Abstract
The identification of new antibacterial targets is urgently needed to address multidrug resistant and latent tuberculosis infection. Sulfur metabolic pathways are essential for survival and the expression of virulence in many pathogenic bacteria, including Mycobacterium tuberculosis. In addition, microbial sulfur metabolic pathways are largely absent in humans and therefore, represent unique targets for therapeutic intervention. In this review, we summarize our current understanding of the enzymes associated with the production of sulfated and reduced sulfur-containing metabolites in Mycobacteria. Small molecule inhibitors of these catalysts represent valuable chemical tools that can be used to investigate the role of sulfur metabolism throughout the Mycobacterial lifecycle and may also represent new leads for drug development. In this light, we also summarize recent progress made in the development of inhibitors of sulfur metabolism enzymes.
Keywords: Tuberculosis, mycobacteria, sulfur metabolism, enzymes, thiols, sulfation, drug design and inhibitors
Mycobacterium tuberculosis
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), is one of the most lethal infectious agents affecting the humans [1, 2]. In 2011, ~10,521 new TB cases were reported in the United States, an incidence of 3.4 cases per 100,000 population [3]. The majority of people afflicted with TB live in developing countries, where lethal synergy with HIV infection also fuels the TB pandemic. According to World Health Organization (WHO) recent report, in 2011, worldwide there were an estimated 8.7 million new cases of TB (13% co-infected with HIV) and 1.4 million people died from TB [4]. Geographically, the burden of TB is highest in Asia and Africa. India and China together account for almost 40% of the world’s TB cases. About 60% of cases are in the South-East Asia and Western Pacific regions. The African region has 24% of the world’s cases and the highest rates of cases and deaths per capita. Almost 80% of TB cases among people living with HIV reside in Africa. There were an estimated 0.5 million cases and 64,000 deaths among children in 2011 [3–6].
M. tuberculosis is difficult to treat, requiring 6–9 months of chemotherapy with a combination of four frontline antibiotics – isoniazid, rifampin, pyrazinamide, and ethambutol [7, 8]. In large part, the lengthy drug therapy is necessary because mycobacteria exist as metabolically diverse population within the human host [8]. Some bacteria will be actively dividing, rendering them susceptible to antibiotic treatment. However, less active subpopulations of mycobacterium also exist in stationary phase or as dormant bacteria leading to latent TB infection [9, 10]. Since TB drugs target biological processes required for bacterial growth (e.g., cell wall biosynthesis), they are far less effective at killing the persistent population [8, 10, 11]. Nonetheless, the treatment for the individuals with latent M. tuberculosis infection begins with diagnosis on the basis of a positive tuberculin skin test or an interferon-γ release assay result and includes use of one of the frontline drugs, isoniazid, rifapentine, or rifampin for 3–9 months [12, 13].
In addition to toxic side effects, the lengthy treatment regime results in poor patient compliance and drug resistant strains are beginning to emerge [5]. According to WHO, worldwide, 3.7% of new cases and 20% of previously treated cases were estimated to have multi-drug resistant TB (MDR-TB). India, China, the Russian Federation and South Africa have almost 60% of the world’s cases of MDR-TB [4]. The highest proportions of TB patients with MDR-TB reside in eastern Europe and central Asia [4]. Taken together, the growing problem of MDR-TB and the lack of drugs that effectively target persistent bacteria, stress the urgent need for identification of new antimicrobial targets [6].
Many fundamental aspects of mycobacterial metabolism and pathogenesis are poorly understood, in part because of the technical difficulties inherent to studying M. tuberculosis. The organism must be manipulated in a biosafety level 3 laboratory, and the slow growth rate (3 weeks for colonies, up to 1 year for completion of animal models) imposes limitations on apparent research productivity. However, the availability of complete mycobacterial genome sequences [14–17] and the maturation of methods for disrupting mycobacterial genes [18–20] have provided tools that can accelerate the discovery of potential drug targets and elucidate metabolic pathways that are essential for mycobacterial survival.
OVERVIEW OF TB INFECTION
M. tuberculosis infection is a complex process that initiates with aerosol inhalation to the host lung [13, 21]. Alveolar macrophages respond to the inhaled pathogen and phagocytocise them. During this process, the infected macrophages release chemokines to recruit the neutrophils, macrophages, NK cells, and γδ-T cells to mount inflammatory response and to wall out the infected macrophages. Activation of the immune system and lung inflammation induced expectoration provides an exit strategy for the bacteria to spread to another host [22, 23]. Thus the macrophages are the building blocks of the granuloma [24–27]. The granuloma is kernel like structure initiated by infected macrophages and surrounded by layers of foamy macrophages, macrophages, lymphocytes and with penetrating blood vessels [28]. During maturation, the number of blood vessels passing through granuloma diminishes and a fibrous sheath develops walling out the infected mycobacteria from rest of the host [28]. Within the context of the granuloma, T-cells can proliferate in response to specific mycobacterial antigens and some may leave the granuloma to reenter the circulation thus, the granuloma is a dynamic structure [28–30]. Recent experiments to study the formation of granuloma in Zebra fish models of infection revealed that mycobacterial RD1-dependent signal induces macrophage migration and facilitates random movement within the granuloma. The dying infected macrophages generate another signal that recruits nearby macrophages for phagocytosis. These studies also showed that RD1-deficient bacteria fail to elicit efficient granuloma formation despite their ability to grow inside infected macrophages [13, 22, 27, 31, 32].
However, less than 10% of infected individuals will develop active TB infection. In the rest, mycobacteria residing within granulomas enter into a persistent or “latent” state characterized by a lack of cell division and a change in basic metabolism [33, 34]. These latent mycobacteria are difficult to eradicate since they are not reliant on machinery targeted by conventional antibiotics [8]. However the granuloma which contains infected mycobacterium, may fail when the immune status of the host changes because of old age, malnutrition or co-infection with HIV which impairs the function of T cells. Following such a change in immune status the granuloma decays, ruptures and spills thousands of viable, infectious bacilli into the airways. This results in the development of a productive cough that facilitates aerosol spread of infectious bacilli [13, 28]. Hence, effective treatment of TB will require efficacy against persistent M. tuberculosis, or at the least a better understanding of the mechanisms underlying immune cell activation, bacterial adaptation and survival within the granuloma [8, 35, 36].
SULFUR AND MYCOBACTERIAL SURVIVAL
To complete its lifecycle, M. tuberculosis must survive within the hostile, nutrient-poor, reactive oxygen/nitrogen rich, di-oxygen deficient environment of the host macrophage [37, 38]. At the same time, M. tuberculosis must activate sufficient immune effector functions to induce granuloma formation in the lung [22, 39–42]. The mechanisms by which bacilli survive the hostile environment and transition in to dormancy are not well understood. A Recent study conducted by Forrellad et al. showed that a large number of different virulence factors have evolved in M. tuberculosis as a response to the host immune reaction [43]. However, genes involved in the metabolism of sulfur have consistently been identified as up-regulated in response to oxidative stress, nutrient starvation and dormancy adaptation (culture conditions that model aspects of mycobacterial life in the granuloma and during macrophage infection [43–53].
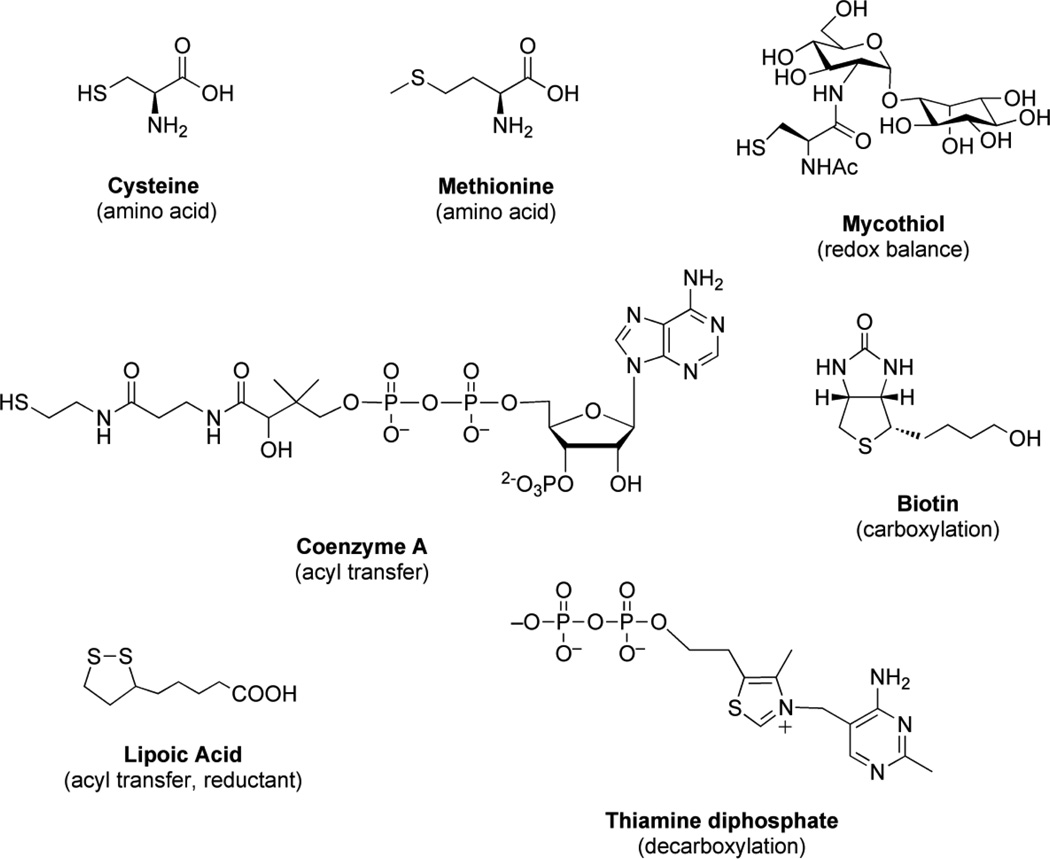
Sulfur is an essential element for life and plays a central role in numerous microbial metabolic processes [54]. In its reduced form, sulfur is used in the biosynthesis of the amino acids cysteine and methionine. Cysteine is incorporated into biomolecules such as proteins, coenzymes, and mycothiol (the mycobacterial equivalent of glutathione) [See Fig. 1]. Found in all actinomycetes, mycothiol regulates cellular redox status and is essential for M. tuberculosis survival [55–58]. Another reduced sulfur-containing metabolite, coenzyme A (CoA), is heavily utilized for lipid metabolism and biosynthesis of mycolic acid, which is an important constituent in mycobacterial cell wall and plays major role in antigenicity of the pathogen [59]. In its oxidized form, sulfur is present as a sulfuryl moiety (–SO3−) that can modify hydroxyls and amines in proteins, polysaccharides and lipids [See Fig. 2] [49, 50]. Sulfated glycolipids of mycobacterium are very closely related to the virulence of the pathogen. For example, Sulfolipid-1 is present only in virulent species of mycobacterium [60]. On the other hand, sulfated menaquinone, S881, suppresses bacterial virulence [61, 62]. Hence, acquisition and metabolism of sulfur are essential for mycobacterial virulence and survival. The identification of new antibacterial targets is essential to address MDR- and latent-TB infection [63, 64]. Toward this end, mycobacterial sulfur metabolism represents a promising new area for anti-TB therapy [62, 65, 66]. Numerous studies have validated amino acid biosynthetic pathways and downstream metabolites as antimicrobial targets [67–70] and sulfur metabolic pathways are required for the expression of virulence in many pathogenic bacteria [71–74]. In particular, mutants in mycobacterial sulfur metabolism genes are severely impaired in their ability to persist and cause disease [49, 50, 73, 75–77]. Furthermore, most of the microbial sulfur metabolic pathways are absent in humans and therefore, represent unique targets for therapeutic intervention. In this review, we focus on the enzymes associated with the production of sulfated and reduced sulfur-containing metabolites in Mycobacteria. Small molecule inhibitors of these catalysts represent valuable chemical tools that can be used to investigate the role of sulfur metabolism in M. tuberculosis survival and may also represent new leads for drug development. In this light, we also highlight major efforts devoted towards inhibitor discovery of mycobacterial sulfur metabolic pathways.
Figure 1.
Reduced sulfur–containing metabolities in mycobacteria.
Figure 2.
Sulfated metabolites in mycobacteria.
SULFATE ASSIMILATION IN MYCOBACTERIA
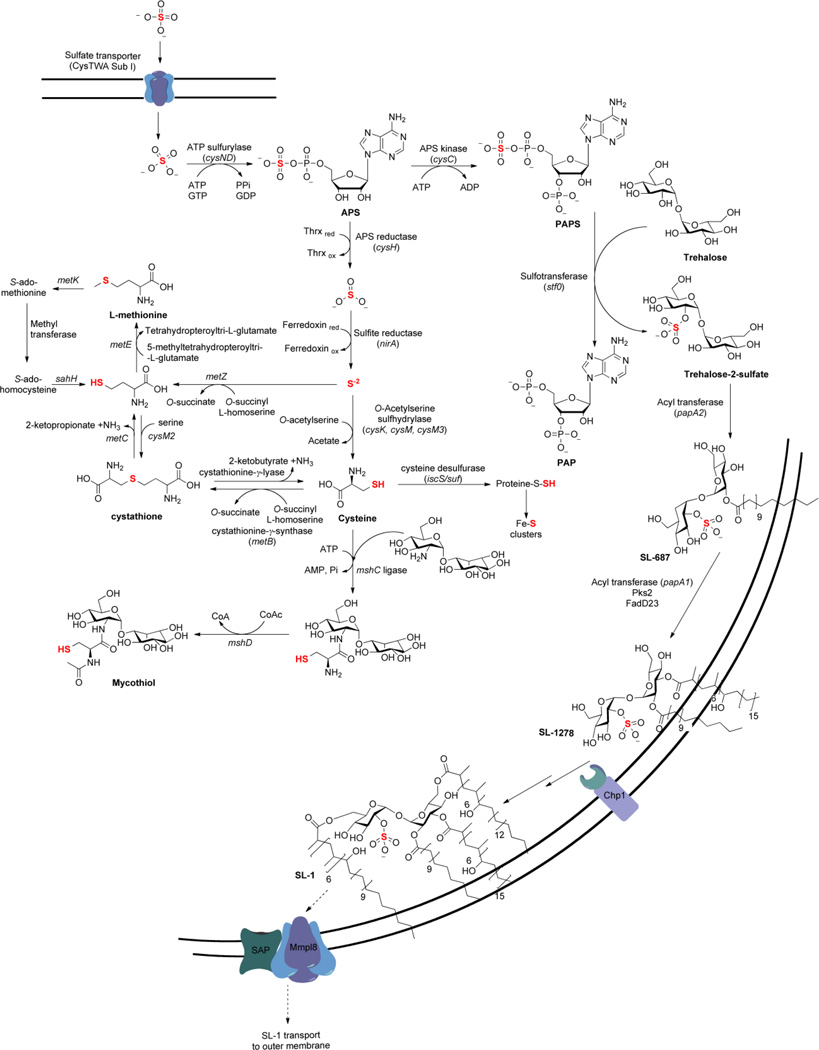
Sulfate assimilation begins with the active transport of inorganic sulfate (SO42−) across the mycobacterial cell membrane by the cysTWA SubI ABC transporter complex [see Fig. 3] [78, 79]. Once sulfate is imported, it gets activated by ATP sulfurylase (encoded by cysND) via adenylation to produce adenosine-5’-phosphosulfate (APS) [47, 66, 80]. In mycobacteria, APS lies at a metabolic branch point [66]. For sulfation of biomolecules such as proteins, lipids and polysaccharides, APS is phosphorylated at the 3’-hydroxyl by APS kinase (encoded by cysC) to form 3’-phosphoadenosine-5’-phosphosulfate (PAPS), the universal sulfate donor for sulfotransferases (STs) [66, 80–82]. Transfer of –SO3− to hydroxyl or amino functionalities of biomolecules plays important roles in regulation of cell-cell communication and metabolism [60]. Alternatively, for production of reduced sulfur-containing metabolites, the sulfate moiety in APS is reduced to sulfite (SO32−) by APS reductase (gene product of cysH) [66, 73, 83]. Sulfite is further reduced to sulfide (S2−) by sulfite reductase (encoded by nirA) [84] and results in the form of sulfur that is used for the biosynthesis of sulfur-containing metabolites including cysteine, methionine, coenzymes, and mycothiol [54, 55, 85]. Each branch of sulfate assimilation is discussed in terms of the available genetic and biochemical data below.
Figure 3.
The sulfate assimilation pathway in mycobacteria.
SULFATE IMPORT AND ACTIVATION
Present at 300–500 µM, inorganic sulfate is the fourth most abundant anion in human plasma [86]. Sulfate transporters have been identified in all major human tissues investigated to date, and of particular relevance to the intracellular lifestyle of M. tuberculosis, the existence of endosomal-associated transporters has also been demonstrated [86]. The genes encoding the cysTWA SubI ABC transporter complex in mycobacteria have been identified by homology to Escherichia coli and Salmonella typhimurium [78], are essential [49], robustly up-regulated during oxidative stress [45], dormancy adaptation [44], and expressed in macrophages [51]. Consistent with this annotation, cysA or subI mutants (∆cysA or ∆subI, respectively) in M. bovis bacillus Calmette-Guérin (BCG) – an attenuated, vaccine strain of M. bovis – are compromised in their ability to transport sulfate [78, 87]. When grown in media supplemented with casamino acids, the rate of sulfate transport in ∆cysA is ~1.1% relative to wild-type M. bovis BCG [78]. The minor amount of transport is not enough to meet bacterial sulfur requirements and hence, these sulfate transport mutants are auxotrophic for reduced sulfur.
Interestingly, no significant difference in the number of viable bacilli was observed in the organs of mice infected with ∆cysA and wild-type M. bovis BCG up to 63 days post-infection [78]. These data indicate that M. bovis BCG may scavenge sufficient amounts of reduced sulfur from the host for survival. However, an important question raised from the findings of this study is whether the sulfur requirements for an attenuated M. bovis strain reflect those of M. tuberculosis known to elicit a more potent host immune response [22, 29, 40]. It is also possible that the mycobacterial genome encodes for an additional sulfate transporter which is not expressed under culture conditions, but is specifically up-regulated during infection [88, 89]. In support of this hypothesis, mRNA array analysis has shown significant up-regulation of hypothetical proteins from Rv1739c and Rv1707 [51, 78] 24 h post infection of activated macrophages in response to nitric oxide [46] or hypoxia [52]. Rv1739c expression in E. coli has been shown to enhance sulfate uptake, though complementation of the M. bovis cysA mutant with the Rv1739cgene was not sufficient to restore sulfate prototrophy [90]. In contrast, little is known about the Rv1707 gene product [62]. However these gene products are believed to be associated with inner membrane of M. tuberculosis [78, 91, 92]. It has recently been established that mycobacteria have an outer membrane [91], which is the primary permeability barrier to overcome the transport of any nutrient molecule. In line with these findings, recent studies conducted by Song et al. demonstrate that specific outer membrane proteins, called porins, are responsible for transport of inorganic anions like sulfate and nitrate in M. tuberculosis [93, 94] and in M. bovis BCG [92, 95]. From these findings, it can be inferred that the porins and cysTWA SubI should align to allow the transport of anions from outside the cell to inside of the cell. However, additional studies will be required to confirm the mechanism for the sulfate transport and its relevance to sulfate acquisition in vivo.
Once sulfate is transported to the cytosol, ATP sulfurylase (encoded by cysD) catalyzes the first committed step in sulfate assimilation [see Fig. 3] [47, 80]. In this reaction, the adenylyl moiety of adenosine 5’-triphosphate (ATP) is coupled to sulfate. The product that results, APS, contains a unique high-energy phosphoric-sulfuric acid anhydride bond, the biologically activated form of sulfate [85]. Formation of APS is energetically unfavorable (Keq of 10−7 – 10−8 near physiological conditions) [85] and in prokaryotes, the hydrolysis of guanosine 5’-triphosphate (GTP) is coupled to sulfurylation of ATP to surmount this energetic hurdle [96]. The GTPase (encoded by cysN) forms a heterodimer with ATP sulfurylase (cysD) and synthesis of APS is driven 1.1×106-fold further during GTP hydrolysis [80, 97]. Notably, eukaryotic ATP sulfurylases do not bear any sequence or structural similarity to their prokaryotic counterparts, nor do they employ aGTPase for PAPS biosynthesis [77]. These mechanistic and structural differences, in particular the unique G protein subunit, could be exploited to develop small molecule inhibitors of bacterial sulfate activation [88]. In this direction, Pinto et al. showed that vaccination with ATP sulfurylase conferred significant protection against murine TB and boosted BCG-induced protective immunity in the lung [98] thereby demonstrating that components of sulfate assimilation pathway are promising candidates for inclusion in new vaccines to control TB in humans.
The final step in PAPS biosynthesis is catalyzed by APS kinase (encoded by cysC) [66, 82]. In this reaction, ATP is utilized to phosphorylate the 3’-hydroxyl of APS. Depending on the organism, APS kinase can be encoded as a separate protein or as a fusion with ATP sulfurylase, without significant variation in catalytic mechanism [66, 99]. Most eukaryotes (including those of humans) encode for ATP sulfurylase (cysD) and APS kinase (cysC) on a single polypeptide. In M. tuberculosis, however, APS kinase (cysC) is genetically fused to the GTPase subunit (cysN) of ATP sulfurylase [66]. The APS kinase domain of M. tuberculosis cysNC was identified through sequence homology and confirmed by genetic complementation [66]. In a subsequent report, a mutant strain of M. tuberculosis that removes the APS kinase domain of the bifunctional cysNC gene was constructed [82]. As expected, the cysC knockout (∆cysC) strain was able to grow on sulfate as a sole sulfur source (indicating a functional ATP sulfurylase), but was unable to synthesize PAPS [82].
Fusion of APS kinase to the GTPase domain of ATP sulfurylase raised the interesting possibility of substrate channeling between subunits [88, 99]. In this scenario, the final product PAPS, and not the APS intermediate, would be released into solution. Leyh et al. have recently tested this hypothesis for M. tuberculosis ATP sulfurylase [99]. Although PAPS synthesis is 5,800 times more efficient than APS synthesis [80], these studies demonstrate that APS is not channeled from the M. tuberculosis adenylyltransferase to the APS kinase domain [99], consistent with the domain arrangement proposed from a recent crystal structure of the cysNC complex [97].
Collectively, cysNC and cysD proteins form a multifunctional enzyme complex ~300 KDa (consistent with a trimer of CysNC•D heterodimers), referred to as the sulfate-activating complex (SAC) [47, 80, 100]. In M. tuberculosis, expression of the SAC operon is induced by conditions likely to be encountered by pathogenic mycobacteria within the macrophage, including sulfur limitation, oxidative stress, and is repressed by cysteine [45, 47]. The SAC operon is also up-regulated during stationary phase growth, an in vitro model of persistent M. tuberculosis infection [44]. M. tuberculosis SAC gene expression is also augmented within the intracellular environment of the macrophage [51, 101]. Taken together, these data are consistent with increased activity of sulfate activating enzymes and flux through the sulfate assimilation pathway during mycobacterial infection.
REGULATION OF SULFUR METABOLISM
Transcriptional regulation
Upon infection, M. tuberculosis undergoes phagocytosis by alveolar macrophages exposing the pathogen to nutrient poor and oxidative environment. This stress induces several genes in M. tuberculosis related to sulfate assimilation pathway to counteract against the oxidative stress and transition to dormancy [44, 47, 102, 103]. Genes that coordinate the sulfate transport and the first few steps of sulfate assimilation are over expressed when M. tuberculosis is subjected to variety of stress conditions. For example, cysT was induced following by stimulus with hydrogen peroxide. Nutrient starvation conditions induced cysA1, cysT, cysW, subI genes that encode for sulfate transporter complex. Also cysD which encodes ATP sulfurylase, and cysNC, which encodes for bifunctional APS kinase, are induced in response to environmental challenges [104]. Hypoxia mediated induction of cysD, cysNC, cysK2, cysM, cysT and subsequent activation of ATP sulfurylase and cysteine biosynthesis indicate genetic level regulation of sulfate assimilation to defend against reactive species in the granuloma [105].
Some frontline antibiotics used to treat TB also induce genes of the sulfate assimilation pathway. For example, menadione (a vitamin K precursor that promotes the production of reactive oxygen species) induces cysA1, cysT, cysW, subI genes [106, 107], Vancomycin (peptidoglycan biosynthesis inhibitor) induces cysK2, cysD, cysNC [62, 108], and Chloropyrazinamide (fatty acid biosynthesis inhibitor) induces cysNC [62, 106]. These data suggest that genes involved in sulfite assimilation respond to antibiotics used in current treatment regimes.
Sigma (σ) factors are bacterial transcription initiation factors, which direct the RNA polymerases to specific promoter sites and regulates the expression of housekeeping genes. It is proposed that one or more σ factors play a role in modulating the various stress adaptive genes to cope with unfavorable conditions in the granuloma [109]. One such factor is σH (SigH), which is induced in response to heat shock, oxidative stress, pH variation and phagocytosis [109–113]. It has been shown that σH regulates the transcription of several sulfate metabolism genes (cysA1, cysT, cysW, cysD and cysNC) following diamide treatment [62, 114] and is required for virulence in animal models of TB infection [115]. The σH factor is auto-regulated by its own promoter at the transcriptional level by anti-sigma factor RshA [116–118]. An analogous type of regulation was identified in Streptomyces coelicolor [119]. Site directed mutagenesis studies and deuterium exchange mass spectrometry conducted by Kumar et al. showed that these opposing sigma factors communicate through salt bridges [117]. Disruption of such communication creates a positive feedback loop that leads to rapid and strong induction of SigH-regulated gene expression [116]. It has been shown that partial inhibition of the SigH-RshA interaction is also possible when RshA is phosphorylated by protein kinase B, and is shown to result in partial activation of SigH [115]. It is not clear why mycobacterium utilizes partial activation of SigH, compared to complete activation. It is possible that regulatory mechanism is used to fine-tune the response to adverse environmental conditions.
Biochemical regulation
In addition to regulation at the transcriptional level, several additional proteins are involved in the sulfate import and activation. Recent studies demonstrated that mycobacterium possesses an outer membrane, which is the primary barrier for transport of molecules across the membrane [91]. The outer membrane was shown to contain specific channels called porins which allow the passage of specific anions through the inner membrane [92–95]. The primary sulfate transporter is an ABC transporter encoded by subI-cysTWA1 operon [78] located at the inner membrane and is expressed in response to oxidative stress and phagocytosis. Genetic disruption of cysA1 gene in M. bovis results in complete inhibition of sulfate uptake and renders the mutant auxotrophic to methionine characteristics. However, this mutation does not result in bacterial death [78]. This observation indicates the presence of alternate sulfate transporters that are activated during infection, or implies the existence of other biochemical pathways that feed into the cysteine biosynthetic pathway. Interestingly, M. tuberculosis is shown to be expressing two additional putative sulfate transporters, Rv1739c and Rv1707 [51, 90]. In E. coli, Rv1739c is shown to be associated with increased up take of sulfate. However, when the M. boviscysA1 mutant was complemented with the Rv1739c gene, sulfate prototrophy was not restored [90]. This suggests that the pathogen relies on alternative biochemical pathway to generate cysteine during critical times. In agreement with this hypothesis, recent biochemical studies established an alternative route for cysteine biosynthesis in M. tuberculosis, which utilizes the O-phosphoserine as carbon skeleton [120, 121]. It is possible that this pathway may be contributing to the survival of the mutant cysA1 strain of M. bovis.
Adenosine-5’-phosphosulfate (APS) reducatse, cysH, catalyzes the first committed step in sulfate assimilation pathway. CysH catalyzes the formation sulfite and adenosine-5’-phosphate (AMP) from APS [83]. The resulting sulfite is further converted into sulfide, which is the required form of sulfur for biosynthesis of cysteine, mycothiol and other sulfur-containing metabolites. It has been shown that AMP is a potent inhibitor of APS reductase activity [122] which may indicate that this reaction byproduct negatively regulates cysH to control the downstream biosynthesis of sulfur-containing metabolites. The 3’-phosphoadenosine-5’-phosphatase, cysQ, is another regulator of M. tuberculosis sulfur metabolism. CysQ dephosphorylates both 3’-phosphoadenosine-5’-phosphate (PAP), and its counterpart3’-phosphoadenosine-5’-phosphosulfate (PAPS) which is utilized in the sulfation of biomolecules [62, 123]. It has also been shown that PAP is an inhibitor of at least one sulfotransferase [124] and that PAPS accumulation being cytotoxic [125]. Recent studies demonstrate that cysQ activity is inhibited by alkali metal cations in vitro at physiological concentrations in Streptococcus mutans [126]. Taken together, these studies demonstrate that the dephosphorylation of PAP and PAPS by cysQ regulates sulfation and balances sulfur utilization in defense mechanisms.
Sulfotransferases and sulfatases are the major enzymes responsible for sulfate transfer and removal processes. It has been reported that the sulfotransferases, stf0 and stf3, from M. tuberculosis are involved in the biosynthesis of outer envelope molecules termed SL-1 and S881 [127, 128]. Sulfatases hydrolyze sulfate esters from sulfated proteins, peptides and arylsulfates [129]. The M. tuberculosis genome encodes six type 1 sulfatases which are characterized as having a unique active site formylglycine residue used for catalysis. The formylglycine is either co- or post-translationally installed in the active site by the formylglycine activating enzyme. Recent studies identified a sulfatase, atsG, which possesses aryl sulfatase activity. However, the precise role of this sulfatase is not yet established [130]. In the absence of formylglycine activating enzyme, M. tuberculosis retains sulfatase activity indicating that M. tuberculosis possesses formylglycine independent sulfatases [62, 131]. Given the importance of sulfur compounds under stress conditions, it is possible that the mycobacterial sulfatases may play a role in scavenging the residual sulfate from the nonessential metabolites.
SULFOTRANSFERASES AND SULFATION
Sulfotransferases (STs), are the enzymes that install sulfate esters, transfer sulfate from PAPS (produced by the SAC) to a hydroxyl or, less frequently, to an amide moiety on glycoproteins, glycolipids and metabolites [see Fig. 3] [132]. Sulfated metabolites are abundant in higher eukaryotes, particularly mammals, where they function primarily in cell-cell communication. For example, sulfated glycoproteins mediate interactions of leukocytes with endothelial cells at sites of chronic inflammation, sulfated peptides such as hirudin and cholecystokinin act as hormones, and sulfated glycolipids are involved in neuronal development [133, 134]. In contrast, reports of sulfated metabolites in prokaryotes have been rare. In 1992, Long et al. reported the first functionally characterized sulfated metabolite from the prokaryotic world – the nodulation factor NodRm-1 from Sinorhizobium meliloti [135]. This sulfated glycolipid is secreted from the bacterium and acts on host plant cell receptors thereby initiating symbiotic infection [136].
Among pathogenic bacteria, only one family has been reported to produce sulfated metabolites – the Mycobacteria. More than 40 years ago, Goren and coworkers isolated an abundant sulfated glycolipid from the M. tuberculosis cell wall and characterized its structure as shown in Fig. 2 [137, 138]. Termed as sulfolipid-1 or SL-1, this compound has only been observed in the tuberculosis complex; it is absent in non-pathogenic mycobacteria such as M. smegmatis. Comprising a trehalose-2-sulfate (T2S) core modified with four fatty acyl groups, SL-1 accounts for almost 1% of the dry weight of M. tuberculosis. Early studies found a correlation between the abundance of SL-1 and the virulence of different clinical M. tuberculosis isolates [139, 140] and its location in the outer envelope has prompted speculation that it may be involved in host-pathogen interactions [141]. The possible link between SL-1 and M. tuberculosis virulence led to the search for the exact functions of SL-1. Indeed. SL-1 has been attributed to altering phagosome-lysosome fusion, disrupting oxidative phosphorylation and controlling cytokine and reactive oxygen species produced by human leukocytes in cell culture models [142–147]. However, the biosynthetic pathway for SL-1 has recently been elucidated [88, 147–150] and knockout studies of SL-1 biosynthesis (∆stf0) have revealed that M. tuberculosis strains lacking SL-1 exhibited enhanced intracellular survival in human but not in murine macrophages suggesting a role for SL-1 in M. tuberculosis virulence in host specificity [151, 152].
Recent genetic and biochemical studies identified two integral membrane proteins, Chp1 and Sap (corresponding to gene loci Rv3822 and Rv3821) associated with transfer of acyl group regioselectively to SL-1278 in two successive reactions to yield tetra acetylated product, SL-1 (see Fig 2)[147]. These data indicate that Chp1 aids in the biosynthesis of SL-1 within the cytosolic compartment. Then Sap, together with MmpL8 (sulfolipid transporter) transports SL-1 across the membrane. The mechanism Chp1 localization and the coupling of biosynthesis/transport via MmpL-8 indicate formation of complex macromolecular protein complex to facilitate the function. However, the precise mechanism by which MmpL-8 and Sap transport SL-1 is unknown [61, 153].
In addition to SL-1, other novel sulfated metabolites have also been identified in M. tuberculosis using an innovative metabolomic approach that combines genetic engineering, metabolic labeling with a stable sulfur isotope (34SO42−) together with mass spectrometry analysis [154] [see Fig. 2]. Structurally distinct sulfated metabolites have also been identified in several other mycobacterial species, including M. smegmatis, M. fortuitum, and the HIV-associated opportunistic pathogen M. avium [see Fig. 2] [154–157]. Interestingly, in M. avium a sulfated cell wall glycopeptidolipid was recently found to be up-regulated in HIV patients with acquired drug resistance [155]. Significant work remains to fully characterize and elucidate the biological significance of sulfated metabolites found in mycobacteria. A major step toward this objective is to define the biosynthetic pathways of mycobacterial sulfated metabolites, including the STs responsible for installing the sulfuryl moiety.
In 2002, an analysis of mycobacterial genomes reported by Mougous et al. revealed a large family of open reading frames with homology to human carbohydrate sulfotransferases [132]. The predicted proteins shared regions of sequence homology associated with binding to their common substrate, PAPS. Presently, four such genes have been identified in M. tuberculosis (annotated as stf0–3) and the M. avium genome encodes nine putative STs (stf0, 1, 4–10) [88]. To date, of the 11 predicted STs found in mycobacterial genomes, genetic and biochemical studies have only been reported for stf0, stf3 and stf9.
Stf0 is present in a number of other pathogenic bacteria and initiates the biosynthesis of SL-1 by sulfating the disaccharide, trehalose, to form T2S [see Fig. 2 and 3] [150]. The structure of stf0 in complex with trehalose has recently been reported and has revealed several interesting features [150]. In the presence of trehalose, stf0 forms a dimer both in solution and in the crystal structure. Moreover, stf0-bound trehalose participates in the dimer interface, with hydroxyl groups from a glucose residue bound in one monomer forming interactions with the other monomer. Residues involved in substrate binding and dimerization have been identified, along with a possible general base (i.e., Glu36) that may facilitate nucleophilic attack of the 2’-hydroxyl group on PAPS. A panel of synthetic glucose and trehalose analogs has also been tested for binding and it was found that any modification to the parent disaccharide compromises substrate sulfation [150]. A kinetic study of the enzyme using MS revealed the order of substrates binding which is consistent with a random sequential mechanism involving a ternary complex with both PAPS [or 3’-phosphoadenosine-5’-phosphate, (PAP)] and trehalose (or T2S) bound in the active site [158].
Stf3 may play a regulatory role in M. tuberculosis virulence [159]. In a mouse model of TB infection, a mutant strain in which stf3 was disrupted (∆stf3) was unable to produce a sulfated molecule termed, as “S881”. Interestingly, when compared to wild-type M. tuberculosis, ∆stf3 exhibited a hyper virulent phenotype indicating that stf3 may negatively regulate virulence through the synthesis and cell surface localization of S881 [128].
Stf9 shows higher similarity to human heparan sulfate 3-O-sulfotransferase isoforms compared to bacterial STs [160]. Stf9 possesses the characteristic of PAPS binding motif inherent to sulfotransferases and can transfer a sulfate group from p-nitrophenolsulfate onto 3'-phosphoadenosine-5'-phosphate. Stf9 is also capable of transferring a sulfate group from PAPS onto certain acceptor substrates in E. coli. [161]. Recently the crystal structure of stf9 in complex with a sulfate ion was solved and a possible mechanism for sulfation was proposed [160]. Despite this advance, the actual substrate for stf9 remains unknown. No other relatives of the remaining Stf family members are found in any other prokaryotic genomes, suggesting that they are unique to mycobacteria. Substrates for the majority of mycobacterial STs remain to be elucidated.
Historically, sulfotransferase assays have often been conducted using 35S labeled substrate in combination with chromatography, electrophoresis, or filter binding [162–165]. Non-radioactive assays using spectrophotometry and mass spectrometry have also been reported [166–168]. Recently, Prather et al. developed a universal phosphatase-coupled sulfotransferase assay. In this method, Golgi-resident PAP-specific 3-phosphatase (gPAPP) is coupled to a sulfotransferase reaction by release of 3′-phosphate from PAP. The released phosphate is then detected by malachite green [169]. The enzyme kinetics of gPAPP allowed them to calculate coupling rate (i.e. the ratio of product-to-signal conversion) of the coupled reaction. Using this method, Michaelis–Menten constants were obtained for human carbohydrate sulfotransferase (CHST10) and cytosolic sulfotransferase (SULT1C4) with the substrates phenolphthalein glucuronic acid and α-naphthol, respectively. The activities obtained with the method were also validated by performing simultaneous radioisotope assays [169]. Thus this assay eliminates the requirement for radio-labeled substrates and should accelerate drug discovery campaigns for sulfotransferase targets.
ST Inhibitor Discovery
Although the roles of sulfated metabolites in the mycobacterial lifecycle remain under investigation ([88] and references therein), the analogy to sulfation in higher eukaryotes is compelling. The challenges to defining their role in mycobacterial infection and survival are two-fold: (1) the collection of sulfated metabolites must be identified and structurally characterized; and, (2) the biosynthetic pathway of the sulfated metabolites must be elucidated. In addition to traditional genetic approaches, small molecule inhibitors of STs in mycobacteria would also be useful tools to dissect their physiological roles. In addition, since STs play critical biological roles in higher eukaryotes and are implicated in several disease states, they also represent promising therapeutic targets [133, 170]. Since prokaryotic STs have not been discovered until relatively recently, the majority of research and inhibitor discovery has focused primarily on eukaryotic STs. Nonetheless, these studies can serve as a platform for mycobacterial ST inhibitor design and the most fruitful efforts to date have been highlighted.
There are two classes of STs - cytosolic and Golgi-resident enzymes [132, 133, 171]. In general, cytosolic STs sulfonate small molecules such as hormones and bioamines while membrane-bound STs prefer larger substrates such as proteins and carbohydrates. STs have also been further classified according to their functional role into estrogen STs (EST), heparin STs, tyrosyl protein STs (TPST), N-Acetyl glucosamine 6-O-ST and carbohydrate STs. The first crystal structure to be elucidated was that of murine estrogen sulfotransferase (mEST) in 1997 [172] and since then, structures of nine other STs have been characterized. These include cytosolic STs such as Phenol ST (SULT1A1) [173], catecholamine ST (SULT1A3) [174], mycobacterial stf0 [150] and Golgi-resident STs (GSTs) such as heparan N-deacetylase-N-ST-1 (NDST-1) [175]. Structures of STs in complex with PAPS or PAP reveal a conserved nature of the cofactor binding site, suggesting that STs share a similar mechanisms of sulfuryl transfer. The catalytic site of each ST must also accommodate diverse substrates and these differences in specificity are reflected in the substrate-binding site of each ST [170].
Bisubstrate analogs
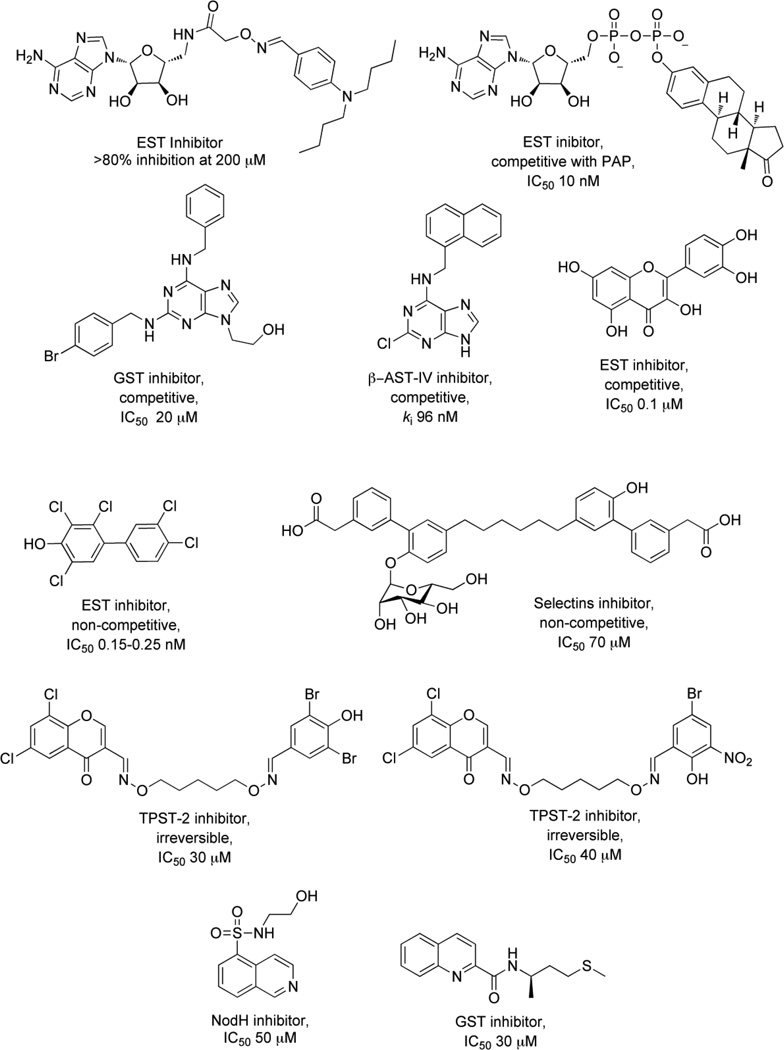
To investigate molecules that inhibit both the PAPS- and substrate–binding domains of STs simultaneously, synthetic bisubstrate analogs have been employed [176, 177]. Compounds were designed to incorporate elements from the cofactor, PAPS and the substrate, providing specificity via critical interactions within both binding pockets of the enzyme [178]. Inhibitor potency is achieved from the entropic advantage of linking structures that mimic each substrate. On screening a 447 member 3’-phosphoadenosine library, several bisubstrate-based compounds were identified (1) [176], (2) [177] as inhibitors of EST (see Fig. 4). The activities of these compounds were comparable to some of the other compounds known to be inhibitors of EST including polychlorinated biphenols (3), discovered by testing a large number of hydroxylated polychlorinated biphenyl metabolites [179] and dietary agents like Quercetin (4), identified from a study investigating the inhibitory effects of natural flavonoids on EST activity (see Fig. 4) [180].
Figure 4.
Sulfotransferase (ST) inhibitors
Similar substrate-emulating approaches have also been used to design inhibitors for E-, P- and L-selectins, all prime targets for anti-inflammatory drug discovery [181]. GSTs are involved in biosynthesis of the L-selectin ligand, 6’-sulfo sialyl Lewis X [182]. The sulfonation of sialyl Lewis X motif by GST leads to a strong interaction with receptors on L-selectin cell adhesion molecules resulting in a potent anti-inflammatory response. A “glycomimetic” strategy was used to design inhibitors for these STs. In this approach, the inhibitors retained structural and functional aspects of the natural ligands, but were designed to be synthetically more feasible [183]. One selectin antagonist (5), was identified using this strategy and it is currently under clinical trials (see Fig.4) [184].
Kinase-Derived Inhibitors
The “kinase inhibitor” approach exploits the similarity between reactions catalyzed by STs and kinases. Since STs and kinases use adenosine-based donor nucleotides to transfer an anionic moiety onto their respective substrates (PAPS for STs and ATP for kinases), it was proposed that ATP derivatives might also function as ST inhibitors [133, 185]. Furthermore, the hydrophobic adenine binding pockets of EST [172, 186] and heparin N-sulfotransferases [175] are similar to those of several kinases. A 2, 6, 9-trisubstituted purine library [187], originally designed to target cyclin dependent kinase 2, was tested for inhibitory activity with carbohydrate STs. Of the 139 compounds screened, the six most potent purines exhibited half maximal inhibitory concentrations (IC50s) that ranged from 20 – 40 µM (6) [185], with five of them having a common benzyl substituent at N6 (see Fig. 4). Though these inhibitors showed selectivity for carbohydrate STs, achieving selectivity over kinases still remains a challenge. A high throughput screen of 35,000 purine and pyrimidine analogs has also identified a potent inhibitor of β–arylsulfotransferases (β-AST-IV) (7) [188] (see Fig. 4).
A second class of kinase inhibitors, isoquinoline sulfonamides, has also been tested for inhibitory activity against a panel of STs consisting of EST, NodH, GST-2 [189]. Isoquinoline sulfonamide inhibitors were developed after a crystal structure of cyclic adenosine-5’-phosphate (cAMP) dependent protein kinase in complex with isoquinoline showed that the heterocycle moiety was bound in the subsite occupied by the adenine ring of ATP. Among 100 isoquinoline and quinoline derivatives screened, the most active compounds inhibited single enzyme selectively with modest IC50 values in the range of 30 – 100 µM (8, 9) [170, 190] (see Fig. 4).
Combinatorial Target-Guided Ligand Assembly
In this strategy, a library of ligands or ‘monomers’ carry a common chemical handle to facilitate their combinatorial assembly [190]. In the first round, monomers were screened against the ST target at concentrations of 1 mM or higher. Compounds that demonstrated inhibitory activity were then used to construct a library of ‘dimers’ via an oxime linkage, and were screened for inhibitory activities. This approach resulted in the identification of two of the first known inhibitors of Golgi-resident tyrosyl protein ST-2 (TPST-2) (10, 11) [190] (see Fig. 4).
ST inhibitors identified in the studies above are a promising start in drug discovery efforts. However, to date the majority of ST inhibitor compounds possess fairly modest IC50s, are neither “drug-like”, nor suffer from a lack of specificity. Recent advances in structure-based drug design and high-throughput screening should greatly facilitate the discovery of new inhibitors for STs and other sulfonucleotide-binding enzymes.
OXIDATIVE MACROPHAGE ANTIMICROBIAL ACTIVITY
In order to replicate and persist in its human host, M. tuberculosis must survive within the hostile environment of the macrophage, where bactericidal oxidants – superoxide (O2·−) and nitric oxide (NO·) – are generated in response to infection [191]. Two enzymes, nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) and inducible nitric oxide synthase (NOS2), are largely responsible for production of these reactive oxygen and nitrogen intermediates (termed as ROI and RNI, respectively) [192, 193].
NADPH oxidase is a membrane protein that generates O2·− by transferring electrons from NADPH inside the cell across the phagosomal membrane; the electrons are coupled to molecular oxygen to produce O2·− [194]. Subsequently, O2·− can accept an electron spontaneously or can be reduced by superoxide dismutase (SOD) to form hydrogen peroxide (H2O2) [195]. In turn, H2O2 can oxidize cellular targets or can be converted into the highly damaging hydroxyl radical (OH·) through the iron-catalyzed Fenton-Haber-Weiss reaction in which solvent accessible ferrous iron (Fe+2) is oxidized by H2O2 to yield (OH·) [196]. Because Fe+2 is capable of localizing to DNA, proteins and lipids, generation of OH· may occur in the immediate vicinity of these biomolecules. Thusly generated hydroxyl radicals indiscriminately attack nearby proteins, lipids, iron-sulfur clusters and DNA in a cytotoxic or mutagenic manner to induce cell death. Unfortunately, there does not exist any known enzyme to detoxify the cellular hydroxyl radicals [38]. It has been shown that Fe+2 can specifically bind to sequences that exist in the operator sites and promoter regions of DNA where the iron regulatory transcription factor, Fur, binds and has been implicated in the iron homeostasis [197, 198]. Thus, these regions are at high risk for oxidative modifications through hydroxyl radicals.
In the NOS2 reaction, the guanidino nitrogen of arginine undergoes a five-electron oxidation via a N-ω-hydroxy-L-arginine (NOHLA) intermediate to yield ·NO [199]. The reaction of ·NO with biological thiols can lead to S-nitrosylation, or to sulfenic acid, or to disulfide formation. However all of these forms are potentially reversible changes [200]. The combination of the two oxidant-generating systems can also exert a synergistic effect in bacterial killing as macrophages can generate O2·− simultaneously with ·NO, yielding the more reactive peroxynitrite (ONOO−) [201]. Peroxynitrite has pKa of 6.8 at 37 °C and peroxynitrous acid (OONOH) is unstable with half life of under 1.0 second and readily decomposes to give OH· and nitrogen dioxide (·NO2) [200, 202]. Peroxynitrite is a highly reactive oxidant capable of oxidizing deoxyribose and dimethylsulfoxide with high yields at acidic pH [200]. It has been shown that peroxynitrite anion oxidizes sulfhydryls 103 times faster than H2O2 and proposed that this reaction might be the important mechanism of oxygen radical mediated toxicity [202]. The other product of peroxynitrite decomposition is nitrogen dioxide which is also a strong oxidant with significant cytotoxic potential [200]. A consequence of NADPH oxidase and NOS2 enzymatic activities and the resulting “oxidative burst” is that phagocytosed bacteria are killed by oxidative damage to a range of protein and DNA targets [191, 199, 203].
In mice, activation of macrophages induces production of NOS2 and phagosomal NADPH oxidase, via ligation of toll-like receptors (TLRs), or via stimulation by the cytokines IFN-γ or TNF-α [204, 205]. In mouse models of TB, numerous studies have demonstrated that NOS2 plays an essential role in controlling persistent infection. Macrophages can inhibit mycobacterial growth via NOS2-generated RNI, inhibition of NOS2 during persistent infection leads to reactivation of disease, and NOS2 gene-disrupted mice are extremely susceptible to TB infection [204, 205]. More recently, a proteomics study has identified proteins in M. tuberculosis that are targeted by RNI stress [206]. Notably, many essential metabolic and antioxidant defense enzymes are among those proteins found modified for RNI.
While good evidence exists for ROI-mediated bacterial killing of other bacterial, fungal and parasitic pathogens, their bactericidal effect on mycobacteria has been less clear. Studies demonstrate that M. tuberculosis resists killing by ROI in vitro and that mice with defects in p47 or gp91 subunits of phagocyte NADPH oxidase (Phox) are also relatively resistant to TB infection [204, 207]. However, NADPH oxidase is highly active during the persistent phase of M. tuberculosis infection in mice [208]. This observation suggests that M. tuberculosis must possess extremely effective detoxification pathways to counter ROI stress. Consistent with this hypothesis, mice deficient in the KatG catalase-peroxidase survived longer than pg91phox-deficient mice [208]. More recently, it was shown that macrophages deficient in early stages of Phox assembly exhibited reduced bacterial killing, correlating with decreased production of ROI [209]. Taken together, these observations indicate that survival of M. tuberculosis within macrophages depends upon the ability of the bacteria to counter oxidative assault.
Mycobacteria produce enzymes such as SOD, peroxidases, catalases, and nitrosothiol reductases to help counteract the effect of ROI/RNI and promote intracellular survival and persistence in the host [191, 210–212]. The analysis of the M. tuberculosis genome has revealed that M. tuberculosis lacks classical redox sensors such as fumarate/nitrate reduction regulator (FNR), oxygen binding heme protein, FixL, and peroxide stress induced transcriptional regulatory protein OxyR [213]. However, recent studies have established that M. tuberculosis possesses some unique redox sensors, such as heme-based DosS and DosT. These sensors can detect different types of redox stress, including hypoxia, nitric oxide, carbon monoxide, and the intracellular redox environment [213]. In addition to enzymatic detoxification of ROI and RNI, reduced sulfur-containing metabolites are an essential component of bacterial antioxidant defense systems [214–218]. Specifically in mycobacteria, low molecular-weight thiols such as mycothiol [see Fig. 1], play a central role in maintaining a reduced cellular environment [214, 219]. Proper redox homeostasis is essential for normal cellular function and to mitigate the effects of oxidative stress. Hence, the metabolic route used for the production of reduced sulfur-containing metabolites [see Fig. 3] is predicted to be important for mycobacterial survival [88, 220, 221]. Consistent with this hypothesis, expression of mycobacterial genes involved in reductive sulfate assimilation is induced by oxidative stress within the environment of the macrophage [47, 222–230].
THE ROLE OF ROI/RNI IN MODULATING CIDAL ACTIVITY OF ANTIMYCOBACTERIAL DRUGS
Bacteria growing aerobically generate ROS/RNS as metabolic byproducts, which are detoxified by specific intracellular mechanisms. Recent studies with E. coli showed that amplification of endogenous ROS/RNS production and inhibition of detoxification/repair by manipulating metabolism increase the sensitivity of the pathogens to antibiotics [231]. During in vivo infection, M. tuberculosis is also exposed to varying amounts of oxygen at different stages ranging from 100 mm Hg in the alveolus to 60 mm Hg in normal lung and 3mm Hg in the center of granuloma [232]. The survival of mycobacteria at 3 mm Hg in granuloma indicates that the pathogen balances the severe hypoxia imposed by the host immune system with its own defense mechanisms. Any shift in the pathogen’s redox balance towards higher intracellular ROS/RNS could exceed the threshold level for ROS/RNS tolerance. Thus, there is a possibility to improve the bactericidal activity of the antibacterial agents by selectively modulating pathogen ROS/RNS metabolism.
Recent studies conducted by Bulatovic et al. support the hypothesis that increased oxidative stress may augment the susceptibility of M. tuberculosis to Isoniazid (INH) [233]. INH is the first line antibiotic used to treat TB. Notably, INH is a pro-drug and requires oxidative activation by the catalase-peroxidase hemoprotein, KatG [234]. When INH is combined with clofazimine and/or plumbagin (compounds capable of generating super oxide), it lowers the MIC of INH for M. tuberculosis H37Rv [233]. Biochemical studies indicate that mutations in the KatG result in impaired activation of INH and, thereby, the pathogen becomes resistant to INH. Interestingly, however, clofazimine and plumbagin, in combination with INH are able to inhibit an S315T KatG mutant of M. tuberculosis. Since clofazimine alone has antimycobacterial activity, these studies raise the possibility of using both drugs in combination to treat TB [233].
Like INH, Pyrazinamide (PZA), is also a frontline antitubercular drug and requires activation by nicotinamidase/pyrazinamidase of M. tuberculosis for conversion into the active pyrazinoic acid (POA) [235]. Recent studies indicate that pyrazinamide treatment induces the production of hydroxyl radicals and other ROS in bacteria under slightly acidic pH (~6.0–6.5), analogous to that observed in macrophages of the granuloma [236]. Thus Kim et al. have shown that up-regulation of hydroxyl radicals by INH and PZA may initiate autophagy for effective antimycobacterial action [236]. ROS release during chemotherapy has been observed in both TB patients and experimental studies. A combination of antibiotics, INH, PZA and rifampin significantly increased plasma allantoin, an ROS marker, in TB patients [237]. Another study of TB infection in animal models has shown that INH and rifampin induce a significant respiratory burst involving NADPH oxidase and NOS [238] and the generated ROS are implicated in bactericidal activity in vivo [236, 239–242]. Thus, ROS generation may be induced by antimycobacterial drugs and that ROS production can be augmented using different drug combinations.
One challenge in TB treatment is to eradicate the persistent population. Recent biochemical studies conducted by Grant et al. have demonstrated that persistent cells constitute a distinct subpopulation within the larger culture population. They found that survival of persistent cells requires a small (i.e. ~20%) drop in the dissolved oxygen. If the dissolved oxygen is maintained at high levels this population is killed over time. Higher dissolved oxygen may increase ROS production during infection, leading to the killing persisters [239]. Supporting this hypothesis, they found that the hydroxyl radical scavenger, thiourea, protects persisters at high levels of dissolved oxygen. Conversely, clofazimine, which produces ROS, successfully eradicates the persistent population [239]. These data suggest that persistent population has differential susceptibility to antibiotic-induced hydroxyl radicals compared with the larger antibiotic susceptible population.
There is a growing evidence for the critical role of ROS and oxidative damage in bactericidal action of antibiotics. This understanding of how bactericidal antibiotics result in cell death raises the hypothesis that drug tolerance may be mediated by pathogens own abilities to detoxify the ROS and the surrounding oxygen levels [239]. Thus, next generation approaches to antibiotic discovery may focus on identifying small molecules that potentiate hydroxyl radical formation or inhibit the molecular mechanisms that detoxify the intracellular oxidative stress. Such drugs might play a major role in eradicating the persistent population of M. tuberculosis from the host.
SULFATE REDUCTION
APS reductase (encoded by cysH) catalyzes the first committed step in the biosynthesis of reduced sulfur compounds [see Fig. 3]. In this reaction, APS is reduced to SO32− and adenosine-5’-phosphate (AMP) [243]. Thioredoxin (Trx), a 12.7 kDa protein with a redox active disulfide bond, supplies the reducing potential necessary for this two-electron reduction [244]. The SO32− product of this reaction is reduced further to S2−, which is used for the biosynthesis of reduced sulfur-containing metabolites, such as cysteine, methionine, CoA, iron-sulfur clusters and mycothiol [245, 246] [see Fig. 1]. Consistent with its important metabolic role, APS reductase was identified in a screen for essential genes in M. bovis BCG [226] and cysH was actively expressed during the dormant phase of M. tuberculosis and in the environment of the macrophage [222, 229].
Humans do not reduce sulfate for de novo cysteine biosynthesis and therefore, do not have a CysH equivalent. Thus, APS reductase may be an attractive drug target if the enzyme is required for bacterial survival or virulence in vivo [88, 220, 221, 247]. To test this hypothesis, Senaratne et al. generated an M. tuberculosis mutant strain lacking cysH (∆cysH) [220]. As predicted, the mutant strain was auxotrophic for cysteine and could only be grown in media supplemented with this amino acid, methionine or glutathione (from which cysteine can be generated catabolically). The cysH mutant exhibited attenuated virulence in BALB/c and C57BL/6 immunocompetent mice. Growth kinetics in the lungs, spleen and liver of mice infected with ∆cysH or wild-type M. tuberculosis were also quantified. Strikingly, the number of colony-forming units recovered from the ∆cysH mutant mirrored those of wild-type M. tuberculosis during the acute stage of infection [up to 16 days post-infection (pi)]. However, the number of viable bacteria in the mutant became significantly less (i.e., by 3 orders of magnitude) coincident with the emergence of adaptive TH1-mediated immunity and the induction of persistence in the mouse (between 16 and 42 days pi) [248]. In addition, ∆cysH was highly compromised in the liver, where the host’s oxidative antimicrobial response is thought to play an especially important role in antimicrobial defense. Since the replication of ∆cysH in mouse tissues during the first 16 days persistent infection was identical to that of wild-type, these data suggest that mouse tissues can provide M. tuberculosis with sufficient reduced sulfur-containing amino acids (e.g., cysteine and methionine), for initial growth (see discussion below) [88, 220, 249, 250]. Hence, APS reductase activity appears to be dispensable during the acute phase of infection, but indispensable in the later, the persistence phase where access to or supply of reduced sulfur-containing nutrients becomes limiting [220].
As discussed above, NOS2 plays a vital role in controlling persistent M. tuberculosis infection in mice [251–253]. In order to determine and test the role of APS reductase in protecting the bacteria against the effects of NOS2, NOS2−/− mice were infected with wild-type and ∆cysH M tuberculosis [220]. In contrast to the observation made in wild-type mice, ∆cysH did not lose viability after the first 21 days pi in NOS−/− mice; all mice succumbed to infection within 26 to 31 days. Thus, ∆cysH is significantly more virulent in the absence of NOS2. Taken together, these studies indicate that APS reductase plays a central role in protecting M. tuberculosis against the effects of reactive nitrogen species produced by NOS2 and is critical for bacterial survival in the persistence phase of infection in mice [220]. Furthermore, a follow-up study demonstrates that immunization of mice with ∆cysH generates protection equivalent to that of the BCG vaccine in mice infected with M. tuberculosis [254].
Attenuation of ∆cysH in a mouse model of M. tuberculosis infection and the importance of APS reductase in mycobacterial persistence further motivated investigation of the molecular details of the reaction catalyzed by APS reductase [220]. Biochemical, spectroscopic, mass spectrometry and structural investigation of APS reductase support a two-step mechanism, in which APS undergoes nucleophilic attack by an absolutely conserved cysteine to form an enzyme S-sulfocysteine intermediate, E-Cys-Sγ–SO3− [220, 247, 255–257]. Positively charged amino acids in the active site, including His252, Lys145, Arg237, and Arg240, are likely candidates for stabilization of the thiolate in the active site [258]. In a subsequent step, SO32− is released in a Trx-dependent reaction. During the catalytic cycle, nucleophilic attack at Sγ atom of the S-sulfocysteine intermediate results in the transient formation of a mixed disulfide between Trx and APS reductase, with concomitant release of sulfite. The structure of this complex has recently been reported and reveals a unique protein-protein interface as a potential candidate for disruption for small molecules or peptide inhibitors [259].
In addition to the conserved catalytic cysteine, the primary sequence of APS reductase is also distinguished by the presence of a conserved iron-sulfur cluster motif, -CysCys-X~80-CysXXCys- [221, 255]. Biochemical studies demonstrate that the four cysteines in this motif coordinate a [4Fe-4S] cluster, and that this cofactor is essential for catalysis [247, 255]. The first structure of an assimilatory APS reductase was recently reported, with its [4Fe-4S] cluster intact and APS bound in the active site [256]. Consistent with prior biochemical observations, the structure revealed that APS binds in close proximity to the iron-sulfur center. Progress in this area has been hampered by the failure to generate a paramagnetic state of the [4Fe-4S] cluster that can be studied by electron paramagnetic resonance (EPR) spectroscopy. Recently Bhave et al. overcame this bottleneck and reported the EPR characterization of M. tuberculosis APR in the [4Fe-4S+] state and identified an essential role for the active site residue Lys-144, whose side chain interacts with both the iron-sulfur cluster and the sulfate group of adenosine 5’-phosphosulfate. On the basis of the data, the co-factor is believed to play a role in pre-organizing active site residues and in substrate activation [260, 261]. Thus compounds that target the metal site and/or nucleotide-binding site may represent promising approaches toward rational inhibitor design. This approach is actively being explored, as well as inhibitors that target the Trx-APS reductase interface and will be reported in due course [262].
The final step in sulfate reduction, the six electron reduction of SO32− to S2−, is catalyzed by sulfite reductase (encoded by nirA) [see Fig. 3] [263]. Like cysH, nirA is an essential gene [226] and is active during the dormant phase of M. tuberculosis [222, 229]. The sulfite reductase in M. tuberculosis belongs to the family of ferredoxin-dependent sulfite/nitrite reductases [263]. These enzymes contain a [4Fe-4S] center and a siroheme. In this reaction, the external electron donor (likely ferredoxin) binds transiently to sulfite reductase and transfers electrons to the [4Fe-4S] center, one by one. Subsequently, sulfite reduction is accomplished by transferring electrons from the cluster to the siroheme, which coordinates the sulfite substrate. In 2005, Schnell and coworkers reported the structure of M. tuberculosis nirA [263]. Interestingly, the structure depicts a covalent bond between the side chains of residues Tyr69 and Cys161 adjacent to the siroheme in the active site of sulfite reductase. Site-directed mutagenesis of either residue impairs catalytic activity, though their involvement in the mechanism of sulfite reduction is presently unknown [263]. However, recent site directed mutagenesis studies by Smith et al. indicate that the first three protons come from solvent, either as part of the HSO3− anion or from ordered active site waters. While the last three come from Lys215, Arg153 and Lys217, whereas Asn149 and Arg153 play a role in the structure of the flexible loop that controls anion binding and release. Arg83 is primarily responsible for siroheme binding. Together, the study revealed specific roles for each active site residue in anion binding and in coupled proton transfer that facilitates electron transfer for reduction of sulfite to sulfide [264].
CYSTEINE BIOSYNTHESIS
De novo cysteine biosynthesis in mycobacterium occurs via condensation of S2− with O-acetyl-L-serine (cysE, a serine acetyl transferase, catalyzes the condensation of serine with acetyl group to form O-acetyl-L-serine which acts as the source of the carbon skeleton for biosynthesis of cysteine) by O-acetylserine sulfhydrylase [245, 246] [see Fig. 3]. The M. tuberculosis genome contains three O-acetylserine sulfhydrylase genes, cysM, cysK and cysM3 that can catalyze this reaction. Notably, cysE and cysM are essential for survival in a mouse model of M. tuberculosis infection or in primary macrophages, respectively [225, 228]; cysM is also up-regulated under oxidative stress conditions [223]. Cysteine is an important intermediate in biosynthesis of many important sulfur containing metabolites such as methionine, mycothiol, iron-sulfur clusters and other co-factors. Perhaps to avoid the toxicity as a result of accumulation of high levels of cysteine, M. tuberculosis might be evolved to convert and store the excessive cysteine into less reactive methionine and non-toxic mycothiol.
Alternative cysteine biosynthesis in M. tuberculosis
In 2005, Burns et al. presented an in vitro evidence for an additional pathway to make cysteine from sulfide [see Fig. 5] [265]. The cysM (Rv1336)-dependent pathway utilizes O-phospho-L-serine (OPS) and a sulfide carrier protein, cysO (Rv1335)-thiocarboxylate (cysO-SH), resulting in a cysO-cysteine adduct that is hydrolyzed by the carboxypeptidase mec, (Rv1334) releasing L-cysteine and regenerating CysO (15). OPS is synthesized from 3-phosphoglycerate (3PG) in two steps by serA (serA1, Rv2996c; serA2, Rv0728c) and serC (Rv0884c) [121, 266]. However, the precise sulfur source for the sulfur carrier protein remains unclear. Recent mechanistic and kinetic studies revealed that cysM proceeds through a stable α-aminoacrylate intermediate and showed that the cysM has 500-fold greater specificity for O-phospho-L-serine than for O-acetyl-L-serine, suggesting that O-phospho-L-serine is the likely substrate in vivo and the carbon skeleton donor in this cysteine biosynthetic pathway [267].
Figure 5.
Alternate cysteine biosynthetic pathway.
The presence of this pathway in M. tuberculosis is also supported by the analysis of the genome of this pathogen, which reveals the necessary genes for biosynthesis of phosphoserine, such as D-3-phosphoglycerate dehydrogenase SerA1, (Rv2996c) [268] and phosphoserine aminotransferase serC (Rv0884c) present in the genome. A transposon mutagenesis study further suggested that serA1 and serC, are essential for M. tuberculosis [50]. The proteins cysM (Rv1336), cysO (Rv1335), and mec (Rv1334) operating in the OPS-dependent cysteine biosynthesis pathway are encoded within the same transcriptional unit in the H37Rv genome [120]. This operon organization is a common feature of several species in the Actinomycetales group comprising Corynebacteria, Streptomyces, and Mycobacteria, again suggesting that this pathway is also operational in species other than M. tuberculosis [121].
An appealing feature of this pathway is that a protein-bound thiocarboxylate would be much more stable to oxidative species in the macrophage, relative to free sulfide [265]. Analysis of mRNA expression demonstrates that each of these genes is upregulated during exposure to toxic oxidants [223]. The existence of an alternative pathway for cysteine biosynthesis, independent of O-acetyl serine as carbon skeleton, has implications for attempts to inhibit biosynthesis of this metabolite as a means of pathogen control. Inhibition of only one of these pathways may not be sufficient to kill this pathogen, as already indicated by cysM mutants that are attenuated in macrophages but still survive [48]. Complete inhibition of this pathway thus may require at least two inhibitors for the two different branches of biosynthesis of this amino acid [121].
Like most organisms, mycobacteria do not have large pools of free cysteines [249]. Once cysteine is produced it is rapidly utilized in protein synthesis, or for the biosynthesis of methionine and reduced sulfur containing Fe-S cofactors [See Fig. 1]. The most abundant thiol metabolite in mycobacteria (present in millimolar concentrations) is mycothiol [269]. Found in all actinomycetes, mycothiol is essential for M. tuberculosis survival and intracellular levels of this thiol are associated with changes in resistance to antibiotics and oxidative stress [219].
BIOSYNTHESIS OF IRON-SULFUR CLUSTER
Fe-S cofactors are involved in the electron transfer, enzymatic catalysis, maintenance of protein structure, and regulation of gene expression. Eukaryotic iron-sulfur clusters biosynthesis necessitates mitochondrial components. In vivo [Fe-S] proteins depend on a dedicated machinery to assemble the [Fe-S] cluster and transfer it to the apoprotein. Bacteria possess at least three different pathways for iron-sulfur clusters biosynthesis [270–275]. All pathways utilize a cysteine desulfurase which cleaves the sulfur atom from cysteine for donation to the scaffold protein. In turn, the scaffold protein receives iron atoms from iron donors and assembles the different conformations of the [Fe-S] clusters. Thusly prepared [Fe-S] clusters are then transferred to the apoprotein target. Depending on the organism, type of cluster and transfer mechanism, additional components are utilized to aid in the [Fe-S] cluster installation processes [266, 272].
Among the three systems, the so-called ISC (iron-sulfur-cluster-formation) system encoded by isc operon iscR-SUA-hsc BA-fdx may be the most common route under physiological conditions [276]. Another system, NIF, encoded by nif operon is tailored for nitrogenase maturation. Relatively little is known about the systems involved in [Fe-S] cluster formation in actinobacteria. However, the SUF (suppressors mobilization of sulfur) system encoded by suf ABCDSE operon functions under oxidative stress or iron starvation conditions, which are frequently encountered by M. tuberculosis [76]. M. tuberculosis SUF system is encoded by an operon comprised of Rv1460–Rv1466. This operon includes seven genes and SufB, the most conserved protein encoded by Rv1461, contains an intein, which self excises and rejoins the remaining fragments to convert into its active form [277, 278]. Unspliced SufB cannot interact with other components in the SUF system and is inactive during iron-sulfur cluster protein assembly. Thus, SufB maturation is an interesting target for inhibitors to block iron sulfur cluster biosynthesis in M. tuberculosis [279–281].
The ISC system consists of cysteine desulfurization enzymes iscS (sulfur donor), frataxin (iron donor), iscU and iscA (scaffold proteins), thioredoxin (reduced oxidant), and chaperones. In addition to the scaffold and sulfur donor proteins, the isc gene encodes two heat shock chaperones and contains the gene cysE, needed for cysteine biosynthesis [282]. However, the iron donor of NIF and SUF system remains unclear. Despite the complexities, NIF, ISC, and SUF share important components such as, sulfur donor (cysteine desulfurization enzyme), iron-sulfur cluster scaffold protein and catalyze the biosynthesis of iron-sulfur clusters in a very similar fashion [277, 283].
MYCOTHIOL
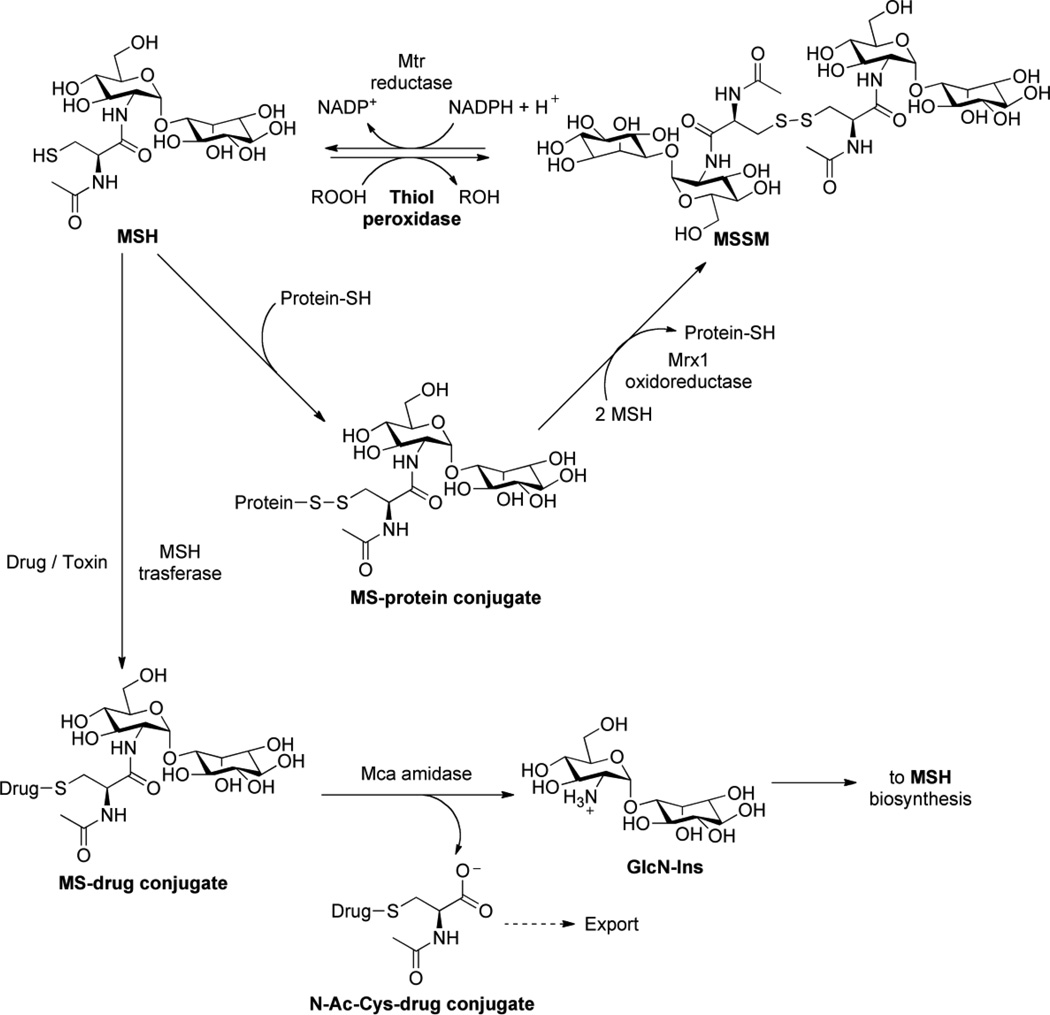
Mycothiol (MSH) or 1D-myo-inosityl 2-(N-acetyl-L-cysteinyl)amido-2-deoxy-α-D-glucopyranoside, is an unusual conjugate of N-acetylcysteine (AcCys) with 1D-myo-inosityl 2-acetamido-2-deoxy-α-D-glucopyranoside (GlcNAc-Ins) [see Fig. 6], and is the major low-molecular mass thiol in most actinomycetes, including mycobacteria [269]. MSH is the functional equivalent of glutathione (GSH) in mycobacteria [219, 284] and is associated with the protection of M. tuberculosis from toxic oxidants and antibiotics [214]. Interestingly, the thiol in MSH undergoes copper-ion catalyzed auto oxidation 30-fold more slowly than cysteine and 7-fold more slowly than glutathione [285]. Thus, high concentrations of cellular MSH may increase the capacity of actinomycetes to mitigate the negative effects of oxidative stress.
Figure 6.
Mycothiol biosynthetic pathway.
Apart from protection against toxic oxidants, M. tuberculosis relies upon MSH for growth in an oxygen-rich environment and for establishing the pattern of resistance to isoniazid and rifampin [214]. While previous reviews on MSH give a detailed overview of the MSH biochemistry [219] and MSH-dependent proteins [284], the purpose of this section is to highlight research avenues that would help clarify the functional role of MSH in the mycobacterial lifecycle and highlight promising drug targets in MSH metabolism.
Overview of Mycothiol Biosynthesis
Over a series of seminal papers, R. C. Fahey, G. L. Newton and Y. Av-Gay have elucidated the biosynthetic pathway of MSH [see Fig. 6]. Production of MSH begins from the biosynthesis of 1L-myo-inositol 1-phosphate (1L-Ins-1-P), produced from glucose-6-phosphate in a reaction catalyzed by inositol-1-phosphate synthase (Ino1) [286]. From this precursor, five enzymes catalyze the conversion of 1L-Ins-1-P to MSH. In the first step, a glycosyltransferase, mshA, catalyzes the reaction between a UDP-N-acetylglucosamine (UDP-GlcNAc) and 1L-Ins-1-P, generating UDP and 1-O-(2-acetamido-2-deoxy-α-D-glucopyranosyl)-D-myo-inositol 3-phosphate (GlcNAc-Ins-P) [286]. A phosphatase, as yet uncharacterized, but designated mshA2, dephosphorylates GlcNAc-Ins-P to produce 1-O-(2-acetamido-2-deoxy-α-D-glucopyranosyl)-D-myo-inositol (GlcNAc-Ins), the substrate for MshB [286]. In the next step, GlcNAc-Ins is deacetylated by mshB to yield 1-O-(2-amino-1-deoxy-α-D-glucopyranosyl)-D-myo-inositol (GlcN-Ins) [287]. Subsequently, mshC catalyzes the ATP-dependent ligation of L-cysteine to GlcN-Ins to produce 1-O-[[(2R)-2-amino-3-mercapto-1-oxopropyl]amino]-2-deoxy-α-D-glucopyranosyl)-D-myo-inositol (Cys-GlcN-Ins) [288]. In the final step, N-acetylation of Cys-GlcN-Ins with acetyl-CoA is catalyzed by mshD to afford MSH [289]. The total chemical synthesis of MSH has also been reported [290, 291].
The genes encoding the enzymes responsible for MSH biosynthesis have been identified using a variety of methods including transposon [292] and chemical mutagenesis [287, 293, 294]. In turn, these mutants have been utilized to determine the indispensability of the respective genes in the biosynthesis of MSH and their consequence on the viability of mycobacteria [295–297]. Significant progress in the biochemical characterization of these enzymes has also been made [287, 289, 292, 293, 296, 298].
Mycothiol Biosynthetic Enzymes
The gene encoding the glycosyltransferase, mshA was first identified as a transposon mutant in M. smegmatis that did not produce measurable amounts of GlcNAc-Ins and MSH [292]. By virtue of homology, mshA belongs to the known CAZy family 4 glycosyltransferases, [292, 299] which include a number of sucrose synthases, mannosyl transferases and GlcNAc transferases. This classification strongly suggested that mshA is the glucosyltransferase required for the biosynthesis of GlcNAc-Ins. M. smegmatis and M. tuberculosis mshA sequences were shown to be 75% identical over a 446-residue overlap. The M. tuberculosis mshA ortholog, Rv0486, complemented the mutant phenotype in M. smegmatis, thereby confirming its function. In M. smegmatis [292] and M. tuberculosis [77], mshA is essential for production of GlcNAc-Ins and therefore, for MSH synthesis. Interestingly, transposon mutants in mshA are viable in M. smegmatis [292], whereas in M. tuberculosis mshA is essential for growth [77]. The gene encoding the phosphatase, mshA2, remains to be identified.
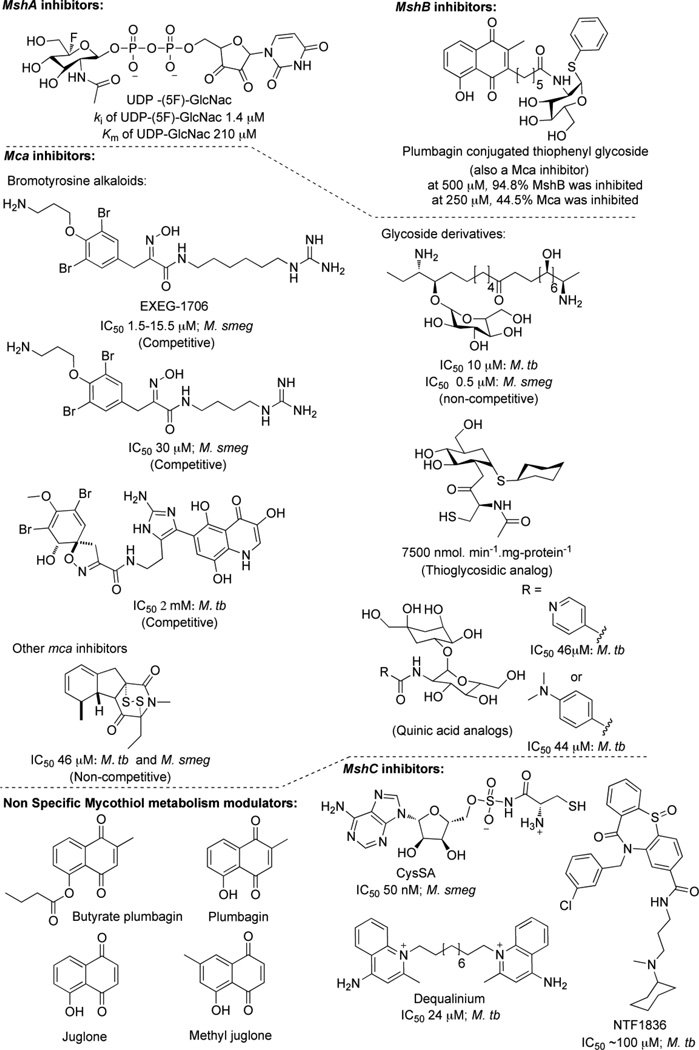
MshB was the first gene identified in the MSH biosynthetic pathway [287]. The deacetylase is encoded by the M. tuberculosis open reading frame Rv1170 and was first discovered as a homolog of Rv1082, a mycothiol S-conjugate amidase (Mca). Although mshB does exhibit some amidase activity, deacetylation of GlcNAc-Ins is the preferred reaction [287]. Characterization and crystallographic studies have revealed that MshB is a Zn2+ metalloprotein and that deacetylase activity is dependent on the presence of a divalent metal cation [300, 301].
Disruption of mshB results in decreased production of MSH (limited to about 5–10% of the parental M. smegmatis strain [302] and up to 20% that of the parental M. tuberculosis strain during log-phase growth, increasing to 100% of the wild-type MSH levels during the stationery phase [214]). Hence, MSH synthesis is not abolished in mshB mutants and, in the absence of mshB, MSH biosynthesis is accomplished via an alternative deacetylase activity that produces modest levels of GlcN-Ins [214, 302]. Under culture conditions, the amount of MSH produced in mshB mutants during log phase growth is sufficient to provide MSH-dependent resistance to moderate oxidative stress. In addition, since normal quantities of MSH are produced in MshB mutants during stationary phase, it was not possible to examine the role of MSH during dormancy-like conditions in these studies.
The role of mshC involving ATP-dependent ligation of L-cysteine with GlcN-Ins was first elucidated by Bornemann and coworkers [288]. First identified in M. smegmatis [298], homologs of mshC have been identified in Streptomyces coelicolor A3, Corynebacterium striatum [269] and orthologs of M. tuberculosis MshC (Rv2130c) were also found in M. leprae [15], M. bovis [17], and in M. avium [303]. Interestingly, the enzyme encoded by mshC appears to have evolved by gene duplication of the cysteinyl-tRNA synthetase, cysS (Rv3580c) as evidenced by their similar mechanism of action [298]. In the reaction catalyzed by mshC, the 2’ amine of GlcN-Ins carries out nucleophilic attack of an activated cysteinyl-AMP intermediate to produce Cys-GlcN-Ins. Presumably, a general base removes a proton from the amino group leading to the formation of a tetrahedral intermediate, which decomposes to form the amide [298].
In M. smegmatis, chemical and transposon mutants lacking mshC activity do not produce detectible amounts of MSH [218]. In the chemical mutants, mshC was sequenced and a point mutation (Leu205Pro) was identified. This region in mshC is largely conserved among actinomycetes and hence, the Leu205Pro substitution was concluded to be responsible for the lack of mshC activity in the mutant [218]. In contrast to M. smegmatis that does not require MSH for growth, a targeted disruption of mshC in M. tuberculosis Erdman produced no viable clones possessing either the disrupted mshC gene or reduced levels of MSH. Thus, the mshC gene is required for MSH production and is essential for M. tuberculosis Erdman survival [304]. The differences in the responses of the mutants between the two strains of mycobacteria could be attributed to the fact that M. smegmatis has a larger genome (7 vs. 4.4 Mb) relative to M. tuberculosis and therefore, includes genes that facilitate its growth in the absence of MSH [305].
MshD catalyzes the final step in MSH biosynthesis. In this reaction, Cys-GlcN-Ins is acetylated using acetyl-CoA [288]. MshD was identified during the characterization of an M. smegmatis transposon mutant lacking the transacetylase activity required for MSH biosynthesis. Sequencing from the site of insertion identified the gene that encodes for mycothiol synthase or mshD. A homology search revealed an mshD ortholog in M. tuberculosis as Rv0819 which exhibits MSH synthase activity when expressed in E. coli [289]. A crystal structure of mshD from M. tuberculosis showed structural homology to the GNAT family of N-acetyltransferases [306–308].
MshD mutants in M. smegmatis produce high levels of Cys-GlcN-Ins along with two other thiols, N-formyl-Cys-GlcN-Ins (fCys-GlcN-Ins) and N-succinyl-Cys-GlcN-Ins (succ-Cys-GlcN-Ins) and ~1% the amount of MSH found in the wild-type strain [305, 309]. These data suggest that in the absence of mshD, mycobacteria can utilize closely related analogs of MSH such as fCys-GlcN-Ins to maintain a reducing environment in the cells [305, 309]. This hypothesis is further supported by the observation that mshD transposon mutants in M. smegmatis are as resistant to peroxide-induced oxidative stress as their parental strain [309]. On the other hand, M. tuberculosis mshD mutants appear to grow poorly under other stress conditions such as low-pH media or in the absence of catalase and oleic acid [305].
MSH and Antibiotic Resistance
The formation of MSH-adducts of various anti-mycobacterial agents like cerulenin and rifamycin S [310] suggests that M. tuberculosis can use MSH in detoxification reactions [see Fig. 7] [293]. As yet an unidentified MSH-S-transferase is believed to catalyze the formation of the MSH-drug adduct [311]. MSH-S-conjugate amidase (discussed below) then catalyzes the hydrolysis of the MSH-drug adduct to produce a mercapturic acid containing the drug moiety, which is excreted from the cell [293]. Alternatively, since the oxidation state of cell wall components could alter cell wall permeability, MSH may also confer antibiotic resistance by influencing the overall cellular redox state [214]. Experiments with mshC mutants demonstrate that MSH, and not any biosynthetic intermediate en route to MSH, is critical for antibiotic and peroxide resistance [218]. Mutants lacking various MSH biosynthetic enzymes support the idea of MSH-related resistance to antibiotics [214, 289, 292, 294]. Independent null deletion mutants of all four genes involved in mycothiol biosynthesis pathway (mshA, mshB, mshC, and mshD) were generated in M. smegmatis and analyzed for MSH levels and isoniazid (INH) and ethionamide (ETH) resistance. The mshA and mshC single deletion mutants were not capable of producing MSH and found to be resistant to INH, whereas mshB deletion only decreased MSH levels and was sensitive to INH suggesting that MSH biosynthesis is essential for INH sensitivity in M. smegmatis [312]. Further evidence was gained by deleting the gene encoding the MSH S-conjugate amidase, mca, from the ∆mshB null mutant. The double mutant ∆mca∆mshB of M. smegmatis resulted in complete loss of MSH production and was resistant to INH [312]. However, there is considerable difference in redox balance between M. tuberculosis and M. smegmatis. M. tuberculosis ∆mshA and ∆mshD strains require exogenous catalase to grow in vitro [313], whereas M. smegmatis MSH synthetic mutants grow without the addition of catalase indicating the difference in the redox homeostasis between the two species [305, 314]. The mshA, mshC, and mshB single deletion mutants of M. smegmatis were also found to be resistant to ETH, indicating that ETH activity is modulated by MSH levels [312, 313]. Interestingly, M. smegmatis ∆mshD strain is devoid of MSH but is still sensitive to INH and ETH. It was found that in M. smegmatis mshD::Tn5 mutant or in M. tuberculosis ∆mshD mutant, the reduced form of thiol is novel N-formyl-cys-GlcN-Ins which substitutes for MSH in M. smegmatis and likely mediates the drug sensitivity [305, 312]. Taken together, it can be inferred from these studies that mycothiol is essential for INH and ETH activity against M. tuberculosis.
Figure 7.
Mycothiol mediated detoxification pathway.
Mycothiol-dependent Detoxification
Biological degradation of one carbon compounds such as methane, methylated amines and sulfur compounds leads to accumulation of formaldehyde. At lower concentrations, formaldehyde is toxic to cells and suggests the existence of a detoxification mechanism in M. tuberculosis. To this end, it has been proposed that MSH or MSH-dependent enzymes are involved in protecting mycobacteria from oxidants/toxins [218, 294] and have led to the study of enzymes that utilize MSH as a cofactor or a substrate for their activity. NAD/MSH-dependent formaldehyde dehydrogenase (mscR) was the first enzyme identified as using MSH as a cofactor [315] and is discussed at length in a recent review [284]. Like glutathione, mycothiol spontaneously reacts with formaldehyde to form an S-hydroxymethylmycothiol adduct that is converted by the NAD/MSH dependent formaldehyde dehydrogenase (mscR encoded by Rv2259) to a mycothiol formate ester. Then an aldehyde dehydrogense becomes likely to convert mycothiol formate ester into CO2, carbonate ester and mycothiol. Modeling studies reveal that the active sites of GSH- and MSH-dependent formaldehydede hydrogenases have distinct binding sites and are specific to their substrates. Recently, Vogt et al. reported that mscR also operates as mycothiol-nitroso reductase, indicating that this enzyme is involved in the protection against oxidative stress posed by reactive nitrogen species. Besides mscR, two other important enzymes involved in MSH metabolism and detoxification are mycothione reductase (mtr) and Mycothiol-S-conjugate Amidase (mca) [see Fig. 6], discussed below.
Mycothione Reductase
To maintain a large cellular pool of reduced MSH, mycothione reductase catalyzes the reduction of oxidized MSH also known as mycothione (MSSM) [see Fig. 6] [284]. M. tuberculosis MSH disulfide reductase (mtr, encoded by Rv2855) was identified by homology to glutathione reductases [316, 317]. Mtr is a member of the pyridine nucleotide-disulfide reductase super family. The reductase is a homodimeric flavoprotein disulfide isomerase and requires FAD as a cofactor [284, 316]. NADPH reduces FAD, which then transfers reducing equivalents to the redox-active disulfide in mtr to generate a stable two-electron reduced enzyme [316, 317]. Subsequently, mtr reduces the disulfide in MSSM via dithiol-disulfide interchange, with concomitant oxidation of NADPH [316, 317].
Phenotypic characterization of an actinomycete mtr mutant has not been reported to date and genome-wide transposon mutagenesis has yielded conflicting results. In one study, a transposon mutant in M. tuberculosis mtr was reported to be viable [318]. In contrast, another study using high-density Himar-1 transposon mutagenesis reported that mtr is essential for M. tuberculosis survival [227]. One possible explanation for these conflicting data could be the relative importance of (or requirement for) mtr in MSH reduction during different stages of growth. Transcriptional analysis of M. bovis BCG reveals that mtr mRNA is actively transcribed during exponential bacterial growth [295]. In the same study, mtr mRNA expression was absent in the stationery phase suggesting that mtr might only be required to maintain the redox balance during intense periods of metabolic activity (e.g., during the growth phase) [295]. However, another study found high MSH levels throughout the growth cycle, including the stationery phase [302]. These findings suggest that, in the absence of mtr, another thiol reductase might reduce MSSM [284]. Additional experiments will be required to clarify the importance of mtr in MSH reduction throughout the mycobacterium lifecycle and to determine whether or not it is essential for bacterial viability.
Mycothiol-S-conjugate Amidase
In mycobacteria, mycothiol-S-conjugate Amidase (mca) plays a major role in electrophone detoxification [see Fig. 6] [293]. This enzyme was discovered in connection with its ability to detoxify a thiol-specific fluorescent alkylating agent, monobromobimane (mBBr), a compound commonly used for the quantitative determination of thiols. mBBr binds to MSH forming an MSH-mBBr adduct, MSmB, and can be cleaved by mca to produce glucosaminyl inositol and acetyl cysteinyl bimane, a mercapturic acid which is rapidly excreted from the cell [293]. Mca was first purified from M. smegmatis and was found to have an ortholog in the M. tuberculosis genome, Rv1082, identified by N-terminal amino acid sequencing [293]. Studies probing the substrate specificity of mca indicate that the enzyme specifically recognizes the MSH moiety in the conjugate, but is relatively non-specific for the group attached to the sulfur in the MSH-toxin conjugate [293].
Mca and mshB exhibit an overall sequence identity of 32% [300]. Interestingly, in vitro studies indicate that mshB possesses amidase activity with MSH substrate [293]. Moreover, mca can function as a deacetylase [286, 293] and partially restores MSH production when introduced into an M. Smegmatis mshB mutant [302]. Based on the sequence identity between mca and mshB and the crystal structure of mshB, a model for the active site of mca has been proposed [300, 301]. With the exception of Lys19 in mca replaced by Ser20 in mshB, other critical catalytic residues, such as the zinc-binding site and an aspartate are perfectly conserved. The Lys to Ser alteration may play an important role in disaccharide binding [300]; a crystal structure of mca will be important to define the MSH binding site.
Apart from the mBBr model substrate, the substrates for mca include the MSH conjugate of cerulenin, an antibiotic that inhibits fatty acid synthetase and other antibiotic adducts. Mca homologs have been found in several antibiotic biosynthesis operons such as those for avermectin (Streptomyces avermitilis) and eythromycin (Saccharopolyspora erythrae) [219, 319]. In addition, it has been demonstrated that MSH forms a conjugate with Rifamycin SV and this complex is a substrate for M. tuberculosis mca [310]. Treatment of mca mutant and wild-type M. smegmatis strains with Rifamycin SV showed that the MSH-Rifamycin SV adduct is converted to mercapturic acid only in the wild-type [297]. Taken together, these findings demonstrate that MSH and mca in mycobacteria work together to detoxify antibiotics [219].
Drug Targets in Mycothiol Metabolism
Mca plays a critical role in mycobacterial detoxification of antibiotics. Therefore, inhibitors of Mca could enhance the sensitivity of MSH-producing bacteria to antibiotics, establishing Mca as a promising new drug target. Toward this end, 1,500 natural product extracts and synthetic libraries were screened to identify lead compounds [320–322]. Two classes of bromotyrosine-derived natural products were competitive inhibitors of Mca. Non-competitive inhibitors were also identified in this screen [see Fig. 8 mca inhibitors (non-competitive)]. These results motivated the total synthesis of a competitive inhibitor [see Fig. 8 mca inhibitors] that inhibits mca with an IC50 value of 30 µM [323].
Figure 8.
Mycothiol metabolism inhibitors.
Recently, a series of compounds based on the structure of the natural product bromotyrosine inhibitor was synthesized and screened against mycobacteria and other gram-positive bacteria [324]. One of the lead compounds identified from this study termed, EXEG1706 [see Fig. 8 mca inhibitors], exhibited low minimum inhibitory concentrations (1.5 – 15.5µg ml−1) for M. smegmatis, M. bovis and against methicillin-sensitive and methicillin-resistant Staphylococcus aureus, and S. aureus. However, this class of compounds was also active against mycobacterial mca mutant strains and against gram-positive bacteria that do not produce MSH. Thus, in addition to mca, it appears that these compounds inhibit other protein targets in vivo. Another approach used to identify mca inhibitors has been the synthesis of MSH analogs. Synthesis of simplified thioglycosidic analogs of MSH [see Fig. 8] [325] and a variety of amide-functionalized MSH analogs synthesized from quinic acid led to the identification of inhibitors with modest inhibitory activities (IC50 values around 50 µM) [326] [see Fig. 8].
Naphtoquinone derivatives like plumbagin, juglone and alkyl plumbagin derivatives were shown to inhibit M. tuberculosis growth. Since napthoquinone derivatives were known to interact with electron transport or related biochemical reactions [327],Gammon et al. utilized these moieties to create plumbagin conjugated glucopyranoside derivatives as inhibitors of mca and mshB [328]. The maximum potent inhibition of mca and mshB was observed when the plumbagin moiety was separated by 5-carbon spacer from glucopyranoside moiety [328]. However, testing of the molecule in disease models of M. tuberculosis is yet to be reported.
In addition to mca, other possible drug targets that could block MSH biosynthesis are the enzymes encoded by mshA and mshC (both essential genes in M. tuberculosis [77, 304]). The identification of inhibitors for mshC has been initiated [286] and recently Newton et al. reported a novel inhibitor, NTF1836, inhibiting MshC in micromolar range (IC50 85µM)[329]. They also reported that inhibition of M. tuberculosis growth by NTF1836 was accompanied by a decrease in mycothiol and an increase in GlcN-Ins cellular levels, which is consistent with the targeting mshC. Moreover, NTF1836 was also found to inhibit non-replicating M. tuberculosis in the carbon starvation model of latency [329]. Subsequent structure activity relationship studies revealed that five structurally related compounds to NTF1836 have similar activity towards clinical strains of M. tuberculosis and paved the way for designing second generation MshC inhibitors [329]. In a similar effort by Gutierrez-Lugo et al., dequalinium chloride was identified through high-throughput screening, inhibiting M. tuberculosis mshC with an IC50 of 24µM. Further studies showed dequalinium chloride as an ATP-competitive inhibitor of mshC which inhibits the growth of M. tuberculosis under aerobic and anaerobic conditions [330]. In another study, Gutierrez-Lugo et al. screened two groups of known aminoacyl tRNA synthetase inhibitors for inhibition of M. tuberculosis mshC including aminoacyl adenosine analogs and natural products. Using enzyme assays, isothermal titration calorimetry and NMR of this group showed that M. Smegmatis mshC can be selectively inhibited by cysteinyl sulfamoyl adenosine (CysSA) with an IC50 of 10nM [331]. Derivatives of naphthoquinone, juglone and plumbagin were shown to be subversive substrates of mycothiol disulfide reductase having anti-tubercular activity. 7-methyljuglone derivatives are the most potent with MIC of 0.5 μg/ml against M. tuberculosis [332]. However, the lack of correlation between anti-tubercular activity and mycothiol disulfide reductase activity suggests that these compounds are not selective for mycothiol disulfide reductase and instead target multiple enzymes in vivo. Identification of inhibitors for another UDP-GlcNAc-dependent glycosyltransferase, murG [333] suggests that mshA is also likely to be a druggable target. Blanchard et al. recently reported the three-dimensional structure and basic kinetic characterization of the retaining glycosyltransferase mshA from Corynebacterium glutamicum (CgmshA) [334]. The same group recently reported a slow-binding competitive inhibitor, UDP-(5F)-GlcNAc, for CgmshA with Ki 1.6µM [335]. Although high-density transposon mutagenesis studies have identified mshD as nonessential for the growth of M. tuberculosis in minimal culture medium [227], the survival of M. tuberculosis mshD mutants is severely compromised in activated and non-activated macrophages [225]. Thus, mshD could be a promising drug target and further analysis of this mutant in animal models of TB infection may be warranted.
METHIONINE BIOSYNTHESIS AND REVERSE TRANSSULFURATION
In M. tuberculosis, Rv1079 (annotated as metB) encodes a bi-functional cysteine γ-lyase (CGL)-cystathionine γ-synthase (CGS) enzyme, converts cysteine to cystathionine [249]. Cystathionine is then transformed into methionine by two subsequent reactions: MetC (Rv3340) converts cystathionine into homocysteine [249, 336] and metE/metH (Rv1133c/Rv2124c) catalyzes methylation of homocysteine to produce methionine [249, 337].
Interestingly, mutation of metB in M. tuberculosis results in a prototrophic methionine mutant [338]. In other words, metB is not absolutely required for methionine production. This finding can be explained by the action of metZ (Rv0391), an O-succinylhomoserine sulfurylase, which bypasses the requirement for metB and metC by condensing S2− with O-succinylhomoserine to produce homocysteine directly [see Fig. 3]. In mice, a ∆metB strain was slightly attenuated [338]. However, no differences in bacterial load in the lungs, liver or spleen, were observed between the metB mutant and wild-type M. tuberculosis in immunocompetent mice up to 80 days post-infection [338]. This growth phenotype contrasts that of cysH mutants in M. tuberculosis where viability decreases significantly, specifically during the persistence phase of infection [220].
The interesting observation that defects in sulfate transport or its reduction could be rescued by methionine supplementation suggested that a functional reverse transsulfuration pathway [see Fig. 3], used to produce cysteine from methionine, was present in mycobacteria [221, 249, 250]. Indeed, the existence of this pathway has recently been confirmed [249]. Although a methionine transporter has not yet been identified in mycobacteria, an apparent Km of 80 µM for the transporter has been estimated in M. bovis BCG [250] and the estimated concentration of methionine in humans is 20–27 µM [339].
Once methionine is transported into the bacteria, three enzymatic steps are required for conversion into homocysteine [see Fig. 7] [246]. In the first step, S-denosyl methionine transferase or S-adenosyl methionine synthase which is denoted by metK, catalyzes the conversion of methionine to S-adenosine-L-methionine [340]. S-adenosyl-L-methionine dependent methyl transferase (Rv2118c) catalyzes the conversion of S-adenosyl-L-methionine to S-adenosine homocysteine [341]. S-adenosyl-L-homocysteine hydrolase (sahH) catalyzes the reversible hydrolysis of S-adenosyl-L-homocysteine into free adenosine and homocysteine and maintains the intracellular balance between S-adenosylhomocysteine and S-adenosylmethionine [342]. Adenosyl homocysteine hydrolase has been validated as a “druggable” target [343], is essential for growth in vitro and is up-regulated in mouse models of TB infection [344]. Subsequently, cysM2 (Rv1077) converts homocysteine to cystathionine and the bifunctional enzyme, Rv1079 (annotated as metB) encodes a bifunctional cysteine γ-lyase (CGL)-cystathionine γ-synthase (CGS) enzyme catalyzes the cystathionine to cysteine [249]. Thus, M. tuberculosis can shuttle the sulfur source between the important sulfur containing metabolites to meet cellular demands. This pathway also provides support for M. tuberculosis survival against oxidative stress by scavenging the methionine from host cell. Also, sulfate assimilation in M. tuberculosis appears to be dynamic and can quickly adapt environmental changes.
OTHER SOURCES OF REDUCED SULFUR
Consistent with the requirement for sulfur in mycobacterial survival, the ability of mycobacteria to scavenge reduced sulfur from its host has been confirmed in M. bovis BCG and in M. tuberculosis [220, 249, 250]. Several potential sources of reduced sulfur in the human host are discussed below.
Cysteine and Cystine
Mutation of cysH in M. smegmatis and M. tuberculosis produces a cysteine auxotroph and this defect can be rescued by the addition of cysteine to the growth medium [220, 221]. The finding that growth of ∆cysH mutant can be restored by the addition of cysteine suggests that cysteine or cystine (the oxidized form of cysteine) can be transported into M. smegmatis and M. tuberculosis. In addition, when [35S] cysteine is added to a growing culture of M tuberculosis, more than 70% of the radioactive sulfur taken up by the bacteria is found in methionine, also consistent with import of cysteine [249]. While genes that encode the cysteine/cystine transporter in mycobacteria have not yet been identified, cysteine and cystine uptake systems have been characterized in other prokaryotes [345, 346]. In humans, cystine is the preferred form of cysteine for the synthesis of glutathione in macrophages and is present in plasma at ~25 – 35 µM [347].
Genetic screens for amino acid auxotrophs in M. bovis BCG (an attenuated version of bovine bacillus) have not isolated cysteine auxotrophs [87, 250]. In the first report, only three auxotrophs were identified, one for methionine and two for leucine [87]. A subsequent study isolated two auxotrophs, both for methionine [250]. Since isolation of mycobacterial auxotrophs depends on the growth medium composition [348], it is possible that the use of casamino acids to rescue the growth of transposon mutants in these studies selects only a small subset of amino-acid auxotrophs. Consistent with this hypothesis, the approximate concentration of sulfur-containing amino acids in 1% (w/v) casamino acids is expected to be ~900 µM methionine and ~60 µM cystine.
The methionine auxotrophs identified by transposon mutagenesis in M. bovis BCG are mapped to genes in the sulfate assimilation pathway, in particular to sulfate transport genes, subI [87] and cysA [250]. Since sulfate serves as the precursor for cysteine synthesis, defects in the sulfate assimilation pathway should result in cysteine auxotrophy. Surprisingly, however, growth of M. bovis BCG subI or cysA mutants could not be rescued by supplementation with cysteine (in contrast to the cysH knockout in M. smegmatis and M. tuberculosis) and instead, required methionine supplementation. In addition to the inability of cysteine to rescue defects in sulfate transport, the same study also reported that wild-type M. bovis BCG grew slowly on growth media supplemented with 0.3 mM cysteine and not at all in the presence of 0.5 mM cysteine. In contrast, toxicity has not been observed in wild-type strains of M. smegmatis [221] or M. tuberculosis [220] grown in the presence of 1 – 2 mM cysteine. Hence, it is possible that mutation of sulfate transport genes, subI or cysA, impacts cysteine/cystine import directly or that M. bovis BCG does not transport cysteine/cystine efficiently. Further investigations into the differences between mycobacterial strains, growth media and other critical factors, such as inoculum densities, for the requirements of sulfur-containing compounds are warranted.
Glutathione
In mycobacteria, a large amount of the reduced sulfur in cells is used to make mycothiol, the dominant low molecular weight thiol used to maintain redox equilibrium and scavenge reactive oxygen species in the cell [269]. Similarly, GSH – a tripeptide, γ-glutamylcysteinylglycine found in many prokaryotes and eukaryotes – is also present at high intracellular levels [284] and may provide a source of reduced sulfur for mycobacteria in the host. Estimates of GSH concentration in human cells and macrophages range from 1 – 7 mM [349, 350]. An analog of GSH, nitrosoglutathione (GSNO), is bactericidal in M. bovis [351] and M. tuberculosis [352]. Use of GSNO has facilitated identification and characterization of the ABC transporter dipeptide permease (Dpp, Rv3663 – Rv3666) responsible for GSH catabolism and utilization [351, 352]. Interestingly, GSH is not transported into mycobacterial cells as the tripeptide, but rather as the dipeptide, Cys-Gly [352]. Hence, import of GSH involves proteolysis by a γ-glutamyl transpeptidase (ggtA, Rv0773c) and subsequent transport via dpp. Consistent with the proposed route of GSH catabolism and import, mutants in the transpeptidase or the permease are resistant to the toxic effects of GSNO [352]. In culture, it has been reported that GSH exhibits bacteriostatic activity at a concentration of 5 mM [353]. This effect appears to be mediated intracellularly since mutations in the dpp or ggtA relieve this phenomenon [353].
Recently Guerra et al. characterized the mechanisms by which glutathione (GSH)-enhanced natural killer cells inhibit the growth of M. tuberculosis inside human monocytes. In healthy individuals, treatment of natural killer cells with N-acetyl cysteine (NAC), a GSH pro-drug in conjunction with cytokines such as interleukin (IL)-2 + IL-12, resulted in enhanced expression of natural killer cell cytotoxic ligands (FasL and CD40L) with subsequent inhibition in the intracellular growth of M. tuberculosis. Neutralization of FasL and CD40L in IL-2, IL-12 and NAC-treated natural killer cells resulted in abrogation in the growth inhibition of M. tuberculosis inside monocytes. Interestingly, the levels of GSH are decreased significantly in natural killer cells derived from individuals with HIV infection compared to healthy subjects and this decrease is correlated with several fold increase in growth of M. tuberculosis inside monocytes [354].
OUTLOOK
The emergence of antibiotic resistance and the problem of mycobacterial persistence in M. tuberculosis urgently stress the need for new target identification. Toward this end, mycobacterial sulfur metabolic pathways represent a promising new area for anti-TB therapy. In the last several years, excellent progress has been made, leading to the identification and validation of several potential drug targets in sulfate assimilation and MSH metabolism. At the same time, many aspects of mycobacterial sulfur metabolism remain poorly understood and represent exciting areas of new or continued investigation. Significant work remains to validate additional targets, improve inhibitor potency for existing targets and to further define the roles that sulfated and many reduced sulfur-containing metabolites play in mycobacterial virulence and persistence. Finally, a wide variety of microbes including Pseudomonas aeruginosa, Bacillus anthracis, and Yersinia pestis also relies on unique sulfur metabolic pathways for their own survival. Hence, in the fight against multidrug resistant microbes, investigation of microbial sulfur metabolism in mycobacteria and other pathogens should be fertile scientific ground in the years to come.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (GM087638 to K.S.C.)
ABBREVIATIONS
- TB
Tuberculosis
- MDR
Multidrug resistant
- CoA
Coenzyme A
- –SO3−
Sulfuryl moiety
- SO42−
Sulfate
- APS
Adenosine-5’-phosphosulfate
- PAPS
3’-Phosphoadenosine-5’-phosphosulfate
- SO32−
Sulfite
- S2−
Sulfide
- BCG
Bacillus Calmette-Guérin
- ATP
Adenosine 5’-triphosphate
- GTP
Guanosine 5’-triphosphate
- SAC
Sulfate-activating complex
- ST
Sulfotransferase
- SL-1
Sulfolipid-1
- T2S
Trehalose-2-sulfate
- PAP
3’-Phosphoadenosine-5’-phosphate
- EST
Estrogen ST
- TPST
Tyrosyl protein ST
- GST
Golgi-resident ST
- β-AST
β–arylsulfotransferases
- cAMP
Cyclic adenosine-5’-phosphate
- IC50
Half maximal inhibitory concentration
- O2·−
Superoxide
- NO·
Nitric oxide
- NADPH oxidase
Nicotinamide adenine dinucleotide phosphate-oxidase
- NOS2
Inducible nitric oxide synthase
- ROI
Reactive oxygen intermediate
- RNI
Reactive nitrogen intermediate
- SOD
Superoxide dismutase
- H2O2
Hydrogen peroxide
- OH·
Hydroxyl radical
- NOHLA
N-ω-hydroxy-L-arginine
- ONOO−
Peroxynitrite
- TLR
Toll-like receptor
- Phox
Phagocyte NADPH oxidase
- AMP
Adenosine-5’-phosphate
- Trx
Thioredoxin
- Cys-Sγ–SO3−
S-sulfocysteine
- [4Fe-4S]
Four iron-four sulfur cluster
- MSH
Mycothiol
- AcCys
N-acetylcysteine
- GlcNAc-Ins
1D-myo-inosityl 2-acetamido-2-deoxy-α-D-glucopyranoside
- GSH
Glutathione
- 1L-Ins-1-P
1L-myo-inositol 1-phosphate
- UDP-GlcNAc
UDP-N-acetylglucosamine
- GlcNAc-Ins-P
1-O-(2-acetamido-2-deoxy-α-D-glucopyranosyl)-D-myo-inositol 3-phosphate
- GlcNAc-Ins
1-O-(2-acetamido-2-deoxy-α-D-glucopyranosyl)-D-myo-inositol
- GlcN-Ins
1D -myo-inosityl-2-amino-2-deoxy-α-D-glucopyranoside
- Cys-GlcN-Ins
1-O-[[(2R)-2-amino-3-mercapto-1-oxopropyl] amino]-2-deoxy-α-D- (glucopyranosyl)-D-myo-inositol
- fCys-GlcN- Ins
N-formyl-Cys-GlcN-Ins
- succ-Cys-GlcN- Ins
N-succinyl-Cys-GlcN-Ins
- MSSM
Mycothione
- mBBr
Monobromobimane
- CGL
Cysteine γ-lyase
- CGS
Cystathionine γ-synthase
- GSNO
Nitrosoglutathione
Footnotes
This manuscript is an updated version of previously published manuscript entitled "Drug targets in mycobacterial sulfur metabolism" with the following bibliography.
Bhave, D. P.; Muse, W. B., 3rd; Carroll, K. S., Drug targets in mycobacterial sulfur metabolism. Infect. Disord. Drug Targets 2007, 7 (2), 140–58.
REFERENCES
- 1.Corbett El WCJ, Walker N, Maher D, Williams BG, Raviglioni MC, Dye C. The growing burden of tuberculosis: Global trends and interactions with the hiv epidemic. Arch. Intern. Med. 2003;163(9):1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 2.Dye C SS, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. JAMA. 1999;282(7):677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 3.Centre for Disease Control and prevention. [Accessed Februvary 1st 2013];Trends in Tuberculosis-United States. 2011 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6111a2.htm.
- 4.World Health Organization. [Accessed May 8, 2013];Global tuberculosis report 2012: Executive Summary. http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf.
- 5.Espinal MA. The global situation of MDR-TB. Tuberculosis. 2003;83(1–3):44–51. doi: 10.1016/s1472-9792(02)00058-6. [DOI] [PubMed] [Google Scholar]
- 6.Duncan K. Progress in TB drug development and what is still needed. Tuberculosis. 2003;83(1-3):201–207. doi: 10.1016/s1472-9792(02)00076-8. [DOI] [PubMed] [Google Scholar]
- 7.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: Treatment of Tuberculosis. Am. J. Respir. Crit. Care Med. 2003;167(4):603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y. The magic bullets and tuberculosis drug targets. Annu. Rev. Pharmacol. Toxicol. 2005;45(1):529–564. doi: 10.1146/annurev.pharmtox.45.120403.100120. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y. Persistent and dormant tubercle bacilli and latent tuberculosis. Front. Biosci. 2004;9:1136–1156. doi: 10.2741/1291. [DOI] [PubMed] [Google Scholar]
- 10.Mitchison DA. Basic Mechanisms of Chemotherapy. CHEST Journal. 1979;76(6_Supplement):771–781. doi: 10.1378/chest.76.6_supplement.771. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y. Mechanisms of drug resistance in mycobacterium tuberculosis Front. Biosci. 2004;9:975–994. doi: 10.2741/1289. [DOI] [PubMed] [Google Scholar]
- 12.Chee CB-E, Sester M, Zhang W, Lange C. Diagnosis and treatment of latent infection with Mycobacterium tuberculosis. Respirology. 2013;18(2):205–216. doi: 10.1111/resp.12002. [DOI] [PubMed] [Google Scholar]
- 13.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 2012;12(5):352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 14.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 15.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honore N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. Massive gene decay in the leprosy bacillus. Nature. 2001;409(6823):1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Bannantine JP, Zhang Q, Amonsin A, May BJ, Alt D, Banerji N, Kanjilal S, Kapur V. The complete genome sequence of Mycobacterium avium subspecies paratuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2005;102(35):12344–12349. doi: 10.1073/pnas.0505662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garnier T, Eiglmeier K, Camus JC, Medina N, Mansoor H, Pryor M, Duthoy S, Grondin S, Lacroix C, Monsempe C, Simon S, Harris B, Atkin R, Doggett J, Mayes R, Keating L, Wheeler PR, Parkhill J, Barrell BG, Cole ST, Gordon SV, Hewinson RG. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. U. S. A. 2003;100(13):7877–7882. doi: 10.1073/pnas.1130426100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glickman MS, Cahill SM, Jacobs WR. The Mycobacterium tuberculosis cmaA2 Gene Encodes a Mycolic Acid trans-Cyclopropane Synthetase. J. Biol. Chem. 2001;276(3):2228–2233. doi: 10.1074/jbc.C000652200. [DOI] [PubMed] [Google Scholar]
- 19.Parish T, Stoker NG. glnE Is an Essential Gene inMycobacterium tuberculosis. J. Bacteriol. 2000;182(20):5715–5720. doi: 10.1128/jb.182.20.5715-5720.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Kessel JC, Hatfull GF. Recombineering in Mycobacterium tuberculosis. Nat. Methods. 2007;4(2):147–152. doi: 10.1038/nmeth996. [DOI] [PubMed] [Google Scholar]
- 21.Flynn JL, Chan J. Tuberculosis: Latency and Reactivation. Infect. Immun. 2001;69(7):4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houben ENG, Nguyen L, Pieters J. Interaction of pathogenic mycobacteria with the host immune system. Curr. Opin. Microbiol. 2006;9(1):76–85. doi: 10.1016/j.mib.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Lin PL, Flynn JL. Understanding Latent Tuberculosis: A Moving Target. The Journal of Immunology. 2010;185(1):15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehlers S, Schaible UE. The granuloma in tuberculosis: Dynamics of a host-pathogen collusion. Frontiers in Immunology. 2013;3 doi: 10.3389/fimmu.2012.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockhart E, Green AM, Flynn JL. IL-17 Production Is Dominated by γδ T Cells rather than CD4 T Cells during Mycobacterium tuberculosis Infection. The Journal of Immunology. 2006;177(7):4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 26.Eum S-Y, Kong J-H, Hong M-S, Lee Y-J, Kim J-H, Hwang S-H, Cho S-N, Via LE, Barry IIICE. Neutrophils Are the Predominant Infected Phagocytic Cells in the Airways of Patients With Active Pulmonary TB. Chest. 2010;137(1):122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vergne I, Chua J, Lee H-H, Lucas M, Belisle J, Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2005;102(11):4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell DG. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 2007;5(1):39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 29.Lazarevic V, Nolt D, Flynn JL. Long-Term Control of Mycobacterium tuberculosis Infection Is Mediated by Dynamic Immune Responses. J. Immunol. 2005;175(2):1107–1117. doi: 10.4049/jimmunol.175.2.1107. [DOI] [PubMed] [Google Scholar]
- 30.Cosma CL, Humbert O, Ramakrishnan L. Superinfecting mycobacteria home to established tuberculous granulomas. Nat. Immunol. 2004;5(8):828–835. doi: 10.1038/ni1091. [DOI] [PubMed] [Google Scholar]
- 31.Davis JM, Ramakrishnan L. The Role of the Granuloma in Expansion and Dissemination of Early Tuberculous Infection. Cell. 2009;136(1):37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkman HE, Clay H, Beery D, Chang JCW, Sherman DR, Ramakrishnan L. Tuberculous Granuloma Formation Is Enhanced by a Mycobacterium Virulence Determinant. PLoS Biol. 2004;2(11):e367. doi: 10.1371/journal.pbio.0020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boshoff HIM, Barry CE. Tuberculosis metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 2005;3(1):70–80. doi: 10.1038/nrmicro1065. [DOI] [PubMed] [Google Scholar]
- 34.Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect. Dis. 2003;3(9):578–590. doi: 10.1016/s1473-3099(03)00741-2. [DOI] [PubMed] [Google Scholar]
- 35.Ulrichs T, Kaufmann SHE. New insights into the function of granulomas in human tuberculosis. J. Pathol. 2006;208(2):261–269. doi: 10.1002/path.1906. [DOI] [PubMed] [Google Scholar]
- 36.Volokhov DV, Chizhikov VE, Denkin S, Zhang Y. The Mycobacteria Protocols. 2008;465:1–24. [Google Scholar]
- 37.Saunders BM, Cooper AM. Restraining mycobacteria: Role of granulomas in mycobacterial infections. Immunol. Cell Biol. 2000;78(4):334–341. doi: 10.1046/j.1440-1711.2000.00933.x. [DOI] [PubMed] [Google Scholar]
- 38.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. U. S. A. 2000;97(16):8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginsberg AM, Spigelman M. Challenges in tuberculosis drug research and development. Nat. Med. 2007;13(3):290–294. doi: 10.1038/nm0307-290. [DOI] [PubMed] [Google Scholar]
- 40.Flynn JL, Chan J. Immunology of Tuberculosis. Annu. Rev. Immunol. 2001;19(1):93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 41.Lienhardt C, Raviglione M, Spigelman M, Hafner R, Jaramillo E, Hoelscher M, Zumla A, Gheuens J. New Drugs for the Treatment of Tuberculosis: Needs, Challenges, Promise, and Prospects for the Future. J. Infect. Dis. 2012;205(suppl 2):S241–S249. doi: 10.1093/infdis/jis034. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y. Drug Resistant and Persistent Tuberculosis: Mechanisms and Drug Development. In: Dougherty TJ, Pucci MJ, editors. Antibiotic Discovery and Development. US: Springer; 2012. pp. 719–749. [Google Scholar]
- 43.Forrellad MA, Klepp LI, Gioffré A, Sabio y García J, Morbidoni HR, Santangelo MdlP, Cataldi AA, Bigi F. Virulence factors of the Mycobacterium tuberculosis complex. Virulence. 2013;4(1):3–66. doi: 10.4161/viru.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hampshire T, Soneji S, Bacon J, James BW, Hinds J, Laing K, Stabler RA, Marsh PD, Butcher PD. Stationary phase gene expression of Mycobacterium tuberculosis following a progressive nutrient depletion: a model for persistent organisms? Tuberculosis. 2004;84(3-4):228–238. doi: 10.1016/j.tube.2003.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manganelli R, Fattorini L, Tan D, Iona E, Orefici G, Altavilla G, Cusatelli P, Smith I. The Extra Cytoplasmic Function Sigma Factor σE Is Essential for Mycobacterium tuberculosis Virulence in Mice. Infect. Immun. 2004;72(5):3038–3041. doi: 10.1128/IAI.72.5.3038-3041.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohno H, Zhu G, Mohan VP, Chu D, Kohno S, Jacobs WR, Chan J. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell. Microbiol. 2003;5(9):637–648. doi: 10.1046/j.1462-5822.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- 47.Pinto R, Tang QX, Britton WJ, Leyh TS, Triccas JA. The Mycobacterium tuberculosis cysD and cysNC genes form a stress-induced operon that encodes a tri-functional sulfate-activating complex. Microbiology. 2004;150(6):1681–1686. doi: 10.1099/mic.0.26894-0. [DOI] [PubMed] [Google Scholar]
- 48.Rengarajan J, Bloom BR, Rubin EJ. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. U. S. A. 2005;102(23):8327–8332. doi: 10.1073/pnas.0503272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sassetti CM, Boyd DH, Rubin EJ. Comprehensive identification of conditionally essential genes in mycobacteria. Proc. Natl. Acad. Sci. U. S. A. 2001;98(22):12712–12717. doi: 10.1073/pnas.231275498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. U. S. A. 2003;100(22):12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. The Journal of Experimental Medicine. 2003;198(5):693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding α-crystallin. Proceedings of the National Academy of Sciences. 2001;98(13):7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trauner A, Lougheed KEA, Bennett MH, Hingley-Wilson SM, Williams HD. The Dormancy Regulator DosR Controls Ribosome Stability in Hypoxic Mycobacteria. J. Biol. Chem. 2012;287(28):24053–24063. doi: 10.1074/jbc.M112.364851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kredich NM. Biosynthesis of cysteine. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 514–527. [Google Scholar]
- 55.Newton GL, Ta P, Fahey RC. A Mycothiol Synthase Mutant of Mycobacterium smegmatis Produces Novel Thiols and Has an Altered Thiol Redox Status. J. Bacteriol. 2005;187(21):7309–7316. doi: 10.1128/JB.187.21.7309-7316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gengenbacher M, Kaufmann SHE. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol. Rev. 2012;36(3):514–532. doi: 10.1111/j.1574-6976.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pinto R, Leotta L, Shanahan ER, West NP, Leyh TS, Britton W, Triccas JA. Host Cell–Induced Components of the Sulfate Assimilation Pathway Are Major Protective Antigens of Mycobacterium tuberculosis. J. Infect. Dis. 2013;207(5):778–785. doi: 10.1093/infdis/jis751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar A, Farhana A, Guidry L, Saini V, Hondalus M, Steyn AJ. Redox homeostasis in mycobacteria: the key to tuberculosis control? Expert. Rev. Mol. Med. 2011;16(13) doi: 10.1017/S1462399411002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takayama K, Wang C, Besra GS. Pathway to Synthesis and Processing of Mycolic Acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 2005;18(1):81–101. doi: 10.1128/CMR.18.1.81-101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mougous JD, Green RE, Williams SJ, Brenner SE, Bertozzi CR. Sulfotransferases and Sulfatases in Mycobacteria. Chem. Biol. 2002;9(7):767–776. doi: 10.1016/s1074-5521(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 61.Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Böhmer G, Prandi J, Mori L, Puzo G, De Libero G. Diacylated Sulfoglycolipids Are Novel Mycobacterial Antigens Stimulating CD1-restricted T Cells during Infection with Mycobacterium tuberculosis. J. Exp. Med. 2004;199(5):649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hatzios SK, Bertozzi CR. The Regulation of Sulfur Metabolism in Mycobacterium tuberculosis. PLoS Pathog. 2011;7(7):e1002036. doi: 10.1371/journal.ppat.1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nathan C. Antibiotics at the crossroads. Nature. 2004;431(7011):899–902. doi: 10.1038/431899a. [DOI] [PubMed] [Google Scholar]
- 64.Casenghi M, Cole ST, Nathan CF. New Approaches to Filling the Gap in Tuberculosis Drug Discovery. PLoS Med. 2007;4(11):e293. doi: 10.1371/journal.pmed.0040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schelle MW, Bertozzi CR. Sulfate Metabolism in Mycobacteria. Chembiochem. 2006;7(10):1516–1524. doi: 10.1002/cbic.200600224. [DOI] [PubMed] [Google Scholar]
- 66.Williams SJ, Senaratne RH, Mougous JD, Riley LW, Bertozzi CR. 5′-Adenosinephosphosulfate Lies at a Metabolic Branch Point in Mycobacteria. J. Biol. Chem. 2002;277(36):32606–32615. doi: 10.1074/jbc.M204613200. [DOI] [PubMed] [Google Scholar]
- 67.Aoki Y, Yamamoto M, Hosseini-Mazinani SM, Koshikawa N, Sugimoto K, Arisawa M. Antifungal azoxybacilin exhibits activity by inhibiting gene expression of sulfite reductase. Antimicrob. Agents Chemother. 1996;40(1):127–132. doi: 10.1128/aac.40.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ejim LJ, Blanchard JE, Koteva KP, Sumerfield R, Elowe NH, Chechetto JD, Brown ED, Junop MS, Wright GD. Inhibitors of Bacterial Cystathionine β-Lyase: Leads for New Antimicrobial Agents and Probes of Enzyme Structure and Function. J. Med. Chem. 2007;50(4):755–764. doi: 10.1021/jm061132r. [DOI] [PubMed] [Google Scholar]
- 69.Jacques SL, Mirza IA, Ejim L, Koteva K, Hughes DW, Green K, Kinach R, Honek JF, Lai HK, Berghuis AM, Wright GD. Enzyme-Assisted Suicide: Molecular Basis for the Antifungal Activity of 5-Hydroxy-4-Oxonorvaline by Potent Inhibition of Homoserine Dehydrogenase. Chem. Biol. 2003;10(10):989–995. doi: 10.1016/j.chembiol.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 70.Kugler M, Loeffler W, Rapp C, Kern A, Jung G. Rhizocticin A, an antifungal phosphono-oligopeptide of Bacillus subtilis ATCC 6633: biological properties. Arch. Microbiol. 1990;153(3):276–281. doi: 10.1007/BF00249082. [DOI] [PubMed] [Google Scholar]
- 71.Łochowska A, Iwanicka-Nowicka R, Zielak A, Modelewska A, Thomas MS, Hryniewicz MM. Regulation of Sulfur Assimilation Pathways in Burkholderia cenocepacia through Control of Genes by the SsuR Transcription Factor. J. Bacteriol. 2011;193(8):1843–1853. doi: 10.1128/JB.00483-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lestrate P, Delrue RM, Danese I, Didembourg C, Taminiau B, Mertens P, De Bolle X, Tibor A, Tang CM, Letesson JJ. Identification and characterization of in vivo attenuated mutants of Brucella melitensis. Mol. Microbiol. 2000;38(3):543–551. doi: 10.1046/j.1365-2958.2000.02150.x. [DOI] [PubMed] [Google Scholar]
- 73.Senaratne RH, De Silva AD, Williams SJ, Mougous JD, Reader JR, Zhang T, Chan S, Sidders B, Lee DH, Chan J, Bertozzi CR, Riley LW. 5′-Adenosinephosphosulphate reductase (CysH) protects Mycobacterium tuberculosis against free radicals during chronic infection phase in mice. Mol. Microbiol. 2006;59(6):1744–1753. doi: 10.1111/j.1365-2958.2006.05075.x. [DOI] [PubMed] [Google Scholar]
- 74.Yang Z, Pascon RC, Alspaugh A, Cox GM, McCusker JH. Molecular and genetic analysis of the Cryptococcus neoformans MET3 gene and a met3 mutant. Microbiology. 2002;148(8):2617–2625. doi: 10.1099/00221287-148-8-2617. [DOI] [PubMed] [Google Scholar]
- 75.Sareen D, Newton GL, Fahey RC, Buchmeier NA. Mycothiol Is Essential for Growth of Mycobacterium tuberculosis Erdman. J. Bacteriol. 2003;185(22):6736–6740. doi: 10.1128/JB.185.22.6736-6740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huet G, Daffé M, Saves I. Identification of the Mycobacterium tuberculosis SUF Machinery as the Exclusive Mycobacterial System of [Fe-S] Cluster Assembly: Evidence for Its Implication in the Pathogen's Survival. J. Bacteriol. 2005;187(17):6137–6146. doi: 10.1128/JB.187.17.6137-6146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buchmeier N, Fahey RC. The mshA gene encoding the glycosyltransferase of mycothiol biosynthesis is essential in Mycobacterium tuberculosis Erdman. FEMS Microbiol. Lett. 2006;264(1):74–79. doi: 10.1111/j.1574-6968.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 78.Wooff E, Michell SL, Gordon SV, Chambers MA, Bardarov S, Jacobs WR, Hewinson RG, Wheeler PR. Functional genomics reveals the sole sulphate transporter of the Mycobacterium tuberculosis complex and its relevance to the acquisition of sulphur in vivo. Mol. Microbiol. 2002;43(3):653–663. doi: 10.1046/j.1365-2958.2002.02771.x. [DOI] [PubMed] [Google Scholar]
- 79.Wheeler PRB, JS . Tuberculosis and the Tubercle Baci. ASM press; 2005. [Google Scholar]
- 80.Sun M, Andreassi JL, Liu S, Pinto R, Triccas JA, Leyh TS. The Trifunctional Sulfate-activating Complex (SAC) of Mycobacterium tuberculosis. J. Biol. Chem. 2005;280(9):7861–7866. doi: 10.1074/jbc.M409613200. [DOI] [PubMed] [Google Scholar]
- 81.Zeng L, Shi T, Zhao Q, Xie J. Mycobacterium Sulfur Metabolism and Implications for Novel Drug Targets. Cell Biochem. Biophys. 2012:1–7. doi: 10.1007/s12013-012-9410-x. [DOI] [PubMed] [Google Scholar]
- 82.Mougous JD, Leavell MD, Senaratne RH, Leigh CD, Williams SJ, Riley LW, Leary JA, Bertozzi CR. Discovery of sulfated metabolites in mycobacteria with a genetic and mass spectrometric approach. Proc. Natl. Acad. Sci. U. S. A. 2002;99(26):17037–17042. doi: 10.1073/pnas.252514899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carroll KS, Gao H, Chen H, Stout CD, Leary JA, Bertozzi CR. A Conserved Mechanism for Sulfonucleotide Reduction. PLoS Biol. 2005;3(8):e250. doi: 10.1371/journal.pbio.0030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schnell R, Sandalova T, Hellman U, Lindqvist Y, Schneider G. Siroheme- and [Fe4-S4]-dependent NirA from Mycobacterium tuberculosis Is a Sulfite Reductase with a Covalent Cys-Tyr Bond in the Active Site. J. Biol. Chem. 2005;280(29):27319–27328. doi: 10.1074/jbc.M502560200. [DOI] [PubMed] [Google Scholar]
- 85.Leyh TS. The physical biochemistry and molecular genetics of sulfate activation. Crit. Rev. Biochem. Mol. Biol. 1993;28(6):515–542. doi: 10.3109/10409239309085137. [DOI] [PubMed] [Google Scholar]
- 86.Markovich D. Physiological Roles and Regulation of Mammalian Sulfate Transporters. Physiol. Rev. 2001;81(4):1499–1533. doi: 10.1152/physrev.2001.81.4.1499. [DOI] [PubMed] [Google Scholar]
- 87.McAdam RA, Weisbrod TR, Martin J, Scuderi JD, Brown AM, Cirillo JD, Bloom BR, Jacobs WR., Jr In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect. Immun. 1995;63(3):1004–1012. doi: 10.1128/iai.63.3.1004-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schelle MW, Bertozzi CR. Sulfate metabolism in mycobacteria. Chembiochem. 2006;7(10):1516–1524. doi: 10.1002/cbic.200600224. [DOI] [PubMed] [Google Scholar]
- 89.Raman K, Yeturu K, Chandra N. targetTB: a target identification pipeline for Mycobacterium tuberculosis through an interactome, reactome and genome-scale structural analysis. BMC Syst. Biol. 2008;2(109) doi: 10.1186/1752-0509-2-109. 1752-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zolotarev AS, Unnikrishnan M, Shmukler BE, Clark JS, Vandorpe DH, Grigorieff N, Rubin EJ, Alper SL. Increased sulfate uptake by E. coli overexpressing the SLC26-related SulP protein Rv1739c from Mycobacterium tuberculosis. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2008;149(3):255–266. doi: 10.1016/j.cbpa.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl. Acad. Sci. U. S. A. 2008;105(10):3963–3967. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song H, Niederweis M. Uptake of sulfate but not phosphate by Mycobacterium tuberculosis is slower than that for Mycobacterium smegmatis. J. Bacteriol. 2012;194(5):956–964. doi: 10.1128/JB.06132-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kartmann B, Stenger S, Niederweis M. Porins in the cell wall of Mycobacterium tuberculosis. J. Bacteriol. 1999;181(20):6543–6546. doi: 10.1128/jb.181.20.6543-6546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niederweis M. Nutrient acquisition by mycobacteria. Microbiology. 2008;154(Pt 3):679–692. doi: 10.1099/mic.0.2007/012872-0. [DOI] [PubMed] [Google Scholar]
- 95.Lichtinger T, Heym B, Maier E, Eichner H, Cole ST, Benz R. Evidence for a small anion-selective channel in the cell wall of Mycobacterium bovis BCG besides a wide cation-selective pore. FEBS Lett. 1999;454(3):349–355. doi: 10.1016/s0014-5793(99)00844-3. [DOI] [PubMed] [Google Scholar]
- 96.Liu C, Suo Y, Leyh TS. The Energetic Linkage of GTP Hydrolysis and the Synthesis of Activated Sulfate. Biochemistry (Mosc.) 1994;33(23):7309–7314. doi: 10.1021/bi00189a036. [DOI] [PubMed] [Google Scholar]
- 97.Mougous JD, Lee DH, Hubbard SC, Schelle MW, Vocadlo DJ, Berger JM, Bertozzi CR. Molecular Basis for G Protein Control of the Prokaryotic ATP Sulfurylase. Mol. Cell. 2006;21(1):109–122. doi: 10.1016/j.molcel.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 98.Pinto R, Leotta L, Shanahan ER, West NP, Leyh TS, Britton W, Triccas JA. Host Cell–Induced Components of the Sulfate Assimilation Pathway Are Major Protective Antigens of Mycobacterium tuberculosis. J. Infect. Dis. 2012 doi: 10.1093/infdis/jis751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun M, Leyh TS. Channeling in Sulfate Activating Complexes†. Biochemistry (Mosc.) 2006;45(38):11304–11311. doi: 10.1021/bi060421e. [DOI] [PubMed] [Google Scholar]
- 100.Murphy DJ, Brown JR. Identification of gene targets against dormant phase Mycobacterium tuberculosis infections. BMC Infect. Dis. 2007;7:84. doi: 10.1186/1471-2334-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Triccas JA, Berthet FX, Pelicic V, Gicquel B. Use of fluorescence induction and sucrose counterselection to identify Mycobacterium tuberculosis genes expressed within host cells. Microbiology. 1999;145(Pt 10):2923–2930. doi: 10.1099/00221287-145-10-2923. [DOI] [PubMed] [Google Scholar]
- 102.Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages. J. Exp. Med. 2003;198(5):693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 2002;43(3):717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 104.Sun M, Andreassi JL, II, Liu S, Pinto R, Triccas JA, Leyh TS. The Trifunctional Sulfate-activating Complex (SAC) of Mycobacterium tuberculosis. J. Biol. Chem. 2005;280(9):7861–7866. doi: 10.1074/jbc.M409613200. [DOI] [PubMed] [Google Scholar]
- 105.Rustad TR, Harrell MI, Liao R, Sherman DR. The Enduring Hypoxic Response of Mycobacterium tuberculosis. PLoS ONE. 2008;3(1):e1502. doi: 10.1371/journal.pone.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boshoff HIM, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE. The Transcriptional Responses of Mycobacterium tuberculosis to Inhibitors of Metabolism. J. Biol. Chem. 2004;279(38):40174–40184. doi: 10.1074/jbc.M406796200. [DOI] [PubMed] [Google Scholar]
- 107.Criddle DN, Gillies S, Baumgartner-Wilson HK, Jaffar M, Chinje EC, Passmore S, Chvanov M, Barrow S, Gerasimenko OV, Tepikin AV, Sutton R, Petersen OH. Menadione-induced Reactive Oxygen Species Generation via Redox Cycling Promotes Apoptosis of Murine Pancreatic Acinar Cells. J. Biol. Chem. 2006;281(52):40485–40492. doi: 10.1074/jbc.M607704200. [DOI] [PubMed] [Google Scholar]
- 108.Provvedi R, Boldrin F, Falciani F, Palù G, Manganelli R. Global transcriptional response to vancomycin in Mycobacterium tuberculosis. Microbiology. 2009;155(4):1093–1102. doi: 10.1099/mic.0.024802-0. [DOI] [PubMed] [Google Scholar]
- 109.Gomez JE, Chen JM, Bishai WR. Sigma factors of Mycobacterium tuberculosis. Tuber. Lung Dis. 1997;78(3-4):175–183. doi: 10.1016/s0962-8479(97)90024-1. [DOI] [PubMed] [Google Scholar]
- 110.Kaushal D, Schroeder BG, Tyagi S, Yoshimatsu T, Scott C, Ko C, Carpenter L, Mehrotra J, Manabe YC, Fleischmann RD, Bishai WR. Reduced immunopathology and mortality despite tissue persistence in a Mycobacterium tuberculosis mutant lacking alternative σ factor, SigH. Proc. Natl. Acad. Sci. U. S. A. 2002;99(12):8330–8335. doi: 10.1073/pnas.102055799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manganelli R, Voskuil MI, Schoolnik GK, Dubnau E, Gomez M, Smith I. Role of the extracytoplasmic-function σ Factor σH in Mycobacterium tuberculosis global gene expression. Mol. Microbiol. 2002;45(2):365–374. doi: 10.1046/j.1365-2958.2002.03005.x. [DOI] [PubMed] [Google Scholar]
- 112.Raman S, Song T, Puyang X, Bardarov S, Jacobs WR, Jr, Husson RN. The alternative sigma factor SigH regulates major components of oxidative and heat stress responses in Mycobacterium tuberculosis. J. Bacteriol. 2001;183(20):6119–6125. doi: 10.1128/JB.183.20.6119-6125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rohde KH, Abramovitch RB, Russell DG. Mycobacterium tuberculosis Invasion of Macrophages: Linking Bacterial Gene Expression to Environmental Cues. Cell Host Microbe. 2007;2(5):352–364. doi: 10.1016/j.chom.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 114.Smriti Mehra DK. Functional Genomics Reveals Extended Roles of the Mycobacterium tuberculosis Stress Response Factor σH. J. Bacteriol. 2009;191(12):3965–3980. doi: 10.1128/JB.00064-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park ST, Kang C-M, Husson RN. Regulation of the SigH stress response regulon by an essential protein kinase in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2008;105(35):13105–13110. doi: 10.1073/pnas.0801143105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Song T, Dove SL, Lee KH, Husson RN. RshA, an anti-sigma factor that regulates the activity of the mycobacterial stress response sigma factor SigH. Mol. Microbiol. 2003;50(3):949–959. doi: 10.1046/j.1365-2958.2003.03739.x. [DOI] [PubMed] [Google Scholar]
- 117.Kumar S, Badireddy S, Pal K, Sharma S, Arora C, Garg SK, Alam MS, Agrawal P, Anand GS, Swaminathan K. Interaction of Mycobacterium tuberculosis RshA and SigH Is Mediated by Salt Bridges. PLoS ONE. 2012;7(8):e43676. doi: 10.1371/journal.pone.0043676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Newton GL, Fahey RC. Regulation of mycothiol metabolism by sigma(R) and the thiol redox sensor anti-sigma factor RsrA. Mol. Microbiol. 2008;68(4):805–809. doi: 10.1111/j.1365-2958.2008.06222.x. [DOI] [PubMed] [Google Scholar]
- 119.Park JH, Roe JH. Mycothiol regulates and is regulated by a thiol-specific antisigma factor RsrA and sigma(R) in Streptomyces coelicolor. Mol. Microbiol. 2008;68(4):861–870. doi: 10.1111/j.1365-2958.2008.06191.x. [DOI] [PubMed] [Google Scholar]
- 120.Burns KE, Baumgart S, Dorrestein PC, Zhai H, McLafferty FW, Begley TP. Reconstitution of a New Cysteine Biosynthetic Pathway in Mycobacterium tuberculosis. J. Am. Chem. Soc. 2005;127(33):11602–11603. doi: 10.1021/ja053476x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ågren D, Schnell R, Oehlmann W, Singh M, Schneider G. Cysteine Synthase (CysM) of Mycobacterium tuberculosis Is an O-Phosphoserine Sulfhydrylase. J. Biol. Chem. 2008;283(46):31567–31574. doi: 10.1074/jbc.M804877200. [DOI] [PubMed] [Google Scholar]
- 122.Hong JA, Bhave DP, Carroll KS. Identification of Critical Ligand Binding Determinants in Mycobacterium tuberculosis Adenosine-5′-phosphosulfate Reductase. J. Med. Chem. 2009;52(17):5485–5495. doi: 10.1021/jm900728u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hatzios SK, Iavarone AT, Bertozzi CR. Rv2131c from Mycobacterium tuberculosis Is a CysQ 3′-Phosphoadenosine-5′-phosphatase†. Biochemistry (Mosc.) 2008;47(21):5823–5831. doi: 10.1021/bi702453s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pi N, Hoang MB, Gao H, Mougous JD, Bertozzi CR, Leary JA. Kinetic measurements and mechanism determination of Stf0 sulfotransferase using mass spectrometry. Anal. Biochem. 2005;341(1):94–104. doi: 10.1016/j.ab.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 125.Neuwald AF, KBR, Brikun I, Kulakauskas S, Suziedelis K, Tomcsanyi T, Leyh TS, Berg DE. cysQ, a gene needed for cysteine synthesis in Escherichia coli K-12 only during aerobic growth. J. Bacteriol. 1992;174(2):415–425. doi: 10.1128/jb.174.2.415-425.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang J, Biswas I. 3′-Phosphoadenosine-5′-Phosphate Phosphatase Activity Is Required for Superoxide Stress Tolerance in Streptococcus mutans. J. Bacteriol. 2009;191(13):4330–4340. doi: 10.1128/JB.00184-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mougous JD, Senaratne RH, Petzold CJ, Jain M, Lee DH, Schelle MW, Leavell MD, Cox JS, Leary JA, Riley LW, Bertozzi CR. A sulfated metabolite produced by stf3 negatively regulates the virulence of Mycobacteriumtuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103(11):4258–4263. doi: 10.1073/pnas.0510861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Holsclaw CM, Sogi KM, Gilmore SA, Schelle MW, Leavell MD, Bertozzi CR, Leary JA. Structural Characterization of a Novel Sulfated Menaquinone produced by stf3 from Mycobacterium tuberculosis. ACS Chem. Biol. 2008;3(10):619–624. doi: 10.1021/cb800145r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bojarová P, Williams SJ. Sulfotransferases, sulfatases and formylglycine-generating enzymes: a sulfation fascination. Curr. Opin. Chem. Biol. 2008;12(5):573–581. doi: 10.1016/j.cbpa.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 130.Hossain MM, Kawarabayasi Y, Kimura M, Kakuta Y. Expression and Functional Analysis of a Predicted AtsG Arylsulphatase Identified from Mycobacterium tuberculosis Genomic Data. J. Biochem. (Tokyo) 2009;146(6):767–769. doi: 10.1093/jb/mvp141. [DOI] [PubMed] [Google Scholar]
- 131.Carlson BL, Ballister ER, Skordalakes E, King DS, Breidenbach MA, Gilmore SA, Berger JM, Bertozzi CR. Function and Structure of a Prokaryotic Formylglycine-generating Enzyme. J. Biol. Chem. 2008;283(29):20117–20125. doi: 10.1074/jbc.M800217200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mougous JD, Green RE, Williams SJ, Brenner SE, Bertozzi CR. Sulfotransferases and sulfatases in mycobacteria. Chem. Biol. 2002;9(7):767–776. doi: 10.1016/s1074-5521(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 133.Armstrong JAB, CR Sulfotransferases as targets for therapeutic interventions. Curr. Opin. Drug Discov. Devel. 2000;3:502–515. [PubMed] [Google Scholar]
- 134.Fukuda M, Hiraoka N, Akama TO, Fukuda MN. Carbohydrate-modifying sulfotransferases: structure, function, and pathophysiology. J. Biol. Chem. 2001;276(51):47747–47750. doi: 10.1074/jbc.R100049200. [DOI] [PubMed] [Google Scholar]
- 135.Schwedock JS, Long SR. Rhizobium meliloti genes involved in sulfate activation: the two copies of nodPQ and a new locus, saa. Genetics. 1992;132(4):899–909. doi: 10.1093/genetics/132.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Roche P, Debelle F, Maillet F, Lerouge P, Faucher C, Truchet G, Denarie J, Prome JC. Molecular basis of symbiotic host specificity in Rhizobium meliloti: nodH and nodPQ genes encode the sulfation of lipo-oligosaccharide signals. Cell. 1991;67(6):1131–1143. doi: 10.1016/0092-8674(91)90290-f. [DOI] [PubMed] [Google Scholar]
- 137.Goren MB. Sulfolipid I of Mycobacterium tuberculosis, strain H37Rv. I. Purification and properties. Biochim. Biophys. Acta. 1970;210(1):116–126. doi: 10.1016/0005-2760(70)90067-6. [DOI] [PubMed] [Google Scholar]
- 138.Goren MB, Brokl O, Das BC, Lederer E. Sulfolipid I of Mycobacterium tuberculosis, strain H37RV. Nature of the acyl substituents. Biochemistry (Mosc.) 1971;10(1):72–81. doi: 10.1021/bi00777a012. [DOI] [PubMed] [Google Scholar]
- 139.Gangadharam PR, Cohn ML, Middlebrook G. Infectivity, Pathogenicity And Sulpholipid Fraction Of Some Indian And British Strains Of Tubercle Bacilli. Tubercle. 1963;44:452–455. doi: 10.1016/s0041-3879(63)80087-2. [DOI] [PubMed] [Google Scholar]
- 140.Goren MB, Brokl O, Schaefer WB. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: correlation of virulence with elaboration of sulfatides and strongly acidic lipids. Infect. Immun. 1974;9(1):142–149. doi: 10.1128/iai.9.1.142-149.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Daffe M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv. Microb. Physiol. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 142.Goren MB, Hart PDA, Young MR, Armstrong JA. Prevention of Phagosome-Lysosome Fusion in Cultured Macrophages by Sulfatides of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 1976;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pabst MJ, Gross JM, Brozna JP, Goren MB. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J. Immunol. 1988;140(2):634–640. [PubMed] [Google Scholar]
- 144.Brozna JP, Horan M, Rademacher JM, Pabst KM, Pabst MJ. Monocyte responses to sulfatide from Mycobacterium tuberculosis: inhibition of priming for enhanced release of superoxide, associated with increased secretion of interleukin-1 and tumor necrosis factor alpha, and altered protein phosphorylation. Infect. Immun. 1991;59(8):2542–2548. doi: 10.1128/iai.59.8.2542-2548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang L, Goren MB, Holzer TJ, Andersen BR. Effect of Mycobacterium tuberculosis-derived sulfolipid I on human phagocytic cells. Infect. Immun. 1988;56(11):2876–2883. doi: 10.1128/iai.56.11.2876-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brodin P, Poquet Y, Levillain F, Peguillet I, Larrouy-Maumus G, Gilleron M, Ewann F, Christophe T, Fenistein D, Jang J, Jang M-S, Park S-J, Rauzier J, Carralot J-P, Shrimpton R, Genovesio A, Gonzalo-Asensio JA, Puzo G, Martin C, Brosch R, Stewart GR, Gicquel B, Neyrolles O. High Content Phenotypic Cell-Based Visual Screen Identifies Mycobacterium tuberculosis Acyltrehalose-Containing Glycolipids Involved in Phagosome Remodeling. PLoS Pathog. 2010;6(9):e1001100. doi: 10.1371/journal.ppat.1001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seeliger JC, Holsclaw CM, Schelle MW, Botyanszki Z, Gilmore SA, Tully SE, Niederweis M, Cravatt BF, Leary JA, Bertozzi CR. Elucidation and Chemical Modulation of Sulfolipid-1 Biosynthesis in Mycobacterium tuberculosis. J. Biol. Chem. 2012;287(11):7990–8000. doi: 10.1074/jbc.M111.315473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bhatt K, Gurcha SS, Bhatt A, Besra GS, Jacobs WR., Jr Two polyketide-synthase-associated acyltransferases are required for sulfolipid biosynthesis in Mycobacterium tuberculosis. Microbiology. 2007;153(Pt 2):513–520. doi: 10.1099/mic.0.2006/003103-0. [DOI] [PubMed] [Google Scholar]
- 149.Converse SE, Mougous JD, Leavell MD, Leary JA, Bertozzi CR, Cox JS. MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence. Proc. Natl. Acad. Sci. U. S. A. 2003;100(10):6121–6126. doi: 10.1073/pnas.1030024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mougous JD, Petzold CJ, Senaratne RH, Lee DH, Akey DL, Lin FL, Munchel SE, Pratt MR, Riley LW, Leary JA, Berger JM, Bertozzi CR. Identification, function and structure of the mycobacterial sulfotransferase that initiates sulfolipid-1 biosynthesis. Nat. Struct. Mol. Biol. 2004;11(8):721–729. doi: 10.1038/nsmb802. [DOI] [PubMed] [Google Scholar]
- 151.Gilmore SA, Schelle MW, Holsclaw CM, Leigh CD, Jain M, Cox JS, Leary JA, Bertozzi CR. Sulfolipid-1 Biosynthesis Restricts Mycobacterium tuberculosis Growth in Human Macrophages. ACS Chem. Biol. 2012;7(5):863–870. doi: 10.1021/cb200311s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Desmarais D, Jablonski PE, Fedarko NS, Roberts MF. 2-Sulfotrehalose, a novel osmolyte in haloalkaliphilic archaea. J. Bacteriol. 1997;179(10):3146–3153. doi: 10.1128/jb.179.10.3146-3153.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guiard J, Collmann A, Garcia-Alles LF, Mourey L, Brando T, Mori L, Gilleron M, Prandi J, De Libero G, Puzo G. Fatty Acyl Structures of Mycobacterium tuberculosis Sulfoglycolipid Govern T Cell Response. J. Immunol. 2009;182(11):7030–7037. doi: 10.4049/jimmunol.0804044. [DOI] [PubMed] [Google Scholar]
- 154.Mougous JD, Leavell MD, Senaratne RH, Leigh CD, Williams SJ, Riley LW, Leary JA, Bertozzi CR. Discovery of sulfated metabolites in mycobacteria with a genetic and mass spectrometric approach. Proc. Natl. Acad. Sci. USA. 2002;99(26):17037–17042. doi: 10.1073/pnas.252514899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khoo KH, Jarboe E, Barker A, Torrelles J, Kuo CW, Chatterjee D. Altered expression profile of the surface glycopeptidolipids in drug-resistant clinical isolates of Mycobacterium avium complex. J. Biol. Chem. 1999;274(14):9778–9785. doi: 10.1074/jbc.274.14.9778. [DOI] [PubMed] [Google Scholar]
- 156.Lopez JA, Ludwig EH, McCarthy BJ. Polymorphism of human glycoprotein Ib alpha results from a variable number of tandem repeats of a 13-amino acid sequence in the mucin-like macroglycopeptide region. Structure/function implications. J. Biol. Chem. 1992;267(14):10055–10061. [PubMed] [Google Scholar]
- 157.McCarthy C. Synthesis and release of sulfolipid by Mycobacterium avium during growth and cell division. Infect. Immun. 1976;14(5):1241–1252. doi: 10.1128/iai.14.5.1241-1252.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pi N, Hoang MB, Gao H, Mougous JD, Bertozzi CR, Leary JA. Kinetic measurements and mechanism determination of Stf0 sulfotransferase using mass spectrometry. Anal. Biochem. 2005;341(1):94–104. doi: 10.1016/j.ab.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 159.Mougous JD, Senaratne RH, Petzold CJ, Jain M, Lee DH, Schelle MW, Leavell MD, Cox JS, Leary JA, Riley LW, Bertozzi CR. A sulfated metabolite produced by stf3 negatively regulates the virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 2006;103(11):4258–4263. doi: 10.1073/pnas.0510861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hossain MM, Moriizumi Y, Tanaka S, Kimura M, Kakuta Y. Crystal structure of sulfotransferase STF9 from Mycobacterium avium. Mol. Cell. Biochem. 2012;361(1-2):97–104. doi: 10.1007/s11010-011-1093-x. [DOI] [PubMed] [Google Scholar]
- 161.Hossain MMM, Yuuji, Tanaka Shotaro, Kimura Makoto, Kakuta Yoshimitsu. Molecular cloning, expression, and functional analysis of a predicted sulfotransferase STF9 from Mycobacterium avium. Mol. Cell. Biochem. 2011;350(1):155–162. doi: 10.1007/s11010-010-0693-1. [DOI] [PubMed] [Google Scholar]
- 162.Liu J, Shworak NW, Sinay P, Schwartz JJ, Zhang L, Fritze LMS, Rosenberg RD. Expression of Heparan Sulfate d-Glucosaminyl 3-O-Sulfotransferase Isoforms Reveals Novel Substrate Specificities. J. Biol. Chem. 1999;274(8):5185–5192. doi: 10.1074/jbc.274.8.5185. [DOI] [PubMed] [Google Scholar]
- 163.Shailubhai K, Khai Huynh Q, Boddupalli H, Yu HH, Jacob GS. Purification and Characterization of a Lymph Node Sulfotransferase Responsible for 6-O-Sulfation of the Galactose Residues in 2'-Fucosyllactose and Other Sialyl LewisX-Related Sugars. Biochem. Biophys. Res. Commun. 1999;256(1):170–176. doi: 10.1006/bbrc.1999.0258. [DOI] [PubMed] [Google Scholar]
- 164.Hooper LV, Baenziger JU. Sulfotransferase and Glycosyltransferase Analyses Using a 96-Well Filtration Plate. Anal. Biochem. 1993;212(1):128–133. doi: 10.1006/abio.1993.1301. [DOI] [PubMed] [Google Scholar]
- 165.Wu ZL, Ethen CM, Larson S, Prather B, Jiang W. A versatile polyacrylamide gel electrophoresis based sulfotransferase assay. BMC Biotechnol. 2010;10(11):1472–6750. doi: 10.1186/1472-6750-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen WT, Liu MC, Yang YS. Fluorometric assay for alcohol sulfotransferase. Anal. Biochem. 2005;339(1):54–60. doi: 10.1016/j.ab.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 167.Frame LT, Ozawa S, Nowell SA, Chou HC, DeLongchamp RR, Doerge DR, Lang NP, Kadlubar FF. A simple colorimetric assay for phenotyping the major human thermostable phenol sulfotransferase (SULT1A1) using platelet cytosols. Drug Metab. Dispos. 2000;28(9):1063–1068. [PubMed] [Google Scholar]
- 168.Danan LM, Yu Z, Hoffhines AJ, Moore KL, Leary JA. Mass Spectrometric Kinetic Analysis of Human Tyrosylprotein Sulfotransferase-1 and -2. J. Am. Soc. Mass Spectrom. 2008;19(10):1459–1466. doi: 10.1016/j.jasms.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Prather B, Ethen CM, Machacek M, Wu ZL. Golgi-resident PAP-specific 3′-phosphatase-coupled sulfotransferase assays. Anal. Biochem. 2012;423(1):86–92. doi: 10.1016/j.ab.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 170.Rath VL, Verdugo D, Hemmerich S. Sulfotransferase structural biology and inhibitor discovery. Drug Discov. Today. 2004;9(23):1003–1011. doi: 10.1016/S1359-6446(04)03273-8. [DOI] [PubMed] [Google Scholar]
- 171.Nimmagadda D, Cherala G, Ghatta S. Cytosolic sulfotransferases. Indian J. Exp. Biol. 2006;44(3):171–182. [PubMed] [Google Scholar]
- 172.Kakuta Y, Pedersen LG, Carter CW, Negishi M, Pedersen LC. Crystal structure of estrogen sulphotransferase. Nat. Struct. Biol. 1997;4(11):904–908. doi: 10.1038/nsb1197-904. [DOI] [PubMed] [Google Scholar]
- 173.Gamage NU, Duggleby RG, Barnett AC, Tresillian M, Latham CF, Liyou NE, McManus ME, Martin JL. Structure of a human carcinogen-converting enzyme, SULT1A1. Structural and kinetic implications of substrate inhibition. J. Biol. Chem. 2003;278(9):7655–7662. doi: 10.1074/jbc.M207246200. [DOI] [PubMed] [Google Scholar]
- 174.Bidwell LM, McManus ME, Gaedigk A, Kakuta Y, Negishi M, Pedersen L, Martin JL. Crystal structure of human catecholamine sulfotransferase. J. Mol. Biol. 1999;293(3):521–530. doi: 10.1006/jmbi.1999.3153. [DOI] [PubMed] [Google Scholar]
- 175.Kakuta Y, Sueyoshi T, Negishi M, Pedersen LC. Crystal structure of the sulfotransferase domain of human heparan sulfate N-deacetylase/ N-sulfotransferase 1. J. Biol. Chem. 1999;274(16):10673–10676. doi: 10.1074/jbc.274.16.10673. [DOI] [PubMed] [Google Scholar]
- 176.Armstrong JI, Ge X, Verdugo DE, Winans KA, Leary JA, Bertozzi CR. A library approach to the generation of bisubstrate analogue sulfotransferase inhibitors. Org. Lett. 2001;3(17):2657–2660. doi: 10.1021/ol0162217. [DOI] [PubMed] [Google Scholar]
- 177.Armstrong JI, Verdugo DE, Bertozzi CR. Synthesis of a bisubstrate analogue targeting estrogen sulfotransferase. J. Org. Chem. 2003;68(1):170–173. doi: 10.1021/jo0260443. [DOI] [PubMed] [Google Scholar]
- 178.Radzicka A, Wolfenden R. Transition state and multisubstrate analog inhibitors. Methods Enzymol. 1995;249:284–312. doi: 10.1016/0076-6879(95)49039-6. [DOI] [PubMed] [Google Scholar]
- 179.Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology. 2000;141(5):1897–1900. doi: 10.1210/endo.141.5.7530. [DOI] [PubMed] [Google Scholar]
- 180.Otake Y, Nolan AL, Walle UK, Walle T. Quercetin and resveratrol potently reduce estrogen sulfotransferase activity in normal human mammary epithelial cells. J. Steroid Biochem. Mol. Biol. 2000;73(5):265–270. doi: 10.1016/s0960-0760(00)00073-x. [DOI] [PubMed] [Google Scholar]
- 181.Gonzalez-Amaro R, Sanchez-Madrid F. Cell adhesion molecules: selectins and integrins. Crit. Rev. Immunol. 1999;19(5-6):389–429. [PubMed] [Google Scholar]
- 182.Bistrup A, Bhakta S, Lee JK, Belov YY, Gunn MD, Zuo FR, Huang CC, Kannagi R, Rosen SD, Hemmerich S. Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin. J. Cell Biol. 1999;145(4):899–910. doi: 10.1083/jcb.145.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wong C-H. J. Am. Chem. Soc. 1997;119:8152. doi: 10.1021/ja964290o. [DOI] [PubMed] [Google Scholar]
- 184.Kogan TP, Dupre B, Bui H, McAbee KL, Kassir JM, Scott IL, Hu X, Vanderslice P, Beck PJ, Dixon RA. Novel synthetic inhibitors of selectin-mediated cell adhesion: synthesis of 1,6-bis[3-(3-carboxymethylphenyl)-4-(2-alpha-D-mannopyranosyloxy)phenyl]hexane (TBC1269) J. Med. Chem. 1998;41(7):1099–1111. doi: 10.1021/jm9704917. [DOI] [PubMed] [Google Scholar]
- 185.Armstrong JI, Portley AR, Chang YT, Nierengarten DM, Cook BN, Bowman KG, Bishop A, Gray NS, Shokat KM, Schultz PG, Bertozzi CR. Discovery of Carbohydrate Sulfotransferase Inhibitors from a Kinase-Directed Library Angew. Chem. Int. Ed. Engl. 2000;39(7):1303–1306. doi: 10.1002/(sici)1521-3773(20000403)39:7<1303::aid-anie1303>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 186.Kakuta Y, Petrotchenko EV, Pedersen LC, Negishi M. The sulfuryl transfer mechanism. Crystal structure of a vanadate complex of estrogen sulfotransferase and mutational analysis. J. Biol. Chem. 1998;273(42):27325–27330. doi: 10.1074/jbc.273.42.27325. [DOI] [PubMed] [Google Scholar]
- 187.Chang YT, Gray NS, Rosania GR, Sutherlin DP, Kwon S, Norman TC, Sarohia R, Leost M, Meijer L, Schultz PG. Synthesis and application of functionally diverse 2,6,9-trisubstituted purine libraries as CDK inhibitors. Chem. Biol. 1999;6(6):361–375. doi: 10.1016/S1074-5521(99)80048-9. [DOI] [PubMed] [Google Scholar]
- 188.Chapman E, Ding S, Schultz PG, Wong CH. A potent and highly selective sulfotransferase inhibitor. J. Am. Chem. Soc. 2002;124(49):14524–14525. doi: 10.1021/ja021086u. [DOI] [PubMed] [Google Scholar]
- 189.Verdugo GE. In: Carbohydrate-based Brug Discovery. Wong C-H, editor. Vol. 2. Wiley-VCH; 2003. pp. 781–802. [Google Scholar]
- 190.Kehoe JW, Maly DJ, Verdugo DE, Armstrong JI, Cook BN, Ouyang YB, Moore KL, Ellman JA, Bertozzi CR. Tyrosylprotein sulfotransferase inhibitors generated by combinatorial target-guided ligand assembly. Bioorg. Med. Chem. Lett. 2002;12(3):329–332. doi: 10.1016/s0960-894x(01)00744-2. [DOI] [PubMed] [Google Scholar]
- 191.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA. 2000;97(16):8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75(7):1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 193.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 1995;9(2):202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 194.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci. STKE. 2006;2006(349):re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 195.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 196.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92(9):3007–3017. [PubMed] [Google Scholar]
- 197.Escolar L, Pérez-Martín J, de Lorenzo V. Opening the Iron Box: Transcriptional Metalloregulation by the Fur Protein. J. Bacteriol. 1999;181(20):6223–6229. doi: 10.1128/jb.181.20.6223-6229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Friedman YE, O'Brian MR. A Novel DNA-binding Site for the Ferric Uptake Regulator (Fur) Protein from Bradyrhizobium japonicum. J. Biol. Chem. 2003;278(40):38395–38401. doi: 10.1074/jbc.M306710200. [DOI] [PubMed] [Google Scholar]
- 199.Chan JF, JL . Nitric oxide in Mycobacterium tuberculosis infection. In: Fang FC, editor. Nitric Oxide and Infection. New York: Kluwer Academic and Plenum Publishers; 1999. pp. 281–310. [Google Scholar]
- 200.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proceedings of the National Academy of Sciences. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Padmaja S, Huie RE. The reaction of nitric oxide with organic peroxyl radicals. Biochem. Biophys. Res. Commun. 1993;195(2):539–544. doi: 10.1006/bbrc.1993.2079. [DOI] [PubMed] [Google Scholar]
- 202.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991;266(7):4244–4250. [PubMed] [Google Scholar]
- 203.Schlosser-Silverman E, Elgrably-Weiss M, Rosenshine I, Kohen R, Altuvia S. Characterization of Escherichia coli DNA lesions generated within J774 macrophages. J. Bacteriol. 2000;182(18):5225–5230. doi: 10.1128/jb.182.18.5225-5230.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 1992;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291(5508):1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 206.Rhee KY, Erdjument-Bromage H, Tempst P, Nathan CF. S-nitroso proteome of Mycobacterium tuberculosis: Enzymes of intermediary metabolism and antioxidant defense. Proc. Natl. Acad. Sci. U. S. A. 2005;102(2):467–472. doi: 10.1073/pnas.0406133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cooper AM, Segal BH, Frank AA, Holland SM, Orme IM. Transient loss of resistance to pulmonary tuberculosis in p47(phox−/−) mice. Infect. Immun. 2000;68(3):1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ng VH, Cox JS, Sousa AO, MacMicking JD, McKinney JD. Role of KatG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol. Microbiol. 2004;52(5):1291–1302. doi: 10.1111/j.1365-2958.2004.04078.x. [DOI] [PubMed] [Google Scholar]
- 209.Daniel DS, Dai G, Singh CR, Lindsey DR, Smith AK, Dhandayuthapani S, Hunter RL, Jr, Jagannath C. The reduced bactericidal function of complement C5-deficient murine macrophages is associated with defects in the synthesis and delivery of reactive oxygen radicals to mycobacterial phagosomes. J. Immunol. 2006;177(7):4688–4698. doi: 10.4049/jimmunol.177.7.4688. [DOI] [PubMed] [Google Scholar]
- 210.Dosanjh NS, Rawat M, Chung JH, Av-Gay Y. Thiol specific oxidative stress response in Mycobacteria. FEMS Microbiol. Lett. 2005;249(1):87–94. doi: 10.1016/j.femsle.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 211.Jaeger T, Budde H, Flohe L, Menge U, Singh M, Trujillo M, Radi R. Multiple thioredoxin-mediated routes to detoxify hydroperoxides in Mycobacterium tuberculosis. Arch. Biochem. Biophys. 2004;423(1):182–191. doi: 10.1016/j.abb.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 212.Jaeger T, Flohe L. The thiol-based redox networks of pathogens: unexploited targets in the search for new drugs. Biofactors. 2006;27(1-4):109–120. doi: 10.1002/biof.5520270110. [DOI] [PubMed] [Google Scholar]
- 213.Bhat SA, Singh N, Trivedi A, Kansal P, Gupta P, Kumar A. The mechanism of redox sensing in Mycobacterium tuberculosis. Free Radic. Biol. Med. 2012;53(8):1625–1641. doi: 10.1016/j.freeradbiomed.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 214.Buchmeier NA, Newton GL, Koledin T, Fahey RC. Association of mycothiol with protection of Mycobacterium tuberculosis from toxic oxidants and antibiotics. Mol. Microbiol. 2003;47(6):1723–1732. doi: 10.1046/j.1365-2958.2003.03416.x. [DOI] [PubMed] [Google Scholar]
- 215.Haramaki N, Han D, Handelman GJ, Tritschler HJ, Packer L. Cytosolic and mitochondrial systems for NADH- and NADPH-dependent reduction of alpha-lipoic acid. Free Radic. Biol. Med. 1997;22(3):535–542. doi: 10.1016/s0891-5849(96)00400-5. [DOI] [PubMed] [Google Scholar]
- 216.Kwon YW, Masutani H, Nakamura H, Ishii Y, Yodoi J. Redox regulation of cell growth and cell death. Biol. Chem. 2003;384(7):991–996. doi: 10.1515/BC.2003.111. [DOI] [PubMed] [Google Scholar]
- 217.Muller S. Redox and antioxidant systems of the malaria parasite Plasmodium falciparum. Mol. Microbiol. 2004;53(5):1291–1305. doi: 10.1111/j.1365-2958.2004.04257.x. [DOI] [PubMed] [Google Scholar]
- 218.Rawat M, Newton GL, Ko M, Martinez GJ, Fahey RC, Av-Gay Y. Mycothiol-deficient Mycobacterium smegmatis mutants are hypersensitive to alkylating agents, free radicals, and antibiotics. Antimicrob. Agents Chemother. 2002;46(11):3348–3355. doi: 10.1128/AAC.46.11.3348-3355.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Newton GL, Fahey RC. Mycothiol biochemistry. Arch. Microbiol. 2002;178(6):388–394. doi: 10.1007/s00203-002-0469-4. [DOI] [PubMed] [Google Scholar]
- 220.Senaratne RH, De Silva AD, Williams SJ, Mougous JD, Reader JR, Zhang T, Chan S, Sidders B, Lee DH, Chan J, Bertozzi CR, Riley LW. 5'-Adenosinephosphosulphate reductase (CysH) protects Mycobacterium tuberculosis against free radicals during chronic infection phase in mice. Mol. Microbiol. 2006;59(6):1744–1753. doi: 10.1111/j.1365-2958.2006.05075.x. [DOI] [PubMed] [Google Scholar]
- 221.Williams SJ, Senaratne RH, Mougous JD, Riley LW, Bertozzi CR. 5'-adenosinephosphosulfate lies at a metabolic branch point in mycobacteria. J. Biol. Chem. 2002;277(36):32606–32615. doi: 10.1074/jbc.M204613200. [DOI] [PubMed] [Google Scholar]
- 222.Hampshire T, Soneji S, Bacon J, James BW, Hinds J, Laing K, Stabler RA, Marsh PD, Butcher PD. Stationary phase gene expression of Mycobacterium tuberculosis following a progressive nutrient depletion: a model for persistent organisms? Tuberculosis (Edinb) 2004;84(3-4):228–238. doi: 10.1016/j.tube.2003.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Manganelli R, Voskuil MI, Schoolnik GK, Dubnau E, Gomez M, Smith I. Role of the extracytoplasmic-function sigma factor sigma(H) in Mycobacterium tuberculosis global gene expression. Mol. Microbiol. 2002;45(2):365–374. doi: 10.1046/j.1365-2958.2002.03005.x. [DOI] [PubMed] [Google Scholar]
- 224.Ohno H, Zhu G, Mohan VP, Chu D, Kohno S, Jacobs WR, Jr, Chan J. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell Microbiol. 2003;5(9):637–648. doi: 10.1046/j.1462-5822.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- 225.Rengarajan J, Bloom BR, Rubin EJ. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. USA. 2005;102(23):8327–8332. doi: 10.1073/pnas.0503272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sassetti CM, Boyd DH, Rubin EJ. Comprehensive identification of conditionally essential genes in mycobacteria. Proc. Natl. Acad. Sci. USA. 2001;98(22):12712–12717. doi: 10.1073/pnas.231275498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 2003;48(1):77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 228.Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. USA. 2003;100(22):12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J. Exp. Med. 2003;198(5):693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha - crystallin. Proc. Natl. Acad. Sci. U. S. A. 2001;98(13):7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Brynildsen MP, Winkler JA, Spina CS, MacDonald IC, Collins JJ. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat Biotech. 2013;31(2):160–165. doi: 10.1038/nbt.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE. Tuberculous Granulomas Are Hypoxic in Guinea Pigs, Rabbits, and Nonhuman Primates. Infect. Immun. 2008;76(6):2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bulatovic VM, Wengenack NL, Uhl JR, Hall L, Roberts GD, Cockerill FR, Rusnak F. Oxidative stress increases susceptibility of Mycobacterium tuberculosis to isoniazid. Antimicrob. Agents Chemother. 2002;46(9):2765–2771. doi: 10.1128/AAC.46.9.2765-2771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cade CE, Dlouhy AC, Medzihradszky KF, Salas-Castillo SP, Ghiladi RA. Isoniazid-resistance conferring mutations in Mycobacterium tuberculosis KatG: Catalase, peroxidase, and INH-NADH adduct formation activities. Protein Sci. 2010;19(3):458–474. doi: 10.1002/pro.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int. J. Tuberc. Lung Dis. 2003;7(1):6–21. [PubMed] [Google Scholar]
- 236.Kim J-J, Lee H-M, Shin D-M, Kim W, Yuk J-M, Jin Hyo S, Lee S-H, Cha G-H, Kim J-M, Lee Z-W, Shin Sung J, Yoo H, Park Young K, Park Jin B, Chung J, Yoshimori T, Jo E-K. Host Cell Autophagy Activated by Antibiotics Is Required for Their Effective Antimycobacterial Drug Action. Cell Host Microbe. 2012;11(5):457–468. doi: 10.1016/j.chom.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 237.Walubo A, Smith PJ, Folb PI. Oxidative stress during antituberculous therapy in young and elderly patients. Biomed. Environ. Sci. 1995;8(2):106–113. [PubMed] [Google Scholar]
- 238.Sharma R, Muttil P, Yadav AB, Rath SK, Bajpai VK, Mani U, Misra A. Uptake of inhalable microparticles affects defence responses of macrophages infected with Mycobacterium tuberculosis H37Ra. J. Antimicrob. Chemother. 2007;59(3):499–506. doi: 10.1093/jac/dkl533. [DOI] [PubMed] [Google Scholar]
- 239.Grant SS, Kaufmann BB, Chand NS, Haseley N, Hung DT. Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc. Natl. Acad. Sci. U. S. A. 2012;109(30):12147–12152. doi: 10.1073/pnas.1203735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell. 2007;130(5):797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 241.Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 2007;3(91):13. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang X, Zhao X. Contribution of Oxidative Damage to Antimicrobial Lethality. Antimicrob. Agents Chemother. 2009;53(4):1395–1402. doi: 10.1128/AAC.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lampreia JP, AS, Moura JJG. Adenylylsulfate reductases from sulfate-reducing bacteria. Methods Enzymol. 1994;243:241–260. [Google Scholar]
- 244.Gonzalez Porque P, Baldesten A, Reichard P. The involvement of the thioredoxin system in the reduction of methionine sulfoxide and sulfate. J. Biol. Chem. 1970;245(9):2371–2374. [PubMed] [Google Scholar]
- 245.Kredich NM. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd ed. Niedhardt FC, editor. Vol. 1. Washington, D. C.: ASM Press; 1996. pp. 514–527. [Google Scholar]
- 246.Wheeler PRB, JS . General Metabolism and Biochemical Pathways of Tubercle Bacilli. In: Cole ST, editor. Tuberculosis and the Tubercle Bacillus. Washington D. C.: ASM Press; 2005. pp. 309–339. [Google Scholar]
- 247.Carroll KS, Gao H, Chen H, Stout CD, Leary JA, Bertozzi CR. A conserved mechanism for sulfonucleotide reduction. PLoS Biol. 2005;3(8):e250. doi: 10.1371/journal.pbio.0030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shi L, Jung YJ, Tyagi S, Gennaro ML, North RJ. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl. Acad. Sci. U. S. A. 2003;100(1):241–246. doi: 10.1073/pnas.0136863100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wheeler PR, Coldham NG, Keating L, Gordon SV, Wooff EE, Parish T, Hewinson RG. Functional demonstration of reverse transsulfuration in the Mycobacterium tuberculosis complex reveals that methionine is the preferred sulfur source for pathogenic Mycobacteria. J. Biol. Chem. 2005;280(9):8069–8078. doi: 10.1074/jbc.M412540200. [DOI] [PubMed] [Google Scholar]
- 250.Wooff E, Michell SL, Gordon SV, Chambers MA, Bardarov S, Jacobs WR, Jr, Hewinson RG, Wheeler PR. Functional genomics reveals the sole sulphate transporter of the Mycobacterium tuberculosis complex and its relevance to the acquisition of sulphur in vivo. Mol. Microbiol. 2002;43(3):653–663. doi: 10.1046/j.1365-2958.2002.02771.x. [DOI] [PubMed] [Google Scholar]
- 251.Flynn JL, Chan J. Tuberculosis: latency and reactivation. Infect. Immun. 2001;69(7):4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 253.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 1997;94(10):5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Senaratne RH, Mougous JD, Reader JR, Williams SJ, Zhang T, Bertozzi CR, Riley LW. Vaccine efficacy of an attenuated but persistent Mycobacterium tuberculosis cysH mutant. J. Med. Microbiol. 2007;56(Pt 4):454–458. doi: 10.1099/jmm.0.46983-0. [DOI] [PubMed] [Google Scholar]
- 255.Carroll KS, Gao H, Chen H, Leary JA, Bertozzi CR. Investigation of the iron-sulfur cluster in Mycobacterium tuberculosis APS reductase: implications for substrate binding and catalysis. Biochemistry (Mosc.) 2005;44(44):14647–14657. doi: 10.1021/bi051344a. [DOI] [PubMed] [Google Scholar]
- 256.Chartron J, Carroll KS, Shiau C, Gao H, Leary JA, Bertozzi CR, Stout CD. Substrate recognition, protein dynamics, and iron-sulfur cluster in Pseudomonas aeruginosa adenosine 5'-phosphosulfate reductase. J. Mol. Biol. 2006;364(2):152–169. doi: 10.1016/j.jmb.2006.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gao H, Leary J, Carroll KS, Bertozzi CR, Chen H. Noncovalent complexes of APS reductase from M. tuberculosis: delineating a mechanistic model using ESI-FTICR MS. J. Am. Soc. Mass Spectrom. 2007;18(2):167–178. doi: 10.1016/j.jasms.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hong JA, Carroll KS. Deciphering the role of histidine 252 in mycobacterial adenosine 5'-phosphosulfate (APS) reductase catalysis. J. Biol. Chem. 2011;286(32):28567–28573. doi: 10.1074/jbc.M111.238998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chartron J, Shiau C, Stout CD, Carroll KS. 3'-Phosphoadenosine-5'-phosphosulfate Reductase in Complex with Thioredoxin: A Structural Snapshot in the Catalytic Cycle(,) Biochemistry (Mosc.) 2007 doi: 10.1021/bi700130e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bhave DP, Hong JA, Lee M, Jiang W, Krebs C, Carroll KS. Spectroscopic Studies on the [4Fe-4S] Cluster in Adenosine 5′-Phosphosulfate Reductase from Mycobacterium tuberculosis. J. Biol. Chem. 2011;286(2):1216–1226. doi: 10.1074/jbc.M110.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bhave DP, Han W-G, Pazicni S, Penner-Hahn JE, Carroll KS, Noodleman L. Geometric and Electrostatic Study of the [4Fe-4S] Cluster of Adenosine-5′-Phosphosulfate Reductase from Broken Symmetry Density Functional Calculations and Extended X-ray Absorption Fine Structure Spectroscopy. Inorg. Chem. 2011;50(14):6610–6625. doi: 10.1021/ic200446c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Paritala H, Suzuki Y, Carroll KS. Efficient microwave-assisted solid phase coupling of nucleosides, small library generation, and mild conditions for release of nucleoside derivatives. Tetrahedron Lett. 2013;54(14):1869–1872. doi: 10.1016/j.tetlet.2013.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Schnell R, Sandalova T, Hellman U, Lindqvist Y, Schneider G. Siroheme- and [Fe4-S4]-dependent NirA from Mycobacterium tuberculosis is a sulfite reductase with a covalent Cys-Tyr bond in the active site. J. Biol. Chem. 2005;280(29):27319–27328. doi: 10.1074/jbc.M502560200. [DOI] [PubMed] [Google Scholar]
- 264.Smith KW, Stroupe ME. Mutational Analysis of Sulfite Reductase Hemoprotein Reveals the Mechanism for Coordinated Electron and Proton Transfer. Biochemistry (Mosc.) 2012;51(49):9857–9868. doi: 10.1021/bi300947a. [DOI] [PubMed] [Google Scholar]
- 265.Burns KE, Baumgart S, Dorrestein PC, Zhai H, McLafferty FW, Begley TP. Reconstitution of a new cysteine biosynthetic pathway in Mycobacterium tuberculosis. J. Am. Chem. Soc. 2005;127(33):11602–11603. doi: 10.1021/ja053476x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Liu Y, Beer LL, Whitman WB. Sulfur metabolism in archaea reveals novel processes. Environ. Microbiol. 2012;14(10):2632–2644. doi: 10.1111/j.1462-2920.2012.02783.x. [DOI] [PubMed] [Google Scholar]
- 267.O’Leary SnE, Jurgenson CT, Ealick SE, Begley TP. O-Phospho-l-serine and the Thiocarboxylated Sulfur Carrier Protein CysO-COSH Are Substrates for CysM, a Cysteine Synthase from Mycobacterium tuberculosis†. Biochemistry (Mosc.) 2008;47(44):11606–11615. doi: 10.1021/bi8013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Dey S, Grant GA, Sacchettini JC. Crystal Structure of Mycobacterium tuberculosis D-3-Phosphoglycerate Dehydrogenase: Extreme Asymmetry In A Tetramer of Identical Subunits. J. Biol. Chem. 2005;280(15):14892–14899. doi: 10.1074/jbc.M414489200. [DOI] [PubMed] [Google Scholar]
- 269.Newton GL, Arnold K, Price MS, Sherrill C, Delcardayre SB, Aharonowitz Y, Cohen G, Davies J, Fahey RC, Davis C. Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J. Bacteriol. 1996;178(7):1990–1995. doi: 10.1128/jb.178.7.1990-1995.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, Function, and Formation of Biological Iron-Sulfur Clusters. Annu. Rev. Biochem. 2005;74(1):247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 271.Lill R, Mühlenhoff U. Iron-Sulfur Protein Biogenesis in Eukaryotes: Components and Mechanisms. Annu. Rev. Cell Dev. Biol. 2006;22(1):457–486. doi: 10.1146/annurev.cellbio.22.010305.104538. [DOI] [PubMed] [Google Scholar]
- 272.Fontecave M, Ollagnier-de-Choudens S. Iron–sulfur cluster biosynthesis in bacteria: Mechanisms of cluster assembly and transfer. Arch. Biochem. Biophys. 2008;474(2):226–237. doi: 10.1016/j.abb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 273.Mansy SS, Cowan JA. Iron−Sulfur Cluster Biosynthesis:? Toward an Understanding of Cellular Machinery and Molecular Mechanism. Accounts of Chemical Research. 2004;37(9):719–725. doi: 10.1021/ar0301781. [DOI] [PubMed] [Google Scholar]
- 274.Qi W, Cowan JA. A structural and functional homolog supports a general role for frataxin in cellular iron chemistry. Chem. Commun. 2010;46(5):719–721. doi: 10.1039/b911975b. [DOI] [PubMed] [Google Scholar]
- 275.Ayala-Castro C, Saini A, Outten FW. Fe-S Cluster Assembly Pathways in Bacteria. Microbiol. Mol. Biol. Rev. 2008;72(1):110–125. doi: 10.1128/MMBR.00034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. DNA Microarray-Mediated Transcriptional Profiling of the Escherichia coli Response to Hydrogen Peroxide. J. Bacteriol. 2001;183(15):4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Balk J, Lobréaux S. Biogenesis of iron sulfur proteins in plants. Trends Plant Sci. 2005;10(7):324–331. doi: 10.1016/j.tplants.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 278.Saves I, Lewis L-A, Westrelin F, Warren R, Daffé M, Masson J-M. Specificities and Functions of the recA and pps1 Intein Genes of Mycobacterium tuberculosis and Application for Diagnosis of Tuberculosis. J. Clin. Microbiol. 2002;40(3):943–950. doi: 10.1128/JCM.40.3.943-950.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Adam E, Perler FB. Development of a positive genetic selection system for inhibition of protein splicing using mycobacterial inteins in Escherichia coli DNA gyrase subunit A. J. Mol. Microbiol. Biotechnol. 2002;4(5):479–487. [PubMed] [Google Scholar]
- 280.Paulus H. Inteins as targets for potential antimycobacterial drugs. Front. Biosci. 2003;1(8):s1157–s1165. doi: 10.2741/1195. [DOI] [PubMed] [Google Scholar]
- 281.Huet G, Castaing JP, Fournier D, Daffé M, Saves I. Protein splicing of SufB is crucial for the functionality of the Mycobacterium tuberculosis SUF machinery. J. Bacteriol. 2006;188(9):3412–3414. doi: 10.1128/JB.188.9.3412-3414.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zheng L, Cash VL, Flint DH, Dean DR. Assembly of Iron-Sulfur Clusters: Identification of an iscSUA-hscBA-fdx Gene Cluster From Azotobacter vinelandii. J. Biol. Chem. 1998;273(21):13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- 283.Lill R, Mühlenhoff U. Maturation of Iron-Sulfur Proteins in Eukaryotes: Mechanisms, Connected Processes, and Diseases. Annu. Rev. Biochem. 2008;77(1):669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- 284.Rawat M, Av-Gay Y. Mycothiol-dependent proteins in actinomycetes. FEMS Microbiol. Rev. 2007 doi: 10.1111/j.1574-6976.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- 285.Newton GL, Bewley CA, Dwyer TJ, Horn R, Aharonowitz Y, Cohen G, Davies J, Faulkner DJ, Fahey RC. The structure of U17 isolated from Streptomyces clavuligerus and its properties as an antioxidant thiol. Eur. J. Biochem. 1995;230(2):821–825. doi: 10.1111/j.1432-1033.1995.0821h.x. [DOI] [PubMed] [Google Scholar]
- 286.Newton GL, Ko M, Ta P, Av-Gay Y, Fahey RC. Purification and characterization of Mycobacterium tuberculosis 1D-myo-inosityl-2-acetamido-2-deoxy-alpha-D-glucopyranoside deacetylase, MshB, a mycothiol biosynthetic enzyme. Protein Expr. Purif. 2006;47(2):542–550. doi: 10.1016/j.pep.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 287.Newton GL, Av-Gay Y, Fahey RC. N-Acetyl-1-D-myo-inosityl-2-amino-2-deoxy-alpha-D-glucopyranoside deacetylase (MshB) is a key enzyme in mycothiol biosynthesis. J. Bacteriol. 2000;182(24):6958–6963. doi: 10.1128/jb.182.24.6958-6963.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bornemann C, Jardine MA, Spies HS, Steenkamp DJ. Biosynthesis of mycothiol: elucidation of the sequence of steps in Mycobacterium smegmatis. Biochem. J. 1997;325(Pt 3):623–629. doi: 10.1042/bj3250623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Koledin T, Newton GL, Fahey RC. Identification of the mycothiol synthase gene (mshD) encoding the acetyltransferase producing mycothiol in actinomycetes. Arch. Microbiol. 2002;178(5):331–337. doi: 10.1007/s00203-002-0462-y. [DOI] [PubMed] [Google Scholar]
- 290.Jardine MA, Spies HS, Nkambule CM, Gammon DW, Steenkamp DJ. Synthesis of mycothiol, 1D-1-O-(2-[N-acetyl-L-cysteinyl]amino-2-deoxy-alpha-D-glucopyranosyl)-myo- inositol, principal low molecular mass thiol in the actinomycetes. Bioorg. Med. Chem. 2002;10(4):875–881. doi: 10.1016/s0968-0896(01)00383-2. [DOI] [PubMed] [Google Scholar]
- 291.Lee S, Rosazza JP. First total synthesis of mycothiol and mycothiol disulfide. Org. Lett. 2004;6(3):365–368. doi: 10.1021/ol0362008. [DOI] [PubMed] [Google Scholar]
- 292.Newton GL, Koledin T, Gorovitz B, Rawat M, Fahey RC, Av-Gay Y. The glycosyltransferase gene encoding the enzyme catalyzing the first step of mycothiol biosynthesis (mshA) J. Bacteriol. 2003;185(11):3476–3479. doi: 10.1128/JB.185.11.3476-3479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Newton GL, Av-Gay Y, Fahey RC. A novel mycothiol-dependent detoxification pathway in mycobacteria involving mycothiol S-conjugate amidase. Biochemistry (Mosc.) 2000;39(35):10739–10746. doi: 10.1021/bi000356n. [DOI] [PubMed] [Google Scholar]
- 294.Newton GL, Unson MD, Anderberg SJ, Aguilera JA, Oh NN, delCardayre SB, Av-Gay Y, Fahey RC. Characterization of Mycobacterium smegmatis mutants defective in 1-d-myo-inosityl-2-amino-2-deoxy-alpha-d-glucopyranoside and mycothiol biosynthesis. Biochem. Biophys. Res. Commun. 1999;255(2):239–244. doi: 10.1006/bbrc.1999.0156. [DOI] [PubMed] [Google Scholar]
- 295.Hayward D, Wiid I, van Helden P. Differential expression of mycothiol pathway genes: are they affected by antituberculosis drugs? IUBMB Life. 2004;56(3):131–138. doi: 10.1080/15216540410001674012. [DOI] [PubMed] [Google Scholar]
- 296.Newton GL, Ta P, Bzymek KP, Fahey RC. Biochemistry of the initial steps of mycothiol biosynthesis. J. Biol. Chem. 2006;281(45):33910–33920. doi: 10.1074/jbc.M604724200. [DOI] [PubMed] [Google Scholar]
- 297.Rawat M, Uppal M, Newton G, Steffek M, Fahey RC, Av-Gay Y. Targeted mutagenesis of the Mycobacterium smegmatis mca gene, encoding a mycothiol-dependent detoxification protein. J. Bacteriol. 2004;186(18):6050–6058. doi: 10.1128/JB.186.18.6050-6058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sareen D, Steffek M, Newton GL, Fahey RC. ATP-dependent L-cysteine:1D-myo-inosityl 2-amino-2-deoxy-alpha-D-glucopyranoside ligase, mycothiol biosynthesis enzyme MshC, is related to class I cysteinyl-tRNA synthetases. Biochemistry (Mosc.) 2002;41(22):6885–6890. doi: 10.1021/bi012212u. [DOI] [PubMed] [Google Scholar]
- 299.Campbell JA, Davies GJ, Bulone V, Henrissat B. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem. J. 1997;326(Pt 3):929–939. doi: 10.1042/bj3260929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Maynes JT, Garen C, Cherney MM, Newton G, Arad D, Av-Gay Y, Fahey RC, James MN. The crystal structure of 1-D-myo-inosityl 2-acetamido-2-deoxy-alpha-D-glucopyranoside deacetylase (MshB) from Mycobacterium tuberculosis reveals a zinc hydrolase with a lactate dehydrogenase fold. J. Biol. Chem. 2003;278(47):47166–47170. doi: 10.1074/jbc.M308914200. [DOI] [PubMed] [Google Scholar]
- 301.McCarthy AA, Peterson NA, Knijff R, Baker EN. Crystal structure of MshB from Mycobacterium tuberculosis, a deacetylase involved in mycothiol biosynthesis. J. Mol. Biol. 2004;335(4):1131–1141. doi: 10.1016/j.jmb.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 302.Rawat M, Kovacevic S, Billman-Jacobe H, Av-Gay Y. Inactivation of mshB, a key gene in the mycothiol biosynthesis pathway in Mycobacterium smegmatis. Microbiology. 2003;149(Pt 5):1341–1349. doi: 10.1099/mic.0.26084-0. [DOI] [PubMed] [Google Scholar]
- 303.Li L, Bannantine JP, Zhang Q, Amonsin A, May BJ, Alt D, Banerji N, Kanjilal S, Kapur V. The complete genome sequence of Mycobacterium avium subspecies paratuberculosis. Proc. Natl. Acad. Sci. USA. 2005;102(35):12344–12349. doi: 10.1073/pnas.0505662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sareen D, Newton GL, Fahey RC, Buchmeier NA. Mycothiol is essential for growth of Mycobacterium tuberculosis Erdman. J. Bacteriol. 2003;185(22):6736–6740. doi: 10.1128/JB.185.22.6736-6740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Buchmeier NA, Newton GL, Fahey RC. A mycothiol synthase mutant of Mycobacterium tuberculosis has an altered thiol-disulfide content and limited tolerance to stress. J. Bacteriol. 2006;188(17):6245–6252. doi: 10.1128/JB.00393-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Aoki Y, Kondoh M, Nakamura M, Fujii T, Yamazaki T, Shimada H, Arisawa M. A new methionine antagonist that has antifungal activity: mode of action. J. Antibiot. (Tokyo) 1994;47(8):909–816. doi: 10.7164/antibiotics.47.909. [DOI] [PubMed] [Google Scholar]
- 307.Vetting MW, Roderick SL, Yu M, Blanchard JS. Crystal structure of mycothiol synthase (Rv0819) from Mycobacterium tuberculosis shows structural homology to the GNAT family of N-acetyltransferases. Protein Sci. 2003;12(9):1954–1959. doi: 10.1110/ps.03153703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Vetting MW, Yu M, Rendle PM, Blanchard JS. The substrate-induced conformational change of Mycobacterium tuberculosis mycothiol synthase. J. Biol. Chem. 2006;281(5):2795–2802. doi: 10.1074/jbc.M510798200. [DOI] [PubMed] [Google Scholar]
- 309.Newton GL, Ta P, Fahey RC. A mycothiol synthase mutant of Mycobacterium smegmatis produces novel thiols and has an altered thiol redox status. J. Bacteriol. 2005;187(21):7309–7316. doi: 10.1128/JB.187.21.7309-7316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Steffek M, Newton GL, Av-Gay Y, Fahey RC. Characterization of Mycobacterium tuberculosis mycothiol S-conjugate amidase. Biochemistry (Mosc.) 2003;42(41):12067–12076. doi: 10.1021/bi030080u. [DOI] [PubMed] [Google Scholar]
- 311.Hand CE, Honek JF. Biological chemistry of naturally occurring thiols of microbial and marine origin. J. Nat. Prod. 2005;68(2):293–308. doi: 10.1021/np049685x. [DOI] [PubMed] [Google Scholar]
- 312.Xu X, Vilcheze C, Av-Gay Y, Gomez-Velasco A, Jacobs WR., Jr Precise null deletion mutations of the mycothiol synthesis genes reveal their role in isoniazid and ethionamide resistance in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 2011;55(7):3133–3139. doi: 10.1128/AAC.00020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Vilchèze C, Av-Gay Y, Attarian R, Liu Z, Hazbón MH, Colangeli R, Chen B, Liu W, Alland D, Sacchettini JC, Jacobs WR., Jr Mycothiol biosynthesis is essential for ethionamide susceptibility in Mycobacterium tuberculosis. Mol. Microbiol. 2008;69(5):1316–1329. doi: 10.1111/j.1365-2958.2008.06365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ung KSE, Av-Gay Y. Mycothiol-dependent mycobacterial response to oxidative stress. FEBS Lett. 2006;580(11):2712–2716. doi: 10.1016/j.febslet.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 315.Misset-Smits M, van Ophem PW, Sakuda S, Duine JA. Mycothiol, 1-O-(2'-[N-acetyl-L-cysteinyl]amido-2'-deoxy-alpha-D-glucopyranosyl)-D- myo-inositol, is the factor of NAD/factor-dependent formaldehyde dehydrogenase. FEBS Lett. 1997;409(2):221–222. doi: 10.1016/s0014-5793(97)00510-3. [DOI] [PubMed] [Google Scholar]
- 316.Patel MP, Blanchard JS. Expression, purification, and characterization of Mycobacterium tuberculosis mycothione reductase. Biochemistry (Mosc.) 1999;38(36):11827–11833. doi: 10.1021/bi991025h. [DOI] [PubMed] [Google Scholar]
- 317.Patel MP, Blanchard JS. Mycobacterium tuberculosis mycothione reductase: pH dependence of the kinetic parameters and kinetic isotope effects. Biochemistry (Mosc.) 2001;40(17):5119–5126. doi: 10.1021/bi0029144. [DOI] [PubMed] [Google Scholar]
- 318.McAdam RA, Quan S, Smith DA, Bardarov S, Betts JC, Cook FC, Hooker EU, Lewis AP, Woollard P, Everett MJ, Lukey PT, Bancroft GJ, Jacobs WR, Jr, Duncan K. Characterization of a Mycobacterium tuberculosis H37Rv transposon library reveals insertions in 351 ORFs and mutants with altered virulence. Microbiology. 2002;148(Pt 10):2975–2986. doi: 10.1099/00221287-148-10-2975. [DOI] [PubMed] [Google Scholar]
- 319.Lombo F, Velasco A, Castro A, de la Calle F, Brana AF, Sanchez-Puelles JM, Mendez C, Salas JA. Deciphering the biosynthesis pathway of the antitumor thiocoraline from a marine actinomycete and its expression in two streptomyces species. Chembiochem. 2006;7(2):366–376. doi: 10.1002/cbic.200500325. [DOI] [PubMed] [Google Scholar]
- 320.Nicholas GM, Eckman LL, Newton GL, Fahey RC, Ray S, Bewley CA. Inhibition and kinetics of mycobacterium tuberculosis and mycobacterium smegmatis mycothiol-S-conjugate amidase by natural product inhibitors. Bioorg. Med. Chem. 2003;11(4):601–608. doi: 10.1016/s0968-0896(02)00345-0. [DOI] [PubMed] [Google Scholar]
- 321.Nicholas GM, Eckman LL, Ray S, Hughes RO, Pfefferkorn JA, Barluenga S, Nicolaou KC, Bewley CA. Bromotyrosine-derived natural and synthetic products as inhibitors of mycothiol-S-conjugate amidase. Bioorg. Med. Chem. Lett. 2002;12(17):2487–2490. doi: 10.1016/s0960-894x(02)00385-2. [DOI] [PubMed] [Google Scholar]
- 322.Nicholas GM, Newton GL, Fahey RC, Bewley CA. Novel bromotyrosine alkaloids: inhibitors of mycothiol S-conjugate amidase. Org. Lett. 2001;3(10):1543–1545. doi: 10.1021/ol015845+. [DOI] [PubMed] [Google Scholar]
- 323.Fetterolf B, Bewley CA. Synthesis of a bromotyrosine-derived natural product inhibitor of mycothiol-S-conjugate amidase. Bioorg. Med. Chem. Lett. 2004;14(14):3785–3788. doi: 10.1016/j.bmcl.2004.04.095. [DOI] [PubMed] [Google Scholar]
- 324.Pick N, Rawat M, Arad D, Lan J, Fan J, Kende AS, Av-Gay Y. In vitro properties of antimicrobial bromotyrosine alkaloids. J. Med. Microbiol. 2006;55(Pt 4):407–415. doi: 10.1099/jmm.0.46319-0. [DOI] [PubMed] [Google Scholar]
- 325.Knapp S, Gonzalez S, Myers DS, Eckman LL, Bewley CA. Shortcut to mycothiol analogues. Org. Lett. 2002;4(24):4337–4339. doi: 10.1021/ol0269796. [DOI] [PubMed] [Google Scholar]
- 326.Metaferia BB, Ray S, Smith JA, Bewley CA. Design and synthesis of substrate-mimic inhibitors of mycothiol-S-conjugate amidase from Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2007;17(2):444–447. doi: 10.1016/j.bmcl.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 327.Mathew R, Kruthiventi AK, Prasad JV, Kumar SP, Srinu G, Chatterji D. Inhibition of Mycobacterial Growth by Plumbagin Derivatives. Chem. Biol. Drug Des. 2010;76(1):34–42. doi: 10.1111/j.1747-0285.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 328.Gammon DW, Steenkamp DJ, Mavumengwana V, Marakalala MJ, Mudzunga TT, Hunter R, Munyololo M. Conjugates of plumbagin and phenyl-2-amino-1-thioglucoside inhibit MshB, a deacetylase involved in the biosynthesis of mycothiol. Bioorg. Med. Chem. 2010;18(7):2501–2514. doi: 10.1016/j.bmc.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 329.Newton GL, Buchmeier N, La Clair JJ, Fahey RC. Evaluation of NTF1836 as an inhibitor of the mycothiol biosynthetic enzyme MshC in growing and non-replicating Mycobacterium tuberculosis. Bioorg. Med. Chem. 2011;19(13):3956–3964. doi: 10.1016/j.bmc.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gutierrez-Lugo M-T, Baker H, Shiloach J, Boshoff H, Bewley CA. Dequalinium, a New Inhibitor of Mycobacterium tuberculosis Mycothiol Ligase Identified by High-Throughput Screening. J. Biomol. Screen. 2009;14(6):643–652. doi: 10.1177/1087057109335743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Gutierrez-Lugo MT, Bewley CA. Susceptibility and mode of binding of the Mycobacterium tuberculosis cysteinyl transferase mycothiol ligase to tRNA synthetase inhibitors. Bioorg. Med. Chem. Lett. 2011;21(8):2480–2483. doi: 10.1016/j.bmcl.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Mahapatra A, Mativandlela SP, Binneman B, Fourie PB, Hamilton CJ, Meyer JJ, van der Kooy F, Houghton P, Lall N. Activity of 7-methyljuglone derivatives against Mycobacterium tuberculosis and as subversive substrates for mycothiol disulfide reductase. Bioorg. Med. Chem. 2007;15(24):7638–7646. doi: 10.1016/j.bmc.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 333.Hu Y, Helm JS, Chen L, Ginsberg C, Gross B, Kraybill B, Tiyanont K, Fang X, Wu T, Walker S. Identification of selective inhibitors for the glycosyltransferase MurG via high-throughput screening. Chem. Biol. 2004;11(5):703–711. doi: 10.1016/j.chembiol.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 334.Vetting MW, Frantom PA, Blanchard JS. Structural and enzymatic analysis of MshA from Corynebacterium glutamicum: substrate-assisted catalysis. J. Biol. Chem. 2008;283(23):15834–15844. doi: 10.1074/jbc.M801017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Frantom PA, Coward JK, Blanchard JS. UDP-(5F)-GlcNAc Acts as a Slow-Binding Inhibitor of MshA, a Retaining Glycosyltransferase. J. Am. Chem. Soc. 2010;132(19):6626–6627. doi: 10.1021/ja101231a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Yin J, Garen CR, Bateman K, Yu M, Lyon EZ, Habel J, Kim H, Hung LW, Kim CY, James MN. Expression, purification and preliminary crystallographic analysis of O-acetylhomoserine sulfhydrylase from Mycobacterium tuberculosis. Acta Crystallograph. Sect. F Struct. Biol. Cryst. Commun. 2011;67(Pt 8):959–963. doi: 10.1107/S1744309111017611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Warner DF, Savvi S, Mizrahi V, Dawes SS. A Riboswitch Regulates Expression of the Coenzyme B12-Independent Methionine Synthase in Mycobacterium tuberculosis: Implications for Differential Methionine Synthase Function in Strains H37Rv and CDC1551. J. Bacteriol. 2007;189(9):3655–3659. doi: 10.1128/JB.00040-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Smith DA, Parish T, Stoker NG, Bancroft GJ. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect. Immun. 2001;69(2):1142–1150. doi: 10.1128/IAI.69.2.1142-1150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wheeler PRRC. Tuberculosis. In: Bloom BR, editor. Pathogenesis, Protection and Control. Washington, D. C.: ASM Press; 1994. pp. 353–388. [Google Scholar]
- 340.Berger B, Knodel M. Characterisation of methionine adenosyltransferase from Mycobacterium smegmatis and M. tuberculosis. BMC Microbiol. 2003;3(1):12. doi: 10.1186/1471-2180-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Gupta A, Kumar PH, Dineshkumar TK, Varshney U, Subramanya HS. Crystal structure of Rv2118c: an AdoMet-dependent methyltransferase from Mycobacterium tuberculosis H37Rv. J. Mol. Biol. 2001;312(2):381–391. doi: 10.1006/jmbi.2001.4935. [DOI] [PubMed] [Google Scholar]
- 342.Reddy MCM, Kuppan G, Shetty ND, Owen JL, Ioerger TR, Sacchettini JC. Crystal structures of Mycobacterium tuberculosis S-adenosyl-L-homocysteine hydrolase in ternary complex with substrate and inhibitors. Protein Sci. 2008;17(12):2134–2144. doi: 10.1110/ps.038125.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wolfe MS, Borchardt RT. S-adenosyl-L-homocysteine hydrolase as a target for antiviral chemotherapy. J. Med. Chem. 1991;34(5):1521–1530. doi: 10.1021/jm00109a001. [DOI] [PubMed] [Google Scholar]
- 344.Dubnau E, Chan J, Mohan VP, Smith I. responses of mycobacterium tuberculosis to growth in the mouse lung. Infect. Immun. 2005;73(6):3754–3757. doi: 10.1128/IAI.73.6.3754-3757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Burguiere P, Auger S, Hullo MF, Danchin A, Martin-Verstraete I. Three different systems participate in L-cystine uptake in Bacillus subtilis. J. Bacteriol. 2004;186(15):4875–4884. doi: 10.1128/JB.186.15.4875-4884.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Muller A, Thomas GH, Horler R, Brannigan JA, Blagova E, Levdikov VM, Fogg MJ, Wilson KS, Wilkinson AJ. An ATP-binding cassette-type cysteine transporter in Campylobacter jejuni inferred from the structure of an extracytoplasmic solute receptor protein. Mol. Microbiol. 2005;57(1):143–155. doi: 10.1111/j.1365-2958.2005.04691.x. [DOI] [PubMed] [Google Scholar]
- 347.Wang XF, Cynader MS. Astrocytes provide cysteine to neurons by releasing glutathione. J. Neurochem. 2000;74(4):1434–1442. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- 348.Pavelka MS, Jr, Jacobs WR., Jr Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis bacillus Calmette-Guerin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J. Bacteriol. 1999;181(16):4780–4789. doi: 10.1128/jb.181.16.4780-4789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Eckert KG, Elbers FR, Eyer P. Depletion of mitochondrial coenzyme A and glutathione by 4-dimethylaminophenol and formation of mixed thioethers. Biochem. Pharmacol. 1989;38(19):3253–3259. doi: 10.1016/0006-2952(89)90622-9. [DOI] [PubMed] [Google Scholar]
- 350.Roederer M, Staal FJ, Osada H, Herzenberg LA, Herzenberg LA. CD4 and CD8 T cells with high intracellular glutathione levels are selectively lost as the HIV infection progresses. Int. Immunol. 1991;3(9):933–937. doi: 10.1093/intimm/3.9.933. [DOI] [PubMed] [Google Scholar]
- 351.Green RM, Seth A, Connell ND. A peptide permease mutant of Mycobacterium bovis BCG resistant to the toxic peptides glutathione and S-nitrosoglutathione. Infect. Immun. 2000;68(2):429–436. doi: 10.1128/iai.68.2.429-436.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Dayaram YK, Talaue MT, Connell ND, Venketaraman V. Characterization of a glutathione metabolic mutant of Mycobacterium tuberculosis and its resistance to glutathione and nitrosoglutathione. J. Bacteriol. 2006;188(4):1364–1372. doi: 10.1128/JB.188.4.1364-1372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Venketaraman V, Dayaram YK, Talaue MT, Connell ND. Glutathione and nitrosoglutathione in macrophage defense against Mycobacterium tuberculosis. Infect. Immun. 2005;73(3):1886–1889. doi: 10.1128/IAI.73.3.1886-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Guerra C, Johal K, Morris D, Moreno S, Alvarado O, Gray D, Tanzil M, Pearce D, Venketaraman V. Control of Mycobacterium tuberculosis growth by activated natural killer cells. Clin. Exp. Immunol. 2012;168(1):142–152. doi: 10.1111/j.1365-2249.2011.04552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]