Abstract
Background and Aims
Eutrema parvulum (synonym, Thellungiella parvula) is an extreme halophyte that thrives in high salt concentrations (100–150 mm) and is closely related to Arabidopsis thaliana. The main aim of this study was to determine how E. parvulum uses reactive oxygen species (ROS) production, antioxidant systems and redox regulation of the electron transport system in chloroplasts to tolerate salinity.
Methods
Plants of E. parvulum were grown for 30 d and then treated with either 50, 200 or 300 mm NaCl. Physiological parameters including growth and water relationships were measured. Activities of antioxidant enzymes were determined in whole leaves and chloroplasts. In addition, expressions of chloroplastic redox components such as ferrodoxin thioredoxin reductases (FTR), NADPH thioredoxin reductases (NTRC), thioredoxins (TRXs) and peroxiredoxins (PRXs), as well as genes encoding enzymes of the water–water cycle and proline biosynthesis were measured.
Key Results
Salt treatment affected water relationships negatively and the accumulation of proline was increased by salinity. E. parvulum was able to tolerate 300 mm NaCl over long periods, as evidenced by H2O2 content and lipid peroxidation. While Ca2+ and K+ concentrations were decreased by salinity, Na+ and Cl– concentrations increased. Efficient induction of activities and expressions of water–water cycle enzymes might prevent accumulation of excess ROS in chloroplasts and therefore protect the photosynthetic machinery in E. parvulum. The redox homeostasis in chloroplasts might be achieved by efficient induction of expressions of redox regulatory enzymes such as FTR, NTRC, TRXs and PRXs under salinity.
Conclusions
E. parvulum was able to adapt to osmotic stress by an efficient osmotic adjustment mechanism involving proline and was able to regulate its ion homeostasis. In addition, efficient induction of water–water cycle enzymes and other redox regulatory components such as TRXs and PRXs in chloroplasts were able to protect the chloroplasts from salinity-induced oxidative stress.
Keywords: Alternative electron sink, antioxidant enzymes, chloroplastic redox, Eutrema parvulum, halophyte, oxidative stress, peroxiredoxin, plastid terminal oxidase, proline, salinity, Thellungiella parvula, thioredoxin, water–water cycle
INTRODUCTION
Soil salinity is one of the most prevalent factors that can limit plant growth and yield. It is estimated that salinity affects nearly 20 % of cultivated lands (FAO, 2000). Salinity shows its toxic effects in two different ways. First, it decreases the available water in the soil by decreasing the osmotic potential, therefore making it more difficult for a plant to extract water, causing osmotic stress. Second, excess accumulation of ions can cause damage to cells through toxic effects and cause disruption of the nutrient balance in a plant by affecting uptake of other important elements (Munns and Tester, 2008). In both cases, production of reactive oxygen species (ROS), which are by-products of normal metabolism, is increased due to loss of coordination between different metabolic pathways (Asada, 2006; Noctor et al., 2007). Extreme production of these ROS under salinity can cause damage to molecules such as DNA, proteins and lipids (Apel and Hirt, 2004), which can interrupt normal cellular function, and this chain of events can ultimately lead to cell death (Van Breusegem and Dat, 2006). To cope with excess accumulation of these molecules, plants have evolved a complex antioxidant defence system containing both enzymatic and non-enzymatic components. Enzymatic antioxidants such as superoxide dismutase (SOD), peroxidases (POXs) and ascorbate peroxidases (APXs) are responsible for efficient scavenging of O2.– and H2O2 in cellular compartments. By contrast, ascorbate and β-carotene, which are non-enzymatic antioxidants, act as the main scavengers of 1O2 and OH. (Mittler, 2002; Miller et al., 2010).
Chloroplasts are the main site of ROS production in plant cells and also one of the most susceptible compartments to salinity due to limitation of gas exchange, redox imbalance and overload of the electron transport system (Asada, 2006). Accordingly, plants have evolved a complex network of enzymes in chloroplasts to regulate their redox state and oxidative status. One of the most important defensive mechanisms in chloroplasts is the water–water cycle (Asada, 2006). This cycle involves an array of enzymes [SOD, APX, monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) and glutathione reductase (GR)] that work in coordination to scavenge ROS produced by the Mehler reaction and relax the electron load of the photosystems (Foyer and Noctor, 2009). Besides this, there are other shunts that can divert electrons from photosystems, such as plastid terminal oxidases (PTOXs) and ferrodoxin thioredoxin reductase (FTR) (Foyer and Shigeoka, 2011; Laureau et al., 2013). PTOX transfers electrons from plastoquinone to molecular oxygen to produce water, while FTR transfers electrons to thioredoxin (Trx). Trxs could regulate the activity of other target enzymes such as rubisco activase, sedoheptulose-bisphosphatase (SBPase) and glucose-6-phosphate dehydrogenase (G6PDH) by disulfide bond exchange or reduce peroxiredoxins (Prxs), which are proteins that are used in chloroplasts to counteract oxidative stress (Dietz et al., 2006; Lemaire et al., 2007).
Most of our knowledge of these mechanisms is derived from a glycophytic model plant, arabidopsis. However, arabidopsis may not be suitable to study salinity tolerance mechanisms, as it has not evolved to tolerate high concentrations of salt (Orsini et al., 2010; Ozgur et al., 2013). This situation has raised the idea of investigating halophytic species that are closely related to arabidopsis [arabidopsis-related model species (ARMS)] for this kind of research (Amtmann et al., 2005). The genus Eutrema (synonym, Thellungiella) contains two ARMS, E. halophilum (syn. Thellungiella halophila) and E. parvulum (syn. Thellungiella parvula), both of which have been used for salinity tolerance studies (Amtmann, 2009). Genomes of these two species were sequenced and are publicly available (Oh et al., 2010; Dassanayake et al., 2011; Wu et al., 2012). By using this approach, various processes have been investigated in E. halophilum growing in saline conditions, such as photosynthesis (M'rah et al., 2006; Stepien and Johnson, 2009), proline accumulation and metabolism (M'rah et al., 2007; Ghars et al., 2008, 2012; Radyukina et al., 2011), ion accumulation (M'rah et al., 2006, 2007), K+/Na+ discrimination and their transporters (Volkov et al., 2004; Vera-Estrella et al., 2005; Ghars et al., 2008; Ali et al., 2012), gene expression (Kant et al., 2006), hormonal changes (Arbona et al., 2010), metabolic responses (Arbona et al., 2010; Pedras and Zheng, 2010), phospholipid signalling (Ghars et al., 2012), lipid profiling (Zhang et al., 2013) and transcript structure (Oh et al., 2010) at the whole plant level. Although an array of processes have been investigated in E. halophilum, studies with E. parvulum are very limited, even though E. parvulum can withstand up to 600 mm NaCl (Orsini et al., 2010) and is as valuable a genetic source as E. halophilum for elucidation of salinity tolerance mechanisms. As there are many gaps in knowledge about antioxidant defence and ROS regulation in halophytic plants, especially at the cellular compartment level (see Ozgur et al., 2013), we present a comprehensive picture of chloroplastic redox regulatory mechanisms for the first time in a model halophyte, E. parvulum.
In this study we investigated ROS regulation and antioxidant defence in chloroplasts and shoots of ARMS E. parvulum by combining biochemical and molecular methods. Growth, water relations, ion accumulation, proline content and expression of genes related to its biosynthesis [P5CS (P5C synthase), P5CR (P5C reductase), PRODH (proline dehydrogenase), P5CDH (P5C dehydrogenase)], H2O2 production, and activities and isoenzymes of some antioxidant defence system enzymes, especially water–water cycle enzymes (SOD, APX, MDHAR, DHAR, GR) were investigated. Also, expression of genes encoding water–water cycle enzymes in chloroplasts such as FSD1 (FeSOD1), FSD2 (FeSOD2), FSD3 (FeSOD3), CSD1 (Cu/ZnSOD1), sAPX (stromal APX), tAPX (thylakoidal APX), MDHAR, DHAR and GR was determined. Moreover, the expression of chloroplastic redox regulatory enzymes such as FED (gene encoding ferrodixin), FTR (ferrodoxin thioredoxin reductase), NTRC (NADPH thioredoxin reductase C), TRX-m, TRX-x, PRXq, PRX2e (Prx IIE), 2CPA (2-Cys Prx A) and 2CPB (2-Cys Prx B) of the extreme halophyte E. parvulum under long-term salinity were also determined to understand the redox regulation in chloroplasts as one of the adaptive mechanisms to high salinity.
MATERIALS AND METHODS
Plant growth and stress treatments
Seeds of Eutrema parvulum [(Schrenk) Al-Shehbaz & Warwick; Brassicaceae] [synonym Thellungiella parvula (Schrenk) Al-Shehbaz & O'Kane] were collected from salt flats in Tuz (Salt) Lake (Central Anatolia, Turkey). Plants raised from these seeds were used to provide further seeds, which were used in the experiments. Seeds were surface sterilized with 70 % ethanol for 30 s and 4 % bleach for 10 min and were washed five times with sterile water. Seeds were sown on a 7 : 2 : 1 (peat moss/vermiculite/Perlite) soil mix and were stratified for 2 d at 4 °C in the dark in a plant growth chamber (JSPC-420, JSR) to synchronize germination. Following stratification plants were grown in the same chamber at 22/20 °C (day/night) with a photoperiod of 12 h light/12 h dark and a relative humidity of 60 %. After germination, during the growth period plants were sub-irrigated with half-strength Hoagland solution every other day. After 30 d, plants were treated with incremental doses (50 mm d–1) of NaCl in the Hoagland's solution every other day, to avoid shock and allow adaptation, producing final concentrations for the experimental groups of 0, 50, 200 and 300 mm NaCl. Plants were treated with NaCl for an additional 2 weeks after the maximum concentration (300 mm NaCl) was reached before harvesting.
Growth measurements
Plants were harvested (n = 6) and shoots and roots were separated. Shoot fresh weights (f. wt) were determined and then samples were then dried at 72 °C for 2 d and were weighed again to determine their dry weights (d. wt).
Leaf osmotic potential
Leaf osmotic potential was measured using a Vapro Vapor pressure Osmometer 5520. Leaf samples were collected from at least six different plants.
Relative water content (RWC)
Whole plants and leaves (n = 6 for each) were obtained from each treatment group and f. wt was determined. The shoots and leaves were floated on deionized water for 6 h under low irradiance and then the turgid tissue was quickly blotted to remove excess water and their turgid weights (TW) were determined. d. wt was measured after the leaves were dried in the oven. RWC was calculated using the following formula:
Leaf water loss
For determination of leaf water loss as an indicator of stomatal conductance, leaves were detached from the plants and their weights were immediately measured. After 10 min in the growth chamber, weights of the leaves were measured again and this procedure was repeated again at 20 min. Leaf water loss was calculated from these values as a percentage of the original weight of the leaves. Six leaf replicates were used for this analysis from different plants in each treatment group.
Determination of ion concentrations
Samples (0·25 g d. wt) were ground to fine powder, which was digested with concentrated nitric acid (HNO3) in a microwave system (CEM, Mars 5). The Na+, K+ and Ca2+ in extracts were analysed by inductively coupled plasma atomic emission spectroscopy (ICP-AES; Varian-Vista) (Nyomora et al., 1997). Cl− contents were determined by the AgNO3 titration method as described by Johnson and Ulrich (1959).
Chloroplast isolation
Leaves (5 g) were harvested within 2–4 h of the start of the photoperiod and homogenized in a chloroplast isolation buffer [CIB, 0·1 m Tris–HCl (pH 7·8), 0·3 m sorbitol, 5 mm MgCl2, 10 mm NaCl, 0·1 % bovine serum albumin (BSA)]. The slurry was filtered through four layers of cheesecloth and the filtrate was centrifuged at 1000 g for 6 min at 4 °C. The supernatant was discarded and the pellet was resuspended in CIB. Resuspended chloroplasts were overlaid on a 40 % Percoll solution and centrifuged at 1700g for 7 min at 4 °C. Intact chloroplasts were obtained after this centrifugation as a pellet which was resuspended again in CIB without BSA. Intactness of the chloroplasts was determined by using a ferricyanide reduction test (Lilley et al., 1975). For analysis of enzyme activity and for the native activity gels, chloroplasts were lysed with a lysis solution (10 mm HEPES-KOH (pH 7·2), 0·1 mm EDTA, 1 mm MgCl2, 0·1 % Triton-X 100) for 1 h at 4 °C. Where APX was estimated, 5 mm ascorbate was added into CIB and lysis buffers.
Enzyme extractions and assays
Enzyme extractions were performed at 4 °C. Samples (0·1 g) were ground to a fine powder in liquid nitrogen and then homogenized in 500 μL of 50 mm Tris-HCl, pH 7·8, containing 0·1 mm EDTA, 0·1 % (w/v) Triton-X100, 1 mm phenylmethanesulfonyl fluoride (PMSF) and polyvinylpyrrolidone (PVP; 1 %, w/v). For APX activity determination, 5 mm ascorbate was added to the homogenization buffer. Samples were centrifuged at 10 000 g for 10 min, and supernatants were used for the determination of protein content and enzyme activities. Total soluble protein contents of the enzyme extracts were determined according to Bradford (1976) using BSA as a standard. All spectrophotometric analyses were conducted on a Shimadzu UV 1700 spectrophotometer.
SOD (EC 1·15·1·1) activity was assayed by its ability to inhibit photochemical reduction of nitro blue tetrazolium (NBT) at 560 nm (Beauchamp and Fridovich, 1971). One unit of SOD was defined as the amount of enzyme that inhibits 50 % NBT photoreduction. APX (EC 1·11·1·11) activity was measured according to Nakano and Asada (1981). The assay depends on the decrease in absorbance at 290 nm as ascorbate is oxidized. The concentration of oxidized ascorbate was calculated using an extinction coefficient of 2·8 mm−1 cm−1. One unit of APX was defined as 1 μmol ascorbate oxidized min−1. MDHAR (EC 1·6·5·4) activity was determined according to Arrigoni et al. (1981) and DHAR (EC 1·8·5·1) activity was determined according to Nakano and Asada (1981). GR (EC 1·6·4·2) activity was measured according to Foyer and Halliwell (1976). NADPH oxidation was followed at 340 nm. Activity was calculated using the extinction coefficient of NADPH (6·2 mm−1 cm−1). One unit of GR was defined as 1 μmol GSSG reduced min−1.
Identification of isoenzymes
Samples containing equal amounts of protein were subjected to native polyacrylamide gel electrophoresis as described by Laemmli (1970).
For the separation of SOD isoenzymes 4·5 % stacking and 12·5 % separating gels were used. SOD activity was detected as described by Beauchamp and Fridovich (1973). POX isoforms were detected according to Seevers et al. (1971). The electrophoretic separation was performed on non-denaturating polyacrylamide mini gels using 10 % separating gel under constant current. The gels were loaded with 50 μg protein and incubated for 30 min at 25 °C in 200 mm Na-acetate buffer (pH 5·0) containing 1·3 mm benzidine and 3 % hydrogen peroxide. GR isoforms were detected using 7·5 % separating gels according to Hou et al. (2004). GR isoforms were detected by incubating the gels in a solution containing 10 mm Tris-HCl (pH 7·9), 4 mm GSSG, 1·5 mm NADPH and 2 mm DTNB for 20 min. After a brief rinse with 50 mm Tris-HCl buffer (pH 7·9), GR activity was negatively stained by 1·2 mm MTT and 1·6 mm PMS for 5–10 min at room temperature. Gels were photographed with a Vilber Lourmat gel imaging system and then analysed with BioCapt software (Vilber Lourmat, Marne la Vallée, France).
Determination of H2O2 content
H2O2 was determined according to Cheeseman (2006) using eFOX reagent. This modified ferrous ammonium sulphate/xylenol orange (FOX) assay was used due to its sensitivity, stability and adaptability to a large number of samples. In this assay 1 % ethanol is added to the reagent, which increases its sensitivity to H2O2 by 50 % (i.e. eFOX). Extraction was carried out using ice-cold acetone containing 25 mm H2SO4 for both leaf samples and intact chloroplasts. Samples were then centrifuged for 5 min at 3000 g at 4 °C. eFOX reagent [950 μL of 250 μm ferrous ammonium sulphate, 100 μm xylenol orange, 100 μm sorbitol, 1 % ethanol (v/v)] was used for 50 μL of supernatant. Reaction mixtures were incubated at room temperature for 30 min and then absorbance at 550 and 800 nm was measured. H2O2 concentrations were calculated using a standard curve prepared with known concentrations of H2O2.
Lipid peroxidation
The level of lipid peroxidation in samples was determined in terms of thiobarbituric acid reactive substances (TBARS) according to the method of Madhava Rao and Sresty (2000) from leaf samples and intact chloroplasts. Lipid peroxidation in chloroplasts was normalized using chlorophyll content.
Determination of proline content
Ground leaf samples (0·1 g) were extracted according to Raymond and Smirnoff (2002) with 80 % ethanol for 24 h at 4 °C and extracts were assayed for proline using the acid-ninhydrin method (Bates et al., 1973). Proline contents were calculated using a standard curve prepared with known concentrations of proline.
Quantitative RT-PCR (qRT-PCR)
RNA was isolated from 0·1 g fresh tissue using a Qiagen RNeasy kit according to the manufacturer's instructions. Total RNA was treated with DNase I (Fermentas, Burlington, ON, Canada) to remove residual genomic DNA. Then, reverse transcription was performed (1 μg total RNA for each treatment group) using M-MuLV reverse transcriptase (New England Biolabs, Ipswich, MA, USA). These cDNAs were used as templates for qRT-PCR. The amount of RNA in each reaction was normalized to the E. parvulum Actin7 gene. The conditions for PCR amplification were as follows: 95 °C for 5 min, and 40 cycles at 94 °C for 15 s, 58 °C for 15 s and 72 °C for 30 s. Maxima SYBR Green qPCR Master Mix was used (Thermo Scientific, Waltham, MA, USA) to perform the qRT-PCR. Three independent experiments were performed for qRT-PCR assays with the Bio-Rad iQ5 Real-Time PCR system. qRT-PCR data analyses were performed with Bio-Rad iQ5 software using Pfaffl's model (Pfaffl, 2001). E. parvulum plants grown in the absence of NaCl were used as a reference point and relative expression levels were calculated with respect to this reference value (set to 1) for genes that were studied.
For expression analysis of E. parvulum genes of interest, a BLAST search was done against E. parvulum predicted gene models, CDS version 2.0, using known genes of arabidopsis that play roles in chloroplastic redox regulation. BLAST search results with e value lower than 10–100 were used for further analysis. Protein coding sequences of these genes were analysed using TargetP 1·1 to confirm that they included a chloroplastic signal peptide. Primers and accessions for arabidopsis and Eutrema homologues of the genes can be found in Supplementary Data Table S1. The primers were synthesized by Sentromer DNA Technologies (Istanbul, Turkey).
Statistical analysis
The experiments were carried out twice, and each data point is the mean of three replicates (n = 6). The results were expressed as mean and error bars were used for showing standard error of the mean (±s.e.m.). Groups were compared using Student's t-test.
RESULTS
Growth parameters under salinity
To understand the effects of salinity on the growth of E. parvulum, shoot length, fresh weight and dry weight were determined in 0, 50, 200 and 300 mm NaCl. NaCl at 300 mm decreased the shoot length by 33 % as compared with non-saline conditions. The shoot fresh and dry weights were unaffected by 50 mm NaCl, but 300 mm NaCl decreased them by 39 and 28 %, respectively, as compared with non-saline conditions (Fig. 1).
Fig. 1.
Shoot lengths (A), shoot fresh weights (B) and shoot dry weights (C) of E. parvulum plants treated with 0, 50, 200 and 300 mm NaCl. *Significant difference from 0 mm NaCl at P < 0·05.
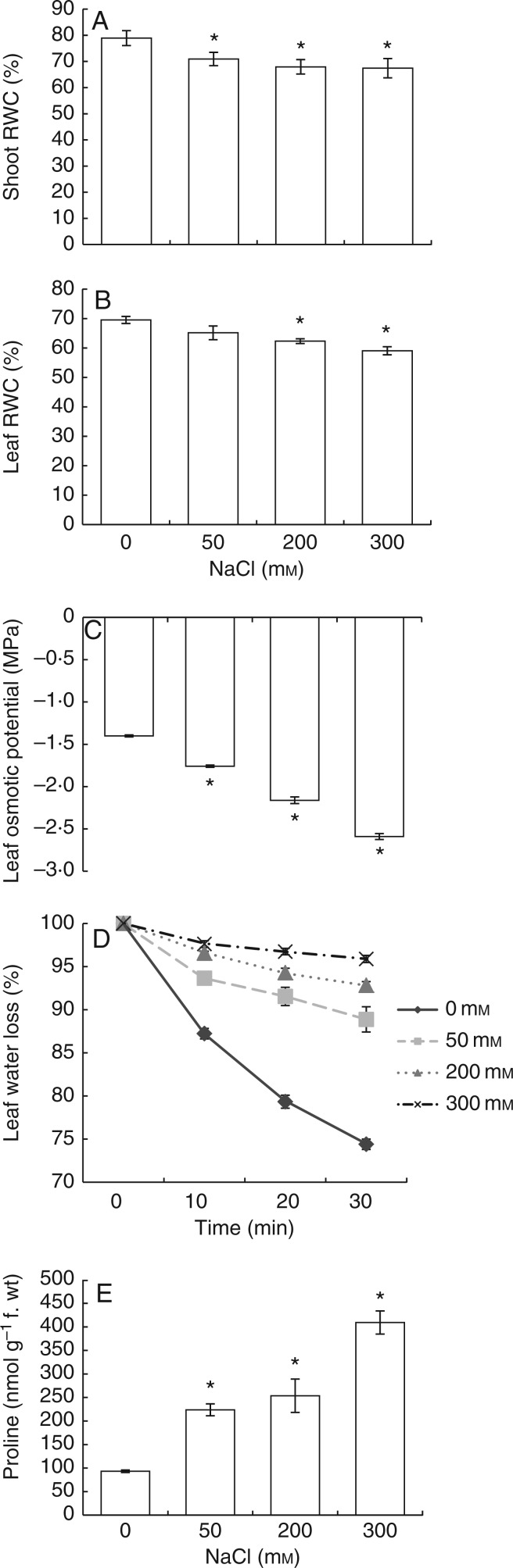
Determination of the water status in E. parvulum as evaluated by osmotic potential, RWC, leaf water loss and proline content
RWC was determined in both shoots and leaves. In shoots, 50 mm NaCl decreased RWC by 8 % whereas 200 and 300 mm NaCl decreased it by 11 % as compared with non-saline conditions (Fig. 2A). The RWC of leaves was also decreased under 50, 200 and 300 mm NaCl by 5, 8 and 11 %, respectively (Fig. 2B). The highest decrease in osmotic potential (by 78 % as compared with non-saline conditions) was measured in 300 mm NaCl; the decrease was only 25 % in 50 mm NaCl (Fig. 2C).
Fig. 2.
Shoot relative water contents (A), leaf relative water contents (B), leaf osmotic potentials (C), leaf water loss (D) and proline concentrations of E. parvulum plants treated with 0, 50, 200 and 300 mm NaCl. *Significant difference from 0 mm NaCl at P < 0·05.
Stomatal closure is a rapid response to salinity to inhibit water loss (Chaves et al., 2011). In our study, leaf water loss was measured to estimate the stomatal closure under salinity. Treatment with 300 mm NaCl caused the lowest water loss (5 % in 300 mm and to 16 % in 0 mm), as can be seen in Fig. 2D.
Salt treatments enhanced the proline concentration (Fig. 2E), with 50 and 200 mm NaCl increasing the values 2·4- and 2·7-fold, respectively, as compared with plants grown in the absence of salt: 300 mm NaCl increased the proline concentration 4·3-fold.
Expression levels of genes involved in proline biosynthesis and degradation
To understand fully the accumulation of proline, expressions of genes that take part in proline biosynthesis and degradation were investigated. As shown in Fig. 3, expressions of P5CS1 and P5CR were markedly increased under 300 mm salinity. P5CS2 showed a slight increase in 200 mm NaCl. Expression of genes that are responsible for degradation of proline, PRODH1 and PRODH2, were decreased. In contrast, expression of the gene responsible for degradation of P5CS, P5CDH, increased in 200 and 300 mm salt.
Fig. 3.
qRT-PCR analysis of expressions of P5CS1 (A, gene encoding P5C synthase 1), P5CS2 (B, P5C synthase 2), P5CR (C, P5C reductase), PRODH1 (D, proline dehydrogenase 1), PRODH2 (E, proline dehydrogenase 2) and P5CDH (F, P5C dehydrogenase) of E. parvulum plants treated with 0, 50, 200 and 300 mm NaCl. (G) A scheme summarizing the functions of genes related to proline biosynthesis.
Ion contents of E. parvulum under 0, 50, 200 and 300 mm NaCl
Figure 4 illustrates the changes in Na+, Cl–, K+ and Ca2+ concentrations for plants grown at different salinities. NaCl at 50 mm enhanced Na+ and Cl– concentrations (48- and 10-fold, respectively) as compared with non-saline conditions) due to the long exposure to salinity. Ca2+ and K+ ion concentrations gradually decreased as the salinity rose from 0 to 300 mm NaCl.
Fig. 4.
Na+, Cl–, K+ and Ca2+ concentrations (A–D) of E. parvulum plants treated with 0, 50, 200 and 300 mm NaCl. *Significant difference from 0 mm NaCl at P < 0·05.
Ratios of ions [K+/Na+, Na+/Cl–, (Na++K+)/Cl–] and net selectivity of K+ over Na+ (SK/Na) were calculated and given in Table 1. Salt treatments decreased the K+/Na+, (Na++K+)/Cl– and SK/Na. There was an increase in Na+/Cl+ in the 50-mm NaCl group as compared with the 0 mm groups, which was caused by an absence of Na+ in Hoagland solution. This ratio dropped from 1·12 to 0·9 with increasing salinity.
Table 1.
K+/Na+, Na+/Cl– and (Na++K+)/Cl– ratios and SK/Na (net K/Na selectivity) of E. parvulum plants treated with 0, 50, 200 and 300 mm NaCl; SK/Na was calculated as the ratio of K+/Na+ in plants divided by K+/Na+ in the medium
| NaCl (mm) | K+/Na+ | Na+/Cl– | (Na++K+)/Cl– | SK/Na |
|---|---|---|---|---|
| 0 | 69·77 | 0·23 | 16·39 | 70·77 |
| 50 | 1·22 | 1·12 | 2·50 | 2·22 |
| 200 | 0·65 | 1·15 | 1·90 | 1·65 |
| 300 | 0·56 | 0·90 | 1·41 | 1·56 |
Effects of stress on photosynthetic machinery
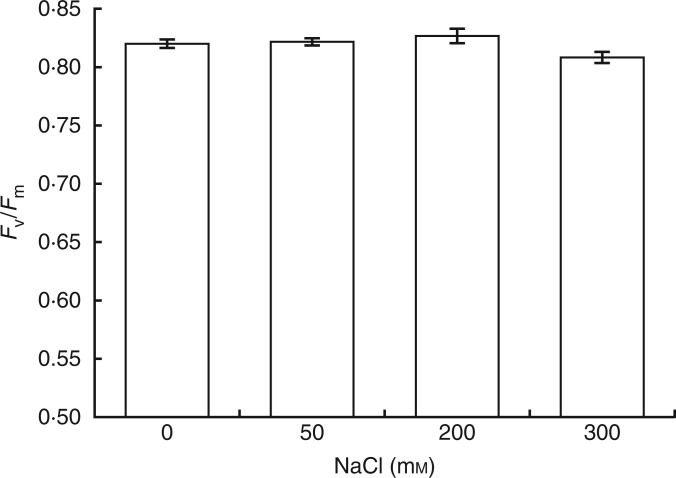
The alteration of the maximum quantum yield of PSII (Fv/Fm) is a good indicator of the state of the photosynthetic machinery, which could be damaged by excess ROS in chloroplasts under salinity. In this study, Fv/Fm ratios were not changed by 50, 200 or 300 mm NaCl (Fig. 5).
Fig. 5.
Maximum quantum yield of PSII (Fv/Fm) of E. parvulum plants treated with 0, 50, 200 and 300 mm NaCl.
Activities of water–water cycle enzymes in chloroplast and shoots
Environmental stress conditions such as salinity cause an over-production of ROS in chloroplasts and disturb photosynthesis. The water–water cycle enzymes (SOD, APX, MDHAR, DHAR and GR) are responsible for scavenging these ROS, especially O2.–. In chloroplasts and in whole cells, SOD activities were increased by salinity; in 300 mm NaCl activity increased by 1·5- and 1·7-fold, while it increased by 1·5- and 1·3-fold in 200 mm as compared with non-saline conditions, respectively (Fig. 6A). Three SOD isoenzymes were detected in chloroplasts, while there were six isoenzymes in whole leaves. Activities of all isoenzymes were increased with increasing salinity, except for SOD2 in chloroplasts isolated from 300 mm NaCl plants.
Fig. 6.
Activities of SOD (A), APX (B), MDHAR (C), DHAR (D), GR (E) and POX (F) enzymes in chloroplasts and whole leaf cells of E. parvulum plants treated with 0, 50, 200 and 300 mm NaCl. *Significant difference from 0 mm NaCl at P < 0·05, chloroplasts and whole leaves compared. Isoenzyme patterns of SOD (G), GR (H) and POX (I) in chloroplasts and whole leaves.
The chloroplastic APX activities gradually increased under salt treatments. The highest activity was observed in 300 mm NaCl, up 2·2-fold higher as compared with chloroplast isolated from plants grown without NaCl. In whole cells, all three salt concentrations increased the activity of APX (Fig. 6B).
In chloroplasts, the activity of MDHAR was increaased by salinity. The activities of MDHAR in chloroplasts from plants grown in 50 and 200 mm NaCl were enhanced by 37 and 120 %, respectively; 300 mm NaCl enhanced MDHAR activity 5·5-fold as compared with chloroplasts of plants grown without salt. In whole cells, the activities of MDHAR were increased in 50, 200 and 300 mm NaCl by 12, 24 and 18 %, as compared with non-saline conditions, respectively (Fig. 6C).
DHAR activity also increased in chloroplasts with increasing salinity; the highest increase was found in 300 mm NaCl – 7-fold as compared with non-saline conditions. DHAR activities also increased in whole cells: 50, 200 and 300 mm NaCl treatments increased activity by 38, 36 and 26 % as compared with non-saline plants (Fig. 6D)
The activities of GR, which is one of the most important components of the ascorbate–GSH cycle, were raised in chloroplasts 2-fold in 200 and 300 mm NaCl treatments as compared with non-saline conditions. In whole cells, the activity of GR was also increased by salinity. Two GR isoenzymes were found in whole cells but only one of them (GR2) was also detected in chloroplasts. The activity of GR1 was increased by salinity in whole cells. Similar to this, activity of GR2 was increased in chloroplasts of E. parvulum with increasing salinity (Fig. 6H).
The activity of H2O2-scavenging enzyme POX
In chloroplasts from plants grown in 50 mm NaCl, the activity of POX1 was decreased by 24 % as compared with chloroplasts from plants in non-saline conditions. In whole cells, gradual enhancement of salinity increased the POX activities so that the highest POX activity was detected under 300 mm NaCl, increased 1·4-fold as compared with non-saline conditions. Two POX isoenzymes (POX1 and POX2) were determined in this study but only POX1 was detected in chloroplasts (Fig. 6F).
Expressions of genes encoding water–water cycle enzymes in chloroplasts
To see how water–water cycle enzymes are regulated at the transcriptional level, expression of some of the genes encoding these enzymes was measured. As shown in Fig. 7, expressions of genes encoding chloroplastic APX, MDHAR, DHAR and GR were increased by salinity. In addition, expressions of three FSD genes and one CSD gene were also determined. Expressions of FSD3 and CSD were induced by salinity, while expression of FSD2 remained unchanged. In contrast, expression of FSD1 was decreased under salt stress.
Fig. 7.
qRT-PCR analysis of expressions of FSD1 (A, gene encoding FeSOD1), FSD2 (B, FeSOD2), FSD3 (C, FeSOD3), CSD (D, Cu/ZnSOD), sAPX (E, stromal APX), tAPX (F, thylakoidal APX), MDHAR (G), DHAR (H) and GR (I) of E. parvulum plants treated with 0, 50, 200 and 300 mm NaCl.
Estimation of lipid peroxidation and H2O2 content in E. parvulum chloroplasts and shoots
Lipid peroxidation, an indicator of oxidative cell damage, was only enhanced in chloroplasts in 300 mm NaCl (1·8-fold), while 50 and 200 mm NaCl had no effect: no significant change was observed at the cell level (Fig. 8A, B).
Fig. 8.
TBARS (A, chloroplasts; B, shoots) and H2O2 (C, chloroplasts; D, shoots) concentrations of E. parvulum plants treated with 0, 50, 200 and 300 mm NaCl. *Significant difference from 0 mm NaCl at P < 0·05.
In chloroplasts, only 300 mm NaCl enhanced the H2O2 concentration (by 1·9-fold) as compared with non-saline conditions. The H2O2 concentration of whole cells did not change significantly (Fig. 8C, D).
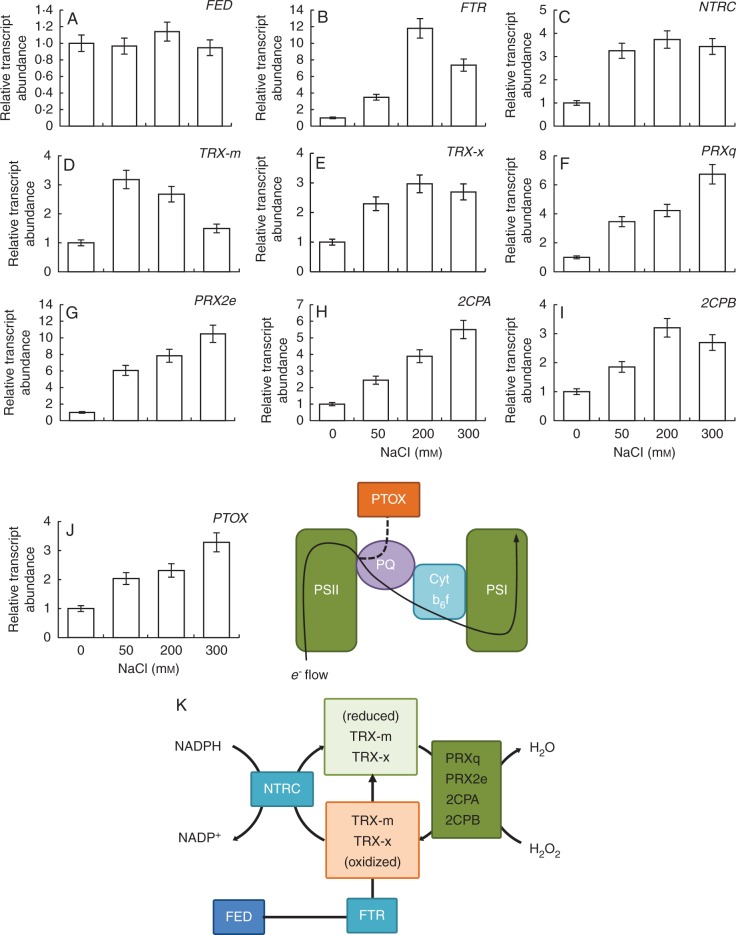
Expression levels of components that regulate redox in chloroplasts.
To evaluate the regulation of redox in chloroplasts, we determined the expression of some of the key components such as FED, FTR, NTRC, TRX-m, TRX-x, PRXq, PRX2e, 2CPA and 2CPB (Fig. 9A–I). Salinity induced the expressions of all these key genes except FED (Fig. 9A). There was no difference in the expression levels of FED by treatments. By contrast, FTR expression reached its highest level in 200 mm NaCl. The relative expression level of NTRC was induced by all treatments as compared with non-saline conditions. In the case of TRX, the expressions of two TRX-related genes (TRX-m and TRX-x) were determined. While 50 mm NaCl provoked the highest expression of TRX-m, TRX-x expressions showed the highest level under 200 mm NaCl.
Fig. 9.
qRT-PCR analysis of expressions of FED (A, gene encoding ferredoxin), FTR (B, ferredoxin thioredoxin reductase), NTRC (C, NADPH thioredoxin reductase C), TRXm (D, thioredoxin m), TRXx (E, thioredoxin x), PRXq (F, peroxiredoxin Q), PRX2e (G, peroxiredoxin IIE), 2CPA (H, 2-cys peroxiredoxin A), 2CPB (I, 2-cys peroxiredoxin B) and PTOX (J, plastid terminal oxidase) of E. parvulum plants treated with 0, 50, 200 and 300 mm NaCl. (K) Scheme summarizing the functions and relationships between the genes investigated in this study.
The expressions of four PRX-related genes, namely PRXq, PRX2e, 2CPA and 2CPB, were increased with an increase in level of salt. A figure summarizing the functions and the relationships between the genes mentioned above is provided in Fig. 9K.
The expression of terminal oxidase (PTOX), which is responsible for electron transfer to molecular oxygen in chloroplasts, similar to the alternative oxidase in mitochondria, was also increased by salinity in E. parvulum (Fig. 9J).
DISCUSSION
The main aim of this study was to reveal how an extreme halophyte, E. parvulum, reacts to salinity by way of ROS production, changes within its antioxidant system and redox regulation of electron transport in its chloroplasts. It is well known that photosynthesis is one of the metabolic events most affected by excess ROS produced under environmental stresses. Any decrease in Fv/Fm is a good indicator of damage to the photosynthetic machinery. Previous studies showed that creating oxidative stress in chloroplasts (by using methyl viologen) can significantly decrease Fv/Fm if there is no adequate antioxidant defence mechanism (Lee et al., 2007; Lim et al., 2007; Kim et al., 2010). In the current study, E. parvulum did not show any apparent damage to its photosynthetic machinery, as evidenced by an unchanged Fv/Fm under high salinity (300 mm NaCl). Therefore, it is important to elucidate that how this halophyte could resist and protect its photosynthetic machinery under such severe environmental conditions. By using biochemical and molecular analysis we sought answers to these questions.
Severe salinity decreased shoot lengths, fresh weights and dry weights in E. parvulum
Although halophytes complete their life cycle under high concentrations of salt where most plant species cannot survive (Flowers and Colmer, 2008) a model glycophyte, Arabidopsis thaliana, cannot tolerate even 50 mm NaCl during its life span. However, a close relative of arabidopsis, E. parvulum, can resist up to 600 mm NaCl (Orsini et al., 2010), although its growth was severely inhibited by 200 mm NaCl (M'rah et al., 2006). In a similar way, Ghars et al. (2008) and Kant et al. (2006) also indicated growth inhibition under 300 and 500 mm NaCl in E. halophilum. In our study, salinity decreased the shoot lengths of E. parvulum, although the fresh and dry weights were unchanged in 50 mm NaCl as compared with non-saline conditions. However, 200 and 300 mm NaCl treatments did reduce these weights. From these results, we conclude that 50 mm NaCl could be counted as a ‘non-stress’ condition for E. parvulum while higher salt concentrations become stressful.
Gradually increased salt treatment affected water relations of E. parvulum negatively and the accumulation of osmolyte proline was increased by salinity
Growth reduction due to long-term exposure to salinity can also be evaluated in terms of its osmotic and ionic effects (Munns and Tester, 2008). The first decrease in RWC was observed in plants treated with 50 mm NaCl. However, at this external salt concentration, growth was unaffected and the plants adjusted osmotically by lowering the osmotic potential of their leaves. As an early response to salinity, plants reduce their stomatal aperture to prevent loss of water from leaves (Chaves et al., 2003). In E. parvulum, we used leaf water loss as an indicator of stomatal conductance and observed reduced water loss starting with 50 mm NaCl treatment. However, this closure in 50 mm NaCl did not affect the growth rate of plants, as evident from shoot d. wt. As a further response, to compensate for the difference in water potential between soil and the plant, plants accumulate compatible solutes and ions in their leaves. In many halophytes such as Centaurea tuzgoluensis, Nitraria tangutorum and Cakile maritima proline accumulation was enhanced by increased salinity treatments (Megdiche et al., 2007; Yıldıztugay et al., 2011; Yang et al., 2013). Accordingly, E. halophilum previously received attention from many researchers due to its ability to accumulate large amounts of proline as a response to salinity and therefore proline metabolism was widely studied in this species (Kant et al., 2006; M'rah et al., 2007; Ghars et al., 2008, 2012; Radyukina et al., 2011). In a comparative study, the proline content of E. parvulum was determined as higher than that of A. thaliana under salinity as well as unstressed conditions (Kant et al., 2006). Similar to E. halophilum, in E. parvulum we have also observed accumulation of significant amounts of proline under 300 mm NaCl.
To gain a better understanding of proline accumulation in E. parvulum, we have investigated the expressions of genes related to its biosynthesis and degradation. In general, our data suggest that under salinity synthesis of proline was increased, while its degradation was decreased, which resulted in net accumulation. In arabidopsis, of the two P5CS genes, it is P5CS1 that responds to abiotic stress and P5CS2 has little or no transcriptional regulation under abiotic stress (Székely et al., 2008). Similarly, we also found that P5CS1 was highly regulated by salt, while salinity had little effect on expression of P5CS2. Expression of PRODH1 decreased more than that of PRODH2, which is again similar to results obtained in arabidopsis under abiotic stress (Verslues and Sharma, 2010). In contrast to PRODH1, expression of P5CDH, which converts P5C to glutamate, was increased. Sharma and Verslues (2010) showed that P5CDH is upregulated by low water potential, which is also consistent with available microarray data (Verslues and Sharma, 2010). As excess P5C can cause tissue damage (Deuschle et al., 2004), an increase in P5CDH might be explained in two ways: (1) to degrade P5C produced by PRODH activity, although this seems unlikely in this situation due to the downregulation of PRODH; and (2) to degrade P5C produced by excess P5CS activity. However, in this situation there needs to be a mechanism to move P5C between cytosol/chloroplast and mitochondria. Proline synthesis uses reducing power (NADPH) in the cytosol and chloroplasts and releases this energy in mitochondria during its degradation. This movement of reducing power can be used to adjust redox balance in the cell between these compartments. Also, proline biosynthesis uses two NADPH, but its degradation produces one FAD and one NADPH, which means there is a net loss of reducing power throughout the proline cycling process. However, for E. parvulum this latter cycling mechanism seems unlikely to work or has little contribution to the stress response considering remarkably decreased PRODH expression. In conclusion proline accumulation not only contributes to osmotic adjustment in E. parvulum, but may also act as an antioxidant to counteract damaging effects of ROS in this species.
While Na+ and Cl– contents were enhanced, Ca2+ and K+ contents decreased under salinity
Plant growth may be reduced by salinity due to its effects on nutrient availability (K+, Ca2+) (Khan et al., 2000; Ben Amor et al., 2005). Yeo (1998) proposed that halophytes are able to protect their K+ contents in a high Na+ environment. A significant decrease in K+/Na+ suggests that there is a large amount of Na+ intake under salinity in E. parvulum plants, as in E. halophilum (M'rah et al., 2006). Net K/Na selectivity (SK/Na) of plants under saline conditions is 7–12 for Brassicaceae, 4–9 for Chenopodiaceae and 11–12 for dicots [see supplementary information 2 in Flowers and Colmer (2008) for a detailed list of SK/Na values]. When compared with these data, the results obtained herein (ranging between 1·6 and 2·2) clearly show that E. parvulum does not discriminate between K+ and Na+ ions as much as others. It is known that some halophytes, especially dicots, can effectively sequester Na+ ions to their vacuoles and compartmentalize the ions to prevent their toxic effects (Flowers and Colmer, 2008). SK/Na values obtained in this study suggest that E. parvulum can use this mechanism very effectively. Sequestering ions in vacuoles requires accumulation of organic solutes to adjust osmotic potential of cytoplasm in accordance with that of vacuoles. Under these circumstances, proline might play an important role if it can be specifically compartmentalized to cytoplasm.
With increasing salinity, Cl– uptake also increased and even surpassed Na+ under 300 mm NaCl treatment (Table 1). However, the (Na++K+)/Cl– ratio was 1·41 even under 300 mm NaCl treatment, similar to values for other dicot halophytes (Flowers and Colmer, 2008).
Ca2+ is an important essential element that plays key roles in growth, development, metabolism and cellular signal transduction (Kudla et al., 2010). Excess Na+ in the growth medium can inhibit uptake of Ca2+ in plants and therefore cause deficiency of this nutrient. High Na+ can also displace Ca2+ ions in the membrane, causing a decrease in its integrity. One of the most important signalling events by Ca2+ during salinity is regulation of the SOS pathway. An increase in cytoplasmic Ca2+ can active SOS3 (Ca binding protein), which regluates SOS1 (Na+/H+ antiporter) via SOS2 and induce a response that would export Na+ out of the cytoplasm (Qiu et al., 2002). In our study, Ca2+ levels decreased with increasing salinity; however, there was no visible Ca2+ deficiency symptom. Similar results were obtained for other halophytes where Ca2+ levels decrease in a similar magnitude (Messedi et al., 2004; Ben Amor et al., 2005). These findings suggest that retention of Ca2+ by plants or thge ability to continue its uptake in a high Na+ environment might help to protect membranes from peroxidation and ensure precise regulation of signalling events.
E. parvulum could cope with oxidative stress induced in 300 mm NaCl as evidenced by H2O2 concentration and lipid peroxidation
Salinity-induced accumulation of ROS (Hernandez and Almansa, 2002) and lipid peroxidation are widely used as a sign of oxidative stress (Mittler, 2002). In our study, H2O2 concentration and lipid peroxidation showed a significant correlation (R2 = 0·882 for whole cells and 0·985 for chloroplasts). In whole cells, lipid peroxidation was not changed by salinity whereas peroxidation increased in chloroplasts isolated from plants of E. parvulum grown in 300 mm NaCl. M'rah et al. (2006) also found that lipid peroxidation is insensitive to salinity in E. halophilum. Levels of malondialdehyde in Crithmum maritimum were also unchanged under 50 or 200 mm NaCl (Ben Amor et al., 2005) and Ellouzi et al. (2011) found that 400 mm NaCl treatment for 72 h did not change the lipid peroxidation level in Cakile maritima. In A. thaliana, however, just 100 mm NaCl treatment for 72 h increased peroxidation approximately 2-fold. Overall, the insensitivity of lipid peroxidation in halophytes mentioned above and E. parvulum to high salinity suggests efficient induction of antioxidant defence seems to protect these plants from damaging effects of oxidative stress.
Efficient induction of water–water cycle enzymes prevented accumulation of excess ROS in chloroplasts, protecting the photosynthetic machinery
When CO2 fixation is limited or inhibited due to severe environmental conditions, electron transport chains can be over reduced due to the decrease in the regeneration of the final electron acceptor NADP in chloroplasts (Rizhsky et al., 2003). Over-reduction of these components may cause production of ROS such as 1O2 by over-excitation of chlorophyll molecules or O2– via the Mehler reaction. O2– is disproportionated to O2 and H2O2 in a reaction catalysed by SOD (Asada, 2006). The H2O2 generated by SOD is reduced to water by APX using ascorbate as electron donor. Oxidized ascorbate is then regenerated in the ascorbate–GSH cycle by the enzymes MDHAR, DHAR and GR (Shimaoka et al., 2003; Asada, 2006). This cycle of events is called the water–water cycle due to electron flow from water in PSII to water in PSI, and is considered to function to dissipate the energy of excess photons (Foyer and Noctor, 2000; Asada, 2006).
In our study, the activities of water–water cycle enzymes (SOD, APX, MDHAR, DHAR and GR) were increased by salt stress. This increase in enzyme activities in chloroplasts implies that O2.– production was increased due to the Mehler reaction. This increase might suggest an overload of chloroplastic electron transport during salinity and, by this means, the electron transport chain was relaxed in E. parvulum. Similar to chloroplasts, the activities of APX, MDHAR, DHAR and GR were also enhanced at the whole cell level showing the importance of Asada–Halliwell–Foyer enzymes in counteracting oxidative stress. Results similar to ours have been reported for Cakile maritima ‘Jerba’: SOD, APX, MDHAR, DHAR and GR activities increased salinities in the range of 0–200 mm NaCl (Ben Amor et al., 2006). These results show that during salinity E. parvulum was able to protect the photosynthetic machinery with efficient use of the water–water cycle.
Redox homeostasis in chloroplasts was achieved by efficient induction of expressions of redox regulatory enzymes such as FTR, NTRC, TRXs and PRXs under salinity
Under salinity, to scavenge excess ROS, an efficient antioxidant defence was induced in E. parvulum chloroplasts. Besides the water–water cycle, the expressions of peroxiredoxin (Prx) genes were significantly increased. Arabidopsis chloroplasts contain four isoforms of Prxs, namely 2-cys PrxA (2CPA), 2-cys PrxB (2CPB), PrxQ and PrxIIE (PRX2e). Besides their role in scavenging H2O2 in chloroplasts, these Prxs can also detoxify alkyl hydroperoxides and peroxinitrite (Dietz et al., 2006). In our study, we investigated the expressions of all four types of Prxs and observed a significant increase under salinity. Horling et al. (2002) showed that expressions of 2CPA, 2CPB and PrxQ decreased under salinity in arabidopsis. Similarly, Dietz et al. (2006) found that expression of 2CPA, 2CPB, PrxQ and Prx2e decreased under salinity according to data obtained from 12 different arabidopsis microarrays. There is, then, a clear contrast in response in Prx expression between the glycophyte arabidopsis and its halophytic relative E. parvulum under salinity. Stepien and Johnson (2009) showed that under saline conditions linear electron flow in chloroplasts of arabidopsis decreased, while cyclic flow increased. A decrease in linear electron flow might cause insufficient reducing power for Trx–Prx and might cause a down-regulation of this system. By contrast, in E. halophilum salinity did not affect the rate of linear electron transport (Stepien and Johnson, 2009). This contrasting response might be the result of different photochemistry strategies employed by glycophytic and halophytic plants under salinity.
Another redox component, thioredoxins (Trxs), can regenerate Prxs in chloroplasts and in arabidopsis 17 isoforms of Trxs were described (Lemaire et al., 2007). Investigating expressions of all these isoforms was beyond the scope of our study, but we selected two isoforms, TRXx and TRXm, that are known to reduce Prxs in chloroplasts. Under saline conditions, expressions of both Trxs were increased, supporting the view that a Prx scavenging system was induced. Similarly, in another halophyte, Spartina alterniflora, it was also found that TRXh and TRXh1-like genes were up-regulated by salt stress (Baisakh et al., 2008). Moreover, TRXh function in the thioredoxin-based redox pathway was induced in the halophytic plant Nitraria sphaerocarpa under salt stress (Chen et al., 2012).
Besides reduction of Prxs, TRXm can also regulate the activity of enzymes involved in carbon metabolism, such as NADP-MDH and G6PDH. TRXm expression decreased in plants treated with 200 and 300 mm NaCl as compared with 50 mm NaCl treatment. By controlling the expression of TRXm, plants might regulate the activities of target photosynthetic enzymes.
Ferredoxin can reduce Trxs with FTR activity in the chloroplasts. FTR expression was increased under all salinity conditions, but it was more prevalent with 200 and 300 mm NaCl treatment, implying an increased demand for electrons from photosystems for the defensive responses and redox homeostasis. In contrast with our results, Yu et al. (2013) obtained a significant decrease in FTR activity of halophyte Puccinellia tenuiflora leaves under Na2CO3 stress, implying a decrease in carbon fixation.
To avoid overload in the chloroplastic electron transport chain, an alternative oxidase-like plastid terminal oxidase (PTOX) can be activated (Stepien and Johnson, 2009). Previously, it was shown that PTOX in E. halophilum was induced by 250 mm NaCl but not in arabidopsis by 100 mm NaCl (Stepien and Johnson, 2009). In our study we have also found a very similar and linear induction trend in expression of PTOX, showing that this response is common to both E. parvulum and E. halophilum. Unchanged FED gene expression, but an increase in demand of electrons by FTR and PTOX, suggests that electrons were diverted from the photosynthetic electron transport chain (NADP reduction) to redox regulation. By this means, both relaxation of the electron transport chain and energy for defensive responses were achieved. Even after NADP reduction by Fd, Trxs can be reduced by NTR for additional reductive power needed by defensive responses or redox homeostasis (Perez-Ruiz et al., 2006). NTRC, formed by an NADPH thioredoxin reductase (NTR), is an NADP-Trx system used to transfer reducing power (NADPH) to the chloroplast detoxification system. Therefore, NTRC is an alternative system for chloroplast protection against oxidative damage, as reported by Perez-Ruiz et al. (2006). It was found that arabidopsis ntrc-knockout mutants have a lower content of photosynthetic pigments and lower rate of CO2 assimilation when compared with wild-type plants. Moreover, damage to the photosynthetic apparatus of these plants was observed due to overload of the electron transport chain (Perez-Ruiz et al., 2006). By contrast, Chae et al. (2012) showed that overexpression of NTRC in arabidopsis enhanced tolerance to heat shock due to a holdase chaperone function of NTRC, whereas NTRC-knockout plants (ntrc1) exhibited a temperature-sensitive phenotype. Toivola et al. (2013) found that overexpression of NTRC promoted plant biomass yield by chloroplast protective pathways controlled by NTRC. Also, in our study, similar to FTR expression, NTRC expression was induced by salinity. It was shown that induction of NTRC (Fig. 9C, summarized in Fig. 10) can protect the chloroplast photosynthetic apparatus, as can be seen in Fig. 5, from salt-induced oxidative damage by blocking overload in the electron transport chain.
Fig. 10.

Scheme summarizing the alternative electron sinks and mechanisms that can relax the electron load on chloroplastic electron transport chain.
Overall, in this study we show that E. parvulum was able to adapt to osmotic stress by an efficient osmotic adjustment mechanism involving proline. In addition, it was shown that efficient induction of water–water cycle enzymes and other redox regulatory components in the chloroplasts, such as PTOX, NTRC, FTR, TRXs and PRXs, were able to protect the chloroplasts from salinity-induced oxidative stress.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjounrals.org and consist of Table S1: primers and accessions for arabidopsis and Eutrema homologues of the genes known to play roles in chloroplastic redox regulation.
ACKNOWLEDGEMENTS
We thank Dr H. Caglar Karakaya for his support with qRT-PCR analysis. This work was a part of EU-COST FA0901 Action.
LITERATURE CITED
- Ali Z, Park HC, Ali A, et al. TsHKT1;2, a HKT1 homolog from the extremophile Arabidopsis relative Thellungiella salsuginea, shows K+ specificity in the presence of NaCl. Plant Physiology. 2012;158:1463–1474. doi: 10.1104/pp.111.193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann A. Learning from evolution: Thellungiella generates new knowledge on essential and critical components of abiotic stress tolerance in plants. Molecular Plant. 2009;2:3–12. doi: 10.1093/mp/ssn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann A, Bohnert HJ, Bressan RA. Abiotic stress and plant genome evolution. Search for new models. Plant Physiology. 2005;138:127–130. doi: 10.1104/pp.105.059972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Arbona V, Argamasilla R, Gomez-Cadenas Common and divergent physiological, hormonal and metabolic responses of Arabidopsis thaliana and Thellungiella halophila to water and salt stress. Journal of Plant Physiology. 2010;167:1342–1350. doi: 10.1016/j.jplph.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Arrigoni O, Dipierro S, Booraccino G. Ascorbate free radical reductase, a key enzyme of the ascorbic acid system. FEBS Letters. 1981;125:242–244. [Google Scholar]
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baisakh N, Subudhi PK, Varadwaj P. Primary responses to salt stress in a halophyte, smooth cordgrass (Spartina alterniflora Loisel.) Functional and Integrative Genomics. 2008;8:287–300. doi: 10.1007/s10142-008-0075-x. [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39:205–207. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Isoenzymes of superoxide dismutase from wheat germ. Biochimica et Biophysica Acta. 1973;317:50–64. doi: 10.1016/0005-2795(73)90198-0. [DOI] [PubMed] [Google Scholar]
- Ben Amor N, Ben Hamed K, Debez A, Grignon C, Abdelly C. Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Science. 2005;168:889–899. [Google Scholar]
- Ben Amor N, Jimenez A, Megdiche W, Lundqvist M, Sevilla F, Abdelly C. Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiologia Plantarum. 2006;126:446–457. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of the protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chae HB, Moon JC, Shin MR, et al. Thioredoxin reductase type C (NTRC) orchestrates enhanced thermotolerance to Arabidopsis by its redox-dependent holdase chaperone function. Molecular Plant. 2012;6:323–336. doi: 10.1093/mp/sss105. [DOI] [PubMed] [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought – from genes to the whole plant. Functional Plant Biology. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Chaves MM, Costa M, Saibo NJM. Recent advances in photosynthesis under drought and salinity. Advances in Botanical Research. 2011;57:49–104. [Google Scholar]
- Cheeseman JM. Hydrogen peroxide concentrations in leaves under natural conditions. Journal of Experimental Botany. 2006;57:2435–2444. doi: 10.1093/jxb/erl004. [DOI] [PubMed] [Google Scholar]
- Chen J, Cheng T, Wang P, et al. Salinity-induced changes in protein expression in the halophytic plant Nitraria sphaerocarpa. Journal of Proteomics. 2012;75:5226–5243. doi: 10.1016/j.jprot.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Dassanayake M, Michie P, Carter G, et al. The genome of the extremophile crucifer Thellungiella parvula. Nature Genetics. 2011;43:913–920. doi: 10.1038/ng.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle K, Funck D, Forlani G, et al. The role of Δ1-pyrroline-5-carboxylate dehydrogenase in proline degradation. The Plant Cell. 2004;16:3413–3425. doi: 10.1105/tpc.104.023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ, Jacob S, Oelze ML, et al. The function of peroxiredoxins in plant organelle redox metabolism. Journal of Experimental Botany. 2006;57:1697–1709. doi: 10.1093/jxb/erj160. [DOI] [PubMed] [Google Scholar]
- Ellouzi H, Ben Hamed K, Cela J, Munne-Bosch S, Abdelly C. Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte) Physiologia Plantarum. 2011;142:128–143. doi: 10.1111/j.1399-3054.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- FAO. FAO land and plant nutrition management service. 2000. http://www.fao.org/ag/agl/agll/spush.
- Flowers J, Colmer TD. Salinity tolerance in halophytes. New Phytologist. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Oxygen processing in photosynthesis: regulation and signalling. New Phytologist. 2000;146:359–388. [Google Scholar]
- Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxidants & Redox Signalling. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiology. 2011;155:93–100. doi: 10.1104/pp.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghars MA, Parre E, Debez A, et al. Comparative salt tolerance analysis between Arabidopsis thaliana and Thellungiella halophila, with special emphasis on K+/Na+ selectivity and proline accumulation. Journal of Plant Physiology. 2008;165:588–599. doi: 10.1016/j.jplph.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Ghars MA, Richard L, Lefebvre-De Vos D, et al. Phospholipases C and D modulate proline accumulation in Thellungiella halophila/salsuginea differently according to the severity of salt or hyperosmotic stress. Plant & Cell Physiology. 2012;53:183–192. doi: 10.1093/pcp/pcr164. [DOI] [PubMed] [Google Scholar]
- Hernandez JA, Almansa MS. Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiologia Plantarum. 2002;115:251–257. doi: 10.1034/j.1399-3054.2002.1150211.x. [DOI] [PubMed] [Google Scholar]
- Horling F, König J, Dietz KJ. Type II peroxiredoxin C, a member of the peroxiredoxin family of Arabidopsis thaliana: its expression and activity in comparison with other peroxiredoxins. Plant Physiology and Biochemistry. 2002;40:491–499. [Google Scholar]
- Hou WC, Liang HJ, Wang CC, Liu DZ. Detection of glutathione reductase after electrophoresis on native or sodium dodecyl sulfate polyacrylamide gels. Electrophoresis. 2004;25:2926–2931. doi: 10.1002/elps.200406041. [DOI] [PubMed] [Google Scholar]
- Johnson CM, Ulrich A. Analytical methods for use in plant analysis. California Agricultural Experiment Station Bulletin. 1959;766:11–35. [Google Scholar]
- Kant S, Kant P, Raveh E, Barak S. Evidence that differential gene expression between the halophyte, Thellungiella halophila, and Arabidopsis thaliana is responsible for higher levels of the compatible osmolyte proline and tight control of Na+ uptake in T. halophila. Plant Cell & Environment. 2006;29:1220–1234. doi: 10.1111/j.1365-3040.2006.01502.x. [DOI] [PubMed] [Google Scholar]
- Khan MA, Ungar IA, Showalters AM. Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte, Atriplex griffithii var. stocksii. Annals of Botany. 2000;85:225–232. [Google Scholar]
- Kim KH, Alam I, Lee KW, et al. Enhanced tolerance of transgenic tall fescue plants overexpressing 2-Cys peroxiredoxin against methyl viologen and heat stresses. Biotechnology Letters. 2010;32:571–576. doi: 10.1007/s10529-009-0185-0. [DOI] [PubMed] [Google Scholar]
- Kudla J, Batistič O, Hashimoto K. Calcium signals: the lead currency of plant information processing. The Plant Cell Online. 2010;22:541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laureau C, De Paepe R, Latouche G. Plastid terminal oxidase (PTOX) has the potential to act as a safety valve for excess excitation energy in the alpine plant species Ranunculus glacialis L. Plant, Cell and Environment. 2013;36:1296–1310. doi: 10.1111/pce.12059. [DOI] [PubMed] [Google Scholar]
- Lee YP, Kim DH, Bang JW, Lee HS, Kwak SS, Kwon SY. Enhanced tolerance to oxidative stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant Cell Reports. 2007;26:591–598. doi: 10.1007/s00299-006-0253-z. [DOI] [PubMed] [Google Scholar]
- Lemaire SD, Michelet L, Zaffagnini M, Massot V, Issakidis-Bourguet E. Thioredoxins in chloroplasts. Current Genetics. 2007;51:343–365. doi: 10.1007/s00294-007-0128-z. [DOI] [PubMed] [Google Scholar]
- Lilley RC, Fitzgerald MP, Rienits KG, Walker DA. Criteria for intactness and the photosynthetic activity of spinach chloroplast preparations. New Phytologist. 1975;75:1–10. [Google Scholar]
- Lim S, Kim YH, Kim SH, et al. Enhanced tolerance of transgenic sweetpotato plants that express both CuZnSOD and APX in chloroplasts to methyl viologen-mediated oxidative stress and chilling. Molecular Breeding. 2007;19:227–239. [Google Scholar]
- M'rah S, Ouerghi Z, Berthomieu C, et al. Effects of NaCl on the growth, ion accumulation and photosynthetic parameters of Thellungiella halophila. Journal of Plant Physiology. 2006;163:1022–1031. doi: 10.1016/j.jplph.2005.07.015. [DOI] [PubMed] [Google Scholar]
- M'rah S, Ouerghi Z, Eymery F, et al. Efficiency of biochemical protection against toxic effects of accumulated salt differentiates Thellungiella halophila from Arabidopsis thaliana. Journal of Plant Physiology. 2007;164:375–384. doi: 10.1016/j.jplph.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Madhava Rao KV, Sresty TVS. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Science. 2000;157:113–128. doi: 10.1016/s0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- Megdiche W, Ben Amor N, Debez A, et al. Salt tolerance of the annual halophyte Cakile maritima as affected by the provenance and the developmental stage. Acta Physiolgiae Plantarum. 2007;29:375–384. [Google Scholar]
- Messedi D, Labidi N, Grignon C, Abdelly C. Limits imposed by salt to the growth of the halophyte Sesuvium portulacastrum. Journal of Plant Nutrition and Soil Science. 2004;167:720–725. [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell and Environment. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Reviews of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology. 1981;22:867–880. [Google Scholar]
- Noctor G, De Paepe R, Foyer CH. Mitochondrial redox biology and homeostasis in plants. Trends in Plant Science. 2007;12:125–134. doi: 10.1016/j.tplants.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Nyomora AMS, Sah RN, Brown PH, Miller RO. Boron determination in biological materials by inductively coupled plasma atomic emission and mass spectrophotometry: effects of sample dissolution methods. Fresenius Journal of Analytical Chemistry. 1997;357:1185–1191. [Google Scholar]
- Oh DH, Dassanayake M, Haas JS, Kropornika A, et al. Genome structures and halophyte-specific gene expression of the extremophile Thellungiella parvula in comparison with Thellungiella salsuginea (Thellungiella halophila) and Arabidopsis. Plant Physiology. 2010;154:1040–1052. doi: 10.1104/pp.110.163923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini F, D'Urzo MP, Inan G, et al. A comparative study of salt tolerance parameters in 11 wild relatives of Arabidopsis thaliana. Journal of Experimental Botany. 2010;61:3787–3798. doi: 10.1093/jxb/erq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgur R, Uzilday B, Sekmen AH, Turkan I. Reactive oxygen species regulation and antioxidant defence in halophytes. Functional Plant Biology. 2013;40:832–847. doi: 10.1071/FP12389. [DOI] [PubMed] [Google Scholar]
- Pedras MS, Zheng QA. Metabolic responses of Thellungiella halophila/salsuginea to biotic and abiotic stresses: metabolite profiles and quantitative analyses. Phytochemistry. 2010;71:581–589. doi: 10.1016/j.phytochem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Perez-Ruiz JM, Spinola MC, Kirchsteiger K, Moreno J, Sahraway M, Cejudo FJ. Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. The Plant Cell. 2006;18:2356–2368. doi: 10.1105/tpc.106.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membraneNa+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8436–8441. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyukina NL, Ivanov YV, Kartashov AV, Pashkovskiy PP, Shevyakova NI, Kuznetsov VV. Regulation of gene expression governing proline metabolism in Thellungiella salsuginea by NaCl and paraquat. Russian Journal of Plant Physiology. 2011;58:643–652. [Google Scholar]
- Raymond MJ, Smirnoff N. Proline metabolism and transport in maize seedlings at low water potential. Annals of Botany. 2002;89:813–823. doi: 10.1093/aob/mcf082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Mittler R. The water–water cycle ıs essential for chloroplast protection in the absence of stress. The Journal of Biological Chemistry. 2003;278:38921–38925. doi: 10.1074/jbc.M304987200. [DOI] [PubMed] [Google Scholar]
- Seevers PM, Daly JM, Catedral FF. The role of peroxidase isoenzymes in resistance to wheat stem rust disease. Plant Physiology. 1971;48:353–360. doi: 10.1104/pp.48.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Verslues PE. Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant, Cell and Environment. 2010;33:1838–1851. doi: 10.1111/j.1365-3040.2010.02188.x. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Miyake C, Yokota A. Mechanism of the reaction catalyzed by dehydroascorbate reductase from spinach chloroplasts. The FEBS Journal. 2003;270:921–928. doi: 10.1046/j.1432-1033.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- Stepien P, Johnson GN. Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: role of the plastid terminal oxidase as an alternative electron sink. Plant Physiology. 2009;149:1154–1165. doi: 10.1104/pp.108.132407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Székely G, Ábrahám E, Cséplő Á, et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. The Plant Journal. 2008;53:11–28. doi: 10.1111/j.1365-313X.2007.03318.x. [DOI] [PubMed] [Google Scholar]
- Toivola J, Nikkanen L, Dahlström KM, Salminen TA, Lepistö A. Overexpression of chloroplast NADPH-dependent thioredoxin reductase in Arabidopsis enhances leaf growth and elucidates in vivo function of reductase and thioredoxin domains. Frontiers in Plant Science. 2013;4:1–18. doi: 10.3389/fpls.2013.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiology. 2006;141:384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Estrella R, Barkla BJ, Garcia Ramirez L, Pantoja O. Salt stress in Thellungiella halophila activates Na+ transport mechanisms required for salinity tolerance. Plant Physiology. 2005;139:1507–1517. doi: 10.1104/pp.105.067850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Sharma S. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists; 2010. Proline metabolism and its implications for plant-environment interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov V, Wang B, Dominy PJ, Fricke W, Amtmann A. Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, possesses effective mechanisms to discriminate between potassium and sodium. Plant Cell & Environment. 2004;27:1–14. [Google Scholar]
- Wu Hj, Zhang Z, Wang JY, et al. Insights into salt tolerance from the genome of Thellungiella salsuginea. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12219–12224. doi: 10.1073/pnas.1209954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang F, Li X, Shi R, Lu J. Signal regulation of proline metabolism in callus of the halophyte Nitraria tangutorum Bobr. grown under salinity stress. Plant Cell Tissue Organ Culture. 2013;112:33–42. [Google Scholar]
- Yeo A. Molecular biology of salt tolerance in the context of whole-plant physiology. Journal of Experimental Botany. 1998;49:915–929. [Google Scholar]
- Yıldıztugay E, Sekmen AH, Turkan I, Kucukoduk M. Elucidation of physiological and biochemical mechanisms of an endemic halophyte Centaurea tuzgoluensis under salt stress. Plant Physiology and Biochemistry. 2011;49:816–824. doi: 10.1016/j.plaphy.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Yu J, Chen S, Wang T, Sun G, Dai S. Comparative proteomic analysis of Puccinellia tenuiflora leaves under Na2CO3 stress. International Journal of Molecular Sciences. 2013;14:1740–1762. doi: 10.3390/ijms14011740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Wang RP, Zhang FJ, Tao FQ, Li WQ. Lipid profiling and tolerance to low-temperature stress in Thellungiella salsuginea in comparison with Arabidopsis thaliana. Biologia Plantarum. 2013;57:149–153. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.