Abstract
Parainfluenza virus type 3 (PIV-3) can cause severe respiratory illness among hematopoietic cell transplantation (HCT) recipients. Factors associated with PIV-3–specific antibody level, and the association between PIV-3 antibody levels and clinical outcomes in HCT recipients who acquire PIV-3 infection, are unknown. We evaluated PIV-3-specific hemagglutination inhibition antibody levels and clinical outcomes among 172 patients with PIV-3 infection following HCT. In a multivariable linear regression model, high post-transplantation antibody levels were independently associated with higher pre-transplantation recipient titer (mean difference 0.38 [95% CI, 0.26, 0.50], p<0.001). Significant associations between pre-HCT antibody titers in both patients and donors and occurrence of lower respiratory tract disease (LRD) after HCT were not observed. In conclusion, low pre-transplantation titers are associated with low antibody levels after HCT. The relationship between PIV-3 antibody levels and outcomes remain uncertain. Further study is needed to prospectively evaluate the dynamics of PIV-3–specific antibody responses and the relative contribution of PIV-3–specific antibody to protection from infection acquisition and progression to LRD.
Keywords: parainfluenza virus, hemagglutination inhibition antibody, lower respiratory tract disease, allogeneic hematopoietic cell transplantation
INTRODUCTION
Parainfluenza virus type 3 (PIV-3), the most commonly detected type of PIV, occasionally causes outbreaks among hematopoietic cell transplantation (HCT) recipients.1–6 Approximately 30–40% of recipients who experience PIV-3 upper respiratory tract infection (URI) progress to lower respiratory tract disease (LRD), often associated with high mortality.7–13 Historically, risk factors for PIV LRD include lymphopenia, high dose corticosteroid use, myeloablative conditioning, infection early after HCT, and respiratory co-pathogens.9–11, 14, 15
Humoral immunity is known to be important for prevention of severe respiratory viral infections. Although several studies have shown that normalization of IgG level may require up to one year after HCT,16, 17 little is known about antibody production after HCT, especially antigen-specific antibodies. With respect to virus-specific antibody titers and outcome, some studies of respiratory syncytial virus (RSV) infection have demonstrated that high serum antibody titers correlate with protection from RSV infection and progression to severe disease in community-acquired infection.18–22 However, limited data exist regarding the role of antibody titer and PIV infection in HCT recipients.
Evidence from studies in non-transplant populations suggests that humoral immunity may act to modify the severity of PIV infection. Infants born with high titers of maternal neutralizing antibody to PIV-3 are generally protected against primary infection during the first months of life.23 Studies in children have shown the risk of re-infection, severity of respiratory illness, and duration of virus shedding are inversely related to levels of circulating serum antibody before infection.24, 25 Although some heterologous antibody to PIV serogroups may occur with acute infection,26 protection is typically type-specific.27 Thus, it is reasonable to hypothesize that a protective effect may occur from PIV-3 type-specific antibody in HCT recipients.
Our objectives were to identify factors influencing the levels of PIV-3-specific hemagglutination inhibition (HAI) antibodies following HCT, and to examine associations between PIV-3-specific antibody titer and outcomes of PIV-3 infection among HCT recipients with post-transplantation PIV-3 infection.
PATIENTS AND METHODS
Study design
We retrospectively evaluated 172 patients who had a first episode of PIV-3 infection following allogeneic HCT at Fred Hutchinson Cancer Research Center (FHCRC) between 1992 and 2009 and an available serum sample at the time of PIV-3 infection. We also collected information regarding demographic and transplant characteristics for all other allogeneic HCT recipients with PIV-3 infection during the same time period. PIV-3 infection was defined as PIV-3 detection in any respiratory sample by viral culture, direct fluorescent antibody test, and/or polymerase chain reaction (PCR). The Institutional Review Board at FHCRC approved the study.
Definitions
PIV-3 URI was defined as PIV-3 detection from an upper respiratory sample, including nasal wash (n = 90 patients), nasopharyngeal swab (n = 50), maxillary sinus (n = 3), or sputum (n = 3) in patients with signs and/or symptoms of URI. PIV-3 LRD was defined as PIV-3 detection in bronchoalveolar lavage (BAL) (n = 41), lung biopsy (n = 3), or autopsy (n = 2) samples in patients with lower respiratory tract symptoms and/or new pulmonary infiltrates. It is standard practice at FHCRC for bronchoscopy to be performed, if feasible, in patients with any lower respiratory tract symptoms or radiographic findings of lower respiratory tract disease. Progression from URI to LRD was defined among patients who initially presented with URI as subsequent development of LRD. Underlying disease risk groups at transplantation were classified as either standard or high risk based on a previous report.28 Myeloablative conditioning regimens consisted mainly of high-dose cyclophosphamide and busulfan or fractionated total body irradiation (TBI) (≥ 12Gy). Reduced intensity conditioning regimens consisted mainly of fludarabine with a single fraction of TBI (2 Gy). A co-pathogen was defined as a pathogenic bacteria, fungus, or opportunistic virus detected by PCR, culture or direct staining methods obtained from concomitant upper or lower respiratory samples.
Laboratory methods
We tested PIV-3 antibody titers in sera stored in a repository containing prospectively collected specimens. Sera tested in this study consisted of pre-transplantation sera from donor and transplant recipient pairs, as well as sera obtained from recipients within 3 weeks prior to diagnosis of PIV-3 infection (URI and/or LRD). PIV-3 antibody titers were measured by HAI assay, using viral HA derived from the PIV-3 Washington/57 strain.29, 30
Statistical analysis
We compared patient characteristics across the following groups: patients with URI alone, progression to LRD, and LRD alone, as well as between the study cohort with serum tested by HAI and the allogeneic HCT recipients with PIV-3 infection during the same time period who were not in the study. We used Fisher’s exact or chi-square tests for categorical variables, and Wilcoxon rank sum or Kruskal-Wallis tests for continuous variables, as appropriate. Linear regression was used to identify factors influencing mean HAI titers after HCT. Variables with p ≤ 0.1 in univariable models were included in the multivariable model. The correlation between recipient and donor antibody titer before HCT was evaluated using the Pearson correlation coefficient. The probability of LRD was estimated by cumulative incidence curves from the time of HCT, treating death as a competing risk. The log-rank test was used to compare hazards of time-to-event outcomes between high and low pre-HCT antibody titers. Cross-sectional associations between risk factors present prior to infection with occurrence of LRD were evaluated using logistic regression models. A multivariable logistic regression model was created with pre-HCT recipient titer included a priori and variables with p ≤ 0.1 in univariable models added in stepwise fashion to identify confounders for inclusion in the final model, keeping factors that modified the effect of pre-HCT titer by >10% and limiting to 4 covariates due to numbers of events. Among subjects with a URI, factors associated with progression from URI to LRD were evaluated using Cox proportional hazards models using time from URI. Recipient titer at diagnosis was included in the multivariable model a priori, and variables with p ≤ 0.1 in univariable models were evaluated in bivariable models to determine relevant confounding factors. Two-sided p values < 0.05 were considered statistically significant. Analyses were performed using SAS 9.3.
RESULTS
Patient characteristics
Among the 172 patients with PIV-3 infection and available serum for HAI testing, 126 (73%) had URI alone, 20 (12%) progressed from URI to LRD, and 26 (15%) had LRD without detection of prior URI. Serum samples were collected a median of 4 days (range, 0–21 days) before diagnosis of PIV-3 infection. Patient characteristics are outlined in Table 1. The median age at HCT was 41.1 years (range, 1 – 73 years). The median time between HCT and PIV-3 infection was 68.5 days (range, 3 – 1678 days) and all but four were diagnosed within one year after transplantation. The patients with URI alone were more frequently transplanted from a related donor and had a higher lymphocyte count at diagnosis of PIV-3 infection (Table 1). Median antibody titers are shown in Table 1.
Table 1.
Demographic and clinical characteristics of patients in PIV-3 antibody cohort (N=172)
| Total (n=172) |
URI alone (n=126) |
Progression to LRD (n=20) |
LRD alone (n=26) |
p value | |
|---|---|---|---|---|---|
| Sex | 0.45 | ||||
| Male | 111 (65) | 78 (62) | 15 (75) | 18 (69) | |
| Female | 61 (35) | 48 (38) | 5 (25) | 8 (31) | |
| Age at HCT, year | 0.17 | ||||
| ≤ 20 | 26 (15) | 20 (16) | 0 (0) | 6 (23) | |
| 21–60 | 130 (76) | 93 (74) | 19 (95) | 18 (69) | |
| > 60 | 16 (9) | 13 (10) | 1 (5) | 2 (8) | |
| Disease risk at HCT | 0.54 | ||||
| Standard | 101 (59) | 77 (61) | 11 (55) | 13 (50) | |
| High | 71 (41) | 49 (39) | 9 (45) | 13 (50) | |
| Transplant year | 0.07 | ||||
| 1992–2000 | 99 (58) | 66 (52) | 15 (75) | 18 (69) | |
| 2001–2009 | 73 (42) | 60 (48) | 5 (25) | 8 (31) | |
| Stem cell source | 0.29 | ||||
| Bone marrow | 97 (56) | 67 (53) | 14 (70) | 16 (62) | |
| Peripheral blood stem cell | 72 (42) | 57 (45) | 5 (25) | 10 (38) | |
| Cord blood | 3 (2) | 2 (2) | 1 (5) | 0 (0) | |
| Donor type | 0.045 | ||||
| Related | 104 (60) | 83 (66) | 8 (40) | 13 (50) | |
| Unrelated | 68 (40) | 43 (34) | 12 (60) | 13 (50) | |
| Conditioning regimen | 0.32 | ||||
| MA including high-dose TBI (≥ 12Gy) | 75 (44) | 50 (40) | 11 (55) | 14 (54) | |
| MA ± low-dose TBI (≤ 2Gy) | 63 (37) | 47 (37) | 6 (30) | 10 (38) | |
| Reduced intensitya | 34 (20) | 29 (23) | 3 (15) | 2 (8) | |
| GVHD prophylaxisb | 0.18 | ||||
| MTX + CNI | 109 (63) | 76 (60) | 14 (70) | 19 (73) | |
| MMF + CNI | 41 (24) | 34 (27) | 5 (25) | 2 (8) | |
| Other | 22 (13) | 16 (13) | 1 (5) | 5 (19) | |
| Days between HCT and infection | 0.24 | ||||
| ≤ 30 | 33 (19) | 22 (17) | 4 (20) | 7 (27) | |
| 31–365 | 135 (78) | 102 (81) | 16 (80) | 17 (65) | |
| > 365 | 4 (2) | 2 (2) | 0 (0) | 2 (8) | |
| Lymphocyte count at diagnosisc | 0.042 | ||||
| > 500 ×106/L | 62 (36) | 49 (39) | 5 (25) | 8 (31) | |
| 100–500 ×106/L | 83 (48) | 64 (51) | 8 (40) | 11 (42) | |
| < 100 ×106/L | 26 (15) | 12 (10) | 7 (35) | 7 (27) | |
| Co-pathogen | 0.11 | ||||
| None | 146 (85) | 111 (88) | 16 (80) | 19 (73) | |
| Any pathogen | 26 (15) | 15 (12) | 4 (20) | 7 (27) | |
| Steroid use at diagnosis | 0.22 | ||||
| None | 54 (31) | 43 (34) | 5 (25) | 6 (23) | |
| < 1 mg/kg | 37 (22) | 28 (22) | 3 (15) | 6 (23) | |
| 1–2 mg/kg | 71 (41) | 51 (40) | 10 (50) | 10 (38) | |
| > 2 mg/kg | 10 (6) | 4 (3) | 2 (10) | 4 (15) | |
| Intravenous immunoglobulin before diagnosisd | 0.98 | ||||
| None | 128 (75) | 95 (75) | 14 (74) | 19 (76) | |
| Low-dosee | 42 (25) | 31 (25) | 5 (26) | 6 (24) | |
| Donor antibody titer before HCT (Log2)f | 7 (4–9) | 7 (4–9) | 7 (5–9) | 7 (5–8) | 0.86 |
| Patient antibody titer before HCT (Log2)f | 6 (0–10) | 6 (0–10) | 6 (5–9) | 6 (4–9) | 0.31 |
All values are indicated as the number (percentage).
Reduced intensity regimens consisted mainly of fludarabine with 2 Gy TBI.
MTX + CNI group includes MTX alone (n=4), MMF + CNI includes MMF alone (n=1), Other includes CNI alone and CNI+steroid.
Data missing for 1 patient with URI alone.
Two patients were excluded because one received high-dose intravenous immunoglobulin and another received a blinded study drug before PIV infection.
To maintain levels of >400mg/dl, as needed.
Values are indicated as the median (range).
URI: upper respiratory tract infection, LRD; lower respiratory tract disease, HCT: hematopoietic cell transplantation, MA: myeloablative, TBI: total body irradiation, MTX: methotrexate, CNI: calcineurin inhibitor, MMF: mycophenolate mofetil
In the same time period (1992 to 2009), 178 allogeneic HCT recipients had PIV-3 infections without serum testing. The comparison between 172 patients in the cohort with antibody testing and the patients without antibody testing showed that patients in the antibody cohort were older (41 years [range, 1 – 73] vs. 37 years [range, 0 – 74], p=0.011), more likely to receive related donor transplants (60% vs. 43%, p=0.001), and had PIV-3 infection earlier after HCT (69 days [range, 3 – 1678] vs. 78 days [range, 1 – 3140], p=0.002) (Supplemental Table).
Factors influencing post-transplantation antibody titers
HAI serum antibody titers against PIV-3 before HCT were measured in 166 of 172 patients, while serum was tested from only 47 donors. Sera from all 172 patients were measured before PIV-3 infection. In a multivariable model, higher pre-HCT recipient antibody titer was associated with high antibody titer after HCT (adjusted mean difference 0.38 [95% CI 0.26, 0.50], p < 0.001) (Table 2). Although significant in univariable analysis, age at HCT, conditioning regimen, and GHVD prophylaxis were not significantly associated with antibody level after HCT in the multivariable model (Table 2). In the subset of subjects with both pre-HCT donor and recipient antibody titers available (N=41), pre-HCT donor antibody titer was evaluated in a separate multivariable model adjusted for age at HCT, conditioning regimen, and recipient antibody titer. Pre-HCT donor antibody titer was not significantly associated with recipient antibody level after HCT (adjusted mean difference −0.04 [95% CI −0.27, 0.20], p=0.76); pre-HCT donor antibody is excluded from the multivariable model in Table 2 because of the reduced sample size with donor titer available.
Table 2.
Factors affecting PIV-3 antibody titer after hematopoietic cell transplantation (N=172)
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean differencea | 95% CI | p value | Mean differencea | 95% CI | p value | |
| Sex | ||||||
| Male | 1 | |||||
| Female | 0.09 | −0.29, 0.47 | 0.65 | |||
| Age at HCT, yearb | 0.005 | 0.004, 0.025 | 0.01 | 0.006 | −0.005, 0.018 | 0.27 |
| Disease risk at HCT | ||||||
| Standard | 1 | |||||
| High | 0.09 | −0.28, 0.46 | 0.62 | |||
| Transplant year | ||||||
| 1992–2000 | 1 | |||||
| 2001–2009 | 0.11 | −0.26, 0.47 | 0.56 | |||
| Stem cell source | ||||||
| Bone marrow | 1 | |||||
| Peripheral blood stem cell/Cord blood | −0.07 | −0.43, 0.30 | 0.73 | |||
| Donor type | ||||||
| Related | 1 | |||||
| Unrelated | −0.07 | −0.44, 0.31 | 0.73 | |||
| Conditioning regimen | ||||||
| MA including high-dose TBI (≥ 12Gy) | 1 | 1 | ||||
| MA ± low-dose TBI (≤ 2Gy)/Reduced intensityc | 0.42 | 0.06, 0.78 | 0.02 | 0.30 | −0.07, 0.68 | 0.11 |
| GVHD prophylaxisd | ||||||
| MMF + CNI | 1 | 1 | ||||
| MTX + CNI | −0.08 | −0.51, 0.35 | 0.71 | −0.15 | −0.61, 0.31 | 0.52 |
| Other | −0.66 | −1.28, 0.04 | 0.04 | −0.46 | −1.11, 0.18 | 0.16 |
| Days between HCT and PIV-3 infectionb | 0.0000 | −0.0011, 0.001 | 0.93 | |||
| Lymphocyte count at diagnosis | ||||||
| ≥ 100 ×106/L | 1 | |||||
| < 100 ×106/L | 0.01 | −0.50, 0.51 | 0.97 | |||
| Co-pathogen | ||||||
| None | 1 | |||||
| Any pathogen | 0.27 | −0.24, 0.77 | 0.30 | |||
| Steroid use at diagnosis | ||||||
| ≤ 2 mg/kg | 1 | |||||
| > 2 mg/kg | 0.58 | −0.19, 1.35 | 0.14 | |||
| Intravenous immunoglobulin before diagnosise | ||||||
| None | 1 | |||||
| Low-dosef | 0.21 | −0.22, 0.63 | 0.34 | |||
| Donor antibody titer before HCT (Log2)b,g | 0.26 | −0.03, 0.54 | 0.08 | |||
| Patient antibody titer before HCT (Log2)b | 0.38 | 0.26, 0.50 | < 0.001 | 0.38 | 0.26, 0.50 | < 0.001 |
Mean difference = Mean (other groups) – Mean (reference group).
Analyzed as a continous variable.
Reduced intensity regimens consisted mainly of fludarabine with 2 Gy TBI.
MTX + CNI group includes MTX alone (n=4), MMF + CNI includes MMF alone (n=1), Other includes CNI alone and CNI + steroid.
Two patients were excluded because one received high-dose intravenous immunoglobulin and another received a blinded study drug before PIV infection.
To maintain levels of >400mg/dl, as needed.
N=41 subjects with both pre-HCT recipient and donor titer available, thus donor titer not included in full multivariable model. In adjusted analyses for this smaller group, donor titer was not significantly associated with the outcome.
HCT: hematopoietic cell transplantation, MA: myeloablative conditioning, TBI: total body irradiation, CNI: calcineurin inhibitor, MMF: mycophenolate mofetil, MTX: methotrexate
Antibody titers in patients with URI and LRD
The median antibody titer before HCT and the median donor antibody titer were compared between patients with URI alone and with LRD, and were similar in each group (left two in Figure 1). Moreover, the median recipient antibody titer before HCT was similar to that of healthy donors. Among 41 donor-recipient serum pairs available, 27 patients had related donors; antibody titers between related donor and recipient pairs had strong correlation (Pearson correlation coefficient 0.73). The median antibody titers at diagnosis (URI or LRD) were similar (second from the right in Figure 1).
Figure 1.
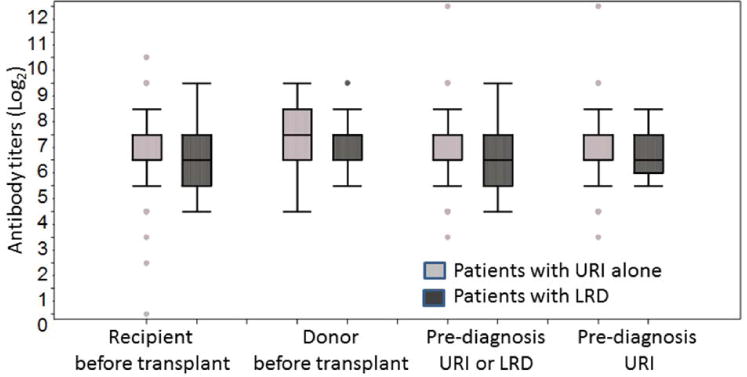
Distribution of PIV-3 HAI antibody titers (expressed as reciprocal log2 values) for patients with URI alone and LRD. Each group indicates patient antibody titer: Left, before transplantation (Median titer (Log2): 6.0 [n = 124] and 6.0 [n=42] in patients with URI alone and with LRD, respectively, p = 0.66); Second from the left, donor antibody titer before transplantation (Median titer (Log2): 7.0 [n = 31] and 7.0 [n=16], p = 0.81); Third from the left, patient antibody titer before diagnosis (URI or LRD) (Median titer (Log2): 6.0 [n = 126] and 6.0 [n=46], p = 0.74); Right, before URI (Median titer (Log2): 6.0 [n = 126] and 6.5 [n=20], p = 0.57). The median is indicated by the center line, and the first and third quartile define the upper and lower edges of the box. The extending lines illustrate the extreme values (to 1.5 times the inter-quartile range from the upper or lower quartiles) and outlines are plotted individually.
To assess for an effect of seasonality on antibody levels, we compared pre-transplantation antibody titers in summer (May to July) and winter (November to January) samples and found no significant differences. (Recipient median titer (Log2) was 6.0 [n = 57] and 6.0 [n=36] in samples collected in summer and winter, respectively, p = 0.29; donor median titer (Log2) was 7.0 [n = 17] and 7.0 [n = 10] in samples collected in summer and winter, respectively, p = 0.26.)
Timing and factors associated with LRD
Next, we examined the timing of LRD occurrence during the three months after HCT within this population. Patients with pre-HCT antibody titers ≤ 5 (Log2) (value closest to the lowest quartile with 23% titers is ≤ 5) had a higher cumulative incidence of LRD than those with pre-HCT antibody titers > 5 (p = 0.11 at day 30, p = 0.12 at day 90) (Figure 2A). Donor antibody titers were evaluated comparing titers ≤ 5 (11th percentile) and > 5, and titers of ≤ 6 (43rd percentile) and > 6, and were not associated with the incidence of LRD in either analysis (p = 0.57 at day 30 and p = 0.43 at day 90, and p = 0.64 at day 30 and p = 0.89 at day 90, respectively).
Figure 2.
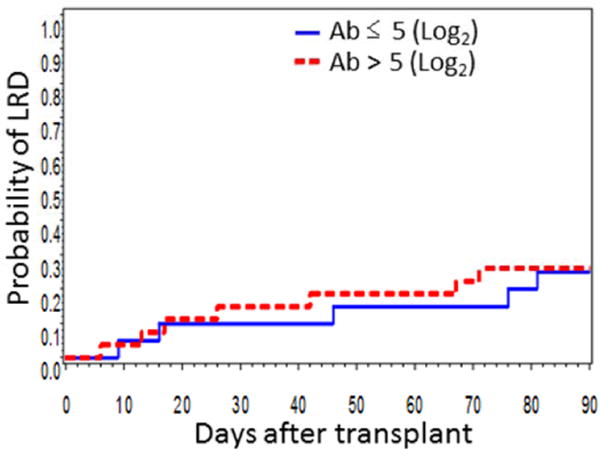
Cumulative incidence of PIV-3 LRD by patient pre-transplantation HAI antibody titers above or below 5 (Log2) (closest to the lowest quartile) (day 30, p = 0.11; day 90, p = 0.12) (There were 166 patients at risk by day 30 and 160 patients at risk by day 90). URI: upper respiratory tract infection; LRD: lower respiratory tract disease; Ab: antibody.
In univariable logistic regression analysis, neither pre-HCT recipient antibody nor donor antibody analyzed as a continuous variable was associated with the presence of LRD (Table 3). In a multivariable model, pre-HCT recipient antibody titer above 5 (Log2) was not significantly associated with occurrence of LRD (adjusted OR 0.50 [95% CI 0.21, 1.22], p = 0.13) (Table 3). Transplant year 1992–2000, lymphocyte count <100 ×106/L at diagnosis, and presence of a co-pathogen were all significantly associated with LRD (Table 3).
Table 3.
Factors associated with PIV-3 lower respiratory tract disease (N=172)
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p value | Odds Ratio | 95% CI | p value | |
| Disease risk at HCT | ||||||
| Standard | 1 | |||||
| High | 4.12 | 1.73, 9.79 | 0.29 | |||
| Transplant year | ||||||
| 1992–2000 | 1 | 1 | ||||
| 2001–2009 | 0.43 | 0.21, 0.90 | 0.03 | 0.42 | 0.18, 0.97 | 0.04 |
| Stem cell source | ||||||
| Bone marrow | 1 | |||||
| Peripheral blood stem cell/Cord blood | 0.61 | 0.30, 1.22 | 0.16 | |||
| Conditioning regimen | ||||||
| MA including high-dose TBI (≥ 12Gy) | 1 | |||||
| MA ± low-dose TBI (≤ 2Gy)/Reduced intens | 0.55 | 0.28, 1.09 | 0.09 | |||
| Lymphocyte count at diagnosis | ||||||
| ≥ 100 ×106/L | 1 | 1 | ||||
| < 100 ×106/L | 4.12 | 1.73, 9.79 | 0.001 | 3.38 | 1.32, 8.69 | 0.01 |
| Steroid use at diagnosis | ||||||
| ≤ 2 mg/kg | 1 | 1 | ||||
| > 2 mg/kg | 4.57 | 1.23, 17.0 | 0.02 | 2.87 | 0.66, 12.6 | 0.16 |
| Co-pathogen | ||||||
| None | 1 | 1 | ||||
| Any pathogen | 2.33 | 0.98, 5.53 | 0.06 | 2.96 | 1.06, 8.31 | 0.04 |
| Days between HCT and PIV-3 infectionb | 1 | 1.00, 1.00 | 0.69 | |||
| Donor antibody titer before HCT (Log2)b | 0.92 | 0.55, 1.53 | 0.74 | |||
| Patient antibody titer before HCT (Log2)b | 1.01 | 0.79, 1.30 | 0.92 | |||
| Patient antibody titer before HCT (Log2) | ||||||
| ≤ 5 | 1 | 1 | ||||
| > 5 | 0.66 | 0.30, 1.47 | 0.31 | 0.50 | 0.21, 1.22 | 0.13 |
Reduced intensity regimens consisted of fludarabine with 2 Gy TBI.
Analyzed as a continuous variable.
HCT: hematopoietic cell transplantation, MA: myeloablative conditioning, TBI: total body irradiation
Progression to LRD
Among 146 patients with PIV-3 URI, 20 (14%) progressed to LRD with a median progression time of 7 days (range, 1 – 21 days). The median antibody titers at URI diagnosis were similar between patients with URI alone and in those who progressed from URI to LRD; right in Figure 1). Moreover, antibody titer prior to URI was not a significant risk factor for progression from URI to LRD (HR 1.09 [95% CI 0.77, 1.53], p = 0.62). Pre-URI antibody titers did not reach significance after adjusting for any other factors including lymphocyte count at URI diagnosis, transplant year, and steroid use at URI diagnosis (data not shown).
DISCUSSION
This study suggests that, among subjects who developed PIV-3 infection, titers of PIV type 3-specific HAI antibodies after HCT were associated with their pre-HCT recipient antibody titer. The level of PIV-3 recipient antibody titer before HCT was similar to that of donor antibody titer, and was not significantly associated with occurrence of LRD after HCT.
Our study showed that pre-HCT recipient antibody titers, but not donor titers, or age, conditioning regimen, or GVHD prophylaxis, are associated with post-HCT antibody titer in patients who developed PIV-3 infection after HCT. Only a few reports have indicated recovery time of total serum IgG levels after HCT, which may be one year or more.16, 17 In some patients, recipient-type immunoglobulins are detected even years after HCT.31 Moreover, limited data on the change of antigen-specific antibody after HCT have been reported. Because post-transplantation antibody titers were mostly obtained within one year after HCT in this study, recipient-derived plasma cells may play an important role in this period.
Our data showed that the pre-transplantation antibody titers in HCT recipients with PIV-3 infection are similar to those in healthy donors. Although most patients had chemotherapy for underlying disease before beginning the transplantation process, antibody titers were not affected. This result suggests that following chemotherapy, patients have a similar risk of acquisition of respiratory viral infections as do immunocompetent persons, although progression to LRD or mortality may occur in HCT recipients with important risk factors, such as severe myelosuppression or co-infections.8–11, 14 Antibody titers in related donor and recipient pairs showed a strong correlation, suggestive of similar exposure history.
To our knowledge, there are no previous studies that describe the relationship between PIV-3 HAI titers associated with PIV-3 URI and PIV-3 LRD in HCT recipients. Our study suggested that there is no association between patient and donor antibody before HCT and occurrence of LRD among HCT recipients. No association was found between pre-diagnosis antibody titer in patients and progression from URI to LRD. A recent study of outcomes of PIV LRD after HCT did not show an effect of high dose intravenous immunoglobulin (IVIG) on mortality after PIV LRD,7 although this analysis was limited by sample size and the amount of PIV-specific antibody in these IVIG products is unknown. Although data suggest that serum antibody is protective against PIV acquisition and disease severity in healthy infants and children,23, 24 mechanisms of protection may differ for HCT patients. Our data did show significant associations with lymphopenia and presence of a co-pathogen at PIV-3 diagnosis, both previously reported as risk factors for PIV LRD.
Some RSV studies suggest that high RSV-specific antibody titer is related to a low incidence of RSV infection and progression from URI to LRD in non-transplant settings,18–21 although an analysis in HCT recipients did not find association between pre- and post-transplantation donor and recipient RSV subtype-specific neutralizing antibody levels and LRD progression.32 Likewise, recent papers have demonstrated an apparent lack of effect of immunoglobulin and RSV-specific monoclonal antibody (palivizumab) on outcomes among HCT patients with RSV LRD.28
This study has several limitations. Since the patients in this retrospective observational study were selected based on availability of sera for laboratory testing, certain subsets of our samples were limited in number and some differences were observed in donor type, age, and timing of PIV-3 infection after HCT in patients in the PIV-3 antibody cohort compared with other patients transplanted during the study period. The small sample size, especially for donor antibody titer, may have restricted our ability to reach statistical significance in some analyses. Another limitation is that there was no control group of uninfected HCT recipients. Since all patients in this cohort had PIV-3 infection, the prospective relationship between pre-transplantation antibody titer and acquisition of PIV-3 infection was not evaluated; rather the results here focus on comparisons of characteristics between groups of infected subjects (URI alone vs. LRD or groups defined by titer levels). Finally, the definition of LRD in this study is based on the detection of PIV-3 in the lung.7 Because standard practice at our center includes a low threshold to perform bronchoscopy in patients with lower respiratory tract symptoms or radiographic findings of lower respiratory tract disease, we feel the definition of LRD based on PIV-3 detection in BAL is reasonable. However, some bias by attending physicians as to whether bronchoscopy was performed was likely inevitable.
In conclusion, we found that higher pre-transplantation HAI antibody titer against PIV-3 in HCT recipients is associated with higher HAI antibody titers after HCT among PIV-3 infected subjects. Our study did not find significant associations between pre- or post-transplantation HAI titers of antibodies against PIV-3 in HCT recipients and the occurrence of, or progression to, PIV-3 LRD after transplantation or URI, respectively. Further prospective studies to evaluate the dynamics of PIV-3–specific antibody responses and the relative contribution of PIV-3–specific antibody (as well as type-specific antibody for other PIV subtypes) to protection from infection acquisition and progression to LRD in HCT recipients are warranted. Given the high morbidity and mortality associated with PIV infection after HCT, and the critical dearth of novel and effective therapies, there is a need for a multi-faceted approach to treating PIV. Based on our lack of associations with HAI antibody titers, a focus on antiviral therapeutics and interventions directed at cell-mediated rather than humoral immunity may be the highest priority for therapeutic intervention for PIV infections in HCT recipients.
Supplementary Material
Acknowledgments
We thank Chris Davis, George Counts, and Zachary Stednick for data management, Tera Matson and Elsa Garnace for laboratory assistance, and Ikuyo Imayama for statistical advice. This work was partially supported by NIH grants CA18029, CA15704, HL081595, HL93294, K23HL091059, and L40AI071572. S.S. is a recipient of a fellowship from the Joel Meyers Memorial Fund. A.P.C. also received support from the Seattle Children’s Center for Clinical and Translational Research and CTSA grant ULI RR025014.
Footnotes
CONFLICT OF INTEREST
M.B. and S.S. received research support from Ansun Biopharma. All other authors declare no competing financial interests.
References
- 1.Nichols WG, Erdman DD, Han A, Zukerman C, Corey L, Boeckh M. Prolonged outbreak of human parainfluenza virus 3 infection in a stem cell transplant outpatient department: insights from molecular epidemiologic analysis. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2004;10(1):58–64. doi: 10.1016/j.bbmt.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Maziarz RT, Sridharan P, Slater S, Meyers G, Post M, Erdman DD, et al. Control of an outbreak of human parainfluenza virus 3 in hematopoietic stem cell transplant recipients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(2):192–8. doi: 10.1016/j.bbmt.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodson A, Kasliwal M, Streetly M, MacMahon E, Raj K. A parainfluenza-3 outbreak in a SCT unit: sepsis with multi-organ failure and multiple co-pathogens are associated with increased mortality. Bone marrow transplantation. 2011;46(12):1545–50. doi: 10.1038/bmt.2010.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sydnor ER, Greer A, Budd AP, Pehar M, Munshaw S, Neofytos D, et al. An outbreak of human parainfluenza virus 3 infection in an outpatient hematopoietic stem cell transplantation clinic. American journal of infection control. 2012;40(7):601–5. doi: 10.1016/j.ajic.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Cortez KJ, Erdman DD, Peret TC, Gill VJ, Childs R, Barrett AJ, et al. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. The Journal of infectious diseases. 2001;184(9):1093–7. doi: 10.1086/322041. [DOI] [PubMed] [Google Scholar]
- 6.Jalal H, Bibby DF, Bennett J, Sampson RE, Brink NS, MacKinnon S, et al. Molecular investigations of an outbreak of parainfluenza virus type 3 and respiratory syncytial virus infections in a hematology unit. Journal of clinical microbiology. 2007;45(6):1690–6. doi: 10.1128/JCM.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo S, Xie H, Campbell AP, Kuypers JM, Leisenring WM, Englund JA, et al. Parainfluenza Virus Lower Respiratory Tract Disease after Hematopoietic Cell Transplantation: Viral Detection in the Lung Predicts Outcome. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 doi: 10.1093/cid/ciu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ustun C, Slaby J, Shanley RM, Vydra J, Smith AR, Wagner JE, et al. Human parainfluenza virus infection after hematopoietic stem cell transplantation: risk factors, management, mortality, and changes over time. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(10):1580–8. doi: 10.1016/j.bbmt.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chemaly RF, Hanmod SS, Rathod DB, Ghantoji SS, Jiang Y, Doshi A, et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood. 2012;119(12):2738–45. doi: 10.1182/blood-2011-08-371112. quiz 2969. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan A, Wang C, Yang J, Shenep JL, Leung WH, Hayden RT. Symptomatic parainfluenza virus infections in children undergoing hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(10):1520–7. doi: 10.1016/j.bbmt.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98(3):573–8. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 12.Elizaga J, Olavarria E, Apperley J, Goldman J, Ward K. Parainfluenza virus 3 infection after stem cell transplant: relevance to outcome of rapid diagnosis and ribavirin treatment. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2001;32(3):413–8. doi: 10.1086/318498. [DOI] [PubMed] [Google Scholar]
- 13.Lewis VA, Champlin R, Englund J, Couch R, Goodrich JM, Rolston K, et al. Respiratory disease due to parainfluenza virus in adult bone marrow transplant recipients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1996;23(5):1033–7. doi: 10.1093/clinids/23.5.1033. [DOI] [PubMed] [Google Scholar]
- 14.Schiffer JT, Kirby K, Sandmaier B, Storb R, Corey L, Boeckh M. Timing and severity of community acquired respiratory virus infections after myeloablative versus non-myeloablative hematopoietic stem cell transplantation. Haematologica. 2009;94(8):1101–8. doi: 10.3324/haematol.2008.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakrabarti S, Avivi I, Mackinnon S, Ward K, Kottaridis PD, Osman H, et al. Respiratory virus infections in transplant recipients after reduced-intensity conditioning with Campath-1H: high incidence but low mortality. British journal of haematology. 2002;119(4):1125–32. doi: 10.1046/j.1365-2141.2002.03992.x. [DOI] [PubMed] [Google Scholar]
- 16.Storek J, Dawson MA, Storer B, Stevens-Ayers T, Maloney DG, Marr KA, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97(11):3380–9. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 17.Bosch M, Dhadda M, Hoegh-Petersen M, Liu Y, Hagel LM, Podgorny P, et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy. 2012;14(10):1258–75. doi: 10.3109/14653249.2012.715243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falsey AR, Walsh EE. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. The Journal of infectious diseases. 1998;177(2):463–6. doi: 10.1086/517376. [DOI] [PubMed] [Google Scholar]
- 19.Luchsinger V, Piedra PA, Ruiz M, Zunino E, Martinez MA, Machado C, et al. Role of neutralizing antibodies in adults with community-acquired pneumonia by respiratory syncytial virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(7):905–12. doi: 10.1093/cid/cir955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. The Journal of infectious diseases. 2004;189(2):233–8. doi: 10.1086/380907. [DOI] [PubMed] [Google Scholar]
- 21.Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine. 2003;21(24):3479–82. doi: 10.1016/s0264-410x(03)00355-4. [DOI] [PubMed] [Google Scholar]
- 22.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102(3 Pt 1):531–7. [PubMed] [Google Scholar]
- 23.Glezen WP, Frank AL, Taber LH, Kasel JA. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. The Journal of infectious diseases. 1984;150(6):851–7. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- 24.Kasel JA, Frank AL, Keitel WA, Taber LH, Glezen WP. Acquisition of serum antibodies to specific viral glycoproteins of parainfluenza virus 3 in children. Journal of virology. 1984;52(3):828–32. doi: 10.1128/jvi.52.3.828-832.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glezen WP, Denny FW. Parainfluenza viruses. In: E AS, K RA, editors. Viral Infections of Humans. 1997. [Google Scholar]
- 26.Henrickson KJ. Parainfluenza viruses. Clinical microbiology reviews. 2003;16(2):242–64. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moscona A. Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease. The Journal of clinical investigation. 2005;115(7):1688–98. doi: 10.1172/JCI25669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo S, Campbell AP, Xie H, Chien JW, Leisenring WM, Englund JA, et al. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(4):589–96. doi: 10.1016/j.bbmt.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clements ML, Belshe RB, King J, Newman F, Westblom TU, Tierney EL, et al. Evaluation of bovine, cold-adapted human, and wild-type human parainfluenza type 3 viruses in adult volunteers and in chimpanzees. Journal of clinical microbiology. 1991;29(6):1175–82. doi: 10.1128/jcm.29.6.1175-1182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karron RA, Thumar B, Schappell E, Surman S, Murphy BR, Collins PL, et al. Evaluation of two chimeric bovine-human parainfluenza virus type 3 vaccines in infants and young children. Vaccine. 2012;30(26):3975–81. doi: 10.1016/j.vaccine.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Current opinion in hematology. 2012;19(4):324–35. doi: 10.1097/MOH.0b013e328353bc7d. [DOI] [PubMed] [Google Scholar]
- 32.Kim YJ, Guthrie KA, Waghmare A, Walsh EE, Falsey AR, Kuypers J, et al. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. The Journal of infectious diseases. 2014;209(8):1195–204. doi: 10.1093/infdis/jit832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


