Abstract
For more than 50 years the Free Radical Theory served as the paradigm guiding most investigations of ageing. However, recent studies in a variety of organisms have identified conceptual and practical limitations to this theory. Some of these limitations are related to the recent discovery that caloric restriction and other experimental manipulations promote longevity by inducing hormesis effects in association with increased reactive oxygen species (ROS). The beneficial role of ROS in lifespan extension is consistent with the essential role of these molecules in cell signalling. However, the identity of specific forms of ROS that promote longevity remains unclear. In this article, we argue that in several model systems, hydrogen peroxide plays a crucial role in the induction of hormesis.
Keywords: hormesis, ROS signalling, oxidative damage, caloric restriction, longevity, ageing
Introduction
Hormesis is an adaptive response to a variety of oxidative and other stresses that renders cells/organisms resistant to higher (and normally harmful) doses of the same stressing agent (Martins, et al., 2011). The hormesis effects of reactive oxygen species (ROS) and their impact on ageing have emerged as one of the most important topics for understanding ageing as reflected in a number of recently published review articles (Hekimi, et al., 2011, Ristow & Schmeisser, 2011, Calabrese, 2012, Labunskyy & Gladyshev, 2012, Maryanovich & Gross, 2012). This review will address the question of the specific form of ROS that trigger hormesis. Recently, we established hydrogen peroxide as a hormesis trigger and extends chronological lifespan (CLS) in budding yeast (Mesquita, et al., 2010). Nevertheless, more recent work on hormesis claims that superoxide anions are the triggers of hormesis effects. In this review, we decided to “bring the case to the court” and to present the evidence supporting superoxide anions or hydrogen peroxide as triggers of hormetic effects. Arguments will be presented showing that hydrogen peroxide may be a general inducer of hormesis effects downstream of the superoxide anions through their conversion to hydrogen peroxide by superoxide dismutases. The evidence that hydrogen peroxide may induce quiescence, which is emerging as an important determinant of longevity will be also discussed.
ROS as “bad guys” – guilty as charged?
In the never-ending battle between processes that maintain cellular homeostasis and factors that disrupt this equilibrium, ROS usually have been viewed as “bad guys”. The deleterious effects of ROS are particularly relevant to the longstanding “free radical theory of ageing”, which posits that oxidative stress and oxidative damage to macromolecules are primary determinants of ageing. Despite the prominent position this theory has attained in more than 50 years of efforts to understand ageing, as has also been noted by others recently (Lapointe & Hekimi, 2010, Hekimi, et al., 2011), mounting evidence suggests that this theory is at best incomplete.
It has long been recognized that ROS cause complex and irreversible damage to cellular constituents that impair cellular homeostasis (reviewed in (de Grey, 2006). Oxidative damage is related to the high reactivity of molecular oxygen and its intermediates, which can lead to oxidative modifications of proteins, lipids and DNA. One of the primary sources of ROS is mitochondria, and ROS damage to mtDNA has been implicated in a number of pathological conditions, including ageing. mtDNA damage can lead to aberrant mitochondrial function that can cause “vicious cycles” of ROS production, which amplify damage. ROS-induced damage to mitochondria eventually became the basis for reformulation of this theory as the “mitochondrial free radical theory”.
The results of numerous studies performed over a period of decades provide support for the free radical theory. For example, oxidatively damaged molecules accumulate in aged post-mitotic cells in species as diverse as yeast and humans. These molecules include misfolded proteins, protein carbonyls and lipofuscin all of which slowly accumulate with age. Lipid peroxidation also increases with age and disrupts cellular functions occurring in biomembranes. A role for oxidative damage to DNA in ageing is consistent with accumulating evidence that genome instability and the rate at which this instability occurs increase with age. The free radical theory of ageing is also consistent with numerous studies in a variety of organisms showing that inhibition of antioxidant defences shortens lifespan. Additional support for the theory is provided by the observation that ROS inhibit telomerase and promote telomere erosion, a well-recognized component of ageing in eukaryotes (Passos, et al., 2007). In the aggregate, the evidence that ROS and oxidative damage are important factors in ageing is compelling in many respects.
The case against the “free radical theory of ageing”
In spite of the compelling evidence that ROS are guilty of disrupting cellular homeostasis, in recent years major cracks have appeared in the defence of the free radical theory. Evidence against the free radical theory has surfaced in studies of a variety of model organisms employed to investigate ageing. For example, we have reported, the surprising observation that in the model organism Saccharomyces cerevisiae (budding yeast), hydrogen peroxide promotes chronological longevity, which is defined as the length of time that yeast cells survive in stationary phase cultures. The longevity effects of hydrogen peroxide occur in response to caloric restriction or inactivation of catalases and in cells ectopically exposed to low levels of hydrogen peroxide (Mesquita, et al., 2010). Our experiments provided additional evidence against the free radical theory as well - the longevity phenotype of catalase mutants was accompanied by elevated damage in the form of protein carbonyls and lipofuscin (Mesquita, et al., 2010). Similarly, elevated intracellular levels of ROS induced by 2-deoxy-D-glucose, which mimics caloric restriction by inhibiting glucose metabolism, were reported to extend the lifespan of worms (Caenorhabditis elegans) (Schulz, et al., 2007, Hekimi, et al., 2011). Impaired insulin/IGF-1 signalling was also recently reported to induce a transient increase in ROS that extends worm lifespan (Zarse, et al., 2012). These reports are relevant to the well-established role that growth signalling by glucose and other factors plays in ageing in all organisms.
Additional evidence against the free radical theory was provided by the observation that exposure of long-lived age-1 worms, but not wild type worms, to high oxygen tension extends lifespan in parallel with decreased mitochondrial ROS and fewer protein carbonyls (Yanase, et al., 2002). These findings imply that the longevity effects of high oxygen in age-1 worms are related to an initial increase in superoxide levels. Increased levels of superoxide anions have also been implicated in longevity effects in wild type worms. For example, the lifespan of wild type worms is extended by exposure to low concentrations of the superoxide-generating compound juglone (Cypser & Johnson, 2002). Elevated superoxide anion levels induced by mutations in the nuo-6 and isp-1 genes encoding subunits of complex I and III of the respiratory chain or by exposing worms to the superoxide-generating compound paraquat also extend the lifespan of wild type worms (Yang & Hekimi, 2010). The absence of an effect in wild type worms exposed to hyperoxic conditions may reflect an upper limit of superoxide anions that promote longevity, which may have been exceeded in these conditions. These and other findings recently reported in studies of more complex organisms (Perez, et al., 2009) argue for acquittal of the charges against ROS brought by the free radical theory as “bud guys” that promote ageing.
The verdict: guilty of some, but not all charges
Although hydrogen peroxide enhances chronological lifespan (CLS) in caloric restricted budding yeast cells, this occurs in parallel with a decrease in superoxide anions. This decrease is caused by induction by hydrogen peroxide of the activity of the cytosolic Cu/Zn-dependent and the mitochondrial Mn-dependent superoxide dismutases, Sod1p and Sod2p respectively (Mesquita, et al., 2010). The induction of SOD activity by hydrogen peroxide is consistent with the earlier demonstration that budding yeast CLS is extended by SOD overexpression (Fabrizio, et al., 2004, Harris, et al., 2005). These findings are also consistent with earlier reports that sub-lethal concentrations of hydrogen peroxide induce transcription of both the SOD1 and SOD2 genes (Godon, et al., 1998, Gasch, et al., 2000) as well as an increase in levels of the corresponding proteins (Godon, et al., 1998). Transactivation of SOD1 and SOD2 is mediated in part by the transcriptional regulator Yap1p (Yahara, 1996). It is well documented that hydrogen peroxide modifies Yap1p as part of a regulatory mechanism associated with Yap1p induction of a large number of genes (Delaunay, et al., 2000, Delaunay, et al., 2002).
The induction of superoxide dismutase transcription and/or activity by hydrogen peroxide is a highly conserved regulatory mechanism. For example, hydrogen peroxide induces sodA transcription in Escherichia coli (Semchyshyn, 2009), as well as the transcription and activity of MnSOD in rat cells (Yoshioka, et al., 1994). Recent data, obtained in S. cerevisiae, also suggests that not only the interaction between hydrogen peroxide and cellular proteins plays a role in signalling transduction but that also thiol peroxidases could sense and transfer oxidative signals to signalling proteins regulating transcription (Fomenko, et al., 2011). Thus, not only the regulatory mechanism of hydrogen peroxide seems to be conserved, but also its role in promoting longevity may be conserved as well. Consistent with this possibility, the replicative lifespan of human skin keratinocytes is extended by ectopic exposure to low concentrations of hydrogen peroxide (Yokoo, et al., 2004). This effect is accompanied by an increase in telomere length (Yokoo, et al., 2004). In mammalian cells, telomere elongation is inhibited by superoxide anions (Passos, et al., 2007) and telomere maintenance is enhanced by SOD activity (Serra, et al., 2003). It is possible, therefore, that the replicative lifespan-extending effect of hydrogen peroxide in human keratinocytes is partially related to hydrogen peroxide-induced SOD activity that reduces superoxide anions.
A transient increase in intracellular hydrogen peroxide has also been implicated in lifespan extension associated with impaired insulin/IGF-1 signalling in C. elegans, which disrupts glucose uptake and up regulates superoxide dismutases and other oxidative stress defences, leading to a subsequent decline in overall levels of ROS (Zarse, et al., 2012). Increased hydrogen peroxide also underlies the pro-survival effects of inactivating catalases or ectopic exposure to hydrogen peroxide in mutant C. elegans dauer larvae (Xie & Roy, 2012). The beneficial effects of hydrogen peroxide reported in this latter study were attributed to the induction of HIF-1-dependent alterations in lipid metabolism. Elevated intracellular levels of hydrogen peroxide may also underlie the switch from short-lived “worker” ants to longer-lived reproductive gamergates in the eusocial insect Harpegnathos saltator. This switch is accompanied by a substantial decrease in catalase activity, which is expected to elevate intracellular levels of hydrogen peroxide (Schneider et al., 2011).
The longevity-promoting effects of caloric restriction induced by hydrogen peroxide in stationary phase budding yeast cells are accompanied by an enhanced quiescence state indicated by the reduced frequency of budded cells (Weinberger, et al., 2007, Weinberger, et al., 2010). This reflects the less frequent entry of calorie-restricted stationary phase cells into S phase (Weinberger, et al., 2013), where they suffer replication stress (Murakami, et al., 2012, Weinberger, et al., 2013). Even in the absence of caloric restriction, hydrogen peroxide levels increase in parallel with the reduction in superoxide levels as budding yeast cells approach stationary phase (Mesquita, et al., 2010, Pan, et al., 2011). This suggests that the regulation of SODs by hydrogen peroxide is a general feature of quiescence in budding yeast that is enhanced by caloric restriction. A similar reciprocal relationship between increased levels of hydrogen peroxide and decreased levels of superoxide anions have been reported in cultured mouse cells as they approach quiescence due to contact inhibition (Sarsour, et al., 2008). Furthermore, disrupting this relationship by inactivating MnSOD, which elevates levels of superoxide and decreases levels of hydrogen peroxide, inhibits quiescence as indicated by a higher fraction of cells that remained in S phase followed by cell death (Sarsour, et al., 2008). Quiescence is also emerging as an important component of longevity in mammalian cells (Chakkalakal, et al., 2012). Although a causal role for hydrogen peroxide in maintaining quiescence in mammalian cells has not been addressed, the studies described above clearly suggest this possibility.
Hydrogen peroxide and/or superoxide anions - the hormesis police?
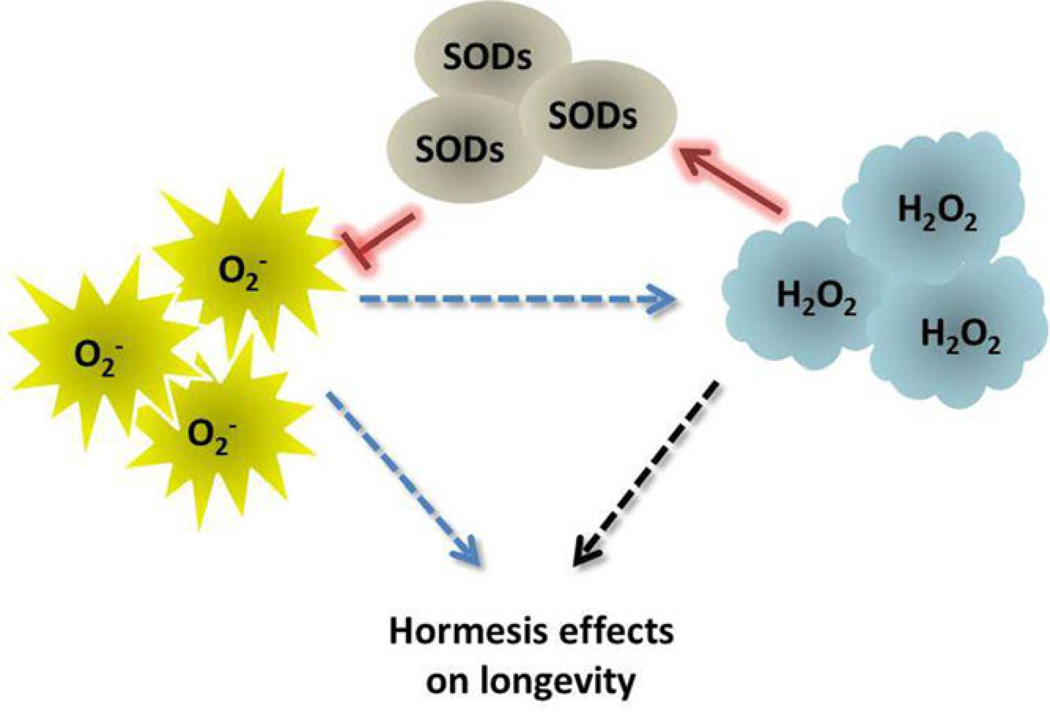
All of these findings point to superoxide anions as a guilty party in the loss of cellular homeostasis that underlies ageing, and to low levels of hydrogen peroxide as the “sheriff” that keeps superoxide anions in check. The critical role of hydrogen peroxide in policing levels of superoxide anions conforms to current concepts of a phenomenon called hormesis, which is defined as the induction of adaptive responses to mild stresses that protect against higher levels of stress (Calabrese, 2012). However, the results of some studies suggest that not all superoxide anions are bad guys that need to be restrained by oxidative stress defences - similar to hydrogen peroxide, superoxide anions are also capable of enhancing lifespan (Figure 1). This includes, for example, the longevity-promoting effect of hyperoxia in age-1 worms, as well as the lifespan-extending effects in wild type worms of the compounds juglone and paraquat and of mutations in the nuo-6 and isp-1 genes described above (Yang & Hekimi, 2010). In budding yeast, inactivation of TOR signalling by deletion of the TOR1 gene extends CLS by increasing superoxide anions early in CLS experiments (Pan, et al., 2011). Consistent with a role for superoxide anions in lifespan extension of tor1Δ cells, the superoxide-generating compound menadione mimics the longevity effects of TOR1 inactivation. Also consistent with this model, uncoupling of mitochondrial respiration from oxidative phosphorylation with dinitrophenol, which inhibits the production of superoxide anions, also attenuates the lifespan-extending effect of TOR1 deletion (Pan, et al., 2011).
Figure 1. Both hydrogen peroxide and superoxide anions have been implicated in hormesis effects that promote longevity.
Whether superoxide anions can induce hormesis effects directly or indirectly (blue dashed arrows) via their conversion into hydrogen peroxide by SODs remains unclear. Importantly, the levels of superoxide anions and hydrogen peroxide are regulated by SOD activity. Hydrogen peroxide increases and promotes SOD activity that decreases superoxide levels (red arrows), thus creating a feedback regulatory mechanism that promotes hormesis effects by maintaining hydrogen peroxide at homeostatic levels.
The lifespan-extending effects of ectopic exposure to hydrogen peroxide detected in budding yeast (Mesquita, et al., 2010) and worms (Xie & Roy, 2012) definitively establishes this form of ROS as a bona fide inducer of hormesis. Although it has been suggested that the effects of ROS on lifespan are conceptually quite distinct from hormesis effects (Hekimi, et al., 2011), the effects of hydrogen peroxide on lifespan were detected in parallel with adaptive responses that elevated SOD activity (Mesquita, et al., 2010) or altered lipid metabolism (Xie & Roy, 2012), which clearly meets the definition of hormesis. It is important to keep in mind that one source of intracellular hydrogen peroxide is the dismutation of superoxide anions by SODs. Therefore, the hormesis effects of superoxide anions reported in some of the studies cited above may in fact be triggered by hydrogen peroxide produced from superoxide anions by SOD activity (Figure 1). Consistent with this possibility, caloric restriction induced by switching exponentially proliferating cultures to a lower concentration of glucose at the beginning of yeast chronological ageing experiments induces an initial increase in superoxide anions (Weinberger, et al., 2010), which is followed by the sustained increase in hydrogen peroxide that triggers SOD activity and reduced levels of superoxide anions (Mesquita, et al., 2010). The early burst of superoxide anions induced by caloric restriction in these experiments likely reflects an increase in respiration rate first reported in calorie-restricted cells several years ago (Lin, et al., 2002).
However, the increased CLS triggered by inactivating TOR1 is accompanied by a decrease, rather than increase, in hydrogen peroxide levels (Pan, et al., 2011). A decrease in intracellular hydrogen peroxide also occurs in concert with chronological lifespan extension in stationary phase cells when SCH9, a downstream effector of TOR1 signalling, is inactivated (Weinberger, et al., 2010). Similarly, the increase in superoxide anions in long-lived nuo-6 and isp-1 mutant worms described above was detected in the absence of an increase in hydrogen peroxide or other forms of ROS (Yang & Hekimi, 2010). These results point to superoxide anions as a different branch of the hormesis police that extend lifespan in the absence of hydrogen peroxide effects.
The specific forms of ROS that trigger hormesis effects in laboratory experiments may be related to how specific experimental manipulations impact a myriad of signalling pathways that interact with one another in complex ways. In fact, although a number of antioxidant and repair processes have been characterized, the mechanisms whereby cells are damaged by particular oxidants or protect themselves from damage remain to be identified (Thorpe, et al., 2004).
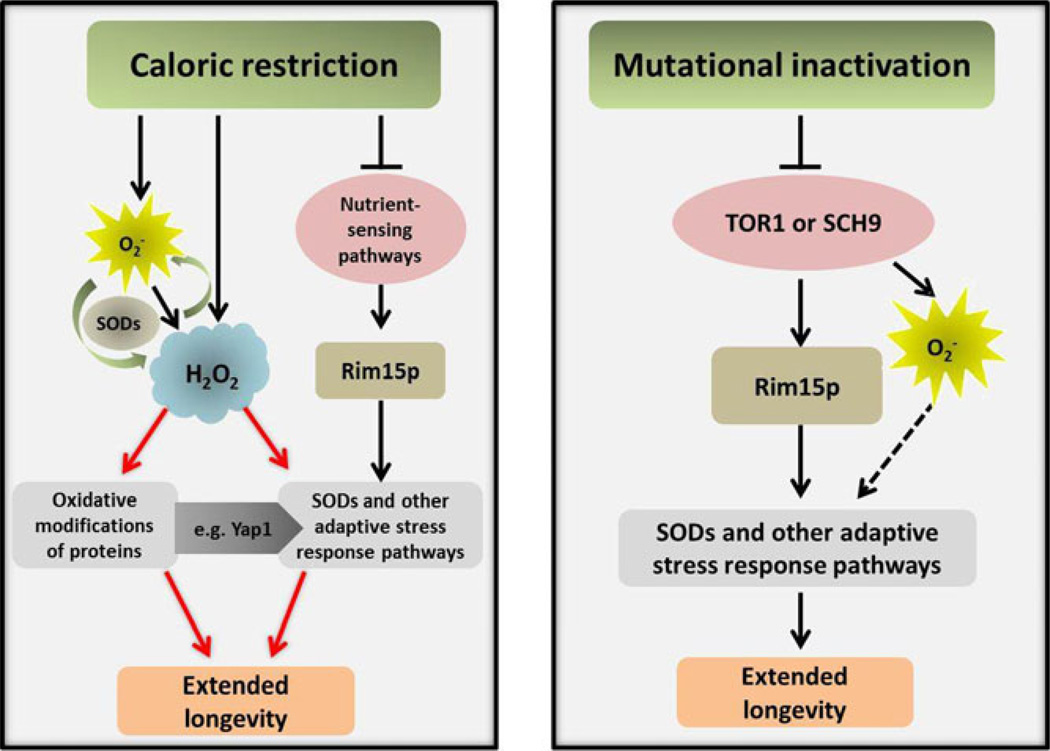
The parallels between hormetic effects of caloric restriction or mutational inactivation of growth signalling pathways in budding yeast chronological ageing experiments may be informative in this regard (Figure 2). Caloric restriction extends CLS in part by attenuating Tor1p-Sch9p -dependent glucose signalling, which leads to the activation of Rim15p and oxidative stress defences regulated by this transcriptional activator (Wei, et al., 2008). As noted above, caloric restriction also increases intracellular hydrogen peroxide, which also induces oxidative stress defences (Mesquita, et al., 2010), but independently of Rim15p (Weinberger, et al., 2010). The increase in hydrogen peroxide in calorie-restricted cells may be triggered by the increase in superoxide anions that occurs before cells transition into stationary phase (Weinberger, et al., 2010), although other possible sources of hydrogen peroxide exist. Mutational inactivation of Tor1p-Sch9p (Figure 2) also induces Rim15p-dependent oxidative stress defences, as well as an increase in intracellular superoxide anions, which may occur independently of Rim15p. This increase may induce SODs and other oxidative stress defences and/or altered mitochondrial function (Pan, et al., 2011). Ultimately, the effects of both types of experimental manipulations converge on a reduction in superoxide anions in cells in stationary phase that promotes longevity (Figure 2).
Figure 2. Pathways mediating hormesis extension of CLS in budding yeast triggered by caloric restriction or mutational inactivation of growth signalling pathways.
Dotted lines represent hypothetical pathways. Although O2− is dismutated to H2O2, whether this is the source of H2O2 in calorie-restricted cells has not yet been tested and other potential sources exist, such as peroxisomes. Hydrogen peroxide modification of the transcriptional activator Yap1p (Georgiou, 2002) likely contributes to the induction of SODs (Mesquita, et al., 2010) and other adaptive stress response pathways. It is likely that H2O2 also induces oxidative stress responses by modifying other proteins, including those regulating epigenetic effects that also contribute to longevity, as described in mammalian cells (reviewed in (Maryanovich & Gross, 2012). Although indirect evidence suggests that O2− triggers oxidative stress defences when Tor1p-Sch9p signalling is mutational inactivated, this possibility also has not been tested directly.
In the context of these parallels and the fact that both types of experimental manipulations result in reduced Tor1p-Sch9p signalling, it’s puzzling that mutational activation of Sch9p or Tor1p does not lead to an increase in hydrogen peroxide, similar to caloric restriction. Apparently, in addition to down regulating Tor1p and Sch9p signalling, caloric restriction triggers additional events that increase intracellular levels of hydrogen peroxide. We propose that this occurs as part of a mechanism that amplifies the hormetic effects of mutationally inactivating a subset of signalling pathways in laboratory experiments. Outside the laboratory, the depletion of nutrients likely triggers in yeast and other organisms a deeper and more sustained response that requires hydrogen peroxide to efficiently drive cells into and maintain a quiescent state, where cells are protected from deleterious effects of superoxide anions and a paucity of nutrients on DNA replication (Weinberger, et al., 2010). According to this model, superoxide anions have been justly convicted as the bad guys, although the initial increase in their numbers triggered by caloric restriction may alert cells to the need to round them up. This becomes the job of hydrogen peroxide, which may serve as the true hormesis police that promote longevity in organisms faced with stresses that more closely resemble those they encounter in their natural environment. Future studies will determine whether this verdict is corrected.
Acknowledgments
The authors wish to thank Molly Burhans for preparing the figures. This work was supported by Fundação para a Ciência e Tecnologia (FCT) and COMPETE/QREN/EU (PTDC/BIA-MIC/114116/2009), a grant from the Roswell Park Alliance Foundation and by a National Cancer Institute Support Grant (P30CA016056) to Roswell Park Cancer Institute.
Footnotes
Authors have no conflict of interest to declare.
References
- Calabrese EJ. Hormesis: improving predictions in the low-dose zone. EXS. 2012;101:551–564. doi: 10.1007/978-3-7643-8340-4_19. [DOI] [PubMed] [Google Scholar]
- Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol A Biol Sci Med Sci. 2002;57:B109–114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- de Grey AD. Free radicals in aging: causal complexity and its biomedical implications. Free Radic Res. 2006;40:1244–1249. doi: 10.1080/10715760600913176. [DOI] [PubMed] [Google Scholar]
- Delaunay A, Isnard AD, Toledano MB. H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 2000;19:5157–5166. doi: 10.1093/emboj/19.19.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–481. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Battistella L, Vardavas R, et al. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J Cell Biol. 2004;166:1055–1067. doi: 10.1083/jcb.200404002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomenko DE, Koc A, Agisheva N, et al. Thiol peroxidases mediate specific genome-wide regulation of gene expression in response to hydrogen peroxide. Proc Natl Acad Sci U S A. 2011;108:2729–2734. doi: 10.1073/pnas.1010721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou G. How to flip the (redox) switch. Cell. 2002;111:607–610. doi: 10.1016/s0092-8674(02)01165-0. [DOI] [PubMed] [Google Scholar]
- Godon C, Lagniel G, Lee J, et al. The H2O2 stimulon in Saccharomyces cerevisiae. J Biol Chem. 1998;273:22480–22489. doi: 10.1074/jbc.273.35.22480. [DOI] [PubMed] [Google Scholar]
- Harris N, Bachler M, Costa V, Mollapour M, Moradas-Ferreira P, Piper PW. Overexpressed Sod1p acts either to reduce or to increase the lifespans and stress resistance of yeast, depending on whether it is Cu(2+)-deficient or an active Cu,Zn- superoxide dismutase. Aging Cell. 2005;4:41–52. doi: 10.1111/j.1474-9726.2005.00142.x. [DOI] [PubMed] [Google Scholar]
- Hekimi S, Lapointe J, Wen Y. Taking a "good" look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labunskyy VM, Gladyshev VN. Role of Reactive Oxygen Species-Mediated Signaling in Aging. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J, Hekimi S. When a theory of aging ages badly. Cell Mol Life Sci. 2010;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Martins I, Galluzzi L, Kroemer G. Hormesis, cell death and aging. Aging (Albany NY) 2011;3:821–828. doi: 10.18632/aging.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanovich M, Gross A. A ROS rheostat for cell fate regulation. Trends Cell Biol. 2012 doi: 10.1016/j.tcb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Mesquita A, Weinberger M, Silva A, et al. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc Natl Acad Sci U S A. 2010;107:15123–15128. doi: 10.1073/pnas.1004432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami C, Delaney JR, Chou A, et al. pH neutralization protects against reduction in replicative lifespan following chronological aging in yeast. Cell Cycle. 2012;11:3087–3096. doi: 10.4161/cc.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011;13:668–678. doi: 10.1016/j.cmet.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Saretzki G, von Zglinicki T. DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. 2007;35:7505–7513. doi: 10.1093/nar/gkm893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Saretzki G, Ahmed S, et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5:e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Sarsour EH, Venkataraman S, Kalen AL, Oberley LW, Goswami PC. Manganese superoxide dismutase activity regulates transitions between quiescent and proliferative growth. Aging Cell. 2008;7:405–417. doi: 10.1111/j.1474-9726.2008.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Semchyshyn H. Hydrogen peroxide-induced response in E. coli and S. cerevisiae: Different stages of the flow of the genetic information. Cent Eur J Biol. 2009;4:142–153. [Google Scholar]
- Serra V, von Zglinicki T, Lorenz M, Saretzki G. Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. J Biol Chem. 2003;278:6824–6830. doi: 10.1074/jbc.M207939200. [DOI] [PubMed] [Google Scholar]
- Thorpe GW, Fong CS, Alic N, Higgins VJ Dawes IW. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc Natl Acad Sci U S A. 2004;101:6564–6569. doi: 10.1073/pnas.0305888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L Longo VD. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4: e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Sampaio-Marques B, Ludovico P Burhans WC. DNA replication stress-induced loss of reproductive capacity in S. cerevisiae and its inhibition by caloric restriction. Cell Cycle. 2013;12:1189–1200. doi: 10.4161/cc.24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Mesquita A, Caroll T, et al. Growth signaling promotes chronological aging in budding yeast by inducing superoxide anions that inhibit quiescence. Aging (Albany NY) 2010;2:709–726. doi: 10.18632/aging.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Feng L, Paul A, et al. DNA replication stress is a determinant of chronological lifespan in budding yeast. PLoS One. 2007;2:e748. doi: 10.1371/journal.pone.0000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Roy R. Increased levels of hydrogen peroxide induce a HIF-1-dependent modification of lipid metabolism in AMPK compromised C. elegans dauer larvae. Cell Metab. 2012;16:322–335. doi: 10.1016/j.cmet.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Yahara I. Stress-inducible cellular responses. Introduction. EXS 77. 1996 XI-XII. [PubMed] [Google Scholar]
- Yanase S, Yasuda K Ishii N. Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech Ageing Dev. 2002;123:1579–1587. doi: 10.1016/s0047-6374(02)00093-3. [DOI] [PubMed] [Google Scholar]
- Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo S, Furumoto K, Hiyama E Miwa N. Slow-down of age-dependent telomere shortening is executed in human skin keratinocytes by hormesis-like-effects of trace hydrogen peroxide or by anti-oxidative effects of pro-vitamin C in common concurrently with reduction of intracellular oxidative stress. J Cell Biochem. 2004;93:588–597. doi: 10.1002/jcb.20208. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Homma T, Meyrick B, Takeda M, Moore-Jarrett T, Kon V Ichikawa I. Oxidants induce transcriptional activation of manganese superoxide dismutase in glomerular cells. Kidney Int. 1994;46:405–413. doi: 10.1038/ki.1994.288. [DOI] [PubMed] [Google Scholar]
- Zarse K, Schmeisser S, Groth M, et al. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012;15:451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]