Highlights
-
•
Dicarboxylate or 2-oxoglutarate carriers are expressed in Lactococcus lactis.
-
•
Glutathione is not a competitive substrate of dicarboxylate or 2-oxoglutarate carriers.
-
•
Dicarboxylate and 2-oxoglutarate carriers do not transport glutathione.
-
•
The identity of the mitochondrial glutathione transporter is unknown.
Abbreviations: AAC, mitochondrial ADP/ATP carrier; CiC, mitochondrial citrate carrier; DIC, mitochondrial dicarboxylate carrier; GSH, glutathione; GSSG, glutathione disulphide; NEM, N-ethylmaleimide; OGC, mitochondrial 2-oxoglutarate carrier; TBS, Tris-buffered saline
Keywords: Mitochondrial carriers, Glutathione transport, Competitive inhibition, Reactive oxygen species, Metabolite exchange, Lactococcus lactis
Abstract
Glutathione carries out vital protective roles within mitochondria, but is synthesised in the cytosol. Previous studies have suggested that the mitochondrial dicarboxylate and 2-oxoglutarate carriers were responsible for glutathione uptake. We set out to characterise the putative glutathione transport by using fused membrane vesicles of Lactococcus lactis overexpressing the dicarboxylate and 2-oxoglutarate carriers. Although transport of the canonical substrates could be measured readily, an excess of glutathione did not compete for substrate uptake nor could transport of glutathione be measured directly. Thus these mitochondrial carriers do not transport glutathione and the identity of the mitochondrial glutathione transporter remains unknown.
1. Introduction
Mitochondria are dynamic subcellular organelles that rely on the import of nuclear-encoded proteins and the transport of metabolites and essential cofactors across the relatively impermeable inner membrane [1]. Dysfunctional mitochondrial transport is linked to a range of human pathologies [2,3]. Glutathione (GSH) is an essential small peptide present throughout the cell that has vital protective roles within mitochondria [4,5]. The mitochondrial GSH concentration is 1–5 mM and this pool exists predominantly (95–99%) in the reduced form due to NADPH-dependent glutathione reductase, whereas the remainder is in the oxidised glutathione disulphide (GSSG) form [5–7]. GSH protects against damage by degrading peroxides, detoxifying electrophiles and by interacting with protein thiols to prevent oxidative damage and mediate redox signalling (Fig. 1A) [5]. These roles are particularly vital in mitochondria, as GSH depletion within the organelle greatly increases oxidative damage, leading to cell dysfunction and death [8]. Thus the mitochondrial GSH pool plays a key role in preventing pathologies, such as neurological disorders, ischaemia/reperfusion injury and alcoholic liver disease [4,9,10].
Fig. 1.
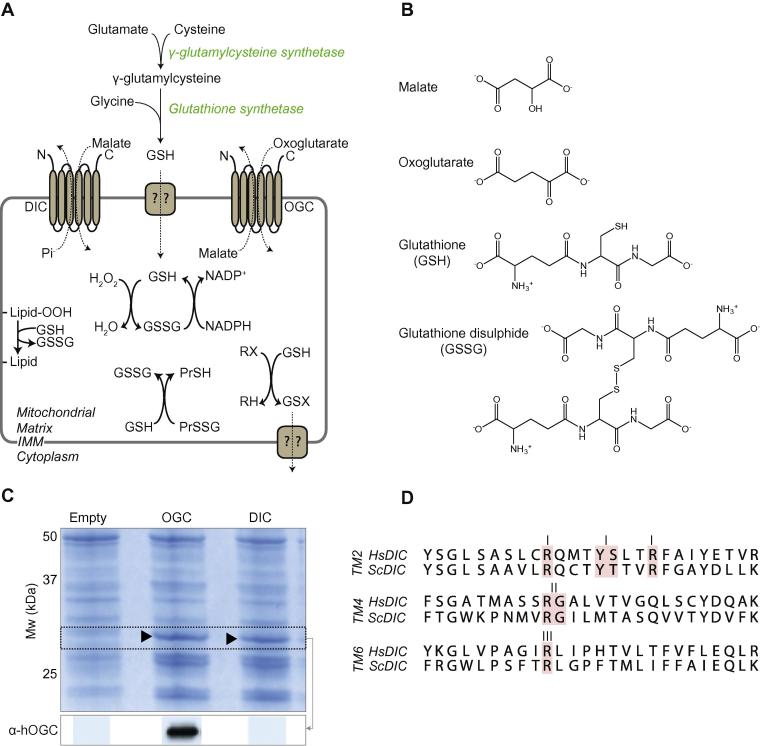
(A) GSH is synthesised in the cytoplasm and transported into the mitochondria where it is involved in protective processes. The dicarboxylate carrier (DIC), left, and the 2-oxoglutarate carrier (OGC), right, are shown transporting their canonical substrates. GSH is involved in the protection against lipid peroxidation, hydrogen peroxide, and protein thiol oxidation. (B) Chemical structures of DIC/OGC substrates along with GSH and GSSG. (C) Expression of DIC and OGC in L. lactis membranes. Bands at ∼32 kDa can be observed on SDS–PAGE gels stained with Coomassie. Immunoblotting against human OGC highlighted a specific band in the membrane fraction, but no antibody was available to probe S. cerevisiae DIC. (D) Alignments of the transmembrane α-helices 2, 4 and 6 from S. cerevisiae DIC and Homo sapiens DIC. Residues involved in substrate binding are highlighted in red [34–36].
GSH is exclusively made in the cytosol by γ-glutamylcysteine synthetase and glutathione synthetase (Fig. 1A) [11], and therefore its transport across the mitochondrial inner membrane is required [6]. Previous studies have suggested that mitochondrial GSH transport was carried out by two members of the mitochondrial carrier family (SLC25), the mitochondrial dicarboxylate carrier (DIC; SLC25A10) and the 2-oxoglutarate carrier (OGC; SLC25A11) [12–19]. In mammals the mitochondrial carrier family consists of 53 members, about half of which have been functionally characterised [2]. All family members have six transmembrane helices, N and C termini that protrude into the intermembrane space (Fig. 1A) and a tripartite sequence repeat of about 100 amino acids [20]. The overall structural fold of the carriers consist of six transmembrane α-helices arranged in a threefold pseudo-symmetrical way [21–23]. There are two sets of salt bridges on the matrix and cytoplasmic side of the carriers that regulate access to a central substrate-binding site for the exchange of metabolites across the mitochondrial inner membrane [24]. The mitochondrial carrier family transports many anion metabolites, such as citrate, malate, glutamate, ornithine and 2-oxoglutarate, so they are plausible candidates for the import of GSH (Fig. 1B).
Early studies reported mitochondrial GSH transport in isolated rat liver mitochondria through both high affinity (Km = 60 μM, Vmax = 0.5 nmol min−1 mg protein−1) and low affinity transport systems (Km = 5.4 mM, Vmax = 5.9 nmol min−1 mg protein−1) [25,26] that required a proton motive force. Subsequent studies have suggested that DIC and OGC were involved in mitochondrial glutathione transport. These studies used a variety of systems, such as Xenopus laevis oocytes [27], isolated kidney mitochondria and mitoplasts [13,14], and reconstituted proteoliposomes [16], but the GSH transport activity of these carriers was not fully explored. Therefore, we set out to characterise in detail the GSH transport capabilities of the DIC and OGC by using the well-established Lactococcus lactis system for overexpression and characterisation of members of the mitochondrial carrier family [28,29].
The OGC and DIC carriers were overexpressed in the cytoplasmic membrane of L. lactis. Isolated membrane vesicles were fused with liposomes and loaded with substrate to measure the exchange with radiolabelled metabolites [21]. Both OGC and DIC transported their canonical substrates, 2-oxoglutarate/malate and phosphate/malate, respectively. Surprisingly however, substrate competition assays with excess GSH had no effect on the transport rate in contrast to known substrates or inhibitors. Furthermore, there was no detectable transport of [35S]-GSH by these carriers. Together these data demonstrate that DIC and OGC do not transport GSH.
2. Materials and methods
2.1. Materials
All materials were from Sigma–Aldrich, unless otherwise stated. M17 media (ForMedium) contained 5% (w/v) pancreatic digest of casein, 5% (w/v) soy peptone, 5% (w/v) beef extract, 2.5% (w/v) yeast extract, 0.5% (w/v) ascorbic acid, 0.25% (w/v) magnesium sulphate, 19% (w/v) disodium-β-glycerophosphate. SM17 plates consisted of M17 broth supplemented with 0.5 M sucrose and 1.5% (w/v) agar. Radiochemicals were from American Radiolabelled Chemicals and Perkin Elmer. Primers were from Sigma–Aldrich.
2.2. Molecular cloning
Codon-optimised genes for OGC (Homo sapiens; Uniprot ID: Q02978), DIC (H. sapiens; Uniprot ID: Q9UBX3, Saccharomyces cerevisiae; Uniprot ID: Q06143), CiC (H. sapiens, Uniprot ID: P53007) and AAC (Myceliophthora thermophila: Uniprot ID: G2QNH0) were synthesised by GenScript. For expression in L. lactis, the genes were cloned into the expression vector pNZ8048 and transformed into the electrocompetent L. lactis strain NZ9000. Successful transformants were selected on SM17 plates containing 5 μg mL−1 chloramphenicol, and confirmed by DNA sequencing.
2.3. Cell growth and membrane isolation
Pre-cultures of L. lactis were obtained by inoculating M17 medium supplemented with 1% (w/v) glucose and 5 μg mL−1 chloramphenicol from glycerol stocks and incubating the cultures overnight at 30 °C with no aeration. The OD600 was measured and cells diluted to a starting OD600 of 0.1 in fresh M17 medium supplemented with 1% (w/v) glucose and 5 μg mL−1 chloramphenicol. Cells were grown at 30 °C with no aeration until the OD600 reached 0.5. The expression of the recombinant proteins was induced by addition of nisin A with a dilution of 1:10 000 of spent M17 medium from the nisin A secreting L. lactis strain NZ9700. The cells were grown for a further 2 h at 30 °C, harvested by centrifugation (6000×g, 10 min, 4 °C), resuspended in Tris-buffered saline, pH 7.4 (TBS) and collected by centrifugation as before. The cells were resuspended in TBS buffer and disrupted mechanically with a cell disruptor (Constant Cell Disruption Systems) at 33 000 psi. Whole cells and debris were removed by centrifugation (10 800×g, 15 min, 4 °C), and membranes were collected by ultracentrifugation (138 000×g, 1 h, 4 °C). Pellets were resuspended in TBS buffer to a total protein concentration of approximately 5 mg mL−1 and stored in liquid nitrogen.
2.4. Fusion of membrane vesicles and liposomes
Escherichia coli polar lipid extract and egg yolk phosphatidylcholine (Avanti Polar Lipids) were mixed in a weight ratio of 3:1. The lipids were resuspended in TBS buffer to a final concentration of 20 mg mL−1 and frozen in liquid nitrogen. For membrane fusions, 1 mg L. lactis membranes were mixed with 5 mg liposomes, diluted to a final volume of 900 μL with TBS, and fused by seven cycles of freezing in liquid nitrogen and thawing at room temperature before storage in liquid nitrogen. The membrane vesicle fusions were thawed, and internal substrate added to a final concentration of 5 mM. Vesicles were extruded 11 times through a 1 µm pore size polycarbonate filter, passed through a pre-equilibrated PD10 column (GE Healthcare) to remove external substrate, and collected in 1.6 mL TBS buffer.
2.5. Transport assays
Transport assays were carried out using a Hamilton MicroLab Star robot (Hamilton Robotics Ltd.). Transport of radiolabeled substrate was initiated by the addition of 100 μL TBS buffer with 1.5 μM [14C]-malate (2.22 GBq mmol−1) or 1.5 μM [35S]-GSH (16.946 TBq mmol−1) to 5 μg fused membranes in a MultiScreenHTS-HA 96-well filter plate (pore size = 0.45 µm Millipore). The transport was stopped at 0, 10, 20, 30, 45 s, 1, 2.5, 5, 7.5, 10 and 15 min by the addition of 200 μL ice-cold TBS buffer and filtering using a vacuum manifold, followed by an additional wash step with 200 μL ice-cold TBS buffer. Levels of radioactivity in the vesicles were measured by the addition of 200 μL MicroScint-20 (Perkin Elmer) and by quantifying the amount of radioactivity with the TopCount scintillation counter (Perkin Elmer). Initial rates were determined from the linear part of the uptake curves. External compounds were added at a final concentration of 10 mM, with the exception of NEM, which was added at 1 mM.
2.6. SDS–PAGE and immunoblotting
To assess protein expression, 10 μg L. lactis membranes were loaded onto 12% Mini-Protean Precast Tris–Glycine gels (BioRad) and run at 120 V. Gels were stained in Imperial Protein Stain (Thermo Scientific). Immunoblotting was carried out after semi-dry transfer of SDS–PAGE gels onto PVDF membrane. Membranes were incubated in 3% milk containing 1:10 000 primary rabbit anti-OGC antibody for 1 h at RT. After washing, membranes were incubated in 1:10000 goat anti-rabbit secondary antibody conjugated to horse radish peroxidase for 1 h at RT. Antibody-labelled proteins were detected using the ECL reagent Western blot detection kit (GE Healthcare), following the instructions of the manufacturer, and visualised by developing the exposed film.
2.7. N-terminal modification by PCR
Primers corresponding to N-terminal regions of mitochondrial carriers known to express in L. lactis were designed. Extension PCR was performed using KOD Hot Start DNA Polymerase kit (Merck Millipore) on the human DIC pUC57 plasmid from GenScript. Previously it has been shown that modifications of the N-terminus of mitochondrial carriers can improve their expression levels and activity [28]. To achieve this, primers corresponding to N-terminal truncations were designed for the AAC and CiC carriers (Δ amino acid residues 2–19 and Δ amino acid residues 2–20, for AAC and CiC, respectively). PCR was carried out on the pUC57 plasmid containing AAC or CiC. Altered genes were then cloned into L. lactis as previously described.
2.8. Statistics
Statistical tests were undertaken in Prism (GraphPad). ANOVAs with post-Bonferroni tests were performed on initial rate data to test for differences. All other statistical analyses were performed using Student’s t-test with details given in the figure legends.
3. Results and discussion
3.1. Expression of OGC and DIC
Genes encoding human OGC and DIC were cloned into expression vector pNZ8048 and transformed into L. lactis. A control strain was made by transforming L. lactis with pNZ8048 lacking a transporter gene. Expression of OGC could be observed in membranes separated by SDS–PAGE and was confirmed by Western blotting (Fig. 1C). In contrast, expression of human DIC was very low (data not shown) and transport activity for its canonical substrate malate, measured as malate/[14C]-malate exchange, was negligible (Fig. S1). As the N-terminal region is critical for successful expression and protein folding of mitochondrial carriers in L. lactis [28], N-termini from three mitochondrial carrier proteins that are well expressed in L. lactis were added to the N-terminal terminus of human DIC by extension PCR, and the resultant gene was cloned and expressed in L. lactis. However, none of the modified genes showed significant expression (data not shown) or [14C]-malate/malate exchange in malate-loaded vesicles (Fig. S1). Therefore, we focussed on the Saccharomyces cerevisiae DIC [31], which is ∼40% identical to human DIC, with high sequence similarity in the functionally important salt bridge networks and substrate binding site [24] (Fig. 1D). In yeast, GSH is also made exclusively in the cytosol and then transported into mitochondria [30]. Therefore, S. cerevisiae DIC could be used to assess whether the mitochondrial dicarboxylate carrier can transport GSH. The gene coding for S. cerevisiae DIC was cloned into pNZ8048 and transformed into L. lactis. S. cerevisiae DIC was expressed in the cytoplasmic membrane (Fig. 1C) and was able to transport its canonical substrates.
3.2. Metabolite transport assays
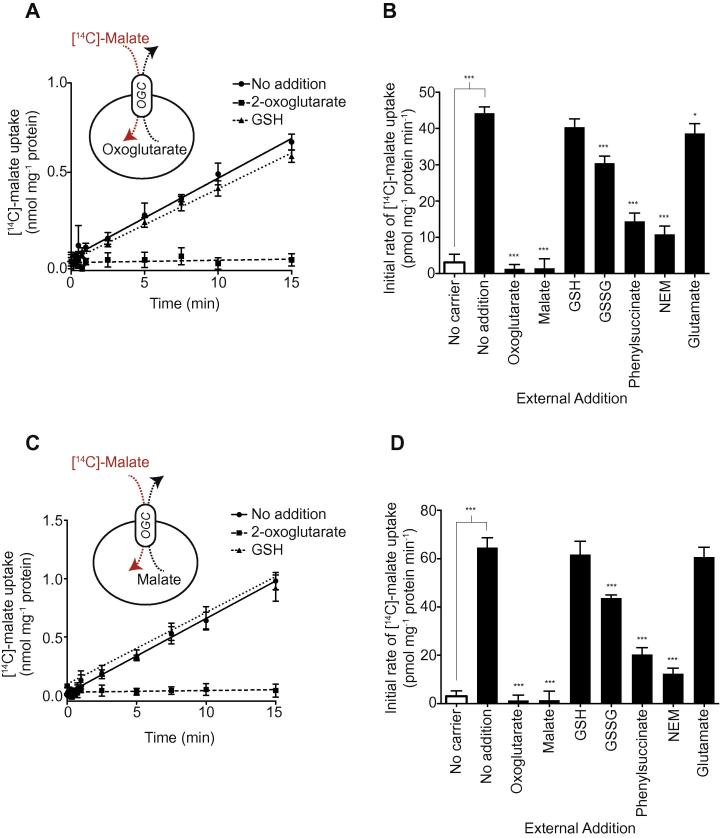
To determine the transport activity and substrate specificity of the OGC, L. lactis membranes containing OGC were fused with liposomes preloaded with 5 mM internal substrate (2-oxoglutarate or malate) and transport was initiated with 1.5 μM [14C]-malate (Fig. 2A and C). Heteroexchange of 2-oxoglutarate for [14C]-malate was quenched by excess of unlabelled 2-oxoglutarate added externally, whereas addition of excess GSH had no effect on transport (Fig. 2A). Transport experiments were carried out in the presence or absence of external substrates and inhibitors [32] and the results showed that the initial transport rate was quenched by excess 2-oxoglutarate or malate and by known OGC inhibitors phenylsuccinate and N-ethylmaleimide (NEM), whereas GSH had no effect. Excess glutamate had a minor competitive effect on transport (Fig. 2B). Together these data show that GSH is not a competitive substrate of OGC. Addition of supraphysiological excess of GSSG had a small effect on [14C]-malate uptake, but this is likely to be due to reaction of GSSG with available protein thiols, disrupting transport in a similar way to other sulphydryl reagents [5,33]. Similar experiments were carried out with OGC vesicles that had been preloaded with 5 mM malate to assess malate/[14C]-malate homoexchange (Fig. 2C). Exchange of [14C]-malate for internal malate occurred readily under these conditions and was blocked by 2-oxoglutarate, while excess GSH again had no effect (Fig. 2C). Competitor and inhibitor experiments of malate/[14C]-malate homo-exchange also showed no effect of GSH (Fig. 2D). We conclude that excess GSH does not compete with the canonical OGC transport process.
Fig. 2.
OGC-mediated transport of its canonical substrates is unaffected by excess GSH. (A) Malate/2-oxoglutarate hetero-exchange in the absence and presence of excess 2-oxoglutarate and GSH. Fused membranes expressing OGC were pre-loaded with 5 mM 2-oxoglutarate and incubated with 1.5 μM [14C]-malate for 15 min, after which external radio-labelled substrate was removed at the indicated time points (circles). The effects of externally added 10 mM 2-oxoglutarate (squares) or 10 mM GSH (triangles) are shown. (B) Effect of substrates, GSH, GSSG, glutamate and known inhibitors on the initial malate/2-oxoxglutarate exchange rate. The initial exchange rates were calculated from the first 60 s of transport. (C) Malate/malate homo-exchange by OGC in the absence and presence of excess 2-oxoglutarate and GSH. As in A, but the fused membranes were preloaded with 5 mM malate rather than 2-oxoglutarate. (D) Effect of substrates, GSH, GSSG, glutamate and known inhibitors on the initial malate/malate exchange rate of OGC. The initial exchange rates were calculated from the first 60 s of transport. Data are represented by the mean ± S.D. (n = 4). ∗∗∗P < 0.001, ∗P < 0.05 for effect of external compound by using ANOVA, or comparison of transport in empty control vesicles and OGC vesicles by Student’s t-test.
Transport of the canonical substrates by DIC was assessed by preloading the fused membranes with 5 mM phosphate or malate, followed by measurement of [14C]-malate uptake (Fig. 3A and C). Heteroexchange of phosphate for [14C]-malate was quenched by excess of the substrate succinate, whereas excess GSH had no effect (Fig. 3A). Competitive inhibition was analysed further by measuring the initial rates of transport in the presence of different substrates and inhibitors of OGC (Fig. 3B). Heteroexchange rates were decreased by excess of the substrate succinate and by the known inhibitors butylmalonate and NEM, confirming that the DIC was working correctly (Fig. 3B). However, excess GSH or glutamate had no effect on transport, indicating that these substrates cannot compete effectively for transport. The small effect of GSSG is most likely due to protein thiol modification, as was found for OGC. Malate/[14C]-malate homoexchange through the DIC was established by loading the DIC-vesicles with 5 mM malate (Fig. 3C). Homoexchange was again abolished by succinate, butylmalonate and NEM, but not by GSH, GSSG or glutamate (Fig. 3D). Therefore, GSH is not a competitive substrate of the canonical transport activity of DIC.
Fig. 3.
DIC-mediated transport of its canonical substrates is unaffected by excess GSH. (A) Malate/phosphate hetero-exchange in the absence and presence of excess succinate and GSH. Fused membranes expressing DIC were pre-loaded with 5 mM phosphate and incubated with 1.5 μM [14C]-malate for 15 min, after which external radio-labelled substrate was removed at the indicated time points (circles). The effects of externally added 10 mM succinate (squares) or of 10 mM GSH (triangles) are shown. (B) Effect of substrates, GSH, GSSG, glutamate and known inhibitors on the initial malate/phosphate exchange rate. The initial exchange rates were calculated from the first 60 s of transport. (C) Malate/malate homo-exchange by DIC in the absence and presence of excess succinate and GSH. As in A, but the fused membranes were preloaded with 5 mM malate rather than phosphate. (D) Effect of substrates, GSH, GSSG, glutamate and known inhibitors on the initial malate/malate exchange rate of DIC. The initial exchange rates were calculated from the first 60 s of transport. Data are represented by the mean and standard deviation (n = 4). ∗∗∗P < 0.001, ∗P < 0.05 for effect of external substrate by ANOVA, or comparison of transport in empty vesicles and OGC vesicles by Student’s t-test. NEM = N-ethylmaleimide.
To investigate whether the small effect of GSSG on transport by DIC and OGC was specific or non-specific, we explored the effect of GSSG on the transport activity of two other mitochondrial carriers. Addition of 10 mM GSSG affected the exchange of citrate/[14C]-citrate by the mitochondrial citrate carrier (CiC) slightly (Fig. S2A), but not the ADP/[14C]-ADP exchange catalysed by the mitochondrial ADP/ATP carrier (AAC) (Fig. S2B). As the OGC, DIC and CiC all have cysteine residues that could be modified by GSSG, whereas the AAC does not, these findings are consistent with GSSG affecting transport by non-specific thiol modification, making it unlikely that DIC or OGC are affected by GSSG through substrate competition. We conclude that GSH cannot compete with the canonical substrates of OGC and DIC [34–36], even when present in ∼6700-fold excess.
3.3. Direct measurement of GSH transport
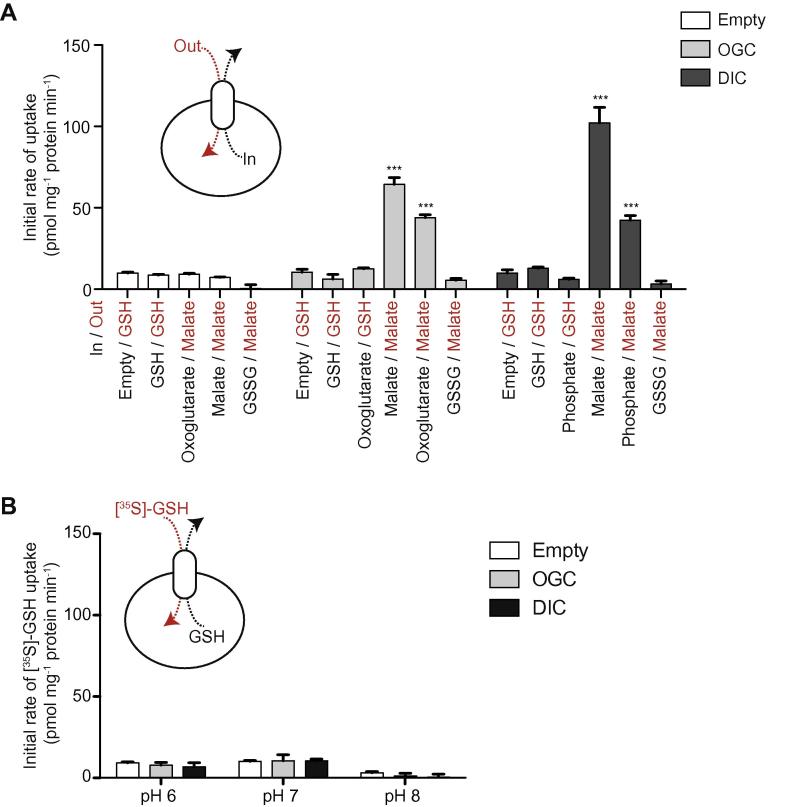
The above experiments showed that GSH does not compete with transport of the canonical substrates of DIC or OGC. However, the possibility remains that these carriers transport small amounts of GSH. To assess this we measured direct transport of [35S]-GSH. Fused membranes of the control strain did not have GSH/[35S]-GSH exchange activity (Fig. 4A). When OGC vesicles were assessed for GSH/[35S]-GSH exchange or 2-oxoglutarate/[35S]-GSH exchange, there was also no measurable uptake. Similarly, no [35S]-GSH uptake was found for DIC, whether the fused membranes were pre-loaded with GSH for GSH/[35S]-GSH exchange, or with phosphate for phosphate/[35S]-GSH exchange (Fig. 4A). Finally, when fused membranes expressing DIC and OGC were pre-loaded with 5 mM GSSG no uptake of [14C]-malate in exchange for GSSG could be observed (Fig. 4A), even though the specific exchange of the canonical substrates could be measured readily. These data indicate that OGC and DIC are not involved in GSH transport.
Fig. 4.
DIC or OGC do not transport GSH (A) [35S]-GSH uptake by fused membranes of the control (white bars), OGC-expressing (grey bars) and DIC-expressing (black bars) strains. The fused membranes were internally loaded with 5 mM of the indicated substrates (in) and incubated with 1.5 μM of the indicated external radio-labelled substrate (out) for 15 min. The initial exchange rates were determined over the first 60 s. (B) [35S]-GSH uptake at different pH values. Fused membranes of the control (white bar), OGC-expressing (grey bars) and DIC-expressing (black bars) strains were preloaded with 5 mM GSH and incubated with 1.5 μM [35S]-GSH to initiate exchange. The initial exchange rates were determined in the first 60 s time interval. Data are represented by the mean ± S.D. (n = 4). ∗∗∗P < 0.001 for comparison of transport of radiolabelled substrate by control vesicles against OGC/DIC vesicles by ANOVA.
3.4. Effect of pH on transport of GSH by DIC and OGC
The charge of GSH is pH-dependent due to the pKa of ∼8 of the cysteine moiety and could be a factor in the transport of GSH by DIC or OGC. Therefore, we carried out experiments at different pH values (Fig. 4B). Vesicles containing DIC or OGC were preloaded with 5 mM GSH and the initial rate of uptake of [35S]-GSH was measured at pH 6, 7 or 8 (Fig. 4B). In none of these conditions could uptake of GSH be observed, indicating that pH was not a relevant factor.
4. Conclusions
The transport of GSH from the cytoplasm to mitochondria is essential and had been suggested to be catalysed by the OGC and DIC [12–19], but the transport process was poorly characterised. The identification of the DIC and OGC as the mitochondrial GSH transporter(s) was based on experiments in relatively complex systems where the characterisation of the individual transport processes was difficult. In isolated kidney mitochondria and mitoplasts [13,14] GSH uptake was inhibited by dicarboxylates and by inhibitors of the DIC and OGC [13,14]. However, many other transport processes were also affected, and the DIC inhibitor butylmalonate only affected transport in the presence of antimycin A [14]. One study was carried out with purified OGC reconstituted into liposomes following expression in E. coli [16,37]. However, mitochondrial carriers expressed in E. coli can only be reconstituted with relatively low yields and thus high substrate gradients are required for their characterisation [38]. The transport of GSH by the reconstituted OGC did not have the typical transport properties of mitochondrial carriers [37–42], as transport ceased after two min [16]. Studies of the DIC and OGC overexpressed within cells led to a number of interesting effects [15,16,18], but these changes are likely to be secondary consequences of metabolite redistribution between the mitochondria and the cytosol, and cannot be taken as evidence for changes in GSH transport. In contrast, the L. lactis system provides a relatively simple and well established system to characterise mitochondrial carriers and other membrane proteins [28,29,32]. Using this approach we have shown that DIC and OGC do not transport GSH and thus the mitochondrial GSH transporter still needs to be identified.
Acknowledgements
This work was supported by the Medical Research Council (MRC, UK). We are grateful to Prof. Roland Lill for helpful discussions.
Contributor Information
Edmund R.S. Kunji, Email: ek@mrc-mbu.cam.ac.uk.
Michael P. Murphy, Email: mpm@mrc-mbu.cam.ac.uk.
Appendix A. Supplementary data
This document contains figures.
References
- 1.Kunji E.R.S. Structural and mechanistic aspects of mitochondrial transport proteins. Comp. Biophys. 2012;8:174–205. [Google Scholar]
- 2.Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol. Aspects Med. 2013;34:465–484. doi: 10.1016/j.mam.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Palmieri F. Diseases caused by defects of mitochondrial carriers: a review. Biochim. Biophys. Acta. 2008;1777:564–578. doi: 10.1016/j.bbabio.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Mari M., Morales A., Colell A., Garcia-Ruiz C., Fernandez-Checa J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy M.P. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid. Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 6.Griffith O.W., Meister A. Origin and turnover of mitochondrial glutathione. Proc. Natl. Acad. Sci. 1985;82:4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong J.S., Steinauer K.K., Hornung B., Irish J.M., Lecane P., Birrell G.W., Peehl D.M., Knox S.J. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Diff. 2002;9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 9.Mytilineou C., Kramer B.C., Yabut J.A. Glutathione depletion and oxidative stress. Parkinsonism Relat. Disord. 2002;8:385–387. doi: 10.1016/s1353-8020(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 10.Iguchi Y., Katsuno M., Takagi S., Ishigaki S., Niwa J.-I., Hasegawa M., Tanaka F., Sobue G. Oxidative stress induced by glutathione depletion reproduces pathological modifications of TDP-43 linked to TDP-43 proteinopathies. Neurobiol. Dis. 2012;45:862–870. doi: 10.1016/j.nbd.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Lu S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkins H.M., Marquardt K., Lash L.H., Linseman D.A. Bcl-2 is a novel interacting partner for the 2-oxoglutarate carrier and a key regulator of mitochondrial glutathione. Free Radic. Biol. Med. 2012;52:410–419. doi: 10.1016/j.freeradbiomed.2011.10.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z., Putt D.A., Lash L.H. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Arch. Biochem. Biophys. 2000;373:193–202. doi: 10.1006/abbi.1999.1527. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z., Lash L.H. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J. Pharmacol. Exp. Ther. 1998;285:608–618. [PubMed] [Google Scholar]
- 15.Zhong Q., Putt D.A., Xu F., Lash L.H. Hepatic mitochondrial transport of glutathione: studies in isolated rat liver mitochondria and H4IIE rat hepatoma cells. Arch. Biochem. Biophys. 2008;474:119–127. doi: 10.1016/j.abb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu F., Putt D.A., Matherly L.H., Lash L.H. Modulation of expression of rat mitochondrial 2-oxoglutarate carrier in NRK-52E cells alters mitochondrial transport and accumulation of glutathione and susceptibility to chemically induced apoptosis. J. Pharmacol. Exp. Ther. 2006;316:1175–1186. doi: 10.1124/jpet.105.094599. [DOI] [PubMed] [Google Scholar]
- 17.Benipal B., Lash L.H. Modulation of mitochondrial glutathione status and cellular energetics in primary cultures of proximal tubular cells from remnant kidney of uninephrectomized rats. Biochem. Pharmacol. 2013;85:1379–1388. doi: 10.1016/j.bcp.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Lash L.H., Putt D.A., Matherly L.H. Protection of NRK-52E cells, a rat renal proximal tubular cell line, from chemical-induced apoptosis by overexpression of a mitochondrial glutathione transporter. J. Pharmacol. Exp. Ther. 2002;303:476–486. doi: 10.1124/jpet.102.040220. [DOI] [PubMed] [Google Scholar]
- 19.Coll O., Colell A., Garcia-Ruiz C., Kaplowitz N., Fernandez-Checa J.C. Sensitivity of the 2-oxoglutarate carrier to alcohol intake contributes to mitochondrial glutathione depletion. Hepatology. 2003;38:692–702. doi: 10.1053/jhep.2003.50351. [DOI] [PubMed] [Google Scholar]
- 20.Walker J.E. The mitochondrial transporter family. Curr. Opin. Struct. Biol. 1992;2:519–526. [Google Scholar]
- 21.Pebay-Peyroula E., Dahout-Gonzalez C., Kahn R., Trezeguet V., Lauquin G.J., Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- 22.Kunji E.R.S., Harding M. Projection structure of the atractyloside-inhibited mitochondrial ADP/ATP carrier of Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:36985–36988. doi: 10.1074/jbc.C300304200. [DOI] [PubMed] [Google Scholar]
- 23.Ruprecht J.J., Hellawell A.M., Harding M., Crichton P.G., Mccoy A.J., Kunji E.R.S. Structures of yeast mitochondrial ADP/ATP carriers support a domain-based alternating-access transport mechanism. Proc. Natl. Acad. Sci. USA. 2014;111:E426–E434. doi: 10.1073/pnas.1320692111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson A.J., Overy C., Kunji E.R.S. The mechanism of transport by mitochondrial carriers based on analysis of symmetry. Proc. Natl. Acad. Sci. USA. 2008;105:17766–17771. doi: 10.1073/pnas.0809580105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurosawa K., Hayashi N., Sato N., Kamada T., Tagawa K. Transport of glutathione across the mitochondrial membranes. Biochem. Biophys. Res. Commun. 1990;167:367–372. doi: 10.1016/0006-291x(90)91774-m. [DOI] [PubMed] [Google Scholar]
- 26.Martensson J., Lai J.C., Meister A. High-affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proc. Natl. Acad. Sci. USA. 1990;87:7185–7189. doi: 10.1073/pnas.87.18.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Ruiz C., Morales A., Colell A., Rodes J., Yi J.R., Kaplowitz N., Fernandez-Checa J.C. Evidence that the rat hepatic mitochondrial carrier is distinct from the sinusoidal and canalicular transporters for reduced glutathione. Expression studies in Xenopus laevis oocytes. J. Biol. Chem. 1995;270:15946–15949. doi: 10.1074/jbc.270.27.15946. [DOI] [PubMed] [Google Scholar]
- 28.Monné M., Chan K.W., Slotboom D.J., Kunji E.R. Functional expression of eukaryotic membrane proteins in Lactococcus lactis. Protein Sci. 2005;14:3048–3056. doi: 10.1110/ps.051689905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunji E.R.S., Slotboom D.-J., Poolman B. Lactococcus lactis as host for overproduction of functional membrane proteins. Biochim. Biophys. Acta. 2003;1610:97–108. doi: 10.1016/s0005-2736(02)00712-5. [DOI] [PubMed] [Google Scholar]
- 30.Penninckx M.J. An overview on glutathione in Saccharomyces versus non-conventional yeasts. FEMS Yeast Res. 2002;2:295–305. doi: 10.1016/S1567-1356(02)00081-8. [DOI] [PubMed] [Google Scholar]
- 31.Palmieri L., Runswick M.J., Fiermonte G., Walker J.E., Palmieri F. Yeast mitochondrial carriers: bacterial expression, biochemical identification and metabolic significance. J. Bioenerg. Biomembr. 2000;32:67–77. doi: 10.1023/a:1005564429242. [DOI] [PubMed] [Google Scholar]
- 32.Chan K.W., Slotboom D.-J., Cox S., Embley T.M., Fabre O., van der Giezen M., Harding M., Horner D.S., Kunji E.R.S., León-Avila G., Tovar J. A Novel ADP/ATP transporter in the mitosome of the microaerophilic human parasite entamoeba histolytica. Curr. Biol. 2005;15:737–742. doi: 10.1016/j.cub.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 33.Bamber L., Slotboom D.-J., Kunji E.R.S. Yeast mitochondrial ADP/ATP carriers are monomeric in detergents as demonstrated by differential affinity purification. J. Mol. Biol. 2007;371:388–395. doi: 10.1016/j.jmb.2007.05.072. [DOI] [PubMed] [Google Scholar]
- 34.Fiermonte G., Dolce V., Arrigoni R., Runswick M.J., Walker J.E., Palmieri F. Organization and sequence of the gene for the human mitochondrial dicarboxylate carrier: evolution of the carrier family. Biochem. J. 1999;344(Pt 3):953–960. [PMC free article] [PubMed] [Google Scholar]
- 35.Iacobazzi V., Palmieri F., Runswick M.J., Walker J.E. Sequences of the human and bovine genes for the mitochondrial 2-oxoglutarate carrier. DNA Seq. 1992;3:79–88. doi: 10.3109/10425179209034000. [DOI] [PubMed] [Google Scholar]
- 36.Kakhniashvili D., Mayor J.A., Gremse D.A., Xu Y., Kaplan R.S. Identification of a novel gene encoding the yeast mitochondrial dicarboxylate transport protein via overexpression, purification, and characterization of its protein product. J. Biol. Chem. 1997;272:4516–4521. doi: 10.1074/jbc.272.7.4516. [DOI] [PubMed] [Google Scholar]
- 37.Palmieri L., Palmieri F., Runswick M.J., Walker J.E. Identification by bacterial expression and functional reconstitution of the yeast genomic sequence encoding the mitochondrial dicarboxylate carrier protein. FEBS Lett. 1996;399:299–302. doi: 10.1016/s0014-5793(96)01350-6. [DOI] [PubMed] [Google Scholar]
- 38.Fiermonte G., Walker J.E., Palmieri F. Abundant bacterial expression and reconstitution of an intrinsic membrane-transport protein from bovine mitochondria. Biochem. J. 1993;294:293–299. doi: 10.1042/bj2940293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrimi G., Di Noia M.A., Marobbio C.M., Fiermonte G., Lasorsa F.M., Palmieri F. Identification of the human mitochondrial S-adenosylmethionine transporter: bacterial expression, reconstitution, functional characterization and tissue distribution. Biochem. J. 2004;379:183–190. doi: 10.1042/BJ20031664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herzig S., Raemy E., Montessuit S., Veuthey J.L., Zamboni N., Westermann B., Kunji E.R.S., Martinou J.C. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;336:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 41.Palmieri L., Pardo B., Larsorsa F.M., del Arco A., Kobayashi K., Iijima M., Runswick M.J., Walker J.E., Saheki T., Satrústegui J., Palmieri F. Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20:5060–5069. doi: 10.1093/emboj/20.18.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiermonte G., Palmieri L., Todisco S., Agrimi G., Palmieri F., Walker J.E. Identification of the mitochondrial glutamate transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution of two human isoforms. J. Biol. Chem. 2002;277:19289–19294. doi: 10.1074/jbc.M201572200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document contains figures.