Abstract
Background and Aims Limonium is a well-known example of a group of plants that is taxonomically complex due to certain biological characteristics that hamper species' delineation. The closely related polyploid species Limonium vulgare Mill., L. humile Mill. and L. narbonense Mill. are defined species and can be used for studying patterns of morphological and reproductive variation. The first two taxa are usually found in Atlantic Europe and the third in the Mediterranean region, but a number of intermediate morphological forms may be present alongside typical examples of these species. This study attempts to elucidate morphological, floral and karyological diversity representative of these taxa in the Iberian Peninsula.
Methods The extent of morphological differentiation was tested through comparison of 197 specimens from both Portugal and Spain using 17 descriptive morphological characters and 19 diagnostic morphometric characters. Analyses of floral morphisms (heterostyly and pollen–stigma dimorphism) and karyological determinations were also conducted.
Key Results and Conclusions Discriminant analysis using morphometric variables reliably assigned individuals in natural populations to their respective groups. In addition, the results provide the first direct evidence that L. narbonense and a new species, Limonium maritimum Caperta, Cortinhas, Paes, Guara, Espírito-Santo and Erben, sp. nov., related to L. vulgare are present on Portuguese coasts. Most of these species are found together in mixed populations, especially L. vulgare and L. narbonense. It is hypothesized that taxonomic biodiversity found in sites where distinct species co-occur facilitates the evolutionary processes of hybridization, introgression and apomixis. This study therefore contributes to the elucidation of the taxonomic diversity in L. vulgare-related species and may also help in implementing future conservation programmes to maintain the evolutionary processes generating biodiversity.
Keywords: Halophyte; heterostyly; karyological polymorphisms; Limonium humile; L. maritimum; L. narbonense; L. vulgare; morphometry; pollen–stigma dimorphism; polyploidy; Plumbaginaceae, taxonomy
INTRODUCTION
Taxonomically complex groups (TCGs) such as the halophytic Limonium spp. (Plumbaginaceae), typically found in coastal areas and saline steppes (Erben, 1993; Kubitzki, 1993; van der Maarel and van der Maarel-Versluys, 1996), harbour significant biodiversity. In some of these TCGs, some form of uniparental reproduction (e.g. selfing, apomixis or gynogenesis) is usually present, and hybridization occurs to some degree among its members, whose biological diversity defies simple classification into discrete species (Palacios et al., 2000; Lledó et al., 2005). Therefore, conserving biodiversity within these TCGs is essential because diversity is generated and maintained by facilitating the evolutionary interactions among their members (i.e. hybridization and introgression), rather than by preserving the taxonomic entities that such evolution produces (Ennos et al., 2005). However, due to the biological characteristics they present, there are difficulties in delimitating taxa. Therefore, clarifying taxonomic biodiversity of well-represented taxa from the European coasts but where taxonomic uncertainties currently exist is crucial for their conservation.
In the TCG Limonium, notably high levels of karyological polymorphisms linked with polyploidy (spanning triploids to octoploids) and aneuploidy are found (Dolcher and Pignatti, 1971; Erben 1978, 1979; Dawson, 1990; Arrigoni and Diana, 1993; Georgakopoulou et al., 2006; Castro and Rosselló, 2007; Róis et al., 2012). Triploid species seem to be highly concentrated in the western Mediterranean region, while tetraploid species and taxa with higher ploidy levels occur mainly in the Atlantic coasts and in the eastern Mediterranean region (Cowan, 1998). Moreover, descriptive morphological (e.g. Pignatti, 1971, 1972), morphometric and sequence data from both plastid and nuclear DNA (Lledó et al., 1998, 2001, 2005; Palacios et al., 2000) have been used to elucidate the enormous taxonomic complexity of this genus. The closely related polyploid saltmarsh species, L. vulgare Mill. (the type of the genus; known as Statice limonium L.; Statice, nom. rej. vs. Armeria; Greuter et al., 2000), L. humile Mill. and L. narbonense Mill., have long been recognized as distinct species (Erben, 1993). Limonium vulgare is known so far as a tetraploid (2n = 4x = 36) and L. humile as a hexaploid (2n = 6x = 54) (Dawson, 1990); on the other hand, the L. narbonense species complex comprises four species with diverse ploidy levels, namely L. hirsuticalyx (2n = 2x = 18), L. narbonense (2n = 4x = 36) (Erben, 1979; Artelari, 1992; Georgakopoulou et al., 2006), L. breviopetalum (2n = 6x = 54) and L. pagasaeum (2n = 8x = 72) (S. Brullo and M. Erben, unpubl. data). Limonium vulgare and L. humile occur in Atlantic Europe (Boorman, 1967, 1971; Erben, 1993; Dawson and Ingrouille, 1995), whereas L. narbonense has a patchy distribution in the Mediterranean coastal region (Erben, 1993; Crespo and Lledó, 1998; Pandža et al., 2007).
The presence of salt glands in plants from these species allows them to remove excess salts in the soil water (Flowers and Colmer, 2008; Grigore et al., 2014). For example, in L. vulgare and L. narbonense, salt glands are below the surface in leaves and sepals, and at the surface of the petals (Ana R. Pina, Generosa Teixeira and Ana D. Caperta, unpubl. data). Fascinating reproductive systems associated with floral polymorphisms such as heterostyly and pollen–stigma dimorphisms are also found in species of Limonium (Baker 1948, 1953a, 1966). Of these, heterostyly is a morphological and reproductive polymorphism in which plant populations are composed of two (distyly) or three (tristyly) floral morphs that differ reciprocally in the height of their sytles and anthers, thus preventing self- and intramorph fertilization (Ganders, 1979). Also, pollen and stigma dimorphisms in which two pollen grain types (A- and B-pollen) that differ in their exine surface patterns and germinate on distinct stigma types (cob-like and papillose) are frequent (Baker, 1948, 1953a, 1966; Nowicke and Skvarla, 1977). Both flower heteromorphies are linked with a sporophytic self-incompatibility system preventing self-fertilization. Thus, in monomorphic populations, self-incompatible plants can only produce seeds through apomixis (asexual reproduction via seeds) (Baker, 1966; Richards, 1997). For example, in L. vulgare, meiotic (sexual) embryo sacs are formed (D'Amato, 1940) while in triploid (2n = 3x = 27) Statice oleaefolia var. confusa, meiotic embryo sacs together with apomictic (non-meiotic) embryo sacs are produced (D'Amato, 1949).
Due to the geographical location of Portugal, at the confluence between the Atlantic Ocean and the Mediterranean Sea, wide coastal areas provide an exceptional opportunity to study L. vulgare and related taxa that, according to the findings of previous authors, do not share distribution areas (Baker, 1953a, 1966; Erben, 1993; Dawson and Ingrouille, 1995). Both L. vulgare and L. humile are recognized in saltmarshes in Portugal (Erben, 1978; Franco, 1984; Costa et al., 2012). Furthermore, earlier morphological studies by some of the authors (A.D.C. and M.E., unpublished data) showed that these species present variants in the coasts of Portugal. However, knowledge of the geographic distribution, morphological variation, ploidy levels and reproductive characters is lacking. In this study, detailed biometric surveys, floral morphisms and karyological analysis revealed, for the first time, that mixed populations of L. vulgare and related taxa occur along the Portuguese coasts.
MATERIALS AND METHODS
Study species
Limonium vulgare is a perennial rossulate chamaephyte with short spikes with densely arranged spikelets (Salmon, 1905a; Erben, 1993). In contrast to L. vulgare, L. humile is more loosely branched with long spikes and sparse spikelets (Salmon, 1905b; Erben, 1993), and L. narbonense forms taller plants with wider leaves and a longer scape than L. vulgare (Erben, 1993). These latter two species are putative obligate outcrossers (Baker, 1948, 1953a, b; Erben, 1979; Georgakopolou et al., 2006), while L. humile is a probable facultative inbreeder (Boorman, 1968; Dawson and Ingrouille, 1995).
Study area, plant sampling and growth conditions
Field surveys were carried out along Portuguese continental coasts during July to September in 2010 and 2011. Specimens were collected in populations located in Tejo (T), Sado (S) and Mira (M) estuaries, in the coastal lagoons of Aveiro (Ria de Aveiro, A) and Ria Formosa (F), and in Veiga beach (Viana do Castelo, V). As populations were variable in size, different numbers of plants were collected at different sites (details in Table 1). All populations were tagged with a Global Positioning System and mapped using ArcGIS Desktop 10.0 (ESRI).
Table 1.
Site locations surveyed for Limonium vulgare and related taxa
| Population | Site location/Province | Geographic co-ordinates | n |
|---|---|---|---|
| Viana (V) | Praia da Veiga, Areosa/Minho | 41·72836/–8·87155 | 9 |
| Aveiro (A) | Gafanha do Carmo-Encarnação/Beira Litoral | 40·62213/–8·73697 | 15 |
| Cais da Bestida, Torreira/Beira Litoral | 40·7599/–8·67680 | 2 | |
| Tijosa, Ovar/Beira Litoral | 40·82073/–8·6497 | 2 | |
| Ribeira da Aldeia, Estarreja/Beira Litoral | 40·80145/–8·63625 | 5 | |
| Quintas do Norte/Beira Litoral | 40·79529/–8·67325 | 2 | |
| Boco/Beira Litoral | 40·58948/–8·68797 | 9 | |
| Tejo (T) | Sítio das Hortas, Alcochete/Estremadura | 38·76044/–8·93741 | 7 |
| Entroncamento, Alcochete/Estremadura | 38·74879/–8·92208 | 2 | |
| Amora/Estremadura | 38·62670/–9·11028 | 3 | |
| Sado (S) | Carrasqueira/Estremadura | 38·41218/–8·75293 | 4 |
| Mouriscas/Estremadura | 38·52844/–8·80435 | 8 | |
| Tróia/Estremadura | 38·46995/–8·86770 | 9 | |
| Gâmbia/Estremadura | 38·54851/–8·75816 | 2 | |
| Salinas de Monte Novo/Estremadura | 38·44548/–8·70153 | 2 | |
| Mira (M) | Vila Nova de Mil Fontes/Alentejo | 37·72775/–8·77093 | 6 |
| Casa Branca/Alentejo | 37·66634/–8·72009 | 12 | |
| Moinho da Asneira/Alentejo | 37·73069/–8·75450 | 4 | |
| Formosa (F) | Pedras D'el Rei/Algarve | 37·08613/–7·66277 | 5 |
Specimens were sampled in the provinces of Minho (North), Beira Litoral (West), Estremadura (West), Alentejo (Southwest) and Algarve (South).
n, number of individuals sampled.
A total of 108 plants were sampled from 19 sites, then pressed, dried, labelled correspondingly and deposited in João de Carvalho e Vasconcellos Herbarium, Instituto Superior de Agronomia – LISI (abbreviation according to Holmgren, 1990). In some populations, seeds were collected for establishing experimental collections.
Descriptive morphological and morphometric analysis
Descriptive morphological (qualitative) and morphometric (quantitative) data were used in this study in both specimens collected in natural populations and reference specimens from herbaria collections. Reference specimens identified as L. vulgare (n = 17), L. narbonense (n = 17) or L. humile (n = 13), as well as specimens collected in natural populations (n = 108) deposited in herbaria of Spain and Portugal, namely Herbário da Universidade do Porto – PO, Herbário da Universidade de Coimbra – COI, Herbário do Museu Nacional de História Natural – LISU, Herbário João de Carvalho e Vasconcellos do Instituto Superior de Agronomia – LISI, Herbário da Estação Agronómica Nacional – LISE, Herbario del Real Jardín Botánico de Madrid – MA, Herbario del Jardín Botánico de la Universidad de Valencia – VAL and Herbario de la Sociedad de Ciencias Aranzadi – ARAN, were used (abbreviations according to Holmgren, 1990) (see Appendices 1 and 2).
For each specimen, 17 descriptive morphological characters were scored and 19 quantitative characters measured (Table 2) as previously reported for other Limonium spp. (Erben, 1978, 1993; Ingrouille, 1984; Ingrouille and Stace, 1986; Dawson and Ingrouille, 1995). For descriptive characters, a matrix was generated using the observed data, and matrix elements corresponded to the number of individuals that, in a given population, present a particular character. Using this matrix, correspondence analysis (CA) (Foucart, 1982; Podani, 1994) using NTSYS-PC v. 2.21 software (Rohlf, 2009) was conducted. To confirm populations with characters in equivalent proportions, χ2 tests of independence were carried out (Sokal and Rohlf, 2012).
Table 2.
Morphological descriptive and morphometric characters used for discrimination of Limonium vulgare-related taxa and collected individuals
| Morphological character* (Code) | (1) Present | (0) Absent | Units |
|---|---|---|---|
| Apex of inner bract centre (AIBC) | Yes | No apex | |
| Calyx and inner bract size relation (CIBSR) | Calyx > inner bract | Calyx < inner bract | |
| Calyx with trichomes (CT) | Yes | No | |
| Extension of the apex of inner bract centre (EAIBC) | Toward the margin | Below the margin | |
| First order sterile branches (SB1) | Yes | No | |
| Inflorescence type (IT) | G type* | Other | |
| Inner bract centre (IBC) | Membranaceous | Fleshy | |
| Leaf apex (LA) | yes | No | |
| Leaf shape (LS) | Oblongo-lanceolate | Other | |
| Margin of inner bract (MIB) | Hyaline | Non-hyaline | |
| Margin of inner bract centre (MIBC) | Dentate | Non-dentate | |
| Margin of middle bract (MMB) | Hyaline | Non-hyaline | |
| Margin of outer bract (MOB) | Hyaline | Non-hyaline | |
| Presence of salt (PS) | Yes | No | |
| Regularity of inner bract centre (RIBC) | Irregular | Regular | |
| Second order sterile branches (SB2) | Yes | No | |
| Venation (Vs) | Peninerved | Other | |
| Lamina length (LL) | cm | ||
| Maximum calyx length (MCL) | mm | ||
| Maximum distance of spikelets (MDS) | mm | ||
| Maximum inner bract length (MIBL) | mm | ||
| Maximum inner bract width (MIBW) | mm | ||
| Maximum limb/tube ratio (MLTR) | NA | ||
| Maximum lamina width (MLW) | cm | ||
| Maximum middle bract length (MMBL) | mm | ||
| Maximum middle bract width (MMBW) | mm | ||
| Maximum number of florets per spikelet (MNFS) | NA | ||
| Maximum number of spikelets per cm (MNSC) | NA | ||
| Maximum outer bract length (MOBL) | mm | ||
| Maximum outer bract width (MOBW) | mm | ||
| Maximum spike length (MSL) | cm | ||
| Petal length (PL) | mm | ||
| Scape first ramification angle (SFRA) | sin | ||
| Scape height (SH) | cm | ||
| Scape height to the first ramification (SHFR) | cm | ||
| Stalk length (SL) | cm |
*Acronyms according to Erben (1993), Dawson and Ingrouille (1995) and Róis et al. (2013).
NA, not applicable.
The morphometric variables were first tested for deviations from a normal distribution using a Kolmogorov–Smirnov test (Zar, 2010; Sokal and Rohlf, 2012). Non-parametric Kruskal–Wallis analyses of variance were also used (Zar, 2010; Sokal and Rohlf, 2012). To test possible affinities between the sampled specimens, standarized principal component analysis (PCA) was carried out on a matrix with the 197 cases corresponding to reference specimens, specimens from distinct beach/estuaries/lagoons and the quantitative characters (morphometric variables) using the NTSYS-PC v. 2.21 software (Rohlf, 2009). Complementary, canonical discriminant analysis (CDA) was conducted with SPSS 20 for Windows on the same matrix of morphometric data.
Determinations of floral morphs and seed germination analysis
A total of 97 dried specimens were used for floral morph determinations. Briefly, herbarium materials were first re-hydrated in distilled water for 15 min. Flower morph determinations were carried out by observing differences in the lengths of the pistil and stamens (heterostyly) as well as stigma and pollen types, as previously described (Baker, 1948; Erben, 1978). The dissected organs were covered with water or with a drop of basic fuchsin (0·05 %)–hydroalcoholic glycerin solution as described in Suárez-García et al. (2009). Preparations of pollen and stigmas were observed using an optical microscope (Leitz hm-lux 3). Statistical analysis of data was performed through CA (Foucart, 1982; Podani, 1994) using NTSYS-PC v. 2.21 software (Rohlf, 2009). χ2 tests of independence were used (Zar, 2010; Sokal and Rohlf, 2012) to test similarities among populations in relation to the typologies analysed.
Estimations of seed set and germination tests were performed as described in Róis et al. (2012).
Karyotyping
Seven plants from experimental collections established using seeds from Aveiro (A) and Tejo (T) populations were used for karyotyping. Root tips were excised and then treated with a 2 mm 8-hydroxyquinoline solution for 2·5 h at 4 °C in the dark and subsequently for 2·5 h at room temperature to induce metaphases, as described in Róis et al. (2012). Then, root tips were fixed in 3:1 (v/v) absolute ethanol/glacial acetic acid fresh solution overnight and stored at –20 °C until used. Next, root tips were digested with a 2 % cellulase (Sigma), 2 % cellulase ‘Onozuka R-10’ (Serva) and 2 % pectinase (Sigma) enzyme solution in Enzyme Buffer (40 mL of 0·1 m citric acid-1-hydrate and 60 mL of 0·1 m sodium citrate dihydrate; pH 4·8) for 3 h at 37 °C, as described in Caperta et al. (2008). Squashes were made in 60 % acetic acid, and preparations were counterstained with 4′,6-diamidino-2-phenylindole hydrochloride (DAPI) (1 mg mL–1) diluted in Citifluor antifadent mounting solutions (Agar Scientific). Cell preparations were observed using a Zeiss Axioskop 2 fluorescence microscope. Images were collected with an AxioCam MRc5 digital camera (Zeiss) and further processed using Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA, USA). Chromosome counts were made on microphotographs of mitotic metaphase spreads with the same degree of chromosomal condensation in at least three individuals from each population.
RESULTS
Morphometric rather than descriptive morphological characters discriminate among species populations
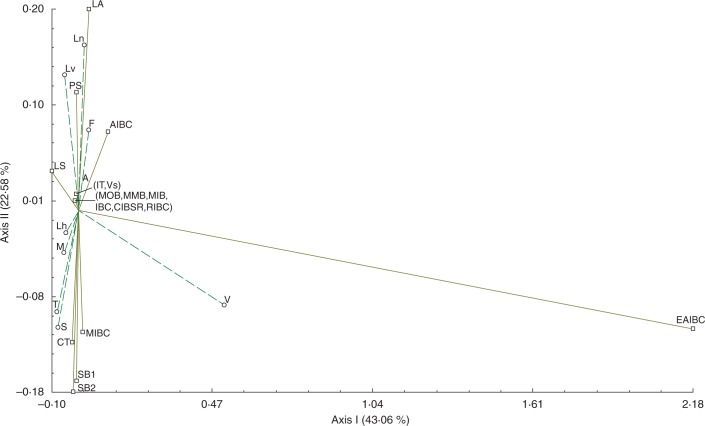
Analysis of morphological data revealed that the characters CIBSR, IBC, IT, LS, MIB, MMB, MOB, PS, RIBC and Vs (see Table 2 for a list of characters and their acronyms) were practically coincident in all populations (>60 %), whereas AIBC, CT, EAIBC, LA, MIBC, SB1 and SB2 were less coincident (<50 %) (Table 3). Corrsepondence analysis of these variables accounted for 65·64 % of the variation in the first two dimensions (axes), and 56·83 % in the first and third dimensions (axes) (Fig. 1). In most populations, the specimens studied exhibited equivalent proportions of the characters analysed (χ2 = 97·379; P = 0·9798). An exception to this was the V population which was significantly (χ2 = 39·814; P < 0·001) different from the reference specimens L. narbonense and L. vulgare and other populations. The first dimension also separated EAIBC in relation to all other variables (χ2 = 38·951; P < 0·001), infrequent in most specimens, but present in all V individuals.
Table 3.
Number and percentage of qualitative characters based on their presence in reference species Limonium vulgare, L. humile and L. narbonense and in collected individuals from natural populations
| Character | L. humile (n = 13) | L. narbonense (n = 18) | L. vulgare (n = 16) | Aveiro (n = 52) | Mira (n = 22) | Ria Formosa (n = 17) | Sado (n = 30) | Tejo (n = 20) | Viana (n = 9) |
|---|---|---|---|---|---|---|---|---|---|
| IT | 13 (100) | 18 (100) | 16 (100) | 52 (100) | 21 (95·45) | 17 (100) | 29 (96·67) | 20 (100) | 9 (100) |
| Vs | 11 (84·62) | 18 (100) | 16 (100) | 52 (100) | 22 (100) | 17 (100) | 29 (96·67) | 20 (100) | 9 (100) |
| LS | 11 (84·62) | 18 (100) | 16 (100) | 52 (100) | 22 (100) | 17 (100) | 29 (96·67) | 20 (100) | 5 (55·56) |
| LA | 8 (61·54) | 17 (94·44) | 15 (93·75) | 50 (96·15) | 20 (90·91) | 16 (94·12) | 10 (33·33) | 16 (80) | 7 (77·78) |
| PS | 10 (76·92) | 18 (100) | 16 (100) | 47 (90·38) | 22 (100) | 12 (70·59) | 21 (70) | 12 (60) | 7 (77·78) |
| SB1 | 5 (38·46) | 4 (22·22) | 6 (37·5) | 25 (48·08) | 17 (77·27) | 4 (23·53) | 11 (36·67) | 11 (55) | 5 (55·56) |
| SB2 | 8 (61·54) | 10 (55·56) | 10 (62·5) | 34 (65·38) | 20 (90·91) | 6 (35·29) | 24 (80) | 19 (95) | 8 (88·89) |
| MOB | 13 (100) | 18 (100) | 16 (100) | 52 (100) | 21 (95·45) | 17 (100) | 30 (100) | 20 (100) | 9 (100) |
| MMB | 13 (100) | 18 (100) | 16 (100) | 52 (100) | 21 (95·45) | 17 (100) | 30 (100) | 20 (100) | 9 (100) |
| MIB | 13 (100) | 18 (100) | 16 (100) | 52 (100) | 21 (95·45) | 17 (100) | 30 (100) | 20 (100) | 9 (100) |
| MIBC | 12 (92·31) | 15 (83·33) | 4 (25) | 52 (100) | 21 (95·45) | 17 (100) | 30 (100) | 20 (100) | 9 (100) |
| RIBC | 12 (92·31) | 18 (100) | 16 (100) | 52 (100) | 21 (95·45) | 17 (100) | 30 (100) | 20 (100) | 9 (100) |
| IBC | 13 (100) | 18 (100) | 16 (100) | 52 (100) | 21 (95·45) | 17 (100) | 30 (100) | 20 (100) | 9 (100) |
| AIBC | 10 (76·92) | 10 (55·56) | 13 (81·25) | 41 (78·85) | 16 (72·73) | 14 (82·35) | 17 (56·67) | 8 (40) | 9 (100) |
| EAIBC | 0 (0) | 1 (5·56) | 0 (0) | 2 (3·85) | 0 (0) | 1 (5·88) | 0 (0) | 0 (0) | 4 (44·44) |
| CIBSR | 13 (100) | 18 (100) | 16 (100) | 52 (100) | 21 (95·45) | 17 (100) | 30 (100) | 20 (100) | 9 (100) |
| CT | 13 (100) | 5 (27·78) | 15 (93·75) | 52 (100) | 21 (95·45) | 17 (100) | 30 (100) | 20 (100) | 9 (100) |
Details of acronyms are given in Table 2.
n, number of individuals.
Fig. 1.
The first two axes of correspondence analysis based on 17 qualitative characters. Percentages of total variance explained by the functions are given in parentheses; details of acronyms are given in Table 2.
Among morphometric variables, only two fitted a normal distribution, SH and SHFR, whereas the other nine failed to do so, even after a logarithmic transformation. Non-parametic Kruskal–Wallis analyses of variance showed that the variables LL, MDS, MIBW, MLTR, MLW, MMBL, MMBW, MNSC, MOBL, MOBW, PL, SHFR, SH, SHFRA and SL were significant. Hence, these variables were used to distinguish the reference species and populations studied. The reference species were discriminated by LL, MDS, MCL, MIBL, MLTR, MLW, MMBL, MMBW, MNSC, MOBL, MOBW, MSL, PL, SH, SHFR and SHFRA (Supplementary Data Table S1). In terms of morphometric variation, SH and SHFR were the most variable characters.
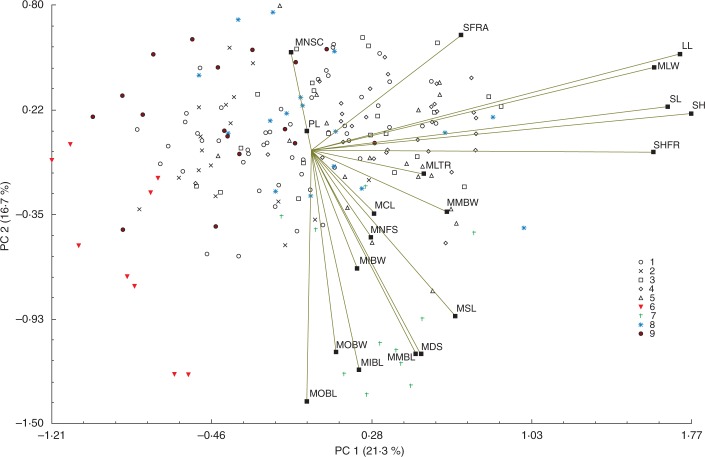
Principal component analysis based on morphometric traits revealed that the accumulated percentage of explained variance was 47·99 %, considering the first three principal components (after applying the ‘broken stick model’ criteria) (Fig. 2). The eigenvalues provided in Supplementary Data Table S2 showed that the amount of variance associated with the first dimension (PC1) was 21·27 % explained by a set of positively correlated variables LL, MLW, SL, SH, and SHFR; 16·74 % in the second dimension (PC2), establishing a gradient between SFRA (positive portion) and MOBL (negative portion) which was positively correlated with MIBL, MMBL, MDS, MSL and MIBW; and 9·88 % in the third dimension (PC3), reflecting a gradient between MSL (positive portion) and MNSC (negative portion) and positively correlated with MMBW and SFRA. Visual inspection of the PCA showed that all reference specimens are positioned in the limits of the arrangement obtained, whereas most specimens from sampled populations were localized in the centre of this arrangement. Specimens from the V population were always represented in the negative portion of the three represented components.
Fig. 2.
The first two axes of principal component analysis based on 19 morphometric characters. Percentages of total variance explained by the functions are given in parentheses; details of acronyms are given in Table 2. 1: Aveiro; 2: Mira; 3: Sado; 4: Tejo; 5: Formosa; 6: Praia da Veiga, Viana do Castelo; 7: L. humile; 8: L. narbonense; 9: L. vulgare.
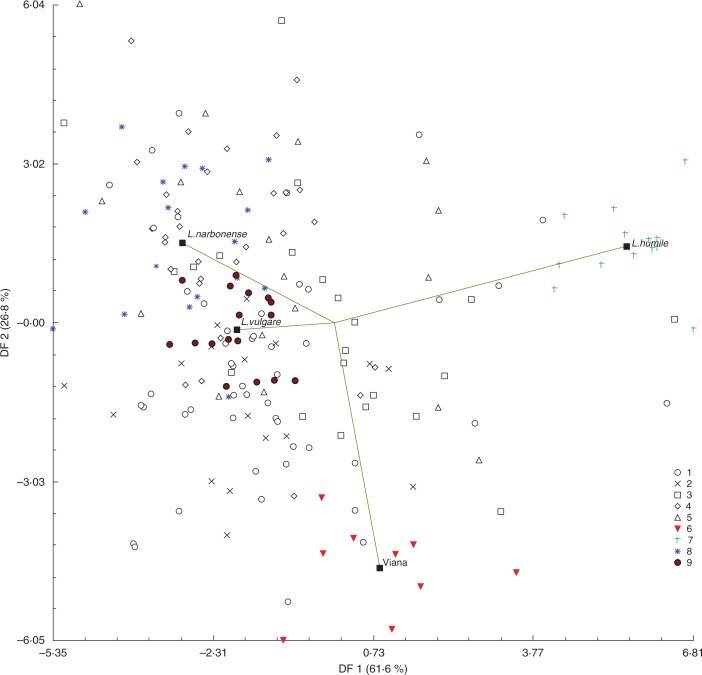
Canonical discriminant analysis of the reference species L. vulgare, L. humile and L. narbonense was perfect for discriminating them (first function 88 %, Wilks's lambda = 0·011; P = 0·000; second function 12 %, Wilks's lambda = 0·251; P = 0·000; Supplementary Data Fig. S1, Tables S3 and S4). The first dimension distinguished L. humile from the other reference species by the characters MIBL, MIBW, MNSC, MOBL and MSL. The second dimension separated L. vulgare from L. narbonense by the rest of the morphometric variables. Since in the PCA, V emerged as an isolated population (highlighted in the PCA; Fig. 2), we performed a second CDA to confirm if V individuals were discriminated into a new group. Thus, when using the three reference species and the V population as references, CDA accounted for 100 % of the variation (62·41 % in the first dimension, 26·04 % in the second dimension and 11·56 % in the third dimension) and correctly assigned individuals to species in 100 % of the cases (n = 197) (Fig. 3; Tables 4 and 5). The first dimension distinguished L. humile from the rest of the reference species and the V population by the characters MDS, MIBL, MLTR, MOBL, MNSC and MSL. The second dimension separated the reference species from specimens of the V population through variables LL, MIBW, MLW, SFRA, SH, SHFR and SL. In this last CDA, 30 individuals were classified in the V group (Tables 4 and 5). Remarkably, most populations were mixed, with L. vulgare being dominant in A (52 %) and F (88 %), and L. narbonense in M (50 %), S (63 %) and T (70 %). On the other hand, V appeared to be a pure population constituted by V specimens only.
Fig. 3.
Canonical discriminant function analyses of morphometric data with pre-defined Limonium humile, L. narbonense and L. vulgare species and specimens from the Viana population. Individuals from each species are represented by coloured symbols. Each species centroid is represented by filled squares. Percentages of total variance explained by the functions are given in parentheses. 1: Aveiro; 2: Mira; 3: Sado; 4: Tejo; 5: Formosa; 6: Praia da Veiga, Viana do Castelo; 7: L. humile; 8: L. narbonense; 9: L. vulgare.
Table 4.
Summary of the canonical discriminant analysis of L. humile, L. narbonense, L. vulgare, Viana specimens and individuals collected in natural populations
| Original group | Predicted group membership classification results |
Total | |||
|---|---|---|---|---|---|
| L. humile | L. narbonense | L. vulgare | Viana | ||
| L. humile | 13 | 0 | 0 | 0 | 13 |
| L. narbonense | 0 | 16 | 1 | 0 | 17 |
| L. vulgare | 0 | 0 | 17 | 0 | 17 |
| Aveiro | 5 | 11 | 27 | 9 | 52 |
| Formosa | 0 | 0 | 15 | 2 | 17 |
| Mira | 2 | 11 | 3 | 6 | 22 |
| Sado | 0 | 19 | 9 | 2 | 30 |
| Tejo | 3 | 14 | 1 | 2 | 20 |
| Viana | 0 | 0 | 0 | 9 | 9 |
Table 5.
Pooled within-groups correlations between discriminating variables and standardized canonical discriminant functions of morphological characters
| Morphometric variable | Function |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| LL | 0·054 | 0·266* | –0·117 |
| MCL | –0·089 | –0·047 | 0·324* |
| MDS | –0·348* | 0·197 | –0·239 |
| MIBL | –0·192* | –0·093 | 0·082 |
| MIBW | –0·091 | 0·131* | 0·012 |
| MLTR | –0·157* | –0·017 | –0·119 |
| MLW | 0·047 | 0·252* | –0·191 |
| MMBL | –0·161 | –0·157 | –0·165* |
| MMBW | –0·034 | 0·088 | –0·249* |
| MNFS | –0·039 | –0·186* | –0·179 |
| MNSC | 0·428* | 0·053 | –0·121 |
| MOBL | –0·150* | –0·060 | –0·060 |
| MOBW | –0·099 | 0·031 | –0·133* |
| MSL | –0·541* | 0·318 | –0·211 |
| PL | –0·040 | –0·011 | 0·527* |
| SFRA | 0·216 | 0·325* | –0·235 |
| SH | –0·084 | 0·468* | –0·236 |
| SHFR | –0·129 | 0·366* | –0·034 |
| SL | –0·044 | 0·258* | 0·017 |
Variables were ordered by absolute size of correlation within function (*).
Details of acronyms are given in table 2.
Floral dimorphic vs. monomorphic populations
Heterostyly analyses revealed that most specimens were heterostylic (75·26 %; Fig. 4A, Table 6). Correspondence analysis of heterostyly–homostyly variables showed that heterostylous and homostylous individuals were significantly and heterogeneously distributed among populations (χ2 = 25·030; P = 0·0053). The first dimension of CA represented 88·93 % of explained variation, while the second dimension accounted for 11·07 % of the variation. A pin flower was the most frequent morphotype in the majority of populations (χ2 = 17·601; P = 0·0034). The V population differed significantly from the rest of the populations since most specimens showed homostylous flowers (χ2 = 18·450; P < 0·001).
Fig. 4.
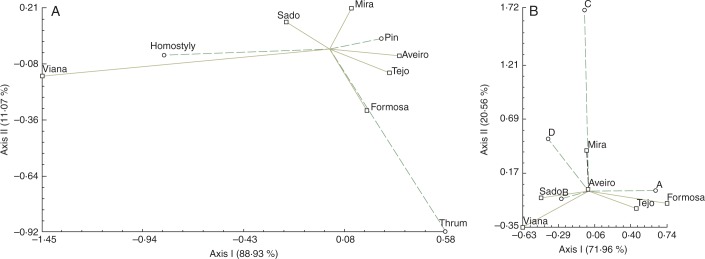
The first two axes of correspondence analysis based on heterostyly and homostyly (A), and on pollen–stigma combinations (B). Percentages of total variance explained by the functions are given in parentheses.
Table 6.
Frequencies (%) of floral heteromorphies in Limonium specimens collected in natural populations
| Population | n | Heterostyly (%) |
Homostyly (%) | Pollen–stigma combinations (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Pin | Thrum | A | B | C | D | |||
| Aveiro | 20 | 17·53 | 1·03 | 2·06 | 6·19 | 12·37 | 0 | 2·06 |
| Formosa | 11 | 8·25 | 1·03 | 2·06 | 7·22 | 4·12 | 0 | 0 |
| Mira | 21 | 17·53 | 0 | 4·12 | 6·19 | 12·37 | 1·03 | 2·06 |
| Sado | 21 | 14·43 | 0 | 7·22 | 2·06 | 17·53 | 0 | 2·06 |
| Tejo | 16 | 13·4 | 1·03 | 2·06 | 8·25 | 8·25 | 0 | 0 |
| Viana | 8 | 1·03 | 0 | 7·22 | 0 | 8·25 | 0 | 0 |
| Total | 97 | 72·16 | 3·09 | 24·74 | 29·9 | 62·89 | 1·03 | 6·19 |
n, number of individuals analysed.
Pollen–stigma analyses revealed that B was the most frequent combination (62·89 %; χ2 = 16·064; P = 0·0067). Only one individual exhibited flowers with combination C (Fig. 4B, Table 6). Correspondence analysis of pollen–stigma combination data showed that they were independently, non-significantly distributed among populations (χ2 = 23·314; P = 0·0777). The first dimension of CA represented 71·96 % of the variation, the second dimension 20·56 %, and the third dimension 7·48 %.
Estimations of seed set revealed that representative individuals from most populations showed that each plant produced >100 seeds per scape which had a low percentage of germination (26·5 %). This was not the case for specimens from the V population in which only two seeds were found, even after three visits in consecutive years (2011, 2012 and 2013) during the fructifying time (mid-July to the end of August). These two seeds, although they appeared to be mature, did not germinate.
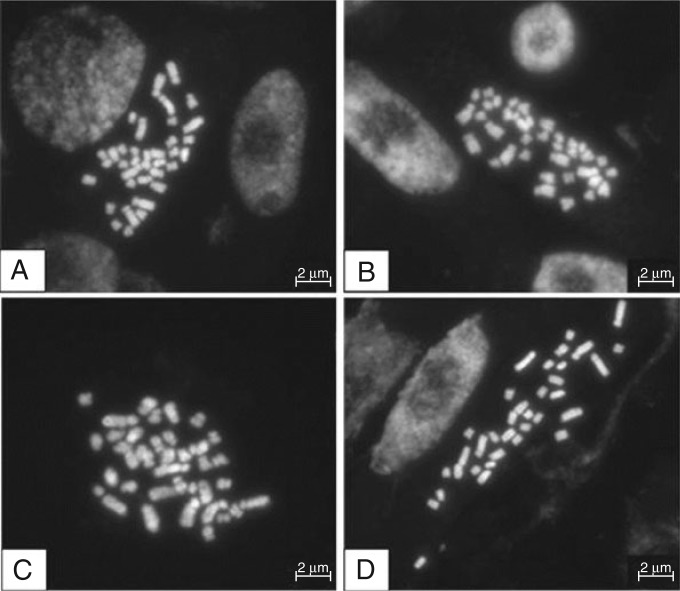
Karyotype analysis revealed that most individuals from the A and T populations had 2n = 4x = 36 chromosomes, but individuals with 2n = 4x = 35 and 38 chromosomes were also found (Fig. 5). In most metaphase cells, four pairs of large sub-metacentric chromosomes, four pairs of medium sub-metacentric chromosomes, three pairs of medium metacentric chromosomes and 14 small metacentric or telocentric chromosomes were exhibited.
Fig. 5.
Mitotic metaphase plates of DAPI-stained metaphase spreads from distinct individuals of Aveiro and Tejo populations. (A, B) Diploid individuals with 2n = 4x = 36. (C, D) Aneuploid individuals with 2n = 4x = 38 or 2n = 4x = 35 chromosomes.
DISCUSSION
Taxonomy has a significant role in delineation and protection of biodiversity (Domínguez Lozano et al., 2007). Studies based on herbarium data provide valuable information that is especially relevant for conservation purposes (Kricsfalusys and Trevisan, 2014) as they allow clarification of species distribution ranges and delimitate ambiguous species, which is the case for Limonium spp. TCG (Ennos et al., 2005). In the study presented here, morphological and reproductive differentiation is found in closely related taxa L. vulgare, L. humile and L. narbonense from the Iberian coasts.
Our results confirm that there is too little variation in morphological descriptive variables to differentiate specimens from distinct populations. Instead, it is clear that morphometric data allow better discrimination between species than morphological variables. Based on a comprehensive revision of species in south-west Europe (Erben, 1978), morphometric data have been employed to examine several species with the same ploidy level, such as the tetraploid species of the L. binervosum complex from western Europe (Ingrouille and Stace, 1986), or species with distinct ploidy levels such as tetraploid L. vulgare and hexaploid L. humile in the British Isles (Dawson and Ingrouille, 1995), and diploid and tetraploid species from L. ovalifolium and L. binervosum complexes in Portugal (Róis et al., 2013), or intraspecific variation in L. dufourii from eastern Spain (Rodríguez et al., 2003).
Data from PCA using morphometric variables reveal that most specimens show similarities with the species used as a reference, except for those from the V population. In the three represented components, these last (V) specimens were always separated from the first ones. Data from CDA using reference species revealed that L. vulgare specimens show higher morphological affinity with L. narbonense specimens than with those of L. humile. These first two species were discriminated from L. humile based primarily on the use of five morphometric variables, i.e. MIBL, MIBW, MNSC, MOBL, and MSL (first dimension). Limonium vulgare and L. narbonense separate from each other based on the remaining 14 morphometric variables (second dimension). In descriptions on Flora Iberica by Erben (1993), the variables MNSC and MLTR are not used to classify L. humile. Also, Erben (1993) did not use the variables MNFS and PL to discriminate L. vulgare from L. narbonense, which we found to be important in the present study.
Remarkably, in the CDA using the three reference species and the V population, a few specimens classified as L. vulgare in the first CDA (three reference species) are assigned to L. narbonense, supporting the view that these two species are morphologically very close. Previous studies have also suggested that these two species are morphologically (Erben, 1993) and phylogenetically (Palacios et al., 2000; Lledó et al., 2005) close, although they do not share habitats in eastern Spain. Our results demonstrate, for the first time, that L. narbonense grows together with L. vulgare and L. humile in Portuguese coasts and that the first two species are prevalent in most populations. Although L. narbonense is considered an unresolved name in the Plant List (http://www.theplantlist.org/), this species has been a focus of taxonomic, cytological, breeding system and genetic studies (Erben, 1993; Crespo and Lledó, 1998; Lledó et al., 2005; Georgakopoulou et al., 2006; Palop-Esteban et al., 2011). Confusion regarding its taxonomic identity is due to the numerous epithets which have been applied to this taxon (Erben, 1993; Crespo and Lledó, 1998). Although L. narbonense presents clear affinities with L. vulgare and L. humile, its taller size and distinct inflorescence structure allow an easy distinction in relation to these species. However, the V population differentiates from the three reference species by the morphometric variables LL, MIBW, MLW, SFRA, SH, SHFR and SL (Table 7).
Table 7.
Diagnostic morphometric characters among Limonium maritimum and its closest relatives L. humile, L. narbonense and L. vulgare
| L. maritimum | L. humile* | L. narbonense* | L. vulgare* | |
|---|---|---|---|---|
| LL | 2·7–6·4 | 6·2–26 | 7·0–27·5 | 3·5–17·0 |
| MIBW | 2·0–3·0 | 2·5–4·0 | 2·0–3·5 | 2–3·0 |
| MLW | 1·0–1·8 | 1·2–5·0 | 1·4–6·8 | 1·1–4·6 |
| SFRA | 0·1–0·4 | 0·2–0·5 | 0·4–1·0 | 0·3–0·7 |
| SH | 13·0–26·5 | 44·0–72·5 | 24·5–92·0 | 14·5–57·5 |
| SHFR | 5·4–9·0 | 19·0–40·0 | 6·0–40·3 | 6·0–36·0 |
| SL | 0·5–3·0 | 2·5–16·0 | 2·0–17·0 | 2·0–19·0 |
Acronyms and units are given in Table 2.
*According to Erben (1993).
It is interesting to verify that the differential characteristics of specimens from the V population are not restricted to their geographical distribution, because specimens of other populations, in particular, Aveiro and Mira, show these characteristics. In fact, except for Viana, most populations are mixed in the sense that individuals from distinct species are present in the same site. This indicates that there is no correlation between taximetry and geographic distribution, as the closest populations are not necessarily the most similar, nor are the populations furthest away the most distinct. In the morphometric studies by Dawson and Ingrouille (1995) for L. humile and L. vulgare in the British Isles, this situation was also observed.
Heterostyly, although not typical in Limonium, is present in L. vulgare (Baker, 1948, 1966), whereas pollen–stigma dimorphism associated with a sporophytic self-incompatibility system is common in Limonium spp. (Baker, 1966). Due to these flower heteromorphies which may facilitate cross-fertilization, L. vulgare and L. narbonense appear to be obligate outcrossers (Baker, 1948; Erben, 1979; Dawson and Ingrouille, 1995; Georgakopoulou et al., 2006) whereas L. humile seems to be a facultative inbreeder (Dawson and Ingrouille, 1995). Here, analysis of flower heteromorphies in the collected specimens reveal that two flower morphs (pin and thrum) and pollen–stigma dimorphisms exist within most populations. Thus, self-incompatible individuals within each of these populations may cross-fertilize. Cytoembryological development studies show that meiotic (sexual) embryo sacs are formed in L. vulgare (D'Amato, 1940). Interestingly, at least in the British Isles, the first two species grow mixed together in some saltmarshes, and morphological variants can be found in some of these sites (Boorman, 1966, 1967; Dawson, 1990; Dawson and Ingrouille, 1995). In this study, we hypothesize that hybridization between L. vulgare and L. narbonense is possible due to their breeding systems. In fact, morphological hybrids L. humile × L. vulgare and L. vulgare × L. narbonense have been described (Erben, 1993). Also, in L. vulgare and L. narbonense mixed populations (e.g. A and T), balanced tetraploids (2n = 4x = 36) and unbalanced aneuploid tetraploids (2n = 4x = 35 and 38) are found. Therefore, we cannot exclude that at least some of these aneuploid individuals could reproduce through apomixis. In fact, in both triploid and tetraploid Limonium spp., apomitic embryo sacs have been observed (D'Amato, 1940, 1949). In contrast to specimens from other populations, V specimens only show homostylic flowers with a unique pollen–stigma morph (B). As this combination is self-incompatible, our results suggest that at least these individuals might reproduce through apomixis. Collectively, our morphometric and flower morph data help to delineate a new taxon in Limonium TCG, especially in L. vulgare-related species, widespread along the coast of Portugal, Limonium maritimum Caperta, Cortinhas, Paes, Guara, Espírito-Santo and Erben sp. nova (Table 7; Figs 6 and Fig. 7; Appendix 3).
Fig. 6.
Type specimen of Limonium maritimum Caperta, Cortinhas, Paes, Guara, Espírito-Santo and Erben.
Fig. 7.
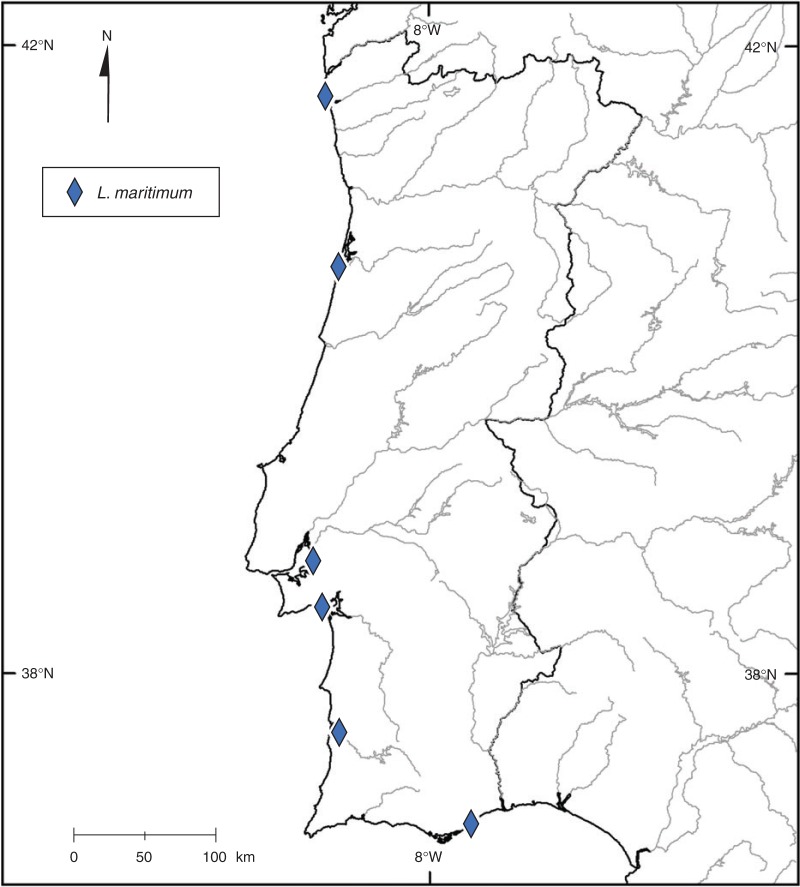
Distribution map of Limonium maritimum. Rhomboids in blue represent populations in which L. maritimum individuals are observed.
In conclusion, for the morphologically closely related Limonium taxa studied, here our data suggested that the species are relatively new and evolving. Further work should be focused on: (1) anatomical and physiological traits that can be used as taxonomical characters; (2) molecular studies in order to differentiate these species genetically; and (3) experimental controlled pollinations to provide evidence for potential hybridization between these taxa.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: mean values of morphometric characters in reference specimens Limonium humile, L. narbonense and L. vulgare, and collected specimens from natural populations. Table S2: Eingenvalues and percentages of variance associated with the first three principal components. Table S3: summary of the canonical discriminant analysis of Limonium spp. reference specimens, and collected specimens from natural populations. Table S4: pooled within-groups correlations between discriminating variables and standardized canonical discriminant functions of morphological characters. Figure S1: canonical discriminant function analyses of morphometric data with pre-defined Limonium humile, L. narbonense and L. vulgare reference species.
ACKNOWLEDGEMENTS
The authors wish to thank Augusta Barão (CBAA) for excellent technical assistance in karyotyping, and anonymous reviewers and Tim Flowers (University of Sussex) for helpful comments and suggestions. A.D.C. (Researcher, CBAA/ISA) and A.S.R. (SFRH/BD/62542/2009 grant) were supported by FUNDAÇÃO PARA A CIÊNCIA E TECNOLOGIA, FCT, Portugal). The project was funded by PEST-OE/AGR/UI0240/2011. A.D.C. designed and co-ordinated the study, and M.G. contributed to the experimental design. A.D.C., A.C. and A.P.P. performed plant sampling. A.C., M.E. and A.P.P. carried out morphometric, floral morphism and karyological analyses. A.C. and M.G. processed the raw data and carried out the statistical analysis. A.D.C., A.C. and M.G. drafted the manuscript. D.E.S. revised the manuscript. All authors read and approved the manuscript. The authors declare that they have no conflicts of interest.
APPENDIX
Appendix 1.
List of specimens used as reference species with voucher sample details
| Species | Herbarium number | Site location/Country | Collection date | Collectors |
|---|---|---|---|---|
| L. humile | ARAN 36106 | Euskal Herria, Gipuzkoa, Zarautz/Spain | 13–09–1986 | P. Garín |
| ARAN 9794 | Euskal Herria, Gipuzkoa, Zarautz, Desembocadura de Iñurritza erreka/Spain | 05–09–1982 | Mª Salaverría | |
| ARAN 36107 | Cantabria, San Vicente de la Barquera/Spain | 21–08–1984 | P. Catalán | |
| ARAN 52971 | Euskal Herria, Pyrénées Atlantiques, Baiona, St. Bernard/Spain | 24–09–1995 | I. Aizpuru | |
| ARAN 36105 | Cantabria, Argoños, Ancillo/Spain | 29–09–1985 | Aizpuru, Catalán and Aedo | |
| ARAN 55151 | Euskal Herria, Gipuzkoa, Zumaia/Spain | 07–08–1987 | J. Elorza | |
| ARAN 47391 | Euskal Herria, Gipuzkoa, Zumaia, Playa de Santiago/Spain | 26–07–1989 | J. L. Terés | |
| ARAN 47390 | ||||
| ARAN 9793 | Euskal Herria, Gipuzkoa, Zarautz, Desembocadura de Iñurritza erreka/Spain | 24–07–1981 | Lizaur y Salaverría | |
| MA 617149 | Cantabria: marisma de Rubín, junto a Abaño, San Vicente de la Barquera/Spain | 23–07–1983 | C. Aedo | |
| MA 681337 | Miengo, Mogro/Spain | 15–09–1995 | NA* | |
| MA 681334 | Cicero (Bárcena de Cicero)/Spain | 29–08–1985 | Herrá y Loricente | |
| MA 681335 | Euskal Herria, Gipuzkoa, Zarautz/Spain | 06–07–1973 | NA | |
| L. narbonense | MA 695629 | Ayamonte/Spain | 25–09–2011 | E. Sánchez Gullón |
| MA 394690 | Castellón, Oropesa del Mar: Punta del Faro/Spain | 08–08–1984 | García-Villaraco | |
| MA 426143 | Valencia: El Saler/Spain | 28–10–1981 | J. B. Peris | |
| VAL149647 | Alicante: Pego (la marina alta)/Spain | 08–12–1990 | R. Pérez | |
| VAL149560 | Alicante: Dénia (la marina alta)/Spain | 27–07–1991 | ||
| VAL149649 | Alicante: Pego (la marina alta)/Spain | 08–10–1987 | ||
| VAL 20801 | Valencia: Marjal de Oliva/Spain | 14–09–1987 | Pilar Soriano | |
| VAL 9382 | Castellón: Cabanes (la plana alta), Torrelasal/Spain | 07–08–1981 | NA | |
| VAL19384 | Castellón: Orpesa (Laplana Alta), Morro de Gós/Spain | 09–09–1989 | J. Tirado and C. Villaescusa | |
| VAL 19383 | Castelló: Cabanes (La plana Alta), Platja Torrelasal/Spain | 15–06–1989 | ||
| VAL187540 | Castelló: Xilxes (La Plana Baixa)/Spain | 01–11–1993 | A. Olivares | |
| VAL 21202 | Castelló: Orpesa (La plana alta), Far d' Orpesa/Spain | 27–07–1988 | A. Aguilella, I. Baeza and J. Riera | |
| VAL 19385 | Castelló: Castelló de la Plana (La Plana Alta), Aerodromo/Spain | 09–09–1989 | NA | |
| L. narbonense | VAL 27396 | Castelló: Torreblanca (La Plana Alta), Colt de Tomàs / Spain | 25–08–1992 | J. Tirado & C. Villaescusa, |
| VAL 27372 | ||||
| VAL 28341 | Castelló: Orpesa (La Plana Alta)/Spain | 27–08–1992 | ||
| VAL 19386 | Castelló: Alcalá de Xivert (El Baix Maestrat) Cap i Corp / Spain | 04–05–1991 | Villaescusa & Tirado | |
| VAL 172185 | Tarragona: Tamarit de Mar, La Mora / Spain | 18–09–1948 | C. Aedo | |
| L. vulgare | MA 348673 | La Coruña: Carnota, playa de Carnota / Spain | 10–08–1982 | S. Castroviejo |
| MA 459699 | La Coruña: Carballo, Playa de Baldayo / Spain | 29–07–1987 | E. Lago & C. Ferreiro, A. Paz | |
| MA 595187 | La Coruña: Carnota, marismas de Carnota / Spain | 30–07–1995 | R.I.Louzán | |
| MA 470215 | Vizcaya: Ria de Guernica, marismas de Axpe/Spain | 08–08–1946 | NA* | |
| MA 681330 | Soano (Arnuero)/Spain | 07–08–1981 | NA | |
| MA 593015 | Ria de Soano a Quejo/Spain | 06–08–1990 | C. Aseguinolaza and P. M. Echebarria | |
| MA 289828 | Algarve: Ilha de Tavira/Portugal | 20–04–1920 | Malato Belizetal | |
| MA 371528 | Beja, Vila Nova Mil Fontes, Rio Mira/Portugal | 23–04–1984 | M. Luceño | |
| MA 310871 | Beira Litoral: Bunheiro: Bestida/Portugal | 10–07–1975 | M. Beliz and J. A. Guerra | |
| MA 372195 | Aveiro, Ria de Aveiro, Murtosa/Portugal | 20–08–1984 | M. Luceño | |
| MA 372194 | ||||
| MA 289830 | Beira Litoral: Bunheiro: Bestida/Portugal | 10–07–1975 | M. Beliz and J. A. Guerra | |
| VAL 154052 | NA/UK | 1827 | Watson | |
| VAL 154054 | Nord Beveland, Kamperland/The Netherlands | September 31 | NA | |
| VAL 154053 | Gironde, Arcachon/France | August 30 | NA | |
| VAL 973674 | Cantabria, Oriñon, Ría de Oriñón/Spain | 14–09–1991 | J. Aparício, T. Pérez and P. Urrutia |
*NA, voucher without details.
Appendix 2.
List of taxa sampled in Portugal with voucher herbarium specimen details
| Herbarium | Herbarium number | Site location | Collection date | Collectors | Species† |
|---|---|---|---|---|---|
| Herbário da Estação Agronómica Nacional – LISE | LISE – 45358 | Aveiro, Murtosa | 23–08–1954 | Bento V. Rainha | L. maritimum |
| LISE – 6588 | Aveiro, Brunheiro | 11–09–1939 | A. R. Pinto da Silva | L. narbonense | |
| LISE – 4730 | Aveiro, Barra de Aveiro | 27–07–1938 | W. Rothmaler | L. maritimum | |
| LISE – 40159 | Póvoa de Santa Iria | 26–08–1942 | C. Fontes and Manuel da Silva | L. narbonense | |
| LISE – 40625 | Sacavém | 16–09–1942 | Manuel da Silva | L. narbonense | |
| LISE – 58240 | Barreiro | 23–07–1958 | Bento V. Rainha | L. narbonense | |
| LISE-94940 | Setúbal, rio Sado | 15–7–1977 | Manuel da Silva | L. vulgare | |
| LISE-5145 | Setúbal, Península | 17–9–1938 | W. Rothmaler | L. maritimum | |
| LISE – 94568 | Ilha da Armona | 13–7–1929 | M. H. Ramos Lopes | L. vulgare | |
| LISE – 92632 | Faro, Ilha das Lebres | 6–1916 | F. Mendes | L. maritimum | |
| Herbário João de Carvalho e Vasconcellos do Instituto Superior de Agronomia – LISI | LISI – 41897/1999 | Sé, ría Formosa | 08–07–1982 | J. G. Pedro and F. Nascimento | L. maritimum |
| LISI – 46618/1999 | NA* | 15–07–1988 | José Carlos Costa | L. vulgare | |
| LISI – 348/2010 | Quarteira, Praia do Ancão | 17–10–2009 | A. Caperta and A. R. Antunes | L. maritimum | |
| LISI – 46188/1999 | Panasqueira | 13–05–1988 | José Carlos Costa | L. narbonense | |
| LISI – 47146/1999 | Ilha da Armona | 05–08–1988 | L. narbonense | ||
| LISI – 46148/1999 | Fuzeta, Bias do Sul | 11–05–1988 | L. narbonense | ||
| LISI – 47129/1999 | Conceição, Cabanas de Tavira, Ribeira do Almargem | 04–08–1988 | L. vulgare | ||
| LISI – 813/2010 | Pedras D'el Rei | 05–07–2010 | A. Caperta, S. Róis and A. Paes | L. maritimum | |
| LISI – 259/2010 | 16–05–2010 | A. Caperta and S. Róis | L. maritimum | ||
| LISI – 552/2010 | 05–07–2010 | A. Caperta, S. Róis and A. Paes | L. vulgare | ||
| LISI – 553/2010 | L. vulgare | ||||
| LISI – 554/2010 | L. vulgare | ||||
| LISI-41781/1999 | Reserva Natural de Castro Marim,Venta-Moinhos | 22–06–1982 | M. Lousã | L. narbonense | |
| LISI-36273/1999 | Reserva Natural de Castro Marim | 11–07–1978 | L. narbonense | ||
| Herbário do Museu Nacional de História Natural – LISU | LISU – 150927 | Ribatejo, Pancas,ríoSorraia | 08–07–1982 | M. Correia, H. Bacelar and J. Cardoso | L. narbonense |
| LISU – 138887 | Ribatejo, Pancas | 30–09–1982 | J. Alves and C. Duarte | L. maritimum | |
| LISU – 29143 | Alcochete | 09–1883 | Pereira Coutinho | L. narbonense | |
| LISU – 29148 | Barreiro | 09–1883 | A. R. da Cunha | L. vulgare | |
| LISU – 29146 | Alcácer do Sal | 15–09–1980 | J. Daveau | L. narbonense | |
| LISU – 139914 | Faro, Ilha das Lebres | 06–1916 | F. Mendes | L. vulgare | |
| LISU – 29150 | 06–1916 | F. Mendes | L. narbonense | ||
| Herbário da Universidade de Coimbra – COI | COI – 20 | Vagos | 09–06–1961 | J. Paiva | L. maritimum |
| COI – 217 | Ria de Aveiro, Barra de Aveiro | 04–07–1967 | J. Ormonde & R. Rodrigues | L. vulgare | |
| COI – 1030 | Aveiro, Murtosa | 1978 | A.Marques | L. vulgare | |
| COI – 1340 | Aveiro, Gafanha da Encarnação | 18–06–1979 | A.Marques | L. vulgare | |
| COI – 1491 | Aveiro, Murtosa | 03–12–1979 | A.Marques | L. narbonense | |
| COI – 13866 | Aveiro, Barra de Aveiro | 27–07–1938 | W. Rothmaler | L. vulgare | |
| COI – 13759 | Aveiro | 29–07–1976 | Alexandrino Matos, Manuel & Alves | L. vulgare | |
| COI – 845 | Sacavém | NA | NA | L. maritimum | |
| COI – 66 | Setúbal, Pântanos da Cotovia | 08–1900 | A.Luisier | L. vulgare | |
| NA* | 09–1906 | A.Luisier | L. narbonense | ||
| Herbário da Universidade do Porto – PO | PO – 6448 G.S. | Estarreja | 08–1984 | A. Egas Moniz | L. vulgare |
| PO – 6449 G.S. | Ria de Aveiro | 08–1898 | Gonçalo Sampaio | L. maritimum | |
| PO – 6450 G.S. | Ílhavo | 30–07–1901 | L. vulgare | ||
| PO- 6451 G.S. | Ria de Aveiro: Gafanha | 09–1898 | L. vulgare | ||
| PO – 52837 | Ílhavo | 21–7–1987 | A. Serra, Armando and Loureiro | L. maritimum | |
| PO – 18798 | Ovar: Marinha | 30–11–1958 | Martins d'Alte and G. Costa | L. narbonense | |
| PO – 18801 | Ílhavo | 27–06–1964 | A. Rozeira and G. Costa | L. narbonense | |
| PO – 18802 | Ria de Aveiro | 28–07–1964 | A. Rozeira | L. maritimum | |
| PO – 18803 | Ílhavo: Gafanha da Nazaré | 16–08–1965 | A. Rozeira | L. maritimum | |
| PO – 18799 | Faro, Salinas | 17–06–1962 | A. Rozeira, K. Koepp and G. Costa | L. vulgare |
*NA, voucher without details.
†Classification results after canonical discriminant analysis.
Appendix 3 Description of Limonium maritimum Caperta, Cortinhas, Paes, Guara, Espírito-Santo and Erben sp. nov.
Type: Portugal, Mi; Viana do Castelo: Praia da Veiga, Areosa. Alt. 0–1 m, UTM: 29 T NG11, 24–08–2013, Ana Caperta (holotype: LISI-1020/2013, isotype: Herb. Erben)
Planta perennis, glabra, viridis, 13–26·5 cm alta, foliis 2·7–6·4 × 1–1·8 cm, oblanceolatis, apice obtusis ad acutis, aristatis, pinnatis, caulibus paucis, ramis sterilibus absentibus vel paucissimis, spicis 0·7–4·0 cm longis, spiculis (1-) 2–3-floris, remotis ad 4–5 in 1 centimetro dispositis, bractea inferiore 2·0–3·5 × 1·0–2·0 mm, tiangulari-ovata, bractea media 2·5–5·5 × 1·0–2·0 mm, anguste triangulari-ovata, bractea superiore 3·0–6·0 × 2·0–3·0 mm, anguste elliptica, mucronata, calyce obconico, 5·0–6·5 mm longo, tubo unilateraliter sparsim longe piloso. Corolla violacea.
Perennial plant, glabrous, forming a sub-shrub 13–26·5 cm tall, with few erect stems and a robust tap root. Caudices 8·0–15 cm long, branched, living leaves in rosettes at apices. Leaves fleshy, grey-green, glaucous, 2·7–6·4 × 1·0–1·8 cm, oblanceolate, apex obtuse at acute, mucronate, with one central nerve and some pinnately branching lateral nerves, gradually tapering into the petiole; the majority withered at anthesis. Stems 5·4–9·0 cm long, rugose, nearly straight to slightly flexuous, with branching normally in the middle of each of the stems. Inflorescence corymbose or obtrullate in outline. Sterile branches absent or very few. Fertile branches 7·6–17·5 cm long, straight, directed obliquely upwards, forming branching angels of 5–24 °, longer branches in the upper half divided. Spikes 0·7–4·0 cm long, straight, erect to directed obliquely upwards. Spikelets composed of (1-) 2–3 flowers, remotely arranged with 4–5 per cm, adpressed in the axis of spices. Outer bract 2·0–3·5 × 1·0–2·0 mm, triangular-ovate, acute, mucronate; bract margin membranous; central part fleshy, acuminate. Middle bract membranous, 2·5–5·5 × 1·0–2·0 mm, narrowly triangular-ovate. Inner bract 3·0–6·0 × 2·0–3·0 mm, narrowly elliptic, normally mucronate; bract margin broadly membranous; central part fleshy, green, oblong, acuminate. Calyx obconical, 5·0–6·5 mm long; calyx tube on one half sparsely, long hairy, with five ribs ending at the base of the lobes. Corolla violet.
LITERATURE CITED
- Arrigoni PV, Diana S. Contribution à la connaissance du genre Limonium en Corse. Candollea. 1993;482:631–677. [Google Scholar]
- Artelari R. Kamari G, Felber F, Garbari F, editors. Reports 51–55. Mediterranean chromosome number reports 2. 1992;2:229–232. Flora Mediterranea. [Google Scholar]
- Baker HG. Dimorphism and monomorphism in the Plumbaginaceae. 1. A survey of the family. Annals of Botany. 1948;12:207–219. [Google Scholar]
- Baker HG. Dimorphism and monomorphism in the Plumbaginaceae. 2. Pollen and stigmata in the genus Limonium. Annals of Botany. 1953a;17:433–445. [Google Scholar]
- Baker HG. Dimorphism and monomorphism in the Plumbaginaceae. 3. Correlation of geographical distribution patterns with dimorphism and monomorphism in Limonium. Annals of Botany. 1953b;17:615–627. [Google Scholar]
- Baker HG. The evolution, functioning and breakdown of heteromorphic incompatibility systems. I. The Plumbaginaceae. Evolution. 1966;20:349–368. doi: 10.1111/j.1558-5646.1966.tb03371.x. [DOI] [PubMed] [Google Scholar]
- Boorman LA. Experimental studies in the genus Limonium. UK: University of Oxford; 1966. PhD Thesis. [Google Scholar]
- Boorman LA. Journal of Ecology. Vol. 55. –; 1967. Limonium vulgare Miller and L. humile Miller, in the biological flora of the British Isles; pp. 221–232. [Google Scholar]
- Boorman LA. Some aspects of the reproductive biology of Limonium vulgare and L. humile. Annals of Botany. 1968;32:803–807. [Google Scholar]
- Boorman LA. Studies in salt marsh ecology with special reference to the genus Limonium. Journal of Ecology. 1971;59:103–120. [Google Scholar]
- Caperta AD, Rosa M, Delgado M, et al. Distribution patterns of phosphorylated Thr 3 and Thr 32 of histone H3 in plant mitosis and meiosis. Cytogenetic and Genome Research. 2008;122:73–79. doi: 10.1159/000151319. [DOI] [PubMed] [Google Scholar]
- Castro M, Rosselló JA. Karyology of Limonium (Plumbaginaceae) species from the Balearic Islands and the western Iberian Peninsula. Botanical Journal of the Linnean Society. 2007;155:257–272. [Google Scholar]
- Costa JC, Neto C, Aguiar C, et al. Vascular plant communities in Portugal Continental, the Azores and Madeira. Global Geobotany. 2012;2:1–180. [Google Scholar]
- Cowan R, Ingrouille MJ, Lledó MD. The taxonomic treatment of agamosperms in the genus Limonium Mill. Plumbaginaceae. Folia Geobotanica. 1998;33:353–366. [Google Scholar]
- Crespo Villalba MB, Lledó MD. El genero Limonium Mill. (Plumbaginaceae) en la Comunidad Valenciana: taxonomia y conservacion. Alicante: Generalitat Valenciana, Conselleria de Medio Ambiente; 1998. [Google Scholar]
- D'Amato F. Contributo all'embriologia delle Plumbaginaceae. Nuovo Giornale Botanico Italiano. 1940;47:349–382. [Google Scholar]
- D'Amato F. Triploidia e apomissia in Statice oleafolia Scop. var. confusa Godr. Caryologia. 1949;401:157–161. [Google Scholar]
- Dawson HJ. Chromosome numbers in two Limonium species. Watsonia. 1990;18:82–84. [Google Scholar]
- Dawson HJ, Ingrouille MJ. A biometric survey of Limonium vulgare Miller and L. humile Miller in the British Isles. Watsonia. 1995;20:239–254. [Google Scholar]
- Dolcher T, Pignatti S. Un'ipotesi sull'evoluzione dei Limonium del bacino mediterraneo. Nuovo Giornale Botanico Italiano. 1971;105:95–107. [Google Scholar]
- Domínguez Lozano F, Saiz JCM, Ollero HS, Schwartz MW. Effects of dynamic taxonomy on rare species and conservation listing: insights from the Iberian vascular flora. Biodiversity Conservation. 2007;16:4039–4050. [Google Scholar]
- Ennos RA, French GC, Hollingsworth PM. Conserving taxonomic complexity. Trends in Ecology and Evolution. 2005;20:164–168. doi: 10.1016/j.tree.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Erben M. Die Gattung Limonium im südwestmediterranen Raum. Mitteilungen der botanischen staatssammlung München. 1978;14:361–626. [Google Scholar]
- Erben M. Karyotype differentiation and its consequences in Mediterranean Limonium. Webbia. 1979;34:409–417. [Google Scholar]
- Erben M. Limonium Mill. In: Castroviejo S, Aedo C, Cirujano S, editors. Flora Iberica 3. Madrid: CSIC; 1993. pp. 2–143. [Google Scholar]
- Flowers T, Colmer T. Salinity tolerance in halophytes. New Phytologist. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Foucart T. Analyse factorielle Programmation sur micro-ordinateurs. 2nd edn. Paris: Masson; 1982. [Google Scholar]
- Franco JA. Nova Flora de Portugal (Continente e Açores) II. Lisboa: Edição do Autor; 1984. [Google Scholar]
- Ganders FR. The biology of heterostyly. New Zealand Journal of Botany. 1979;17:607–635. [Google Scholar]
- Georgakopoulou A, Manousou S, Artelari R, Georgiou O. Breeding systems and cytology in Greek populations of five Limonium species Plumbaginaceae. Willdenowia. 2006;362:741–750. [Google Scholar]
- Greuter W, McNeill J, Barrie FR, et al. International Code of Botanical Nomenclature (Saint Louis Code) adopted by the 16th International Botanical Congress, St Louis, Missouri, USA, July-August 1999. Regnum Vegetabile. 2000;138:1–474. [Google Scholar]
- Grigore M-N, Ivanescu L, Toma C. Halophytes: an integrative anatomical study. New York: Springer; 2014. [Google Scholar]
- Holmgren PK, Holmgren NH, Barnett LC. Index Herbariorum. Part I: the herbaria of the world, 8th edn. Regnum Vegetabile. 1990;120:1–693. [Google Scholar]
- Ingrouille MJ. A taxometric analysis of Limonium Plumbaginaceae in western-Europe. Plant Systematics and Evolution. 1984;147:103–118. [Google Scholar]
- Ingrouille MJ, Stace CA. The Limonium binervosum aggregate Plumbaginaceae in the British Isles. Botanical Journal of the Linnean Society. 1986;92:177–217. [Google Scholar]
- Kricsfalusys VV, Trevisan N. Prioritizing regionally rare plant species for conservation using herbarium data. Biodiversity Conservation. 2014;23:39–61. [Google Scholar]
- Kubitzki K. Plumbaginaceae. In: Kubitzki K, Rohwer JG, Bittrich V, editors. The families and genera of vascular plants 2. Berlin: Springer; 1993. pp. 523–530. [Google Scholar]
- Lledó MD, Crespo MB, Cameron KM, Fay MF, Chase MW. Systematics of Plumbaginaceae based upon cladistic analysis of rbcL sequence data. Systematic Botany. 1998;23:21–29. [Google Scholar]
- Lledó MD, Karis PO, Crespo MB, Fay MF, Chase MW. Phylogenetic position and taxonomic status of the genus Aegialitis and subfamilies Staticoideae and Plumbaginoideae (Plumbaginaceae): evidence from plastid DNA sequences and morphology. Plant Systematics and Evolution. 2001;229:107–124. [Google Scholar]
- Lledó MD, Crespo MB, Fay MF, Chase MW. Molecular phylogenetics of Limonium and related genera Plumbaginaceae: biogeographical and systematic implications. American Journal of Botany. 2005;92:1189–1198. doi: 10.3732/ajb.92.7.1189. [DOI] [PubMed] [Google Scholar]
- van der Maarel E, van der Maarel-Versluys M. Distribution and conservation status of littoral vascular plant species along the European coasts. Journal of Coastal Conservation. 1996;2:73–92. [Google Scholar]
- Nowicke J, Skvarla J. Pollen morphology and the relationship of the Plumbaginaceae, Polygonaceae, and Primulaceae to the Order Centrospermae. Smithsonian Contributions to Botany. 1977;37:1–64. [Google Scholar]
- Palacios C, Rosselló JA, González-Candelas F. Study of the evolutionary relationships among Limonium species Plumbaginaceae using nuclear and cytoplasmic molecular markers. Molecular Phylogenetics and Evolution. 2000;14:232–249. doi: 10.1006/mpev.1999.0690. [DOI] [PubMed] [Google Scholar]
- Palop-Esteban M, Segarra-Moragues J, González-Candelas F. Polyploid origin, genetic diversity and population structure in the tetraploid sea lavender Limonium narbonense Miller (Plumbaginaceae) from eastern Spain. Genetica. 2011;139:1309–1322. doi: 10.1007/s10709-012-9632-2. [DOI] [PubMed] [Google Scholar]
- Pandža M, Franjić J, Škvorc Ž. The salt marsh vegetation on the East Adriatic coast. Biologia. 2007;62:24–31. [Google Scholar]
- Pignatti S. Studi sui Limonium, VIII. In: Heywood VH, editor. Florae Europaea. Notulae systematicae ad Flora Europaeam spectantes. Botanical Journal of the Linnean Society. 1971. 64: 353–381. [Google Scholar]
- Pignatti S. Limonium. In: Tutin TG, Heywood VH, Burges NA, editors. Flora Europaea 3. Cambridge: Cambridge University Press; 1972. pp. 38–50. [Google Scholar]
- Podani J. Multivariate data analysis in ecology and systematics: a methodological guide to the SYN-TAX 5·0 package. The Hague: SPB Publishing; 1994. [Google Scholar]
- Richards AJ. Plant breeding systems. 2nd edn. London: Chapman and Hall; 1997. [Google Scholar]
- Rodríguez S, Palop ML, Palacios C, González-Candelas F. Molecular and morphological differentiation in Limonium dufourii (Plumbaginaceae), an endangered Mediterranean plant. Conservation Genetics. 2003;43:383–391. [Google Scholar]
- Rohlf FJ. New York: Exeter Software: Setauket; 2009. NTSYSS pc: numerical taxonomy system ver. 2.21c. [Google Scholar]
- Róis AS, Teixeira G, Sharbel TF, et al. Male fertility versus sterility, cytotype, and DNA quantitative variation in seed production in diploid and tetraploid sea lavenders Limonium sp. (Plumbaginaceae) reveal diversity in reproduction modes. Sexual Plant Reproduction. 2012;25:305–318. doi: 10.1007/s00497-012-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Róis AS, López CMR, Cortinhas A, et al. Epigenetic rather than genetic factors may explain phenotypic divergence between coastal populations of diploid and tetraploid Limonium spp. (Plumbaginaceae) in Portugal. BMC Plant Biology. 2013;13:305–318. doi: 10.1186/1471-2229-13-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon CE. Notes on Limonium, III – Limonium vulgare Mill. Journal of Botany. 1905a;43:5–14. [Google Scholar]
- Salmon CE. Notes on Limonium, IV – Limonium humile. Journal of Botany. 1905b;43:54–59. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry The principles and practice of statistics in biological research. 4th edn. New York: W. H. Freeman and Company; 2012. [Google Scholar]
- Suárez-García C, Pérez de Paz J, Febles R, Caujapé-Castells J. Genetic diversity and floral dimorphism in Limonium dendroides (Plumbaginaceae), a woody Canarian species on the way of extinction. Plant Systematics and Evolution. 2009;280:105–117. [Google Scholar]
- Zar JH. Biostatistical analysis. 5th edn. Upper Saddle River, NJ: Pearson Education International; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.