Abstract
Background As important components in saline agriculture, halophytes can help to provide food for a growing world population. In addition to being potential crops in their own right, halophytes are also potential sources of salt-resistance genes that might help plant breeders and molecular biologists increase the salt tolerance of conventional crop plants. One especially promising halophyte is Suaeda salsa, a euhalophytic herb that occurs both on inland saline soils and in the intertidal zone. The species produces dimorphic seeds: black seeds are sensitive to salinity and remain dormant in light under high salt concentrations, while brown seeds can germinate under high salinity (e.g. 600 mm NaCl) regardless of light. Consequently, the species is useful for studying the mechanisms by which dimorphic seeds are adapted to saline environments. S. salsa has succulent leaves and is highly salt tolerant (e.g. its optimal NaCl concentration for growth is 200 mm). A series of S. salsa genes related to salt tolerance have been cloned and their functions tested: these include SsNHX1, SsHKT1, SsAPX, SsCAT1, SsP5CS and SsBADH. The species is economically important because its fresh branches have high value as a vegetable, and its seed oil is edible and rich in unsaturated fatty acids. Because it can remove salts and heavy metals from saline soils, S. salsa can also be used in the restoration of salinized or contaminated saline land.
Scope Because of its economic and ecological value in saline agriculture, S. salsa is one of the most important halophytes in China. In this review, the value of S. salsa as a source of food, medicine and forage is discussed. Its uses in the restoration of salinized or contaminated land and as a source of salt-resistance genes are also considered.
Keywords: Amaranthaceae, dimorphic seeds, food, forage, germination, halophyte, heavy metal, medicine, saline agriculture, salt-resistance genes, salt tolerance, Suaeda salsa, succulence
INTRODUCTION
More than 800 million ha of land worldwide, or about 6 % of the world's total land area, are salt-affected (Munns, 2005). Global annual losses in agricultural production from salt-affected land are in excess of US$12 billion and rising. At the same time, the increasing amount of arable land being lost to urban sprawl is forcing agricultural production into marginal areas (Shabala, 2013). One tool that can be useful for dealing with salt-affected land is halophytic plants.
Le Houérou (1993) estimated that there are 5000–6000 halophytic species, which represent about 2 % of all angiosperms. Flowers and Colmer (2008) defined halophytes as those plants that are able to survive and reproduce in environments where the salt concentration is ≥200 mm NaCl, which reduced the number of halophytes to about 1 % of the world flora. Such differences highlight the arbitrary nature of the definitions. Among halophytic species, only a small number (fewer than 500 species) are tolerant of seawater salinity (Flowers et al., 2010); most halophytes can resist only lower salt concentrations. In a saline agricultural system, the salinity of the soil is perhaps half that of seawater (Rozema and Flowers, 2008), and so it seems possible that some halophytes could be developed as salt-tolerant crops. A better understanding of how halophytes tolerate saline soils could also help plant breeders and molecular biologists increase the salt tolerance of conventional crop plants (Glenn and Brown, 1999).
According to a survey of plants that are able to grow and reproduce when salt concentrations range from 0·8 to 4·2 % based on dry soil, China contains about 587 halophytic species (Zhao et al., 2011). Halophytic species are currently widely studied in China because of their value for the development of saline agriculture. An example is the euhalophytic herb Suaeda salsa. Euhalophytes, which can dilute salt within their succulent leaves or stems and thus have high salt tolerance, are valuable for understanding how dicotyledonous plants can tolerate salt (Flowers and Colmer, 2008; Huchzermeyer and Flowers, 2013; Rozema and Schat, 2013). The potential of several highly salt-tolerant, succulent halophytes in the Amaranthaceae (especially species of Suaeda and Salicornia) for use in saline agriculture has been reported in a series of papers (Rozema and Flowers, 2008; Glenn et al., 2013; Rozema and Schat, 2013; Ventura and Sagi, 2013). S. salsa is highly salt tolerant; the optimal NaCl concentration for its growth is 200 mm, and it grows as well with 400 mm NaCl as with 10 mm NaCl (Song et al., 2009). A series of genes related to S. salsa salt tolerance have been cloned and their functions tested, i.e. the species is a promising model for understanding salt tolerance. At the same time, fresh branches of the species have substantial value as a vegetable, and its seed oil is edible and is rich in unsaturated fatty acids, and thus the species can be used for developing saline agriculture. Plant breeders in China have been attempting to increase S. salsa seed yield in saline soils (Shao et al., 2004). Here, we review the value of S. salsa from economic and ecological perspectives. We also consider its value as a model halophyte in the Amaranthaceae.
BIOLOGICAL AND ECOLOGICAL CHARACTERISTICS OF S. SALSA
Suaeda salsa Pall. (Caryophyllales, Amaranthaceae, Chenopodioideae) is a leaf-succulent halophytic herb with high salt tolerance during germination and seedling stages (W. Li et al., 2005; Song et al., 2008). The species is distributed in Europe and Asia. Although S. salsa in China grows in areas that differ greatly in geography and ecology, it grows better in littoral saline soils, for example the Yellow River Delta, than in saline inland soils of arid zones, such as the Zhunger Basin in China (X. Li et al., 2012). S. salsa cannot be found in soils that contain <5 g of salt kg–1 dry soil, and the optimal salt concentration for its growth is between 15 and 20 g kg–1 dry soil (Gu, 1999). When the salt concentration in soil exceeds 20 g kg–1 dry soil in inland saline soil or in the intertidal zone, S. salsa forms a monospecific community (Table 1) (Gu, 1999). The species occurs both in coastal salt marshes and in saline inland sites in China (Song et al., 2008). S. salsa leaves and stems are green in inland saline soils but red–violet in the intertidal zone during the entire growth period; the red–violet colour is due to the accumulation of betacyanin (Fig. 1). S. salsa seeds germinate in late April. The plants flower from July onwards, and seeds begin to mature in late September (Gu, 1999). An adult plant that is representative of the inland S. salsa population is shown in Fig. 2.
Table 1.
The abundance of Suaeda salsa and accompanying plant species as related to soil salt content (reproduced from Gu, 1999)
| Salt content (g kg–1 dry soil) | Suaeda salsa | Aeluropus littoralis | Imperata cylindricα | Artemisia spp. | Phragmites australis |
|---|---|---|---|---|---|
| <5 | + | + + | + + + | + + + | |
| 5–10 | + | + + | + + + | + + + | + + + |
| 10–15 | + + | + + + | + + | + + | + |
| 15–20 | + + + | + | |||
| 20–30 | + + | ||||
| >30 | + |
Plus signs indicate the abundance of the plant species.
Fig. 1.
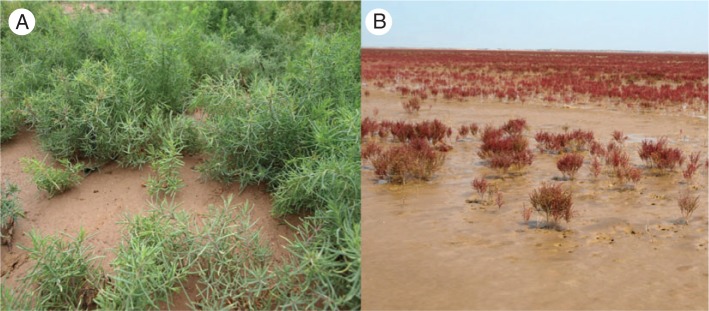
Suaeda salsa growing at an inland site with saline soil (A) and at an intertidal zone (B).
Fig. 2.
A Suaeda salsa plant growing from seed of a plant occurring in inland saline soil. The above-ground plant (A), the flowering branches (B) and flower (C). The bar in C indicates 0·5 mm.
DIMORPHIC SEEDS
Germination and dormancy
The term seed heteromorphism refers to the production by individual plants of seeds with different form or behaviour (Gul et al., 2013). Seed heteromorphism has been reported in 26 families, 129 genera and 292 species of angiosperms; these plants are mainly members of the Asteraceae and Chenopodiaceae (now the Amaranthaceae) that grow in highly variable environments such as arid regions, semi-arid regions, deserts and saline soils. Seed heteromorphism is generally considered to be an adaptive strategy that enables plants to escape from sib competition and to tolerate a changeable environment (L. Wang et al., 2010).
Seed dimorphism and polymorphism are known for many halophytic species, for example Atriplex patens (He and Li, 1995), Suaeda moquinii (Khan et al., 2001), Suaeda aralocaspica (L. Wang et al., 2008) and Suaeda acuminata (H. L. Wang et al., 2012). S. salsa also produces dimorphic seeds (W. Li et al., 2005; Song et al., 2008), i.e. soft brown seeds and hard black seeds. Brown seeds absorbed water more quickly than black seeds; brown seeds have a higher germination rate than black seeds under salinity, while black seeds are more sensitive than brown seeds to salinity in the absence of light; the optimal NaCl concentration for germination is about 400 mm for brown seeds and about 100 mm for black seeds (W. Li et al., 2005). In S. acuminata, the two seed types (brown vs. black seed) absorbed water at different rates with brown seeds imbibing water faster; germination percentages of brown seeds were significantly higher than those of black seeds in all temperature and light regimes tested (H. L. Wang et al., 2012). This indicates that heteromorphic seeds present different germination characteristic to help their mother plants adapt to a changeable environment.
After S. salsa seeds were stored for 1 year at approximately 20 °C and 30–40 % relative humidity, germination decreased for brown seeds but increased for black seeds regardless of salinity. Stratification and gibberellic acid (GA4) treatments improved germination of black seeds but had little effect on brown seeds (W. Li et al., 2008). The latter authors hypothesized that GA4 may be the active form of endogenous gibberellin that releases dormancy and promotes germination of black seeds of S. salsa, and that certain environmental factors (e.g. stratification, soil salinity and storage) may affect S. salsa seed germination by affecting the biosynthesis of gibberellins, especially GA4 (W. Li et al., 2008). In another desert annual halophyte, S. acuminata, germination of black seeds was promoted by exogenous gibberellic acid (GA3) but not by 8 weeks of cold stratification (H. L. Wang et al., 2012). In non-dormant S. acuminata brown seeds, contents of zeatin riboside (ZR), GA3 and abscisic acid (ABA) were significantly higher than in dormant black seeds, while contents of indole-3-acetic acid (IAA) were significantly higher in black than in brown seeds (H. L. Wang et al., 2012). These results indicated that interactions among ZR, ABA and GA3 may affect the dormancy of both seed types. The role of endogenous hormones in the dormancy and germination of dimorphic S. salsa seeds warrants further investigation.
Seed development under salinity
In a greenhouse sand-culture experiment, X. Li et al. (2011) found that salinity during the seed maturation stage benefited S. salsa in that it maintained brown seed (black seed of S. salsa was not used in the literature of this section) viability and ensured seedling emergence and population establishment. A preliminary study showed that, at 500 mm NaCl, the PG (phosphatidylglycerol) and SQDG (sulfoquinovosyldiacylglycerol) levels and the DGDG/MGDG (digalactosyldiacylglycerol/monogalactosyldiacylglycerol) ratio were higher in germinated brown seeds whose source plants had been cultured with 500 mm rather than with 1 mm NaCl. When brown seeds were incubated with 600 mm NaCl, the conductivity and malondialdehyde (MDA) concentration in the embryos were greater if the source plants had been cultured in 1 mm rather than in 500 mm NaCl. These results indicate that salinity during seed maturation may increase the salt tolerance of seeds by changing the lipid composition of membranes (Zhou et al., 2014). The mechanism that explains why the viability of S. salsa seeds relies on salt during plant culture remains to be determined.
Seed photosynthesis allows biosynthetic fluxes by providing ATP and oxygen, which are readily used for respiration and biosynthesis (Weber et al., 2005). Rolletschek et al. (2003) showed that oxygen production in maturing cotyledons of Vicia faba was able to reach 50 % of atmospheric oxygen concentration within approx. 5 min. In a field investigation, X. Li et al. (2012) reported that oxygen production was greater in maturing embryos of S. salsa brown seeds in the intertidal population than in the inland population (the oxygen production in fresh embryos was 4 times greater per embryo, and 3 times greater per gram fresh weight of embryo for the intertidal population than for the inland population), which may be related to the greater proportion of brown seeds in the intertidal than in the inland population (Song et al., 2008).
Cotyledons in embryos of maturing brown seeds of the inland and intertidal populations of S. salsa are green and contain chlorophyll, although the chlorophyll content in the embryos is much lower in mature seeds than in maturing seeds of both populations (X. Li et al., 2012). However, the embryos of mature S. salsa seeds did not photosynthesize during germination (X. Li et al., 2012). In contrast, cotyledons of mature seeds of the desert halophyte Suaeda physophora are dark green and photosynthetically active during germination (Y. Li et al., 2008; S. R. Zhang et al., 2010). S. physophora is a common plant species in the Zhunger Basin, Xinjiang, north-western China, which is one of the driest regions in the world (Song et al., 2005). Chlorophyll in mature seeds of S. physophora may help the species to establish in saline and arid regions. In a field investigation, Zhang (2009) identified about 20 halophytic species that contain chlorophyll in the cotyledons of mature seeds. All of these species, which include Salsola foliosa, S. acuminata, Halogeton glomeratus and S. physophora, are succulent halophytes in the Amaranthaceae and are distributed in the inland saline arid zone in China. However, none of the halophytes in littoral saline soils in China, such as S. salsa, Suaeda glauca and Kochia scoparia, contains chlorophyll in the cotyledons of mature seeds. The question arises: Why do mature seeds of halophytes such as S. salsa in littoral saline soils contain very little chlorophyll while mature seeds of halophytes such as S. physophora in the inland saline arid zone of the Zhunger Basin contain substantial chlorophyll (Fig. 3)? The probable answer relates to differences in moisture availability. Suitable conditions for germination in the Zhunger Basin are limited to short periods in early spring when there is snowmelt or intermittent rainfall. Because moisture availability is limited, seeds must germinate as soon as possible. Thus, green cotyledons may benefit these species in that they enhance seedling growth and establishment under arid conditions (S. R. Zhang et al., 2010). In the littoral saline soils, by contrast, there is more rainfall and water is not so limiting as in arid regions. Thus, the rapid growth and establishment of S. salsa seedlings does not seem to depend on photosynthesis during germination.
Fig. 3.
Morphology of the embryos in Suaeda physophora and Suaeda salsa seeds. The embryo in a mature S. physophora seed without moistening (A) and after moistening with distilled water for 12 h (B). The embryo in a mature brown S. salsa seed from saline inland soil without moistening (C) and after moistening with distilled water for 10 h (D). Scale bars = 1 mm. The photographs in A and B are reproduced from S. R. Zhang et al. (2010), the photograph in C is reproduced from Song et al. (2008) and the photograph in D is reproduced from X. Li et al. (2012).
SOURCES OF SALT-TOLERANCE GENES
Na+ accumulation in vacuoles
As noted earlier, S. salsa is highly salt tolerant and is a good source for genes that confer salt tolerance. A series of S. salsa genes related to salt tolerance have been cloned and their functions tested (Table 2). By affecting the accumulation of inorganic cations and anions, some of these genes help regulate the osmotic balance in the vacuole, a very important feature of halophytes (Short and Colmer, 1999). The roots of Suaeda maritima have two low-affinity Na+-uptake pathways: pathway 1 regulates Na+ uptake under low salinity (e.g. 25 mm NaCl), and pathway 2 regulates Na+ uptake under high salinity (e.g. 150 mm NaCl). Pathway 1 might be mediated by a high-affinity K transporter-type transporter, and pathway 2 by an AKT1-type channel (S. M. Wang et al., 2007). The Na+/H+ antiporter in the tonoplast plays a central role in salinity tolerance, and its activity is tightly controlled by the electrochemical H+-gradient across the tonoplast, a gradient that is generated by V-H+-ATPase and V-H+-PPase (Lv et al., 2012). Efficient vacuolar sequestration of cytotoxic Na+ is considered an important feature of halophytes. This sequestration requires that Na+ be pumped into the vacuole against the electrochemical gradient and that Na+ in the vacuole be prevented from leaking back into the cytosol (Bonales-Alatorre et al., 2013). The NHX Na+/H+ antiporters are thought to contribute to the vacuolar compartmentalization of Na+ (Hasegawa, 2013). As indicated by SEMeX-ray and TEMeX-ray microanalyses, Na+ in Salicornia europaea is predominantly compartmentalized in the vacuoles of shoot endodermal cells. Accordingly, the transcript amounts of SeNHX1, SeVHA-A and SeVP1 (responsible for Na+/H+ antiporter, V-H+-ATPase and V-H+-PPase, respectively, in S. europaea) in shoots increase significantly with an increase in NaCl concentration, suggesting that these genes and their encoded proteins are important for Na+ sequestration in vacuoles (Lv et al., 2012). In S. salasa, northern blot analysis indicated that SsNHX1 expression in leaves was up-regulated by 500 mm NaCl stress. The SsNHX1 product is probably an Na+/H+ antiporter that may play important roles in the salt tolerance of S. salsa (Ma et al., 2004). Further study showed that, when exposed to 300 mm NaCl, tomato (Solanum esculentum) transformed with SsNHX1 accumulated less leaf cytosolic Na+ and maintained a higher K+/Na+ ratio than the wild-type; the dry weight, the net photosynthetic rate (Pn) and chlorophyll concentration in leaves were also much higher in transgenic plants than the wild-type (Zhao, 2006). A similar result was shown in rice (Oryza sativa L. ‘Zhonghua No.11’) transformed with SsNHX1 under saline conditions (Zhao et al., 2006). For example, the K+/Na+ ratio in leaves of transgenic rice plants was much higher than the wild-type at 300 mm NaCl; Pn and Fv/Fm ratio declined in both experimental lines under salinity, but a remarkable decrease occurred in the wild-type compared with the transformed plants after exposure to 300 mm NaCl; for example, there was a 91·7 and 18·4 % reduction of the Pn and Fv/Fm ratio, respectively in wild-type, whereas only 54·4 and 7·5 %, respectively in transgenic rice plants (Zhao et al., 2006). These results indicate that SsNHX1 contributes to salt tolerance. Meanwhile, under salt stress the up-regulation of the vacuolar H+-ATPase (V-H+-ATPase) genes, SsVHA-H and SsVHA-B, and the increase in the activity of V-H+-ATPase provide the proton driving force for sequestering Na+ in leaf vacuoles of S. salsa (P. H. Li et al., 2004; Y. Li et al., 2006).
Table 2.
Cloned genes in Suaeda salsa and their probable functions
| Gene | Probable function | Reference(s) |
|---|---|---|
| SsHKT1 | K+ uptake under K+ starvation or salt stress | Shao et al. (2006) |
| SsNHX1 | Na+/H+ antiporter across the tonoplast membrane | Ma et al. (2004) |
| SsVHA-H | Encoding H subunit of V-H+-ATPase | Y. Li et al. (2006) |
| SsVHA-B | Encoding B subunit of V-H+-ATPase | P. H. Li et al. (2004) |
| SsSOS1 | Na+/H+ antiporter across the plasma membrane | S. Y. Wang et al. (2013), Duan et al. (2013) |
| SsPIP | Encoding an aquaporin located in plasma membrane | Liu (2003) |
| SsCAX1 | Ca2+/H+ antiporter at tonoplast | Han et al. (2011) |
| SsP5CS | Encoding Δ1-pyrroline-5-carboxylate synthase involved in proline synthesis | P. P. Wang et al. (2002a) |
| SsBADH | Encoding betaine aldehyde dehydrogenase involved in betaine synthesis | S. H. Wang et al. (2007) |
| SsCMO | Encoding choline monooxygenase involved in betaine synthesis | S. H. Wang et al. (2007) |
| SsINPS | Encoding myo-inositol-1-phosphate (I-1-P) synthase involved in myo-inositol synthesis | P. P. Wang et al. (2002b) |
| SsGST | Encoding glutathione S-transferase | L. P. Wang et al. (2002), Qi et al. (2004) |
| SsCAT1 | Encoding catalase | Ma et al. (2003) |
| SsCAT2 | Encoding catalase | Ma et al. (2003) |
| SsAPX | Encoding ascorbate peroxidase | Ma et al. (2002) |
| Ss.sAPX | Encoding stroma ascorbate peroxidase | K. Li et al. (2012) |
| SsTypA1 | Encoding a member of the TypA/BipA GTPase gene family involved in oxidative stress | F. Wang et al. (2008) |
| SsPrxQ | Encoding peroxiredoxin Q involved in oxidative stress under salinity and low temperature | Guo et al. (2004) |
| SsMAPKK | Encoding mitogen-activated protein kinase kinase involved in resistance to high salinity, low temperature and drought | Yin (2003) |
| SsNCEDI | Encoding 9-cis-epoxyearotenoid dioxygenase involved in ABA synthesis | Cao (2004) |
| SsDREB | Transcription factor involved in drought and salt resistance | Liu et al. (2011) |
| SsEF-1α | Encoding an alpha subunit of translation elongation factor-1 alpha, which is involved in resistance to high salinity, low temperature, osmotic stress and oxidative stress | Sun et al. (2004) |
Na+ and Cl− exclusion by roots
The ability to regulate Na+ or Cl– uptake and transport to the shoot is crucial for salt tolerance in plants (Greenway and Munns, 1980; Tester and Davenport, 2003; Munns, 2005). In S. maritima, comparison of the external Na+ with that in the xylem indicated that Na+ was more strongly excluded from the transpiration stream as salinity increased (Clipson and Flowers, 1987). In a hydroponic experiment, the ability of roots to exclude Na+ and Cl− was greater under saline conditions in an intertidal population than in an inland population of S. salsa. Moreover, X-ray microanalysis showed that S. salsa roots of the intertidal population accumulated more [Na+] and [Cl−] in both the cortex and the stele than the roots of the inland population (Song et al., 2011). The results may explain why leaves of the intertidal population accumulated less Na+ and Cl− than the leaves of the inland population (Song et al., 2011).
Na+ efflux across the plasma membrane is attributed to the Salt-Overly-Sensitive1 (SOS1) Na+/H+ antiporter. SOS1 functions in root and shoot cells, and mediates Na+ efflux to the apoplast against the electrochemical potential via secondary active transport driven by the H+ gradient across the plasma membrane established by H+-ATPase. The expression of SsSOS1 in roots, stems and leaves is induced by salt stress (Duan et al., 2013; S. Y. Wang et al., 2013). Researchers have suggested that SsSOS1 may mediate Na+ efflux in leaves and roots but reduce Na+ long-distance transfer in stems, which may minimize Na+ toxicity (Duan et al., 2013).
In comparison with Na+, less attention has been paid to Cl− homeostasis under salinity. The uptake of Cl− from low external concentrations is thought to be via Cl− : 2H+ symport, but at the higher concentrations at which halophytes normally grow influx is likely to be via an anion channel; in this case, the Cl− concentration remains low in the cytoplasm if the membrane is depolarized (Flowers and Colmer, 2008). The cytoplasmic concentration of Cl− in S. maritima has been estimated to be 86 mm, about half that for Na+ (Flowers and Yeo, 1988). If this difference in concentration existed across the root plasma membrane, the Nernst potential would be about −33 mV. Uptake into the vacuole could be mediated by H+ exchange. Recently, a bumetanide-sensitive cation–Cl− co-transporter has been discovered in Arabidopsis and warrants further investigation in halophytes. However, there are no data obtained by direct measurement using ion-specific microelectrodes in the cells of S. maritima (Flowers and Colmer, 2008). In both inland and intertidal populations of S. salsa, the concentration of Cl− in leaves was about 30–40 % lower than that of Na+ based on tissue water (Song et al., 2011). However, the physiological and molecular mechanisms of Cl− uptake and transfer in S. salsa under salinity remain unclear.
K+ homeostasis
HKT class 1 (HKT1) proteins typically exhibit high Na+ selectivity, while class 2 (HKT2) proteins are more selective for K+ than Na+ or are non-selective (Hasegawa, 2013). Recently, molecular experiments reveal that the sustained activation of K (+) outward current may be the result of an unexpected O2·–1 post-transcriptional regulation of the guard cell outward-rectifying K (+) (GORK) channels. This consists of a probable new mode of regulating the processing of the GORK mRNA, in a reactive oxygen species (ROS)-dependent manner, which allows sustained K (+) effluxes during programmed cell death (PCD). These results provide new mechanistic insights into K (+) channel regulation during an oxidative stress response (Tran et al., 2013).
In S. salsa, SsHKT1 proteins increase under conditions of K+ starvation or salt stress, which suggests that SsHKT1 is important for K+ uptake, especially under low K+ and high salinity (Shao et al., 2006). Further study demonstrated that transgenic Arabidopsis plants overexpressing SsHKT1;1 showed enhanced salt tolerance and increased shoot K+ concentrations, whereas no significant changes in shoot Na+ concentrations were observed. The K+ transporters in the roots selectively mediated K+ uptake irrespective of external Na+, and their inhibitor N-ethylmaleimide (NEM) did not affect Na+ uptake at low K+ in S. salsa. The results provide strong in vivo evidence that SsHKT1;1 mainly acts as a potassium transporter in heterologous expression systems and in S. salsa. The results also indicate that SsHKT1;1 contributes to salt tolerance by helping maintain cytosolic cation homeostasis in general and by helping maintain K+ nutrition under salinity in particular (Shao et al., 2014).
The role of Ca2+ in maintaining K+ and Na+ homeostasis
Ca2+ is an important cation in plant responses to stress. Many stresses induce the increase of cytosolic Ca2+ concentrations (Kaplan et al., 2006). Ca2+ can activate high-affinity K+ uptake, substantially increasing K+-selective uptake over Na+-selective uptake (Hasegawa, 2013). Ca2+ can ameliorate Na+ toxicity in plants by decreasing Na+ influx via non-selective cation channels; elevated external Ca2+ concentrations also inhibit Na+-induced K+ efflux via outwardly directed, K+-permeable channels (Shabala et al., 2006). Therefore, Ca2+ is very important in maintaining K+ and Na+ homeostasis under salinity. In contrast to AtCAX1, which is inactive under salinity, SsCAX1 might play an important role in lowering the cytosolic Ca2+ burst induced by salt stress. First, SsCAX1 expression and protein levels were both increased markedly to sequester exogenous Ca2+ in vacuoles in the presence of 100 mm CaCl2 and 100 mm NaCl. In addition, salt stress significantly induced the transcription of V-H+-ATPase subunit c and increased the activities of V-H+-ATPase, which provided more energy to the Ca2+/H+ antiporter as well as to the Na+/H+ antiporter. As a result, cytosolic Ca2+ and Na+ homeostasis were maintained (Han et al., 2011).
Succulence
An important characteristic of halophytes is succulence, which is associated with an increase in cell size, a decrease in surface area per tissue volume and a high water content per unit surface area (Waisel, 1972). Succulence in leaves or stems is an adaptive feature that enables dicotyledonous halophytes to regulate their internal ion concentrations (Short and Colmer, 1999). Some findings suggest that, like increased light and aridity, Na+ can induce an increase in succulence (Jennings, 1968); the author suggested that these three factors exert the same effect on plant cells, i.e. they all bring about an increase in ATP synthesis (Jennings, 1968). V-H+-ATPase can provide energy to the tonoplast Na+/H+ antiporter, which can be induced to increase Na+ in vacuoles (Han et al., 2011). Uptake of Na+ and Cl– into halophyte cells may occur via gated cation and anion channels or even vesicles (Glenn and Brown, 1999). Electron micrographs of the succulent euhalophytes S. europaea and Suaeda arcuata growing with 400 mm NaCl showed pinocytic invaginations on the cell membrane and vesicular bodies in the vacuoles, which indicated that ion transport from the apoplast to the vacuole in above-ground organs of salt-accumulating halophytes is carried out by means of pinocytosis (Kurkova and Balnokin, 1994). Accumulation of Na+ and Cl– in the vacuole by the tonoplast Na+/H+ antiporter or vesicular transport can decrease cell water potential, which benefits water uptake of plants under salinity and which therefore increases stem or leaf succulence. However, the actual mechanism remains unclear. Many extremely salt-tolerant plant species are stem- or leaf-succulent euhalophytes. For example, the halophyte S. maritima grows optimally at moderate salinities (about 200 mm NaCl), and the optimal growth is accompanied by an increase in succulence and other morphological changes (Yeo and Flowers, 1980; Hajibagheri et al., 1984). When S. salsa plants from inland saline soil and from an intertidal zone were exposed to 600 mm NaCl, leaf water content was >8 and 10 mL g–1 dry weight, respectively (Song et al., 2009). When S. aralocaspica, Suaeda eltonica and Suaeda heterophylla were treated with salinity, the cross-sectional area of leaves increased, and there was a noticeable increase in the size of chlorenchyma and other cells. However, how cells regulate their size is largely unknown (Park et al., 2009).
Aquaporins (AQPs) facilitate water movement across bio-membranes. Currently, two types of plant AQPs have received the most attention, and these are referred to as plasma membrane AQPs (PIPs) and tonoplast AQPs (TIPs) based on their subcellular localization. The transcriptional level of SsPIP (Southern blot analysis showed that there was only one copy of SsPIP in the S. salsa genome) in S. salsa leaves was increased after treatment with 400 mm NaCl (Liu, 2003). Immunoblot analyses of PIP located in plasma membrane-enriched fractions of S. salsa seedlings also showed a significant increase under salinity (Qi et al., 2009). The results suggest that AQPs may correlate with the increase in leaf succulence of S. salsa under salinity (Qi et al., 2009). However, further research is needed to clarify AQP activity under salinity.
Salinity tolerance is a physiologically and genetically complex trait (Flowers, 2004). Because succulence is an important morphological adaptation that increases salinity tolerance, a better understanding of the physiological and molecular processes underlying the succulence induced by salinity may help plant breeders and molecular biologists increase the salt tolerance of conventional crop plants.
Osmotic stress tolerance
All halophytes must meet the challenge of osmotic adjustment in response to a low external water potential, and osmotic adjustment involves both inorganic and organic solutes (Munns, 2005; Flowers and Colmer, 2008; Munns and Tester, 2008). Glycinebetaine and proline are two important organic osmotica involved in osmotic adjustment in certain halophytes (Flowers and Colmer, 2008). The halophyte S. maritima accumulated glycinebetaine when grown in the presence of NaCl, and glycinebetaine is considered to act as a non-toxic cytoplasmic osmoticum that maintains the intracellular osmotic balance between the cytoplasm and the NaCl in the vacuole (Flowers and Hall, 1978; Hall et al., 1978). When another leaf-succulent halophyte, Suaeda fruticosa, was treated with 600 mm NaCl, the glycinebetaine content in shoots was 220 mm based on tissue water, suggesting that glycinebetaine plays a role in the osmotic adjustment (Khan et al., 2000). At 200 mm NaCl, the average level of glycinebetaine in salt-treated plants was 33 mm kg–1 fresh weight in leaves of S. aralocaspica, S. eltonica and S. heterophylla, and if all glycinebetaine accumulated in the cytoplasm, the concentration would be 10 times higher than that or about 330 mm. Thus, if glycinebetaine accumulates in the cytoplasm, it could significantly contribute to the cytoplasmic osmotic potential (Park et al., 2009). Glycinebetaine, however, often accumulates specifically in the chloroplasts of chenopods such as Spinacia okracea (Hanson et al., 1985) and not in the whole cytoplasm. In S. salsa treated with 400 mm NaCl, the glycinebetaine concentration exceeded 25 mm kg–1 fresh weight and was inferred to play an important role in S. salsa osmotic adjustment (Liu et al., 1994).
Under salinity, the contribution of proline to osmotic potential (Ψs) was <0·2 % in S. physophora (Song et al., 2006) and <0·5 % in S. salsa (Zhang and Zhao, 1998). S. maritima accumulates higher levels of proline in its leaves under salt conditions than S. europaea. However, the glycinebetaine content in leaves is greater in S. europaea than in S. maritima (Moghaieb et al., 2004). Tipirdamaz et al. (2006) suggested that ‘species that behaved as glycinebetaine accumulators contained little proline and vice versa’. Thus, the cytoplasmic osmoticum glycinebetaine may play a more important role than proline in osmotic adjustment in S. salsa under high-salinity conditions (Song et al., 2006). It follows that the role of compatible solutes in osmotic adjustment seems to vary among halophytes.
Besides their role in osmotic adjustment, compatible solutes may also have many other roles including the protection of enzymes and membranes, and ROS scavenging (Bohnert and Shen, 1999). They are even potent blockers of ROS-activated K+ channels (Cuin and Shabala, 2005, 2007). For example, low (0·5–5 mm) concentrations of exogenously supplied proline or glycinebetaine significantly reduced NaCl-induced K+ efflux from barley roots in a dose–response manner (Cuin and Shabala, 2005). Low (5 mm) concentrations of glycinebetaine and proline significantly reduced OH•-induced K+ efflux. A significant reduction in K+ efflux was found using osmolytes for which free radical scavenging activity had and had not been demonstrated. These results indicate that, in addition to free radical scavenging, glycinebetaine and proline must have other roles in mitigating the damaging effects of oxidative stress (Cuin and Shabala, 2007).
Two genes involved in glycinebetaine synthesis have been molecularly characterized in several halophytes in the genus Suaeda. Western blots showed that the protein levels of choline monooxygenase (CMO) and glycinebetaine aldehyde dehydrogenase (BADH), enzymes that catalyse the synthesis of glycinebetaine from choline, increase under salt stress in S. aralocaspica (Park et al., 2009). In S. salsa, SsBADH and SsCMO were cloned and their functions evaluated. Transgenic tobacco with SsCMO exhibited an increase in tolerance to salt stress (S. H. Wang et al., 2007). Moreover, SsP5CS (involved in proline synthesis) and SsINPS (involved in myo-inositol synthesis) in S. salsa were cloned, and the expression levels of SsP5CS (P. P. Wang et al., 2002a) and SsINPS were upregulated by salinity (P. P. Wang et al., 2002b).
Oxidative stress
High salinity can lead to the formation of ROS, and timely scavenging of ROS is critical in plant salt tolerance. Halophytes are equipped with powerful antioxidant systems, including enzymatic and non-enzymatic components, which are thought to protect important biomolecules such as lipoproteins and DNA from the damage caused by ROS (Oueslati et al., 2012a). The activity of both catalase (CAT) and superoxide dismutase (SOD) in S. maritima increased significantly 10 d after the initial application of 255 mm NaCl; treatment with 255 mm NaCl also resulted in the synthesis of new isoforms of both CAT and SOD (Mallik et al., 2011). In S. salsa, activities of Mn-SOD and several isoforms of Fe-SOD and CuZn-SOD were detected in leaf extracts, and the results indicated that these SOD isoforms may increase the tolerance to oxidative stress under salinity (B. S. Wang et al., 2004). Several genes involved in oxidative stress tolerance of S. salsa, such as SsGST, SsPrxQ, SsCAT1, SsCAT2, SsAPX, Ss.sAPX and SsTypA1, have been cloned and their functions tested (Ma et al., 2002, 2003; L. P. Wang et al., 2002; Guo et al., 2004; Qi et al., 2004; F. Wang et al., 2008; K. Li et al., 2012). For example, the overexpression of Ss.sAPX (a gene of the stromal APX in S. salsa) increased the germination, cotyledon growth, survival rate, and salt tolerance in transgenic Arabidopsis plants (K. Li et al., 2012). Under salinity, the transgenic plants had longer roots, higher total chlorophyll content, higher total APX activity, less cell membrane damage (as indicated by MDA levels) and lower H2O2 content than the wild-type. The results suggest that Ss.sAPX may play an important role in the protection against salt-induced oxidative stress in higher plants (K. Li et al., 2012).
UTILIZATION AS FOOD, MEDICINE, FORAGE AND BIOENERGY
As food
With the rapid growth of human populations and the irreversible spread of soil salinization in arid and semi-arid regions, the competition for fresh water and arable land among agricultural, domestic and industrial interests has increased tremendously (Rozema and Flowers, 2008). The problems resulting from reduced availability of fresh water and non-saline land might be partially reduced by the domestication of halophytes, because many of these salt-adapted plants can be used as vegetables and might also be developed as oilseed crops (Weber et al., 2007; Rozema and Schat, 2013). Several halophytes in the genus Suaeda have the potential to become oilseed crops. For example, the seed oil content of S. aralocaspica was >29 % on a dry weight basis, and both black and brown seeds contain about 93 % unsaturated fatty acids, with linoleic (>68 %) and oleic acid (>20 %) being the most abundant (L. Wang et al., 2012). Its high seed oil content and significant percentage of polyunsaturated fatty acids make the halophyte S. aralocaspica a promising source of high-quality edible oil (L. Wang et al., 2012). Weber et al. (2007) evaluated the seeds of six halophytes (Arthrocnemum indicum, Alhaji maurorum, Cressa cretica, Halopyrum mucronatum, Haloxylon stocksii and S. fruticosa) as sources of edible oil. The results indicated that the seeds of these halophytes and of S. fruticosa in particular (the oils of this species contained 74 % unsaturated fatty acids) could be used as a source of oil for human consumption. This suggests that Suaeda spp. have great potential as oilseed crops.
The seed yield of S. salsa is about 2000kg ha–1 in saline soils where the soil salt content is about 5 g kg–1 dry soil. Plant breeders and other researchers in China have been attempting to increase the plant biomass and seed yield of S. salsa in saline soil. For example, the artificial cultivation of S. salsa can increase its seed yield one- to two-fold, while breeding for high yield can increase S. salsa seed yield two- to three-fold; and the excessive saline water irrigation can increase the shoot biomass of S. Salsa efficiently (Shao et al., 2004). The oil content is >20 % in dry seeds of S. salsa. The oil contains seven kinds of fatty acids, and unsaturated fatty acids account for 90·7 %, with linoleic acid, oleic acid and linolenic acid accounting for 68·7, 13·9, and 4·2 %, respectively (Table 3). Therefore, S. salsa produces a high-quality oil that is fit for human consumption (Li and Fan, 2010). Besides Suaeda spp., Salicornia bigelovii is also thought to have potential as a high-quality oil-seed crop. The species can produce about 2000kg ha–1 of seeds over a 200-d growing cycle (Rozema and Flowers, 2008).
Table 3.
The composition and relative content of fatty acids in the seed oil of Suaeda salsa (reproduced from Gu, 1999)
| Fatty acid | Molecular formula | Relative content (%) |
|---|---|---|
| Terephthalic acid | C8H8O4 | 0·82 |
| 11-Hexadecenoic acid | C16H30O2 | 0·45 |
| Palmitoleic acid | C16H30O2 | 3·36 |
| Palmitic acid | C16H32O2 | 6·59 |
| Linoleic acid | C18H32O2 | 68·74 |
| Oleic acid | C18H34O2 | 13·93 |
| Stearic acid | C18H36O2 | 1·93 |
| Linolenic acid | C18H30O2 | 4·17 |
When grown in saline inland soils, S. salsa is green (Fig. 1A), and the fresh branches are highly valued as a vegetable. The contents of protein, crude fibre, carotenoids, amino acids and vitamin C in fresh branches of S. salsa are 2·3 mg g–1 f. wt, 63·7 mg g–1 f. wt, 106·5 μg g–1 f. wt, 30·4 μg g–1 f. wt and 1·3 mg 100 g–1 f. wt, respectively. The content of nitrate, by contrast, is only 1·13 μg g–1 f. wt, i.e. the fresh branches of S. salsa are safe for human consumption with respect to nitrates (Zhao et al., 2010). Like S. salsa, species of the annual genus Salicornia and of the perennial genus Sarcocornia can also be used as leafy vegetable crops, not only because of their salty taste but also because of their high nutritional value in terms of minerals and antioxidant vitamins, such as vitamin C and β-carotene. In addition, total polyphenol, β-carotene and ureides, all of which have antioxidant activity, increased in Salicornia and Sarcocornia spp. as the percentage of seawater used for irrigation increased (Ventura et al., 2011).
As medicine
Many halophytes have high contents of polyphenols and other bioactive compounds and are promising sources of pharmaceuticals. Mesembryanthemum edule, for example, is a traditional remedy for treatment of fungal and bacterial infections, sinusitis, diarrhoea, infantile eczema and tuberculosis. Tamarix gallica has been used as an astringent, detergent, diuretic, expectorant and laxative (Oueslatia et al., 2012a). Oueslatia et al. (2012b) assessed the in vitro and ex vivo antioxidant activities of acetone extracts of S. fruticosa, Suaeda pruinosa, Suaeda mollis and S. maritima in Tunisia. The results showed that S. mollis has the highest DPPH• scavenging ability followed by S. pruinosa, S. fruticosa and S. maritima, while S. fruticosa had the highest antioxidant ability (as determined by the inhibition of β-carotene bleaching) and a high total antioxidant capacity (Oueslatia et al., 2012b). These results show that antioxidant activity differs among Suaeda species. In another paper, Oueslatia et al. (2012a) reported that the edible halophyte S. fruticosa is a valuable source of antioxidants that have novel anti-inflammatory and anti-cancer capacities. Astragalin (kaempferol-3-O-glucoside), which was isolated from extracts of Suaeda asparagoides, can function as an antioxidant in biological systems, particularly on skin exposed to solar radiation, and can protect cellular membranes against ROS (Park et al., 2012). An experiment with S. maritima showed that maritima-supplemented diets protect haematological and biochemical properties (total protein, glucose and calcium levels), improve innate immunity, and protect against Miamiensis avidus infection in the olive flounder (Harikrishnan et al., 2012). Salicornia plants are also a promising source of pharmaceutical compounds. For example, isorhamnetin 3-O-β-d-glucopyranoside isolated from Salicornia herbacea protects against ROS-induced cellular damage (Kong et al., 2009).
Leaves and stems of S. salsa in the intertidal zone are red–violet (Fig. 1B) and during the entire growth period they accumulate betacyanin. Betacyanins are probably involved in the regulation of ROS in stressed plants (C. Q. Wang et al., 2006). Betalains have been used as natural additives for food, drug and cosmetic products. The content of flavanols in S. salsa is much higher in July (98·8 mg g–1 d. wt) than in other months, and July extracts have the highest antioxidant activity in vitro (Z. S. Zhang et al., 2010). In inland saline sites, the average Se content is 10 times greater in fresh branches of S. salsa (0·02 mg kg–1 f. wt) than in non-halophytic crops (Gu, 1999). Therefore, the species is regarded as a potential resource for cancer prevention (Ding et al., 2008). Because of its high content of unsaturated fatty acids, the seed oil of S. salsa is used to decrease blood sugar and blood pressure, to dilate blood vessels, to prevent heart disease and to develop disease immunity (Ding et al., 2008).
Fourteen fatty acid methyl esters have been identified in the product formulated from the extracts of S. salsa seedlings, and hexadecanoic acid methyl ester (25·9 %), 9,12-octadecandienoic acid methyl ester (28·3 %) and 9,12,15-octadecatrienoic acid methyl ester (9·0 %) are major extract components. Ten fatty acid methyl esters dominated by 9,12-octadecadienoic acid methyl ester (83·4 %) are present in the extracts of seeds. These extracts evidently inhibit ear swelling of normal and adrenalectomized mice, decrease vascular permeability and suppress the formation of granuloma. The anti-inflammatory activity of the extracts from seeds and seedlings results from the suppression of inflammatory mediators, the decrease in the content of MDA from inflammatory exudates and accordingly the increase in catalase activity (Zheng et al., 2003). Therefore, S. salsa is valued as a source of medicine.
As forage and bioenergy
Halophytes can be used as forage. Sheep and goats fed diets containing Salicornia, Suaeda and Atriplex gained as much weight as those whose diets included hay without halophytes (Glenn et al., 1998). Moreover, the quality of the meat of the experimental animals was unaffected by a diet rich in halophytes (Glenn et al., 1998). Chenopods generally contain high contents of crude protein, sulphur and minerals, which are critical to ruminant health. Halophytes, however, also contain oxalate and can generate mineral toxicities and deficiencies, which are obviously harmful to animals (Norman et al., 2013). The high salt content of halophytes limits the amount an animal can eat. Increasing sodium in the diet significantly decreases feed intake, digestibility, live weight gain and wool growth either as a main effect or through an interaction with potassium (Masters et al., 2005). The strategy is to incorporate halophytes as part of a mixed diet for livestock, replacing conventional hay forage with halophytes to make up between 30 and 50 % of the total food intake of sheep and goats (Glenn and Brown, 1999). Like S. salsa branches, S. salsa seed meal has a high protein content (about 27 %). This means that both branches and seed meal of S. salsa can be used as forage; a mixed diet for livestock can contain about 10 % of S. salsa hay (Ding et al., 2008).
Regarding bioenergy, S. salsa oil can be used as a raw material to produce fatty acid methyl esters, which in turn can be used to produce biodiesel; about 97 % of S. salsa oil can be converted to fatty acid methyl esters (Yang et al., 2008). In addition, the air-dried S. salsa plant is a potential feedstock for bio-ethanol production and high value-added products. Xylose yield and glucose yield from air-dried S. salsa plants reach a maximum of 18·48 and 25·49 g per 100 g of raw material, respectively (S. X. Li et al., 2013).
UTILIZATION IN THE RESTORATION OF SALINIZED OR CONTAMINATED LAND
In the restoration of contaminated land
The large volumes of wastewater discharged from coastal aquaculture projects can be a serious source of pollution. Untreated effluent may damage coastal ecosystems (Brown et al., 1999). Under high-salinity irrigation, halophytes can be used as biofilters to remove nutrients from saline aquaculture wastewater. In a greenhouse experiment, for example, the succulent salt marsh species Suaeda esteroa and S. bigelovii performed better than the desert saltbush Atriplex barclayana in removing total nitrogen, inorganic nitrogen, and total and soluble reactive phosphorus (Brown et al., 1999). In Salicornia brachiata, quantitative reverse-transcriptase PCR showed that the SbMT-2 gene is up-regulated by stresses induced by zinc, copper, salt, heat and drought. Escherichia coli cells expressing recombinant SbMT-2 were more tolerant than non-transformed cells to Zn2+, Cu2+ and Cd2+; the fusion protein SbMT-2 showed the highest affinity to zinc ions followed by copper and cadmium ions. These results indicated that the SbMT-2 protein has metal-binding characteristics and could therefore by useful in detoxification of heavy metals (Chaturvedi et al., 2012). S. brachiata has inherent resilience of varying abiotic tolerance, and therefore the SbMT-2 gene could be a potential candidate to be used for enhanced metal tolerance and heavy metal phytoremediation under saline conditions (Chaturvedi et al., 2012). This indicates that certain succulent salt marsh species can be used as promising biofilters to remove nutrients from saline aquaculture wastewater.
In a field investigation, accumulation of copper (Cu), chromium (Cr), lead (Pb) and arsenic (As) was greater for S. salsa than for Phragmites australis, Spartina alterniflora or Typha orientali; the content of the four metals in S. salsa shoots was about 100, 40, 5 and 2 μg g–1 d. wt, respectively (Gao et al., 2010). Relative to salt-sensitive plants, halophytes such as S. salsa offer a greater potential for phytoremediation with respect to reducing the levels of toxic metals from saline soils. Using the scanning ion-selective electrode technique, L. Z. Li et al. (2012) analysed the pattern and rate of Cd2+ fluxes at different regions of the S. salsa root apex. The results indicated that Cd2+ influx into roots can be significantly suppressed by pretreatment with the Ca2+-channel blockers LaCl3 and verapamil. This means that Cd2+ may enter roots through non-selective Ca2+ channels, which suggests a theoretical basis for the phytoremediation of Cd2+ contamination by S salsa in saline coastal soils. Wu et al. (2012) found that the expression of INPS, CMO, BADH, CAT and Gpx (glutathione peroxidase) was elevated in the above-ground parts of S. salsa after combined Hg and salinity exposure. For Hg-treated S. salsa plants, the metabolic profiles of extracts from the above-ground parts of seedlings showed clear increases in the amino acids valine, leucine, isoleucine, threonine, alanine, glutamine and phenylalanine. These results indicate that Hg may induce oxidative stress and disturb protein bio-degradation and energy metabolism in S. salsa.
In the restoration of salinized land
Halophytes are ideally suited for the revegetation and remediation of salt-affected land (Shabala, 2013). Atriplex nummularia, for example, can achieve a biomass of 20–30 t ha–1 year–1 and accumulate between 20 and 40 % NaCl in its dry matter when irrigated with saline water. S. fruticosa can remove >2·5 t of salts ha–1 in a single harvest of the aerial parts of the plant each year (Shabala, 2013). S. salsa can also efficiently absorb and accumulate salts from saline soils. If planted at a density of 15 plants m–2 and if harvested at the end of the growing season, S. salsa could potentially remove 3–4 t of Na+ ha–1 from saline soil, suggesting that it might be used to improve the quality of saline soils (Zhao, 1991).
In a field study at an inland site, the planting of S. salsa increased soil organic matter by 43 % and increased total soil nitrogen by 18 % relative to soil that was not planted (Lin et al., 2005). In the same study, the planting of S. salsa increased numbers of actinomyces and fungi five- and 16-fold, respectively. This indicates that the planting of S. salsa has potential for improving coastal saline soils.
CONCLUSIONS
Although genetic engineering may generate crops for saline land, the selection of useful transgenes is hampered by a fundamental lack of knowledge about the mechanisms of salt resistance in halophytes (Rozema and Schat, 2013). The genetic background is better described in non-halophytes such as Arabidopsis (Bevan et al., 2001) and rice (Huang et al., 2013) than in euhalophytes, but Arabidopsis, rice and other non-halophytes cannot survive on saline land. The genomes of the halophytes Eutrema halophilum (Deng et al., 2009) and E. parvulum (Dassanayake et al., 2011) have been reported, and although these two species have been promulgated as valuable model halophytes (Deng et al., 2009; Dassanayake et al., 2011), their salt tolerance is lower than that of the euhalophytes S. salsa (Song et al., 2009) and S. europaea (Lv et al., 2012). Euhalophytes within the Amaranthaceae offer the best opportunity to understand how dicotyledonous plants can tolerate salt (Flowers and Colmer, 2008; Huchzermeyer and Flowers, 2013; Rozema and Schat, 2013). Therefore, elucidating the genetic background of certain model euhalophytes (e.g. S. salsa and S. europaea) is important. Regarding the utilization of S. salsa, further work is needed to increase S. salsa seed yield and to evaluate the value of S. salsa in the restoration of contaminated saline soils. The latter research should include the combined study of the physiological and molecular mechanisms of heavy metal uptake and transfer within S. salsa plants and the efficiency at which S. salsa removes heavy metals from saline soils in the field.
ACKNOWLEDGMENTS
We thank Professor Timothy J. Flowers (Department of Biology and Environmental Science, School of Life Sciences, University of Sussex) for his constructive suggestions and critical revision of the manuscript. The financial support of the National Natural Science Research Foundation of China (31370420), the Natural Science Research Foundation of Shandong Province (ZR2010CM005) and the Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province is greatly appreciated.
LITERATURE CITED
- Bevan M, Mayer K, White O, et al. Sequence and analysis of the Arabidopsis genome. Current Opinion in Plant Biology. 2001;4:105–110. doi: 10.1016/s1369-5266(00)00144-8. [DOI] [PubMed] [Google Scholar]
- Bohnert HJ, Shen B. Transformation and compatible solutes. Scientia Horticulturae. 1999;78:237–260. [Google Scholar]
- Bonales-Alatorre E, Pottosin I, Shabala L, et al. Differential activity of plasma and vacuolar membrane transporters contributes to genotypic differences in salinity tolerance in a halophyte species, Chenopodium quinoa. International Journal of Molecular Sciences. 2013;14:9267–9285. doi: 10.3390/ijms14059267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JJ, Glenn EP, Fitzsimmons KM, Smith SE. Halophytes for the treatment of saline aquaculture effluent. Aquaculture. 1999;175:255–268. [Google Scholar]
- Cao YR. China: Shandong Normal University; 2004. Molecular cloning and founctional analysis of SsNCEDI gene in Suaeda salsa. MSc thesis [in Chinese] [Google Scholar]
- Chaturvedi AK, Mishra A, Tiwari V, Jha B. Cloning and transcript analysis of type 2 metallothionein gene (SbMT-2) from extreme halophyte Salicornia brachiata and its heterologous expression in E. coli. Gene. 2012;499:280–287. doi: 10.1016/j.gene.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Clipson NJW, Flowers TJ. Salt tolerance in the halophyte Suaeda maritima (L.) Dum – the effect of salinity on the concentration of sodium in the xylem. New Phytologist. 1987;105:359–366. doi: 10.1111/j.1469-8137.1987.tb00873.x. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Shabala S. Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant and Cell Physiology. 2005;46:1924–1933. doi: 10.1093/pcp/pci205. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Shabala S. Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant, Cell and Environment. 2007;30:875–885. doi: 10.1111/j.1365-3040.2007.01674.x. [DOI] [PubMed] [Google Scholar]
- Dassanayake M, Oh DH, Haas JS, et al. The genome of the extremophile crucifer Thellungiella parvula. Nature Genetics. 2011;43:913–918. doi: 10.1038/ng.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng ZY, Li Y, Xia R, et al. Structural analysis of 83-kb genomic DNA from Thellungiella halophila: sequence features and microcolinearity between salt cress and Arabidopsis thaliana. Genomics. 2009;94:324–332. doi: 10.1016/j.ygeno.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Ding HR, Hong Z, Yang ZQ, Wang MW, Wang K, Zhu XM. Progress of study on halophyte Suaeda salsa. Acta Agriculturae Jiangxi. 2008;20:35–37. [in Chinese] [Google Scholar]
- Duan HR, Wang SY, Wang Li, Zhang JL, Wang SM. Expression analysis of plasma membrane Na+/H+ antiporter gene (SsSOS1) from halophyte Suaeda salsa. 2013. http://www.paper.edu.cn [in Chinese]
- Flowers TJ. Improving crop salt tolerance. Journal of Experimental Botany. 2004;55:307–319. doi: 10.1093/jxb/erh003. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytologist. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Hall JL. Salt tolerance in the halophyte, Suaeda rnaritima L. Dum.: the influence of the salinity of the culture solution on the content of various organic compounds. Annals of Botany. 1978;42:1057–1063. [Google Scholar]
- Flowers TJ, Yeo AR. Ion relation of salt tolerance. In: Baker DA, Hall JL, editors. Solute transport in plant cells and tissues. Harlow, UK: Longman Scientific and Technical; 1988. pp. 392–413. [Google Scholar]
- Flowers TJ, Galal HK, Bromham L. Evolution of halophytes: multiple origins of salt tolerance in land plants. Functional Plant Biology. 2010;37:604–612. [Google Scholar]
- Gao YF, Li XQ, Dong GC, Li F, Wang YN, Qie H. Purification of several salt marsh plants to the coastal wetlands in the estuary of Yellow River. Journal of Anhui Agriculture Science. 2010;38:19499–19501. [in Chinese] [Google Scholar]
- Glenn EP, Brown JJ. Salt tolerance and crop potential of halophytes. Critical Reviews in Plant Sciences. 1999;18:227–255. [Google Scholar]
- Glenn EP, Brown JJ, O'Leary JW. Irrigating crops with seawater. Scientific American. 1998;279:76–81. [Google Scholar]
- Glenn EP, Anday T, Chaturvedi R, et al. Three halophytes for saline-water agriculture: an oilseed, a forage and a grain crop. Environmental and Experimental Botany. 2013;92:110–121. [Google Scholar]
- Greenway H, Munns R. Mechanisms of salt tolerance in nonhalophytes. Annual Review of Plant Biology. 1980;31:149–190. [Google Scholar]
- Gu FT. Research in exploiting the green series of edibles-Suaeda Salsa. Journal of Binzhou Edueation College. 1999;5:43–48. [in Chinese] [Google Scholar]
- Gul B, Ansari R, Flowers TJ, Khan MA. Germination strategies of halophyte seeds under salinity. Environmental and Experimental Botany. 2013;92:4–18. [Google Scholar]
- Guo XL, Cao YR, Cao ZY, Zhao YX, Zhang H. Molecular cloning and characterization of a stress-induced peroxiredoxin Q gene in halophyte Suaeda salsa. Plant Science. 2004;167:969–975. [Google Scholar]
- Hajibagheri MA, Hall JL, Flowers TJ. Stereological analysis of leaf cells of the halophyte Suaeda maritima (L.) Dum. Journal of Experimental Botany. 1984;35:1547–1557. [Google Scholar]
- Hall JL, Harvey DMR, Flowers TJ. Evidence for the cytoplasmic localization of betaine in leaf cells of Suaeda maritima. Planta. 1978;140:59–62. doi: 10.1007/BF00389380. [DOI] [PubMed] [Google Scholar]
- Han N, Shao Q, Bao HY, Wang BS. Cloning and characterization of a Ca2+/H+ antiporter from halophyte Suaeda salsa L. Plant Molecular Biology Reporter. 2011;29:449–457. [Google Scholar]
- Hanson AD, May AM, Grumet R, Bode J, Jamieson GC, Rhodes D. Betaine synthesis in chenopods: localization in chloroplasts. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:3678–3682. doi: 10.1073/pnas.82.11.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikrishnan R, Kim JS, Kim MC, et al. Effect of dietary supplementation with Suaeda maritima on blood physiology, innate immune response, and disease resistance in olive flounder against Miamiensis avidus. Experimental Parasitology. 2012;131:195–203. doi: 10.1016/j.exppara.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Hasegawa PM. Sodium (Na+) homeostasis and salt tolerance of plants. Environmental and Experimental Botany. 2013;92:19–31. [Google Scholar]
- He XQ, Li FZ. Seed morphology of Atriplex L. from China and its taxonomic significance. Bulletin of Botanical Research. 1995;15:65–71. [in Chinese] [Google Scholar]
- Huang XH, Lu TT, Han B. Resequencing rice genomes: an emerging new era of rice genomics. Trends in Genetics. 2013;29:225–232. doi: 10.1016/j.tig.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Huchzermeyer B, Flowers T. Putting halophytes to work – genetics, biochemistry and physiology. Functional Plant Biology. 2013;40:5–8. doi: 10.1071/FPv40n9_FO. [DOI] [PubMed] [Google Scholar]
- Jennings DH. Halophytes, succulence and sodium in plants-a unified theory. New Phytologist. 1968;67:899–911. [Google Scholar]
- Kaplan B, Davydov O, Knight H, et al. Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. Plant Cell. 2006;18:2733–2748. doi: 10.1105/tpc.106.042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Ungar IA, Showalter AM. The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. Journal of Arid Environments. 2000;45:73–84. [Google Scholar]
- Khan MA, Gul B, Weber DJ. Germination of dimorphic seeds of Suaeda moquinii under high salinity stress. Australian Journal of Botany. 2001;49:185–192. [Google Scholar]
- Kong CS, Kim JA, Zhong-Ji Qian ZJ, et al. Protective effect of isorhamnetin 3-O-β-D-glucopyranoside from Salicornia herbacea against oxidation-induced cell damage. Food and Chemical Toxicology. 2009;47:1914–1920. doi: 10.1016/j.fct.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Kurkova EB, Balnokin YV. Pinocytosis and its possible role in ion transport in halophyte salt-accumulating organ cells. Fiziologiyai Biokhimiya Kul'turnykh Rastenii (Moscow) 1994;41:578–582. [Google Scholar]
- Le Houérou HN. Salt-tolerant plants for the arid regions of the Meterranean isoclimatic Zone. In: Lieth H, Masoon A, Al, editors. Towards the national use of high salinity tolerant plants. Vol 1. Dordrecht: Kluwer; 1993. pp. 403–422. [Google Scholar]
- Li HS, Fan YX. Extraction and characteristics analysis of Suaeda salsa seed oil. China Oils and Fats. 2010;35:74–76. [in Chinese] [Google Scholar]
- Li K, Pang CH, Ding F, Sui N, Feng ZT, Wang BS. Overexpression of Suaeda salsa stroma ascorbate peroxidase in Arabidopsis. South African Journal of Botany. 2012;78:235–245. [Google Scholar]
- Li LZ, Liu XL, Peijnenburg WJ, et al. Pathways of cadmium fluxes in the root of the halophyte Suaeda salsa. Ecotoxicology and Environmental Safety. 2012;75:1–7. doi: 10.1016/j.ecoenv.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Li PH, Wang ZL, Zhang H, Wang BS. Cloning and expression ananysis of the B subunit of V-H+-ATPase in the leaves of Suaeda salsa under NaCl stress. Acta Botanica Sinica. 2004;46:93–99. [Google Scholar]
- Li SX, Li JH, Hu XT, et al. Study on enzymatic saccharification of Suaeda salsa as a new potential feedstock for bio-ethanol production. Journal of the Taiwan Institute of Chemical Engineers. 2013;44:904–910. [Google Scholar]
- Li W, An P, Liu X. Effect of storage, stratification, temperature and gibberellins on germination of dimorphic seeds of Suaeda salsa under saline conditions. Seed Science and Technology. 2008;36:122–132. [Google Scholar]
- Li WQ, Liu XJ, Khan MA, Yamaguchi S. The effect of plant growth regulators, nitric oxide, nitrite and light on the germination of dimorphic seeds of Suaeda salsa under saline conditions. Journal of Plant Research. 2005;118:207–214. doi: 10.1007/s10265-005-0212-8. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang XD, Song J, Fan H, Feng G, Wang BS. Accumulation of ions during seed development under controlled saline conditions of two Suaeda salsa populations is related to their adaptation to saline environments. Plant and Soil. 2011;341:99–107. [Google Scholar]
- Li X, Liu Y, Chen M, et al. Relationships between ion and chlorophyll accumulation in seeds and adaptation to saline environments in Suaeda salsa populations. Plant Biosystems. 2012;146:142–149. [Google Scholar]
- Li YY, Li PH, Wang BS. Cloning and expression of subunit H of V-H+-ATPase in vacuole membrane in the leaves of the halophyte Suaeda salsa under salt stress. Acta Botanica Boreali-Occidentalia Sinica. 2006;26:63–67. [in Chinese] [Google Scholar]
- Li Y, Zhang SR, Song J, Wu CX, Tian CY, Feng G. Chlorophyll in desiccated seeds of a euhalophyte, Suaeda physophora, and its significancy in plant adaptation to salinity during germination. Science in China Series C. 2008;51:410–417. doi: 10.1007/s11427-008-0055-3. [DOI] [PubMed] [Google Scholar]
- Lin XZ, Shen JH, Liu KZ, Huang XH. Study on remediation effects of Suaeda salsa L. planting on coastal saline soil. Advances in Marine Science. 2005;23:65–69. [in Chinese] [Google Scholar]
- Liu H. China: Shandong Normal University; 2003. Molecular cloning and differential expression of SsPIP gene in suaeda salsa under salinity stress setup the system of soybean transformation. MSc thesis [in Chinese] [Google Scholar]
- Liu JR, Yi YJ, Zhao KF. Effects of salinity on ion contents, betaine level and betaine-aldehyde dehydrogenase activity in seepweed (Suaeda salsa) seedlings. Acta Botanica Sinica. 1994;36:622–626. [in Chinese] [Google Scholar]
- Liu XX, Sun XB, Wang XE, Ma HX. Cloning and expression analysis of SsDREB gene from Suaeda salsa L. Journal of Nuclear Agriculture Science. 2011;25:684–691. [in Chinese] [Google Scholar]
- Lv SL, Jiang P, Chen XY, Fan PX, Wang XC, Li YX. Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiology and Biochemistry. 2012;51:47–52. doi: 10.1016/j.plaphy.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Ma CL, Wang PP, Cao ZY, Zhao YX, Zhang H. cDNA cloning and gene expression of APX in Suaeda salsa in response to salt stress. Journal of Plant Physiology and Molecular Biology. 2002;28:261–266. [in Chinese] [Google Scholar]
- Ma CL, Wang PP, Cao ZY, Zhao YX, Zhang H. Cloning and differentiai gene expression of two catalases in Suaeda salsa in response to salt stresss. Acta Botanica Sicina. 2003;45:93–97. [Google Scholar]
- Ma XL, Zhang Q, Shi HZ, et al. Molecular cloning and different expression of a vacuolar Na+/H+ antiporter gene in Suaeda salsa under salt stress. Biologia Plantarum. 2004;48:219–225. [Google Scholar]
- Mallik S, Nayak M, Sahu BB, Panigrahi AK, Shaw BP. Response of antioxidant enzymes to high NaCl concentration in different salt-tolerant plants. Biologia Plantarum. 2011;55:191–195. [Google Scholar]
- Masters DG, Rintoul AJ, Dynes RA, Pearce KL, Norman HC. Feed intake and production in sheep fed diets high in sodium and potassium. Australian Journal of Agricultural Research. 2005;56:427–434. [Google Scholar]
- Moghaieb REA, Saneoka H, Kounosuke Fujita K. Effect of salinity on osmotic adjustment, glycinebetaine accumulation and the betaine aldehyde dehydrogenase gene expression in two halophytic plants, Salicornia europaea and Suaeda maritima. Plant Science. 2004;166:1345–1349. [Google Scholar]
- Munns R. Genes and salt tolerance: bringing them together. New Phytologist. 2005;167:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Norman HC, Masters DG, Barrett-Lennard EG. Halophytes as forages in saline landscapes: interactions between plant genotype and environment change their feeding value to ruminants. Environmental and Experimental Botany. 2013;92:96–109. [Google Scholar]
- Oueslatia S, Ksouri R, Falleh H, Pichette A, Abdelly C, Legault J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chemistry. 2012a;132:943–947. [Google Scholar]
- Oueslatia S, Trabelsi N, Boulaaba M, Legault J, Abdelly C, Ksouri R. Evaluation of antioxidant activities of the edible and medicinal Suaeda species and related phenolic compounds. Industrial Crops and Products. 2012b;36:513–518. [Google Scholar]
- Park J, Okita TW, Edwards GE. Salt tolerant mechanisms in single-cell C4 species Bienertia sinuspersici and Suaeda aralocaspica (Chenopodiaceae) Plant Science. 2009;176:616–626. [Google Scholar]
- Park SN, Kim SY, Lim GN, Jo NR, Lee MH. In vitro skin permeation and cellular protective effects of flavonoids isolated from Suaeda asparagoides extracts. Journal of Industrial and Engineering Chemistry. 2012;18:680–683. [Google Scholar]
- Qi CH, Chen M, Song J, Wang BS. Increase in aquaporin activity is involved in leaf succulence of the euhalophyte Suaeda salsa, under salinity. Plant Science. 2009;176:200–205. [Google Scholar]
- Qi YC, Zhang SM, Wang LP, Wang MD, Zhang H. Overexpression of GST gene accelerates the growth of transgenic Arabidopsis under salt stress. Journal of Plant Physiology and Molecular Biology. 2004;30:517–522. [in Chinese] [PubMed] [Google Scholar]
- Rolletschek H, Weber H, Borisjuk L. Energy status and its control on embryogenesis of legumes: embryo photosynthesis contributes to oxygen supply and is coupled to biosynthetic fluxes. Plant Physiology. 2003;132:1196–1206. doi: 10.1104/pp.102.017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozema J, Flowers T. Crops for a salinized world. Science. 2008;32:1478–1480. doi: 10.1126/science.1168572. [DOI] [PubMed] [Google Scholar]
- Rozema J, Schat H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environmental and Experimental Botany. 2013;92:83–95. [Google Scholar]
- Shabala S. Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany. 2013;112:1209–1221. doi: 10.1093/aob/mct205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Demidchik V, Shabala L, et al. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiology. 2006;141:1653–1665. doi: 10.1104/pp.106.082388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao QL, Xie XD, Zhang FS, Cui HW, Cao ZY. A preliminary study on the artificial cultivation and breeding selection of Suaeda salsa. Chinese Journal of Eco-Agriculture. 2004;12:47–49. [in Chinese] [Google Scholar]
- Shao Q, Han N, Ding TL, Wang BS. Polyclonal antibody preparation and expression analysis of high-affinity K+ transporter SsHKT1. Journal of Wuhan Botanical Research. 2006;24:292–297. [in Chinese] [Google Scholar]
- Shao Q, Han N, Ding TL, Zhou F, Wang BS. SsHKT1;1 is a potassium transporter of a C3 halophyte Suaeda salsa involving in salt tolerance. Functional Plant Biology. 2014;41:790–802. doi: 10.1071/FP13265. [DOI] [PubMed] [Google Scholar]
- Short DC, Colmer TD. Salt tolerance in the halophyte Halosarcia pergranulata subsp. Pergranulata. Annals of Botany. 1999;83:207–213. [Google Scholar]
- Song J, Feng G, Tian CY, Zhang FS. Strategies for adaptation of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum to saline environment during seed germination stage. Annals of Botany. 2005;96:399–405. doi: 10.1093/aob/mci196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Ding XD, Feng G, Zhang FS. Nutritional and osmotic roles of nitrate in a euhalophyte and xerophyte in saline conditions. New Phytologist. 2006;171:357–366. doi: 10.1111/j.1469-8137.2006.01748.x. [DOI] [PubMed] [Google Scholar]
- Song J, Fan H, Zhao YY, Jia YH, Du XH, Wang BS. Effect of salinity on germination, seedling emergence, seedling growth and ion accumulation of a euhalophyte Suaeda salsa in an intertidal zone and on saline inland. Aquatic Botany. 2008;88:331–337. [Google Scholar]
- Song J, Chen M, Feng G, Jia YH, Wang BS, Zhang FS. Effect of salinity on growth, ion accumulation and the roles of ions in osmotic adjustment of two populations of Suaeda salsa. Plant and Soil. 2009;314:133–141. [Google Scholar]
- Song J, Shi GW, Gao B, Fan H, Wang BS. Waterlogging and salinity effects on two Suaeda salsa populations. Physiologia Plantarum. 2011;141:343–351. doi: 10.1111/j.1399-3054.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- Sun W, Li Y, Zhao YX, Zhang H. Isolation and characterizing of a cDNA clone encoding an elongation factor EF-1α from halophyte Suaeda salsa. Acta Botanica Boreali-Occidentalia Sinica. 2004;24:1657–1661. [in Chinese] [Google Scholar]
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Annals of Botany. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipirdamaz R, Gagneul D, Duhaze C, et al. Clustering of halophytes from an inland salt marsh in Turkey according to their ability to accumulate sodium and nitrogenous osmolytes. Environmental and Experimental Botany. 2006;57:139–153. [Google Scholar]
- Tran D, El-Maarouf-Bouteau H, Rossi M, et al. Post-transcriptional regulation of GORK channels by superoxide anion contributes to increases in outward-rectifying K+ currents. New Phytologist. 2013;198:1039–1048. doi: 10.1111/nph.12226. [DOI] [PubMed] [Google Scholar]
- Ventura Y, Sagi M. Halophyte crop cultivation: the case for Salicornia and Sarcocornia. Environmental and Experimental Botany. 2013;92:144–153. [Google Scholar]
- Ventura Y, Wuddineh WA, Myrzabayeva M, et al. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Scientia Horticulturae. 2011;128:189–196. [Google Scholar]
- Waisel Y. Biology of halophytes. London: Academic Press; 1972. [Google Scholar]
- Wang BS, Lüttge U, Ratajczak R. Specific regulation of SOD isoforms by NaCl and osmotic stress in leaves of the C3 halophyte Suaeda salsa L. Journal of Plant Physiology. 2004;161:285–293. doi: 10.1078/0176-1617-01123. [DOI] [PubMed] [Google Scholar]
- Wang CQ, Zhao JQ, Chen M, Wang BS. Identification of betacyanin and effects of environmental factors on its accumulation in halophyte Suaeda salsa. Journal of Plant Physiology and Molecular Biology. 2006;32:195–201. [in Chinese] [PubMed] [Google Scholar]
- Wang F, Zhong NQ, Gao P, Wang GL, Wang HY, Xia GX. SsTypA1, a chloroplast-specific TypA/BipA-type GTPase from the halophytic plant Suaeda salsa, plays a role in oxidative stress tolerance. Plant, Cell and Environment. 2008;31:982–994. doi: 10.1111/j.1365-3040.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- Wang HL, Wang L, Tian CY, Huang ZY. Germination dimorphism in Suaeda acuminata: a new combination of dormancy types for heteromorphic seeds. South African Journal of Botany. 2012;78:270–275. [Google Scholar]
- Wang L, Huang ZY, Baskin CC, Baskin JM, Dong M. Germination of dimorphic seeds of the desert annual halophyte Suaeda aralocaspica (Chenopodiaceae), a C4 plant without Kranz anatomy. Annals of Botany. 2008;102:757–769. doi: 10.1093/aob/mcn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dong M, Huang ZY. Review of research on seed heteromorphism and its ecological significance. Chinese Journal of Plant Ecology. 2010;34:578–590. [in Chinese] [Google Scholar]
- Wang L, Zhao ZY, Zhang K, Tian CY. Oil content and fatty acid composition of dimorphic seeds of desert halophyte Suaeda aralocaspica. African Journal of Agricultural Research. 2012;7:1910–1914. [Google Scholar]
- Wang LP, Qi YC, Zhao YX, Zhang H. Cloning and sequencing of GST Gene of Suaeda salsa and its expression characteristics. Journal of Plant Physiology and Molecular Biology. 2002;28:133–136. [in Chinese] [Google Scholar]
- Wang PP, Ma CL, Zhao KF, Zhao YX, Zhang H. Isolation and characterizing of a Δ1-pyrroline-5-carboxylate synthase gene in Suaeda salsa under slinity stress. Journal of Shandong Normal University (Natural Science) 2002a;17:59–62. [in Chinese] [Google Scholar]
- Wang PP, Ma CL, Cao ZY, Zhao YX, Zhang H. Molecular cloning and differential expression of amyo-inositol-1-phosphate synthase gene in Suaeda salsa under salinity stress. Journal of Plant Physiology and Molecular Biology. 2002b;28:175–180. [in Chinese] [Google Scholar]
- Wang SH, Tao JM, Zhang HM, Zhang Z. Molecular cloning of betaine synthetase in Suaeda salsa and construction of plant coexpression vector in a single open reading frame with the 2A region of FMDV. Acta Botanica Boreali-Occidentalia Sinica. 2007;27:215–222. [in Chinese] [Google Scholar]
- Wang SM, Zhang JL, Flowers TJ. Low-affinity Na+ uptake in the halophyte Suaeda maritima. Plant Physiology. 2007;145:559–571. doi: 10.1104/pp.107.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Duan HR, Wang Li, Zhang JL, Wang SM. Molecular cloning and sequence analysis of plasma membrane Na+/H+ antiporter gene (SsSOS1) from halophyte Suaeda salsa. 2013. http://www.paper.edu.cn [in Chinese]
- Weber DJ, Ansari R, Gul B, Khan MA. Potential of halophytes as source of edible oil. Journal of Arid Environments. 2007;68:315–321. [Google Scholar]
- Weber H, Borisjuk L, Wobus U. Molecular physiology of legume seed development. Annual Review of Plant Biology. 2005;56:253–79. doi: 10.1146/annurev.arplant.56.032604.144201. [DOI] [PubMed] [Google Scholar]
- Wu HF, Liu XL, Zhao JM, Yu JB. Toxicological responses in halophyte Suaeda salsa to mercury under environmentally relevant salinity. Ecotoxicology and Environmental Safety. 2012;85:64–71. doi: 10.1016/j.ecoenv.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Yang QL, Zhu F, Zhang CS, Yu SL, Qin S. Optimization of technological process for synthesizing biodiesel from Suaeda salsa. Modern Chemical Industry. 2008;28:43–46. [in Chinese] [Google Scholar]
- Yeo AR, Flowers TJ. Salt tolerance in the halophyte Suaeda maritima L. Dum.: evaluation of the effect of salinity upon growth. Journal of Experimental Botany. 1980;31:1171–1183. [Google Scholar]
- Yin HB. Molecular cloning and function characterization of SsVP and SsMAPKK genes in Suaeda salsa. China: Shandong Normal University; 2003. MSc thesis [in Chinese] [Google Scholar]
- Zhang HY, Zhao KF. Effects of salt and water stresses on osmotic adjustment of Suaeda salsa seedlings. Acta Botanica Sinica. 1998;40:56–61. [in Chinese] [Google Scholar]
- Zhang SR. China: China Agriculture University; 2009. The roles of cotyledonal chlorophyll in dehydrated seed of some halophytes in plant adaptation to arid environment. PhD thesis [in Chinese] [Google Scholar]
- Zhang SR, Song J, Wang H, Feng G. Effect of salinity on seed germination, ion content and photosynthesis of cotyledons in halophytes or xerophyte growing in central Asia. Journal of Plant Ecology. 2010;3:259–267. [Google Scholar]
- Zhang ZS, Mu H, Zhao L, Zhao L, Qian J. Study on antioxidant activity of Suaeda Salsa extract. Food Research and Development. 2010;31:4–8. [in Chinese] [Google Scholar]
- Zhao FY, Zhang XJ, Li PH, Zhao YX, Zhang H. Co-expression of the Suaeda salsa SsNHX1 and Arabidopsis AVP1 confer greater salt tolerance to transgenic rice than the single SsNHX1. Molecular Breeding. 2006;17:341–353. [Google Scholar]
- Zhao HL, Ma YZ, Li JX, Piao L. Study on edible value of Suaeda salsa (L.) Pall. Journal of Anhui Agriculture Science. 2010;38:14350–14351. [in Chinese] [Google Scholar]
- Zhao KF. Desalinization of saline soils by Suaeda salsa. Plant and Soil. 1991;135:303–305. [Google Scholar]
- Zhao KF, Song J, Feng G, Zhao M, Liu JP. Species, types, distribution, and economic potential of halophytes in China. Plant and Soil. 2011;342:495–509. [Google Scholar]
- Zhao SQ. China: Shandong Normal University; 2006. Studies on transforming tomato by SsNHX1 gene. MSc thesis [in Chinese] [Google Scholar]
- Zheng WF, Chen CF, Li W. Chemical composition and anti-inflammation of methanol/chloroform extracts from seeds and seedlings of Suaeda salsa (L.) Pall. Chinese Traditional Patent Medicine. 2003;25:997–1002. [in Chinese] [Google Scholar]
- Zhou JC, Fu TT, Sui N, et al. The role of salinity in seed maturation of the euhalophyte Suaeda salsa. 2014. Plant Biosystems(in press )