Abstract
Background and Aims The activity of H+-ATPase is essential for energizing the plasma membrane. It provides the driving force for potassium retention and uptake through voltage-gated channels and for Na+ exclusion via Na+/H+ exchangers. Both of these traits are central to plant salinity tolerance; however, whether the increased activity of H+-ATPase is a constitutive trait in halophyte species and whether this activity is upregulated at either the transcriptional or post-translation level remain disputed.
Methods The kinetics of salt-induced net H+, Na+ and K+ fluxes, membrane potential and AHA1/2/3 expression changes in the roots of two halophyte species, Atriplex lentiformis (saltbush) and Chenopodium quinoa (quinoa), were compared with data obtained from Arabidopsis thaliana roots.
Key Results Intrinsic (steady-state) membrane potential values were more negative in A. lentiformis and C. quinoa compared with arabidopsis (−144 ± 3·3, −138 ± 5·4 and −128 ± 3·3 mV, respectively). Treatment with 100 mm NaCl depolarized the root plasma membrane, an effect that was much stronger in arabidopsis. The extent of plasma membrane depolarization positively correlated with NaCl-induced stimulation of vanadate-sensitive H+ efflux, Na+ efflux and K+ retention in roots (quinoa > saltbush > arabidopsis). NaCl-induced stimulation of H+ efflux was most pronounced in the root elongation zone. In contrast, H+-ATPase AHA transcript levels were much higher in arabidopsis compared with quinoa plants, and 100 mm NaCl treatment led to a further 3-fold increase in AHA1 and AHA2 transcripts in arabidopsis but not in quinoa.
Conclusions Enhanced salinity tolerance in the halophyte species studied here is not related to the constitutively higher AHA transcript levels in the root epidermis, but to the plant’s ability to rapidly upregulate plasma membrane H+-ATPase upon salinity treatment. This is necessary for assisting plants to maintain highly negative membrane potential values and to exclude Na+, or enable better K+ retention in the cytosol under saline conditions.
Keywords: AHA expression, Arabidopsis thaliana, Atriplex lentiformis, Chenopodium quinoa, H+ fluxes, K+ fluxes, Na+ fluxes, membrane potential, H+-ATPase, halophyte, salinity tolerance, saltbush
INTRODUCTION
Soil salinity is one of the major environmental stresses affecting crop growth worldwide. It is estimated that an area of ∼ 950 million hectares of arable land globally, including 250 million hectares of irrigated land, is affected by salinity (Ruan et al., 2010). The expected demand for food (Schmidhuber and Tubiello, 2007), good quality water (Hanjra and Qureshi, 2010) and biofuels (Valentine et al., 2012) for human use in an era of climate change puts enormous strain on current food production. To manage these challenges, domestication and cultivation of halophyte crops (e.g. Chenopodium quinoa), as well as improvements in salinity tolerance in existing glycophyte crops, could expand food production into salt-affected marginal lands and at the same time allow the use of brackish and saline waters for irrigation (Shabala, 2013). Although the cultivation of halophytes in saline lands is relatively straightforward, the range of species is rather limited (Flowers et al., 2010). Improving the salt tolerance of existing crop species is not progressing because the genetic variability within existing crop species, including land races, is limited (Colmer et al., 2005). Hence, new salt-tolerance traits or genes must be identified in halophytes and introduced into existing crop species of high agronomic value. Comparing the performance of glycophytes with that of halophytes is the necessary first step towards achieving this goal.
The growth of plants in saline environments depends on their ability to cope with the major constraints (osmotic, ionic and oxidative stresses) imposed by salinity. While the growth of glycophytes is severely hampered at salt concentrations exceeding 100 mm NaCl (or even lower), such concentrations are considered optimal for halophyte growth (reviewed in Greenway and Munns, 1980; Munns and Tester, 2008). Many halophytes use Na+ ions as a cheap osmoticum for osmotic adjustment (e.g. Hariadi et al., 2011), are able to adopt efficient Na+ excretion (e.g. salt glands) and compartmentalization (both internally into vacuoles and externally into salt bladders) mechanisms (reviewed in Shabala, 2013) and have superior reactive oxygen species (ROS) homeostasis (reviewed in Ozgur et al., 2013; Bose et al., 2014a) during salt stress. Several independent studies have established that most, if not all, of the salt tolerance mechanisms employed by halophytes are similar to those employed by glycophytes (Sengupta and Majumder, 2010; Bartels and Dinakar, 2013). Nonetheless, although the salt tolerance mechanisms are similar, halophytes may either constitutively turn on salt tolerance mechanisms or exhibit subtle changes in their transcriptional and post-transcriptional regulation, which creates large variation in the salt tolerance levels between glycophytes and halophytes (Oh et al., 2010; Kosova et al., 2013). However, the specific details of this process remain obscure and await elucidation.
In contrast to glycophytes, many halophytes are classified as Na+ includers; they store large amounts of Na+ in either leaf vacuoles or epidermal bladders, when the latter are present (Osmond et al., 1969; Flowers and Colmer, 2008). Nonetheless, excluding salt by preventing Na+ entry has also been noted in some halophytes (e.g. Thellungiella halophila) (Wang et al., 2006). To restrict Na+ entry or to transport Na+ into specific locations in the shoot, Na+ uptake and xylem loading in roots must be strictly regulated. However, the information pertinent to Na+ entry and transport in roots of halophytes is ambiguous (Flowers and Colmer, 2008). This is because all the methods employed so far have either very poor resolution (Na+ depletion, whole-plant Na+ content analysis) or are able to quantify only the unidirectional transport of Na+ (22Na+ uptake studies) into root tissue (Cuin et al., 2011). However, most researchers agree that salinity stress tolerance is not conferred by the difference in unidirectional Na+ uptake (Davenport et al., 1997, 2005), but rather by the magnitude of the net Na+ flux, determined predominantly by genotypic difference in the plant’s ability to actively pump Na+ back into the rhizosphere (Cuin et al., 2011) and/or sequester it in vacuoles (Apse and Blumwald, 2007). This problem can be addressed when Na+-selective microelectrodes are used. Until recently, this approach was hampered by the lack of suitable Na+ liquid ion exchangers with good ability to differentiate between Na+ and K+ (for details see Chen et al., 2005). Introduction of the calixarene-based microelectrode with improved Na+ selectivity (Jayakannan et al., 2011) unlocks good prospects for dissecting Na+ entry and efflux pathways and understanding of their regulation in the roots of halophytes.
It is generally accepted that salinity stress induces H+-pumping capacity in plant tissues, primarily to energize Na+/H+ exchanger activity, and that this capacity is stronger in halophyte species (Niu et al., 1993; Ayala et al., 1996; Wu and Seliskar, 1998; Vera-Estrella et al., 2005; Shabala and Mackay, 2011). However, most reports have focused on the leaf rather than on root tissues and have usually been targeted to the tonoplast rather than the plasma membrane pumps. Furthermore, the reports are often controversial. While salt treatment increased plasma membrane H+-ATPase transport activity in T. halophila leaves (Vera-Estrella et al., 2005), in Aster tripolium a pronounced decline in plasma membrane P-ATPase activity was reported after 1 d of salinity exposure (Ramani et al., 2006). In Salicornia bigelovii, the highest plasma membrane H+-ATPase activity was observed in plants grown at optimal (200 mm NaCl) salt concentration (Ayala et al., 1996), while in another halophyte, Plantago maritima, this treatment caused a decrease in plasma membrane H+-ATPase activity in leaves (Bruggemann and Janiesch, 1989). In glycophytes, salinity tolerance in barley was related to higher root H+-ATPase activity (Chen et al., 2007a), but barley plants were not capable of maintaining their membrane potential and preventing NaCl-induced membrane depolarization, while salt-sensitive pea plants did have this ability (Bose et al., 2014b). Thus, the link between salinity tolerance and H+ pumping is not that straightforward and requires thorough investigation.
Maintaining K+ homeostasis is central to the survival of both glycophytes and halophytes in saline environments (Volkov et al., 2004; Volkov and Amtmann, 2006); salt stress can induce cell death by depleting the cytosolic K+ concentration beyond its threshold level (Anschütz et al., 2014; Shabala and Pottosin, 2014). However, the concept of the ‘lower the K+ efflux the higher the tolerance’ is often not applicable when comparing different species. Wheat is known to be much more salt-sensitive than barley, yet a screen of nearly 50 wheat genotypes showed that even the most sensitive wheat varieties had much better ability to prevent NaCl-induced K+ efflux compared with barley cultivars (for a comparison see Chen et al., 2007b; Cuin et al., 2009) in short-term experiments. The main modes of salt-induced K+ loss in root tissue are through depolarization-activated K+ outward-rectifying (KOR) channels and ROS-activated non-selective cation channels (NSCCs) (Shabala and Cuin, 2008). So, do halophytes have better control over salt-induced K+ loss? If so, which of the above pathways plays the most crucial role?
The main aim of this work was to address these knowledge gaps by comparing the kinetics of ion fluxes and membrane potential maintenance during salt stress between two halophyte species, Atriplex lentiformis and Chenopodium quinoa, and comparing the response to measurements from a glycophyte species, Arabidopsis thaliana. The results of P-ATPase relative gene expression (AHA-like transcript abundance), viability staining, ion flux, membrane potential and pharmacological experiments revealed that the roots of halophytes are able to activate plasma membrane H+-ATPase within minutes of sensing Na+ entry, but without increasing the transcriptional abundance of AHA1/2/3. This enhanced H+-ATPase activity fuels Na+ efflux and prevents K+ efflux through the depolarization-activated KOR channels. By contrast, salt-induced stimulation of the H+-ATPase activity, activation of Na+ efflux and prevention of K+ loss were absent in the roots of arabidopsis, even though the expression levels of AHA1/2/3 were high in this species.
MATERIALS AND METHODS
Plant materials and growth conditions
Arabidopsis thaliana (Col-0) seeds were surface-sterilized with commercial bleach for 10 min, washed three times with sterile water and then sown in 90-mm Petri dishes containing solid basal salt medium (BSM: 0·2 mm KCl + 0·1 mM CaCl2, pH 5·5, unbuffered) with 0·8 % (w/v) agar. Petri dishes containing the seeds were kept at 4 °C for 2 d to break dormancy. Quinoa (Chenopodium quinoa cv KVL52) seeds were surface-sterilized as above, sown on paper rolls wetted with BSM solution and kept in the dark for a day to initiate germination. Saltbush (Atriplex lentiformis) seeds were soaked in deionized water overnight, then sown on paper rolls wetted with BSM solution. Seeds were then kept in the dark for 9 d until germinated. Once germination was initiated, the seeds of all species were transferred to a growth chamber with a 14/10-h day/night regime at 150 µmol m−2 s−1 irradiation and grown at 24 ± 1 °C. Plants 5 d old were used in experiments.
For experiments on mature plants, arabidopsis plants were grown on solid agar medium containing full-strength Murashige–Skoog (MS) solution for 20 d prior to measurements. For the same purpose, quinoa plants were grown on paper rolls wetted with the full-strength MS solution media under the conditions described above.
Viability staining
Control and salt-treated roots of 5-d-old seedlings were double-stained with fluorescein diacetate (FDA)–propidium iodide (PI) and observed under a fluorescence microscope prior to and after 1 and 4 h of treatment with 100 mm NaCl, essentially as described by Bose et al. (2014b).
Net ion flux measurements
Net fluxes of H+, K+ and Na+ were measured non-invasively using the Microelectrode Ion Flux Estimation technique (MIFE™; University of Tasmania, Hobart, Australia). The basic principles of MIFE™ measurement are described elsewhere (Newman, 2001) and the specific details pertinent to microelectrode fabrication, calibration and measurements are available in our previous publications (e.g. Shabala et al., 2006). The Na+ fluxes were measured using calixarene-based microelectrodes as described by Jayakannan et al. (2011, 2013). Ion-selective microelectrodes with a Nernst slope response of <50 mV per decade were discarded. Experiments were conducted at room temperature (24 ± 1 °C) under ambient light.
Unless specified, all flux measurements were carried out in the elongation zone (between ∼ 200 and ∼ 600 µm from the root cap) and mature root zone ( ∼ 5 mm from the root apex) (Supplementary Data Fig. S1). Roots of a 5-d-old intact seedlings were immobilized in the measuring chamber and pre-conditioned in BSM for 30 min as described elsewhere (Bose et al., 2013). After pre-conditioning, steady-state ion fluxes were recorded over a period of 5 min. Then, an NaCl-containing double stock solution was applied and mixed to reach the required final NaCl concentration of 100 mm. The resulting transient H+, K+ and Na+ fluxes were measured for up to 25 min. For pharmacology experiments, quinoa seedlings were pre-treated with 1 mm vanadate (a P-type H+-ATPase inhibitor) for 1 h before 100 mm NaCl treatment. Fluxes of between four and 12 individual seedlings were averaged for every plant species, root zone and treatment combination.
In experiments with longer-term salinity exposure, 5-d-old arabidopsis and quinoa seedlings were exposed to salt stress on the BSM background. At the 24- and 72-h time points, salt-induced steady-state H+ and Na+ fluxes were measured for 5 min in the elongation and mature root zones. Fluxes from eight to 12 seedlings for each species were measured and averaged.
To capture the variability in Na+ fluxes along the longitudinal root axis, salt-induced transient Na+ fluxes were also measured in the basal mature root zone ( ∼15 mm from the base of the stem) of 5-d-old quinoa seedlings (Supplementary Data Fig. S2).
To eliminate a possible contribution of growth method on ion flux response patterns, quinoa seedlings were grown using both the paper roll and the agar medium method (as above), and fluxes from the elongation zone were then measured and compared (Supplementary Data Fig. S3). No difference in ion flux response kinetics, either qualitative or quantitative, was observed, suggesting that either method can be used and compared with the other.
Membrane potential measurements
Conventional KCl-filled Ag/AgCl microelectrodes were used for membrane potential measurements in the elongation and mature root zones (Bose et al., 2013, 2014b). The roots of an intact 5-d-old seedling were immobilized and pre-conditioned as described above. Resting membrane potential measurements were recorded for 1 min before adding 100 mm NaCl to the BSM solution. The resulting change in transient membrane potential was continuously monitored for up to 30 min. Membrane potential values of seven or eight individual seedlings were averaged for each plant species, root zone and treatment combination.
Real-time RT–PCR analysis for AHA1/2/3 expression
Uniform 5-d-old arabidopsis and quinoa seedlings were exposed to 100 mm NaCl stress. At the indicated time points, 100 mg of roots on a fresh weight basis were harvested and used immediately for RT–PCR analysis. Total RNA from roots was isolated by grinding in liquid nitrogen until a fine powder appeared and using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA (2 µg) was reverse-transcribed using oligo(dT) primer and avian myeloblastosis virus reverse transcriptase XL (TaKaRa, Dalian, China). Real-time quantitative RT–PCR reactions were performed using a Mastercycler® realplex real-time PCR system (Eppendorf, Hamburg, Germany) with SYBR® Premix Ex Taq™ (TaKaRa Bio, China) according to the manufacturer’s instructions. The experiment was repeated on three separate occasions (i.e. on three batches of plants grown at different times), with three biological replicates each time, always with consistent results. Since the exact primers and accession codes for quinoa were not available, the conserved sequence of the AHA genes of arabidopsis was used (Supplementary Data Table S1). The expression levels of the genes are presented as values relative to the corresponding control samples under the indicated conditions, with normalization of data to the geometric average of the internal control gene Actin2/7, previously verified as a housekeeping gene for both Chenopodium (Cooper, 2001) and Atriplex (Sadder and Al-Doss, 2014) species.
Statistical analysis
Data are presented as mean ± SE. The standard least significant difference test at P ≤ 0·05 level was used to determine statistical significances of differences between mean values.
RESULTS
Salt stress affects the viability of arabidopsis but not quinoa or saltbush roots
Numerous reports have demonstrated that 100 mm NaCl reduces the shoot growth of arabidopsis by up to 70 % of that of control plants (e.g. Jayakannan et al., 2013). In contrast, shoot growth of saltbush and quinoa is enhanced by 100 mm NaCl treatment compared with no salt treatment (e.g. Redondo-Gomez et al., 2007; Hariadi et al., 2011). Although the effect of salt stress on shoot growth of these plant species is well established in the literature, information about the roots is lacking. To address this knowledge gap, the effect of 100 mm NaCl treatment on the viability of roots was assessed using the FDA–PI double staining method. The results showed that the elongation zone of arabidopsis started losing viability within an hour of salt stress and most of the cells in this zone were severely damaged after 4 h of salt exposure (as indicated by the red colour in Fig. 1). At the same time, roots of quinoa and saltbush were unaffected at either time point (indicated by the green colour in Fig. 1).
Fig. 1.

Viability staining of arabidopsis, saltbush and quinoa roots in response to 100 mm NaCl. The fluorescein diacetate (FDA)–propidium iodide (PI) double staining method was used to image the viability of root cells. One (of six) typical images is shown for each treatment/species.
Halophyte species have higher Na+ exclusion ability
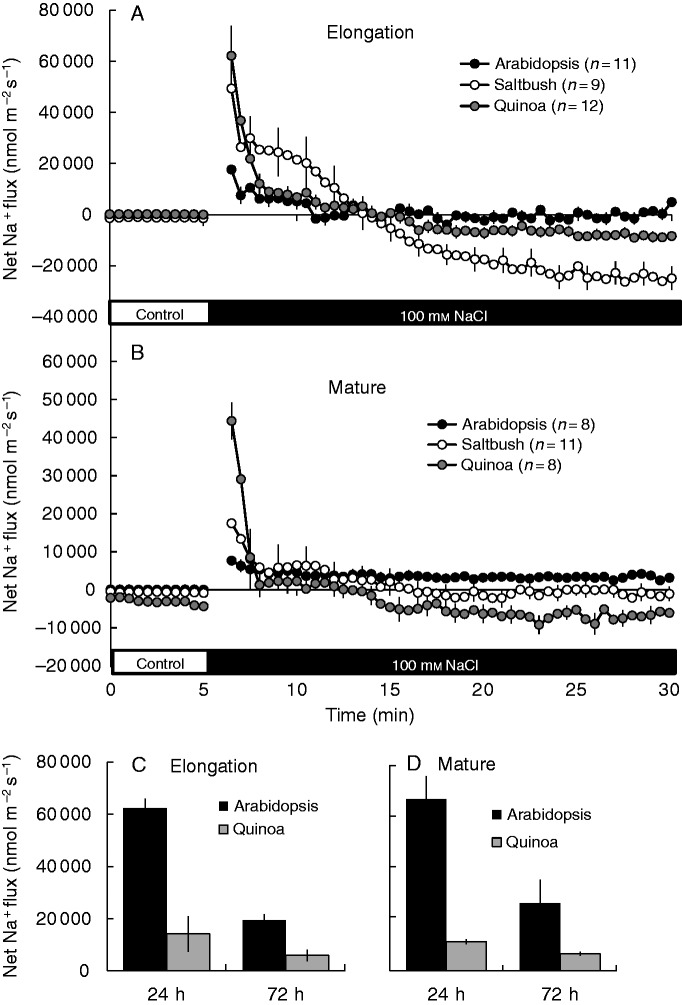
Adding 100 mm NaCl to the bath solution caused an immediate transient increase in Na+ influx into both root zones of all species studied (Fig. 2). This Na+ influx was higher (2- to 3- fold in the elongation zone and 2- to 5-fold in the mature zone) in quinoa and saltbush than in arabidopsis. After the initial Na+ uptake, both halophytes showed the ability to export significant amounts of Na+ from the root tissue (net Na+ efflux from 15 min onwards; Fig. 2), with a higher net Na+ efflux measured from the elongation zone. The net Na+ efflux was not observed in root cells located close to the root base (Supplementary Data Fig. S2), suggesting tissue-specific expression of appropriate transporters. This is consistent with the general consensus that salt overly sensitive (SOS1) Na+/H+ exchangers are located predominantly in the root apex (Shi et al., 2000). Net Na+ efflux from the root apex also showed pronounced time-dependence; it was not detected after 24 h of salinity exposure (Fig. 2C, D). Net Na+ flux in arabidopsis was not statistically different from zero in the elongation zone in short-term experiments (Fig. 2A) and was directed inwards in the mature root zone (Fig. 2B). Long-term exposure resulted in a substantial shift towards massive net Na+ uptake in both zones (Fig. 2C, D). Regardless of the root zone or the length of exposure, net Na+ uptake in arabidopsis exceeded that in quinoa several-fold (Fig. 2C, D).
Fig. 2.
(A, B) Transient net Na+ fluxes measured from the elongation (A) and mature (B) root zones of arabidopsis, saltbush and quinoa in response to 100 mm NaCl. Salt stress was applied at the fifth minute. (C, D) Steady-state net Na+ fluxes measured from the elongation (C) and mature (D) regions of quinoa and arabidopsis roots after long-term (72 h) salinity exposure. Data are mean ± s.e. (n = 8–12). In all MIFE figures the s.e. bars are only shown for every third data point.
Salt-induced plasma membrane depolarization is higher in arabidopsis than in halophytes
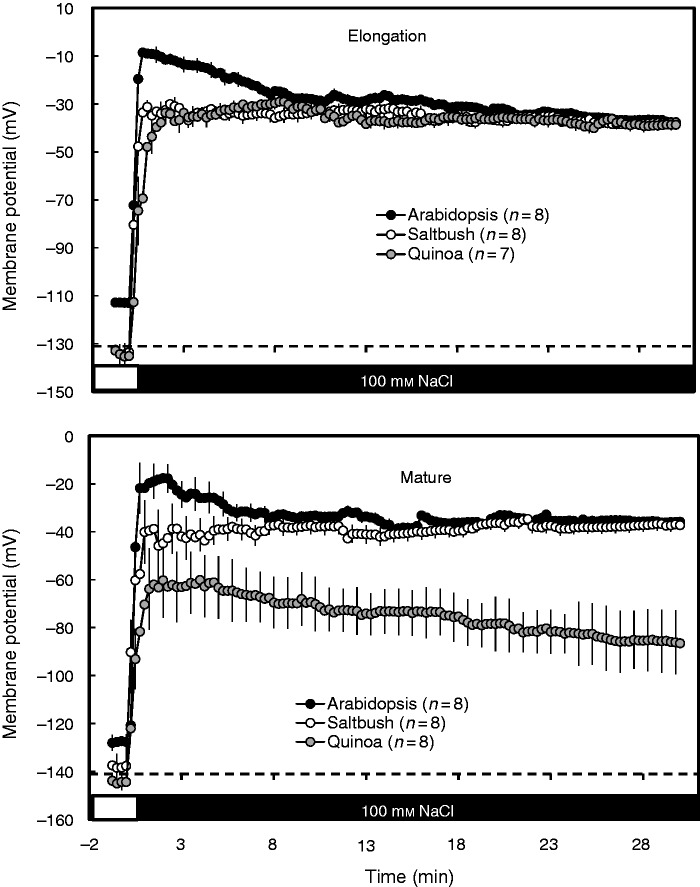
The resting membrane potential of epidermal cells in the elongation and mature root zones under control conditions was more negative in halophytes (−134 ± 4 mV in the elongation zone, −141 ± 2 mV in the mature zone) than in arabidopsis (−112 ± 1 mV in the elongation zone, −127 ± 2 mV in the mature zone) (Fig. 3). As in our previous observations (Bose et al., 2013; Jayakannan et al., 2013), 100 mm NaCl treatment significantly (P < 0·01) depolarized the plasma membrane within a minute in all three species examined, albeit by different magnitudes (Fig. 3). The initial salt-induced depolarization (after 1–8 min) was significantly higher in the roots of arabidopsis compared with the roots of both halophyte species, with the peak depolarization values changing in the sequence arabidopsis > saltbush > quinoa. After 8 min, there was little difference between different species in the root elongation zone, whereas in the mature zone quinoa roots maintained a more negative potential than the roots of saltbush and arabidopsis throughout the measuring period (Fig. 3).
Fig. 3.
Transient membrane potential values measured in root epidermal cells in the elongation and mature zones of arabidopsis, saltbush and quinoa in response to 100 mm NaCl. Salt stress was applied at time zero. Data are mean ± s.e. (n = 7–8).
Halophytes lose less K+ during salt stress than does arabidopsis
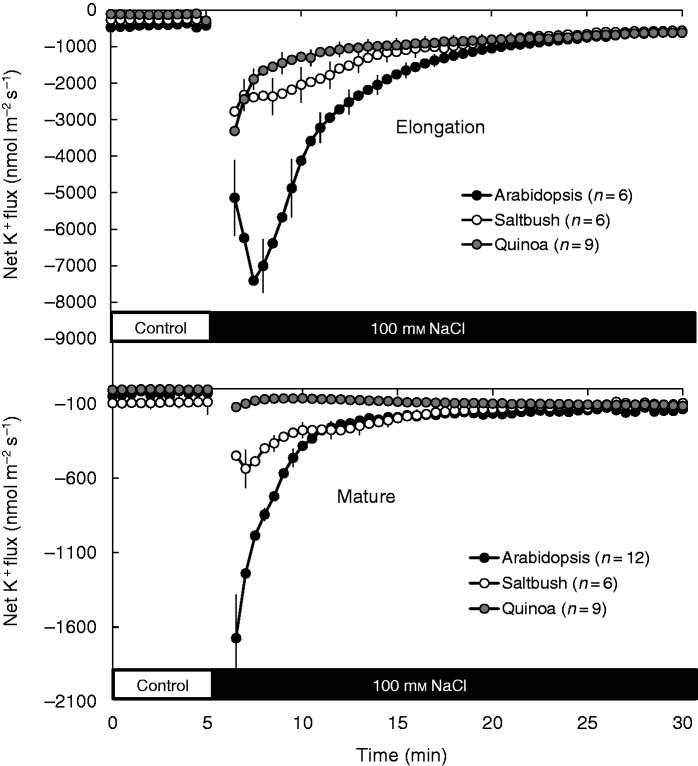
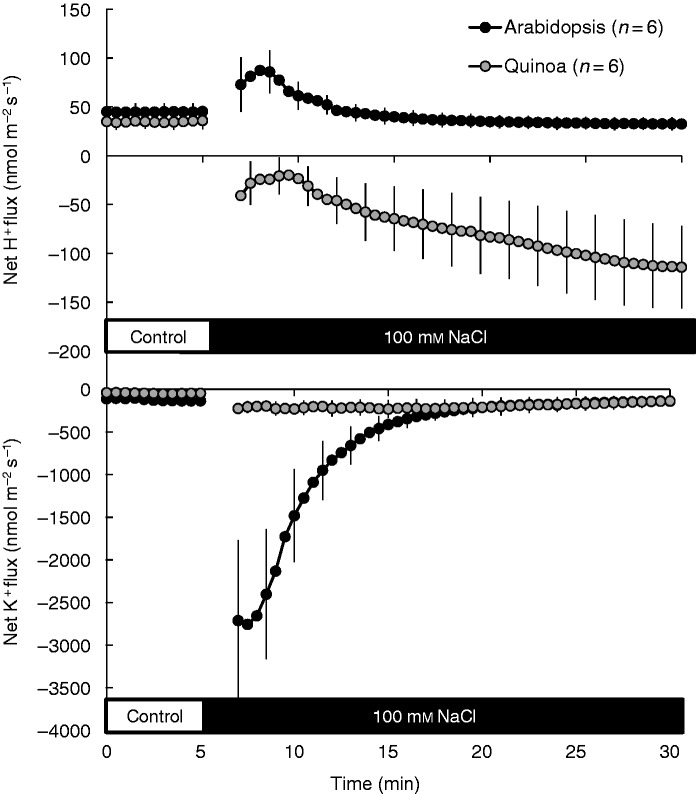
All three species showed a small net K+ efflux in both the elongation and mature root zones prior to salt exposure (Fig. 4), with no significant (P < 0·05) difference between species. Consistent with our previous publications (Bose et al., 2013, 2014b), acute salt stress induced K+ efflux from both root zones of the species examined, but with very different magnitudes (Fig. 4). In the elongation zone, the total amount of K+ lost by arabidopsis roots was 2-fold higher than that lost by quinoa and saltbush roots (100 ± 5 versus 56 ± 4 and 48 ± 3 µmol m−2, respectively, over the 20 min of stress exposure). In the mature zone, peak K+ efflux declined in the sequence arabidopsis > saltbush>quinoa with a 13:4:1 ratio (Fig. 4B), strongly resembling the membrane depolarization sequence (Fig. 3B).
Fig. 4.
Transient net K+ fluxes measured from epidermal cells in the elongation and mature root zones of arabidopsis, saltbush and quinoa in response to 100 mm NaCl. Salt stress was applied at the fifth minute. Data are mean ± s.e. (n = 6–12).
In many species, most of the salt-induced K+ efflux is mediated by depolarization-activated K+ channels (Jayakannan et al., 2013). Accordingly, a linear correlation was plotted (Fig. 5) between the peak K+ efflux values (data from Fig. 4) and the respective membrane potential values (data from Fig. 3) measured from each individual plant in the root elongation zone. This showed that greater salt-induced depolarization is indeed responsible for the higher K+ loss from arabidopsis compared with halophyte roots (Fig. 5).
Fig. 5.
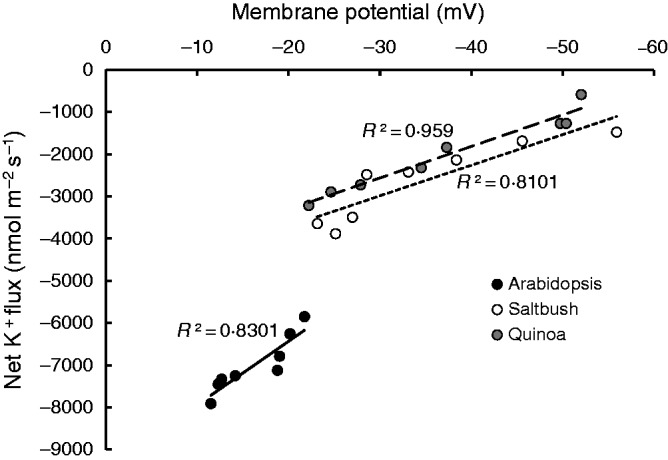
Linear correlation between the peak of the transient net K+ efflux and the most depolarized state of membrane potential values measured in the root elongation zone of arabidopsis, saltbush and quinoa in response to 100 mm NaCl in short-term kinetic experiments. Each point represents the value for one individual seedling within a species.
NaCl induces massive vanadate-sensitive H+ efflux from halophyte but not from arabidopsis roots
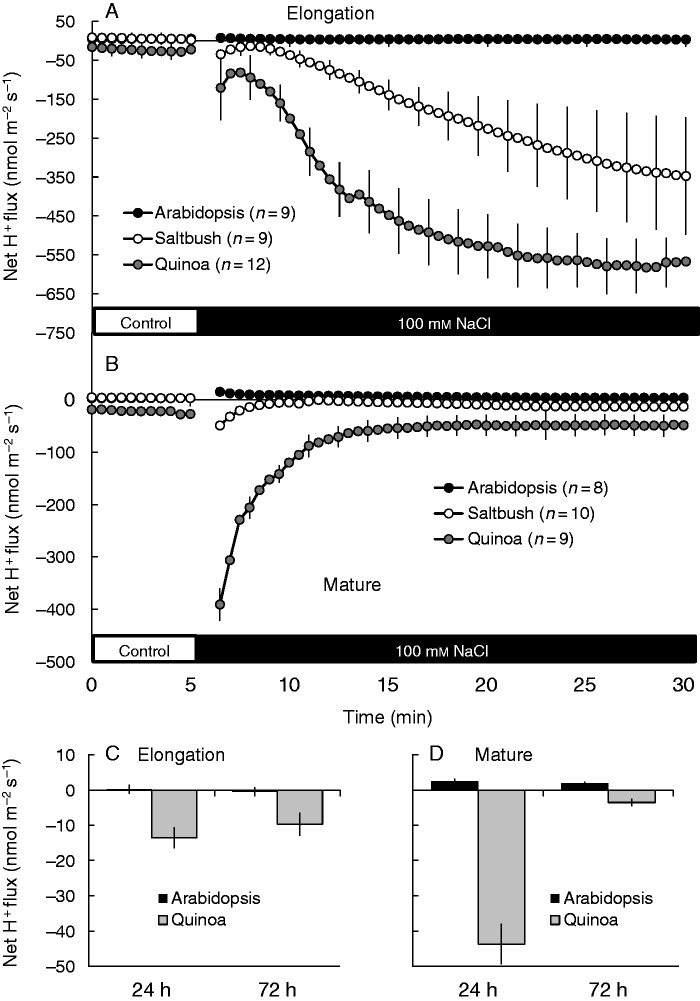
Under control conditions, net H+ fluxes from plants were close to zero in saltbush and arabidopsis, while in quinoa a small but significant (P < 0·05) net H+ efflux was measured in both root zones (Fig. 6A, B). Application of 100 mm NaCl induced massive H+ efflux from halophyte roots, but not from arabidopsis (Fig. 6A, B). In all species, salt-induced H+ efflux was higher in the elongation zone than in the mature zone (Fig. 6A, B). Overall, NaCl-induced H+ efflux decreased in the following sequence: quinoa > saltbush > arabidopsis (Fig. 6A, B). The observed NaCl-induced stimulation of H+ pumping in halophytes was long-lasting, occurring for several days after salinity exposure (illustrated for quinoa in Fig. 6C, D). The growing condition (paper roll or agar medium) did not alter salt-induced H+ efflux (Supplementary Data Fig. S3). This ability of halophyte species to respond to salinity by increasing the rate of H+ pumping was independent of plant age and was also observed in roots of mature plants (Fig. 7A). In full agreement with our data for young seedlings (Fig. 4), mature quinoa roots were also able to retain much more K+ compared with arabidopsis (Fig. 7B).
Fig. 6.
(A, B) Transient net H+ fluxes measured from epidermal cells of the elongation (A) and mature (B) root zones of arabidopsis, saltbush and quinoa in response to 100 mm NaCl. Salt stress was applied at the fifth minute. (C, D) Steady-state net H+ fluxes measured from the elongation (C) and mature (D) region of quinoa and arabidopsis roots after long-term (72 h) salinity exposure. Data are mean ± s.e. (n = 8–12).
Fig. 7.
Transient net H+ (A) and K+ (B) fluxes measured from the mature zone of old ( ∼ 20 d) arabidopsis and quinoa roots in response to addition of 100 mm NaCl at 5 min. Data are mean ± s.e. (n = 6).
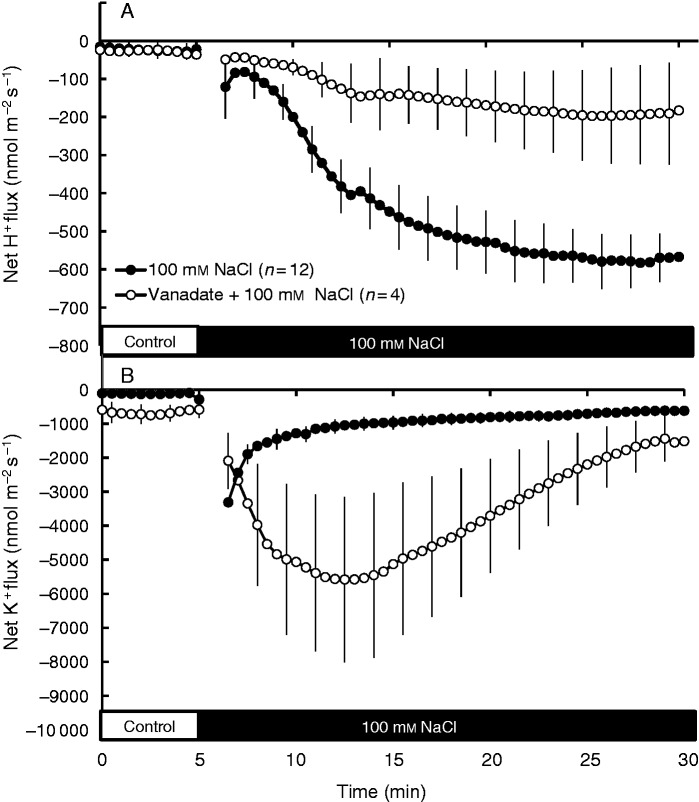
To determine whether the salt-induced H+ efflux originated from plasma membrane H+-ATPase activity, quinoa roots were pre-treated with 1 mm sodium orthovanadate (a known inhibitor of the P-type plasma membrane H+-ATPase) for 1 h before applying salt stress. The results showed that vanadate was able to decrease salt-induced H+ efflux by 75–80 % (Fig. 8A). Interestingly, vanadate pre-treatment also increased salt-induced K+ efflux by 2- to 3-fold (Fig. 8B), supporting the notion that the observed NaCl-induced K+ leak from quinoa roots was mediated predominantly by voltage-gated channels controlled by the plasma membrane H+-ATPase.
Fig. 8.
The effect of 1 mm vanadate, a known H+-ATPase inhibitor, on 100 mm NaCl-induced fluxes of H+ (A) and K+ (B) ions measured in the root elongation zone of quinoa. Quinoa seedlings were pre-treated with 1 mm sodium orthovanadate for 1 h. Salt stress was applied at the fifth minute. Data are mean ± s.e. (n = 4–12).
Higher NaCl-induced H+-ATPase activity in halophytes is not related to constitutively higher AHA expression
Analysis of the relative expression of AHA1/2/3 transcript levels for various H+-ATPase isoforms revealed that expression levels of AHA1 and AHA2 were constitutively higher in the roots of arabidopsis compared with the roots of quinoa, while AHA3 expression levels were similar in the two species (Fig. 9). Salinity stress (100 mm NaCl) did not alter AHA1/2/3 expression in quinoa, but resulted in a 3- to 5-fold increase in AHA1/2/3 expression levels in arabidopsis within an hour of salt treatment. After 24 h of salt stress, AHA1 and AHA2 transcript levels had returned to their original values in arabidopsis while AHA3 transcript levels remained elevated (Fig. 9).
Fig. 9.

Plasma membrane H+-ATPase (AHA1/2/3-like) transcript abundances in roots of arabidopsis and quinoa in response to 100 mm NaCl. Each bar represent the mean ± s.e. (n = 3). Bars labelled with the same letter are not significantly different at P < 0·05.
DISCUSSION
Halophytes activate H+-ATPase to fuel Na+ efflux through the Na+/H+ exchanger during acute salt stress
Sodium entry is a thermodynamically passive process, controlled predominantly by the electric potential difference across the plasma membrane (Blumwald et al., 2000). Given that the roots of halophytes (−134 ± 4 mV in the elongation zone and −141 ± 2 mV in the mature zone) have a more negative membrane potential than arabidopsis (−112 ± 1 mV in the elongation zone and −127 ± 2 mV in the mature zone) (Fig. 3), the driving force for Na+ uptake is greater in the roots of halophytes than in arabidopsis roots. Consequently, the initial Na+ influx in the roots of halophytes was 2- to 3-fold higher than in arabidopsis (Fig. 2A, B). This enhanced initial Na+ uptake could aid the roots of halophytes in sensing Na+ entry via the resulting elevation in cytosolic Ca2+, cGMP and hydrogen peroxide production (Bose et al., 2011; Maathuis, 2014). This could enable their superior ability compared with the glycophyte counterpart to activate H+ efflux by means of H+-ATPases, so energizing Na+ efflux through SOS1 exchangers (Hassidim et al., 1990; Maughan et al., 2009). Indeed, roots of both halophyte species activated vanadate-sensitive H+ efflux (Figs 6 and 8A) upon sensing Na+ entry, so fuelling Na+ efflux within 10 min of salt addition (Fig. 2).
Salt-induced plasma membrane H+-ATPase activity is responsible for the prevention of K+ loss from the roots of saltbush and quinoa during salt stress
The entry of Na+ ions into the root cell, through either NSCCs or high-affinity K+ transporters, depolarizes the plasma membrane (Fig. 3), which makes K+ uptake through K+ inward rectifying channels thermodynamically impossible (Shabala and Cuin, 2008). Furthermore, salt-induced plasma membrane depolarization activates K+ efflux (Fig. 4) through depolarization-activated KOR channels (Jayakannan et al., 2013). In addition, K+ may also leak through NSCCs as a result of the accumulation of ROS during salt stress (Bose et al., 2014b). These processes deplete the K+ pool available for numerous metabolic functions, resulting in the shutdown of metabolic functions and eventual cell death (Anschütz et al., 2014). Even if the reported K+ fluxes are transient, the amount of K+ lost may be large enough to disturb cell metabolism. To make our point, we did some model calculations based on the measured fluxes reported in this work and root cell geometry in arabidopsis. Assuming the cell is a cylinder 50 µm long with a diameter of 14 µm, the cell volume is 385 × 10−18 m3. Assuming cytosol constitutes 5 % of the cell’s volume and the cytosolic K+ concentration is 100 mm, each cell contains ∼ 38·5 pmol K+ in its cytosol. From Fig. 4A, the average K+ flux in the elongation zone during the first 25 min is ∼ 2100 nmol m−2 s−1. The cell surface area is 2·5 × 10−9 m2. Thus, the total amount of K+ leaked over 25 min will be ∼7·9 pmol. This is >20 % of the total cytosolic K+ content. We believe this amount is large enough to disturb cell metabolism. Thus, in order to live and complete their life cycle in a saline environment, halophytes must possess mechanisms that efficiently control K+ loss through KOR channels and NSCCs during salt stress. Since halophytes exercise efficient mechanisms to prevent ROS production during salt stress (Bose et al., 2014a), the K+ loss via ROS-activated NSCCs will be negligible, if it occurs at all. Nonetheless, to the best of our knowledge there are no reports of how halophytes control K+ loss through depolarization-activated KOR channels. Here we report that the roots of saltbush and quinoa are proficient in preventing K+ loss through such KOR channels. Two lines of evidence support this claim. First, salt-induced plasma membrane depolarization (Fig. 3) and the corresponding K+ efflux (Fig. 4) are positively correlated (R2 ≥ 0·81; Fig. 5) in all three species. The magnitudes of this salt-induced depolarization and K+ efflux are much lower in the roots of saltbush and quinoa compared with arabidopsis. The ability of the salt-tolerant T. halophila to maintain membrane potential at more negative values than arabidopsis supports this notion (Volkov and Amtmann, 2006). Secondly, the pivotal contribution of H+-ATPases in regulating the plasma membrane potential during salt stress is well established in diverse plant species (Palmgren, 2001; Chen et al., 2007a; Jayakannan et al., 2013). Pre-treating the roots of quinoa with 1 mm vanadate, a known inhibitor of the P-type H+-ATPase, significantly decreased the extent of the salt-induced H+ extrusion and potential salt-induced K+ efflux (Fig. 7), implying that the roots of halophytes rely heavily on H+-ATPases for the regulation of the plasma membrane potential during salt stress.
Regulation of H+-ATPase activity but not higher expression of AHA1/2/3 -like transcripts by quinoa and saltbush contributes to salt tolerance
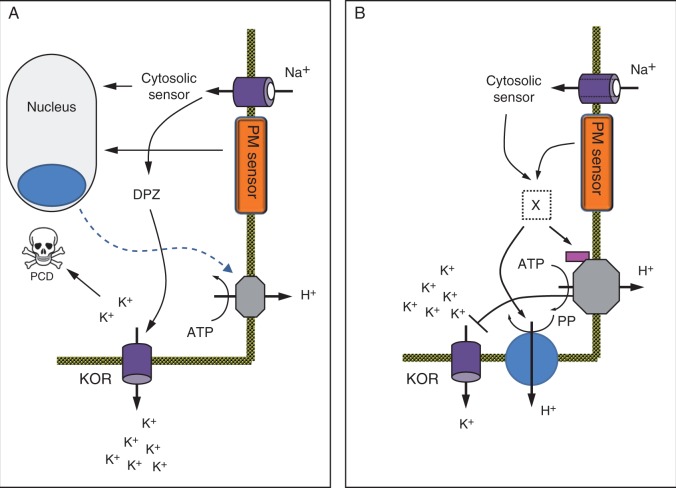
Many studies comparing glycophytes with halophytes suggest that, in most cases, salt-responsive genes are constitutively up- or downregulated in halophytes (e.g. Kant et al., 2006; Edelist et al., 2009). In the present study, salt-induced H+ efflux was observed only from the roots of saltbush and quinoa, not from arabidopsis (Fig. 6). Furthermore, 1 mm vanadate, a P-type H+-ATPase inhibitor, was able to decrease the magnitude of salt-induced H+ efflux from the roots of quinoa, providing solid evidence that H+-ATPase is rapidly and strongly stimulated by NaCl treatment. A study comparing the glycophytic species tobacco (Nicotiana tabacum) with the halophyte Atriplex nummularia revealed that accumulation of plasma membrane H+-ATPase mRNA in the roots of the halophyte was substantially greater than that in the roots of the glycophyte during NaCl stress (Niu et al., 1993). Thus, we envisaged that the relative expression of the ATPase isoforms (AHA1/2/3) may be higher in the roots of quinoa than in the roots of arabidopsis. Surprisingly, AHA transcript levels were much higher in arabidopsis compared with quinoa plants, and 100 mm NaCl treatment led to a further 3- to 5-fold increase in AHA1 and AHA2 transcripts in arabidopsis, but not in quinoa (Fig. 9). This observation can be explained by the fact that 100 mm NaCl treatment is not a stress for halophytes; it is considered an optimal concentration for growth in quinoa (Redondo-Gomez et al., 2007; Hariadi et al., 2011). Hence, halophytes are not in need of increased AHA transcripts during low to moderate levels of salinity stress and it may be predicted that the AHA transcript level may increase at higher salt concentrations (e.g. >300 mm NaCl). Consistent with this prediction, H+-ATPase activity in the halophyte Spartina patens was stimulated by salt stress up to 340 mm NaCl, with no change in the amount of H+-ATPase protein (Wu and Seliskar, 1998). In salt-tolerant glycophytes such as barley, a 2·5-fold difference in root plasma membrane H+-ATPase activity between cultivars with contrasting salt tolerance was not related to the difference in the amount of the actual protein (Chen et al., 2007a), implying post-translational regulation. It has been suggested that Na+ can directly stimulate H+-ATPase activity through the inactivation of the autoinhibitory domain of the C-terminal portion of plasma membrane H+-ATPase (Wu and Seliskar, 1998). Further examination of NaCl-induced H+-ATPase activity revealed that NaCl is able to stimulate H+-ATPase activity in vivo, but inhibit it in vitro in two different halophytes, Spartina patens and Salicornia bigelovii (Ayala et al., 1996; Wu and Seliskar, 1998). This strongly implies that NaCl requires some unidentified cytoplasmic factor ‘X’ to stimulate H+-ATPase during salt stress (Fig. 10). Factor ‘X’ may operate in a similar way to the PATROL1 protein identified in guard cells, which senses environmental changes and accordingly participates in the reversible translocation of AHA1 from endosomes to the plasma membrane (Hashimoto-Sugimoto et al., 2013). This possibility needs to be investigated in future studies.
Fig. 10.
Proposed model depicting the differences in regulation of the plasma membrane H+-ATPase activity between arabidopsis (A) and halophytes (B). In both species, Na+ transport across the plasma membrane results in significant membrane depolarization, activating outward-rectifying K+-permeable channels (KOR), leading to depletion of the cytosolic K+ pool. In halophyte species (B), this depolarization is prevented by the instantaneous and strong upregulation of plasma membrane H+-ATPase and H+-PPase, the result of a rapid (within seconds) signal from either the plasma membrane or cytosolic Na+ sensor to the plasma membrane H+-ATPase. This signalling is mediated by some unknown second messenger (labelled X). No transcriptional activation is required. In arabidopsis (A), such direct signalling from the Na+ sensor to H+-ATPase is either absent or is inefficient. Here, salt stress is signalled to the nucleus, where it triggers the expression of AHA genes and the formation of H+-ATPase. As the process takes many hours, arabidopsis plants are unable to prevent NaCl-induced membrane depolarization during the initial stages of salt stress. This results in a loss of substantial amounts of K+ and the cell’s viability (Figs 1 and 4).
Since the roots of halophytes activate H+-ATPases, one could argue that such H+-ATPase activity would jeopardize the energy status of the plant, resulting in poor performance in saline environments (e.g. Bose et al., 2014b). However, the roots of halophytes deal with this problem by increasing the affinity for the substrate (lower Km) to achieve maximum H+ extrusion (higher Vmax) upon sensing Na+ ions (Wu and Seliskar, 1998). Consequently, halophytes may be able to negate salt stress better than glycophytes. Another interesting aspect that may warrant further investigation is the fact that the vanadate-sensitive component of H+ efflux activated by salinity was only 75–80 % (Fig. 8). Could it be that the remaining 20–25 % may originate from an H+-pump driven by pyrophosphatase? Heterologous expression of H+-pyrophosphatase (H+-PPase) from the halophytic T. halophila in tobacco enhanced the salt tolerance of tobacco (Gao et al., 2006). Until now, energy-conserving H+-PPases have been suggested to be present only at the tonoplast. However, there is some evidence that suggests that, as in prokaryotes (Baykov et al., 2013), H+-PPase may also be present at the plasma membrane of plants (reviewed in Gaxiola et al., 2012). Importantly, type I H+-PPase is vanadate-insensitive (Rea and Poole, 1985) and its activity depends on the cytosolic K+ concentration (Gaxiola et al., 2012). Given that halophytes retain more K+ than glycophytes during salt stress and that 20–25 % of salt-induced H+ efflux is vanadate-insensitive, it is reasonable to speculate that H+-PPase activity localized at the plasma membrane is higher in halophytes during salt stress.
A model (Fig. 10) comparing the responses of arabidopsis (Fig. 10A) and halophytes (Fig. 10B) is proposed to summarize the results of the relative gene expression studies, viability staining, and ion flux, membrane potential and pharmacological experiments. In halophytes (Fig. 10B), the entry of Na+ is rapidly (within seconds) sensed by some unknown second messenger (labelled ‘X’ in the model), present either at the plasma membrane or in the cytosol. This rapidly stimulates both H+-ATPase and H+-PPase activity. No increase in the transcriptional abundance of AHA1/2/3 is required. Such enhanced H+ extrusion energizes Na+ efflux though the Na+/H+ exchanger and also decreases the magnitude of plasma membrane depolarization, so preventing K+ loss through KOR channels. Hence, the roots of halophytes are efficient in maintaining K+ homeostasis as well as their viability (Fig. 1) during salt stress. In arabidopsis (Fig. 10A), such instantaneous sensing of Na+ entry to signal H+-ATPase activation is either absent or inefficient. Here, salt stress is signalled to the nucleus, where it triggers expression of AHA genes and the formation of H+-ATPase. As the process takes many hours, arabidopsis plants are unable to prevent K+ loss through depolarization-activated KOR channels in the initial stages of salt stress. As a result, the roots of arabidopsis lose their viability within a few hours of salt stress (Fig. 1), with a long-term consequence for plant phenotype.
The data reported here included only two halophyte species. More species should be studied before the above conclusions can be extrapolated to all halophytes. Also, one could argue that H+-ATPase pumping may be an energy-demanding exercise that will come at a high carbon cost, so transgenic crops with higher rates of H+ pumping may incur substantial yield penalties, especially under control conditions. This problem could potentially be overcome by using stress-induced promoters that are activated only under saline conditions. Furthermore, our data suggest that it is not only the expression level but also the differences in pump regulation that cause the diversity in tolerance. Consequently, before any recommendations can be made to breeders, the mechanism of the post-translational regulation of plasma membrane H+-ATPase activation (i.e. the identity of the putative messenger X in Fig. 10) must be revealed in direct experiments using genetic manipulation of candidate genes in the signalling/regulatory pathway.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: visualization of Arabidopsis thaliana and Chenopodium quinoa root zones measured in the experiments. Figure S2: transient Na+ fluxes measured from epidermal root cells of the mature root zone at different locations (apical and basal) along the longitudinal root axis of 5-d-old C. quinoa seedlings in response to 100 mm NaCl. Figure S3: transient H+ fluxes in response to 100 mm NaCl measured from the elongation zone of 5-d-old C. quinoa seedlings grown using either the paper roll or the agar medium method. Table S1: sequences of primers for real-time RT-PCR.
ACKNOWLEDGEMENTS
This work was supported by an Australian Research Council Discovery grant to S.S., and by Fundamental Research Funds for the Central Universities (KYTZ201402) and a National Natural Science Foundation of China (J1210056 and J1310015) grant to W.S. Dr Tracey Cuin (University of Wurzburg) is greatly acknowledged for her assistance in editing this article.
LITERATURE CITED
- Anschütz U, Becker D, Shabala S. 2014. Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. Journal of Plant Physiology 171: 670–687. [DOI] [PubMed] [Google Scholar]
- Apse MP, Blumwald E. 2007. Na+ transport in plants. FEBS Letters 581: 2247–2254. [DOI] [PubMed] [Google Scholar]
- Ayala F, O’Leary JW, Schumaker KS. 1996. Increased vacuolar and plasma membrane H+-ATPase activities in Salicornia bigelovii Torr. in response to NaCl. Journal of Experimental Botany 47: 25–32. [DOI] [PubMed] [Google Scholar]
- Bartels D, Dinakar C. 2013. Balancing salinity stress responses in halophytes and non-halophytes: a comparison between Thellungiella and Arabidopsis thaliana. Functional Plant Biology 40: 819–831. [DOI] [PubMed] [Google Scholar]
- Baykov AA, Malinen AM, Luoto HH, Lahti R. 2013. Pyrophosphate-fueled Na+ and H+ transport in prokaryotes. Microbiology and Molecular Biology Reviews 77: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E, Aharon GS, Apse MP. 2000. Sodium transport in plant cells. Biochimica et Biophysica Acta 1465: 140–151. [DOI] [PubMed] [Google Scholar]
- Bose J, Pottosin I, Shabala SS, Palmgren MG, Shabala S. 2011. Calcium efflux systems in stress signalling and adaptation in plants. Frontiers in Plant Science 2: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Xie Y, Shen W, Shabala S. 2013. Haem oxygenase modifies salinity tolerance in arabidopsis by controlling K+ retention via regulation of the plasma membrane H+-ATPase and by altering SOS1 transcript levels in roots. Journal of Experimental Botany 64: 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Rodrigo-Moreno A, Shabala S. 2014a. ROS homeostasis in halophytes in the context of salinity stress tolerance. Journal of Experimental Botany 65: 1241–1257. [DOI] [PubMed] [Google Scholar]
- Bose J, Shabala L, Pottosin I, et al. 2014b. Kinetics of xylem loading, membrane potential maintenance, and sensitivity of K+-permeable channels to reactive oxygen species: physiological traits that differentiate salinity tolerance between pea and barley. Plant, Cell and Environment 37: 589–600. [DOI] [PubMed] [Google Scholar]
- Bruggemann W, Janiesch P. 1989. Comparison of plasma-membrane ATPase from salt-treated and salt-free grown Plantago maritima L. Journal of Plant Physiology 134: 20–25. [Google Scholar]
- Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S. 2005. Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant, Cell and Environment 28: 1230–1246. [Google Scholar]
- Chen ZH, Pottosin II, Cuin TA, et al. 2007a. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiology 145: 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Zhou MX, Newman IA, Mendham NJ, Zhang GP, Shabala S. 2007b. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Functional Plant Biology 34: 150–162. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Munns R, Flowers TJ. 2005. Improving salt tolerance of wheat and barley: future prospects. Australian Journal of Experimental Agriculture 45: 1425–1443. [Google Scholar]
- Cooper B. 2001. Collateral gene expression changes induced by distinct plant viruses during the hypersensitive resistance reaction in Chenopodium amaranticolor. Plant Journal 26: 339–349. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Tian Y, Betts SA, Chalmandrier R, Shabala S. 2009. Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Functional Plant Biology 36: 1110–1119. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Bose J, Stefano G, et al. 2011. Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: in planta quantification methods. Plant, Cell and Environment 34: 947–961. [DOI] [PubMed] [Google Scholar]
- Davenport R, James RA, Zakrisson-Plogander A, Tester M, Munns R. 2005. Control of sodium transport in durum wheat. Plant Physiology 137: 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport RJ, Reid RJ, Smith FA. 1997. Sodium-calcium interactions in two wheat species differing in salinity tolerance. Physiologia Plantarum 99: 323–327. [Google Scholar]
- Edelist C, Raffoux X, Falque M, et al. 2009. Differential expression of candidate salt-tolerance genes in the halophyte Helianthus paradoxus and its glycophyte progenitors H. annuus and H. petiolaris (Asteraceae). American Journal of Botany 96: 1830–1838. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Colmer TD. 2008. Salinity tolerance in halophytes. New Phytologist 179: 945–963. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Galal HK, Bromham L. 2010. Evolution of halophytes: multiple origins of salt tolerance in land plants. Functional Plant Biology 37: 604–612. [Google Scholar]
- Gao F, Gao Q, Duan X, Yue G, Yang A, Zhang J. 2006. Cloning of an H+-PPase gene from Thellungiella halophila and its heterologous expression to improve tobacco salt tolerance. Journal of Experimental Botany 57: 3259–3270. [DOI] [PubMed] [Google Scholar]
- Gaxiola RA, Sanchez CA, Paez-Valencia J, Ayre BG, Elser JJ. 2012. Genetic manipulation of a “vacuolar” H+-PPase: from salt tolerance to yield enhancement under phosphorus-deficient soils. Plant Physiology 159: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway H, Munns R. 1980. Mechanisms of salt tolerance in nonhalophytes. Annual Review of Plant Physiology 31: 149–190. [Google Scholar]
- Hanjra MA, Qureshi ME. 2010. Global water crisis and future food security in an era of climate change. Food Policy 35: 365–377. [Google Scholar]
- Hariadi Y, Marandon K, Tian Y, Jacobsen SE, Shabala S. 2011. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. Journal of Experimental Botany 62: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Sugimoto M, Higaki T, Yaeno T, et al. 2013. A Munc13-like protein in arabidopsis mediates H+-ATPase translocation that is essential for stomatal responses. Nature Communications 4: doi:10.1038/ncomms3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassidim M, Braun Y, Lerner HR, Reinhold L. 1990. Na+/H+ and K+/H+ antiport in root membrane vesicles isolated from the halophyte atriplex and the glycophyte cotton. Plant Physiology 94: 1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakannan M, Babourina O, Rengel Z. 2011. Improved measurements of Na+ fluxes in plants using calixarene-based microelectrodes. Journal of Plant Physiology 168: 1045–1051. [DOI] [PubMed] [Google Scholar]
- Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S. 2013. Salicylic acid improves salinity tolerance in arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. Journal of Experimental Botany 64: 2255–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S, Kant P, Raveh E, Barak S. 2006. Evidence that differential gene expression between the halophyte, Thellungiella halophila, and Arabidopsis thaliana is responsible for higher levels of the compatible osmolyte proline and tight control of Na+ uptake in T. halophila. Plant, Cell and Environment 29: 1220–1234. [DOI] [PubMed] [Google Scholar]
- Kosova K, Prasil IT, Vitamvas P. 2013. Protein contribution to plant salinity response and tolerance acquisition. International Journal of Molecular Sciences 14: 6757–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM. 2014. Sodium in plants: perception, signalling, and regulation of sodium fluxes. Journal of Experimental Botany 65: 849–858. [DOI] [PubMed] [Google Scholar]
- Maughan PJ, Turner TB, Coleman CE, et al. 2009. Characterization of Salt Overly Sensitive 1 (SOS1) gene homoeologs in quinoa (Chenopodium quinoa Willd.). Genome 52: 647–657. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59: 651–681. [DOI] [PubMed] [Google Scholar]
- Newman IA. 2001. Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant, Cell and Environment 24: 1–14. [DOI] [PubMed] [Google Scholar]
- Niu XM, Narasimhan ML, Salzman RA, Bressan RA, Hasegawa PM. 1993. NaCl regulation of plasma-membrane H+-ATPase gene expression in a glycophyte and a halophyte. Plant Physiology 103: 713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DH, Dassanayake M, Haas JS, et al. 2010. Genome structures and halophyte-specific gene expression of the extremophile Thellungiella parvula in comparison with Thellungiella salsuginea (Thellungiella halophila) and Arabidopsis. Plant Physiology 154: 1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C, Lüttge U, West K, Pallaghy C, Shacher-Hill B. 1969. Ion absorption in Atriplex leaf tissue II. Secretion of ions to epidermal bladders. Australian Journal of Biological Sciences 22: 797–814. [Google Scholar]
- Ozgur R, Uzilday B, Sekmen AH, Turkan I. 2013. Reactive oxygen species regulation and antioxidant defence in halophytes. Functional Plant Biology 40: 832–847. [DOI] [PubMed] [Google Scholar]
- Palmgren MG. 2001. Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annual Review of Plant Biology 52: 817–845. [DOI] [PubMed] [Google Scholar]
- Ramani B, Reeck T, Debez A, et al. 2006. Aster tripolium L. and Sesuvium portulacastrum L.: two halophytes, two strategies to survive in saline habitats. Plant Physiology and Biochemistry 44: 395–408. [DOI] [PubMed] [Google Scholar]
- Rea PA, Poole RJ. 1985. Proton-translocating inorganic pyrophosphatase in red beet (Beta vulgaris L.) tonoplast vesicles. Plant Physiology 77: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo-Gomez S, Mateos-Naranjo E, Davy AJ, et al. 2007. Growth and photosynthetic responses to salinity of the salt-marsh shrub Atriplex portulacoides. Annals of Botany 100: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan C-J, da Silva JAT, Mopper S, Qin P, Lutts S. 2010. Halophyte improvement for a salinized world. Critical Reviews in Plant Sciences 29: 329–359. [Google Scholar]
- Sadder MT, Al-Doss AA. 2014. Characterization of dehydrin AhDHN from Mediterranean saltbush (Atriplex halimus). Turkish Journal of Biology 38: 469–477. [Google Scholar]
- Schmidhuber J, Tubiello FN. 2007. Global food security under climate change. Proceedings of the National Academy of Sciences of the USA 104: 19703–19708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Majumder AL. 2010. Porteresia coarctata (Roxb.) Tateoka, a wild rice: a potential model for studying salt-stress biology in rice. Plant, Cell and Environment 33: 526–542. [DOI] [PubMed] [Google Scholar]
- Shabala L, McMeekin TA, Ross T, Shabala S. 2006. Non-invasive microelectrode ion flux measurements to study adaptive responses of microorganisms to the environment. FEMS Microbiology Reviews 30: 472–486. [DOI] [PubMed] [Google Scholar]
- Shabala S. 2013. Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany 112: 1209–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Cuin TA. 2008. Potassium transport and plant salt tolerance. Physiologia Plantarum 133: 651–669. [DOI] [PubMed] [Google Scholar]
- Shabala S, Mackay A. 2011. Ion transport in halophytes. Advances in Botanical Research 57: 151–199. [Google Scholar]
- Shabala S, Pottosin I. 2014. Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiologia Plantarum. doi: 10.1111/ppl.12165. [DOI] [PubMed] [Google Scholar]
- Shi HZ, Ishitani M, Kim CS, Zhu JK. 2000. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proceedings of the National Academy of Sciences of the USA 97: 6896–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J, Clifton-Brown J, Hastings A, Robson P, Allison G, Smith P. 2012. Food vs. fuel: the use of land for lignocellulosic ‘next generation’energy crops that minimize competition with primary food production. Global Change Biology Bioenergy 4: 1–19. [Google Scholar]
- Vera-Estrella R, Barkla BJ, Garcia-Ramirez L, Pantoja O. 2005. Salt stress in Thellungiella halophila activates Na+ transport mechanisms required for salinity tolerance. Plant Physiology 139: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov V, Amtmann A. 2006. Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, has specific root ion-channel features supporting K+/Na+ homeostasis under salinity stress. Plant Journal 48: 342–353. [DOI] [PubMed] [Google Scholar]
- Volkov V, Wang B, Dominy PJ, Fricke W, Amtmann A. 2004. Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, possesses effective mechanisms to discriminate between potassium and sodium. Plant, Cell and Environment 27: 1–14. [Google Scholar]
- Wang B, Davenport RJ, Volkov V, Amtmann A. 2006. Low unidirectional sodium influx into root cells restricts net sodium accumulation in Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana. Journal of Experimental Botany 57: 1161–1170. [DOI] [PubMed] [Google Scholar]
- Wu J, Seliskar DM. 1998. Salinity adaptation of plasma membrane H+-ATPase in the salt marsh plant Spartina patens: ATP hydrolysis and enzyme kinetics. Journal of Experimental Botany 49: 1005–1013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.