biosolids. Sci Total Environ. 2007;384:221–228. doi: 10.1016/j.scitotenv.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Dhawan A, Bajpayee M, Parmar D. Comet assay: A reliable tool for the assessment of DNA damage in different models. Cell Biol Toxicol. 2009;25:5–32. doi: 10.1007/s10565-008-9072-z. [DOI] [PubMed] [Google Scholar]
- 28.Davis EF, Stapleton HM. Photodegradation pathways of nonabrominated diphenyl ethers, 2-ethylhexyltetrabromobenzoate, and di(2-ethylhexyl)tetrabromophthalate: Identifying potential markers of photodegradation. Environ Sci Technol. 2009;43:5739–5746. doi: 10.1021/es901019w. [DOI] [PubMed] [Google Scholar]
- 29.Ito Y, Yokota H, Wang R, Yamanoshita O, Ichihara G, Wang H, Kurata Y, Takagi K, Nakajima T. Species differences in the metabolism of di(2-ethylhexyl) phthalate (DEHP) in several organs of mice, rats, and marmosets. Arch Toxicol. 2005;79:147–154. doi: 10.1007/s00204-004-0615-7. [DOI] [PubMed] [Google Scholar]
- 30.Koch H, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79:367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- 31.Kavlock R, Boeckelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T. NTP Center for the evaluation of risks to human reproduction: Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl)phthalate. Reprod Toxicol. 2002;16:529–653. doi: 10.1016/s0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 32.Albro PW, Lavenhar SR. Metabolism of di(2-ethylhexyl)phthalate. Drug Metab Rev. 1989;21:13–34. doi: 10.3109/03602538909029953. [DOI] [PubMed] [Google Scholar]
- 33.Cheng SW, Randall K, Kotchevar AT. In vitro metabolism studies of Polybrominated diphenyl ethers using rat and human liver microsomes. Am J Biochem Biotechnol. 2008;4:295–303. [Google Scholar]
- 34.Park SY, Choi J. Cytotoxicity, genotoxicity and ecotoxicity assay using human cell and environmental species for the screening of the risk from pollutant exposure. Environ Int. 2007;33:817–822. doi: 10.1016/j.envint.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 35.He P, Wang AG, Xia T, Gao P, Niu Q, Guo LJ, Xu BY, Chen XM. Mechanism of the neurotoxic effect of PBDE-47 and interaction of PBDE-47 and PCB153 in enhancing toxicity in SH-SY5Y cells. Neurotoxicology. 2009;30:10–15. doi: 10.1016/j.neuro.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Riva C, Binelli A, Cogni D, Provini A. Evaluation of DNA damage induced by decabromodiphenyl ether (BDE-209) in hemocytes of Dreissena polymorpha using the Comet and micronucleus assays. Environ Mol Mutagen. 2007;48:735–743. doi: 10.1002/em.20353. [DOI] [PubMed] [Google Scholar]
- 37.Wahl M, Lahni B, Guenther R, Kuch B, Yang L, Straehle U, Strack S, Weiss C. A technical mixture of 2,2′,4,4′-tetrabromo diphenyl ether (BDE47) and brominated furans triggers aryl hydrocarbon receptor (AhR) mediated gene expression and toxicity. Chemosphere. 2008;73:209–215. doi: 10.1016/j.chemosphere.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Ito Y, Nakajima T. PPAR alpha- and DEHP-induced cancers. PPAR Res. 2008;2008:1–11. doi: 10.1155/2008/759716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agency for Toxic Substances and Disease Registry . Toxicology profile for di(2-ethylhexyl) phthalate. Atlanta, GA, USA: 2002. [PubMed] [Google Scholar]