Abstract
Objective
LRP1, a multifunctional protein involved in endocytosis and cell signaling pathways, leads to a range of vascular pathologies when deleted in vascular smooth muscle cells (SMCs). The purpose of this study was to determine whether LRP1 deletion in SMCs influenced AngII-induced arterial pathologies.
Approach and Results
LRP1 protein abundance was equivalent in selected arterial-regions, but SMC-specific LRP1 depletion had no effect on abdominal and ascending aortic diameters in young mice. To determine the effects of LRP1 deficiency on AngII vascular responses, SMC-specific LRP1 (smLRP1) +/+ and -/- mice were infused with saline, AngII, or norepinephrine (NE). Several smLRP-/- mice died of superior mesenteric arterial (SMA) rupture during AngII infusion. In surviving mice, AngII profoundly augmented SMA dilation in smLRP1-/- mice. SMA dilation was blood pressure-dependent as demonstrated by a similar response during NE infusion. SMA dilation was also associated with profound macrophage accumulation, but minimal elastin fragmentation. AngII infusion led to no significant differences in abdominal aorta diameters between smLRP1+/+ and -/- mice. In contrast, ascending aortic dilation was exacerbated markedly in AngII-infused smLRP1-/- mice, but NE had no significant effect on either aortic region. Ascending aortas of smLRP1-/- mice infused with AngII had minimal macrophage accumulation but significantly increased elastin fragmentation and mRNA abundance of several LRP1 ligands including MMP-2 and uPA.
Conclusions
smLRP1 deficiency had no effect on AngII-induced abdominal aortic aneurysm formation. Conversely, AngII infusion in smLRP1-/- mice exacerbated SMA and ascending aorta dilation. Dilation in these two regions had differential association with blood pressure and divergent pathologic characteristics.
Keywords: LRP1, aortic aneurysm, angiotensin II
Introduction
LRP1 is a multifunctional member of the LDL receptor gene family that interacts with numerous ligands.1 Ligand engagement can result in either removal of the ligand from the extracellular environment or stimulation of specific intracellular signaling pathways.2 Determination of the contribution of LRP1 to vascular diseases was hindered initially by the embryonic lethality of whole body deletion.3 Subsequent vascular studies have demonstrated a role for LRP1 using cell-specific deletion, with particular emphasis on macrophages and smooth muscle cells (SMC). LRP1 deficiency in either of these cell types augments atherosclerosis in hypercholesterolemic mice and neointimal formation.4-8 SMC-specific deletion of LRP1 (smLRP1-/-) in aged mice also leads to several vascular phenotypes including extended aortic length, aberrant superior mesenteric artery structure, and ascending aorta dilation.4,9,10
Recent studies have suggested a role for LRP1 in human vascular pathologies, particularly aortic aneurysms. Genome wide association studies have implicated the rs1466535 polymorphism of the LRP1 gene with abdominal aortic aneurysms (AAAs), although the two studies differ in which allele confers risk.11,12 A role for LRP1 in human AAAs has also been inferred by reduced abundance of LRP1 protein in aneurysmal tissue.13 In addition to an implied role in AAAs, exome sequencing of LRP1 identified a missense mutation in patients with thoracic aortic aneurysms that are afflicted with Marfan syndrome.14 These recent studies provide the basis for a potential role of LRP1 within aortic aneurysm formation.
Numerous studies have demonstrated a role for angiotensin II (AngII) in several vascular pathologies, particularly atherosclerosis and aortic aneurysms.15-17 AngII is one of the few mediators that regulates abundance of LRP1 protein in SMCs.18 In addition, AngII regulates expression of many LRP1 ligands, some of which have been implicated in aneurysm formation and compromised vascular integrity. These include PAI-1,19 TGF-β,20 selected MMPs 21 and connective tissue growth factor.22 Given the potential for AngII to augment many LRP1 ligands that affect vascular integrity, we speculated that LRP1 deficiency would influence vascular pathologies formed during chronic infusion of AngII.
The purpose of this study was to determine whether the absence of SMC LRP1 influenced AngII-induced arterial pathologies. The primary expectation was that smLRP1 deficiency would promote AngII-induced AAAs. However, this was not observed. In contrast, AngII infusion profoundly augmented aneurysms in both the superior mesenteric artery (SMA) and ascending aorta. Despite the commonality of arterial dilation, the two regions differed markedly in response to elevated blood pressure and tissue pathology.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
LRP1 Abundance in Arterial Vasculature
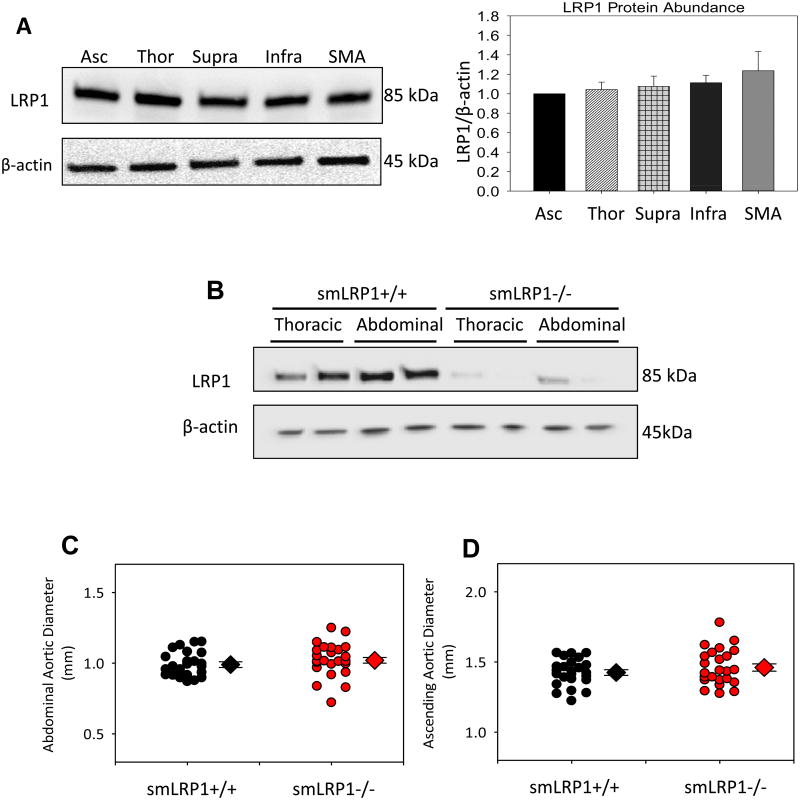
We first determined the abundance of LRP1 protein in selected arterial regions. LRP1 protein abundance was found to be uniform across all regions of the aorta and superior mesenteric artery (SMA; Figure 1A). Regional abundance of LRP1 protein was also determined in LRP1+/+ and -/- mice to confirm that SM22-driven Cre effectively mediated recombination of the homozygous LRP1 flox/flox gene to prominently deplete the LRP1 gene in vascular SMCs (Figure 1B) as described previously.9
Figure 1. LRP1 protein was uniformly abundant in arterial vasculature, and its absence in SMCs did not lead to aortic pathologies in young mice.
A. Representative immunoblot of LRP1 protein abundance in ascending (Asc), thoracic (Thor), suprarenal (Supra), infrarenal (Infr) aorta, and superior mesenteric artery (SMA). β-actin was used as a loading control. Bar graph depicts LRP1/β-actin ratio quantified for each region. Histobars represent group means and errors are SEMs (n=4 per group). B. Representative immunoblot of vascular SMCs harvested from thoracic and abdominal aortas of smLRP1+/+ and -/- mice. β-actin was used as a loading control. C,D. Maximum suprarenal and ascending aortic diameter measured by ultrasound in 8 week old mice (n= 24 per group).
Baseline aortic measurements were acquired prior to initiating infusions while smLRP1+/+ and -/- mice were approximately 8 weeks of age and were predicted to have no overt vascular pathology. In agreement with this prediction, smLRP1 genotype had no significant difference in ascending or abdominal aortic diameters (Figure 1C,D).
SMC Depletion of LRP1 Exacerbated SMA Dilation in a Blood Pressure-dependent Manner
Following baseline measurements, smLRP1+/+ and -/- mice were infused with either saline or AngII (1,000 ng/kg/day) for 28 days. Chronic AngII infusion increased systolic blood pressure in all mice, with no significant difference between genotypes (Supplementary Table I). Several smLRP1-/- mice died during AngII infusion. Upon necropsy, mesenteric hematomas were noted (Figure 2A,B). In surviving mice, in vivo MRI demonstrated that AngII-infused smLRP1-/- mice had pronounced SMA dilation (Figure 2C, Supplemental Figure IA,B). Ex vivo measurements following 28 days of infusion demonstrated that SMA diameters of saline-infused smLRP1+/+ and -/- mice were not significantly different (Figure 3A,B). In contrast, SMA diameters were dilated markedly during infusion of AngII (P<0.05; Figure 3A,B).
Figure 2. AngII infusion in smLRP1-/- mice promoted SMA dilation and rupture.
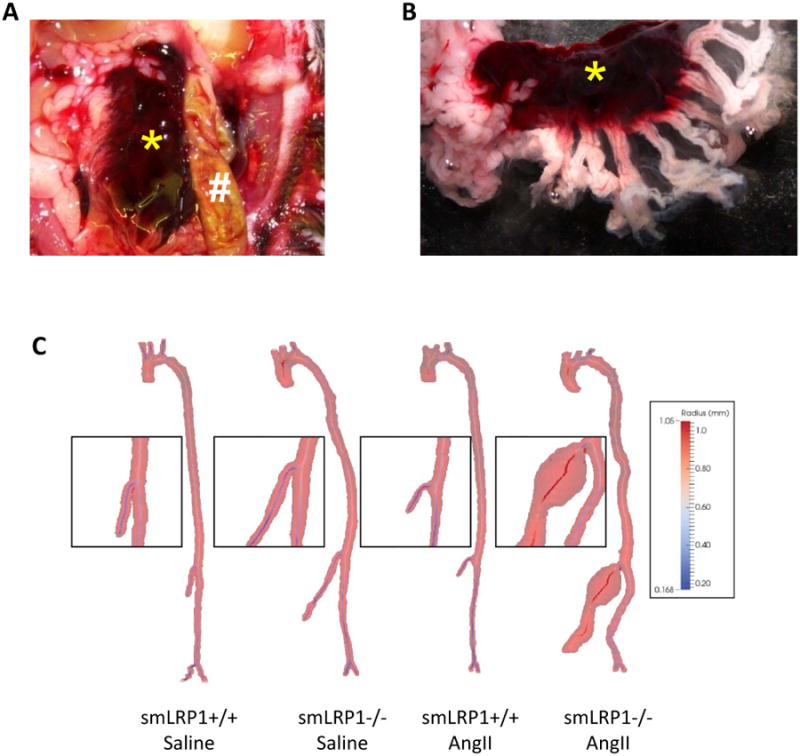
A and B. Rupture of SMA caused a mesenteric hematoma. * denotes hematoma within the mesenteric fat pad. # denotes small intestine. C. Representative 3 dimensional MRI images performed using a 7.0-T Clinscan system.
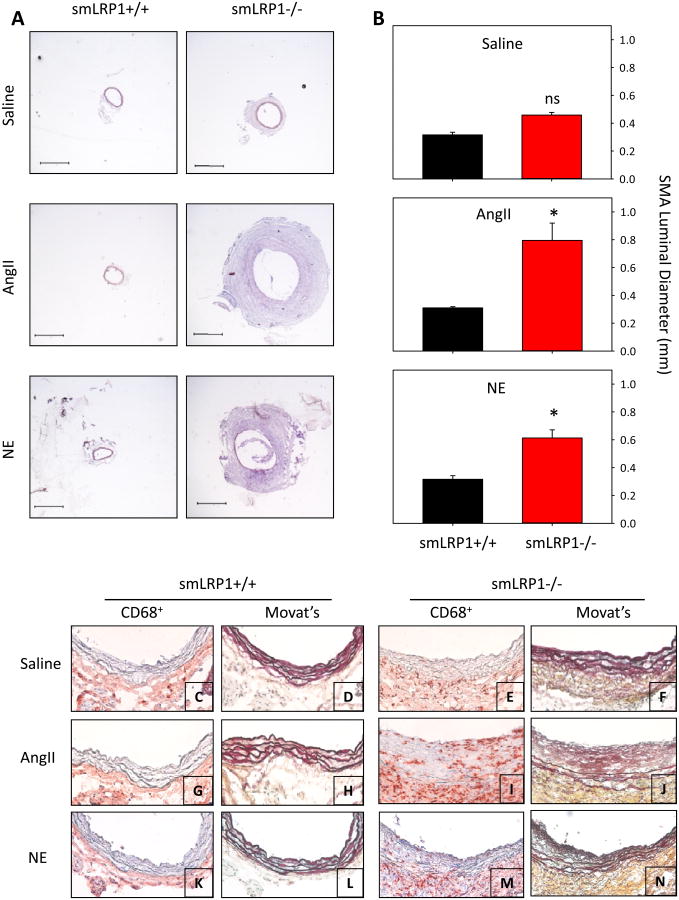
Figure 3. Increased systolic blood pressure promoted SMA dilation characterized by macrophage accumulation in smLRP1-/- mice.
A. Hematoxylin and eosin stained SMA cross sections were obtained 0.2 mm from bifurcation with aorta. smLRP1+/+ and smLRP1-/- were infused with saline, AngII, or NE. Bar represents 400 μm. B. Bar graphs of SMA dilation following saline, AngII, or NE infusion. Histobars represent means and errors are SEMs (n=5 per group). * denotes P<0.05 by two way ANOVA with multiple comparisons post hoc Holm-Sidak test by all genotypes and infusion groups. “ns” represents no statistical significance. C-N. Representative SMA micrographs of smLRP1+/+ (C,D,G,H,K,L) and -/- mice (E,F,I,J,M,N) infused with saline (C,D,E,F), AngII (G,H,I,J), or NE (K,L,M,N). Cross sections of SMA were immunostained for CD68+ cells (C,E,G,I,K,M) and stained with Movat's (D,F,H,J,L,N). CD68+ cells are red and elastin stains black. Sections are oriented with the lumen at the top of the image.
Since AngII infusion increased systolic blood pressure, we determined its contribution to the pronounced SMA dilation. smLRP1+/+ and -/- mice were infused with either saline or NE (5.6 mg/kg/day) for 28 days. As described previously,23,24 this infusion rate of NE produced systolic blood pressure increases that were equivalent to AngII infusion (Supplemental Table I). As with AngII infusion, NE infusion promoted pronounced SMA dilation (Figure 3A,B).
Dilated SMA tissue was characterized by increased accumulation of CD68+ cells in the adventitia and media of smLRP1-/- mice infused with either AngII or NE (Figure 3C,E,G,I,K,M). In contrast, no CD68+ cells were detected in the media SMAs of smLRP1+/+ mice that had been infused with saline, AngII or NE, although there was a sparse presence of CD68+ cells in the adventitia (Figure 3C,G,K). Movat's staining demonstrated a relatively modest, but significant, increase in elastin fragmentation in SMAs of smLRP1-/- mice following infusion of both AngII and NE (Figure 3D,F,H,J,L,N and Supplemental Figure II). In addition, AngII infusion promoted the development of a pronounced neointima (Figure 3J).
Region-specific Effects of SMC Depletion of LRP1 on Aortic Aneurysms
Aortic images were acquired in mice the day before termination using MRI. These images demonstrated that aortas were tortuous in smLRP1-/- mice infused with AngII (Supplemental Figure IC,D, IIIA). Aortic elongation in AngII-infused smLRP1-/- mice was confirmed by ex vivo measurements, even though mice had the same body length (Supplemental Figure IIIB,C). The aortic elongation was due to increased length of both the thoracic and abdominal aorta (Supplemental Figure IIID,E).
Aortic aneurysmal pathology was examined after 28 days of infusion. Against expectations, smLRP1-/- had no effect on AngII-induced abdominal aortic aneurysm formation as measured in vivo by ultrasound and ex vivo by measurement of diameters of suprarenal aortas (Supplemental Figure IV).
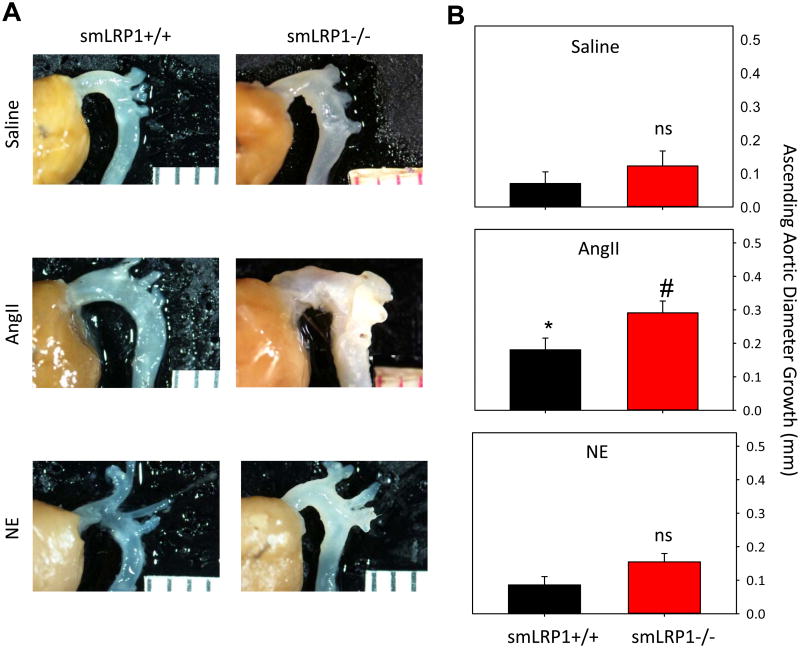
Dimensions of the ascending aorta were also measured in both smLRP1+/+ and -/- mice. As described previously,17,25 AngII infusion significantly increased ascending aortic diameter in smLRP1+/+ mice independent of blood pressure elevation. This dilation was significantly exacerbated in smLRP1-/- mice infused with AngII (P<0.05; Figure 4A,B). Unlike SMA dilation, development of dilation in the ascending aorta was independent of systolic blood pressure since NE infusion did not significantly enhance expansion of ascending aortas from smLRP1-/- mice (Figure 4B).
Figure 4. smLRP1-/- exacerbated AngII-induced ascending aortic aneurysm formation independent of systolic blood pressure.
A. Representative intact aortic arches of smLRP1+/+ and -/- infused with saline, AngII, or NE. B. Ascending aortic diameter growth in saline, AngII or NE-infused smLRP1+/+ and -/- mice. Histobars represent group means (n= 5 per group) and errors are SEM. * represents P<0.05 for AngII-infused smLRP1+/+ mice compared to saline or NE infused smLRP1+/+ or -/- mice by two way ANOVA. Multiple comparisons were performed using Holm-Sidak test. # denotes P<0.05 for AngII-infused smLRP1-/- versus all other groups by two way ANOVA. “ns” denotes no statistical significance when comparing within a given infusion group.
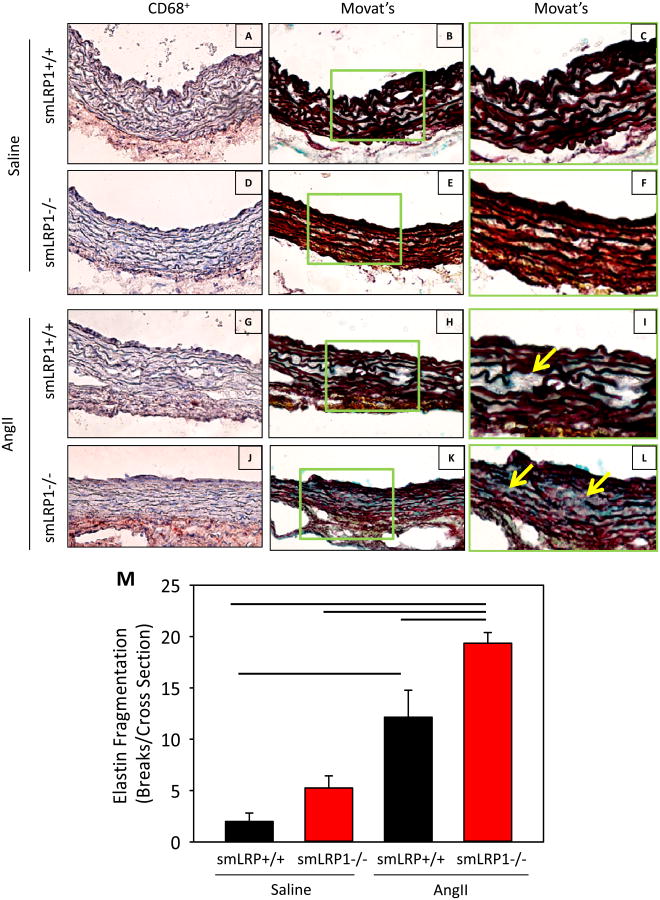
Concomitant with luminal expansion were pathological changes in the media of ascending aortas after 28 days of AngII infusion. No significant increase in CD68+ cell accumulation was discernible in aortic media of any group (Figure 5A,D,G,J). However, CD68+ cell accumulation was visible in adventitia of the ascending aorta in AngII-infused smLRP1-/- mice (Figure 5J). Elastin fragmentation was increased greatly in AngII-infused smLRP1+/+ and -/- mice compared to saline-infused mice. AngII-infused smLRP1-/- mice had the most significant increase in elastin disruption compared to all other groups (Figure 5B,C,E,F,H,I,K-M).
Figure 5. Ascending aortic sections from AngII-infused smLRP1-/- mice have increased elastin fragmentation.
A-L. Representative ascending aortic sections of smLRP1+/+ (A,B,C,G,H,I) or -/- mice (D,E,F,J,K,L) infused with saline or AngII infusion. Macrophage accumulation was identified by CD68+ cells (A,D,G,J) and elastin fragmentation was examined by Movat's (B,E,H,K). Additional higher magnification of Movat's stained sections is seen in the green boxes (C,F,I,L). Sections are oriented with the lumen at the top of the image. Yellow arrows indicated regions of marked elastin fragmentation. M. Elastin fragmentation counted in cross sections of ascending aortas from saline or AngII-infused smLRP1+/+ and -/- mice. Histobars represent group means (n=4 per group) and errors are SEM. Lines represent P<0.05 by two way ANOVA followed by a post hoc Holm-Sidak test.
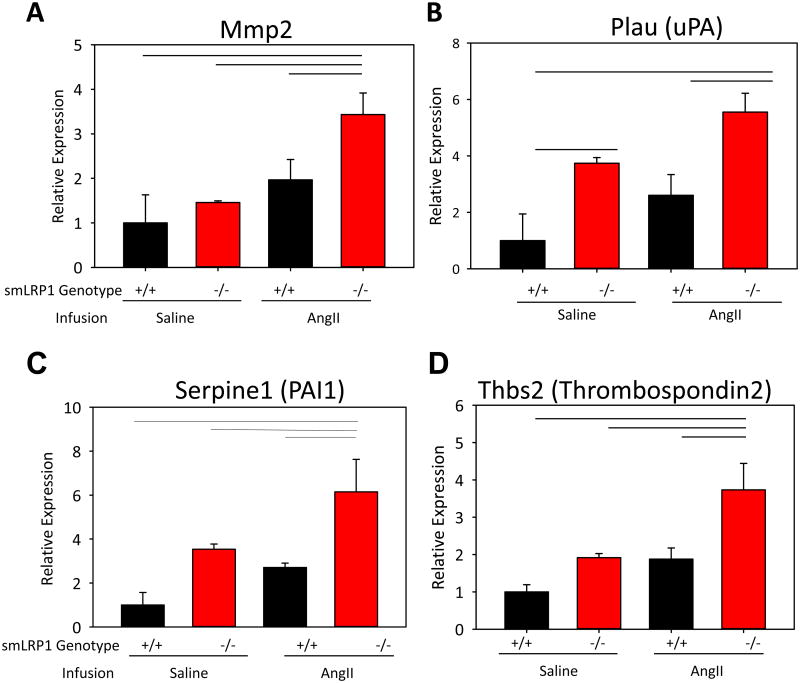
AngII Upregulated Expression of LRP1 Ligands in Ascending Aortas of smLRP1-/- Mice
To identify a potential mechanism by which AngII exacerbates smLRP1-/- ascending aortic dilation, we quantified abundance of known LRP1 ligands. In these experiments, mRNA was extracted from ascending aortas of saline or AngII-infused smLRP1+/+ and -/- mice and analyzed for mRNA abundance of LRP1 ligands. Given that NE infusion in smLRP1-/- mice did not produce ascending aortic dilation, we did not analyze expression of LRP1 ligands within these mice (Supplemental Table II). mRNA of several extracellular proteases including MMP-2 and uPA were increased significantly in AngII-infused smLRP1-/- ascending aortas (Figure 6A,B). Furthermore, mRNA abundance of PAI-1 and thrombospondin 2were increased (Figure 6C, D).
Figure 6. Increased abundance of LRP1 ligands in ascending aortas following AngII infusion.
A-E. Total mRNA, isolated from the ascending aorta of smLRP1+/+ or -/- mice following 28 days of saline or AngII infusion, was subjected to quantitative reverse transcription-polymerase chain reaction analyses. β-actin was used as the internal control (n=4 per group). Bars represent group means and errors are SEM. Lines represent P<0.05 by two way ANOVA followed by post hoc Holm-Sidak test.
Discussion
AngII infusion into mice promotes formation of aneurysms in the abdominal and ascending aortic regions, and augments atherosclerosis when associated with hypercholesterolemia.15-17 Although these vascular pathologies are due to the interaction of AngII with AT1a receptors,26-28 it has yet to be defined which cell type is stimulated by AngII to form these diseases, except in the case of deleting endothelial AT1a receptors on development of ascending aortic aneurysms.26,29,30 LRP1 is expressed in many cell types where it exerts protective effects from vascular pathology. AngII regulates many LRP1 ligands, but the interactions of AngII, LRP1 and vascular disease have not been studied in vivo. The present study demonstrated that LRP1 protein abundance was equivalent throughout all arterial regions examined. Surprisingly, deletion of LRP1 in SMCs had no effect on AAA; while promoting AngII-induced SMA and ascending aortic aneurysms by disparate mechanisms.
Many studies have reported aortic aneurysm formation during the initial few days of AngII infusion. Death is commonly attributable to rupture of the suprarenal or ascending aorta.31,32 While arterial rupture also occurred in a limited number of smLRP1-/- mice during AngII infusion, this was attributed to SMA rupture rather than compromise of aortic integrity. Although SMA enlargement has been described previously in aged smLRP1 -/- mice,4,33 it has not been documented during AngII infusion. To further define the differences between SMA and aortic aneurysms in smLRP1-/- mice, we investigated the effects of hypertension on aneurysmal disease within these arterial regions. It has been previously demonstrated that increases of blood pressure are not directly involved in AngII augmented aneurysmal formation of the suprarenal and ascending aortic regions. This conclusion was primarily based on comparisons to pathology in mice with hemodynamic equivalent infusions of NE.16,23,25 Using the same strategy in this study, hemodynamic equivalent infusions of either AngII or NE promoted a similar extent of dilation of the SMA; while infusion of NE did not promote ascending or abdominal aortic dilation. Therefore, the role of blood pressure on SMA dilation differs from aneurysm formation in aorta.
Furthermore, the pathological characteristics differed between tissues from AngII-induced SMA dilation and aorta aneurysm. SMAs retrieved from smLRP1-/- mice with increased systolic blood pressure had greatly expanded thickness, much of which was due to an area on the luminal aspect that was not encased by elastin layers and was assumed to be neointimal. A markedly thickened SMA has also been demonstrated in hypercholesterolemic smLRP1-/- mice through an undefined mechanism.4,33 Mechanistic studies have focused on LRP1 signaling through PDGFR-β, and its antagonism by a tyrosine kinase inhibitor, Gleevec, decreased atherosclerosis. However, the deletion of PDGFR-β had no effect on SMA dilation.33
SMAs from AngII-infused smLRP1-/- mice also contained abundant macrophages in all regions of tissue sections. Elastin fragmentation products have been invoked as a potential mechanism of promoting macrophage infiltration.34 Therefore, the presence of elastin fragmentation in AngII and NE infused mice could be responsible for the pronounced macrophage accumulation. However, this relationship differed in the ascending aorta of AngII-infused mice where there was extensive elastin fragmentation but minimal vascular macrophage accumulation. The cause and consequence of macrophage accumulation in the SMA need further study.
Previous studies in mice have inferred that LRP1 deficiency led to formation of abdominal aortic aneurysms,4 but did not provide direct evidence. In humans, LRP1 has been linked to the development of abdominal aortic aneurysms based on genetic associations and changes of LRP1 protein abundance in aneurysm tissue 11-13. Therefore, it was predicted that SMC deletion of LRP1 would promote development of AAAs in normocholesterolemic mice during AngII infusion. Since normocholesterolemic mice usually have a low incidence of AngII-induced AAAs,35-37 they provided a model to determine augmentation of AAAs. Contrary to this prediction, LRP1 absence did not lead to overt AAA formation during AngII infusion, or any measurable change in aortic diameters compared to LRP1 proficient mice. LRP1 was effectively deleted in this aortic region, so the absence of alteration in abdominal aortic aneurysm pathology was not attributable to any technical shortcomings. This lack of effect may not necessarily contradict the inference of LRP1 in human AAA, which is derived from weak associations that have generated discrepant allelic associations.
There has been considerable interest on the role of AngII in development of ascending aortic aneurysms, both experimentally and clinically.38 Interest was stimulated primarily by demonstrating that an AT1 receptor antagonist, losartan, prevented thoracic aortic aneurysms in mice harboring a C1039G fibrillin-1 mutation.39 Conversely, AngII infusion promotes expansion of the ascending aorta in both normo- and hypercholesterolemic mice.17,25 SMC-specific LRP1 deficiency also promotes expansion of the aortic root and descending aorta in aged mice.10 The present study demonstrated that lack of SMC LRP1 greatly augmented AngII-induced ascending aortic expansion. As described for AngII-induced ascending aortic dilation in LRP1 proficient mice,25 NE infusion at rates that induced equivalent systolic blood pressure increases did not mimic effects of AngII. Also, although LRP1 deficiency greatly augmented rates of ascending aortic expansion, there was no profound difference in pathological characteristics of tissue sections. These were characterized by profound elastin degradation, but a relative paucity of medial macrophage accumulation. Other characteristics include the lack of any neointimal formation and a predominance of medial changes on the adventitial aspect of the aorta. Overall, while concomitant AngII infusion and SMC-specific LRP1 deficiency promote dilation of the ascending aorta and SMA, the disparate roles of blood pressure and pathological appearances imply very different mechanisms for aneurysm formation.
The mechanisms by which LRP1 deficiency enhances selected AngII-induced vascular pathologies is unclear. AngII generates vascular pathologies through stimulation of AT1a receptors, but deletion of this receptor in SMCs had no effect on AngII-induced atherosclerosis and aneurysm.29 The pronounced enhancement of AngII-induced vascular pathologies in the absence of SMC LRP1 is likely due to augmentation of ligands that interact with LRP1. The current study has demonstrated increased mRNA abundance for several proteases that have been indicated in aneurysm formation. The marked elastin fragmentation in the ascending aorta may be due to increased activity of extracellular matrix proteases. However, a shortcoming of this analysis is that these measurements were performed on tissue from mice that had being chronically infused with AngII. Therefore, it is unclear whether the increased abundance of specific mRNAs is a cause or consequence of aneurysm formation. Also, previous studies using smLRP1-/- mice noted changes in protein abundance that were not mimicked by increased mRNA abundance, implying that other proteases were increased in aneurysmal tissue.9 Furthermore, while we have defined mRNA abundance for these proteases and arterial tissue, LRP1 ligands could have originated from extravascular sources. Defining the role of these proteases will require future studies that systematically delete these genes in mice with SMC-specific deletion of LRP1.
Overall, these studies demonstrate that the activity of LRP1 in SMCs is a major factor regulating development of AngII-induced vascular pathologies. These studies also demonstrate regional disparate effects of elevating systolic blood pressure. It will be of interest in future studies to determine the specific LRP1 ligands that are enhanced by AngII to compromise vascular integrity.
Supplementary Material
Figure I: Representative MR images from smLRP1-/- mice demonstrated profound dilation of the SMA and tortuosity of the aortic arch. Representative MRI of aortas from AngII-infused smLRP1+/+ and -/- mice. A,B. MRI transverse scan of the abdominal aorta (ˆ). * denotes SMA. C,D. Oblique scan of the aortic arch.
Figure II: SMA dilation was associated with modest elastin fragmentation. Bar graph of elastin fragmentation counted in cross sections of SMAs from Saline, AngII or NE-infused smLRP1+/+ and -/- mice. Histobars represent group means (n=4 per group) and errors are SEM. Lines denote P<0.05 by two way ANOVA followed by a post hoc Holm-Sidak test.
Figure III: smLRP1-/- resulted in an increased aortic length. A. Representative MRI of aortas from smLRP1+/+ and -/- mice. Black lines indicate boundaries of regional aortic length measurements. B. Overall aortic length was measured ex vivo from left subclavian artery to bifurcation. C. Mouse body length was measured from snout to anus. Circles represent individual mice (n=8-16 per group). Diamonds are group means and error bars are SEM. D-E. The aortic length was further divided into regions with the thoracic + suprarenal aortic region (measured from left subclavian artery to left renal artery) and infrarenal aortic region (measured from the left renal artery to bifurcation). * denotes P<0.01. Statistical comparisons were performed using Student's t test.
Figure IV: smLRP1-/- did not affect AngII-induced AAA formation. A. Maximum suprarenal abdominal aortic diameters in vivo were measured by ultrasound. B. Maximal suprarenal abdominal aortic diameters were measured ex vivo. Circles represent individual mice (n=4-14 per group). Diamonds are group means and error bars are SEM. No statistical differences were detected among groups by two way ANOVA with Holm-Sidak test multiple comparisons.
Supplemental Table I: Systolic blood pressure measurements.
Supplemental Table II: Relative abundance of mRNA in the ascending aorta using a gene expression assay plate assay.
Supplemental Table III: Taqman Gene Expression Assays of LRP1 Ligands
Significance.
LRP1 is a multi-functional protein that engages many ligands to perform endocytosis and signaling. It has been implicated in vascular pathologies in both humans and mice. AngII is well known to promote experimental vascular pathologies. In this study, the effects of LRP1 deletion in smooth muscle cells were determined during AngII-induced vascular pathologies. Unexpectedly, AngII infusion led to deaths due to SMA rupture that was associated with pronounced dilation of this vessel in smLRP-/- mice. Also unexpectedly, there was no effect of smooth muscle cell deficiency of LRP1 on formation of abdominal aortic aneurysms during AngII infusion. However, the absence of LRP1 led to a major promotion of ascending aortic dilation during AngII infusion. Therefore, AngII infusion in mice deficient of LRP1 in smooth muscle cells had a highly regional effect on development of vascular pathologies.
Acknowledgments
Sources of Funding: This research was supported by NIH grants HL107319 (AD), HL114379 (DKS) and HL120388 (DKS). Frank M. Davis is supported by a Sarnoff Cardiovascular Foundation Fellowship.
Abbreviations
- AAA
Abdominal Aortic Aneurysm
- AngII
Angiotensin II
- LRP1
Low-density lipoprotein receptor-related protein 1
- MRI
Magnetic resonance imaging
- NE
Norepinephrine
- SMC
Smooth muscle cells
- smLRP1-/-
Smooth muscle cell specific LRP1 deficiency
Footnotes
Disclosures: None.
References
- 1.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strickland DK, Au DT, Cunfer P, Muratoglu SC. Low-density lipoprotein receptor-related protein-1: role in the regulation of vascular integrity. Arterioscler Thromb Vasc Biol. 2014;34:487–498. doi: 10.1161/ATVBAHA.113.301924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71:411–421. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- 4.Boucher P, Gotthardt M, Li WP, Anderson RGW, Herz J. LRP: Role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 5.Hu L, Boesten LS, May P, Herz J, Bovenschen N, Huisman MV, Berbee JF, Havekes LM, van Vlijmen BJ, Tamsma JT. Macrophage low-density lipoprotein receptor-related protein deficiency enhances atherosclerosis in ApoE/LDLR double knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2710–2715. doi: 10.1161/01.ATV.0000249641.96896.e6. [DOI] [PubMed] [Google Scholar]
- 6.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res. 2007;100:670–677. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 7.Basford JE, Moore ZW, Zhou L, Herz J, Hui DY. Smooth muscle LDL receptor-related protein-1 inactivation reduces vascular reactivity and promotes injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2009;29:1772–1778. doi: 10.1161/ATVBAHA.109.194357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muratoglu SC, Belgrave S, Lillis AP, Migliorini M, Robinson S, Smith E, Zhang L, Strickland DK. Macrophage LRP1 suppresses neo-intima formation during vascular remodeling by modulating the TGF-beta signaling pathway. PLoS One. 2011;6:e28846. doi: 10.1371/journal.pone.0028846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muratoglu SC, Belgrave S, Hampton B, Migliorini M, Coksaygan T, Chen L, Mikhailenko I, Strickland DK. LRP1 protects the vasculature by regulating levels of connective tissue growth factor and HtrA1. Arterioscler Thromb Vasc Biol. 2013;33:2137–2146. doi: 10.1161/ATVBAHA.113.301893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basford JE, Koch S, Anjak A, Singh VP, Krause EG, Robbins N, Weintraub NL, Hui DY, Rubinstein J. Smooth muscle LDL receptor-related protein-1 deletion induces aortic insufficiency and promotes vascular cardiomyopathy in mice. PLoS One. 2013;8:e82026. doi: 10.1371/journal.pone.0082026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bown MJ, Jones GT, Harrison SC, et al. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am J Hum Genet. 2011;89:619–627. doi: 10.1016/j.ajhg.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galora S, Saracini C, Pratesi G, Sticchi E, Pulli R, Pratesi C, Abbate R, Giusti B. Association of rs1466535 LRP1 but not rs3019885 SLC30A8 and rs6674171 TDRD10 gene polymorphisms with abdominal aortic aneurysm in Italian patients. J Vasc Surg. 2014 doi: 10.1016/j.jvs.2013.10.090. [DOI] [PubMed] [Google Scholar]
- 13.Chan CY, Chan YC, Cheuk BL, Cheng SW. A pilot study on low-density lipoprotein receptor-related protein-1 in Chinese patients with abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2013;46:549–556. doi: 10.1016/j.ejvs.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Yu J, Wang K, Wang B, Wang M, Zhang S, Qin S, Yu Z. Exome sequencing identified new mutations in a Marfan syndrome family. Diagn Pathol. 2014;9:25. doi: 10.1186/1746-1596-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 17.Daugherty A, Rateri DL, Charo IF, Owens AP, Howatt DA, Cassis LA. Angiotensin II infusion promotes ascending aortic aneurysms: attenuation by CCR2 deficiency in apoE-/- mice. Clin Sci (Lond) 2010;118:681–689. doi: 10.1042/CS20090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sendra J, Llorente-Cortes V, Costales P, Huesca-Gomez C, Badimon L. Angiotensin II upregulates LDL receptor-related protein (LRP1) expression in the vascular wall: a new pro-atherogenic mechanism of hypertension. Cardiovasc Res. 2008;78:581–589. doi: 10.1093/cvr/cvn043. [DOI] [PubMed] [Google Scholar]
- 19.Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian V, Golledge J, Heywood EB, Bruemmer D, Daugherty A. Regulation of peroxisome proliferator-activated receptor-gamma by angiotensin II via transforming growth factor-beta1-activated p38 mitogen-activated protein kinase in aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2012;32:397–405. doi: 10.1161/ATVBAHA.111.239897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, Struber M, Haverich A, Bavendiek U, Drexler H, Schieffer B. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun. 2005;328:183–188. doi: 10.1016/j.bbrc.2004.12.152. [DOI] [PubMed] [Google Scholar]
- 22.Iwanciw D, Rehm M, Porst M, Goppelt-Struebe M. Induction of connective tissue growth factor by angiotensin II: integration of signaling pathways. Arterioscler Thromb Vasc Biol. 2003;23:1782–1787. doi: 10.1161/01.ATV.0000092913.60428.E6. [DOI] [PubMed] [Google Scholar]
- 23.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. Angiotensin II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owens AP, 3rd, Subramanian V, Moorleghen JJ, Guo Z, McNamara CA, Cassis LA, Daugherty A. Angiotensin II induces a region-specific hyperplasia of the ascending aorta through regulation of inhibitor of differentiation 3. Circ Res. 2010;106:611–619. doi: 10.1161/CIRCRESAHA.109.212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rateri DL, Davis F, Balakrishnan A, Howatt DA, Moorleghen JJ, O'Connor W, Charnigo R, Cassis LA, Daugherty A. Angiotensin II induces region-specific medial disruption during evolution of ascending aortic aneurysms. Am J Pathol. 2014;184:2586–2595. doi: 10.1016/j.ajpath.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 27.Poduri A, Owens AP, 3rd, Howatt DA, Moorleghen JJ, Balakrishnan A, Cassis LA, Daugherty A. Regional Variation in Aortic AT1b Receptor mRNA Abundance Is Associated with Contractility but Unrelated to Atherosclerosis and Aortic Aneurysms. PLoS One. 2012;7:e48462. doi: 10.1371/journal.pone.0048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daugherty A, Rateri DL, Howatt DA, Charnigo R, Cassis LA. PD123319 augments angiotensin II-induced abdominal aortic aneurysms through an AT2 receptor-independent mechanism. PLoS One. 2013;8:e61849. doi: 10.1371/journal.pone.0061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rateri DL, Moorleghen JJ, Knight V, Balakrishnan A, Howatt DA, Cassis LA, Daugherty A. Depletion of endothelial or smooth muscle cell-specific angiotensin II type 1a receptors does not influence aortic aneurysms or atherosclerosis in LDL receptor deficient mice. PLoS One. 2012;7:e51483. doi: 10.1371/journal.pone.0051483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor-/- mice. Circ Res. 2011;108:574–581. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 32.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L, Takayama Y, Boucher P, Tallquist MD, Herz J. LRP1 regulates architecture of the vascular wall by controlling PDGFRbeta-dependent phosphatidylinositol 3-kinase activation. PLoS One. 2009;4:e6922. doi: 10.1371/journal.pone.0006922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980;66:859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng GG, Martin-McNulty B, Sukovich DA, Freay A, Halks-Miller M, Thinnes T, Loskutoff DJ, Carmeliet P, Dole WP, Wang YX. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–517. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 36.King VL, Trivedi D, Gitlin JM, Loftin CD. Selective cyclooxygenase-2 inhibition with celecoxib decreases angiotensin II-induced abdominal aortic aneurysm formation in mice. Arterioscler Thromb Vasc Biol. 2006;26:1137–1143. doi: 10.1161/01.ATV.0000216119.79008.ac. [DOI] [PubMed] [Google Scholar]
- 37.Uchida HA, Poduri A, Subramanian V, Cassis LA, Daugherty A. Urokinase-type plasminogen activator deficiency in bone marrow-derived cells augments rupture of angiotensin II-induced abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011;31:2845–2852. doi: 10.1161/ATVBAHA.111.234997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moltzer E, Essers J, van Esch JH, Roos-Hesselink JW, Danser AH. The role of the renin-angiotensin system in thoracic aortic aneurysms: Clinical implications. Pharmacol Ther. 2011;131:50–60. doi: 10.1016/j.pharmthera.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure I: Representative MR images from smLRP1-/- mice demonstrated profound dilation of the SMA and tortuosity of the aortic arch. Representative MRI of aortas from AngII-infused smLRP1+/+ and -/- mice. A,B. MRI transverse scan of the abdominal aorta (ˆ). * denotes SMA. C,D. Oblique scan of the aortic arch.
Figure II: SMA dilation was associated with modest elastin fragmentation. Bar graph of elastin fragmentation counted in cross sections of SMAs from Saline, AngII or NE-infused smLRP1+/+ and -/- mice. Histobars represent group means (n=4 per group) and errors are SEM. Lines denote P<0.05 by two way ANOVA followed by a post hoc Holm-Sidak test.
Figure III: smLRP1-/- resulted in an increased aortic length. A. Representative MRI of aortas from smLRP1+/+ and -/- mice. Black lines indicate boundaries of regional aortic length measurements. B. Overall aortic length was measured ex vivo from left subclavian artery to bifurcation. C. Mouse body length was measured from snout to anus. Circles represent individual mice (n=8-16 per group). Diamonds are group means and error bars are SEM. D-E. The aortic length was further divided into regions with the thoracic + suprarenal aortic region (measured from left subclavian artery to left renal artery) and infrarenal aortic region (measured from the left renal artery to bifurcation). * denotes P<0.01. Statistical comparisons were performed using Student's t test.
Figure IV: smLRP1-/- did not affect AngII-induced AAA formation. A. Maximum suprarenal abdominal aortic diameters in vivo were measured by ultrasound. B. Maximal suprarenal abdominal aortic diameters were measured ex vivo. Circles represent individual mice (n=4-14 per group). Diamonds are group means and error bars are SEM. No statistical differences were detected among groups by two way ANOVA with Holm-Sidak test multiple comparisons.
Supplemental Table I: Systolic blood pressure measurements.
Supplemental Table II: Relative abundance of mRNA in the ascending aorta using a gene expression assay plate assay.
Supplemental Table III: Taqman Gene Expression Assays of LRP1 Ligands