Abstract
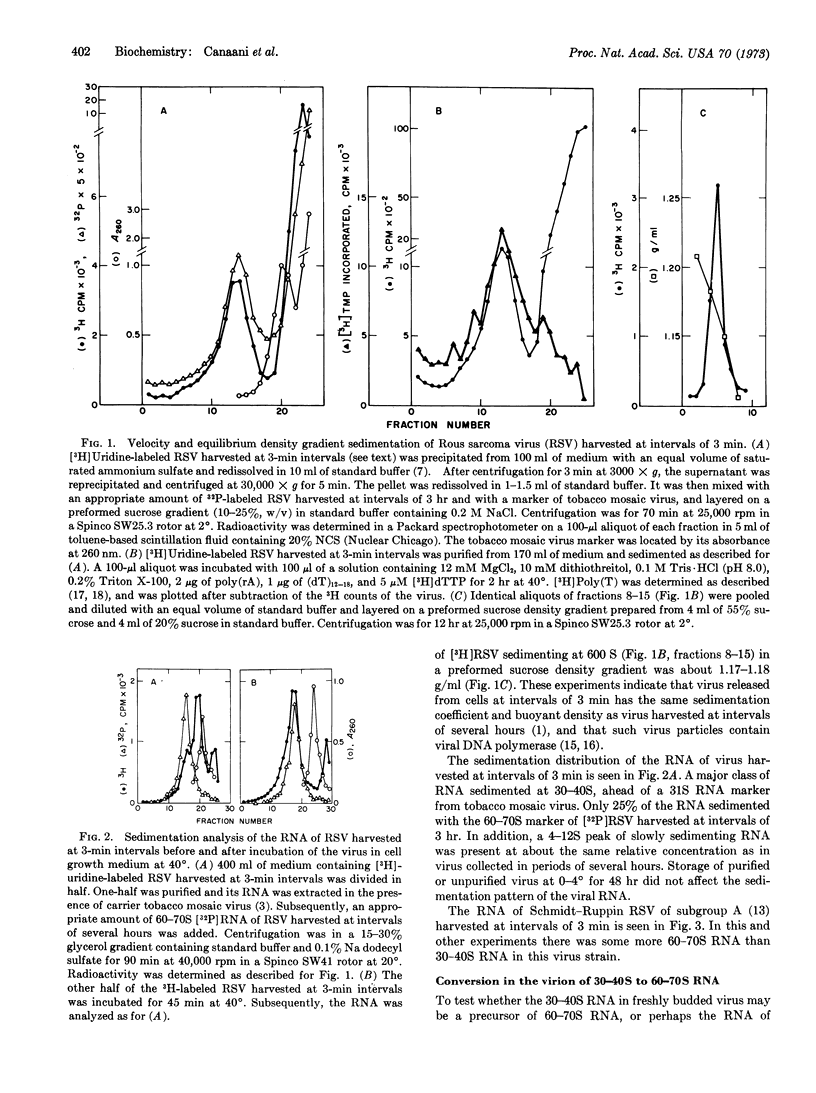
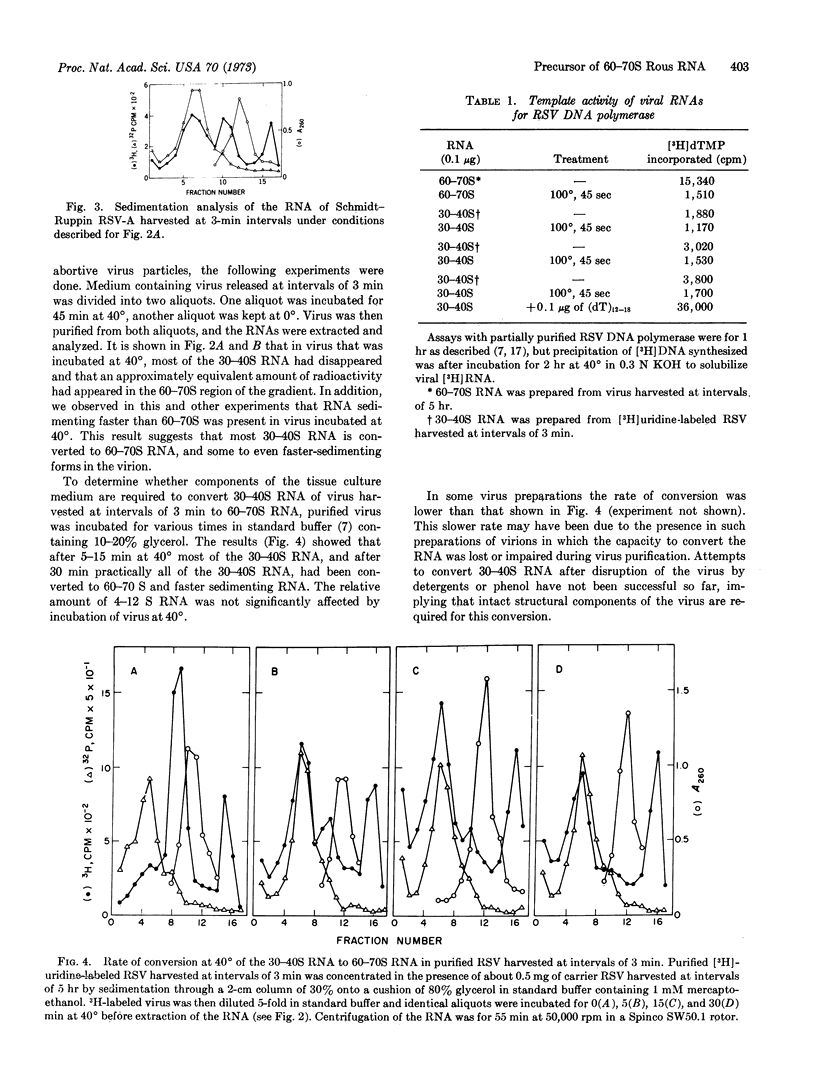
Rous sarcoma virus harvested from cells at intervals of 3 min has the same density, sedimentation coefficient, and DNA polymerase as virus harvested at hourly intervals. The RNA of the Prague strain-C consists of a minor class of 60-70S RNA, a major class 30-40S RNA, and a 4-12S class of RNA present at variable concentration. The RNA of the Schmidt-Ruppin strain-A contains more 60-70S than 30-40S RNA. Upon incubation of virus harvested at 3-min intervals at 40° in cell growth medium or Tris-saline, most of the 30-40S RNA is converted to 60-70S RNA.
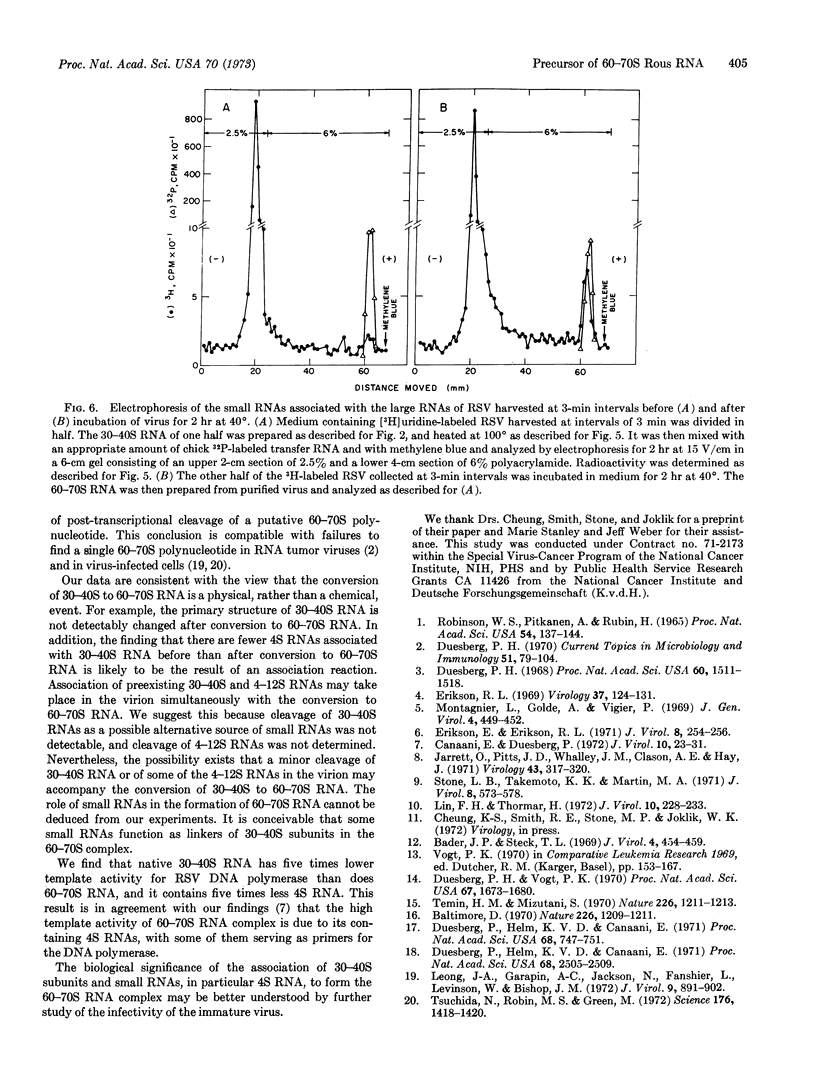
The electrophoretic mobility of the 30-40S RNA of the Rous virus harvested at 3-min intervals is lower than that of the 30-40S subunits of completely dissociated 60-70S RNA; after heating, their mobilities are identical. Heating also releases some small RNAs from 30-40S RNA of virus harvested at 3-min intervals, but five times more 4S RNA is released if the 30-40S RNA is allowed to convert to 60-70S in the virus. The template activity for Rous virus DNA polymerase of the 30-40S RNA of Rous virus harvested at 3-min intervals is about five times lower than that of 60-70S RNA. It is suggested that association of 30-40S RNAs with some RNAs of the 4-12S class may take place simultaneously with their conversion to 60-70S RNA.
Keywords: subunits of tumor virus RNA, RNA-dependent DNA polymerase, RNA primers
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P., Helm K. V., Canaani E. Comparative properties of RNA and DNA templates for the DNA polymerase of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2505–2509. doi: 10.1073/pnas.68.10.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P., Helm K. V., Canaani E. Properties of a soluble DNA polymerase isolated from Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):747–751. doi: 10.1073/pnas.68.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Association of 4S ribonucleic acid with oncornavirus ribonucleic acids. J Virol. 1971 Aug;8(2):254–256. doi: 10.1128/jvi.8.2.254-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Pitts J. D., Whalley J. M., Clason A. E., Hay J. Isolation of the nucleic acid of feline leukemia virus. Virology. 1971 Jan;43(1):317–320. doi: 10.1016/0042-6822(71)90252-2. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Properties of maedi nucleic acid and the presence of ribonucleic acid- and deoxyribonucleic acid-dependent deoxyribonucleic acid polymerase in the virions. J Virol. 1972 Aug;10(2):228–233. doi: 10.1128/jvi.10.2.228-233.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnier L., Goldé A., Vigier P. A possible subunit structure of Rous sarcoma virus RNA. J Gen Virol. 1969 Apr;4(3):449–452. doi: 10.1099/0022-1317-4-3-449. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone L. B., Takemoto K. K., Martin M. A. Physical and biochemical properties of progressive pneumonia virus. J Virol. 1971 Oct;8(4):573–578. doi: 10.1128/jvi.8.4.573-578.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Envelope classification of avian RNA tumor viruses. Bibl Haematol. 1970;(36):153–167. doi: 10.1159/000391704. [DOI] [PubMed] [Google Scholar]



