Abstract
Introduction
Childhood diarrheal illnesses are a major public health problem. In low-income settings data on disease burden and factors associated with diarrheal illnesses are poorly defined, precluding effective prevention programs. This study explores factors associated with recurrent diarrheal illnesses among children in Kabul, Afghanistan.
Methods
A cohort of 1–11 month old infants was followed for 18 months from 2007–2009. Data on diarrheal episodes were gathered through active and passive surveillance. Information on child health, socioeconomics, water and sanitation, and hygiene behaviors was collected. Factors associated with recurrent diarrheal illnesses were analyzed using random effects recurrent events regression models.
Results
3,045 children were enrolled and 2,511 (82%) completed 18-month follow-up. There were 14,998 episodes of diarrheal disease over 4,200 child-years (3.51 episodes/child-year, 95%CI 3.40–3.62). Risk of diarrheal illness during the winter season was 63% lower than the summer season (HR = 0.37, 95%CI 0.35–0.39, P<0.001). Soap for hand washing was available in 72% of households and 11.9% had toilets with septic/canalization. Half of all mothers reported using soap for hand washing. In multivariate analysis diarrheal illness was lower among children born to mothers with post-primary education (aHR = 0.79, 95%CI 0.69–0.91, p = 0.001), from households where maternal hand washing with soap was reported (aHR = 0.83, 95%CI 0.74–0.92, p<0.001) and with improved sanitation facilities (aHR = 0.76, 95%CI 0.63–0.93, p = 0.006). Malnourished children from impoverished households had significantly increased risks for recurrent disease [(aHR = 1.15, 95%CI 1.03–1.29, p = 0.016) and (aHR = 1.20, 95%CI 1.05–1.37, p = 0.006) respectively].
Conclusions
Maternal hand washing and improved sanitation facilities were protective, and represent important prevention points among public health endeavors. The discrepancy between soap availability and utilization suggests barriers to access and knowledge, and programs simultaneously addressing these aspects would likely be beneficial. Enhanced maternal education and economic status were protective in this population and these findings support multi-sector interventions to combat illness.
Trial Registration
www.ClinicalTrials.gov NCT00548379 https://www.clinicaltrials.gov/ct2/show/NCT00548379
Introduction
Diarrheal diseases account for over 700,000 child deaths annually, with 98% of these occurring in low- and middle-income countries (LMIC).[1] In 2010, 1.7 billion cases of diarrhea were estimated to occur in children.[2] Although the mortality and disease incidence have declined, the public health burden in children remains substantial; resulting in malnutrition, impaired development and reduced vaccine efficacy.[3–6]
Infectious diarrhea is caused by a variety of pathogens. In children most cases of moderate-to-severe diarrhoea are attributable to four pathogens: rotavirus, Cryptosporidium, enterotoxigenic E. coli, and Shigella.[7] Diarrhoegenic agents are transmitted through fecal-oral routes, which include: transmission by flies, ingestion of contaminated food, or water, and person-to-person contact.
World Health Organization (WHO) recommendations for prevention of childhood diarrhea promote vaccinations, child nutrition, and interruption of fecal-oral transmission routes. Prevention strategies for interrupting fecal-oral transmission routes focus on hand washing, sanitation, and access to sufficient safe water. Although improved hygiene, water supply and sanitation could prevent 95% of all diarrhea cases,[8] and the fact that safe and water and sanitation are acknowledged as a basic human right,[9] over a 750 million people still lack access to improved water supply, and 2.5 billion people lack access to improved sanitation[10].
Globally, Afghanistan has the fourth highest diarrheal mortality rates and approximately nine percent of all deaths among children 1–59 months of age are due to diarrheal diseases.[11,12] However, with the exception of a small number of studies,[13–15] there exists limited data pertaining to diarrheal illnesses in Afghan children. With multiple transmission routes and etiologic pathogens associated with childhood diarrhea it is key to identify specific factors associated with disease in order to identify setting appropriate effective interventions. This becomes even more important, when financial resources are limited and when depleted post-conflict infrastructure exists, as is the case in Afghanistan, and Kabul in particular.[16] This study aimed to describe the incidence of recurrent diarrhea and identify factors associated with enteric illnesses among children in Kabul, with the goal of informing preventative measures in the region.
Methods
Ethics and reporting
Research approval was obtained from the Ethics and Review Board of the Ministry of Public Health of Afghanistan (Reference: 422328) and the Ethics Committee of the London School of Hygiene and Tropical Medicine (Application no. 5117). Written informed consent was obtained from the mother and father, or other head of household for all enrolled children where the individual was not adequately literate, the details of the consent were read and explained to the consenting caregiver by study staff and all questions were answered. Reporting guidelines for observational studies were followed.[17]
Data collection
Data were collected between November 2007 and June 2009 in five districts from within Kabul, Afghanistan. The districts comprise the central city and adjacent embankment and represent Kabul’s socio-economically deprived inner-city population. All of the districts were within the catchment area of the study hospital. The data was derived from a randomized controlled trial designed to evaluate the impact of supplementation with vitamin D on the incidence of childhood pneumonia and diarrheal illnesses.[18,19] Details on the trial design, household selection, inclusion and exclusion criteria have been presented previously.[18]
A total of 3,045 infants aged one to eleven months and residing in households located within the study districts were enrolled, and followed for 18 months prospectively. An episode of diarrheal illness was defined as a child having three or more loose stools in a 24 hour period.[11] A symptom free period of ≥72 hours was required to define a unique recurrent event otherwise the illness was considered part of the prior diarrheal episode.[20] Data on the occurrence of, and risk factors associated with diarrhea were collected through fortnightly household visits. During visits, children underwent a physical examination, and their health history was gathered by caregiver report. Recall of defecation history was based on the 24-hour period preceding each visit. Passive surveillance of self-referring children was undertaken at the Maiwind Teaching Hospital, the primary health center serving the study districts. During each clinic evaluation trained pediatricians completed standardized data forms assessing the number of loose/liquid stools during the 24-hours prior to presentation. If a study child was absent during a home visit, an absentee form was completed, and information regarding child health and treatment during the period was gathered when the child was contacted in the subsequent visit.
At enrollment, data on household socio-demographic characteristics and infant health were collected. Additional cross-sectional data was gathered during follow-up. Child feeding modality was categorized based on maternal report over one week prior to sampling as exclusive breastfeeding, mixed breastfeeding, or replacement feeding. Nutritional status was assessed using weight-for-age z-scores (WAZ) calculated at four time points during follow-up. Water and sanitation characteristics included information on hand washing practices, household sanitation facilities, food storage and water access and treatment. Impoverished households were defined based on World Bank standards (daily household income per family member utilized).[21]
Data analysis
Data analysis was performed using STATA version 10.0 (College Station, USA). In estimating child-time at risk, participants were censored four days after each episode to account for mean illness duration.[20] Diarrheal episodes with repeat visits were excluded. Children absent from surveillance for greater than 45 days were censored at the time of their last recorded contact, and if subsequently relocated were reentered into follow-up at that time.
To assess for changes in the incidence of diarrheal illness that occur with development, child age groups were stratified as: ≤ 6 months, > 6 months to ≤ 12 months and > 12 months.[22] Children found to have WAZ ≤ -2 during follow-up were classified as malnourished in analyses.[23] To explore the role of climate variation on diarrheal disease a seasonal variable was derived based on months of follow-up.[24]
Distribution of the characteristics of the overall cohort and the subset of children who were lost-to-follow-up were compared. Significant differences between children who completed follow-up and those lost-to-follow-up were assessed using Pearson X2 and independent sample t-tests. The cohort was analyzed using recurrent events Poisson regression modeling. A random effects model was used to account for intra-child clustering of events (participant heterogeneity).[25] Incidence rates of diarrheal illnesses with corresponding 95% confidence intervals (95% CI) were calculated using linear combinations of coefficients.
Variables were explored in univariate recurrent events analyses to calculate hazard ratios (HR) with 95%CI. Multivariate forward stepwise Poisson regression models yielding adjusted hazard rations (aHR) were built utilizing all assessed factors and evaluated through likelihood ratio testing (LRT) with a p value <0.05 considered significant for inclusion. Child age and season were defined a priori to be included in all analyses. Child age was significantly associated with feeding modality, and age categorization was used in multivariate models. Similarly, maternal reports of hand washing with soap before eating, and after toilet use, where highly associated and only post toilet use was used in the recurrent events analyses. Goodness-of-fit of the final multivariate model was assessed through LRT and was found to be robust (p <0.05). The randomization variable was added to the final model to assess for effect on outcomes and no alterations to estimates were found.
Results
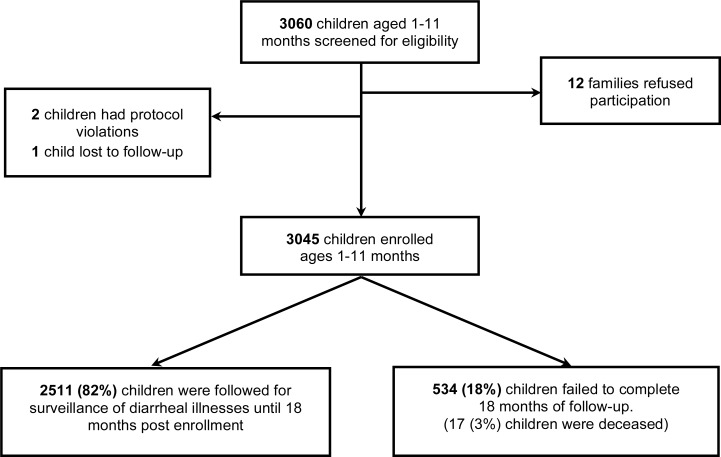
Among the 3,060 infants screened 3,045 were enrolled with 2,511 (82%) followed for 18 months. There were 534 (18%) children lost to follow-up, of which 17 (3%) died (Fig. 1). Ten deaths were attributed to pneumonia/septicemia and seven were due to congenital or accidental causes. There were 14,998 episodes of diarrheal illnesses diagnosed; 6,094 (40.6%) episodes from home visit data, and 8,904 (59.4%) from hospital assessments. Seventy-eight (0.5%) children required inpatient treatment for a diarrheal episode. Cumulative follow-up time was 4200.0 child-years. The observed incidence of diarrheal illness was 3.51 episodes per child-year (95%CI 3.40–3.62).
Fig 1. Study population.
Mean age at enrollment was 6.2 (SD±3.1) months. The majority of children ≤ 6 months were either exclusive breast fed, or received a mixed mode of feeding. Approximately 16% of the children were malnourished, and half of children had received their first measles vaccination at time of enrollment. The majority (81.2%) of mothers had none or only primary education. No significant differences were found between children completing the study and those that were lost-to-follow-up, with the exception of a greater proportion of children completing the study had received their measles vaccination at enrollment (p<0.001).
Approximately half of households obtained water from a piped source and the majority (72.7%) reported no drinking water treatment. Most families (88.1%) reported us of an open/ground toilet (defecation at designated places outside of the home). Although soap was present in over 70% of all household, only 38.8% of mothers reported hand washing with soap after using the toilet (Table 1).
Table 1. Characteristics of the study population.
| Characteristics | Overall Cohort (N = 3045) | Lost-to-follow-up (n = 534) |
|---|---|---|
| n(%)/mean(±SD) | n(%)/mean(±SD) | |
| Child | ||
| Gender | ||
| female | 1455(47.8) | 239(44.8) |
| male | 1590(52.2) | 295(55.2) |
| Age at enrollment (months) | 6.2(±3.1) | 6.3(±3.2) |
| Feeding modality at ≤ 6 months of age | ||
| exclusive breast feeding | 432(43.3%) | 69(43.3%) |
| mixed breast feeding | 533(53.4%) | 73(49.2%) |
| non-breast fed | 33(3.3%) | 6(4.1%) |
| Weight-for-age z-scores | ||
| > -2 | 2325(78.4%) | 456(94.0%) |
| ≤ -2 | 484(16.3%) | 32(6.0%) |
| Sleeps | ||
| alone | 2227(88.1) | 345(89.4) |
| with ≥ 1 other child | 302(11.9) | 41(10.6) |
| Received first measles vaccination | ||
| no | 1570(51.6) | 396(74.2) |
| yes | 1475(48.4) | 138(25.8) |
| Maternal | ||
| Age | ||
| ≤ 15 years | 209(6.9) | 44(8.3) |
| > 15 years | 2833(93.1) | 489(91.7) |
| Highest education | ||
| primary or none | 2473(81.2) | 424(79.4) |
| Secondary or greater | 572(18.8) | 110(20.6) |
| Paternal | ||
| Highest education | ||
| none | 888(29.2) | 175(32.8) |
| primary or greater | 2156(70.8) | 358(67.2) |
| Current employment | ||
| no | 177(5.8) | 42(7.9) |
| Yes | 2868(94.2) | 492(92.1) |
| Impoverished householdsa | ||
| no | 364(20.6) | 16(25.4) |
| Yes | 1406(79.4) | 47(74.6) |
| Water and sanitation | ||
| Water source type | ||
| Piped | 1231(47.5) | 91(36.6) |
| open well | 742(38.6) | 79(31.7) |
| tube well | 618(23.9) | 79(31.7) |
| Drinking water treatment | ||
| None | 1974(72.7) | 184(71.0) |
| Chlorine | 305(11.2) | 32(12.4) |
| Boiling | 436(16.1) | 43(16.6) |
| Water source distance | ||
| piped to home | 691(27.1) | 29(22.1) |
| well in home | 1003(39.3) | 81(61.8) |
| well outside of home | 857(33.6) | 21(16.1) |
| Food storage | ||
| refrigerator | 264(10.7) | 12(5.2) |
| cold water | 579(23.4) | 67(29.0) |
| Nothing | 1627(65.9) | 152(65.8) |
| Toilet type | ||
| open toilet/ground | 2380(88.1) | 530(88.6) |
| toilet (septic/canalization) | 323(11.9) | 68(11.4) |
| Mother reports washing hands with soap prior to eating | ||
| No | 1136(51.6) | 100(42.2) |
| Yes | 1065(48.4) | 137(57.8) |
| Mother reports washing hands with soap post-toilet | ||
| No | 1289(61.2) | 136(60.2) |
| Yes | 817(38.8) | 90(39.8) |
| Number rooms in home | ||
| < 5 | 2654(89.9) | 423(91.6) |
| ≥ 5 | 299(10.1) | 39(8.4) |
a. Impoverished household defined as households that reported living on less than 1.25 US dollars per person per day.[21]
Diarrheal Risk factors
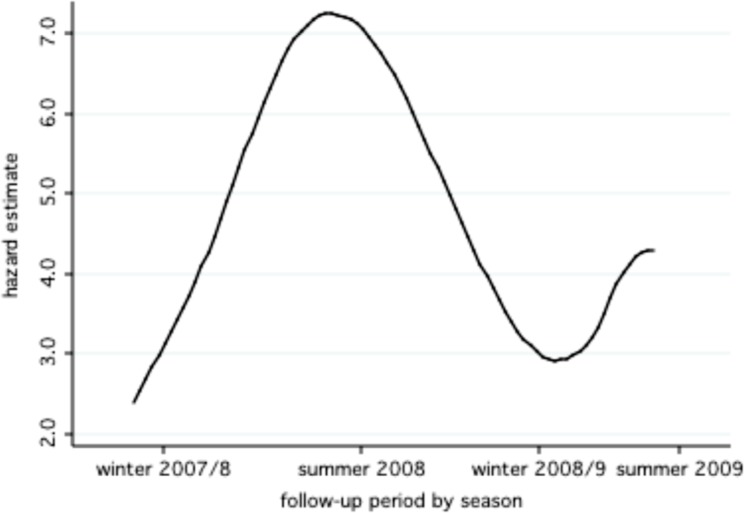
The highest risk of diarrheal illnesses were during the summer months (incidence 5.71 episodes per child-year (95%CI 5.48–5.96) and lowest during winter months (2.12 episodes per child-year (95%CI 2.02–2.21). The seasonal trends in hazards are illustrated in Fig. 2. The risk of diarrhea was 63% lower in winter as compared to summer (HR = 0.37, 95%CI 0.35–0.39, p<0.001).
Fig 2. Estimated hazards for diarrheal illness by seasona. a.
Hazards estimates are adjusted for child age.
Among children less than six months of age the incidence of diarrheal episodes was 2.35 per child-year (95%CI 2.15–2.57). For children aged six months to less than one year, the incidence was 3.89 episodes per child-year (95%CI 3.73–4.06). In univariate analysis, the risk of diarrheal illness was 60% greater among children aged six months to one year in comparison to those less than six months of age. For children greater than one year of age, the incidence of diarrheal illness was 3.48 episodes per child-year (95%CI 3.36–3.60) with a 48% greater risk of recurrent episodes. Malnourished children and those from impoverished households had a greater risk for recurrent illnesses. Children who were born to mothers with greater than primary education had a lower risk recurrent events as compared to those born to mothers with primary education or less.
Among children from homes using wells the risk of diarrheal illnesses was lower in comparison to a piped water source. Water treatment with chlorine was found to confer a reduced risk of diarrheal illnesses however no significant difference was found when treatment with boiling was reported. Food storage with refrigeration, having an in-home well, use of a toilet with septic/canalization and maternal hand washing with soap post-toilet use were all associated with a reduced risk of diarrheal disease (Table 2).
Table 2. Univariate analysis for risk of diarrheal illness.
| Factor | Events | Child-years | Incidence per child-year (95% CI) | HR (95% CI) | P value |
|---|---|---|---|---|---|
| Child | |||||
| Gender | |||||
| Female | 6843 | 2000.0 | 3.16(2.85–3.50) | 1.00 | |
| Male | 8053 | 2200.0 | 3.38(3.23–3.55) | 1.07(1.01–1.14) | 0.034 |
| Age (months) | |||||
| < 6 | 563 | 224.8 | 2.35(2.15–2.57) | 1.00 | |
| > 6 to < 12 | 3913 | 992.6 | 3.89(3.73–4.06) | 1.65(1.51–1.81) | <0.001 |
| > 12 | 10420 | 3000.0 | 3.48(3.36–3.60) | 1.48(1.35–1.62) | <0.001 |
| Weight-for-age z-scores | |||||
| > -2 | 10753 | 3200.0 | 3.37(3.26–3.49) | 1.00 | |
| < -2 | 3861 | 959.1 | 3.98(3.69–4.30) | 1.19(1.10–1.29) | <0.001 |
| Sleeps | |||||
| Alone | 11027 | 3100.0 | 3.48(3.35–3.61) | 1.00 | |
| with >1 other child | 1684 | 424.1 | 3.97(3.60–4.38) | 1.14(1.03–1.27) | 0.014 |
| > 1 measles vaccination | |||||
| no | 7226 | 2000.0 | 3.49(3.34–3.65) | 1.00 | |
| Yes | 7670 | 2200.0 | 3.53(3.38–3.69) | 1.01(0.95–1.08) | 0.76 |
| Maternal | |||||
| Age (years) | |||||
| <15 years | 1144 | 287.6 | 4.32(3.39–5.49) | 1.00 | |
| >15 years | 13743 | 3900.0 | 3.88(3.44–4.37) | 0.90(0.80–1.02) | 0.09 |
| Highest education | |||||
| primary or none | 12909 | 3400.0 | 3.74(3.61–3.87) | 1.00 | |
| post primary | 1987 | 782.0 | 2.51(2.33–2.71) | 0.67(0.62–0.73) | <0.001 |
| Paternal | |||||
| Employed | |||||
| no | 948 | 241.0 | 3.86(3.40–4.40) | 1.00 | |
| Yes | 13948 | 4000.0 | 3.48(3.38–3.61) | 0.90(0.79–1.03) | 0.13 |
| Impoverished householdsa | |||||
| no | 1555 | 542.9 | 2.86(2.61–3.13) | 1.00 | |
| Yes | 7870 | 2100.0 | 3.74(3.58–3.91) | 1.31(1.18–1.45) | <0.001 |
| Water and sanitation | |||||
| Type of water source | |||||
| Piped | 7334 | 1800.0 | 4.02(3.84–4.22) | 1.00 | |
| open well | 3461 | 1100.0 | 3.17(2.98–3.38) | 0.79(0.73–0.85) | <0.001 |
| tube well | 2955 | 899.0 | 3.27(3.06–3.51) | 0.81(0.75–0.88) | <0.001 |
| Drinking water treatment | |||||
| None | 10732 | 2900.0 | 3.69(3.55–3.83) | 1.00 | |
| Chlorine | 1286 | 442.6 | 2.91(2.63–3.22) | 0.79(0.71–0.88) | <0.001 |
| Boiling | 2264 | 642.5 | 3.51(3.24–3.81) | 0.95(0.87–1.04) | 0.27 |
| Distance to water source | |||||
| piped to home | 3804 | 1000.0 | 3.67(3.44–3.91) | 1.00 | |
| well in home | 4428 | 1500.0 | 2.98(2.82–3.15) | 0.81(0.75–0.88) | <0.001 |
| well outside of homed | 5395 | 1300.0 | 4.20(3.97–4.44) | 1.14(1.05–1.24) | 0.002 |
| Food storage | |||||
| Refrigerator | 1174 | 393.1 | 2.99(2.68–3.33) | 1.00 | |
| cold water | 3236 | 843.3 | 3.58(3.43–3.73) | 1.19(1.06–1.34) | 0.003 |
| none used | 8542 | 2400.0 | 3.83(3.57–4.12) | 1.28(1.13–1.47) | <0.001 |
| Toilet type | |||||
| open toilet | 12948 | 3500.0 | 3.69(3.56–3.82) | 1.00 | |
| toilet (septic/canalization) | 1291 | 470.2 | 2.75(2.49–3.03) | 0.75(0.67–0.83) | <0.001 |
| Mother reports washing hands with soap post-toilet use | |||||
| No | 7360 | 1900.0 | 3.89(3.71–4.07) | 1.00 | |
| Yes | 3983 | 1200.0 | 3.32(3.13–3.52) | 0.85(0.79–0.92) | <0.001 |
| Number rooms in home | |||||
| < 5 | 13435 | 3700.0 | 3.57(3.46–3.69) | 1.00 | |
| > 5 | 1283 | 430.4 | 2.97(2.68–3.30) | 0.83(0.75–0.93) | 0.001 |
a. Impoverished household defined as households that reported living on less than 1.25 US dollars per person per day.[21]
In recurrent event multivariate analysis malnourishment and being from an impoverished household were associated with an increased risk of childhood diarrheal illnesses at 15% and 20% respectively (aHR = 1.15, 95%CI 1.03–1.29, p = 0.016 and aHR = 1.20, 95%CI 1.05–1.37, p = 0.006). Level of maternal education, maternal hand washing with soap post-toilet, and use of a toilet with septic/canalization were found to be protective against recurrent illnesses. Maternal education post primary school was associated with 21% lower risk (aHR = 0.79, 95%CI 0.69–0.91, p = 0.001), and hand washing with soap a 17% reduction (aHR = 0.83, 95%CI 0.74–0.92, p<0.001). Children from households using toilets with septic/canalized systems were had a 24% lower risk of diarrheal illnesses (aHR = 0.76, 95%CI 0.63–0.93, p = 0.006).
A trend of reduced risk was found among households using an open well versus a piped water source (aHR = 0.87, 95%CI 0.76–1.00, p = 0.053). No significant association was found in relation to diarrheal illnesses and tube wells. Treatment of drinking water, food storage, distance to water source, sleeping with other children and number of rooms in the home were not significantly associated with risk of diarrheal illness in multivariate analysis (Table 3).
Table 3. Multivariate analysis for risk of diarrheal illness.
| Factor | aHR | (95% CI) | p value |
|---|---|---|---|
| Weight-for-age z-scores | |||
| > -2 | 1.00 | ||
| ≤ -2 | 1.15 | (1.03–1.29) | 0.016 |
| Sleeps | |||
| alone | 1.00 | ||
| with ≥1 other child | 1.05 | (0.90–1.22) | 0.550 |
| Maternal education | |||
| primary or none | 1.00 | ||
| post primary | 0.79 | (0.69–0.91) | 0.001 |
| Mother reports washing hands with soap post-toilet use | |||
| no | 1.00 | ||
| yes | 0.83 | (0.74–0.92) | <0.001 |
| Type of water source | |||
| piped | 1.00 | ||
| open well | 0.87 | (0.76–1.00) | 0.053 |
| tube well | 0.95 | (0.79–1.14) | 0.576 |
| Distance to water source | |||
| piped to home | 1.00 | ||
| well in home | 0.94 | (0.80–1.12) | 0.507 |
| well outside of home | 1.08 | (0.95–1.23) | 0.239 |
| Drinking water treatment | |||
| None | 1.00 | ||
| chlorine | 0.88 | (0.73–1.06) | 0.187 |
| boiling | 0.89 | (0.77–1.03) | 0.106 |
| Toilet type | |||
| open toilet/ground | 1.00 | ||
| toilet (septic/canalized) | 0.76 | (0.63–0.93) | 0.006 |
| Food storage | |||
| refrigerator | 1.00 | ||
| cold water | 0.92 | (0.77–1.10) | 0.359 |
| nothing or fresh foods | 0.99 | (0.81–1.21) | 0.946 |
| Impoverished householdsa | |||
| no | 1.00 | ||
| yes | 1.20 | (1.05–1.37) | 0.006 |
a. Impoverished household defined as households that reported living on less than 1.25 US dollars per person per day.[21]
Discussion
This study provides new findings pertinent to prevention of childhood diarrheal disease in both the global and Afghanistan specific settings. In this cohort the incidence of diarrheal illness was identified in a population that has previously lacked contemporary statistics, and the seasonal profile showed that disease burden is greatest during the winter months. Water and sanitation risk factors in relation to hand washing, and use of improved sanitation facilities were of key importance in mitigating the risk of diarrheal illness in this population.
The overall incidence of disease for children between one and 29 months of age agrees with global childhood estimates.[22] The incidence in this cohort also coincides with research from geographically, and culturally similar regions. In a study from Pakistan an incidence of 3.5 episodes per child-year was found among children less than three years of age.[26] Additionally, a study from Egypt reported an incidence of 3.6 episodes per child-year.[27] In this cohort the lowest incidence of diarrheal illness was found among children less than six months of age and the highest was present among those aged between six months and one year. These trends reproduce age band specific variations in the incidence of diarrheal disease reported in secular analyses of pooled global estimates.[22,28,29] The similarity between the incidences found in this prospective cohort and other works suggests validity in the findings.
As in other settings, in the present analysis greater maternal education was found to have a protective effect against childhood illness.[13,30] Although these data do not allow for identification of the specific aspects of education accounting for the protective effect it is possible that similar to other low-income settings education cultivated more accurate health knowledge, receptivity to health messages and improved communication abilities in this cohort.[31] As enhancement of maternal education in coordinating effective interventions is a component of The integrated Global Action Plan for Pneumonia and Diarrhoea, our findings provide further evidence supporting the importance of maternal education in reducing child morbidity in high burden regions.[32]
In meta-analyses, interventions of hand washing with soap and use of improved sanitation facilities have each been associated with greater than 30% reductions in disease risk.[33,34] In this study, hand washing was associated with a 15% reduction and household use of a toilet with septic/canalization a 24% reduction. Although these reductions are lower than pooled estimates, their agreement with the reported protective trends and significance in multivariate analysis demonstrates their importance in the setting of Kabul.
In this population, maternal hand washing with soap was reported by less than half of mothers however fieldworkers observed soap availability in 72% of households. This discrepancy between availability and utilization indicates that lack of use may be a function of both barriers to access and education. Work aimed at improving hand washing in resource constrained settings has shown that formative research with setting appropriate interventions are crucial in facilitating behavior change.[35] Given these findings prevention programs in this setting would be most efficacious if they addressed both access to, and understanding of soap use in relation to the socio-cultural context of the population.
Open defecation was more common than global estimates from resource-limited settings.[11] This high prevalence and the demonstrated risk reduction with access to improved sanitation facilities highlight the importance of this prevention point in programs addressing diarrheal disease in urban Kabul. Further, previous observational research from Kabul found an association between averted child deaths and latrine improvement programs which is in line with the findings here.[15,34] Setting specific evidence for public health interventions is vital in successful programs, and the results surrounding hand washing with soap and use of improved sanitation facilities should be used to focus diarrheal prevention strategies in Kabul.[36,37]
Rotavirus is postulated to be a major cause of diarrheal illness in Afghanistan.[38,39] In low-income settings, geographically similar to the study setting, rotavirus associated diarrhea predominates during colder seasons.[40] In this cohort the incidence of diarrheal illness was lowest during the winter period. This seasonal observation suggests a non-rotavirus diarrhoegenic predominance, however laboratory samples to identify microbiologic causes of enteric illnesses were not collected and the specific microbial profile in this setting could not be identified. Further microbiological data are required to investigate this finding
A trend of reduced risk of diarrheal illness was found among children from households that accessed water from an open well versus a piped source. This may relate to factors of water quantity and quality. Although improved water sources in the form of piped access have been shown to protect against diarrheal disease,[41] and approximately half of households in this study reported home piped access, no association with disease prevention was found with piped water. In Kabul water delivery is restricted and interruption of municipal water services is common.[42,43] This reduced delivery quantity perpetuates barriers to access and negates the intended benefits of improved water sources, and may explain the findings in this cohort. Alternatively, poor water quality due to contamination of municipal water could account for the observed findings. Therefore further studies to explore these hypotheses and disentangle the impact of water quantity and quality in this setting are needed.
Limitations
Continuous surveillance for diarrheal illness was not done and subsequently, child episodes may have been missed. In this case, the calculated incidence rates would be an underestimation of the true burden of disease and non-differentially bias towards the null. Therefore any risk estimates observed would be an underestimation and thereby strengthening the associations found in this population.[44,45] Methodologically shorter recall periods have been shown to improve the accuracy of report of childhood diarrheal morbidity.[46,47] In the present study recall of stool frequency was limited to one day prior to assessment which should have served to increase the accuracy of identifying episodes of diarrheal disease.[48] Additionally the majority of diagnosed episodes in this study came from the clinic setting suggesting that caregivers were apt to address child illnesses, thereby making the probability of missed episodes less likely. This study gathered data on risk factors through participant reports that have the potential to suffer from recall bias. However, as the results are externally valid reproducing findings from other research settings, such bias is less likely.[33,34,49] Although seasonal variations in diarrhoegenic organisms are described and temporal trends in risks were observed, microbiologic samples were not collected and this limitation precludes etiologic seasonal analysis.[50,51] Subsequent studies should address this through concurrent microbiological sampling to better inform prevention initiatives. Lastly, confounding in relation to breastfeeding cannot be ruled out in this analysis. Although breastfeeding data at recruitment was available and child age as a proxy indicator was used, the study did not provide the ability to assess for the time varying effect of breastfeeding practices that may have altered disease risks throughout follow-up and this is a limitation of the analysis.[52]
Conclusion
This study provides contemporary diarrheal incidence rates and evidence-based findings pertinent to prevention of childhood diarrheal illness in Afghanistan. Enhanced education and economic status were beneficial in this population and these findings are in line with multi-sector public health interventions to combat child illness. Maternal hand washing with soap and household use of improved sanitation facilities were found to be protective against diarrheal illness. Taking into account the low prevalence of hand washing with soap and high prevalence of open toilet use, these factors represent important prevention points for public health endeavors in Kabul that should be prospectively evaluated.
Supporting Information
(DOCX)
(DTA)
Data Availability
All relevant data are included within the Supporting Information files.
Funding Statement
This study was funded by the Wellcome Trust (Grant reference 082476/Z/07/Z, http://wellcome.ac.uk/funding/index/htm) and the Development Partnership in higher education (grant reference code 53, http://www.britishcouncil.org/delphe/htm). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, et al. (2013) Global burden of childhood pneumonia and diarrhoea. Lancet 381: 1405–1416. 10.1016/S0140-6736(13)60222-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhutta ZA, Das JK (2013) Global burden of childhood diarrhea and pneumonia: what can and should be done? Pediatrics 131: 634–636. 10.1542/peds.2012-3737 [DOI] [PubMed] [Google Scholar]
- 3. Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA (2008) Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev 66: 487–505. 10.1111/j.1753-4887.2008.00082.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, et al. (2008) Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 37: 816–830. 10.1093/ije/dyn099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodriguez L, Cervantes E, Ortiz R (2011) Malnutrition and gastrointestinal and respiratory infections in children: a public health problem. Int J Environ Res Public Health 8: 1174–1205. 10.3390/ijerph8041174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levine MM (2010) Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol 8: 129 10.1186/1741-7007-8-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. [DOI] [PubMed]
- 8. Bhutta ZA, Das JK, Walker N, Rizvi A, Campbell H, et al. (2013) Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet 381: 1417–1429. 10.1016/S0140-6736(13)60648-0 [DOI] [PubMed] [Google Scholar]
- 9.United Nations Genarel Assembly: Plenary 108th meeting: General Assembly Adopts resoluation recognizing access to clean water, sanitation as a human right. 2010. Accessed online: 25 March 2014: http://www.un.org/News/Press/docs/2010/ga10967.doc.htm. United Nations: GA/10967: 108th Meeting.
- 10.WHO/UNICEF. Joint Monitoring Programme for Water Supply and Sanitation. Progress on Drinking Water and Sanitation: 2010 Update. Accessed online: 25 March 2014: http://whqlibdoc.who.int/publications/2010/9789241563956_eng_full_text.pdf. Geneva and New York.
- 11.UNICEF: Diarrhoea: Why children are still dying and what can be done. 2009. Accessed online: 25 March 2014: http://whqlibdoc.who.int/publications/2009/9789241598415_eng.pdf?ua=1.
- 12.National Action Plan of Improved Diarrhea Case Management. Islamic Republic of Afghanistan, Ministry of Public Health. 2008. Accessed online: 25 March 2014: http://www.basics.org/documents/National-Action-Plan-of-Improved-Diarrhea-Case-Mgmt_Afghanistan.pdf.
- 13. Mashal T, Takano T, Nakamura K, Kizuki M, Hemat S, et al. (2008) Factors associated with the health and nutritional status of children under 5 years of age in Afghanistan: family behaviour related to women and past experience of war-related hardships. BMC Public Health 8: 301 10.1186/1471-2458-8-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Opryszko MC, Majeed SW, Hansen PM, Myers JA, Baba D, et al. (2010) Water and hygiene interventions to reduce diarrhoea in rural Afghanistan: a randomized controlled study. J Water Health 8: 687–702. 10.2166/wh.2010.121 [DOI] [PubMed] [Google Scholar]
- 15. Meddings DR, Ronald LA, Marion S, Pinera JF, Oppliger A (2004) Cost effectiveness of a latrine revision programme in Kabul, Afghanistan. Bull World Health Organ 82: 281–289. [PMC free article] [PubMed] [Google Scholar]
- 16. Acerra JR, Iskyan K, Qureshi ZA, Sharma RK (2009) Rebuilding the health care system in Afghanistan: an overview of primary care and emergency services. Int J Emerg Med 2: 77–82. 10.1007/s12245-009-0106-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, et al. (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 4: e296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manaseki-Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, et al. (2012) Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 379: 1419–1427. 10.1016/S0140-6736(11)61650-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aluisio AR, Maroof Z, Chandramohan D, Bruce J, Mughal MZ, et al. (2013) Vitamin d3 supplementation and childhood diarrhea: a randomized controlled trial. Pediatrics 132: e832–840. 10.1542/peds.2012-3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morris SS, Cousens SN, Lanata CF, Kirkwood BR (1994) Diarrhoea—defining the episode. Int J Epidemiol 23: 617–623. [DOI] [PubMed] [Google Scholar]
- 21. Ravallion M, Chen S, Sangraula P (2009) Dollar a Day Revisited World Bank Econ Rev 23(2): 163–184. [Google Scholar]
- 22. Fischer Walker CL, Perin J, Aryee MJ, Boschi-Pinto C, Black RE (2012) Diarrhea incidence in low- and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health 12: 220 10.1186/1471-2458-12-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO/UNICEF (2009) WHO child growth standards and the identification of severe acute malnutrition in infants and children. [PubMed]
- 24.UNDP Regional Rural Economic Regeneration Strategies (RRERS) Provincial Profile, Kabul Province. Accessed online: 13 May 2012: http://www.undp.org.af/publications/RRERS/Kabul Provincial Profile.pdf
- 25. Thomsen JL, Parner ET (2006) Methods for analysing recurrent events in health care data. Examples from admissions in Ebeltoft Health Promotion Project. Fam Pract 23: 407–413. [DOI] [PubMed] [Google Scholar]
- 26. Mahmud A, Jalil F, Karlberg J, Lindblad BS (1993) Early child health in Lahore, Pakistan: VII. Diarrhoea. Acta Paediatr Suppl 82 Suppl 390: 79–85. [DOI] [PubMed] [Google Scholar]
- 27. Jousilahti P, Madkour SM, Lambrechts T, Sherwin E (1997) Diarrhoeal disease morbidity and home treatment practices in Egypt . Public Health 111: 5–10. [DOI] [PubMed] [Google Scholar]
- 28. Bern C, Martines J, de Zoysa I, Glass RI (1992) The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull World Health Organ 70: 705–714. [PMC free article] [PubMed] [Google Scholar]
- 29. Kosek M, Bern C, Guerrant RL (2003) The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ 81: 197–204. [PMC free article] [PubMed] [Google Scholar]
- 30. Siziya S, Muula AS, Rudatsikira E (2009) Diarrhoea and acute respiratory infections prevalence and risk factors among under-five children in Iraq in 2000 . Ital J Pediatr 35: 8 10.1186/1824-7288-35-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vikram K, Vanneman R, Desai S (2012) Linkages between maternal education and childhood immunization in India . Soc Sci Med 75: 331–339. 10.1016/j.socscimed.2012.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.(UNICEF, WHO: End preventable deaths: Global Action Plan for Prevention and Control of Pneumonia and Diarrhoea. 2013 Accessed online: 25 march 2014: http://apps.who.int/iris/bitstream/10665/79200/1/9789241505239_eng.pdf).
- 33. Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, et al. (2005) Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis 5: 42–52. [DOI] [PubMed] [Google Scholar]
- 34. Clasen TF, Bostoen K, Schmidt W-P, Boisson S, Fung IC-H, et al. (2010) Interventions to improve disposal of human excreta for preventing diarrhoea. Cochrane Database Syst Rev 6: CD007180 10.1002/14651858.CD007180.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biran A, Schmidt W, Varadharajan KS, Rajaraman D, Kumar R, et al. (2014) Effect of a behaviour-change intervention on handwashing with soap in India (SuperAmma): a cluster-randomised trial. Lancet Global Health 2: e145–154. 10.1016/S2214-109X(13)70160-8 [DOI] [PubMed] [Google Scholar]
- 36. Fielding JE, Briss PA (2006) Promoting evidence-based public health policy: can we have better evidence and more action? Health Aff (Millwood) 25: 969–978. [DOI] [PubMed] [Google Scholar]
- 37. Hausman AJ (2002) Implications of evidence-based practice for community health. Am J Community Psychol 30: 453–467. [DOI] [PubMed] [Google Scholar]
- 38. Glass RI, Bresee JS, Turcios R, Fischer TK, Parashar UD, et al. (2005) Rotavirus vaccines: targeting the developing world. J Infect Dis 192 Suppl 1: S160–166. [DOI] [PubMed] [Google Scholar]
- 39. Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI (2003) Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 9: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel MM, Pitzer VE, Alonso WJ, Vera D, Lopman B, et al. (2013) Global seasonality of rotavirus disease. Pediatr Infect Dis J 32: e134–147. 10.1097/INF.0b013e31827d3b68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clasen TF, Roberts IG, Rabie T, Schmidt WP, Carincross S (2009) Interventions to improve water quality for preventing diarrhoea (Review). The Cochrane Collaboration.Idem [DOI] [PubMed]
- 42.Royal Geographical Society With The Institute of British Geographers: The city of Kabul. Accessed online: 12 May 2012: http://www.unlockingthearchives.rgs.org/resources/documents/Kabul.pdf
- 43. Pinera JF, Reed RA (2009) A tale of two cities: restoring water services in Kabul and Monrovia. Disasters 33: 574–590. 10.1111/j.1467-7717.2008.01088.x [DOI] [PubMed] [Google Scholar]
- 44. Copeland KT, Checkoway H, McMichael AJ, Holbrook RH (1977) Bias due to misclassification in the estimation of relative risk. Am J Epidemiol 105: 488–495. [DOI] [PubMed] [Google Scholar]
- 45. Fosgate GT (2006) Non-differential measurement error does not always bias diagnostic likelihood ratios towards the null. Emerg Themes Epidemiol 3: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramakrishnan R, Venkatarao T, Koya PK, Kamaraj P (1998) Influence of recall period on estimates of diarrhoea morbidity in infants in rural Tamil Nadu. Indian J Public Health 42: 3–6. [PubMed] [Google Scholar]
- 47. Alam N, Henry FJ, Rahaman MM (1989) Reporting errors in one-week diarrhoea recall surveys: experience from a prospective study in rural Bangladesh. Int J Epidemiol 18: 697–700. [DOI] [PubMed] [Google Scholar]
- 48. Lee G, Cama V, Gilman RH, Cabrera L, Saito M, et al. (2010) Comparison of two types of epidemiological surveys aimed at collecting daily clinical symptoms in community-based longitudinal studies. Ann Epidemiol 20: 151–158. 10.1016/j.annepidem.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ejemot RI, Meremikwu JE, Critchley jA (2009) Hand washing for preventing diarrhoea (Review). The Cochrane Collaboration.Idem [DOI] [PubMed]
- 50. Hashizume M, Armstrong B, Wagatsuma Y, Faruque AS, Hayashi T, et al. (2008) Rotavirus infections and climate variability in Dhaka, Bangladesh: a time-series analysis. Epidemiol Infect 136: 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jagai JS, Sarkar R, Castronovo D, Kattula D, McEntee J, et al. (2012) Seasonality of rotavirus in South Asia: a meta-analysis approach assessing associations with temperature, precipitation, and vegetation index. PLoS One 7: e38168 10.1371/journal.pone.0038168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kramer MS, Kakuma R (2012) Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev 8: CD003517 10.1002/14651858.CD003517.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DTA)
Data Availability Statement
All relevant data are included within the Supporting Information files.