Abstract
Background and Aims In order to cope with arid environments, the xerohalophyte Zygophyllum xanthoxylum efficiently compartmentalizes Na+ into vacuoles, mediated by ZxNHX, and maintains stability of K+ in its leaves. However, the function of ZxNHX in controlling Na+ and K+ homeostasis at the whole-plant level remains unclear. In this study, the role of ZxNHX in regulating the expression of genes involved in Na+ and K+ transport and spatial distribution was investigated.
Methods The role of ZxNHX in maintaining Na+ and K+ homeostasis in Z. xanthoxylum was studied using post-transcriptional gene silencing via Agrobacterium-mediated transformation. Transformed plants were grown with or without 50 mm NaCl, and expression levels and physiological parameters were measured.
Key Results It was found that 50 mm NaCl induced a 620 % increase in transcripts of ZxSOS1 but only an 80 % increase in transcripts of ZxHKT1;1 in roots of wild-type (WT) plants. Consequently, the ability of ZxSOS1 to transport Na+ exceeded that of ZxHKT1;1, and Na+ was loaded into the xylem by ZxSOS1 and delivered to the shoots. However, in a ZxNHX-silenced line (L7), the capacity to sequester Na+ into vacuoles of leaves was weakened, which in turn regulated long-distance Na+ transport from roots to shoots. In roots of L7, NaCl (50 mm) increased transcripts of ZxSOS1 by only 10 %, whereas transcripts of ZxHKT1;1 increased by 53 %. Thus, in L7, the transport ability of ZxHKT1;1 for Na+ outweighed that of ZxSOS1. Na+ was unloaded from the xylem stream, consequently reducing Na+ accumulation and relative distribution in leaves, but increasing the relative distribution of Na+ in roots and the net selective transport capacity for K+ over Na+ from roots to shoots compared with the WT. Silencing of ZxNHX also triggered a downregulation of ZxAKT1 and ZxSKOR in roots, resulting in a significant decrease in K+ accumulation in all the tissues in plants grown in 50 mm NaCl. These changes led to a significant reduction in osmotic adjustment, and thus an inhibition of growth in ZxNHX-silenced lines.
Conclusions The results suggest that ZxNHX is essential for controlling Na+, K+ uptake, long-distance transport and their homeostasis at whole-plant level via feedback regulation of the expression of genes involved in Na+, K+ transport. The net result is the maintenance of the characteristic salt accumulation observed in Z. xanthoxylum and the regulation of its normal growth. A model is proposed for the role of ZxNHX in regulating the Na+ transport system in Z. xanthoxylum under saline conditions.
Keywords: Xerohalophyte, tonoplast Na+/H+ antiporter ZxNHX, Na+ and K+ homeostasis, long-distance transport, gene silencing, ZxSOS1, ZxHKT1;1, Zygophyllum xanthoxylum
INTRODUCTION
Drought and salinity are two major abiotic stresses constraining agricultural expansion onto uncultivated land and limiting crop productivity worldwide (Martínez et al., 2003; Flowers, 2004; Yamaguchi and Blumwald, 2005; Shabala, 2013). Approximately one-third of the earth's land surface is threatened by drought, and 30 % of the irrigated areas worldwide have suffered salinity problems (Martínez et al., 2003; Chaves et al., 2009). Most crops are very sensitive to drought and salinity: both generate osmotic stress and a disequilibrium in intracellular ion homeostasis, resulting in cell membrane dysfunction and attenuation of metabolic activity, leading to growth inhibition and even plant death (Munns and Tester, 2008; Zhang et al., 2013). However, xerophytes grown in arid regions and halophytes in saline regions have developed various protective mechanisms for maintaining Na+ and K+ homeostasis to adapt to these harsh environments (Wang et al., 2004a; Flowers and Colmer, 2008; Zhang et al., 2010; Kronzucker and Britto, 2011; Janz and Polle, 2012; Shabala, 2013). Therefore, there is increasing interest in studying the physiological and molecular mechanisms involved in the responses of xerohalophytes to drought and salinity (Yamaguchi and Blumwald, 2005; Shabala, 2013).
Zygophyllum xanthoxylum, a perennial fodder shrub that colonizes arid areas in China and Mongolia, is a salt-accumulating succulent plant with excellent adaptability to adverse arid environments (Liu et al., 1987, 1988; Wu et al., 2011; Ma et al., 2012; Yue et al., 2012). Our previous studies showed that Z. xanthoxylum can accumulate larger quantities of Na+ than K+ for osmotic adjustment even from low salt soils (Wang et al., 2004a). Further investigations revealed that Z. xanthoxylum responded to salt with increased growth and, moreover, became more tolerant to drought in the presence of moderate salinity; the increased tolerance was closely related to high Na+ accumulation in leaves (Ma et al., 2010; Yue et al., 2012). Ma et al. (2012) found that the Na+ concentration in leaves of Z. xanthoxylum was significantly higher (by 64 %) under water deficit than that under well-irrigated conditions and, again under water deficit, was 2·3-fold higher in the presence of additional 50 mm NaCl than in its absence. Accordingly, the contribution of Na+ to the total osmotic potential varied from 8 % in plants under well-irrigated conditions to 13 % in plants subjected to water deficit and, remarkably, to 28 % in plants grown in the presence of 50 mm NaCl under water deficit (Ma et al., 2012). These findings indicated that Z. xanthoxylum could absorb a large quantity of Na+ which was efficiently transported to the leaves, reducing the osmotic potential of cells and increasing the water uptake capacity.
It is well known that high [Na+]ext can disturb K+ uptake, and hence induce K+ deficiency and growth inhibition for many glycophytes: excess Na+ can replace K+ at enzyme binding sites due to similarities in their ionic radii and ion hydration energies, with metabolic consequences (Maathuis and Amtmann, 1999; Wang et al., 2004b; Kronzucker et al., 2006; Shabala and Cuin, 2008; Horie et al., 2009; Wang et al., 2009; Zhang et al., 2013). However, in Z. xanthoxylum, although the Na+ concentration in leaves increased significantly under salt treatment and drought, the K+ concentration in leaves decreased only slightly under salt treatment and remained unchanged under drought (Wu et al., 2011; Ma et al., 2012). These results suggest that Z. xanthoxylum has a strong ability to regulate Na+ and K+ homeostasis, thus maintaining plant growth when subjected to drought and salinity.
In leaves, compartmentalization of Na+ into the vacuoles from the cytosol is a particularly important mechanism not only for improving osmotic adjustment to cope with drought or osmotic stress but also for reducing Na+ toxicity in the cytosol under salinity stress (Munns and Tester, 2008; Shabala, 2013). The tonoplast Na+/H+ antiporters, NHXs, have been suggested to play an important role in sequestration of Na+ into vacuoles to minimize the cytoplasmic Na+ concentration (Apse et al., 1999; 2003; Brini et al., 2007; Bao et al., 2014). Many studies in which NHX genes from various plants species have been overexpressed have shown an increase in salt tolerance (Munns and Tester, 2008). Recent research indicated that AtNHX1 and AtNHX2 in Arabidopsis mediated K+ sequestration into the vacuoles (Leidi et al., 2010; Bassil et al., 2011; Barragán et al., 2012; Andrés et al., 2014). Wu et al. (2011) cloned ZxNHX, the NHX homologous gene from Z. xanthoxylum, and found a positive correlation between upregulation of ZxNHX and Na+ accumulation in leaves of Z. xanthoxylum exposed to salt and drought, suggesting that ZxNHX plays important roles in Na+ accumulation under these conditions (Wu et al., 2011). Janz and Polle (2012) have systematically highlighted the contribution of Na+ in regulating water balance at both the cellular and whole-plant level during the response of Z. xanthoxylum to salt and drought. However, so far the function of NHX proteins has been identified and characterized mainly at a cell or tissue level; its role in regulating Na+ and K+ homeostasis at the whole-plant level remained unclear.
In the current study we have evaluated, using post-transcriptional gene silencing, the role of ZxNHX in Na+ and K+ homeostasis and growth of Z. xanthoxylum. The phenotypes, photosynthesis, Na+ and K+ accumulation and synergistic responses of ZxSOS1, ZxHKT1;1, ZxAKT1 and ZxSKOR in wild-type (WT) and ZxNHX-silenced lines were investigated. The results indicate that ZxNHX plays vital roles in controlling ion uptake and the spatial distribution of Na+ and K+, and hence maintaining Na+ and K+ homeostasis, thereby regulating plant growth.
MATERIALS AND METHODS
Plant materials and growth conditions
Seeds of Zygophyllum xanthoxylum were collected from wild plants in the Alxa Desert in the Inner Mongolia Autonomous Region, China (39°05′N, 105°34′E; elevation 1360 m). After removal of the bracts, seeds were surface sterilized for 1 min in 75 % ethanol (v/v) and rinsed three times with distilled water and then germinated at 25 °C in the dark on filter paper wetted with distilled water. When the plumule emerged, uniform seedlings were transplanted into plugged holes in plastic containers (5 × 5 × 5 cm, one seedling per container) filled with vermiculite irrigated with modified Hoagland nutrient solution containing 2 mm KNO3, 0·5 mm NH4H2PO4, 0·25 mm MgSO4·7H2O, 0·1 mm Ca(NO3)2·4H2O, 50 μm Fe-citrate, 92 μm H3BO3, 18 μm MnCl2·4H2O, 1·6 μm ZnSO4·7H2O, 0·6 μm CuSO4·5H2O and 0·7 μm (NH4)6Mo7O24·4H2O. Solutions were renewed every 3 d. Seedlings were grown in a greenhouse where the temperature was 28 °C/23 °C (day/night), the daily photoperiod was 16/8 h (light/dark; the flux density was approx. 800 μmol m–2 s–1) and relative humidity was about 65 %. Three-week-old plants were used for genetic transformation (Ma et al., 2014).
RNA interference silencing of ZxNHX
Plasmid construction was performed using the procedure described by Ma et al. (2014) with a slight modification. Stable gene silencing via Agrobacterium-mediated transformation was performed using a pHANNIBAL vector (Wesley et al., 2001) designed for producing a hairpin RNA construct of ZxNHX. A 542 bp fragment (nucleotides 421–962) was obtained using primers P1 (Supplementary Data Table S1: XbaI and XhoI restriction sites underlined) and P2 (Table S1: HindIII and KpnI restriction sites underlined). The whole NotI cassette bearing the RNA interference (RNAi) construct was sub-cloned into the corresponding site of the binary vector pART27, under the control of the Cauliflower mosaic virus (CaMV) 35S promoter, which was introduced into Agrobacterium tumefaciens strain GV3101 cells and used for plant transformation of Z. xanthoxylum.
The transformation was performed using the procedure of Weeks et al. (2008) with a slight modification. Briefly, after the cotyledons of 3-week-old seedlings expanded and the apical node emerged, a cotyledon and the apical node were excised and the wound covered with cotton wool which had been dipped in Agrobacterium suspension. The Agrobacterium suspension was obtained using the procedure described by Ma et al. (2014). For the WT control, the Agrobacterium suspension was replaced by distilled water. After 3 h, seedlings were put in the dark for 3 d. Thereafter, seedlings were transferred into a greenhouse and irrigated with modified Hoagland nutrient solution as described above. Five days later, a new apical node emerged from the wound region and formed new shoots 4 weeks later. Individual plants of the WT and each RNAi transgenic line were obtained by propagation from stem cuttings: stem sections with two nodes were excised using a sharp razor blade and the base placed into moist vermiculite under a photoperiod of 16/8 h (light/dark) at 25 ± 2 °C and 90 ± 5 % relative humidity in a growth chamber, and irrigated with modified Hoagland nutrient solution. After about a week, when the adventitious roots formed, seedlings were taken out of the growth chamber and grown in the greenhouse for 3 weeks.
The transformed plants were screened by a PCR assay using pHANNIBAL-specific primers and DNA obtained from leaves in order to detect the presence of the RNAi construct. Positive plants were selected to study the ZxNHX expression level by semi-quantitative reverse transcription–PCR (RT–PCR). Four-week-old plants were used for the following treatments. Plants were treated (1) with modified Hoagland nutrient solution as a control or (2) with modified Hoagland nutrient solution supplemented with 50 mm NaCl. Treatment solutions were changed every day to maintain a constant ion concentration. After 48 h, plants were harvested and total RNA was extracted with a Trizol Kit (Sangon Biotech Co., Ltd, Shanghai, China) following the manufacturer's instructions. RNA samples were quantified by absorbance at 260 nm, and the purity was assessed by the 260/280 nm ratio and on a 1·0 % (w/v) agarose gel stained with ethidium bromide (EtBr). First-strand cDNA was synthesized from 4 μg of total RNA with MMLV reverse transcriptase (Sangon Biotech Co., Ltd). Semi-quantitative RT–PCR was performed with the primer pairs P3 and P4 (Supplementary Data Table S1), which yielded a fragment of 580 bp. ACTIN was used as the internal control in the semi-quantitative RT–PCR. The specific primers of ACTIN that amplified a 598 bp fragment were A1 and A2 (Table S1), designed according to the cDNA sequence of ACTIN from Z. xanthoxylum (GenBank accession no. EU019550). PCR products were separated on 1·2 % (w/v) agarose gels containing EtBr and visualized by AlphaImager (ProteinSimple Inc., Santa Clara, CA, USA) for subsequent analysis. The ratios of the quantity of mRNA for ZxNHX to that for ACTIN were calculated, and results reflect the relative expression level. Experiments were repeated at least three times.
Two ZxNHX-silenced lines (L2 and L7, obtained from cuttings as described above) with differently reduced expression levels of ZxNHX were chosen for further real-time quantitative PCR analysis. Total RNA samples were extracted from leaves, stems and roots of the WT, L2 and L7, respectively, and first-strand cDNA samples were used for real-time quantitative PCR, which was performed on a thermal cycler (ABI PRISM 7500, USA). A specific fragment (170 bp) of ZxNHX was amplified with a pair of primers P5 and P6 (Supplementary Data Table S1). ZxACTIN (GenBank accession no. EU019550) was used for RNA normalization; the specific primers of ZxACTIN that amplified a 176 bp fragment were A3 and A4 (Table S1). SYBR Green PCR master mix (Takara, Biotech Co., Ltd, Dalian, China) was used for 20 μL PCRs as follow: 95 °C for 30 s, and 40 cycles of 95 °C for 5 s and 60 °C for 34 s. Each sample was assayed three times. The relative expression levels (RELs) of each samples were estimated according to the following equation as described by Livak and Schmittgen (2001): REL = 2–ddCt, where the ddCt value was the dCt value of ZxNHX in each sample minus the dCt value of the calibrator. The dCt value of ZxNHX came from the difference between the Ct value of ZxNHX and the Ct value of ZxACTIN in each sample. The dCt value of the calibrator was the mean value that came from the difference between the Ct value of ZxNHX and the Ct value of ZxACTIN in a sample of the WT under control conditions. The Ct value of ZxNHX and ZxACTIN in samples was obtained from the thermal cycler (ABI PRISM 7500, USA).
Determination of growth, water use efficiency, photosynthesis and osmotic potential
Individual plants of the WT, L2 and L7 were obtained by propagation from stem cuttings (ramets) as described above. For physiological analysis, plants were treated with modified Hoagland nutrient solution supplemented or not with 50 mm NaCl for 7 d. Roots were washed twice for 8 min in ice-cold 20 mm CaCl2 to exchange cell wall-bound Na+; leaves and stems were rinsed in deionized water to remove surface salts (Wang et al., 2007) and then tissue fresh weights were determined. Tissues were then immediately dried in an oven at 80 °C. After 72 h, tissue dry weights were measured. Leaf tissue water content was calculated using the following formula: water content = (f. wt – d. wt)/d. wt, where f. wt is leaf fresh weight and d. wt is leaf dry weight.
Leaf area was estimated by the Epson Perfection 4870 photo scanner (Epson America Inc., Long Beach, CA, USA).
Net photosynthesis rate (Pn), stomatal conductance (gs) and transpiration rate (Tr) were measured using an automatic photosynthetic measuring apparatus (GFS-3000; Heinz Walz GmbH, Germany) in the greenhouse. The instantaneous water use efficiency (WUE) was estimated using the formula: WUE = Pn/gs (Liu et al., 2005).
For the leaf osmotic potential (Ψs), leaf samples were frozen in liquid nitrogen. Cell sap was collected by thawing slowly and then Ψs was determined using a cryoscopic osmometer (Osmomat-030, Gonotec GmbH, Berlin, Germany) at 24 °C. The readings (mmol kg−1) were used to calculate the solute potential (Ψs) in MPa with the formula Ψs = –mol of solute × R × K, where R = 0·008314 and K = 298·8.
Assay of Na+ and K+ concentration and calculation of net Na+ uptake rate, Na+ and K+ relative distribution in tissues and the net selective transport capacity for K+ over Na+ (ST)
Na+ and K+ were extracted from dried plant tissues in 100 mm acetic acid at 90 °C for 2 h. Ion analysis was performed using a flame spectrophotometer (2655–00, Cole-Parmer Instrument Co., Vernon Hills, IL, USA).
Net Na+ uptake rate was calculated according to the following equation as described by Wang et al. (2009): net Na+ uptake rate (nmol g f. wt–1 min–1) = (Δ whole-plant Na+ content between salt-treated plants and BT plant)/root f. wt/Δ time, where BT indicates before treatments (Guo et al., 2012; Ma et al., 2014).
The relative distribution of Na+ (or K+) in different tissues was estimated by the formula: Na+ (or K+) relative distribution (%) = Na+ (or K+) content in each tissue/Na+ (or K+) content in the whole plant (Ma et al., 2014).
The contributions of Na+ and K+ to leaf osmotic potential (Ψs) were estimated by the formula C = COP/Ψs × 100 % (Guerrier, 1996), where COP (calculated osmotic potential) was the Ψs values of Na+ and K+ calculated by the Van't Hoff equation as described by Guerrier (1996): COP = –nRT; here n is the number of solute molecules.
The net selective transport capacity for K+ over Na+ (ST) from roots to shoots was estimated according to the following equation: ST = (K+/Na+ in shoots)/(K+/Na+ in roots) (Wang et al., 2004b, 2009; Guo et al., 2012; Ma et al., 2014).
Analysis of the expression level of ZxSOS1, ZxHKT1;1, ZxAKT1 and ZxSKOR in roots of Z. xanthoxylum
Four-week-old WT and ZxNHX-silenced lines (L2, L7) of Z. xanthoxylum were treated with modified Hoagland nutrient solution supplemented or not with 50 mm NaCl for 48 h, then roots were harvested and first-strand cDNA was synthesized by the procedures described above. The expression level of ZxSOS1, ZxHKT1;1, ZxAKT1 and ZxSKOR was analysed by real-time quantitative PCR as described above. A specific fragment (158 bp) of ZxSOS1 was amplified with a pair of primers P7 and P8; the ZxHKT1;1-specific fragment (101 bp) was amplified with primers P9 and P10; the ZxAKT1-specific fragment (111 bp) was amplified with primers P11 and P12; and the ZxSKOR-specific fragment (140 bp) was amplified with primers P13 and P14 (Supplementary Data Table S1). Experiments were repeated at least three times.
Data analysis
Results of growth, ion concentrations and gene expression level are presented as means with standard deviation, and data analysis was performed by one-way analysis of variance (ANOVA) using SPSS 13·0 statistical software (SPSS Inc., Chicago, IL, USA). Duncan's multiple range test was used to detect a difference between means at a significance level of P < 0·05.
RESULTS
ZxNHX silencing induced a significant inhibition of the growth of Z. xanthoxylum
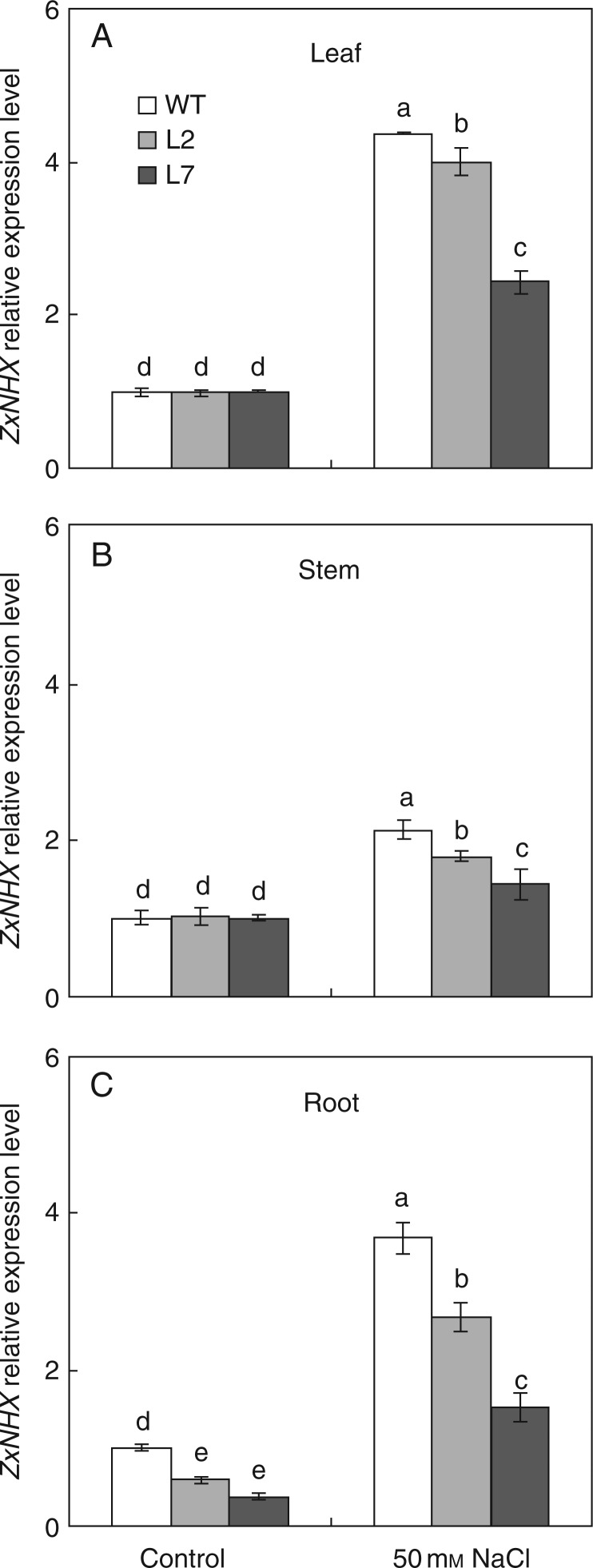
Thirteen transgenic lines harbouring the RNAi construct were identified (data not shown). Since the transcript of ZxNHX was preferentially expressed in leaves and significantly induced by 50 mm NaCl (Wu et al., 2011), the expression levels of ZxNHX in leaves of each transgenic line treated with 50 mm NaCl for 48 h were determined by semi-quantitative RT–PCR (data not shown). Of the 13 positive lines, two ZxNHX-silenced lines (L2 and L7) with different (reduced) expression levels of ZxNHX were chosen for further analysis. The expression levels of ZxNHX in root, stem and leaf tissue of L2 and L7 were further determined by real-time quantitative PCR after plants were treated with 50 mm NaCl for 48 h. As expected, the expression of ZxNHX was induced by 50 mm NaCl in all tissues in the WT, L2 and L7; when grown in 50 mm NaCl, the expression level of ZxNHX in leaves was lower than that of the WT by 8 % for L2 and 45 % for L7 (Fig. 1A), and reduced by 16 and 32 % in stems (Fig. 1B) and by 28 and 59 % in roots (Fig. 1C) for L2 and L7, respectively, in comparison with the WT. Even under control conditions (no additional NaCl), the expression level of ZxNHX in roots was lower by 41 % for L2 and by 62 % for L7 than in the WT (Fig. 1C), indicating that the expression of ZxNHX in all tissues was, to various extents, reduced by RNAi in each transgenic line (Fig. 1).
Fig. 1.
Relative expression level of ZxNHX in leaf (A), stem (B) and root (C) in the 4-week-old wild type (WT) and ZxNHX-silenced lines (L2, L7) of Z. xanthoxylum under control conditions (no additional NaCl) and 50 mm NaCl for 48 h. ACTIN was used as an internal control. Experiments were repeated at least three times (with similar results). Values are means ± s.d. (n = 3) and bars indicate the s.d. Columns with different letters indicate a significant difference at P < 0·05 (Duncan's test).
Phenotypically, ZxNHX-silenced lines differed significantly from the WT under salt treatment and even under control conditions (Fig. 2A). The silencing of ZxNHX triggered a significant reduction in plant dry weight (decreased by 27 % in L7) before treatment with 50 mm NaCl (Fig. 2 B). Under control conditions, dry weight and leaf area of L7 both decreased, by 29 and 19 %, respectively, in comparison with the WT (Fig. 2B, C). Salt treatment (50 mm NaCl) significantly increased dry weight only in the WT compared with its control (Fig. 2B).
Fig. 2.
(A) Morphology, (B) dry weight and (C) leaf area of 4-week-old wild-type (WT) and ZxNHX-silenced lines (L2, L7) of Z. xanthoxylum under control conditions (no additional NaCl) and 50 mm NaCl for 7 d, and (in B) before treatment with 50 mm NaCl. Values in (B, C) are means ± s.d. (n = 4) and bars indicate the s.d. Columns with different letters indicate a significant difference at P < 0·05 (Duncan's test).
We investigated the difference in photosynthetic activities between the WT and ZxNHX-silenced lines. Under control conditions, WUE, net photosynthesis rate (Pn), transpiration rate (Tr) and stomatal conductance (gs) in ZxNHX-silenced lines, especially in L7 which had the highest ZxNHX silencing efficiency, were significantly lower than those in the WT (Fig. 3A–D); the addition of 50 mm NaCl significantly enhanced WUE, Pn, Tr and gs in the WT but only increased Tr and gs in L7 (Fig. 3A–D). In 50 mm NaCl, WUE, Pn, Tr and gs were 77, 80, 10 and 37 % lower in L7 than in the WT (Fig. 3A–D).
Fig. 3.
(A) Water use efficiency (WUE), (B) net photosynthesis rate, (C) transpiration rate (D) and stomatal conductance in 4-week-old wild-type (WT) and ZxNHX-silenced lines (L2, L7) of Z. xanthoxylum under control conditions (no additional NaCl) and 50 mm NaCl for 7 d. Values are means ± s.d. (n = 4) and bars indicate the s.d. Columns with different letters indicate a significant difference at P < 0·05 (Duncan's test).
Under control conditions, although osmotic potential (Ψs) in leaves of L7 was lower (6·0 %) than in those of the WT, no significant difference was observed in leaf tissue water content between L7 and the WT (Table 1). Interestingly, in 50 mm NaCl, L7 possessed a significantly increased Ψs in leaves but a significantly reduced (48 %) leaf tissue water content compared with the WT (Table 1).
Table 1.
Osmotic potential (Ψs) and tissue water content of leaves in 4-week-old wild-type (WT) and ZxNHX-silenced lines (L2, L7) of Z. xanthoxylum under control conditions (no additional NaCl) and 50 mm NaCl for 7 d
| Ψs (control) (MPa) | Ψs (50 mm NaCl) (MPa) | Leaf tissue water content (control) (g g–1 d. wt) | Leaf tissue water content (50 mm NaCl) (g g–1 d. wt) | |
|---|---|---|---|---|
| WT | –1·67 ± 0·03a | –2·16 ± 0·05b | 10·95 ± 1·53a | 11·00 ± 1·67a |
| L2 | –1·74 ± 0·05a | –2·13 ± 0·03b | 10·41 ± 0·94a | 6·64 ± 1·17b |
| L7 | –1·77 ± 0·04b | –2·03 ± 0·03a | 10·26 ± 1·07a | 5·69 ± 0·30b |
Values are means ± s.d. (n = 4).
Columns with different letters indicate significant differences at P < 0·05 (Duncan's test).
These results indicated that ZxNHX silencing significantly inhibited the growth of Z. xanthoxylum under both normal and saline conditions.
ZxNHX silencing altered Na+ and K+ accumulation, uptake and partitioning in plant tissues under saline conditions
We investigated Na+ and K+ accumulation, uptake and partitioning in plant tissues. No significant difference in Na+ and K+ concentrations in any tissues was observed between the WT and ZxNHX-silenced lines under control conditions (Fig. 4A–F). The addition of 50 mm NaCl significantly increased Na+ concentrations but did not affect K+ concentrations in any tissues in the WT (Fig. 4A–F). Na+ concentrations only increased by a relatively small degree in leaves and roots (Fig. 4A–C) but K+ concentrations significantly decreased in all tissues in ZxNHX-silenced lines under 50 mm NaCl compared with controls (Fig. 4D–F).
Fig. 4.
Na+ concentration in leaf (A), stem (B) and root (C), and K+ concentration in leaf (E), stem (F) and root (G) in 4-week-old wild-type (WT) and ZxNHX-silenced lines (L2, L7) of Z. xanthoxylum under control conditions (no additional NaCl) and 50 mm NaCl for 7 d. Values are means ± s.d. (n = 4) and bars indicate the s.d. Columns with different letters indicate a significant difference at P < 0·05 (Duncan's test).
Under control conditions, no significant difference in net Na+ uptake rate and Na+ relative distribution in different tissues was found between the WT and ZxNHX-silenced lines, but L7 exhibited a higher relative distribution of K+ in roots (by 23 %) than the WT (Fig. 5A–C). The addition of 50 mm NaCl significantly increased the net Na+ uptake rate in both WT and ZxNHX-silenced lines, but to a lesser degree in L7 than in the WT (Fig. 5A); under 50 mm NaCl, L7 exhibited a 40 % lower net Na+ uptake rate than the WT (Fig. 5A). When grown in 50 mm NaCl, the relative distribution of Na+ in roots and stems of L7 significantly increased by 25 and 54 %, respectively, but decreased by 10 % in leaves compared with the WT (Fig. 5B). The relative distribution of K+ in roots of L7 was 23 % lower than in the WT (Fig. 5C).
Fig. 5.
Net Na+ uptake rate (A), and Na+ (B) and K+ (C) relative distribution in tissues in 4-week-old wild-type (WT) and ZxNHX-silenced lines (L2, L7) of Z. xanthoxylum under control conditions (no additional NaCl) and 50 mm NaCl for 7 d. Values are means ± s.d. (n = 4) and bars indicate the s.d. Columns with different letters indicate a significant difference at P < 0·05 (Duncan's test).
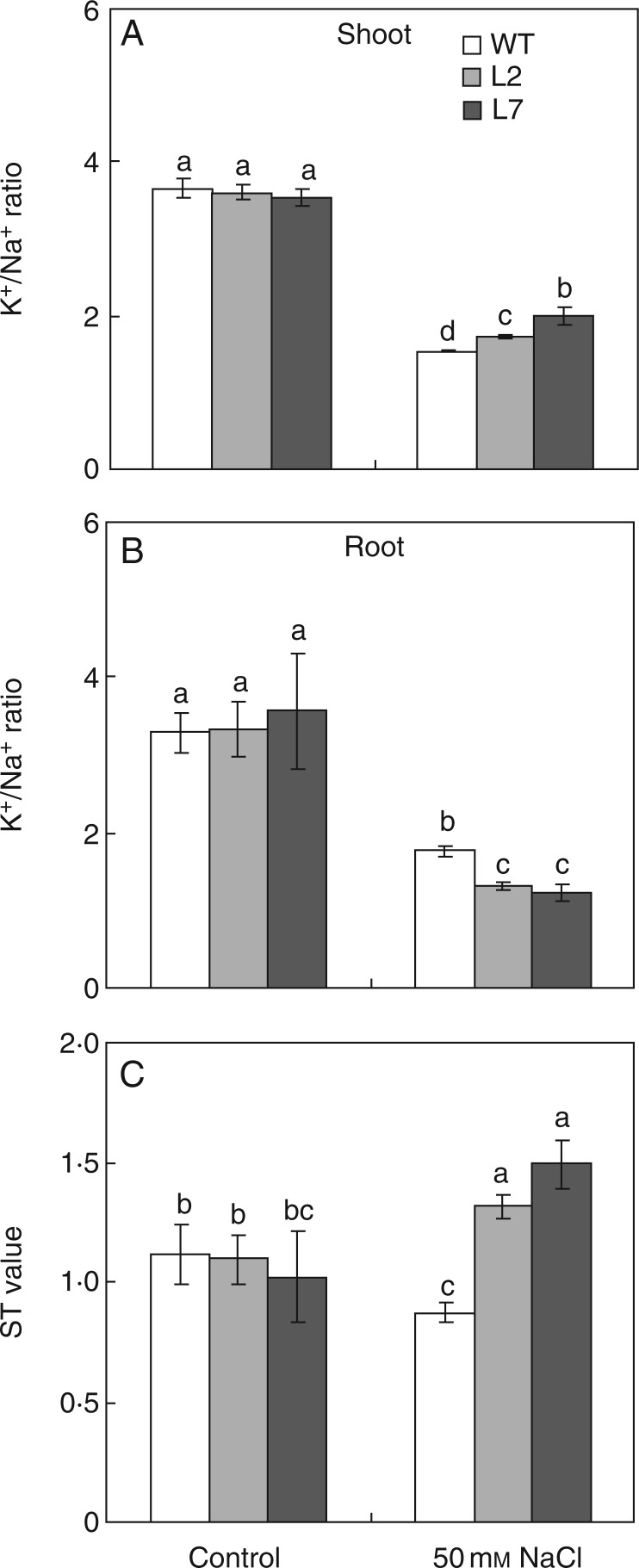
Under control conditions, there was no significant difference in the K+/Na+ ratio in shoots and roots and net selective transport capacity for K+ over Na+ from roots to shoots (ST value) between the WT and ZxNHX-silenced lines (Fig. 6). NaCl (50 mm) triggered a significant decrease in the K+/Na+ ratio in shoots and roots of both the WT and ZxNHX-silenced lines (Fig. 6A, B), but L7 exhibited a higher K+/Na+ ratio (by 30 %) in shoots and a lower K+/Na+ ratio (by 31 %) in roots compared with the WT in 50 mm NaCl (Fig. 6A, B). The addition of 50 mm NaCl significantly reduced the ST value in the WT but increased the ST value in ZxNHX-silenced lines (Fig. 6C).
Fig. 6.
K+/Na+ ratio in shoots (A) and roots (B) and net selective transport capacity for K+ over Na+ from roots to shoots (ST value) (C) in 4-week-old wild-type (WT) and ZxNHX-silenced lines (L2, L7) of Z. xanthoxylum under control conditions (no additional NaCl) and 50 mm NaCl for 7 d. Values are means ± s.d. (n = 4) and bars indicate the s.d. Columns with different letters indicate a significant difference at P < 0·05 (Duncan's test).
ZxNHX silencing altered the expression level of genes involved in Na+ and K+ uptake and transport in roots of Z. xanthoxylum
All the results in the previous section suggested that ZxNHX regulates long-distance transport of Na+ and K+ in Z. xanthoxylum. To explore further the possible molecular mechanisms underlying these observations, the expression levels of genes involved in uptake and transport of Na+ and K+ were analysed in the WT and ZxNHX-silenced lines growing in 50 mm NaCl.
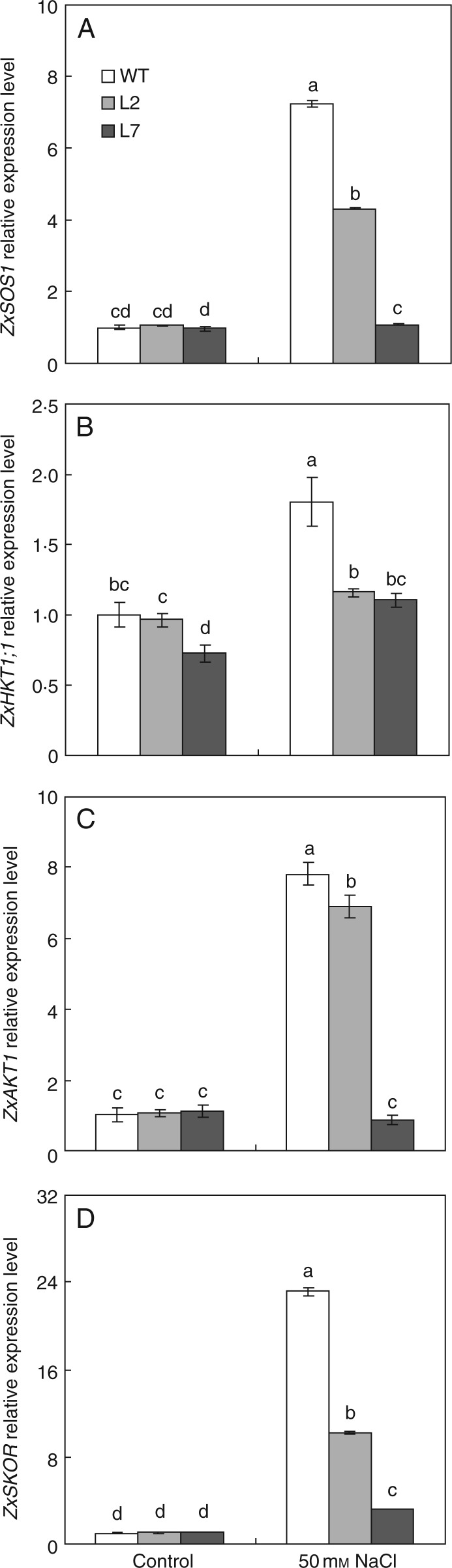
ZxSOS1, encoding a plasma membrane Na+/H+ antiporter, has been proposed as an important transporter for long-distance movement of Na+ from roots to shoots and spatial distribution of Na+ in Z. xanthoxylum (Ma et al., 2014). In the present work, under control conditions, the expression level of ZxSOS1 in roots of ZxNHX-silenced lines was the same as that in the WT; the addition of 50 mm NaCl significantly increased the expression level of ZxSOS1 in roots by 620 % in the WT but only by 10 % in L7, respectively, compared with plants in the absence of salt; under 50 mm NaCl, the expression level of ZxSOS1 in roots was lower by 41 % in L2 and by 85 % in L7 than that in the WT (Fig. 7A). These results suggest that ZxNHX silencing triggered a significant downregulation of ZxSOS1 in roots of Z. xanthoxylum under saline conditions.
Fig. 7.
Synergistic response of (A) ZxSOS1, (B) ZxHKT1;1, (C) ZxAKT1 and (D) ZxSKOR in roots in 4-week-old wild-type (WT) and ZxNHX-silenced lines (L2, L7) of Z. xanthoxylum under control conditions (no additional NaCl) and 50 mm NaCl for 48 h. ACTIN was used as an internal control. Experiments were repeated at least three times (with similar results). Values are means ± s.d. (n = 3) and bars indicate the s.d. Columns with different letters indicate a significant difference at P < 0·05 (Duncan's test).
The high-affinity K+ transporter (HKT) is proposed to play a crucial role in controlling Na+ and K+ homeostasis in shoots (Hauser and Horie, 2010). In Arabidopsis, as well as a direct and vital role in unloading of Na+ from the xylem to the xylem parenchyma cells (XPCs) in roots (Mäser et al., 2002; Gong et al., 2004; Sunarpi et al., 2005; Davenport et al., 2007), AtHKT1;1 is indirectly involved in mediating K+ release from XPCs into xylem vessels since the retrieval of Na+ by AtHKT1;1 possibly causes membrane depolarization of XPCs and triggers K+ penetration into the xylem via membrane depolarization-induced K+ efflux channels such as SKOR (Stelar K+ outward rectifier) (Wegner and Raschke, 1994; Wegner and De Boer, 1997; Gaymard et al., 1998; Hauser and Horie, 2010; Guo et al., 2012). In our current study, under control conditions, the expression level of ZxHKT1;1 in roots in L7 was significantly lower by 28 % that in the WT; the addition of 50 mm NaCl significantly enhanced the expression level of ZxHKT1;1 in roots in both the WT and L7, but the relative increase was greater in the WT (80 %) than in L7 (53 %); under 50 mm NaCl, the expression level of ZxHKT1;1 in roots was significantly lower by 39 % in L7 than in the WT (Fig. 7B). These results indicate that ZxNHX silencing induced an inhibitory effect on the expression of ZxHKT1;1 in roots of Z. xanthoxylum.
It is well known that AKT1, as an inward-rectifying K+ channel, mediates K+ influx into root cells in many plants species (Lagarde et al., 1996; Hirsch et al., 1998; Gierth et al., 2005; Li et al., 2006; Xu et al., 2006; Wang and Wu, 2013). SKOR, a K+ outward rectifying channel, has been proposed to mediate loading of K+ from XPCs into xylem vessels (Wegner and Raschke, 1994; Wegner and De Boer, 1997; Gaymard et al., 1998; Johansson et al., 2006; Liu et al., 2006). In our study, no significant difference in the expression level of ZxAKT1 or ZxSKOR in roots was observed between the WT and ZxNHX-silenced lines under control conditions (Fig. 7C, D). However, 50 mm NaCl induced a significant increase (7-fold) in the expression level of ZxAKT1 in roots of the WT, but there was no change in its expression in L7 (Fig. 7C). The addition of 50 mm NaCl significantly increased the expression level of ZxSKOR in roots in both the WT and ZxNHX-silenced lines, but the relative increase was much greater in the WT (21-fold) than in L7 (2-fold) (Fig. 7D). These results suggested that the expression level of both ZxAKT1 and ZxSKOR in roots was downregulated by ZxNHX silencing under saline conditions.
DISCUSSION
ZxNHX is critical for controlling Na+ and K+ homeostasis by regulating their uptake, transport and spatial distribution in Z. xanthoxylum
Previous studies showed that the tonoplast-located NHXs are critical for minimizing the cytoplasmic Na+ concentration through the sequestration of Na+ in vacuoles in many plants (Apse et al., 1999, 2003; Brini et al., 2007). Recent research indicated that NHX1 and NHX2 from Arabidopsis are involved in the accumulation of K+ into vacuoles of plant cells, thereby decreasing their osmotic potential and driving the uptake of water that generates the turgor pressure necessary for cell expansion and growth (Bassil et al., 2011; Barragán et al., 2012); and in particular the vacuolar accumulation of K+ mediated by NHX1 and NHX2 in guard cells directly regulated stomatal activity (Andrés et al., 2014). Our previous work indicated a positive correlation between upregulation of ZxNHX and Na+ accumulation in leaves of Z. xanthoxylum exposed to salt and drought (Wu et al., 2011), and it was found that the overexpression of ZxNHX significantly enhanced salt and drought tolerance in transgenic Lotus corniculatus by increasing accumulation of cations, especially Na+ (Bao et al., 2014). However, none of these studies addressed the role of NHX proteins in relation to uptake and spatial distribution of Na+ and K+ at the whole-plant level. Our present results showed that ZxNHX silencing triggered a significant decrease in net Na+ uptake rate, Na+ accumulation and relative distribution in leaves, but increased the Na+ distribution in roots and stems in Z. xanthoxylum under 50 mm NaCl (Figs 4 and 5).
Na+ enters roots and is transported to shoots via the transpiration stream in the xylem. As much as 10–15 % of the surface area of xylem vessels is in contact with XPCs (Apse and Blumwald, 2007) and this large surface area can unquestionably accommodate the large quantities of ions and water that pass from roots to shoots (Tester and Davenport, 2003; Apse and Blumwald, 2007). To date, both passive and active models have been proposed in glycophytes for the transport mechanisms involved in Na+ loading into the xylem (Wegner and Raschke, 1994; Shi et al., 2002; Wegner et al., 2011). However, thermodynamically, passive Na+ loading into the xylem (probably via passive, channel-like, transport proteins) would occur only if the membrane potential of XPCs was less negative than –17 mV (Shabala and Mackay, 2011). This seems not to be the case in halophytes since highly negative values (e.g. –130 to –140 mV) for halophyte XPCs have been reported (Anderson et al., 1977). This might leave active Na+ loading operated by SOS1 as the only option in halophytes (Shabala and Mackay, 2011; Adolf et al. 2013) and in plants such as Z. xanthoxylum which possesses a feature typical for salt-accumulating halophytes (Janz and Polle, 2012; Ma et al., 2012). Indeed, it has been suggested that SOS1 and HKT located at the plasma membrane of XPCs may play crucial roles in regulating Na+ transport from roots to shoots by mediating opposite fluxes of Na+ across plasma membranes of XPCs (Olías et al., 2009; Hauser and Horie, 2010). Guo et al. (2012) proposed a model to explain the co-ordinated function of SOS1 and HKT in regulating Na+ and K+ homeostasis in plants. Under mild salinity, when Na+ accumulation in leaves is probably below the capacity of sequestering Na+ into vacuoles (Blumwald et al., 2000), it appears that the transport activities of SOS1 outweigh those of HKT at the plasma membrane of XPCs and Na+ is loaded into the transpiration stream. When Na+ in vacuoles of leaves reaches its maximum concentration, it seems that the transport activities of HKT exceed those of SOS1 at the plasma membrane of XPCs and, thus, Na+ is unloaded from the xylem into XPCs (Guo et al., 2012). For Z. xanthoxylum, Yue et al. (2012) found that 5–100 mm NaCl in the medium significantly enhanced its growth, so (at the optimal salt concentration of 50 mm NaCl) ZxNHX efficiently compartmentalized Na+ into vacuoles of leaves. Simultaneously, this induced a 620 % increase in transcripts of ZxSOS1 but only an 80 % increase in transcripts of ZxHKT1;1 in roots (Fig. 7A, B). Therefore, the transport ability of ZxSOS1 for Na+ exceeded that of ZxHKT1;1 and Na+ was loaded into xylem (Fig. 8A) and delivered rapidly to shoots for osmotic adjustment (Wu et al., 2011; Ma et al., 2012). However, when ZxNHX was silenced in L7, the capacity for sequestering Na+ into vacuoles of leaves was weakened, which in turn regulated Na+ long-distance transport from roots; 50 mm NaCl increased transcripts of ZxSOS1 by only 10 % whereas transcripts of ZxHKT1;1 increased by 53 % in roots of L7 (Fig. 7A, B), and thus the ability of ZxHKT1;1 to transport Na+ outweighed that of ZxSOS1, and Na+ was unloaded from the xylem stream (Fig. 8B). This combination of changes reduced the net Na+ uptake rate (Fig. 5A), Na+ accumulation (Fig. 4A) and relative distribution in leaves (Fig. 5B) by 40, 44 and 10 %, respectively, but increased the Na+ distribution in roots and stems of L7 by 25 and 54 %, respectively (Fig. 5B). Therefore, we propose that ZxNHX not only determines Na+ accumulation in vacuoles of mesophyll cells, but also controls the (opposite) Na+ fluxes across the plasma membranes of XPCs mediated by ZxSOS1 and ZxHKT1;1, thereby regulating Na+ accumulation.
Fig. 8.
Schematic model for the function of ZxNHX in regulating the Na+ transport system in Z. xanthoxylum under saline conditions. (A) In WT plants growing in 50 mm NaCl, ZxNHX efficiently compartmentalized Na+ into vacuoles of leaves. NaCl at 50 mm induced a 620 % increase in transcripts of ZxSOS1 but only an 80 % increase in transcripts of ZxHKT1;1 in roots; consequently, Na+ was loaded into the xylem and delivered to the shoots. (B) In a ZxNHX-silenced line (L7), the transcripts of ZxNHX in leaves were decreased by 45 % compared with the WT, which in turn regulated long-distance Na+ transport from roots to shoots. NaCl at 50 mm increased transcripts of ZxSOS1 by only 10 %, whereas transcripts of ZxHKT1;1 increased by 53 % in roots of L7; thus, Na+ was unloaded from the xylem stream.
Our results also showed that silencing of ZxNHX triggered a significant decrease in K+ accumulation in all the tissues under 50 mm NaCl (Fig. 4D–F). This might be attributed to the downregulated expression of ZxAKT1 and ZxSKOR in roots of ZxNHX-silenced lines (Fig. 7C, D). Downregulated expression of ZxAKT1 and ZxSKOR (Fig. 7C, D) may impair K+ uptake and loading K+ from XPCs into xylem vessels, and thus decrease K+ transport from roots to shoots. As a result, the concentration of K+ decreases in all the tissues (Fig. 4D–F). It has been suggested that SOS1 plays a necessary role in protecting K+ uptake mediated by AKT1, on which growth depends, and the elevated cytoplasmic Na+ level resulting from loss of SOS1 function impairs K+ uptake ability in root cells and compromises K+ nutrition under salt stress (Qi and Spalding, 2004). In our study, downregulated expression of ZxSOS1 in roots of ZxNHX-silenced lines (Fig. 7A) may also be involved in the decrease of K+ accumulation in plants.
Taken together, these data suggested that ZxNHX is involved in regulating uptake and spatial distribution of not only Na+, but also K+, and maintaining their homeostasis by regulating the expression level of ZxSOS1, ZxHKT1;1, ZxAKT1and ZxSKOR in roots.
ZxNHX might be a key factor for maintaining the characteristic of salt accumulation in Z. xanthoxylum under saline conditions
Salt-resistant plants have been categorized into salt-excluding, salt-secreting or salt-accumulating plants according to their physiological response in relation to ion selectivity (Wang et al., 2002; Munns and Tester, 2008). Wang et al. (2002) proposed that the net selective transport capacity for K+ over Na+ from roots to shoots (ST value) is an important indicator distinguishing these three types of plants. Salt-excluding plants have the highest ST value while salt-secreting plants have the lowest, and in salt-accumulating plants the ST value lies between that of salt-excluding and salt-secreting plants (Wang et al., 2002). Based on the evaluation of the ST value, we concluded that Z. xanthoxylum, just like the halophyte Suaeda salsa, is a salt-accumulating plant (Wang et al., 2004). However, ZxNHX-silenced lines possessed a significantly higher ST value than the WT under 50 mm NaCl (Fig. 6C), suggesting that the silencing of ZxNHX could convert Z. xanthoxylum from being a typical salt-accumulating plant to becoming a salt-excluding plant. As discussed above, ZxNHX controls the (opposite) Na+ fluxes across the plasma membranes of XPCs by regulating the expression of ZxSOS1 and ZxHKT1;1 (Figs 7A, B and 8). In salt-accumulating plants like Z. xanthoxylum under saline conditions optimal for their growth, Na+ could be efficiently compartmentalized by ZxNHX into vacuoles of leaves, thus inducing the higher expression level of ZxSOS1 over ZxHKT1;1. Thus, Na+ is loaded into xylem (Fig. 8A), resulting in a relatively lower ST value (Fig. 6C). However, in the ZxNHX-silenced line, the capacity to sequester Na+ by NHX into vacuoles of leaves is relatively weak, and hence, through feedback regulation, the transcription of ZxHKT1;1 is higher than that of ZxSOS1. Consequently, Na+ is unloaded from the xylem stream (Fig. 8 B), leading to a relative higher ST value (Fig. 6C), thus impairing the characteristic of salt accumulation in Z. xanthoxylum. Therefore, we conclude that the activities of NHX, SOS1 and HKT synergistically determine the ST value in plants, and ZxNHX might play a key role in maintaining the characteristic of salt accumulation in Z. xanthoxylum under saline conditions.
ZxNHX might be essential for normal growth and development of Z. xanthoxylum
Our results showed that silencing of ZxNHX had strong negative effects on plant growth; under 50 mm NaCl, dry weight and leaf area were significantly reduced in ZxNHX-silenced lines as compared with the WT (Fig. 2). WUE, Pn, gs and Tr also decreased in ZxNHX-silenced lines in comparison with the WT under 50 mm NaCl (Fig. 3), indicating that ZxNHX is involved in regulation of photosynthetic activity in leaves of Z. xanthoxylum. Ma et al. (2012) found that the significant stimulation of photosynthesis and growth in the presence of salt was strongly related to the enhancement of stomatal conductance. Franks (2006) found that the opening of stomata and leaf area development were strongly dependent on cell turgor, which in turn was determined by osmotic adjustment capacity. In fact, for Z. xanthoxylum, Na+ has been proposed as a beneficial osmoregulatory substance accumulated in leaves for osmotic adjustment and could significantly increase chlorophyll concentrations (Wu et al., 2011; Ma et al., 2012; Yue et al., 2012). In our study, the silencing of ZxNHX triggered a significant decrease in Na+ concentration in leaves (Fig. 4A), which resulted in a significantly increased Ψs under 50 mm NaCl. Consequently, osmotic adjustment capacity decreased and triggered a significant reduction in leaf tissue water content (Table 1) and this inhibited growth of Z. xanthoxylum. We also found that the K+ concentration in all the tissues was significantly lower in ZxNHX-silenced lines than in the WT under 50 mm NaCl (Fig. 4D–F), which might be another important reason why ZxNHX-silenced lines showed growth defects compared with the WT under 50 mm NaCl.
It is worth noting that ZxNHX silencing also carried inhibitory effects on plant growth even under non-saline conditions (Fig. 2). Actually, it has been shown that the tonoplast-localized AtNHX1 and AtNHX2 proteins are essential for active K+ uptake at the tonoplast, for osmotic adjustment and turgor regulation necessary for plant growth and organ development under normal conditions (Bassil et al., 2011; Barragán et al., 2012), and they are also directly involved in the regulation of stomatal activity by mediating K+ accumulation in the vacuoles of guard cells (Andrés et al., 2014). In addition, several other cellular functions of AtNHX have also been suggested, including H+ homeostasis and vesicle trafficking (Rodríguez-Rosales et al., 2009; Bassil et al., 2012; Andrés et al., 2014). Studies to uncover whether ZxNHX is involved in these biological processes for regulating normal plant growth and development would be worthwhile.
In conclusion, our results demonstrate that ZxNHX is essential for controlling Na+ and K+ homeostasis at the whole-plant level through feedback regulation of the expression of genes involved in their transport, thereby maintaining the characteristic of salt accumulation in Z. xanthoxylum and regulating its normal growth.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of Table S1: primer sequences used in this study.
ACKNOWLEDGEMENTS
We are very grateful to Professor Timothy J. Flowers from the University of Sussex, UK, for critically reviewing the manuscript and for valuable suggestions. This work was supported by the National Basic Research Program of China (973 Program, grant no. 2014CB138701), the National Natural Science Foundation of China (grant no. 31170431) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (grant no. 20130211130001).
LITERATURE CITED
- Adolf VI, Jacobsen SE, Shabala S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.) Environmental and Experimental Botany. 2013;92:43–54. [Google Scholar]
- Anderson WP, Willcocks DA, Wright BJ. Electrophysiological measurements on root of Atriplex hastata. Journal of Experimental Botany. 1977;28:894–901. [Google Scholar]
- Andrés Z, Pérez-Hormaeche J, Leidi EO, et al. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proceedings of the National Academy of Sciences, USA; 2014. pp. E1806–E1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apse MP, Aharon GS, Snedden WA, Blumwald E. Overexpression of a vacuolar Na+/H+ antiport confers salt tolerance in Arabidopsis. Science. 1999;285:1256–1258. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- Apse MP, Blumwald E. Na+ transport in plants. FEBS Letters. 2007;581:2247–2254. doi: 10.1016/j.febslet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Apse MP, Sottosanto JB, Blumwald E. Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. The Plant Journal. 2003;36:229–239. doi: 10.1046/j.1365-313x.2003.01871.x. [DOI] [PubMed] [Google Scholar]
- Bao AK, Wang YW, Xi JJ, Liu C, Zhang JL, Wang SM. Co-expression of xerophyte Zygophyllum xanthoxylum ZxNHX and ZxVP1–1 enhances salt and drought tolerance in transgenic Lotus corniculatus by increasing cations accumulation. Functional Plant Biology. 2014;41:203–214. doi: 10.1071/FP13106. [DOI] [PubMed] [Google Scholar]
- Barragán V, Leidi EO, Andrés Z, et al. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. The Plant Cell. 2012;24:1127–1142. doi: 10.1105/tpc.111.095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E, Tajima H, Liang YC, et al. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. The Plant Cell. 2011;23:3482–3497. doi: 10.1105/tpc.111.089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E, Aharon GS, Apse MP. Sodium transport in plant cells. Biochimica et Biophysica Acta. 2000;1465:140–151. doi: 10.1016/s0005-2736(00)00135-8. [DOI] [PubMed] [Google Scholar]
- Brini F, Hanin M, Mezghani I, Berkowitz GA, Masmoudi K. Overexpression of wheat Na+/H+ antiporter TNHX1 and H+-pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. Journal of Experimental Botany. 2007;58:301–308. doi: 10.1093/jxb/erl251. [DOI] [PubMed] [Google Scholar]
- Chaves M, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport RJ, Muñoz-Mayor A, Jha D, Essah PA, Rus A, Tester M. The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant, Cell and Environment. 2007;30:497–507. doi: 10.1111/j.1365-3040.2007.01637.x. [DOI] [PubMed] [Google Scholar]
- Flowers TJ. Improving crop salt tolerance. Journal of Experimental Botany. 2004;55:307–319. doi: 10.1093/jxb/erh003. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytologist. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Franks PJ. Higher rates of leaf gas exchange are associated with higher leaf hydrodynamic pressure gradients. Plant, Cell and Environment. 2006;29:584–592. doi: 10.1111/j.1365-3040.2005.01434.x. [DOI] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, et al. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell. 1998;94:647–655. doi: 10.1016/s0092-8674(00)81606-2. [DOI] [PubMed] [Google Scholar]
- Gierth M, Mäser P, Schroeder JI. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiology. 2005;137:1105–1114. doi: 10.1104/pp.104.057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JM, Waner DA, Horie T, et al. Microarray-based rapid cloning of an ion accumulation deletion mutant in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA; 2004. pp. 15404–15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier G. Fluxes of Na+, K+ and Cl–, and osmotic adjustment in Lycopersicon pimpinellifolium and L. esculentum during short- and long-term exposures to NaCl. Plant Physiology. 1996;97:583–591. [Google Scholar]
- Guo Q, Wang P, Ma Q, Zhang JL, Bao AK, Wang SM. Selective transport capacity for K+ over Na+ is linked to the expression levels of PtSOS1 in halophyte Puccinellia tenuiflora. Functional Plant Biology. 2012;39:1047–1057. doi: 10.1071/FP12174. [DOI] [PubMed] [Google Scholar]
- Hauser F, Horie T. A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant, Cell and Environment. 2010;33:552–565. doi: 10.1111/j.1365-3040.2009.02056.x. [DOI] [PubMed] [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- Horie T, Hauser F, Schroeder J. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends in Plant Science. 2009;14:660–668. doi: 10.1016/j.tplants.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz D, Polle A. Harnessing salt for woody biomass production. Tree Physiology. 2012;32:1–3. doi: 10.1093/treephys/tpr127. [DOI] [PubMed] [Google Scholar]
- Johansson I, Wulfetange K, Porée F, et al. External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. The Plant Journal. 2006;46:269–281. doi: 10.1111/j.1365-313X.2006.02690.x. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Szczerba MW, Moazami-Goudarzi M, Britto DT. The cytosolic Na+:K+ ratio does not explain salinity-induced growth impairment in barley: a dual-tracer study using 42K+ and 24Na+ Plant, Cell and Environment. 2006;29:2228–2237. doi: 10.1111/j.1365-3040.2006.01597.x. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Britto DT. Sodium transport in plants: a critical review. New Phytologist. 2011;189:54–81. doi: 10.1111/j.1469-8137.2010.03540.x. [DOI] [PubMed] [Google Scholar]
- Lagarde D, Basset M, Lepetit M, et al. Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. The Plant Journal. 1996;9:195–203. doi: 10.1046/j.1365-313x.1996.09020195.x. [DOI] [PubMed] [Google Scholar]
- Leidi EO, Barragan V, Rubio L, et al. The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. The Plant Journal. 2010;61:495–506. doi: 10.1111/j.1365-313X.2009.04073.x. [DOI] [PubMed] [Google Scholar]
- Li L, Kim BG, Cheong YH, Pandey GK, Luan S. A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proceedings of the National Academy of Sciences; USA. 2006. pp. 12625–12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FL, Andersen MN, Jacobsen SE, Jensen CR. Stomatal control and water use efficiency of soybean (Glycine max L. Merr.) during progressive soil drying. Environmental and Experimental Botany. 2005;54:33–40. [Google Scholar]
- Liu JQ, Pu JC, Liu XM. Comparative studies on water relations and xeromorphic structures of some plant species in the middle part of the desert zone in China. Acta Botanica Sinica. 1987;29:662–673. [Google Scholar]
- Liu JQ, Li ZJ, Pu JC, Zheng SD. Comparative studies on relationships between proline accumulation and photosynthesis, respiration and chlorophyll content of some plant species in the middle part of the desert zone in China. Acta Botanica Sinica. 1988;30:85–95. [Google Scholar]
- Liu K, Li LG, Luan S. Intracellular K+ sensing of SKOR, a Shaker-type K+ channel from Arabidopsis. The Plant Journal. 2006;46:260–268. doi: 10.1111/j.1365-313X.2006.02689.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma Q, Lou JQ, Wang SM. Effects of Na+ on photosynthetic characteristics of Zygophyllum xanthonylon seedlings under osmotic stress. Acta Prataculturae Sinica. 2010;19:198–203. [Google Scholar]
- Ma Q, Yue LJ, Zhang JL, Wu GQ, Bao AK, Wang SM. Sodium chloride improves photosynthesis and water status in the succulent xerophyte Zygophyllum xanthoxylum. Tree Physiology. 2012;32:4–13. doi: 10.1093/treephys/tpr098. [DOI] [PubMed] [Google Scholar]
- Ma Q, Li YX, Yuan HJ, Hu J, Wei L, Bao AK, Zhang JL, Wang SM. ZxSOS1 is essential for long-distance transport and spatial distribution of Na+ and K+ in the xerophyte Zygophyllum xanthoxylum. Plant and Soil. 2014;374:661–676. [Google Scholar]
- Maathuis FJ, Amtmann A. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Annals of Botany. 1999;84:123–133. [Google Scholar]
- Martínez J, Ledent J, Bajji M, Kinet J, Lutts S. Effect of water stress on growth, Na+ and K+ accumulation and water use efficiency in relation to osmotic adjustment in two populations of Atriplex halimus L. Plant Growth Regulation. 2003;41:63–73. [Google Scholar]
- Mäser P, Eckelman B, Vaidyanathan R, et al. Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Letters. 2002;531:157–161. doi: 10.1016/s0014-5793(02)03488-9. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Olías R, Eljakaoui Z, Li J, et al. The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant, Cell and Environment. 2009;32:904–916. doi: 10.1111/j.1365-3040.2009.01971.x. [DOI] [PubMed] [Google Scholar]
- Qi Z, Spalding EP. Protection of plasma membrane K+ transport by the salt overly sensitive Na+/H+ antiporter during salinity stress. Plant Physiology. 2004;136:2548–2555. doi: 10.1104/pp.104.049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Rosales MP, Gálvez FJ, Huertas R, et al. Plant NHX cation/proton antiporters. Plant Signaling and Behavior. 2009;4:265–276. doi: 10.4161/psb.4.4.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Cuin TA. Potassium transport and plant salt tolerance. Physiologia Plantarum. 2008;133:651–669. doi: 10.1111/j.1399-3054.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- Shabala S, Mackay A. Ion transport in halophytes. Advances in Botanical Research. 2011;57:151–187. [Google Scholar]
- Shabala S. Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany. 2013;112:1209–1221. doi: 10.1093/aob/mct205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HZ, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. The Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunarpi, Horie T, Motoda J, et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. The Plant Journal. 2005;44:928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- Tester M, Davenport RJ. Na+ tolerance and Na+ transport in higher plants. Annals of Botany. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CM, Zhang JL, Liu XS, et al. Puccinellia tenuiflora maintains a low Na+ level under salinity by limiting unidirectional Na+ influx resulting in a high selectivity for K+ over Na+ Plant, Cell and Environment. 2009;32:486–496. doi: 10.1111/j.1365-3040.2009.01942.x. [DOI] [PubMed] [Google Scholar]
- Wang SM, Zheng WJ, Ren JZ, Zhang CL. Selectivity of various types of salt-resistant plants for K+ over Na+ Journal of Arid Environments. 2002;52:457–472. [Google Scholar]
- Wang SM, Wan CG, Wang YR, et al. The characteristics of Na+, K+ and free proline distribution in several drought-resistant plants of the Alxa Desert, China. Journal of Arid Environments. 2004a;56:525–539. [Google Scholar]
- Wang SM, Zhao GQ, Gao YS, Tang ZC, Zhang CL. Puccinellia tenuiflora exhibits stronger selectivity for K+ over Na+ than wheat. Journal of Plant Nutrition. 2004b;27:1841–1857. [Google Scholar]
- Wang SM, Zhang JL, Flowers TJ. Low-affinity Na+ uptake in the halophyte Suaeda maritima. Plant Physiology. 2007;145:559–571. doi: 10.1104/pp.107.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu WH. Potassium transport and signaling in higher plants. Annual Review of Plant Biology. 2013;64:451–476. doi: 10.1146/annurev-arplant-050312-120153. [DOI] [PubMed] [Google Scholar]
- Weeks JT, Ye JS, Rommens CM. Development of an in planta method for transformation of alfalfa (Medicago sativa) Transgenic Research. 2008;17:587–597. doi: 10.1007/s11248-007-9132-9. [DOI] [PubMed] [Google Scholar]
- Wegner LH, De Boer AH. Properties of two outward-rectifying channels in root xylem parenchyma cells suggest a role in K+ homeostasis and long-distance signaling. Plant Physiology. 1997;115:1707–1719. doi: 10.1104/pp.115.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner LH, Raschke K. Ion channels in the xylem parenchyma of barley roots: a procedure to isolate protoplasts from this tissue and a patch-clamp exploration of salt passageways into xylem vessels. Plant Physiology. 1994;105:799–813. doi: 10.1104/pp.105.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner LH, Stefano G, Shabala L, Rossi M, Mancuso S, Shabala S. Sequential depolarization of root cortical and stelar cells induced by an acute salt shock – implications for Na+ and K+ transport into xylem vessels. Plant, Cell and Environment. 2011;34:859–869. doi: 10.1111/j.1365-3040.2011.02291.x. [DOI] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, et al. Construct design for efficient, effective and high-throughput gene silencing in plants. The Plant Journal. 2001;27:581–590. doi: 10.1046/j.1365-313x.2001.01105.x. [DOI] [PubMed] [Google Scholar]
- Wu GQ, Xi JJ, Wang Q, et al. The ZxNHX gene encoding tonoplast Na+/H+ antiporter from the xerophyte Zygophyllum xanthoxylum plays important roles in response to salt and drought. Journal of Plant Physiology. 2011;168:758–767. doi: 10.1016/j.jplph.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, et al. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell. 2006;125:1347–1360. doi: 10.1016/j.cell.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Blumwald E. Developing salt-tolerant crop plants: challenges and opportunities. Trends in Plant Science. 2005;10:615–620. doi: 10.1016/j.tplants.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Yue LJ, Li SX, Ma Q, et al. NaCl stimulates growth and alleviates water stress in the xerophyte Zygophyllum xanthoxylum. Journal of Arid Environments. 2012;87:153–160. [Google Scholar]
- Zhang JL, Flowers TJ, Wang SM. Mechanisms of sodium uptake by roots of higher plants. Plant and Soil. 2010;326:45–60. [Google Scholar]
- Zhang JL, Flowers TJ, Wang SM. Differentiation of low-affinity Na+ uptake pathways and kinetics of the effects of K+ on Na+ uptake in the halophyte Suaeda maritima. Plant and Soil. 2013;368:629–640. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.