Abstract
Background and Aims Many stem-succulent halophytes experience regular or episodic flooding events, which may compromise gas exchange and reduce survival rates. This study assesses submergence tolerance, gas exchange and tissue oxygen (O2) status of two stem-succulent halophytes with different stem diameters and from different elevations of an inland marsh.
Methods Responses to complete submergence in terms of stem internal O2 dynamics, photosynthesis and respiration were studied for the two halophytic stem-succulents Tecticornia auriculata and T. medusa. Plants were submerged in a glasshouse experiment for 3, 6 and 12 d and O2 levels within stems were measured with microelectrodes. Photosynthesis by stems in air after de-submergence was also measured.
Key Results Tecticornia medusa showed 100 % survival in all submergence durations whereas T. auriculata did not survive longer than 6 d of submergence. O2 profiles and time traces showed that when submerged in water at air-equilibrium, the thicker stems of T. medusa were severely hypoxic (close to anoxic) when in darkness, whereas the smaller diameter stems of T. auriculata were moderately hypoxic. During light periods, underwater photosynthesis increased the internal O2 concentrations in the succulent stems of both species. Stems of T. auriculata temporally retained a gas film when first submerged, whereas T. medusa did not. The lower O2 in T. medusa than in T. auriculata when submerged in darkness was largely attributed to a less permeable epidermis. The submergence sensitivity of T. auriculata was associated with swelling and rupturing of the succulent stem tissues, which did not occur in T. medusa.
Conclusions The higher submergence tolerance of T. medusa was not associated with better internal aeration of stems. Rather, this species has poor internal aeration of the succulent stems due to its less permeable epidermis; the low epidermal permeability might be related to resistance to swelling of succulent stem tissues when submerged.
Keywords: Flooding tolerance, gas film, halophyte, oxygen dynamics, submergence tolerance, Tecticornia auriculata, Tecticornia medusa, underwater photosynthesis, underwater respiration, Salicornioideae, samphire.
INTRODUCTION
Complete submergence of plants results in diminished supply of oxygen (O2) as diffusion of gases in water is 10 000 times slower than in air (Armstrong and Drew, 2002). The O2 deficiency, especially at night, can inhibit respiration in tissues that might eventually become anoxic (Gibbs and Greenway, 2003). When shoots are fully submerged there are two potential sources of O2, either from the floodwater or from underwater photosynthesis, so internal O2 levels may fluctuate diurnally depending on incident light, CO2 and O2 in the water column (Pedersen et al., 2004; Winkel et al., 2013). Besides diminishing O2 supply, submergence can also reduce CO2 entry to the shoot for photosynthesis (Voesenek et al., 2006; Colmer et al., 2011), especially if there is a high resistance across the leaf cuticle (Mommer et al., 2005). Internal O2 in succulent halophytes is highly dependent on O2 from photosynthesis during the day and the tissue may become anoxic during the night when there is no light to drive photosynthesis (Pedersen et al., 2006; Colmer et al., 2013). In addition to challenges associated with exchange of gases when underwater, halophytes also have to cope with differences in osmotic potential between the tissue and the floodwater, which could potentially result in swelling due to water entry into the tissues (Colmer and Flowers, 2008). In such conditions, the strong epidermis which may be required to constrain such excessive swelling may further reduce gas exchange rates that are already low due to long diffusion paths and relatively low surface area to volume (SA/V) ratios of succulent stems. Here, we investigated whether gas exchange under water influences submergence tolerance in two contrasting stem-succulent halophytes.
Stem succulents of the Salicornioideae (family Amaranthaceae) typically occupy saline, often flooded habitats around the world. The genus Tecticornia contains perennial, halophytic stem-succulent species that are largely endemic to Australia (Wilson, 1980; Shepherd and Wilson, 2007). Many Tecticornia species grow at ephemeral inland salt lakes, environments characterized by high soil salinity and episodic flooding punctuated by periods of drought. The Tecticornia communities on margins of ephemeral salt lakes in Australia often show patterns of species zonation (English, 2004; English and Colmer, 2011). These field distributions could be explained by Tecticornia species having different tolerances to flooding, salinity and/or drought, but at low marsh elevations in particular plant establishment might be dependent on ability to tolerate submergence (e.g. for small seedlings or for larger plants in the case of deep floods). Furthermore, when these plants are flooded or submerged at some locations, such as for Tecticornia medusa at the Fortescue Marsh in the semi-arid inland of north-western Australia, the water is of low salinity as floods result from cyclonic events and low salinity shallow groundwater (Skrzypek et al., 2013), whereas in other systems the species present can experience saline water submergence (e.g. Tecticornia pergranulata, Pedersen et al., 2006).
The main objective of this study was to elucidate O2 dynamics during submergence in the succulent stems of two Tecticornia species with different field distributions occurring at the Fortescue Marsh. Tecticornia auriculata and T. medusa are two of the dominant Tecticornia species at the Fortescue Marsh, but at different locations along an elevation gradient. At low lying zones, more prone to longer, deeper and more frequent floods, T. medusa forms discrete populations whereas T. auriculata inhabits higher elevations less prone to flooding (L. Moir-Barnetson et al., pers. observ.). We hypothesized that these field distributions could be explained by different submergence tolerance, with T. medusa expected to be more tolerant than T. auriculata. Furthermore, an objective was to elucidate O2 dynamics in stems under different light and submergence conditions to determine whether this could explain any differences in submergence tolerance. These objectives were met by measuring internal O2 levels, photosynthesis under water and in air and underwater respiration by the succulent stems.
MATERIALS AND METHODS
Plant culture
Seeds of Tecticornia auriculata (Paul G.Wilson) K.A.Sheph. & Paul G.Wilson and Tecticornia medusa K.A.Sheph. & S.J.van Leeuwen were collected from plants at the Fortescue Marsh in the Pilbara region of Western Australia (22° 21′S, 119° 20′E). Seeds were germinated on filter paper moistened in half-strength nutrient solution (see below for full-strength composition) in transparent plastic containers at 35 °C light/5 °C dark (12 h/12 h) (cf. Malcolm, 1964). Seedlings were transplanted into compartmented trays containing washed fine-grain white sand kept moist by sub-irrigation with full-strength nutrient solution, and grown initially for 2 months. Plants were then transplanted to larger pots (150 mm high, 80 mm diameter) containing washed fine-grain white sand and grown for a further 5 months with watering every 2 d with full-strength nutrient solution. Watering volume was twice that retained in the sand when at field (i.e. pot) capacity, so that leaching prevented the accumulation of salts. The salinity of the nutrient solution initially contained 10 mm Na+ and was, after the first 5 months in the pots, stepped up to 250 mm NaCl in 50 mm increments every 3 d, and plants were grown in these conditions for a further 10 weeks with watering of the pots daily before submergence experiments were commenced.
The nutrient solution in the present study had previously been used in experiments on other Tecticornia species (English and Colmer, 2011) and was prepared from deionized water and had the following composition (mm): Na+, 10; K+, 10; NH4+, 0·2; Ca2+, 10; Mg2+, 1·0; NO3−, 1·4; Cl−, 18·6; SO42−, 10; HPO42−, 0·5; Fe-EDTA, 0·05; H2BO3−, 0·00625; Mn2+, 0·0005; Zn2+, 0·00005; Cu2+, 0·000125; and Mo2+, 0·000125. pH was adjusted to 6·5 with KOH. The same nutrient solution, adjusted to 250 mm NaCl, was used to irrigate plants after the salinity step-up phase, and during the experiment. The concentration of 250 mm NaCl was used because this level is relevant to substrate concentrations in the field and maximum growth of these species occurs in the range 150–600 mm NaCl (L. Moir-Barnetson, unpubl. res.).
Experimental design
The experimental design consisted of non-submerged controls and three submergence durations (3, 6 or 12 d), all followed by a recovery period of 60 d. The submergence durations were started and ended at 0800–0900 h. The non-submerged controls continued to be watered every day with nutrient solution containing 250 mm NaCl. Pots de-submerged were drained and irrigated every day as in the non-submerged controls for the 60-d recovery period. Plants that were submerged had pellets of slow-release fertilizer (Osmocote® Plus Native Gardens; Scotts Australia, Bella Vista, NSW, Australia) implanted into the sand of the pots as these pots did not receive any additional nutrients when submerged, unlike the drained pots which continued to receive nutrients during irrigation. Pots assigned to submergence treatments were sealed at the bottom and then waterlogged with nutrient solution containing 250 mm NaCl. The pots were covered by a lid with a single hole for the stem base which was sealed with an inert butyl sealant (Terostat® VII, Henkel, Düsseldorf, Germany) to prevent the exchange of submergence water and the rooting solution. The submergence experiment was conducted using a system of Perspex tanks with circulating floodwater (Colmer et al., 2009). There were four replicates of each treatment. The submergence solution was a modified form of the nutrient solution, without N or P to avoid algal growth (Colmer et al., 2009), and this solution was prepared from deionized water and contained (in mm): K2SO4, 5; CaCl2.2H2O, 10; Na2SO4, 4·5; MgSO4.7H2O, 1; the same micronutrients as above and also 1 mm NaHCO3. The pH of the solution was 7·7, giving a free CO2 concentration of 40 µm at 20 °C.
The submergence solution in the tanks was circulated continuously and periodic measurements of dissolved O2 concentration showed it was at air-equilibrium. Submergence solution pH was monitored and adjusted accordingly to 7·7, and the electrical conductivity was also monitored (it remained constant). The water level in the tanks was kept constant by daily addition of deionized water as required. When pots were opened at harvest, the sediment was black and a strong smell of sulphide was detected, indicating that the bulk substrate in the pots was anoxic. Plants were raised in a naturally lit, temperature-controlled glasshouse with average day and night air temperatures of 23 and 16 °C, respectively. The submergence experiments were carried out in October–November.
Morphological and anatomical characteristics
The succulent stems of both species in this study consist of segments, termed ‘articles’. Hand sections were taken at the mid-point of fully expanded articles and observed using a microscope (Zeiss Axioskop II, Carl Zeiss AG, Oberkochen, Germany) to assess the anatomy and photographed with a digital camera (Zeiss AxioCam). The articles consisted of a central cylinder, hydrenchyma (water storage tissue), chlorenchyma (photosynthetically active tissue) and epidermis (cuticle + epidermis cell layer). The thicknesses of these four tissues were determined from images.
Article diameter, net photosynthesis (PN) in air and recovery
Changes in diameter of marked articles on the plants were measured daily using digital callipers and articles were also noted for the occurrence of any rupturing.
PN in air at a quantum flux density of 1500 µmol m−2 s−1 and a CO2 concentration of 400 µL L−1 was measured on excised stems using an infrared gas analyser with a LED light source chamber (LI-6400, Li-Cor, Lincoln, NE, USA). Non-ruptured succulent stems were measured approximately 24 h after the plants had been de-submerged, and after a 60-d recovery period, PN was measured on stems that had previously been submerged and on new stems produced after the de-submergence. PN rates were expressed per unit tissue area.
Underwater PN and dark respiration (RD)
Underwater PN was measured as net O2 production by excised stems. Succulent stems were excised and mounted on mesh within a custom-built 82-mL cylindrical transparent Perspex chamber, which was illuminated with photosynthetically active radiation at 1500 µmol m−2 s−1 at the stem surface. The chamber was filled with freshly made submergence solution as used in the submergence treatments. Measurements were started with dissolved O2 at approximately 50 % of the air equilibrium concentration (mixing solutions bubbled with N2 and air in 1 : 1 volumes) to reduce effects of photorespiration (Pedersen et al., 2013). PN was measured at two different dissolved inorganic carbon (DIC) levels of 1 and 10 mm, obtained by adding NaHCO3 and with pH = 7·7; this corresponded to 40 and 400 µm CO2, respectively. After the PN measurements, the solution was changed with submergence solution containing near air-equilibrium dissolved O2 and 40 µm CO2 and RD was measured as O2 uptake. T. auriculata had visible gas films when submerged (whereas T. medusa did not), so for both species PN and RD were measured ‘with’ and ‘without’ gas films at time 0 (i.e. immediately following submergence) and after 3 d of submergence; the without gas films samples had been brushed with 0·05 % (v/v) Triton X-100 in submergence solution (Raskin and Kende, 1983; Colmer and Pedersen, 2008b). The solution in the chamber was stirred with a magnetic stirrer and the lid was fitted with an O2 electrode (tip diameter = 500 µm, OX-500, Unisense, Aarhus, Denmark) connected to a picoammeter (PA meter) (PA 2000, Unisense). The O2 signal was logged every 10 s using an analogue-to-digital converter (ADC-20, Pico Technology, St Neots, UK) connected to a computer.
Potential CAM activity
Potential CAM activity of stem tissue was evaluated by measurements of tissue titratable acidity at dawn and at dusk. Stems were harvested at dawn and dusk to determine whether tissue acidity was significantly higher at dawn, which would indicate potential CAM activity. An amount of 0·10–0·12 g fresh tissue was ground using a mortar and pestle and following the addition of 5 mL of CO2-free deionized water, the mixture was transferred to a glass vial and titrated with 0·01 m NaOH to a pH endpoint of 6·4 (Keeley and Sandquist, 1991). Samples were taken both from plants in air and from plants that had been submerged for 3 d.
Oxygen profiles and time traces
Radial O2 profiles of succulent stems were measured using an O2 microelectrode (tip diameter = 25 µm, OX-25, Unisense) connected to a PA meter (PA 2000, Unisense) and the electrode was moved in steps of 25 µm using a motorized micromanipulator (MM33, Unisense) connected to a PC via a motor controller (MC-232, Unisense). Succulent stems of submerged or control plants were excised, and the basal end was sealed with petroleum jelly to prevent gas entry via the cut end. Each stem was mounted with rubber bands on a metal mesh in a trough (length 0·25 m) made of 50-mm-diameter Perspex pipe sliced lengthways in half, with caps at each end. The trough was filled with freshly made submergence solution as used in the submergence treatments and stirring was maintained by bubbles from an air stone connected with a tube to an air pump. Diameters of the stems were measured before they were mounted on the mesh and profiles were taken at least half way through the stem. Profiles were taken of stems in air or submerged, in darkness or with light and four replicate profiles were taken for each treatment with typical profiles selected for presentation of results. Light was provided by a fibre optic lamp (model LGPS; Olympus, Tokyo, Japan) at a quantum flux density of 1500 µmol m−2 s−1. After each profile, stems were stored at 5 °C for 2 d and then hand sections were taken using a razor blade to confirm the electrode trace through the central cylinder of the succulent stem.
Time traces of O2 dynamics under changing light and submergence conditions were also conducted using the same set-up as for the profiles described above. A succulent stem was mounted on the mesh and a microelectrode was inserted and moved inwards until the tip reached the central cylinder of the stem. The microelectrode was then pulled back 100 µm so it was positioned within the hydrenchyma and the O2 partial pressure (pO2) was logged every 60 s on a computer. Conditions were then changed in the following order: air and light (1 h), air and darkness (1 h), submerged and darkness (1 h), submerged and light (6 h) and then air and light (1 h). This was repeated for three replicate stems of each species and typical curves were selected for presentation of results.
Statistical analyses
Data were analysed by analysis of variance (ANOVA) using Type III sum of squares with the software Statgraphics XVI centurion version 16.1.11 (StatPoint, Inc., Warrenton, VA, USA). Multiple comparisons of means were performed using the Tukey HSD procedure at the 0·05 significance level. All data were tested for homogeneity of variance by Levene’s test. If necessary, logarithmic transformations were performed to ensure homogeneity of variance, but for clarity all data are presented as untransformed data.
RESULTS
Morphological and anatomical characteristics
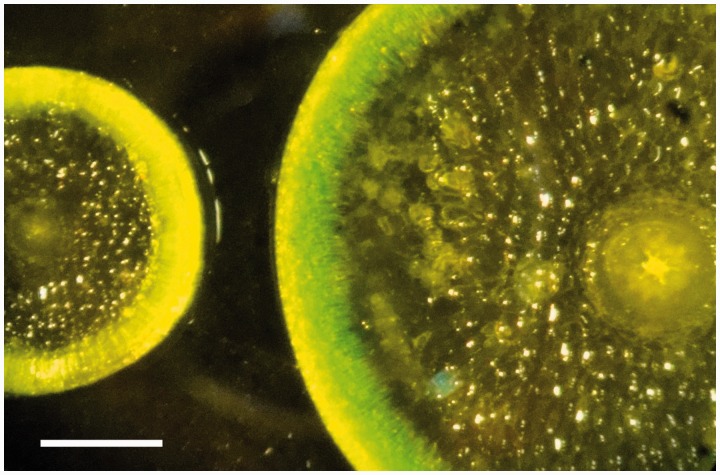
There were significant differences in stem diameter and SA/V between the two species with T. medusa having the highest diameter and lowest ratio (Table 1). Thicknesses of all the different tissues constituting the succulent stems were also highest in T. medusa (Fig. 1). In particular, the hydrenchyma showed a substantial difference between the two species with an average radius of 779 and 2091 µm for T. auriculata and T. medusa, respectively.
Table 1.
Anatomical characteristics of succulent stems in Tecticornia auriculata and T. medusa grown under drained conditions and watered with nutrient solution containing 250 mm NaCl
| Parameter | T. auriculata | T. medusa |
|---|---|---|
| Stem surface area to volume ratio (mm−1) | 1·57 ± 0·03a | 0·77 ± 0·03b |
| Succulent stem radius (µm) | 1424 ± 51a | 3049 ± 72b |
| Central cylinder radius (µm) | 233 ± 20a | 403 ± 16b |
| Hydrenchyma thickness (µm) | 779 ± 41a | 2091 ± 61b |
| Chlorenchyma thickness (µm) | 377 ± 9a | 506 ± 28b |
| Epidermal thickness (µm) | 36 ± 2a | 47 ± 2b |
Fully expanded (i.e. fully elongated) succulent stem articles were measured. Different letters indicate significant differences between columns using ANOVA and Tukey’s HSD post-hoc test (P < 0·05). Values are means ± s.e. (n = 4).
Fig. 1.
Cross sections of Tecticornia auriculata (left) and Tecticornia medusa (right) succulent stem articles (‘fully expanded’) of plants grown under drained conditions and watered with nutrient solution containing 250 mm NaCl. Scale bar, 1 mm.
Submergence recovery and PN in air
A response to submergence in both species was article swelling, but T. auriculata had a significantly higher relative increase in article diameter as early as the first day of submergence (P < 0·01) (Fig. 2). Furthermore, the swelling of articles also led to rupturing of tissue in T. auriculata whereas no rupturing was observed in T. medusa. The rupturing of tissue in T. auriculata started within 2 d of submergence and in the plants that were submerged for 12 d, more than 90 % of the articles had ruptured. When the articles ruptured, they deteriorated and were not able to recover, eventually leading to decomposition of the succulent tissue. Therefore, there were obvious visual differences between the two species when they were de-submerged, with T. medusa looking very similar to non-submerged controls, and T. auriculata having severely swollen articles or ruptured articles starting to decompose. The de-submergence was followed by a recovery period of 60 d, and the two species showed different ability to recover following de-submergence; all T. medusa recovered from all three submergence durations, whereas in T. auriculata three of four, one of four and zero of four plants were able to recover from the 3, 6 and 12 d of submergence, respectively.
Fig. 2.

Diameters of succulent stem articles of Tecticornia auriculata and Tecticornia medusa when completely submerged in a tank system in a glasshouse over 12 s (values are means ± s.e., n = 4). None of the ‘fully expanded’ (i.e. fully elongated) succulent articles tagged for these measurements for T. auriculata remained after 7 d.
No potential CAM activity was found in either of the two species when in air or after 3 d of submergence, as there were no significant differences between titratable acidity of tissues at dawn and at dusk (data not shown).
PN in air was measured on all plants the day after they had been de-submerged and the branches with non-ruptured parts were chosen for these measurements to determine if these had changed in photosynthetic capacity after the submergence durations. T. auriculata had very few intact articles after 12 d of submergence so only two of the four replicates could be measured (Table 2). After a recovery period of 60 d, PN in air was measured again on tissue that had previously been submerged and also on tissue that had been produced after de-submergence. The PN rates in air of non-submerged control plants were in the range 5·6–7·1 µmol m−2 s−1 and there was no significant difference between the two species (Table 2). For plants that had just been de-submerged there was also no difference between the two species in PN in air when compared within each submergence duration. For plants that had recovered for 60 d, however, there were some differences in PN in air between the two species. Tissues that had been submerged had very low PN in T. auriculata with 0·97 µmol m−2 s−1 for the plants that survived 3 d of submergence and 0·89 µmol m−2 s−1 for the single plant that survived 6 d of submergence, whereas in T. medusa the previously submerged articles had significantly higher PN in the range 3·5–3·9 µmol m−2 s−1, even after 12 d of prior submergence. However, the T. auriculata that did survive 3 or 6 d of submergence produced new articles which had high PN rates of 8·1–8·8 µmol m−2 s−1. These rates were significantly higher than the PN of the newly produced articles in T. medusa of 5·7–7·1 µmol m−2 s−1.
Table 2.
Net photosynthesis (PN) (µmol CO2 m−2 s−1) in air of succulent stems of Tecticornia auriculata and Tecticornia medusa after four submergence durations (0, 3, 6 and 12 d) and in three different tissues (De-submerged: measured on tissue the day after de-submergence; Recov. old: measured on tissue which had previously been submerged after a 60-d recovery period; Recov. new: measured on tissue produced after de-submergence and a 60-d recovery period)
| Tissue | Species | #0 d | 3 d | 6 d | 12 d |
|---|---|---|---|---|---|
| De-submerged | T. auriculata | 5·73 ± 0·39 | 2·41 ± 0·51ab | 1·10 ± 0·39a | 0·52 ± 0·30a*** |
| T. medusa | 7·07 ± 0·55 | 4·09 ± 0·49bc | 1·78 ± 0·26a | 1·93 ± 0·57a | |
| Recov. old | T. auriculata | 5·55 ± 0·83 | 0·97 ± 0·43a* | 0·89** | |
| T. medusa | 5·82 ± 0·41 | 3·45 ± 0·51b | 3·85 ± 0·84ab | 3·67 ± 0·42a | |
| Recov. new | T. auriculata | n.a. | 8·84 ± 0·77d* | 8·14** | |
| T. medusa | n.a. | 5·74 ± 0·90c | 6·62 ± 1·03b | 7·09 ± 0·93b |
Different letters indicate significant differences between rows using ANOVA and Tukey’s HSD post-hoc test (P < 0·05). Values are means ± s.e. (n = 4).
*n = 3; **n = 1 (not included in ANOVA); ***n = 2; n.a. = not applicable; #0 days = plant not submerged (i.e. controls). An empty space in the Table means that no plants survived that submergence duration.
Underwater PN and RD
Underwater PN was determined at two different CO2 concentrations (40 and 400 µm). Because T. auriculata had gas films (at least initially) when it was submerged, we measured underwater PN of succulent stems with and without gas films in plants immediately after submergence and 3 d later (gas films were no longer apparent). The underwater PN rates were generally low with values below 1 µmol m−2 s−1 except for T. auriculata with intact gas films in 400 µm CO2 where the average rate was 5·5 µmol m−2 s−1 (Table 3). For plants that had just been submerged (0 d submergence), T. auriculata had higher rates than T. medusa at both CO2 concentrations and for T. auriculata there was also a 10-fold higher PN in stems with intact gas films compared with stems for which the gas films had been removed by prior brushing with dilute Triton X. After 3 d of submergence, when the gas films had visually disappeared in T. auriculata (i.e. gas films did not persist), there was no difference in underwater PN between stems brushed with dilute Triton X just prior to measurements and those that were not brushed. For T. medusa there was no effect of brushing with dilute Triton X either at 0 or at 3 d of submergence, as no gas film was present. Generally, underwater PN rates were higher in 400 µm CO2 compared with 40 µm for both species, and in 400 µm CO2 the remaining succulent stem articles of T. auriculata had higher rates after 6 and 12 d of submergence compared with those of T. medusa.
Table 3.
Underwater net photosynthesis (PN) (µmol O2 m−2 s−1) of succulent stems of Tecticornia auriculata and Tecticornia medusa measured as O2 production in artificial lake water after four submergence durations (0, 3, 6 and 12 d) and at two different CO2 concentrations (40 and 400 µm dissolved CO2)
| CO2 conc. | Species | #0 d | 3 d | 6 d | 12 d |
|---|---|---|---|---|---|
| 40 µm | T. auriculata | 0·89 ± 0·09c | 0·09 ± 0·03a | 0·07 ± 0·01a | 0·03 ± 0·01ab |
| T. auriculata (Triton X-100) | 0·08 ± 0·05ab | 0·11 ± 0·04a | n.m. | n.m. | |
| T. medusa | 0·04 ± 0·02a | 0·10 ± 0·04a | 0·07 ± 0·04a | 0·02 ± 0·01a | |
| T. medusa (Triton X-100) | 0·04 ± 0·02a | 0·12 ± 0·04a | n.m. | n.m. | |
| 400 µm | T. auriculata | 5·54 ± 0·73d | 0·45 ± 0·19b | 0·43 ± 0·08b | 0·40 ± 0·10c |
| T. medusa | 0·62 ± 0·25bc | 0·38 ± 0·04b | 0·18 ± 0·07a | 0·09 ± 0·02b |
At 40 µm CO2 some stems were brushed with 0·05 % (v/v) Triton X-100 solution to remove gas films in the 0 and 3 d submergence treatments. Different letters indicate significant differences between rows using ANOVA and Tukey’s HSD post-hoc test (P < 0·05). Values are means ± s.e. (n = 4). #0 days refers to measurements taken as soon as stems were first submerged; n.m. = not measured.
The presence of gas films on recently submerged T. auriculata enhanced the rates of RD when under water; rates were 0·90 µmol m−2 s−1 for stems with gas films and 0·55 µmol m−2 s−1 in stems without gas films (Table 4). Like with underwater PN, there was no significant effect on RD of brushing with dilute Triton X after 3 d of submergence as the gas films were no longer present. For T. medusa, there was no effect on RD of brushing with dilute Triton X, and the rates were lower in T. medusa (0·23–0·42 µmol m−2 s−1) than in T. auriculata (0·39–0·90 µmol m−2 s−1) at all four submergence durations.
Table 4.
Underwater dark respiration (RD) (µmol O2 m−2 s−1) of succulent stems of Tecticornia auriculata and Tecticornia medusa measured as O2 uptake in artificial lake water after four submergence durations (0, 3, 6 and 12 d)
| Species | #0 d | 3 d | 6 d | 12 d |
|---|---|---|---|---|
| T. auriculata | 0·90 ± 0·02 c | 0·77 ± 0·07b | 0·52 ± 0·04b | 0·39 ± 0·04b |
| T. auriculata (Triton X-100) | 0·55 ± 0·07b | 0·78 ± 0·11b | n.m. | n.m. |
| T. medusa | 0·27 ± 0·01a | 0·42 ± 0·06a | 0·33 ± 0·02a | 0·23 ± 0·02a |
| T. medusa (Triton X-100) | 0·28 ± 0·02a | 0·39 ± 0·04a | n.m. | n.m. |
The medium was near to air-equilibrium dissolved O2 and contained 40 µm CO2. Some stems were brushed with 0·05 % (v/v) Triton X-100 solution to remove gas films in the 0 and 3 d submergence treatments. Different letters indicate significant differences between rows using ANOVA and Tukey’s HSD post-hoc test (P < 0·05). Values are means ± s.e. (n = 4). #0 days refers to measurements taken as soon as stems were first submerged; n.m. = not measured.
Oxygen profiles and time traces
Radial O2 profiles of succulent stems when submerged in water bubbled with air and in darkness showed a steep decline across the epidermis in both species (Fig. 3). In T. auriculata, pO2 remained at around 4 kPa across the chlorenchyma and hydrenchyma and declined to close to zero in the central vascular cylinder. By contrast, in T. medusa pO2 was close to zero all the way across the chlorenchyma, hydrenchyma and the central vascular cylinder when submerged in darkness. In light, there was a steep increase in pO2 across the epidermis and the tissue was above the atmospheric pO2 throughout, in both species, but more so in T. medusa with pO2 up to 35 kPa (Fig. 3). Radial O2 profiles were also taken of stems in air and plants that had been submerged for 3 d and the averages for pO2 at 100–400 µm from the central cylinder were calculated to assess if the longer diffusion path in T. medusa affected the pO2 in the tissue near to the central part of the stems (Table 5). In light, both species had a higher pO2 when submerged compared with when in air, but for T. auriculata the pO2 after 3 d of submergence was closer to that in air (i.e. it had declined from the higher initial values compared with the plants that had just been submerged). In darkness, T. auriculata had the highest pO2 within stems in air, and pO2 was low in stems that had just been submerged (3·8 kPa). In T. medusa pO2 within stems in air was slightly below that in T. auriculata in the same condition, and in stems of T. medusa from both submergence durations pO2 dropped to be close to zero.
Fig. 3.

Radial profiles of O2 partial pressures (pO2) through submerged succulent stem articles of Tecticornia auriculata in darkness (diameter = 2700 µm) (A), Tecticonia auriculata in light (diameter = 2680 µm) (B), Tecticornia medusa in darkness (diameter = 6300 µm) (C) and Tecticornia medusa in light (diameter = 6610 µm) (D). Measurements were taken on a fully elongated article near the middle of an excised stem. The surface of each stem is at 0 µm on the horizontal axes.
Table 5.
Partial pressures of O2 (pO2; kPa) measured in the range 100–400 µm from the central cylinder in succulent stems of Tecticornia auriculata and Tecticornia medusa in air, submerged (i.e. as soon as stems were first submerged) or after 3 d of submergence, in light or darkness
| Species | Treatment | Light | Darkness |
|---|---|---|---|
| T. auriculata | air | 19·9 ± 0·8a | 19·1 ± 0·5e |
| submerged | 26·0 ± 1·7b | 3·8 ± 0·4b | |
| 3 d submerged | 21·4 ± 0·4ab | 7·5 ± 0·6c | |
| T. medusa | air | 26·1 ± 1·5b | 16·9 ± 0·5d |
| submerged | 34·2 ± 0·8c | 0·2 ± 0·1a | |
| 3 d submerged | 32·7 ± 1·4c | 0·5 ± 0·4a |
The mean diameters of stems were 3100 ± 130 and 6700 ± 110 µm for T. auriculata and T. medusa, respectively. Different letters indicate significant differences between columns using ANOVA and Tukey’s HSD post-hoc test (P < 0·05). Values are means ± s.e. (n = 4).
The effects on pO2 of light versus darkness and submergence versus air were also assessed dynamically with the O2 electrode positioned 100 µm from the central cylinder in the hydrenchyma (Fig. 4). When the light was switched off in air, there was a decline in pO2 in both species (steepest in T. medusa), and when the plants were then submerged there was a further decline, again steepest in T. medusa with pO2 close to zero. The subsequent exposure to light resulted in a rapid increase in pO2 with peaks around 50 kPa for both species; the peak was transient and the rate of decline to reach a new steady state was slower in T. medusa than in T. auriculata. In the final phase, the plants were de-submerged and pO2 decreased approximately to levels similar to those in the beginning of the measurements for tissues in air with light.
Fig. 4.

Partial pressures of O2 (pO2) in succulent stem articles of Tecticornia auriculata (A) and Tecticornia medusa (B) measured as the conditions were changed in the following order: air and light (1 h), air and darkness (1 h), submerged and darkness (1 h), submerged and light (6 h), and then air and light (1 h). Measurements were taken on a fully elongated article near the middle of an excised stem.
DISCUSSION
The different SA/V ratios of the two stem-succulent halophytic species prompted us to look at internal O2 dynamics when under water as it seemed intriguing that T. medusa with the thicker stems was more tolerant to submergence than T. auriculata. The radial O2 profiles of stems in darkness and when submerged showed a steep decline across the epidermis in both species, indicating a high resistance to the diffusion of O2 across this outermost layer. The decline was most pronounced in T. medusa, probably because of a less permeable epidermis, which could be because it is thicker than that of T. auriculata (Table 1). The epidermis therefore appears to be the major barrier to O2 supply to tissues within the stems when submerged in darkness, with T. medusa stems being severely hypoxic almost throughout. Similarly, it has been reported that pO2 in the leaf-succulent halophyte Suaeda maritima drops to 0 kPa when submerged during night-time (Colmer et al., 2013). In light, for both Tecticornia species, there was a steep increase in pO2 across the epidermis, as O2 produced in photosynthesis ‘built up’ presumably because of the low permeability of the epidermis. As before, the profiles indicate that T. medusa had the less permeable epidermis of the two species but the gas films on T. auriculata could also contribute to this species having better gas exchange initially after submergence than T. medusa which did not have a gas film. Furthermore, it seemed that the epidermis of T. auriculata became more permeable after 3 d of submergence as pO2 within the stems decreased to around air-equilibrium in light and internal pO2 in darkness increased from 3·8 to 7·5 kPa. This increased permeability in submerged T. auriculata could be caused by the approx. 45 % ‘stretching’ of the epidermis after 3 d as the articles were expanding in volume due to water uptake.
The lower permeability of the epidermis and the lower SA/V of T. medusa resulted in different O2 patterns when light and submergence were manipulated (Fig. 4). A sudden switch to darkness caused a decline in pO2 in both species but steepest in T. medusa and subsequent submergence depleted O2 completely after approx. 20 min in T. medusa because of slow diffusion, whereas T. auriculata still maintained a low pO2 when submerged in darkness. When light was again switched on, internal pO2 increased within a few minutes in both species and reached a peak with values up to 50 kPa. These peaks probably resulted from high photosynthetic activity fuelled by respiratory CO2 that had built up during darkness. As this internal CO2 would become depleted, PN decreased again and reached a quasi-steady state where it depended on slow inward diffusion of CO2 from the water. After the peak in pO2, it took longer to reach a quasi-steady state in T. medusa, presumably indicating that more CO2 had accumulated in this species. Similar peaks in pO2 in submerged tissues have been found in another stem-succulent species, Tecticornia pergranulata (Pedersen et al., 2006), and also in submerged rice a peak was observed which was attributed to CO2 accumulated during darkness (Colmer and Pedersen, 2008a). However, such peaks at the start of the light period have not been observed for seagrasses under field conditions (Greve et al., 2003; Borum et al., 2005), possibly because build-up of respiratory CO2 does not occur to the same extent in aquatic species which have thinner leaves and a more permeable epidermis (no cuticle) enabling better gas exchange with the surrounding water.
We also tested for CAM activity in the two species as this could be a mechanism to enhance uptake of CO2 and hence PN when plants are submerged and limited by CO2 (Pedersen et al., 2011) but we did not find this mechanism to be present. In another study, T. pergranulata and T. indica were tested for CAM and the mechanism was not found in these species (Voznesenskaya et al., 2008).
The slow diffusion of CO2 into the submerged plants would also be expected to affect underwater PN, and this could be overcome by having a gas film, as such a feature has been demonstrated to enhance gas exchange in other terrestrial plants when under water (Colmer and Pedersen, 2008b). We found that when submerged, PN was very low in both species with values below 1 µmol O2 m−2 s−1, except for T. auriculata when it still had gas films present soon after submergence and when in water with 400 µm CO2, which resulted in a PN as high as 5·5 µmol O2 m−2 s−1. The significantly higher PN at 400 µm than at 40 µm CO2 indicates a CO2 limitation, which could be caused by low permeability of the epidermis and slow diffusion across the aqueous diffusive boundary layer. The fact that T. auriculata with intact gas films in 400 µm CO2 had an underwater PN as high as in air suggests that the epidermis is a major limitation to gas exchange, as gas films enhance gas exchange via stomata of submerged leaves (Verboven et al., 2014). Note that the CO2 concentration of 400 µm is high in comparison with levels recorded in salt lakes; for example, Pedersen et al. (2006) found average CO2 concentrations of 18·5 µm in Yenyening Lakes in south-west Australia.
In the present study we also found when in darkness a higher uptake of O2 by the succulent stems of T. auriculata with gas films than without gas films; the gas films are only present temporarily when submerged (Table 4). This effect of gas films indicates that RD was limited by diffusion of O2 into the submerged stems lacking this feature. However, the gas films did not persist and after 3 d there was no difference in RD, or in PN, between stems brushed or not with dilute Triton X. Gas films can enhance O2 uptake during dark periods and improve CO2 uptake during light periods as noted above, and both these beneficial effects have been demonstrated in submerged wetland plants (Colmer and Pedersen, 2008b; Pedersen et al., 2009). Nevertheless, the ecophysiological significance of gas films on these stem-succulent species can be questioned as submergence may often last longer than a few days, and the duration is longer in T. medusa than in T. auriculata habitats, but this feature might aid T. auriculata if submergence is only short term.
Aeration of roots by O2 diffusion from the shoot is common is waterlogged and submerged plants (Armstrong, 1979; Colmer, 2003). In the present study we only measured O2 in stems but it has been demonstrated that root supply of O2 in T. pergranulata is dependent on pO2 in shoots (Pedersen et al., 2006). Pedersen et al. (2006) speculated that the central cylinder of the stems could be a path for longitudinal transport of O2 in T. pergranulata and that the woody part of the stem could be the major area of O2 entry from the water column. In the present study, we did not observe the central cylinder to have a higher pO2 but as we only measured stems with succulent parts, and the cut base was sealed (see Materials and Methods), our results did not reject the hypothesis that O2 enters at the woody parts. For the succulent stems in our study it was apparent that the internal O2 dynamics were determined by PN to a large extent, and pO2 therefore changed dramatically in the plants depending on light conditions. The voluminous nature of the succulent stems and the apparent low permeability of the epidermis are probably the main reasons behind this diurnal pattern, causing the greatest fluctuations in T. medusa, and thus this species seems to be most challenged by O2 depletion during nights when submerged.
There were obvious visual differences between the two species in submergence tolerance as succulent stem articles of T. auriculata started to rupture within 2 d of submergence. The rupturing subsequently resulted in deterioration of the tissues, from which they were not able to recover, so the poor submergence tolerance of T. auriculata is probably related directly to this tissue damage. Both species are halophytes that typically accumulate high concentrations of Na+ and Cl−to maintain low osmotic potentials, allowing water uptake in saline soil conditions (Flowers and Colmer, 2008). It has been shown in a similar glasshouse experiment that in both T. auriculata and T. medusa the succulent stem tissue water concentrations of ions can be high: Na+ (600 mm), Cl− (500 mm) and K+ (150 mm) (L. Moir-Barnetson, unpubl. res.). When the succulent stems become submerged in water of low salinity (10 mm Na+ in the present study), the gradient in osmotic potential could result in water entry and the expansion of succulent articles. The extent of article swelling depends on ion tissue concentrations, epidermis permeability and the strength of the tissue to withstand the increased turgor; the pO2 measurements in the present study indicate a lower epidermal permeability to O2 in T. medusa, which might also be relevant to water uptake. In Sarcocornia perennis submerged in freshwater or low-salinity water, mortality was also attributed to the swelling, rupturing and decomposition of succulent stem articles, and there was no mortality when plants were submerged at higher salinities in the range 600–1286 mm NaCl (Adams and Bate, 1994). These results show that for halophytes it can be more stressful to be submerged in low-saline water in contrast to non-halophytic plants, where the combination of submergence and high salinity is considered to be a more severe stress (Barrett-Lennard, 2003).
Although it can be osmotically stressful for succulent halophytes to be submerged in low-salinity water, the present study showed that T. medusa was able to survive 12 d of submergence without any visual signs of tissue injury, although PN was lower in tissue that had experienced submergence. Another species in the genus, T. pergranulata, survived 56 d of experimental submergence in 10–400 mm NaCl by having a quiescence response during which growth ceased and therefore carbohydrates were conserved and the tissues were in good health afterwards (Colmer et al., 2009). Our measurement of PN in air showed that stem tissues that had been submerged had low PN, even after 60 d of recovery, whereas new stem articles produced after de-submergence were more photosynthetically active. Thus, Tecticornia submergence survival is associated with conservation of carbohydrates and withstanding osmotic stress and subsequent production of new tissue after the flood is over. This ‘quiescence response’ is considered to aid survival of plants either in environments prone to prolonged deep floods or during short transient floods as plants can then best resume growth after the floodwaters recede (Bailey-Serres and Voesenek, 2008).
In summary, the different tolerances to submergence may explain, at least in part, the different field distributions of T. auriculata and T. medusa. At low lying zones, more prone to longer, deeper and more frequent floods, T. medusa forms discrete populations whereas T. auriculata inhabits higher elevations less prone to flooding. However, it seems that maintaining higher stem tissue O2 is not what determines the difference in submergence tolerance of these two stem-succulent halophytes. Instead, the ability to resist the swelling caused by gradients in osmotic potential when these halophytes are submerged in water of low salinity relative to the tissue ion concentrations is apparently more important. The more submergence-tolerant T. medusa had anoxic tissue when succulent stems were submerged in darkness, so this species must be tolerant of transient anoxia. Thus, although adequate internal O2 is considered a key determinant of survival during submergence, plant submergence tolerance also requires additional traits (Bailey-Serres and Voesenek, 2008; Colmer and Voesenek, 2009) and for succulent halophytes tolerance of hypo-osmotic stress and prevention of associated stem tissue swelling are of importance.
ACKNOWLEDGEMENTS
D.K. was supported by a grant from the Villum Kann Rasmussen Foundation. This research was supported by Fortescue Metals Group Ltd and ARC-Linkage Grant LP0882350.
LITERATURE CITED
- Adams JB, Bate GC. 1994. The effect of salinity and inundation on the estuarine macrophyte Sarcocornia perennis (Mill.) A.J. Scott. Aquatic Botany 47: 341–348. [Google Scholar]
- Armstrong W. 1979. Aeration in higher plants. Advances in Botanical Research 7: 225–332. [Google Scholar]
- Armstrong W, Drew M. 2002. Root growth and metabolism under oxygen deficiency. In: Waisel EA, Kafkafi Y, eds. Plant roots: the hidden half. New York: Marcel Dekker, 729–761. [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. 2008. Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology 59: 313–339. [DOI] [PubMed] [Google Scholar]
- Barrett-Lennard EG. 2003. The interaction between waterlogging and salinity in higher plants: causes, consequences and implications. Plant and Soil 253: 35–54. [Google Scholar]
- Borum J, Pedersen O, Greve TM, et al. 2005. The potential role of plant oxygen and sulphide dynamics in die-off events of the tropical seagrass, Thalassia testudinum. Journal of Ecology 93: 148–158. [Google Scholar]
- Colmer TD. 2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment 26: 17–36. [Google Scholar]
- Colmer TD, Flowers TJ. 2008. Flooding tolerance in halophytes. New Phytologist 179: 964–974. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Pedersen O. 2008a. Oxygen dynamics in submerged rice (Oryza sativa). New Phytologist 178: 326–334. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Pedersen O. 2008b. Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist 177: 918–926. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Voesenek LACJ. 2009. Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology 36: 665–681. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Vos H, Pedersen O. 2009. Tolerance of combined submergence and salinity in the halophytic stem-succulent Tecticornia pergranulata. Annals of Botany 103: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD, Winkel A, Pedersen O. 2011. A perspective on underwater photosynthesis in submerged terrestrial wetland plants. AoB Plants 11: doi: 10.1093/aobpla/plr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD, Pedersen O, Wetson AM, Flowers TJ. 2013. Oxygen dynamics in a salt-marsh soil and in Suaeda maritima during tidal submergence. Environmental and Experimental Botany 92: 73–82. [Google Scholar]
- English JP. 2004. Ecophysiology of salt- and waterlogging-tolerance in selected species of Halosarcia. PhD thesis, The University of Western Australia, Perth, Australia [Google Scholar]
- English JP, Colmer TD. 2011. Salinity and waterlogging tolerances in three stem-succulent halophytes (Tecticornia species) from the margins of ephemeral salt lakes. Plant and Soil 348: 379–396. [Google Scholar]
- Flowers TJ, Colmer TD. 2008. Salinity tolerance in halophytes. New Phytologist 179: 945–963. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. 2003. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30: 1–47. [DOI] [PubMed] [Google Scholar]
- Greve TM, Borum J, Pedersen O. 2003. Meristematic oxygen variability in eelgrass (Zostera marina). Limnology and Oceanography 48: 210–216. [Google Scholar]
- Keeley JE, Sandquist DR. 1991. Diurnal photosynthesis cycle in CAM and non-CAM seasonal-pool aquatic macrophytes. Ecology 72: 716–727. [Google Scholar]
- Malcolm CV. 1964. Effects of salt, temperature and seed scarification on germination of two varieties of Arthrocnemum halocnemoides. Journal of the Royal Society of Western Australia 47: 72–74. [Google Scholar]
- Mommer L, Pons TL, Wolters-Arts M, Venema JH, Visser EJW. 2005. Submergence-induced morphological, anatomical, and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiology 139: 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O, Binzer T, Borum J. 2004. Sulphide intrusion in eelgrass (Zostera marina L.). Plant, Cell and Environment 27: 595–602. [Google Scholar]
- Pedersen O, Vos H, Colmer TD. 2006. Oxygen dynamics during submergence in the halophytic stem succulent Halosarcia pergranulata. Plant, Cell and Environment 29: 1388–1399. [DOI] [PubMed] [Google Scholar]
- Pedersen O, Rich SM, Colmer TD. 2009. Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. Plant Journal 58: 147–156. [DOI] [PubMed] [Google Scholar]
- Pedersen O, Rich SM, Pulido C, Cawthray GR, Colmer TD. 2011. Crassulacean acid metabolism enhances underwater photosynthesis and diminishes photorespiration in the aquatic plant Isoetes australis. New Phytologist 190: 332–339. [DOI] [PubMed] [Google Scholar]
- Pedersen O, Colmer TD, Sand-Jensen K. 2013. Underwater photosynthesis of submerged plants – recent advances and methods. Frontiers in Plant Science 4: doi: 10.3389/fpls.2013.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Kende H. 1983. How does deep water rice solve its aeration problem. Plant Physiology 72: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd KA, Wilson PG. 2007. Incorporation of the Australian genera Halosarcia, Pachycornia, Sclerostegia and Tegicornia into Tecticornia (Salicornioideae, Chenopodiaceae). Australian Systematic Botany 20: 319–331. [Google Scholar]
- Skrzypek G, Dogramaci S, Grierson PF. 2013. Geochemical and hydrological processes controlling groundwater salinity of a large inland wetland of northwest Australia. Chemical Geology 357: 164–177. [Google Scholar]
- Verboven P, Pedersen O, Ho QT, Nicolai BM, Colmer TD. 2014. The mechanism of improved aeration due to gas films on leaves of submerged rice. Plant, Cell and Environment 37: 2433–2452. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM. 2006. How plants cope with complete submergence. New Phytologist 170: 213–226. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya EV, Akhani H, Koteyeva NK, et al. 2008. Structural, biochemical, and physiological characterization of photosynthesis in two C4 subspecies of Tecticornia indica and the C3 species Tecticornia pergranulata (Chenopodiaceae). Journal of Experimental Botany 59: 1715–1734. [DOI] [PubMed] [Google Scholar]
- Wilson PG. 1980. A revision of the Australian species of Salicornieae (Chenopodiaceae). Nuytsia 3: 1–154. [Google Scholar]
- Winkel A, Colmer TD, Ismail AM, Pedersen O. 2013. Internal aeration of paddy field rice (Oryza sativa) during complete submergence importance of light and floodwater O2. New Phytologist 197: 1193–1203. [DOI] [PubMed] [Google Scholar]