Abstract
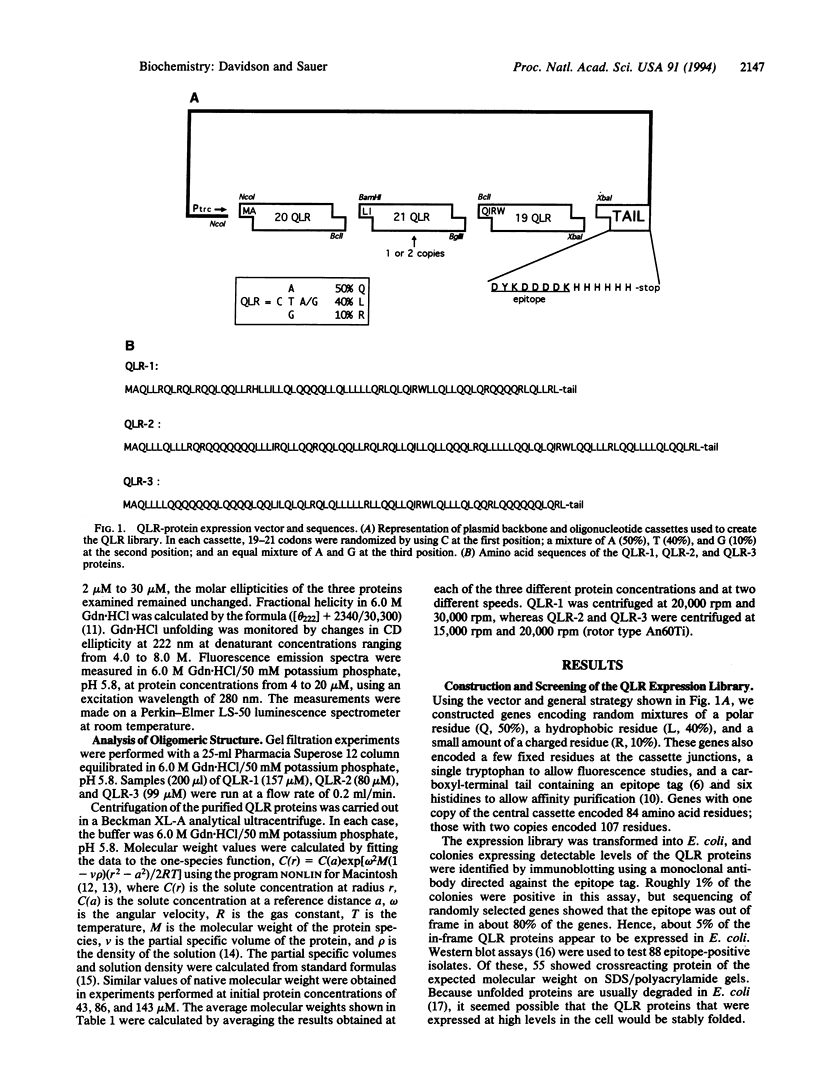
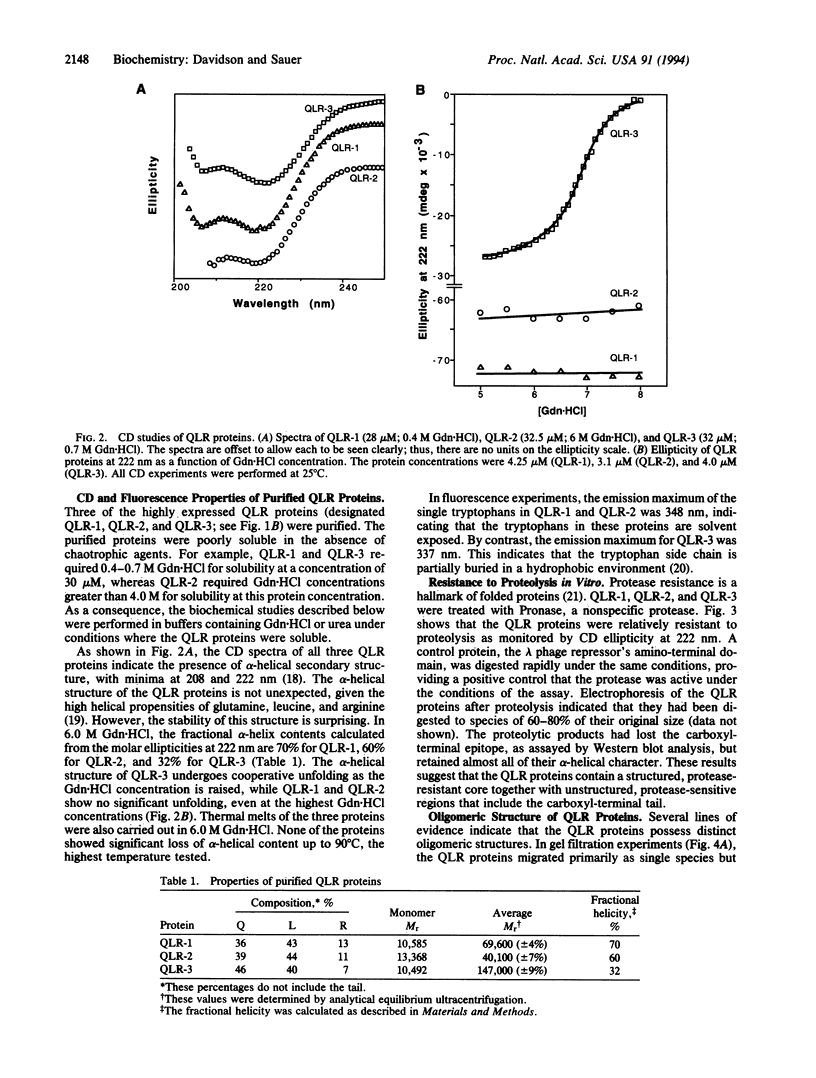
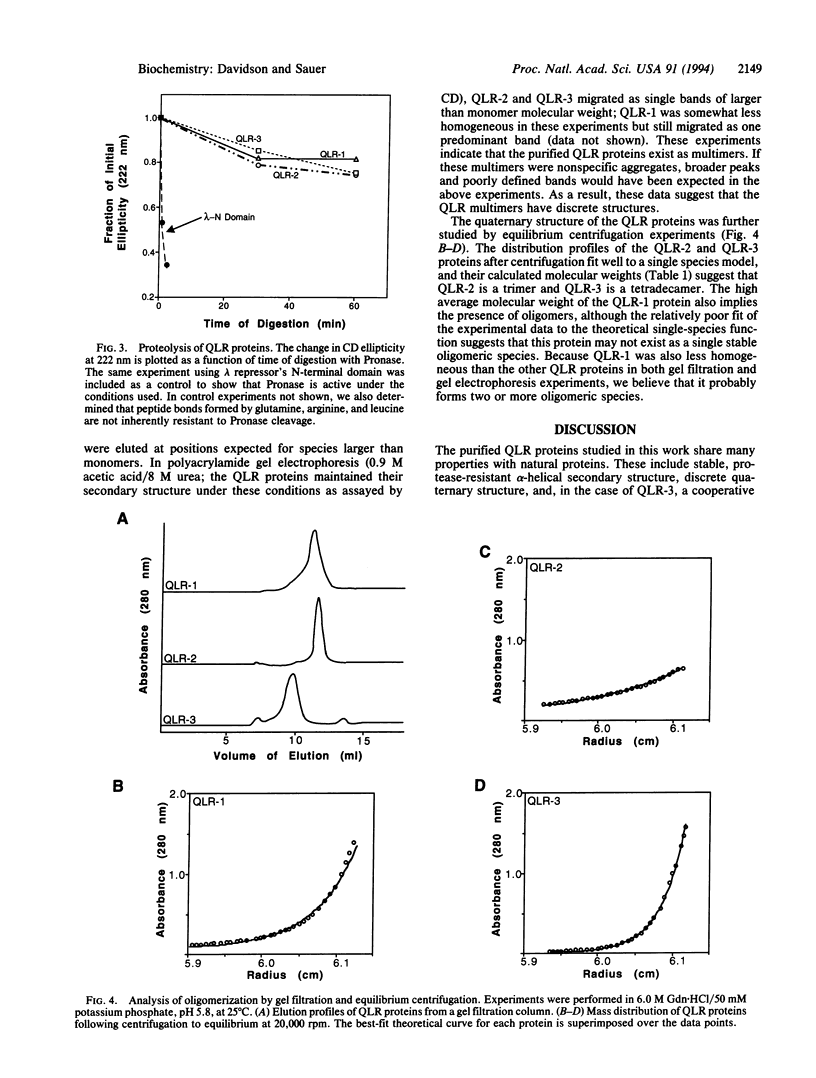
A library of synthetic genes encoding 80- to 100-residue proteins composed mainly of random combinations of glutamine (Q), leucine (L), and arginine (R) has been expressed in Escherichia coli. These genes also encode an epitope tag and six carboxyl-terminal histidines. Screening of this library by immunoblotting showed that 5% of these QLR proteins are expressed at readily detectable levels. Three well-expressed QLR proteins were purified and characterized. Each of these proteins has significant alpha-helical content, is largely resistant to degradation by Pronase, and has a distinct oligomeric structure. In addition, one protein unfolds in a highly cooperative manner. These properties of the QLR proteins demonstrate that they possess folded structures with some native-like properties. The QLR proteins differ from most natural proteins, however, in being remarkably resistant to denaturant-induced and thermal-induced unfolding and in being relatively insoluble in the absence of denaturants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford D., Chothia C., Lesk A. M. Determinants of a protein fold. Unique features of the globin amino acid sequences. J Mol Biol. 1987 Jul 5;196(1):199–216. doi: 10.1016/0022-2836(87)90521-3. [DOI] [PubMed] [Google Scholar]
- Blaber M., Zhang X. J., Matthews B. W. Structural basis of amino acid alpha helix propensity. Science. 1993 Jun 11;260(5114):1637–1640. doi: 10.1126/science.8503008. [DOI] [PubMed] [Google Scholar]
- Breyer R. M., Sauer R. T. Mutational analysis of the fine specificity of binding of monoclonal antibody 51F to lambda repressor. J Biol Chem. 1989 Aug 5;264(22):13355–13360. [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Dill K. A. Dominant forces in protein folding. Biochemistry. 1990 Aug 7;29(31):7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Handel T. M., Williams S. A., DeGrado W. F. Metal ion-dependent modulation of the dynamics of a designed protein. Science. 1993 Aug 13;261(5123):879–885. doi: 10.1126/science.8346440. [DOI] [PubMed] [Google Scholar]
- Hochuli E., Döbeli H., Schacher A. New metal chelate adsorbent selective for proteins and peptides containing neighbouring histidine residues. J Chromatogr. 1987 Dec 18;411:177–184. doi: 10.1016/s0021-9673(00)93969-4. [DOI] [PubMed] [Google Scholar]
- Oliphant A. R., Nussbaum A. L., Struhl K. Cloning of random-sequence oligodeoxynucleotides. Gene. 1986;44(2-3):177–183. doi: 10.1016/0378-1119(86)90180-0. [DOI] [PubMed] [Google Scholar]
- Parsell D. A., Sauer R. T. Induction of a heat shock-like response by unfolded protein in Escherichia coli: dependence on protein level not protein degradation. Genes Dev. 1989 Aug;3(8):1226–1232. doi: 10.1101/gad.3.8.1226. [DOI] [PubMed] [Google Scholar]
- Parsell D. A., Sauer R. T. The structural stability of a protein is an important determinant of its proteolytic susceptibility in Escherichia coli. J Biol Chem. 1989 May 5;264(13):7590–7595. [PubMed] [Google Scholar]
- Regan L., DeGrado W. F. Characterization of a helical protein designed from first principles. Science. 1988 Aug 19;241(4868):976–978. doi: 10.1126/science.3043666. [DOI] [PubMed] [Google Scholar]
- Shakhnovich E. I., Gutin A. M. Implications of thermodynamics of protein folding for evolution of primary sequences. Nature. 1990 Aug 23;346(6286):773–775. doi: 10.1038/346773a0. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. The ultraviolet fluorescence of proteins in neutral solution. Biochem J. 1960 Aug;76:381–388. doi: 10.1042/bj0760381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Waugh D. S., Sauer R. T. Single amino acid substitutions uncouple the DNA binding and strand scission activities of Fok I endonuclease. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9596–9600. doi: 10.1073/pnas.90.20.9596. [DOI] [PMC free article] [PubMed] [Google Scholar]



