Abstract
Background Freshwater comprises about a mere 2·5 % of total global water, of which approximately two-thirds is locked into glaciers at the polar ice caps and on mountains. In conjunction with this, in many instances irrigation with freshwater causes an increase in soil salinity due to overirrigation of agricultural land, inefficient water use and poor drainage of unsuitable soils. The problem of salinity was recognized a long time ago and, due to the importance of irrigated agriculture, numerous efforts have been devoted towards improving crop species for better utilization of saline soils and water. Irrigating plants with saline water is a challenge for practitioners and researchers throughout the world.
Scope Recruiting wild halophytes with economic potential was suggested several decades ago as a way to reduce the damage caused by salinization of soil and water. A range of cultivation systems for the utilization of halophytes have been developed, for the production of biofuel, purification of saline effluent in constructed wetlands, landscaping, cultivation of gourmet vegetables, and more. This review critically analyses past and present halophyte-based production systems in the context of genetics, physiology, agrotechnical issues and product value. There are still difficulties that need to be overcome, such as direct germination in saline conditions or genotype selection. However, more and more research is being directed not only towards determining salt tolerance of halophytes, but also to the improvement of agricultural traits for long-term progress.
Keywords: Agrotechniques, Aster tripolium, biofuel, cash crops, Crithmum maritimum, Euphorbia tirucalii, halophytes, Pennisetum clandestinum, Salicornia, saline agriculture salinity, Sporobolus virginicus, Tamarix jordanis
INTRODUCTION
Water is generally considered a renewable resource, but its availability for human needs is limited by its chemical and physical properties and its global distribution. Freshwater comprises about a mere 2·5 % of total global water, of which approximately two-thirds is locked in glaciers at the polar ice caps and on mountains (Gleick, 2009). Constant accessibility to freshwater from rivers and lakes is of the utmost importance, particularly for irrigated agriculture, industry and households. Almost 20 years ago Postel et al. (1996) estimated that global water use constituted 54 % of accessible runoff, with 65 % of this going to agriculture, frequently for irrigation. The disparity between the geographical distribution of water and that of the human population further aggravates the problem of freshwater scarcity.
In many instances, irrigation causes an increase in soil salinity due to overirrigation of agricultural land, inefficient water use and poor drainage of unsuitable soils. Most known agricultural crop plants are salt-sensitive glycophytes, the growth of which is severely inhibited when grown under saline conditions. Therefore these plants cannot be produced economically with saline water. The United States Department of Agriculture estimates that, worldwide, 10 million hectares of arable land are lost every year to salinity as a result of improper irrigation.
The problem of salinity was recognized a long time ago and, due to the importance of irrigated agriculture, numerous efforts have been devoted towards improving crop species for better utilization of saline soils and water (Malcolm, 1969; Mudie, 1974; Epstein et al., 1980; O'Leary, 1984). The decrease in dry matter production of several crops in response to increasing salinity was estimated by Munns and Tester (2008), revealing a 50 % decrease at 80 mm NaCl for rice (Oryza sativa), at 100 mm NaCl for durum wheat (Triticum turgidum ssp. durum) and at 120 mm NaCl for barley (Hordeum vulgare). Conversely, ‘salt-loving’ halophytes, which occur in the wild throughout the world, live and reproduce in salt marshes, sea shores, estuaries or saline deserts (Epstein et al., 1980). Halophytes are capable of tolerating a wide range of salinities, even beyond seawater concentration (approx. 500 mm NaCl), thus providing a basis for the development of an extensive spectrum of halophytic crops covering large fields of application (Yensen, 2006).
This current review aims to reassess agricultural production systems from the past and in the present that use halophytic plants converted into agricultural crops. The main focus is placed on multifaceted applications ranging from production of animal feed to gourmet vegetables, and from ornamentals to biofuel, sometimes realized by one and the same plant species.
FROM THE PAST TO THE PRESENT DAY
Irrigating agricultural crops with seawater was and still is one of the biggest challenges for farmers and for plant researchers, whose main efforts are directed to discover solutions to the problems. Driven by the need to expand the use of natural resources, Hugo and Elisabeth Boyko, respectively a geophysicist and plant-ecologist, demonstrated in the early 1960s the possibility of cultivating crops with full-strength seawater, and thus paved the way for pioneering research of seawater agriculture (Boyko and Boyko, 1964). They hypothesized that the combination of the high soil permeability to water and the good drainage capability of sandy soils together with the use of excess quantities of seawater may be the basic factors allowing the development of this field of agriculture. To prove the concept, these scientists showed in a small-scale experiment that two plant species with extremely different ecological requirements, Agropyrum junceum and Juncus arabicus, could be grown successfully, for fibre production, on dunes irrigated with various seawater dilutions over a period of about 1·5 years (Boyko and Boyko, 1959). In subsequent experiments it was demonstrated that certain local barley strains could complete their life cycle when irrigated with seawater containing up to 4·2 % salt (Boyko and Boyko, 1964). Nearly simultaneously, similar hypotheses were tested, and the potential of two grasses, Agropyron elongatum and Puccinellia capillaris, was demonstrated for ground cover and for grazing when grown on saline wastelands, (Rogers and Bailey, 1963; Malcolm, 1969).
Nevertheless, the majority of agricultural crops are highly sensitive even to small amounts of sodium chloride. Maas (1990) summarized research that showed that soil salinity even less than 2 dS m–1 resulted in yield reduction of vegetable crops such as beans (Phaseolus vulgaris, yield decline of 19 % per dS m–1), peppers (Capsicum annuum, 14 %), corn (Zea mays, 12 %) and potatoes (Solanum tuberosum, 12 %). Thus, the main ambition of those interested in saline agriculture was to increase the number of crop species that could produce economic yields while growing under unfavourable saline conditions. To realize this goal, two research directions were proposed: (1) crossing of salt-tolerant relatives within sensitive crop lines by traditional breeding methods (Yeo and Flowers, 1989); and (2) domestication of naturally salt-tolerant plants. Many research efforts worldwide were devoted to the first strategy, with high expectations that improved lines could be developed (Epstein et al., 1980). However, breeding for salt tolerance proved to be a difficult task, as this is a multigenetic trait (Flowers and Yeo, 1995; Flowers et al., 2010), and the numerous attempts resulted in only few salt-tolerant lines.
The second route, exploiting the potential of natural halophytes, was followed by another group of researchers, led by Elisabeth Boyko. A 6 year plant introduction programme was conducted oriented mainly toward the development of agricultural and pasture material suitable for dryland conditions (Nurock, 1960). The resulting ecological desert garden was the first milestone for a plant collection of xerophytes and halophytes, particularly those with economic potential (Boyko, 1962). Follow-up research started in 1982 with a new project for systematic collection and evaluation of halophytes for fodder production irrigated with saline water (Pasternak, 1990). About 140 halophytic species were evaluated in a large experimental plot on the Mediterranean coast. The importance of comparing a broad range of halophytes, collected from most of the world's continents, under the same environmental conditions was highlighted. Based on visual observations obtained three times a year during three sequential years (1983–1985), a list was compiled including 78 plant species that could grow successfully even if irrigated with 100 % seawater and an additional 22 species that produced well at 15 % seawater (Pasternak, 1990). The HALOPH database was initialized by this list, then further extended and finally published by Aronson (1989). According to Aronson's criteria, HALOPH included about 1560 plant species that were able to grow when exposed to ECi (electrical conductivity of the irrigation water) of 7–8 dS m–1 during significant portions of their life cycle. One rubric of the HALOPH database was dedicated to the economic uses of the different species, modified from the code developed by G. E. Wickens and co-workers for the Survey of Economic Plants for Arid and Semiarid Lands (SEPASAL), Royal Botanic Gardens, Kew, UK (Aronson, 1989). Aronson's data have since been extended by Menzel and Lieth (2003). An additional version on halophytes and their uses, based on Aronsons' data, was developed by Yensen (http://www.ussl.ars.usda.gov/pls/caliche/Halophyte.query). Most recently, an interactive version, the eHALOPH database, was compiled and can be found at http://www.sussex.ac.uk/affiliates/halophytes.
The initial enthusiasm for growing Atripex spp. successfully with highly saline water and its fodder potential for small ruminants as a rich N source (up to 17 % crude protein under 100 % seawater) was stifled by the very low animal intake (0·3–0·4 kg dry matter d–1) and its low metabolic energy content (approx. 1·5 Mcal kg–1 d. wt) (Pasternak et al., 1985; Pasternak, 1990). Therefore, it was concluded that Atriplex nummularia, although highly salt tolerant, is a poor food source and its use as a fodder crop is limited. Conversely, additional studies conducted in the Mediterranean basin showed that Atriplex spp. could successfully be used as a complementary nutrient source, although energy supplements (e.g. barley) were necessary for small ruminants fed on salt-tolerant forage-based diets (Le Houerou, 1992; El Shaer, 2010). Based on this conclusion, and since halophytic grasses do concentrate salts in their leaves to a lesser extent than dicotyledonous halophytes and thus should have a higher net caloric value, a new line of testing several halophytic grass species at lower irrigation salinity (ECi 9·5 dS m–1) was initiated in the late 1980s (Pasternak, 1990). Indeed, several American accessions of saltgrass (Distichlis spicata) were examined and showed considerable promise for selection as fodder crop for ruminants (Bustan et al., 2005).
Glenn et al. (1999) presented an overview summarizing the dominant research groups conducting field trials with halophytes under saline irrigation, working in arid zones of the USA [‘The Environmental Research Laboratory of the University of Arizona’ (Glenn et al., 1996) and the ‘Halophyte Biotechnology Center’, University of Delaware (Gallagher, 1985)], Australia (Malcolm, 1969) and northern Africa (Le Houerou, 1996). Since then numerous researchers have joined the field of halophyte crop cultivation, not only from dry regions, but also from areas with ample rainfall encountering the problem of salinity on coastal areas or inland salt pans. The groups of Rozema in The Netherlands (de Vos et al., 2013; Katschnig et al., 2013; Rozema and Schat, 2013), Koyro (Koyro et al., 2011) and Papenbrock (Buhmann and Papenbrock, 2013a, b) in Germany, Abdelly in Tunisia (Ksouri et al., 2012) and Khan in Pakistan (Gul and Khan, 2003) introduced additional halophytic species as new cash crops and presented novel growing techniques. In particular, the field of renewable energy and ecological sustainability (e.g. purification of land-based aquaculture effluents) gained importance (Shpigel et al., 2013). There has been a shift from producing halophytes as animal feed or salty vegetables for human consumption, to the mass production of non-food crops. Cultivation of halophytes as ornamental plants for either landscaping or floriculture is still in its infancy. In the field of ornamentals, most efforts are still directed towards testing glycophytes for improved performance under salinity (Cassaniti et al., 2013). Since Boyko (1966) set the important first milestones in saline agriculture, many ideas have been tested and several discarded, but the ‘ne plus ultra’ has yet to be found (http://www.cost.eu/domains_actions/fa/Actions/FA0901). Koyro et al. (2011) presented 12 major uses for halophytes, inter alia food, feed, wood, chemicals, landscaping, ornamentals, industrial raw materials and bioremediation. However, introducing non-domesticated halophytic plant species as agricultural crops with reasonable income for the growers will require the refinement of growing protocols and selection of improved varieties. To date, the scientific literature still focuses mostly on basic research questions dealing with plant salt tolerance mechanisms, and thus refers to small-scale experiments (Koyro et al., 2011). As Boyko concluded in 1966 ‘Agrotechnical details for economic purposes have to be worked out …’; therefore, scaling-up to larger field experiments and intensifying genotype selection directed towards economic crop production should be the primary activities for future directions (Glenn et al., 2013). However, many novel ways for the utilization of halophytes were proposed recently through the COST action ‘Putting Halophytes to Work, From Genes to Ecosystems’ and have made a significant input in advancing these matters for future halophyte growers (http://www.cost.eu/domains_actions/fa/Actions/FA0901).
FODDER-YIELDING HALOPHYTES
High palatability, digestibility and good nutritional value (high protein and low fibre, ash and oxalate contents) signify high fodder quality (El Shaer, 2006). Since salt accumulation reduces the nutritional value and feeding quality of most plants, the future prospects of Atriplex species as useful fodder crops, even in combination with other energy sources, were described as rather limited by Pasternak (1990). A different tactic was proposed by Barrett-Lennard and Setter (2010), who recognized the importance of feeding mixtures of plants, such as A. nummularia, with herbaceous species and annual grasses, which together fulfil the requirements of an effective fodder crop and can be grown at moderate salt levels. This approach has been further discussed by Norman et al. (2013), who highlighted the critical importance of voluntary feed intake (palatability, post-ingestive feedback) and nutritive value (metabolizable energy, salt, antioxidants, toxins) as factors affecting production of livestock. A concluding remark made by Norman et al. (2013) for successfully using the mixed plant system (halophytic grasses and shrubs) was to take advantage of the benefits of using halophytes while managing their negative consequences.
The potential of halophytic grass species as fodders was also investigated by Pasternak (1990) and Bustan et al. (2005). Indeed, although less salt tolerant than species of Atriplex, the ash content of all tested Distichlis spicata accessions never exceeded 11 % of the dry matter, about half the amount found in the salt-accumulating chenopods, highlighting its potential as a fodder crop (Bustan et al., 2005). The protein content of D. spicata varied widely between the accessions, and ranged from a minimum of 9·2 % to a maximum of 18·9 % of dry matter, similar to the protein content reported by Pasternak (1990) for A. nummularia. In Pakistan, cultivating the halophytic species Leptochloa fusca (Kallar grass) not only resulted in high productivity [20 t dry matter ha–1 from 4–5 cuts per year (Mahmood et al., 1994)], but also successfully improved the soil conditions of the existing saline sodic soils, showing increased vegetation growth after a 5 year period (Hollington et al., 2001). The anatomical adaptation of grasses for salt secretion evidently contributes to the maintenance of low leaf salt levels and relatively low (compared with those existing in dicotyledonous halophytes) Na/K ratios (Flowers and Colmer, 2008). Liphschitz et al. (1974) reported the existence of active salt-secreting glands on the leaves of Rhodes Grass (Chloris gayana Kth.). In a similar manner, Bermuda grass (Cynodon dactylon) was determined as a salt-secreting species (Liphschitz and Waisel, 1974; A. Eshel and Y. Waisel, 2007, unpubl. res.), which was confirmed by Hameed et al. (2013) by an increased number of vesicular trichomes for the exclusion of toxic ions. Thus these plants attract interest as important candidates for economic utilization in saline environments (Liphschitz et al., 1974).
Grasses have also been used in combination with other chenopods apart from Atriplex species. Salicornia bigelovii was tested as a protein-rich fodder crop for saline irrigation (Glenn et al., 1991), but its high ash content (up to 39 %) limited its nutritional potential and its fodder quality (Basmaeil et al., 2003; Y. Ventura, J. Miron and M. Sagi, 2012, unpubl. res.). To overcome this problem, Glenn et al. (1992) substituted 50 % of a Rhodes Grass-based fodder with S. bigelovii. Plants were grown on complete seawater and directly after harvest the plant biomass was soaked in seawater. This action reduced the NaCl content by half to approx. 8–13 %, resulting in an ash content of 16·7 %. Glenn et al. (1992) also noted that the protein content of the Salicornia biomass was higher if the seeds were not removed prior to animal feeding. The conclusion drawn from an experiment feeding S. bigelovii straw or seed meal to lambs showed that this halophyte could be used as an acceptable feed substitute in arid coastal regions where fresh water for crop irrigation is limited (Swingle et al., 1996). In another study, the replacement of 12·5 % alfalfa by Salicornia herbacea, the locally occurring Salicornia in Kuwait, in the basal diet resulted in the highest body weight gain and feed consumption of Australian wither lambs, as compared with the other treatments (Abdal, 2009). On the other hand, in a camel feeding trial, the incorporation of 25 % seawater-irrigated S. bigelovii biomass in the diet had an adverse effect on the nutritive value of the feed in comparison with Rhodes Grass diet. In particular, ADF (acid detergent fibre) and NDF (neutral detergent fibre) digestibility decreased by approx. 50 and 20 %, respectively, which might have indicated an increased flow of undigested dietary components out of the rumen (Basmaeil et al., 2003). The usefulness of Salicornia spp. as an animal feed for ruminants seems to be directly related to its salt content, which can be adjusted by the plant portion in the diet. Selection for low-salt-accumulating varieties may increase the feeding value of Salicornia for small ruminants. For other species, Belal and Al-Dosari (1999) successfully included 40 % Salicornia meal in fish (Nile Tilapia) feeds as a replacement for the conventional fish meal diet, with no adverse effect on fish growth and body composition.
NEW GENERATION HALOPHYTES – FOOD AND GOURMET VEGETABLES
Halophytes (e.g. Crithmum maritimum, Portulaca oleracea, Salicornia spp. and Aster tripolium) have been consumed by humans for centuries, and to date are still often gathered from the coastal salt marshes and inland salt pans of Europe (Franke, 1982; Wagenvoort et al., 1989; Davy et al., 2001; Simopoulos, 2004; Tardio et al., 2006). These species are well known for their ability to synthesize secondary metabolites, which have several functions, such as osmolytes and scavengers of reactive oxygen species (Hasegawa et al., 2000). The secondary metabolites include simple and complex sugars, amino acids, quaternary ammonium compounds, polyols and antioxidants (e.g. polyphenols, β-carotene, ascorbic acid and ureides; Parvaiz and Satyawati, 2008; Ventura and Sagi, 2013). Osmolytes can potentially be utilized in functional food, which is defined as having disease-preventing and/or health-promoting benefits (Stuchlík and Žák, 2002; Buhmann and Papenbrock, 2013a). The modern awareness of a healthier diet may promote additional markets for halophytes with high nutritional potential, evident in the rapidly growing consumption of products from some halophytic plants (e.g. quinoa; Panta et al., 2014). Such alternative crops may find niches in the demanding market for novelties, while taking advantage of a range of saline irrigation water sources. Several halophyte products such as Salicornia spp. and Aster tripolium are already being sold as sea vegetables and salad crops on the European markets at comparatively high prices (Böer, 2006). A number of additional halophytes, e.g. Salsola soda, Crambe maritima and Beta maritime, have a great potential to be released as novel sea vegetables to the market. The non-seasonality and year-round availability was an important step in the dissemination of the Salicornia crop and should be realized for any further halophyte vegetable (Böer, 2006, http://www.scribd.com/doc/102170797/Saline-Crops-From-Halophyte-Research-to-Sea-Vegetable-Markets). A broad range of saline water sources and soil salinities, from drainage water originating from sub-surface drainage systems used to lower saline shallow water tables, up to complete seawater, can be used for halophyte vegetable cultivation (Grieve and Suarez, 1997; Ventura et al., 2011a; Ventura and Sagi, 2013).
The South American seed crop quinoa (Chenopodium quinoa) has a long history of cultivation and, within the existing 2500 accessions, some are able to cope with salinity levels present in seawater (approx. 40 dS m–1). Due to its high tolerance not only to salinity but also to other abiotic stresses (drought, frost and wind) and the exceptional nutritional quality of the seeds (rich in vitamins, minerals, essential amino acids and fatty acids), this ‘old crop’ gained interest in the Western world's diet and was nominated to contribute to global food security (Adolf et al., 2013). Presently, in Bolivia, one of the largest quinoa producers, grain yields are low (<0·5 t ha–1), but potential yields may reach 3–5 t ha–1 through optimizing cultivation conditions (Gómez et al., 2011; Adolf et al., 2013).
Although the effect of salinity on secondary metabolites has been extensively studied with respect to plant salt tolerance, these compounds have seldom been referred to in terms of quality parameters of a commercial product: they can enhance the nutritional value of a crop and may differ between plant species and even between genotypes within the same species. The content of secondary metabolites may also be further influenced by agrotechnical practices (Ventura et al., 2011a), which include irrigation water quantity and salinity, plant fertilization, harvest time and cycle, and harvested plant material (young or old leaves) (Ventura et al., 2010, 2011a, 2013). An example of improvement of the quality of a leafy vegetable by plant fertilization was presented by Ventura et al., (2013) for Aster tripolium. This highly salt-tolerant plant species was considered a potential halophytic cash crop and it is already cultivated in pilot projects in The Netherlands, Belgium and Portugal (Lieth and Mochtchenko, 2002; Geissler et al., 2009a). Nevertheless, the introduction of A. triopolium as a salt-resistant vegetable crop met unexpected difficulties (Ventura et al., 2013). When cultivated on soil suitable for saline irrigation (96 % sand, 0·8 % silt, 3·1 % clay, <0·1 % organic matter, pH 8), a specific microelement deficiency that affected product quality (leaf yellowing) was indirectly induced by the high pH. Therefore it is clear that growing protocols need to be adapted to the existing cultivation conditions, taking not only salt tolerance into account.
An additional halophytic species with cash crop potential is Crithmum maritimum, which was known for its antiscorbutic property. In the past, leaves were collected by sailors along the maritime cliffs (Cunsolo et al., 1993). To date, the salt tolerance, secondary metabolite content and antioxidant capacity of Crithmum have been studied extensively (Cunsolo et al., 1993; Guil-Guerrero and Rodríguez-García, 1999; Ben Hamed et al., 2005; Ben Amor et al., 2006; Meot-Duros and Magné, 2009; Atia et al., 2011) using non-domesticated plant material, collected from the wild. A study comparing four ecotypes revealed differences in yield, leaf appearance, polyphenols and ascorbate content (Y. Ventura et al., unpubl. res.). These results highlight the genetic differences and the potential for selection of genoptypes with high yield and high nutritional metabolite content.
The annuals Atriplex hortensis (red orach) and Tetragonia tetragonioides (New Zealand spinach) are both halophytic plant species suggested as spinach substitutes (Wilson et al., 2000; Słupski et al., 2010). Atriplex hortensis has been cultivated for its edible leaves since ancient times and is still grown in kitchen gardens as a pot herb (Wilson et al., 2000). Atriplex hortensis is more salt tolerant than T. tetragonioides (Wilson et al., 2000). In an agronomic study carried out in Israel, early flowering of A. hortensis in the spring season led to a very short harvest period, during which only two sequential harvests could be performed. Shifting to a selective harvest regime, in which only marketable size shoot tops were removed, resulted in five harvest cycles, before flowering occurred. In a greenhouse experiment, maximum yield of 2·2 kg m–2 fresh biomass was obtained under an irrigation salinity of 4 dS m–1 for the attractive variety, Purple Orach, which declined to 1·4 kg m–2 at 8 dS m–1. Since only shoot tops of marketable quality were harvested, no further yield loss was recorded for the final marketable product (M. Myrzabayeva, Z. Alikulov, Y. Ventura and M. Sagi, unpubl. res.). Atriplex hortensis has been recommended as a substitute or supplement to spinach (Spinacea oleracea) due to their similar chemical composition (Carlsson and Clark, 1983). Likewise, T. tetragonioides (New Zealand spinach) was considered to be of good nutritional value, with the exception of sulphur amino acid deficiency. Moreover, culinary and technological processing (blanching–freezing–storage–cooking) caused a significant increase in total amino acid content, except for methionine and cysteine, in this species (Słupski et al., 2010).
Inula crithmoides was proposed as a candidate for saline agriculture by Zaruyk and Baalbaki (1996). This species is traditionally consumed in Lebanon, but is less commonly used in other Mediterranean countries such as Spain and Italy (Zaruyk and Baalbaki, 1996; Guarrera et al., 2006; Tardío et al., 2006). In a potted-plant experiment, growth of I. crithmoides was reduced only at salinities exceeding 20 dS m–1, reaching a yield of 18·3 g d. wt per plant in an 87 d experiment (Zaruyk and Baalbaki, 1996). A 1 year long pot experiment, with monthly sequential harvests, produced a maximum yield of 30 and 6 kg m–2 fresh biomass at 50 mm (approx. 5 dS m–1) and 200 mm NaCl (approx. 20 dS m–1), respectively (Y. Ventura and M. Sagi, unpubl. res.). Marketable yield, defined as undamaged shoot tops, accounted for >50 % of the total fresh biomass at the highest tested salinity (200 mm NaCl). The potted plants could recover after harvest throughout the year, even under prolonged saline irrigation. Leaves of two I. crithmoides genotypes (Ramat Negev and UAE) were tested for their nutritional ingredients, and their fatty acid profile was established (Table 1): the content of a nutritionally important group of unsaturated fatty acids, the long-chain (C16–C20) polyunsaturated fatty acids (PUFAs), particularly those desaturated at either C3 or C6, known as ω3 and ω6 essential fatty acids, was tested (Stuchlík and Žák, 2002). Inula crithmoides contains a high total lipid content with a significant portion of ω3 fatty acids found as 18:3ω3 and 16:3ω3. Compared with other halophytic plants, I. crithmoides ranked second after the ω3 leader Portulaca oleracea: Guil-Guerrero and Rodríguez-García (1999) found total fatty acids to be 3·8 % of dry matter in P. oleracea collected from the wild and 3·4 % in I. crithmoides depending on the genotype and salinity (Table 1), followed by C. maritimum with 3·0 and 2·2 %, for wild collected (Spain) and 50 mm NaCl-irrigated plants from Tunisia, respectively (Guil-Guerrero and Rodríguez-García, 1999; Ben Hamed et al., 2005). Both the genetically inherited high fatty acid content of a certain plant species and irrigation salinity have a significant impact on the total lipid content as well as on the PUFA profile.
Table 1.
Fatty acid methyl ester (FAME) profile (in % of total fatty acids in dry matter) of two Inula crithmoides genotypes (Ramat Negev and UAE) grown in pots irrigated with solutions containing different concentrations of NaCl
| FAME | Salinity (mm NaCl) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Ramat Negev |
UAE |
|||||||
| 0 | 50 | 100 | 200 | 0 | 50 | 100 | 200 | |
| 16:0 | 13·9bc | 14·5ab | 14·7a | 14·8a | 13·2c | 13·4c | 14·5ab | 14·0abc |
| 16:1 | 2·2 | 1·8 | 2·1 | 2·3 | 2·4 | 1·9 | 1·8 | 2·3 |
| 16:2 | 1·0ab | 1·2a | 1·2a | 1·1ab | 0·7b | 0·8ab | 0·8ab | 0·7b |
| 16:3ω3 | 2·4cd | 2·2d | 2·2d | 2.1d | 7·8a | 8·1a | 5·5ab | 4·9bc |
| 18:0 | 3·3ab | 3·3ab | 3·2ab | 3·4a | 2·6bc | 2·6bc | 2·4c | 2·3c |
| 18:1ω9 | 1·8ab | 1·5ab | 1·4b | 1·5ab | 1·7ab | 1·9a | 1·6ab | 1·6ab |
| 18:2 | 28·4a | 28·9a | 28·5a | 29·3a | 24·1b | 26·3ab | 26·9ab | 26·1ab |
| 18:3ω3 | 45·7a | 44·7a | 45·1a | 44·4a | 44·3a | 41·0a | 42·9a | 45·6a |
| Others | 1·4c | 1·8bc | 1·5c | 1·2c | 3·2ab | 3·9a | 3·6a | 2·4abc |
| Total omega-3 | 48·0ab | 46·9b | 47·3b | 46·5b | 52·1a | 49·1ab | 48·4ab | 50·5ab |
| Total fatty acids (% d. wt) | 3·41a | 2·99bc | 2·88c | 2·85c | 3·26ab | 3·12abc | 2·88c | 3·41a |
Values are means of three replicates.
Different letters within the row indicate a significant difference, P < 0·05.
Salicornia and Sarcocornia species, in particular S. bigelovii, have been tested for several applications: gourmet food, animal feed and oils for biodiesel. When grown for human consumption as a gourmet vegetable, only young, vegetative shoots of Salicornia or Sarcocornia should be used. Daylength manipulations and multiple harvesting are agrotechnical practices used in this case to ensure high market value (Ventura et al., 2011a, b). The total lipid content of both Salicornia and Sarcocornia shoots grown in seawater was 21 and 17 mg g–1 dry matter, containing 48·2 % ω3 fatty acids, respectively (Ventura et al., 2011a). Differences between the plant genera (Salicornia and Sarcocornia) and their respective genotypes leave a large gap for further investigation in order to optimize the nutritional value of these plants. Salicornia and Sarcocornia also contain antioxidant compounds, namely polyphenols, β-carotene and ureides. Increasing salinity increased the content of those compounds (Ventura et al., 2011a). The high NaCl content in the shoot may have only a minor impact on the plant's nutritional value, since as a gourmet product it is consumed in small quantities.
ANTINUTRITIONAL FACTORS
In addition to the accumulation of high value nutritional components, halophytes also accumulate undesired factors. Among these antinutritional factors are oxalates, nitrates, phenols, saponins, tannins and salts (Kumar, 1991). Table 2 summarizes antinutritional factors in potential cash crop halophytes and the agrotechnical practices that can be applied in order to decrease their content and improve the product quality.
Table 2.
Antinutritional factors in cash crop halophytes
| Plant species | Antinutritional factor | Agricultural activity to reduce the content | Reference |
|---|---|---|---|
| Portulaca oleracea | Oxalate | Reduce NO3 fertilization in favour of NH4 | Palaniswamy et al. (2002) |
| Salicornia/Sarcocornia | High ash content | Wash harvested biomass in seawater | Glenn et al. (1992) |
| Saponins in seeds | Careful leaching with running water | Glenn et al. (1991); Eganathan et al. (2006) | |
| Aster tripolium | Nitrate accumulation | Adjust iron fertilization | Ventura et al. (2013) |
| Atriplex hortensis | Oxalate, nitrate, | May be decreased during processing. | Carlsson and Clarke (1983) |
| Saponins, phenolics | Would not present problems for ruminants. | ||
| Atriplex nummularia | Oxalate | Reduce NO3 fertilization in favour of NH4. | Al Daini et al. (2013) |
| Selection of low oxalate genotypes |
Purslane (Portulaca oleracea) is considered moderately salt tolerant (EC 6·3 dS m–1 is a threshold for yield reduction) and possesses the highest leaf ω3 fatty acid content (4 mg g–1 wet weight) in green leafy vegetable yet examined (Kumamoto et al., 1990; Simopoulos, 2004). Furthermore, this plant species is cultivated commercially in Mediterranean regions and the fleshy leaves and stems are used in salads (Shannon and Grieve, 1999). Unfortunately, purslane also accumulates large amounts of oxalates (Palaniswamy et al., 2004). The oxalate content in purslane was found to be influenced by salinity (Teixeira and Carvalho, 2009), NO3/NH4 fertilization ratio (Palaniswamy et al., 2002), harvesting stage (Palaniswamy et al., 2001) and variety (Carvalho et al., 2009; Szalai et al., 2010). An increase in ammonium at the expense of nitrate did not influence vegetative biomass parameters significantly, but did lead to a significant decrease in oxalate content in leaves and stem (Palaniswamy et al., 2002). A similar finding of high oxalate levels, which could be counteracted by ammonium nutrition, was described for Atriplex nummularia, a halophytic shrub widely used as forage for ruminant production in saline farming systems in Australia (Al Daini et al., 2013). Ammonium application in A. nummularia not only reduced leaf oxalate levels, but, in contrast to the study of Palaniswamy et al. (2002) in purslane, also significantly decreased the relative growth rate and total plant dry matter. Importantly, the same authors discussed the possibility of genotype selections as useful breeding objectives for achieving low oxalate content. Antinutritive secondary substances (e.g. nitrate and oxalic acid) found in Atriplex oleracea may be reduced during processing and therefore may not diminish the nutritional value of the plant (Carlsson and Clarke, 1983).
Microelement deficiency may result from soil alkalization as a side effect of soil salinization (Grattan and Grieve, 1999). As a secondary effect of low iron availability in sand dune soils (pH 8), nitrate was found to accumulate in leaves of A. tripolium, when irrigated with 50 mm NaCl. Enhanced nitrate reductase activity, induced by the application of iron fertilizer in a chelate form, reduced the leaf nitrate content. Concomitantly, leaf colour was restored after chlorosis, which in turn improved its market value (Ventura et al., 2013).
HALOPHYTES FOR LANDSCAPING AND ORNAMENTALS
Success in the floriculture industry is largely based on the availability of new and attractive ornamental plant varieties, there being a constant demand for novelties (Zaccai, 2002). Due to the primary importance of their external appearance, ornamentals are traditionally irrigated with the highest quality water, as salinity usually affects their yield and quality (Shillo et al., 2002). Valdez-Aguilar et al. (2009) noted that salinity could cause adverse effects on the visual quality of ornamentals including reduced plant growth, distorted flower growth and yield quality, foliar injury and reduced stem length. Still, efforts were made to test a broad range of floricultural glycophytes for their salt tolerance and the possibility of irrigating them with low quality brackish water, recently reviewed by Cassaniti et al. (2013). The application of halophytic plants for landscaping and ornamental purposes seems to be a very attractive solution for the economic utilization of saline soil. Many halophyte species produce attractive flowers. Based on the HALOPH database (Aronson, 1989), Cassaniti and Romano (2011) investigated halophytes native to the Mediterranean region and listed 13 families with about 42 species having ornamental potential.
Besides its value as a vegetable crop, Aster tripolium produces attractive lilac-colour flowers with yellow stamens in the centre (Fig. 1), during its second year of growth. In nature, the flowers appear from July to September. The seeds require a stratification period of 14 d at 4 °C in moist soil before germination (Ramani et al., 2006). Germination was reported to be restricted by salinities >1·5 % NaCl (Ungar, 1995). Both characteristics may have important impact on establishment on saline soils or where only saline water is available for irrigation. Although the salt sensitivity during germination may be an impediment for direct sowing in saline soil, it may be solved through pre-cultivation and subsequent transplanting to the final location. Aster tripolium subsp. tripolium and the smaller subsp. pannonicus (not suitable for vegetable production because of its bitter taste), with distinct morphological differences (leaf size and shape, leaf colour, leaf number and growth habitus; Sági and Erdei, 2002), may have high potential both as cut flowers and for the cultivation in flowering pots. Aster tripolium has been the subject of many investigations concerning its salt tolerance mechanism from several points of view, including growth response (Shennan et al., 1987a), elevated atmospheric CO2 concentrations (Geissler et al., 2009a, b), leaf fatty acid profile (Ramani et al., 2004), stomatal closure (Kerstiens et al., 2002) and ionic regulation (Shennan et al., 1987b). However, no study has analysed the effects of salt on flowering. The genetic material of A. tripolium seems to possess great potential for selection regarding flower appearance (Fig. 1). Therefore, the desired application–purpose, appropriate agrotechniques and salinity levels should be determined in order to direct the flowering period. Post-harvest treatments for cut flowers should also be developed to guarantee quality and shelf-life comparable with those of non-halophyte cut flowers.
Fig. 1.
Aster tripolium flowers. (A) Aster tripolium subsp. pannonicus growing in September in a salt meadow named ‘Nagylapos’, in Törtel, Hungary (photograph by Professor Laszlo Erdei). (B) Inflorescences with increased amount of petals. (C) Flowering branch, kept for 6 d in double-distilled water after cutting. B and C were grown in 5 L plastic pots irrigated with 50 mm NaCl.
MULTIFUNCTIONAL APPLICATIONS OF HALOPHYTES
Crithmum maritimum seems to have the potential to become a multipurpose halophytic cash crop. The aromatic, succulent leaves are appreciated on one hand as a salty vegetable (fresh or pickled), and on the other hand the approx. 30 cm high umbrella-like, delicate inflorescences may be attractive for ornamental purposes. Crithmum maritimum grows in rocky coastal environments in the Mediterranean region, where it is often subjected to sea spray (Ben-Hamed et al., 2004). Indeed, Franke (1982) mentioned that this plant may be used in rock gardens close to the sea, not exclusively for its flowers, but also for its decorative succulent leaves. Special care should be devoted to the germination as the germination of hulled seeds of C. maritimum decreased when exposed to salinity levels >15 dS m–1 (Conesa et al., 2008). Moreover, Atia et al. (2006) reported that germination was significantly inhibited when NaCl concentrations exceeded 50 mm (approx. 5 dS m–1).
Due to their easy cultivation, even at seawater salinity, Salicornia/Sarcocornia have been the subject of numerous research trials which demonstrated their multipurpose applications that include biodiesel, oil, bioremediation, forage, vegetable, probiotic and ornamental usages (Table 3). In addition to Salicornia/Sarcocornia, numerous other halophytes have been listed for more than one application (Table 3). Inula crithmoides, proposed by Zurayk and Baalbaki (1996) as a saline crop, consumed in Lebanon, yields profusions of attractive yellow flowers from July to August, which make it a candidate for flowering pots as well as landscaping. Mesembryanthemum crystallinum is a well-studied model plant for abiotic stresses, which induces the switch from the C3 to CAM photosynthetic mechanism (Bohnert and Cushman, 2000). It can be grown as an ornamental ground cover (Jessop, 1986). Herppich et al. (2008) tested the effects of saline irrigation on plant growth, physiology and leaf quality of a rare vegetable crop of M. crystallinum, commonly known and cultivated in California, India and the southern hemisphere, reporting that irrigation with 150 mm NaCl did not have a negative impact on plant quality.
Table 3.
Halophytic plant species and their multiple application potential
| Scientific name | Uses | References |
|---|---|---|
| Aster tripolium | Vegetable | Ventura et al. (2013) |
| Ornamental | Lieth (2000) | |
| Crithmum maritimum | Vegetable | Meot-Duros and Magné (2009) |
| Ornamental | Ben Dov et al. (1993) | |
| Edible seed oil | Zarrouk et al. (2003) | |
| Inula crithmoides | Food and fodder | Zuryak and Balbakki (1996) |
| Ornamental | Franke (1982) | |
| Mesembryanthemum crystallinum | Vegetable | Herppich et al. (2008) |
| Ornamental | Jessop (1986) | |
| Atriplex hortensis | Vegetable | Wilson et al. (2000) |
| Ornamental | ||
| Salicornia/Sarcocornia spp | Biofuel/oilseed crop | Glenn et al. (1991, 2013) |
| Bioremediation | Webb et al., (2012, 2013); Sphigel et al. (2013) | |
| Forage | Glenn et al. (1992); Imai et al. (2004) | |
| Meal by-product of oil extraction from seeds | Attia et al. (1997) | |
| Vegetable | Ventura and Sagi (2013) | |
| Probiotics | Sarker et al. (2010) | |
| Ornamental | Ventura and Sagi (2013) | |
| Sporobolus virginicus | Ground cover, fodder | A. Eshel and Y. Waisel,(unpubl. res.) |
| Pennisetum clandestinum | Ground cover, fodder | Muscolo et al. (2013) |
| Biomass | Muscolo et al. (2013) |
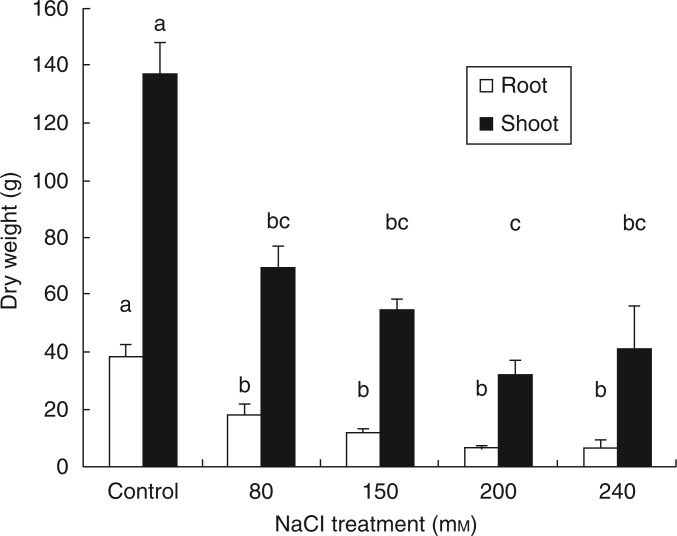
Pennisetum clandestinum and Sporobolus virginicus, salt-tolerant grass species, were investigated for their potential as salt-resistant ground cover and pasture plants with good nutritive properties (Tables 3 and 4). Root, as well as shoot, growth decreased significantly when plants were irrigated with saline water, but no further reduction could be observed among all salt treatments ranging from 80 to 240 mm NaCl (Fig. 2). Moreover, Muscolo et al. (2013) pointed out that P. clandestinum is more resistant to salinity during germination than during vegetative growth, an important characteristic for starting a ground cover crop on marginal, salinized soils. The same authors also mentioned the use of P. clandestinum as a source of renewable bioenergy, as it contains lignocellulotic compounds that could be hydrolysed to produce high amounts of biogas.
Table 4.
Yield of the perennial grasses in September 2006
| Species | Irrigation water | f. wt (t ha–1 month–1) | % d. wt | d. wt (t ha–1 month–1) |
|---|---|---|---|---|
| Sporobolus virginicus | Reclaimed | 4·20 ± 2·18 | 66·87 | 2·81 ± 1·46 |
| Brackish | 5·58 ± 2·68 | 45·88 | 2·56 ± 1·23 | |
| Pennisetum clandestinum | Reclaimed | 7·19 ± 1·20 | 41·17 | 2·96 ± 0·49 |
| Brackish | 9·92 ± 2·61 | 33·57 | 3·33 ± 0·88 |
The salinity of the brackish water ranged between 7 and 10 dS m–1. The salinity of the reclaimed sewage was 2–3 dS m–1. The grasses were cut monthly by a tractor-drawn mechanical harvester (A. Eshel and Y. Waisel, unpubl. res.).
Fig. 2.
Effects of increasing NaCl concentration on dry root and shoot biomass of kikuyu grass (mean ± s.e.). Lower case letters denote statistically significant differences between treatments (Tukey HSD, P < 0·001) (A. Eshel and Y. Waisel, unpubl. res.).
INDUSTRIAL USES OF HALOPHYTES
The utilization of halophytic plants as a source of renewable energy has emerged during the last decade (Rozema and Flowers, 2008). Recently, the use of glycophytic crops, such as maize, sugarcane or soybean, which can be easily used for production of bioethanol or biodiesel, was criticized, because the resource allocation for bioenergy production competes with food production. Furthermore, not only food crops, but also non-food crops such as switchgrass (Panicum virgatum) or short rotation woody perennials including willow and poplar depend on the existing scarce freshwater and arable land resources (Dominguez-Faus et al., 2009). Therefore, the use of halophytes as bioenergy feedstock may be advantageous, since they may occupy a niche of unused marginal or saline lands where irrigation with available saline water is possible.
Eshel and co-workers trialled two desert halophyte plants, Tamarix spp. and Euphorbia tirucalii, for biomass production under extreme desert conditions (Eshel et al., 2010, 2011). Among the four tested Tamarix species, Tamarix jordanis was selected for its high cellulose and low hemicellulose and phenol contents; preferable characteristics for ethanol fermentation (Santi et al., 2014). Tamarix aphylla (erect type) trees produced 52 and 26 t ha–1 organic biomass when irrigated with reclaimed sewage (EC approx. 3 dS m–1) or brine (EC approx. 7–10 dS m–1), respectively. Euphorbia tirucalii, a desert succulent from East Africa, was suggested as a potential biofuel crop by Nobel Laureate Melvin Calvin >40 years ago (Nielsen et al., 1977; Calvin, 1980). In recent experiments it exhibited a 60-fold weight increase 18 months after transplanting, when irrigated with saline sewage (EC 8–10 dSm–1), generating a crop rich in carbon and hydrogen that has potential for being directly converted into biofuel (Eshel et al., 2010).
A second approach is the cultivation of oilseed crops for biodiesel production using direct seawater irrigation, which is readily available in coastal areas of arid regions. Salicornia bigelovii, being highly salt tolerant and producing up to 30 % seed oil, was a primary candidate (Weete et al., 1970; Glenn et al., 1991; Alsaeedi and Elprince, 2000). However, the uneven seed ripening and their small size (0·6–1·2 mg) may result in approx. 50 % grain loss when harvested mechanically (Glenn et al. 2013), emphasizing the need to develop a special mechanical harvest technology for seed harvesting of this plant. Another disadvantage of growing S. bigelovii is the need to grow the plants for a relatively long period, approx. 100 d at cool temperatures, to initiate flowering (Glenn et al., 1998) which is rather difficult in warm areas. However, breeding programmes for S. bigelovii demonstrated that accessions with improved seed and biomass yields could be obtained, indicating that improvement can be achieved over a relatively short period (approx. 5 years) (Zerai et al., 2010).
An additional potential biofuel candidate with positive prospects to become a commercial crop is Kosteletzkya pentacarpos (syn. K. virginica), the seashore mallow, an oilseed-producing perennial halophyte (Gallagher, 1985). Kosteletzkya pentacarpos may be cultivated on large, saline marginal lands that can be irrigated with saline water (Gallagher, 1985). Therefore, (1) the biofuel crop does not compete with land suitable for conventional food crops; (2) utilizes areas of fallow land; and (3) liberates freshwater resources for more vital purposes.
The seeds of K. pentacarpos contain 18–22 % oil, which has a similar composition to cotton-seed oil, an edible oil and currently one of the oils used successfully for biodiesel production. Still, due to limited breeding and selection, the seed yield of K. pentacarpos is relatively low (up to 1500 kg ha–1) as compared with soybean (approx. 2300 kg ha–1) (Moser et al., 2013). Nonetheless, the plants' perennial growth habit should allow for the development of multiple harvests, resulting in increasing yields with plant age due to the larger number of branches produced (www.ceoe.udel.edu/Halophyte/Growing%20Seaside%20Biodiesel%20proposal%20FINAL.pdf). The low disease susceptibility as well as retention of seed (non-shattering; Moser et al., 2013) are additional helpful traits for mechanizing harvesting and efficient phytosanitary control.
THE USE OF CONSTRUCTED WETLANDS FOR MARINE EFFLUENT PURIFICATION
Coastal marine aquaculture development is frequently associated with negative environmental impacts and competition for resources (Bunting and Shpigel, 2009), because marine aquaculture effluents contain particulates, organic matter (including algae), nitrogen and phosphorus. Constructed wetlands, originally designed for effluent purification of non-saline water (Craft, 2005), might be used to deal with pollutants from marine aquaculture. The main principle of constructed wetlands is based on a combination of physical, chemical and biological processes that can be divided into four major elements: (1) plants; (2) soil and sediment; (3) microbial biomass; and (4) an aqueous phase containing contaminants. The inorganic nutrients from marine fish and shrimp culture could serve as fertilizer for plants and promote their growth and development. Since marine effluents are moderate to highly saline, the use of halophytes for their purification is advantageous. The relatively new approach of combining aquaculture and cultivation of halophytes as cash crops is gaining interest, with several recent papers demonstrating the purification capacity of constructed wetlands planted with Salicornia/Sarcocornia under different climatic conditions (Buhmann and Papenbrock, 2013b; Turcios and Papenbrock, 2014) ranging from a warm climate system (Shpigel et al., 2013) to temperate climatic conditions (Webb et al., 2012, 2013). Shpigel et al. (2013) concentrated on the effect of nutrient load under two different hydraulic flow regimes on Salicornia performance as a biofilter and biomass producer. Here, it was concluded that the surface flow regime with Salicornia would probably be more efficient in facilities with low nutrient load (e.g. fish hatcheries), whereas a sub-surface flow regime would be more efficient with high nutrient load (e.g, super-intensive fish farms) (Shpigel et al., 2013). In this study, seawater-grown Salicornia persica yields ranged between 17 and 26 kg m−2 year−1 fresh biomass in all the treatments, comparable with previous investigations [15–26 kg m−2 year−1 f. wt by Glenn et al. (1991); 8–9 kg m−2 year−1 f. wt by Glenn et al. (1998); 1·2–2·4 kg m−2 year−1 d. wt by Lu et al. (2010); and 1·7 kg m−2 year−1 d. wt by Ventura et al. (2011a)]. Year-round Salicornia production in warm water aquaculture systems may have an advantage as compared with seasonal and irregular harvesting, practised throughout much of Western Europe (Bunting and Shpigel, 2009). As a reliable biofilter for waste water treatments throughout the year, the plant can provide income as an animal feed and a source of nutraceuticals. However, further assessments are needed in order to understand fully the market potential of cultured Salicornia, which may change with the use of the crop (gourmet vegetable, animal feed, oilseed crop or for the nutraceuticals industry).
FUTURE PERSPECTIVES
During the last decade, public awareness regarding successful establishment of halophytes has grown (Böer, 2006; Rozema and Flowers, 2008). Nevertheless, scientific documentation of large-scale experiments is limited, and no cultivation protocols are available for halophyte crops, as exist for conventional crops. To date, only one breeding programme has reported selection for yield parameters using Salicornia bigelovii as an example for an oilseed crop (Zerai et al., 2010) and only a small number of investigations have evaluated the potential of the existing genetic material. There is a need for long-term experiments proving the sustainability of halophyte crop production and their economic prospective for future growers. Halophytic crops should undergo the same process undergone by conventional agricultural crops (breeding for improvement of agricultural traits such as yield, taste and mechanical harvesting over extended time periods) so that during short time spans economically profitable and consumer-acceptable products can be attained.
Moreover, halophytic crops can make use of marginal soils and saline irrigation water, both of which are inapplicable for conventional crop production. However, to ensure lasting sustainability of saline agricultural, the correct choice of adequate cultivation systems is of utmost importance. Sandy soils existing in coastal areas or inland sand dunes may be ready available for large-scale halophyte production without the risk of salt contamination occurring on fertile soils through Ca2+/Na+ exchange and subsequent clay dispersion. Likewise, underground freshwater contamination should be avoided by the existence of sufficient deep water tables or adequate drainage. On the other hand, closed cultivation systems, which are separated from the natural soil, may offer a wide range of possible applications: hydroponics, constructed wetlands, artificial growth media (perlite, vermiculite and coconut fibres) and any suitable combination of these.
The almost infinite availability of saline water highlights the importance of halophytes as a source of renewable energy, particularly since they do not compete with glycophytic food crops. There are still difficulties that should be overcome, such as direct germination in saline conditions or genotype selection. However, more and more research is directed not only towards determining salt tolerance of halophytes, but also towards the improvement of agricultural traits for long-term progress (yield, palatability, chemical composition and mechanical harvesting), testing market potential and finally securing farmers' income. Perhaps time will ultimately be the determining factor advancing halophyte cultivation, when prices for fossil and alternative energy resources will exceed the halophyte biofuel production costs.
ACKNOWLEDGEMENTS
The authors acknowledge constructive comments and suggestions by Professor Tim Flowers on this manuscript and thank Professor Laslo Erdei for providing the picture of Aster tripolium subsp. pannonicus. We thank Dr Tali Brunner for her English editing.
LITERATURE CITED
- Abdal MS. Salicornia production in Kuwait World Applied Sciences Journal. 2009. pp. 1033–1038. [Google Scholar]
- Adolf VI, Jacobsen SE, Shabala S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.) Environmental and Experimental Botany. 2013;92:43–54. [Google Scholar]
- Al Daini H, Norman HC, Young P, Barrett-Lennard EG. The source of nitrogen (NH4+ or NO3–) affects the concentration of oxalate in the shoots and the growth of Atriplex nummularia (oldman saltbush) Functional Plant Biology. 2013;40:1057–1064. doi: 10.1071/FP13060. [DOI] [PubMed] [Google Scholar]
- Alsaeedi AH, Elprince AM. Critical phosphorus levels for Salicornia growth. Agricultural Journal. 2000;92:336–345. [Google Scholar]
- Aronson JA. HALOPH: a data base of salt tolerant plants of the world. Tucson, AZ: Arid Land Studies; 1989. University of Arizona. [Google Scholar]
- Atia A, Barhoumi Z, Mokded R, Abdelly C, Smaoui A. Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae) Journal of Medicinal Plants Research. 2011;5:3564–3571. [Google Scholar]
- Atia A, Ben Hamed K, Debez A, Abdelly C. Salt and seawater effect on the germination of Crithmum maritimum. In: Öztürk M, Waisel Y, Khan MA, Görk G, editors. Biosaline agriculture and salinity tolerance in plants. Basel: BirkhauserVerlag; 2006. pp. 29–33. [Google Scholar]
- Attia FM, Alsobayel AA, Kriadees MS, Al Saiady MY, Bayoumi MS. Nutrient composition and feeding value of Salicornia bigelovii torr meal in broiler diets. Animal Feed Science and Technology. 1997;65:257–263. [Google Scholar]
- Barrett-Lennard EG, Setter TL. Developing saline agriculture: moving from traits and genes to systems. Functional Plant Biology. 2010;37:iii–iv. [Google Scholar]
- Basmaeil S, Al-Saiady M, Abouheif MA, Zahran S, El-Shaikh YA. Effect of graded levels of crude protein on nutrient digestibility of rhodesgrass hay or dried Salicornia biomass diets in camels. Journal of King Saud University of Agricultural Science. 2003;2:117–125. [Google Scholar]
- Belal IEH, Al-Dosari M. Replacement of fish meal with Salicornia meal in feed for Nile tilapia Oreochromis niloticus. Journal of the World Aquaculture Society. 1999;30:285–289. [Google Scholar]
- Ben Amor N, Ben Hamed K, Ranieri A, Abdelly C. Kinetics of the antioxidant response to salinity in Crithmum maritimum. In: Öztürk M, Waisel Y, Khan MA, Görk G, editors. Biosaline agriculture and salinity tolerance in plants. Basel: BirkhauserVerlag; 2006. pp. 83–88. [Google Scholar]
- Ben Dov Y, Forti M, Pasternak D. Drought and salt tolerant plants for water conserving gardening. Beer Sheva: The Institutes for Applied Research; 1993. Ben Gurion University of the Negev (in Hebrew) [Google Scholar]
- Ben-Hamed K, Debez A, Chibani F, Abdelly C. Salt response of Crithmum maritimum, an oleaginous halophyte. Tropical Ecology. 2004;45:151–159. [Google Scholar]
- Ben Hamed K, Ben Youssef N, Ranieri A, Zarrouk M, Abdelly C. Changes in content and fatty acid profiles of total lipids and sulfolipids in the halophyte Crithmum maritimum under salt stress. Journal of Plant Physiology. 2005;162:599–602. doi: 10.1016/j.jplph.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Böer B. Halophyte research and development: what needs to be done next? In: Khan MA, Weber DJ, editors. Ecophysiology of high salinity tolerant plants. Berlin: Springer Verlag; 2006. pp. 397–399. [Google Scholar]
- Bohnert HJ, Cushman JC. The ice plant cometh: lessons in abiotic stress tolerance. Journal of Plant Growth Regulators. 2000;19:334–346. [Google Scholar]
- Boyko E. Guide book for the Sam Fryer ecological desert garden. Beer Sheva, Jerusalem: Sivan Press Ltd, 1–44; 1962. The Negev Institute for Arid Zone Research. [Google Scholar]
- Boyko H. Salinity and aridity: new approaches to old problems. The Hague: Dr. W. Junk Publishers; 1966. [Google Scholar]
- Boyko H, Boyko E. Seawater irrigation, a new line of research on a bioclimatic plant–soil complex. International Journal of Bioclimatology and Biometeorology. 1959;3:33–61. [Google Scholar]
- Boyko H, Boyko E. Principles and experiments regarding direct irrigation with highly saline and sea water without desalination. Transactions of the New York Academy of Sciences. 1964;26:1087–1102. [Google Scholar]
- Buhmann A, Papenbrock J. An economic point of view of secondary compounds in halophytes. Functional Plant Biology. 2013a;40:952–967. doi: 10.1071/FP12342. [DOI] [PubMed] [Google Scholar]
- Buhmann A, Papenbrock J. Biofiltering of aquaculture effluents by halophytic plants: basic principles, current uses and future perspectives. Environmental and Experimental Botany. 2013b;92:122–133. [Google Scholar]
- Bunting SW, Shpigel M. Evaluating the economic potential of horizontally integrated land-based marine aquaculture. Aquaculture. 2009;294:43–51. [Google Scholar]
- Bustan A, Pasternak D, Pirogova I, et al. Evaluation of saltgrass as a fodder crop for livestock. Journal of the Science of Food and Agriculture. 2005;85:2077–2084. [Google Scholar]
- Calvin M. Hydrocarbons from plants: analytical methods and observations. Naturwissenschaften. 1980;67:525–533. [Google Scholar]
- Carlsson R, Clarke EMW. Qualitas Plantarum Plant Foods for Human Nutrition. 1983. Atriplex hortensis L. as a leafy vegetable, and as a leaf protein concentrate plant; pp. 127–133. [Google Scholar]
- Carvalho IS, Teixeira M, Brodelius M. Effect of salt stress on purslane and potential health benefits: oxalic acid and fatty acids profile. 2009. UC Davis: Proceedings of the International Plant Nutrition Colloquium XVI. Retrieved from http://www.escholarship.org/uc/item/4cc78714.
- Cassaniti C, Romano D. The use of halophytes for Mediterranean landscaping. Proceedings of the European COST Action FA901. European Journal of Plant Science and Biotechnology. 2011;5:58–63. [Google Scholar]
- Cassaniti C, Romano D, Hop MECM, Flowers TJ. Growing floricultural crops with brackish water. Environmental and Experimental Botany. 2013;92:165–175. [Google Scholar]
- Conesa E, Vicente MJ, Martinez-Sanchez JJ, Munuera M, Franco JA. Germination of Crithmum maritimum under saline conditions. Acta Horticulturae. 2008;782:115–120. [Google Scholar]
- Craft CB. Natural and constructed wetlands. In: Anderson M, editor. Encyclopedia of hydrological sciences. Chichester, UK: John Wiley and Son Ltd; 2005. [Google Scholar]
- Cunsolo F, Ruberto G, Amico V, Piatelli M. Bioactive metabolites from Sicilian marine fennel, Crithmum maritimum. Journal of Natural Products. 1993;56:1598–1600. doi: 10.1021/np50099a022. [DOI] [PubMed] [Google Scholar]
- Davy AJ, Bishop GF, Costa CSB. Salicornia L. (Salicornia pusilla J. Woods, S. ramosissima J. Woods, S. europaea L., S. obscura P.W. Ball & Tutin, S. nitens P.W. Ball & Tutin, S. fragilis P.W. Ball & Tutin, P. W. Ball & Tutin and S. dolichostachya Moss) Journal of Ecology. 2001;89:681–707. [Google Scholar]
- De Vos AC, Broekman R, de Almeida Guerra CC, van Rijsselberghe M, Rozema J. Developing and testing new halophyte crops: a case study of the salt tolerance of two species of the Brassicaceae, Diplotaxis tenuifolia and Cochlearia officinalis. Environmental and Experimental Botany. 2013;92:154–164. [Google Scholar]
- Dominguez-Faus R, Powers SE, Burken JG, Alvarez PJ. The water footprint of biofuels: a drink or drive issue. Environmental Science and Technology. 2009;43:3005–3010. doi: 10.1021/es802162x. [DOI] [PubMed] [Google Scholar]
- Eganathan P, SR Subramanian HM, Latha R, Srinivasa Rao C. Oil analysis in seeds of Salicornia brachiata. Industrial Crops and Products. 2006;23:177–179. [Google Scholar]
- El Shaer HM. Halophytes as cash crops for animal feeds in arid and semi-ard regions. In: Öztürk M, Waisel Y, Khan MA, Görk G, editors. Biosaline agriculture and salinity tolerance in Plants. Basel: BirkhauserVerlag; 2006. pp. 117–128. [Google Scholar]
- El Shaer HM. Halophytes and salt-tolerant plants as potential forage for ruminants in the Near East region. Small Ruminant Research. 2010;91:3–12. [Google Scholar]
- Epstein E, Norylon JD, Rush DW, et al. Crops for saline agriculture: a genetic approach. Science. 1980;210:399–404. doi: 10.1126/science.210.4468.399. [DOI] [PubMed] [Google Scholar]
- Eshel A, Zilberstein A, Alekparov C, et al. Proceedings of the 5th IASME/WSEAS international conference on energy and environment. Recent advances in energy and environment. Cambridge, UK: 2010. Biomass production by desert halophytes: alleviating the pressure on food production; pp. 362–367. February 23–25, 2010. [Google Scholar]
- Eshel A, Oren I, Alekparov C, Eilam T, Zilberstein A. Biomass production by desert halophytes: alleviating the pressure on the scarce resources of arable soil and fresh water. European Journal of Plant Science and Biotechnology. 2011;5:48–53. [Google Scholar]
- Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytologist. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Yeo AR. Breeding for salinity resistance in crop plants – where next. Australian Journal of Plant Physiology. 1995;22:875–884. [Google Scholar]
- Flowers TJ, Galal HK, Bromham L. Evolution of halophytes: multiple origins of salt tolerance in land plants. Functional Plant Biology. 2010;37:604–612. [Google Scholar]
- Franke W. Vitamin C in sea fennel (Crithmum maritimum), an edible wild plant. Economic Botany. 1982;36:163–165. [Google Scholar]
- Gallagher JL. Halophytic crops for cultivation at seawater salinity. Plant and Soil. 1985;89:323–336. [Google Scholar]
- Geissler N, Hussin S, Koyro HW. Elevated atmospheric CO2 concentration ameliorates effects of NaCl salinity on photosynthesis and leaf structure of Aster tripolium L. Journal of Experimental Botany. 2009a;60:137–151. doi: 10.1093/jxb/ern271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler N, Hussin S, Koyro HW. Interactive effects of NaCl salinity and elevated atmospheric CO2 concentration on growth, photosynthesis, water relations and chemical composition of the potential cash crop halophyte Aster tripolium L. Environmental and Experimental Botany. 2009b;65:220–231. [Google Scholar]
- Gleick PH. The world's water 2008–2009. The Biennial Report on Freshwater Resources. Washington, Covelo; London: Island Press; 2009. Peak water; pp. 1–16. [Google Scholar]
- Glenn EP, O'Leary JW, Watson MC, Thompson LT, Kuehl RO. Salicornia bigelovii Torr.: an oilseed halophyte for seawater irrigation. Science. 1991;251:1065–1067. doi: 10.1126/science.251.4997.1065. [DOI] [PubMed] [Google Scholar]
- Glenn EP, Coates WE, Riley JJ, Kuehl RO, Swingle RS. Salicornia bigelovii Torr.: a seawater-irrigated forage for goats. Animal Feed Science and Technology. 1992;40:21–30. [Google Scholar]
- Glenn E, Hicks N, Riley J, Swingle R. Seawater irrigation of halophytes for animal feed. In: Chakour-Allah R, Malcolm CV, Hamdy A, editors. Halophytes and biosaline agriculture. New York: Marcel Dekker; 1996. pp. 221–236. [Google Scholar]
- Glenn EP, Brown J, O'Leary J. Irrigating crops with seawater. Scientific American. 1998;279:56–61. [Google Scholar]
- Glenn EP, Brown JJ, Blumwald E. Salt tolerance and crop potential of halophytes. Critical Review in Plant Science. 1999;18:227–255. [Google Scholar]
- Glenn EP, Anday T, Chaturvedi R, et al. Three halophytes for saline-water agriculture: an oilseed, a forage and a grain crop. Environmental and Experimental Botany. 2013;92:110–121. [Google Scholar]
- Gómez MB, Castro PA, Mignone C, Bertero HD. Can yield potential be increased by manipulation of reproductive partitioning in quinoa (Chenopodium quinoa)? Evidence from gibberellic acid synthesis inhibition using paclobutrazol. Functional Plant Biology. 2011;38:420–430. doi: 10.1071/FP10168. [DOI] [PubMed] [Google Scholar]
- Grattan SR, Grieve CM. Salinity–mineral nutrient relations in horticultural crops. Scientia Horticulturae. 1999;78:127–157. [Google Scholar]
- Grieve CM, Suarez DL. Purslane (Portulaca oleracea L.): a halophytic crop for drainage water reuse system. Plant and Soil. 1997;192:277–283. [Google Scholar]
- Guarrera PM, Salerno G, Caneva G. Food, flavouring and feed plant traditions in the Tyrrhenian sector of Basilicata, Italy. Journal of Ethnobiology and Ethnomedicine. 2006;2:37. doi: 10.1186/1746-4269-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guil-Guerrero JL, Rodríguez-García I. Lipids classes, fatty acids and carotenes of the leaves of six edible wild plants. European Food Research and Technology. 1999;209:313–316. [Google Scholar]
- Gul B, Khan MA. Saline agriculture: promises and prospects for future agriculture in degraded saline lands. In: Azhar A, Siddiqui A, Khan MA, editors. Technology and development in the new millennium. Karachi: 2003. pp. 149–156. [Google Scholar]
- Hameed M, Ashraf M, Naz N, et al. Anatomical adaptions of Cynodon dactylon (L.) Pers. from the salt range (Pakistan) to salinity stress. II Leaf anatomy. Pakistani Journal of Botany. 2013;45(Suppl. 1):133–142. [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Herppich WB, Huyskens-Keil S, Schreiner M. Effects of saline irrigation on growth, physiology and quality of Mesembryanthemum crystallinum L., a rare vegetable crop. Journal of Applied Botany and Food Quality. 2008;82:47–54. [Google Scholar]
- Hollington PA, Hussain Z, Kahlown MA, Abdullah M. Success stories in saline agriculture in Pakistan: from research to production and development. 2001. BAC Saline Agriculture Conference. March 19–21, 2001.
- Imai H, Kinoshita M, Ohishi M. Chemical characterization of glycerolipids and cerebrosides in a halophytic plant, Salicornia europaea L. Journal of Oleo Science. 2004;53:337–341. [Google Scholar]
- Jessop JP. Family – Aizoaceae (Ficoidaceae, Mesembryanthemaceae, Molluginaceae, Tetragoniaceae) In: Jessop JP, Toelken HR, editors. Flora of South Australia Part I, Lycopodiaceae – Rosaceae. Adelaide: South Australian Government Publishing Division; 1986. 383, 415. [Google Scholar]
- Katschnig D, Broekman R, Rozema J. Salt tolerance in the halophyte Salicornia dolichostachya Moss: growth, morphology and physiology. Environmental and Experimental Botany. 2013;92:32–42. [Google Scholar]
- Kerstiens G, Tych W, Robinson MF, Mansfield TA. Sodium-related partial stomatal closure and salt tolerance of Aster tripolium. New Phytologist. 2002;153:509–515. doi: 10.1046/j.0028-646X.2001.00330.x. [DOI] [PubMed] [Google Scholar]
- Koyro HW, Ajmal Khan M, Lieth H. Halophytic crops: a source for the future to reduce the water crisis. Emirates Journal of Food and Agriculture. 2011;23:1–6. [Google Scholar]
- Kumamoto J, Scora RW, Clerx WA, Matsumura M, Layfield D, Grieve CM. Purslane: a potential new vegetable crop rich in omega-3 fatty acid with a controllable sodium chloride content. In: Naqvi HH, Estilai A, Ting IP, editors. Proceedings of the First International Conference on New Industrial Crops and Products. Riverside, Tucson: University of Arizona; 1990. pp. 229–233. [Google Scholar]
- Ksouri R, Smaoui A, Isoda H, Abdelly C. Utilization of halophyte species as new sources of bioactive substances. Journal of Arid Land Studies. 2012;22:41–44. [Google Scholar]
- Kumar R. Anti-nutritional factors, the potential risks of toxicity and methods to alleviate them. In: Speedy A, Pugliese P-L, editors. Legume trees and other fodder trees as protein sources for livestock. 1991. pp. 145–160. Proceedings of the FAO Expert Consultation held at the Malaysian Agricultural Research and Development Institute (MARDI) in Kuala Lumpur, Malaysia. [Google Scholar]
- Le Houérou HN. The role of saltbushes (Atriplex spp.) in arid land rehabilitation in the Mediterranean Basin, a review. Agroforestry Systems. 1992;18:107–148. [Google Scholar]
- Le Houérou HN. Forage halophytes in the Mediterranean basin. In: Chakour-Allah R, Malcolm CV, Hamdy A, editors. Halophytes and biosaline agriculture. New York: Marcel Dekker; 1996. pp. 115–136. [Google Scholar]
- Lieth H. Cashcrop halophytes for future halophyte growers. 2000. EU concerted action project IC 18CT96-0055, final meeting at the beginning of the EXPO 2000. Institute of Environmental Systems Research, University of Osnabrück, Germany. ISSN 09336-3114 No. 20.
- Lieth H, Mochtchenko M, editors. Halophyte uses in different climates. IV. Cashcrop halophytes for future halophyte growers. Progress in Biometeorology 18. Leiden: Backhuys Publishers; 2002. [Google Scholar]
- Liphschitz N, Shomer-Ilan A, Eshel A, Waisel Y. Salt glands on leaves of Rhodes grass (Chloris gayana Kth.) Annals of Botany. 1974;38:459–462. [Google Scholar]
- Liphschitz N, Waisel Y. Existence of salt glands in various genera of the Gramineae. New Phytologist. 1974;73:507–513. [Google Scholar]
- Lu D, Zhang M, Wang S, Cai J, Zhou X, Ahu C. Nutritional characterization and changes in quality of Salicornia bigelovii Torr. during storage. Food Science and Technology. 2010;43:519–524. [Google Scholar]
- Maas EV. Crop salt tolerance. 1990 In: Tanji KK, ed. Agricultural salinity assessment and management. ASCE Manuals and Reports on Engineering Pratice No. 71. New York: American Society of Civil Engineers, 262–304. [Google Scholar]
- Mahmood K, Malik KA, Lodhi MAK, Sheikh KK. Soil–plant relationships in saline wastelands: vegetation, soils, and successional changes, during biological amelioration. Environmental Conservation. 1994;21:236–241. [Google Scholar]
- Malcolm CV. Use of halophytes for forage production on saline wastelands. Journal of the Australian Institute of Agricultural Science. 1969;35:38–49. [Google Scholar]
- Meot-Duros L, Magné C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiology and Biochemistry. 2009;47:37–41. doi: 10.1016/j.plaphy.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Menzel U, Lieth H. HALOPHYTE Database Vers. 2.0 update. In: Lieth H, Mochtchenko M, editors. Cash crop halophytes: recent studies. Dordrecht: Kluwer Academic Publisher, 1–19; 2003. [Google Scholar]
- Moser BR, Dien BS, Seliskar DM, Gallagher JL. Seashore mallow (Kosteletzkya pentacarpos) as a salt-tolerant feedstock for production of biodiesel and ethanol. Renewable Energy. 2013;50:833–839. [Google Scholar]
- Mudie PJ. The potential economic uses of halophytes. In: Reimold RJ, Queen WH, editors. Ecology of halophytes. New York: Academic Press; 1974. pp. 565–597. [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Muscolo A, Panuccio MR, Eshel A. Ecophysiology of Pennisetum clandestinum: a valuable salt tolerant grass. Environmental and Experimental Botany. 2013;92:55–63. [Google Scholar]
- Nielsen PE, Nishimura H, Otvos JW, Calvin M. Plant crops as a source of fuel and hydrocarbon-like materials. Science. 1977;198:942–944. doi: 10.1126/science.198.4320.942. [DOI] [PubMed] [Google Scholar]
- Norman HC, Masters DG, Barrett-Lennard EG. Halophytes as forages in saline landscapes: interactions between plant genotypes and environment change their feeding value to ruminants. Environmental and Experimental Botany. 2013;92:96–109. [Google Scholar]
- Nurock M, editor. The Negev Institute for Arid Zone Research. Jerusalem: Jerusalem Academic Press Ltd; 1960. pp. 20–26. [Google Scholar]
- O'Leary JW. The role of halophytes in irrigated agriculture. In: Staples RC, Toenniessen GH, editors. Salinity tolerance in plants. New York: John Wiley & Sons; 1984. pp. 285–300. [Google Scholar]
- Palaniswamy UR, McAvoy RJ, Bible BB. Stage of harvest and polyunsaturated essential fatty acid concentrations in purslane (Portulaca oleraceae) leaves. Journal of Agricultural Food Chemistry. 2001;49:3490–3493. doi: 10.1021/jf0102113. [DOI] [PubMed] [Google Scholar]
- Palaniswamy U, Bible BB, McAvoy RJ. Effect of nitrate: ammonium nitrogen ratio on oxalate levels of Purslane. In: Janick J, Whipkey A, editors. Trends in new crops and new uses. Alexandria: ASHS Press; 2002. pp. 453–455. [Google Scholar]
- Palaniswamy UR, Bible BB, McAvoy RJ. Oxalic acid concentrations in purslane (Portulaca oleraceae L.) is altered by the stage of harvest and the nitrate to ammonium ratios in hydroponics. Scientia Horticulturae. 2004;102:267–275. [Google Scholar]
- Panta S, Flowers T, Lane P, Doyle R, Haros G, Shabala S. Halophyte agriculture: success stories. Environmental and Experimental Botany. 2014;107:71–83. [Google Scholar]
- Parvaiz A, Satyawati S. Salt stress and phyto-biochemical response of plants – a review. Plant, Soil and Environment. 2008;54:89–99. [Google Scholar]
- Pasternak D. Fodder production with saline water. 1990. p. 173. Project report January 1982–December 1989. BGUN-ARI-35-90.
- Pasternak D, Danon A, Aronson JA, Benjamin RW. Developing the seawater agriculture concept. Plant and Soil. 1985;89:337–348. [Google Scholar]
- Postel SL, Daily GC, Ehrlich PR. Human appropriation of renewable fresh water. Science. 1996;271:785–788. [Google Scholar]
- Ramani B, Zorn H, Papenbrock J. Quantification and fatty acid profiles of sulfolipids in two halophytes and a glycophyte grown under different salt concentrations. Zeitung für Naturforschung. 2004;59c:835–842. doi: 10.1515/znc-2004-11-1212. [DOI] [PubMed] [Google Scholar]
- Ramani B, Reeck T, Debez A, et al. Plant Physiology and Biochemistry. 2006. Aster tripolium L. and Sesuvium portulacastrum L.: two halophytes, two strategies to survive in saline habitats; pp. 395–408. [DOI] [PubMed] [Google Scholar]
- Rogers AL, Bailey ET. Salt tolerance trials with forage plants in south-western Australia. Australian Journal of Experimental Agriculture and Animal Husbandry. 1963;3:125–130. [Google Scholar]
- Rozema J, Flowers TJ. Crops for a salinized world. Science. 2008;322:1478–1480. doi: 10.1126/science.1168572. [DOI] [PubMed] [Google Scholar]
- Rozema J, Schat H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environmental and Experimental Botany. 2013;92:83–95. [Google Scholar]
- Sági B, Erdei L. Distinct physiological characteristics of two subspecies of Aster tripolium L. Acta Biologica Szegediensis. 2002;46:257–258. [Google Scholar]
- Santi G, D'Annibale A, Eshel A, et al. Bioethanol production from xerophilic and salt-resistant Tamarix jordanis biomass. Biomass and Bioenergy. 2014;61:73–81. [Google Scholar]
- Sarker SK, Park S-R, Kim G-M, Yang C-J. Hamcho (Salicornia herbacea) with probiotics as alternative to antibiotic for broiler production. Journal of Medicinal Plants Research. 2010;4:415–420. [Google Scholar]
- Shannon MC, Grieve CM. Tolerance of vegetable crops to salinity. Scientia Horticulturae. 1999;78:5–38. [Google Scholar]
- Shennan C, Hunt R, Macrobbie EAC. Salt tolerance in Aster tripolium L. I. The effect of salinity on growth. Plant, Cell and Environment. 1987a;10:59–65. doi: 10.1111/j.1365-3040.1987.tb02080.x. [DOI] [PubMed] [Google Scholar]
- Shennan C, Hunt R, Macrobbie EAC. Salt tolerance in Aster tripolium. II. Ionic regulation at different salinities. Plant, Cell and Environment. 1987b;10:67–74. doi: 10.1111/j.1365-3040.1987.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Shillo R, Ding M, Pasternak D, Zaccai M. Cultivation of cut flower and bulb species with saline water. Scientia Horticulturae. 2002;92:41–54. [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids and antioxidants in edible wild plants. Biological Research. 2004;37:263–277. doi: 10.4067/s0716-97602004000200013. [DOI] [PubMed] [Google Scholar]
- Shpigel M, Ben-Ezra D, Shauli L, et al. Constructed wetland with Salicornia as a biofilter for mariculture effluents. Aquaculture. 2013;412–413:52–63. [Google Scholar]
- Słupski J, Achrem-Achremowicz J, Lisiewska Z, Korus A. Effect of processing on the amino acid content of New Zealand spinach (Tetragonia tetragonioides Pall. Kuntze) International Journal of Food Science and Technology. 2010;45:1682–1688. [Google Scholar]
- Stuchlík M, Žák S. Vegetable lipids as components of functional foods. Biomedical Papers. 2002;146:3–10. doi: 10.5507/bp.2002.001. [DOI] [PubMed] [Google Scholar]
- Swingle RS, Glenn EP, Squires V. Growth performance of lambs fed mixed diets containing halophyte ingredients. Animal Feed Science and Technology. 1996;63:137–148. [Google Scholar]
- Szalai G, Dai N, Danin A, Dudai N, Barazani O. Effect of nitrogen source in the fertilizing solution on nutritional quality of three members of the Portulaca oleracea aggregate. Journal of the Science of Food and Agriculture. 2010;90:2039–2045. doi: 10.1002/jsfa.4049. [DOI] [PubMed] [Google Scholar]
- Tardio J, Pardo de Santayana M, Morales R. Ethnobotanical review of wild edible plants in Spain. Botanical Journal of the Linnean Society. 2006;152:27–71. [Google Scholar]
- Teixeira M, Carvalho IS. Effects of salt stress on purslane (Portulaca oleracea) nutrition. Annals of Applied Biology. 2009;154:77–86. [Google Scholar]
- Turcios AE, Papenbrock J. Sustainable treatment of aquaculture effluents – what can we learn from the past for the future. Sustainability. 2014;6:836–856. [Google Scholar]
- Ungar IA. Seed germination and seed-bank ecology in halophytes. In: Kigel J, Galili G, editors. Seed development and germination. New York: Marcel Dekker; 1995. pp. 599–628. [Google Scholar]
- Valdez-Aguilar LA, Grieve CM, Poss J. Salinity and alkaline pH in irrigation water affect marigold plants: I. Growth and shoot dry weight partitioning. HortScience. 2009;44:1719–1725. [Google Scholar]
- Ventura Y, Sagi M. Halophyte crop cultivation: the case for Salicornia and Sarcocornia. Environmental and Experimental Botany. 2013;92:144–153. [Google Scholar]
- Ventura Y, Myrzabayeva M, Alikulov Z, Cohen S, Shemer Z, Sagi M. The importance of iron supply during repetitive harvesting of Aster tripolium. Functional Plant Biology. 2013;40:968–976. doi: 10.1071/FP12352. [DOI] [PubMed] [Google Scholar]
- Ventura Y, Wuddineh WA, Ephrath Y, Shpigel M, Sagi M. Molybdenum as an essential element for improving total yield in seawater-grown Salicornia europaea L. Scientia Horticulturae. 2010;126:395–401. [Google Scholar]
- Ventura Y, Wuddineh WA, Myrzabayeva M, et al. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Scientia Horticulturae. 2011a;128:189–196. [Google Scholar]
- Ventura Y, Wuddineh WA, Shpigel M, et al. Effects of day length on flowering and yield production of Salicornia and Sarcocornia species. Scientia Horticulturae. 2011b;130:510–516. [Google Scholar]
- Wagenvoort WA, van de Vooren JG, Brandenburg WA. Plant domestication and the development of sea starwort (Aster tripolium L.) as a new vegetable crop. Acta Horticulturae. 1989;242:115–122. [Google Scholar]
- Webb JM, Quinta R, Papadimitriou S, et al. Halophyte filter beds for treatment of saline wastewater from aquaculture. Water Research. 2012;46:512–514. doi: 10.1016/j.watres.2012.06.034. [DOI] [PubMed] [Google Scholar]
- Webb JM, Quinta R, Papadimitriou S, et al. The effect of halophyte planting density on the efficiency of constructed wetlands for the treatment of wastewater from marine aquaculture. Ecological Engineering. 2013;61:145–153. [Google Scholar]
- Weete JD, Rivers WG, Weber DJ. Hydrocarbon and fatty acid distribution in the halophyte, Salicornia bigelovii. Phytochemistry. 1970;9:2041–2045. [Google Scholar]
- Wilson C, Lesch SM, Grieve CM. Growth stage modulates salinity tolerance of New Zealand Spinach (Tetragonia tetragonioides, Pall) and Red Orach (Atriplex hortensis L.) Annals of Botany. 2000;85:501–509. [Google Scholar]
- Yensen NP. Halophyte uses for the twenty-first century. In: Khan MA, Weber DJ, editors. Ecophysiology of high salinity tolerant plants. Berlin: Springer Verlag; 2006. pp. 367–397. [Google Scholar]
- Yeo AR, Flowers TJ. Selection for physiological characters – examples from breeding for salt tolerance. In: Jones HG, Flowers TJ, Jones MB, editors. Plants under stress: biochemistry, physiology and ecology and their application to plant improvement. New York: Cambridge University Press; 1989. pp. 217–234. [Google Scholar]
- Zaccai M. Floriculture in the Mediterranean region. Acta Horticulturae. 2002;582:165–173. [Google Scholar]
- Zarrouk M, El Almi H, Ben Youssef N, et al. Lipid composition of seeds of local halophytes: Cakile maritime, Zygophyllum album and Crithmum maritimum. In: Lieth H, Mochtchenko M, editors. Cash crop halophytes: recent studies. Dordrecht: Kluwer Academic Publisher; 2003. pp. 121–124. [Google Scholar]
- Zerai DB, Glenn EP, Chatervedi R, et al. Potential for the improvement of Salicornia bigelovii through selective breeding. Ecological Engineering. 2010;36:730–739. [Google Scholar]
- Zurayk R, Baalbaki R. Inula crithmoides: a candidate plant for saline agriculture. Arid Soil Research and Rehabilitation. 1996;10:213–223. [Google Scholar]