Abstract
Diester diterpenoid alkaloids (DDAs), such as aconitine (AC), mesaconitine (MA), and hypaconitine (HA), are both pharmacologically active compounds and toxic ingredients in a traditional Chinese herb, the Aconitum species. Many DDA metabolism studies have been performed to explore mechanisms for reducing toxicity in these compounds and in Aconitum species extracts for safe clinical administration. In this review, we summarize recent progress on the metabolism of toxic AC, MA, and HA and corresponding monoester diterpenoid alkaloids (MDAs) in the gastrointestinal tract and liver in different animal species and humans in vivo and/or in vitro, where these alkaloids are primarily metabolized by cytochrome P450 enzymes, carboxylesterases, and intestinal bacteria, which produces phase I metabolites, ester hydrolysed products, and lipoalkaloids. Furthermore, we classify metabolites detected in the blood and urine, where the aforementioned metabolites are absorbed and excreted. Less toxic MDAs and nontoxic alcohol amines are the primary DDA metabolites detected in the blood. Most other DDAs metabolites produced in the intestine and liver detected in the urine have not been reported in the blood. We propose an explanation for this nonconformity. Finally, taking AC, for instance, we generalize a process of toxicity reduction in the body after oral AC administration for the first time.
1. Introduction
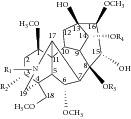
Diester diterpenoid alkaloids (DDAs, Table 1), such as aconitine (AC), mesaconitine (MA), and hypaconitine (HA), are a family of highly toxic alkaloids from the root of a traditional Chinese herb, the Aconitum species (sp.), which has been used clinically for years. Monoester diterpenoid alkaloids (MDAs, Table 1) are the ester hydrolysis products of DDAs at the C-8 position, which are also components of this herb. Both DDAs and MDAs exhibit excellent pharmacological effects, including anti-inflammatory, analgesic, and cardiotonic activities [1, 2].
Table 1.
DDA, MDA, and alcohol amine chemical structures.
| ||||||
|
| ||||||
| Compounds | R1 | R2 | R3 | R4 | Formula | Mass |
|
| ||||||
| DDAs | ||||||
| Aconitine (AC) | Ethyl (Et) | Hydroxy (OH) | Acetyl (Ac) | Benzoyl (Bz) | C34H47NO11 | 645.3149 |
| Mesaconitine (MA) | Methyl (Me) | OH | Ac | Bz | C33H45NO11 | 631.2992 |
| Hypaconitine (HA) | Me | Hydrogen (H) | Ac | Bz | C33H45NO10 | 615.3043 |
| MDAs | ||||||
| Benzoylaconine (BAC) | Et | OH | H | Bz | C32H45NO10 | 603.3043 |
| Benzoylmesaconine (BMA) | Me | OH | H | Bz | C31H43NO10 | 589.2887 |
| Benzoylhypaconine (BHA) | Me | H | H | Bz | C31H43NO9 | 573.2938 |
| Alcohol amines | ||||||
| Aconine | Et | OH | H | H | C25H41NO9 | 499.2781 |
| Mesaconine | Me | OH | H | H | C24H39NO9 | 485.2625 |
| Hypaconine | Me | H | H | H | C24H39NO8 | 469.2676 |
However, these compounds, especially DDAs, have narrow therapeutic windows. For example, a single lethal AC dose for humans is estimated at 2–6 mg [3, 4] with poisoning symptoms, such as hypotension, palpitations, ventricular tachyarrhythmias, asystole, and numbness of the face and limbs [1]. Severe poisoning may occur after improper ingestion of DDA-containing drugs or prescriptions, such as Chuanwu [5], Caowu [6], and Fuzi [7]. Therefore, Aconitum herbs are traditionally boiled or steamed before oral administration to ensure safety [8]. During this process, DDAs are mainly hydrolysed to less toxic MDAs. Further MDA hydrolysis yields almost nontoxic alcohol amines (Table 1), such as aconine, mesaconine, and hypaconine [3, 9, 10]. In contrast with AC, the half-maximal lethal dose (LD50, mg/kg, i.v. mice) of 14-benzoylaconine (BAC) and aconine increases by approximately 38- and 430-fold, respectively [11].
On the other hand, many valuable studies have recently been performed on DDA and MDA metabolism to explore the toxicity reduction mechanisms and obtain information for clinical guidance. In this paper, we review for the first time the metabolites biotransformed in the gastrointestinal tract and liver from toxic AC, MA, and HA of DDAs as well as their corresponding ester hydrolysed products, BAC, 14-benzoylmesaconine (BMA), and 14-benzoylhypaconine (BHA) of MDAs, in different animal species and humans in vivo and in vitro. Furthermore, we classify the metabolites detected in the blood and urine, in which these metabolites are absorbed and excreted. Our study will be fundamental and helpful for further studies on reducing the toxicity of DDA-containing drugs compatible with other medicine based on DDAs absorption and metabolism [12, 13].
2. Metabolism in the Gastrointestinal Tract and Liver
Traditional Chinese prescriptions are commonly prepared through decoction and ingested orally. The active compounds are unavoidably converted in the gastrointestinal tract.
2.1. Metabolism in the Stomach
The stomach provides an acidic environment for drug dissolution and absorption; however, studies on stomach metabolism are typically ignored. Only one study has focused on AC metabolism in the stomach.
In this study, 14 metabolites and 2 ester hydrolysis products are identified in gastric content in rabbits after oral AC administration [14]. Metabolism includes hydroxylation, deoxylation, demethylation, didemethylation/deethylation, and ester exchange at the C-8 position with long chain fatty acids (Table 2). The enzymes responsible for metabolism have not been reported. The aforementioned metabolic process may be catalysed by CYP2C9 and CYP2C8 that are expressed in parietal gastric cells [15] and by bacteria that are located in the human stomach [16].
Table 2.
AC metabolites produced in rabbit stomachs.
| DDAs |
m/z
(ESI+) |
Formula | Identification | Neutral loss (Da), identification of fatty acid | Metabolic procedure | MS detection | References |
|---|---|---|---|---|---|---|---|
| AC | 662 | C34H47NO12 | 2′-Hydroxy AC or 3′-AC (M1)a |
NAb | Rabbits and rats; ig, in vivo. | IT, FT-ICR | [14] |
| 3′-Hydroxy AC or 2′-hydroxy AC (M3)a | |||||||
| 4′-Hydroxy AC (M6)a | |||||||
| 632 | C33H45NO11 | Demethyl AC (M4) | NA | ||||
| 630 | C34H47NO10 | Indaconitine (15-deoxy AC, M5)c | NA | ||||
| Deoxyaconitine (3-deoxy AC, M7) | |||||||
| 618 | C32H43NO11 | Didemethyl AC or N-deethyl AC (M2) |
NA | ||||
| 604 | C32H45NO10 | BAC (hydrolysis product 2) | NA | Rabbits and rats; ig, in vivo. | IT, FT-ICR | ||
| 542 | C27H43NO10 | 14-O-Debenzoyl AC (hydrolysis product 1) | NA | Rabbits and rats; ig, in vivo. | IT, FT-ICR | ||
| 828 | C47H73NO11 | 8-O-Pentadecanoyl BAC (M10) | 242, pentadecanoic acid | Rabbits and rats; ig, in vivo. | IT, FT-ICR | ||
| 842 | C48H75NO11 | 8-O-Palmitoyl BAC (M12) | 256, palmitic acid | ||||
| 864 | C50H73NO11 | 8-O-Linolenoyl BAC (M9) | 278, linolenic acid | ||||
| 866 | C50H75NO11 | 8-O-Linoleoyl BAC (M11) | 280, linoleic acid | ||||
| 868 | C50H77NO11 | 8-O-Oleoyl BAC (M13) | 282, oleic acid | ||||
| 870 | C50H79NO11 | 8-O-Stearoyl BAC (M14) | 284, stearic acid | ||||
| 978 | C58H91NO11 | 8-O-Hexacosandienoyl BAC (M8) | 392, hexacosandienoic acid |
a2′, 3′, and 4′, the position in benzoyl group.
bNot available.
cDeoxy may also be referred to as dehydroxy in the literature.
The ester hydrolysis products at the C-8 and C-14 positions are not only observed in rabbit stomachs but also in acid solutions (negative control). Ester hydrolysis in the stomach may be catalysed by carboxylesterases (CEs) in the gastric mucosa [17] because CE expression has also been reported in the stomach, although CEs are predominantly distributed in the liver, plasma, and intestine [18]. However, this finding also implies that DDAs can be nonenzymatically ester hydrolysed under acidic conditions, which is discussed in Section 5.
In addition, AC, MA, HA, and their hydrolysis products (MDAs and alcohol amines) are detected in gastric contents in a dead female, who was suspected of dying from acute drug poisoning involving Aconitum alkaloids [19]. However, the reference did not indicate whether the hydrolysis products were metabolized from DDAs in the stomach or were originally in the toxicant.
2.2. Metabolism in the Intestine
A large number of bacteria populate the gastrointestinal tract; the bacterial concentration increases distally. The majority of bacteria reside in the colon, where the density approaches 1011-1012 cells/mL, and anaerobic species dominate. This microbiota secretes a diverse array of enzymes that participate in various metabolic processes, such as reduction, hydrolysis, deoxylation, acetylation, deacetylation, and N-demethylation; thus, the intestinal microbiota is important to orally ingested drug metabolism [20, 21]. Notably, hydrolysis catalysed by bacteria is common in glycosides. Based on DDA and MDA structures, ester hydrolysis is likely driven by CEs, which also dominate the intestine [18].
The intestinal bacteria DDA metabolism reviewed herein was mainly performed in vitro through anaerobic incubation in a feces suspension, which included high levels of intestinal bacteria. The intestinal bacteria DDA metabolism is similar to metabolism in the stomach and included hydroxylation, deoxylation, demethylation, demethylation with deoxylation, ester hydrolysis at the C-8 and/or C-14 position, and ester exchange at the C-8 position with short and long chain fatty acids (Table 3). AC metabolites, such as 16-O-demethyl AC, 3-deoxy AC, and 16-O-demethyl-3-deoxy AC, were further converted to deoxylation, demethylation, ester hydrolysis, and ester exchange products (Table 4). These results imply that MDAs, which are DDA ester hydrolysed products, may be metabolized through the same pathway; however, no studies have reported on intestinal MDA metabolism.
Table 3.
Metabolites of AC, MA, and HA converted in intestine.
| DDAs | m/z (ESI+) | Formula | Identification | Neutral loss (Da), identification of fatty acid | Metabolic procedure | MS detection | References |
|---|---|---|---|---|---|---|---|
| AC | 662 | C34H47NO12 | 10-Hydroxy AC | NAa | Rats; intestinal bacteria; anaerobic incubation at pH 7.0, in vitro. | IT | [22] (P4) |
| 632 | C33H45NO11 | 16-O-Demethyl AC* | NA | Rabbits; contents from small intestine and caecum and feces; ig, in vivo. | IT | [23] (M3) | |
| Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] (M1) | |||||
| 630 | C34H47NO10 | Indaconitine (15-deoxy AC)b | Rabbits; contents from small intestine and caecum and feces; ig, in vivo. | IT | [23] (M6) | ||
| Rats; intestinal bacteria; anaerobic incubation at pH 7.0, in vitro. | IT | [22] (P5) | |||||
| Deoxy AC* | NA | Rabbits; contents from small intestine and caecum and feces; ig, in vivo. | IT | [23] (M5) | |||
| Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] (M2) | |||||
| Rats; intestinal bacteria; anaerobic incubation at pH 7.0, in vitro. | IT | [22] (P10) | |||||
| 616 | C33H45NO10 | 16-O-Demethyl-deoxy AC* | NA | Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] (M3) | |
| 604 | C32H45NO10 | BAC | NA | Rabbits; contents from small intestine and caecum and feces; ig, in vivo. | IT | [23] (M2) | |
| Rats; intestinal bacteria; anaerobic incubation, in vitro.c | IT | [25] | |||||
| Rats; intestinal bacteria; anaerobic incubation, in vitro.d | IT | [26] | |||||
| Rats; intestinal bacteria; anaerobic incubation at pH 7.0, in vitro. | IT | [22] (P1) | |||||
| 590 | C31H43NO10 | 16-O-Demethyl BAC | NA | Rabbits; contents from small intestine and caecum and feces; ig, in vivo. | IT | [23] (M1) | |
| 588 | C32H45NO9 | 15-Deoxy BAC | NA | Rats; intestinal bacteria; anaerobic incubation at pH 7.0, in vitro. | IT | [22] (P2) | |
| 586 | C32H43NO9 | Deacetoxy AC | NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.c,d | IT | [25, 26] | |
| 660 | C35H49NO11 | 8-O-Propionyl BAC | 74, propionic acid | Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] | |
| NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.e | IT, MALDI source-FT-ICR | [27] | ||||
| NA | Rats; intestinal bacteria; anaerobic incubation at pH 7.0, in vitro. | IT | [22] (P8) | ||||
| 674 | C36H51NO11 | 8-O-Butyryl BAC | 88, butyric acid | Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] | |
| NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.e | IT, MALDI source-FT-ICR | [27] | ||||
| NA | Rats; intestinal bacteria; anaerobic incubation at pH 7.0, in vitro. | IT | [22] (P9) | ||||
| 688 | C37H53NO11 | 8-O-Valeryl BAC | 102, valeric acid | Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] | |
| NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.e | IT, MALDI source-FT-ICR | [27] | ||||
| 700 | C38H53NO11 | 8-O-Hexenoyl BAC | 114, hexenoic acid | Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] | |
| NA | Rats; intestinal bacteria; anaerobic incubation at pH 7.0, in vitro. | IT | [22] (P7) | ||||
| 690 | C36H51NO12 | 8-O-(3-Hydroxy)-butyryl BAC | NA | Rats; intestinal bacteria; anaerobic incubation at pH 7.0, in vitro. | IT | [22] (P11) | |
| 702 | C38H55NO11 | 8-O-Hexanoyl BAC | 116, hexanoic acid | Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] | |
| 716 | C39H57NO11 | 8-O-Heptanoyl BAC | 130, heptanoic acid | Ibid. | Ibid. | Ibid. | |
| 722 | C40H51NO11 | 8-O-Phenylacetyl BAC | 136, phenylacetic acid | Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] | |
| NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.e | IT, MALDI source-FT-ICR | [27] | ||||
| 728 | C40H57NO11 | 8-O-Octenoyl BAC | NA | Rats; intestinal bacteria; anaerobic incubation at pH 7.0, in vitro. | IT | [22] (P3) | |
| 736 | C41H53NO11 | 8-O-Phenylpropionyl BAC | 150, phenylpropionic acid | Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] | |
| 800 | C45H69NO11 | 8-O-Tridecanoyl BAC | 214, tridecanoic acid | Ibid. | Ibid. | Ibid. | |
| 814 | C46H71NO11 | 8-O-Tetradecanoyl BAC | 228, tetradecanoic acid | Ibid. | Ibid. | Ibid. | |
| 828 | C47H73NO11 | 8-O-Pentadecanoyl BAC | 242, pentadecanoic acid | Ibid. | Ibid. | Ibid. | |
| 842 | C48H75NO11 | 8-O-Palmitoyl BAC | 256, palmitic acid | Ibid. | Ibid. | Ibid. | |
| 854 | C49H75NO11 | 8-O-Heptadecenoyl BAC | 268, heptadecenoic acid | Ibid. | Ibid. | Ibid. | |
| 856 | C49H77NO11 | 8-O-(Methyl)-palmitoyl BAC | 270, methyl palmitic acid | Ibid. | Ibid. | Ibid. | |
| 866 | C50H75NO11 | 8-O-Linoleyl BAC | 280, linoleic acid | Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] | |
| NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.c,d | IT | [25, 26] | ||||
| 868 | C50H77NO11 | 8-O-Oleoyl BAC | 282, oleic acid | Human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [24] | |
| 870 | C50H79NO11 | 8-O-Stearoyl BAC | 284, stearic acid | Ibid. | Ibid. | Ibid. | |
| 882 | C51H79NO11 | 8-O-(9)-Nonadecenoyl BAC | 296, nonadecene | Ibid. | Ibid. | Ibid. | |
| 886 | C50H79NO12 | 8-O-(3-Hydroxy)-stearoyl BAC | 300, 3-hydroxy stearic acid | Ibid. | Ibid. | Ibid. | |
| 954 | C56H91NO11 | 8-O-Tetracosanoyl BAC | 368, tetracosanoic acid | Ibid. | Ibid. | Ibid. | |
| 962 | C57H87NO11 | 8-O-Pentacosatrienoyl BAC | 376, pentacosatrienoic acid | Ibid. | Ibid. | Ibid. | |
|
| |||||||
| MA | 590 | C31H43NO10 | BMA | NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.c,d | IT | [25, 26] |
| 572 | C31H41NO9 | Deacetoxy MA | NA | Ibid. | Ibid. | Ibid. | |
| 660 | C35H49NO11 | 8-O-Butyryl BMA | NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.e | IT, MALDI source-FT-ICR | [27] | |
| 674 | C36H51NO11 | 8-O-Valeryl BMA | NA | Ibid. | Ibid. | Ibid. | |
| 852 | C49H73NO11 | 8-O-Linoleyl BMA | NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.c,d | IT | [25, 26] | |
|
| |||||||
| HA | 574 | C31H43NO9 | BHA | NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.e | IT, MALDI source-FT-ICR | [27] |
| Rats; intestinal bacteria; anaerobic incubation, in vitro.c,d | IT | [25, 26] | |||||
| 556 | C31H41NO8 | Deacetoxy HA | NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.c,d | IT | [25, 26] | |
| 630 | C34H47NO10 | 8-O-Propionyl BHA | NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.e | IT, MALDI source-FT-ICR | [27] | |
| 644 | C35H49NO10 | 8-O-Butyryl BHA | NA | Ibid. | Ibid. | Ibid. | |
| 658 | C36H51NO10 | 8-O-Valeryl BHA | NA | Ibid. | Ibid. | Ibid. | |
| 692 | C39H49NO10 | 8-O-Phenylacetyl BHA | NA | Ibid. | Ibid. | Ibid. | |
| 836 | C49H73NO10 | 8-O-Linoleyl BHA | NA | Rats; intestinal bacteria; anaerobic incubation, in vitro.c,d | IT | [25, 26] | |
aNot available.
bDeoxy may also be referred to as dehydroxy in the literature.
cDDA was produced through decoction of Aconiti Radix Cocta with Fritillariae Thunbergii Bulbus, Pinelliae Rhizoma Preparatum, and Ampelopsis Radix.
It is not clear whether these compounds were directly metabolized from DDAs or were originally ingested.
dDDA was produced through decoction of Aconiti Lateralis Radix Praeparata with Glycyrrhizae Radix and Rhizome as well as with Atractylodis Macrocephalae Rhizoma.
It is not clear whether these compounds were directly metabolized from DDAs or were originally ingested.
eIn addition to AC and HA monomers, DDAs were also generated from ethyl alcohol extraction ofRadix Aconiti.
It is not clear whether these compounds were directly metabolized from DDAs or were originally ingested.
*These metabolites were further biotransformed in the intestine. Metabolites of these intermediate products are listed in Table 4.
Table 4.
Further biotransformation of intestinal AC metabolites in the intestine.
| m/z (ESI+) | Formula | Identification | Neutral loss (Da), identification of fatty acid |
Metabolic procedure | MS detection | References |
|---|---|---|---|---|---|---|
| 618 | C32H43NO11 | 1,16-Didemethyl AC (M1) | NAa | 16-O-Demethyl AC (C33H45NO11, 632) from AC; human; intestinal bacteria; anaerobic incubation, in vitro. | IT, FT-ICR | [28] |
| 616 | C33H45NO10 | 16-O-Demethyl-3-deoxy AC (M2)b | NA | |||
| 602 | C32H43NO10 | 1,16-Didemethyl-3-deoxy AC (M3) | NA | |||
| 590 | C31H43NO10 | 16-O-Demethyl BAC (M4) | NA | |||
| 486 | C24H39NO9 | 16-O-Demethyl aconine (M5) | NA | |||
| 646 | C34H47NO11 | 16-O-Demethyl-8-O-propionyl BAC | 74, propionic acid | |||
| 660 | C35H49NO11 | 16-O-Demethyl-8-O-butyryl BAC | 88, butyric acid | |||
| 674 | C36H51NO11 | 16-O-Demethyl-8-O-valeryl BAC | 102, valeric acid | |||
| 16-O-Demethyl-8-O-(methyl)-butyryl BAC | 102, methyl butyric acid | |||||
| 696 | C38H49NO11 | 16-O-Demethyl-8-O-heptatrienoyl BAC | 124, heptatrienoic acid | |||
| 698 | C38H51NO11 | 16-O-Demethyl-8-O-heptadienoyl BAC | 126, heptadienoic acid | |||
| 700 | C38H53NO11 | 16-O-Demethyl-8-O-heptenoyl BAC | 128, heptenoic acid | |||
| 702 | C38H55NO11 | 16-O-Demethyl-8-O-heptanoyl BAC | 130, heptanoic acid | |||
| 710 | C39H51NO11 | 16-O-Demethyl-8-O-octatrienoyl BAC | 138, octatrienoic acid | |||
| 716 | C39H57NO11 | 16-O-Demethyl-8-O-octanoyl BAC | 144, octanoic acid | |||
| 730 | C40H59NO11 | 16-O-Demethyl-8-O-nonanoyl BAC | 158, nonanoic acid | |||
| 736 | C41H53NO11 | 16-O-Demethyl-8-O-decatetraenoyl BAC | 164, decatetraenoic acid | |||
| 762 | C43H55NO11 | 16-O-Demethyl-8-O-dodecapentaenoyl BAC | 190, dodecapentaenoic acid | |||
| 764 | C43H57NO11 | 16-O-Demethyl-8-O-dodecatetraenoyl BAC | 192, dodecatetraenoic acid | |||
| 766 | C43H59NO11 | 16-O-Demethyl-8-O-dodecatrienoyl BAC | 194, dodecatrienoic acid | |||
| 778 | C44H59NO11 | 16-O-Demethyl-8-O-tridecatetraenoyl BAC | 206, tridecatetraenoic acid | |||
| 786 | C44H67NO11 | 16-O-Demethyl-8-O-(methyl)-dodecanoyl BAC | 214, methyl dodecanoic acid | |||
| 800 | C45H69NO11 | 16-O-Demethyl-8-O-retradecanoyl BAC | 228, tetradecanoic acid | |||
| 854 | C49H75NO11 | 16-O-Demethyl-8-O-oleoyl BAC | 282, oleic acid | |||
| 856 | C49H77NO11 | 16-O-Demethyl-8-O-stearoyl BAC | 284, stearic acid | |||
| 870 | C50H79NO11 | 16-O-Demethyl-8-O-(methyl)-stearoyl BAC | 298, methyl stearic acid | |||
| 884 | C51H81NO11 | 16-O-Demethyl-8-O-arachidyl BAC | 312, arachidic acid | |||
| 898 | C52H83NO11 | 16-O-Demethyl-8-O-heneicosanoyl BAC | 326, heneicosanoic acid | |||
| 926 | C54H87NO11 | 16-O-Demethyl-8-O-tricosanoyl BAC | 354, tricosanoic acid | |||
|
| ||||||
| 616 | C33H45NO10 | 16-O-Demethyl-3-deoxy AC (M1) | NA | 3-Deoxy AC (C34H47NO10, 630) from AC; human; intestinal bacteria; anaerobic incubation, in vitro. |
IT, FT-ICR | [29] |
| 614 | C34H47NO9 | 1,13-Dideoxy AC (M2) | NA | |||
| 588 | C32H45NO9 | 3-Deoxy BAC (M3) | NA | |||
| 484 | C25H41NO8 | 3-Deoxy aconine (M4) | NA | |||
| 644 | C35H49NO10 | 3-Deoxy-8-O-propionyl BAC | 74, propionic acid | |||
| 658 | C36H51NO10 | 3-Deoxy-8-O-butyryl BAC | 88, butyric acid | |||
| 700 | C39H57NO10 | 3-Deoxy-8-O-heptanoyl BAC | 130, heptanoic acid | |||
| 702 | C38H55NO11 | 3-Deoxy-8-O-(2-methyl-3-hydroxy)-valeryl BAC | 132, 2-methyl-3-hydroxy valeric acid |
|||
| 714 | C40H59NO10 | 3-Deoxy-8-O-octanoyl BAC | 144, octanoic acid | |||
| 730 | C40H59NO11 | 3-Deoxy-8-O-(3-hydroxy)-octanoyl BAC | 160, 3-hydroxy octanoic acid | |||
| 746 | C43H55NO10 | 3-Deoxy-8-O-undecapentaenoyl BAC | 176, undecapentaenoic acid | |||
| 762 | C44H59NO10 | 3-Deoxy-8-O-dodecatetraenoyl BAC | 192, dodecatetraenoic acid | |||
| 786 | C44H67NO11 | 3-Deoxy-8-O-(hydroxy)-dodecanoyl BAC | 216, hydroxy dodecanoic acid | |||
| 800 | C45H69NO11 | 3-Deoxy-8-O-(hydroxy)-tridecanoyl BAC | 230, hydroxy tridecanoic acid | |||
| 814 | C46H71NO11 | 3-Deoxy-8-O-(3-hydroxy)-tetradecanoyl BAC | 244, hydroxy tetradecanoic acid | |||
| 828 | C47H73NO11 | 3-Deoxy-8-O-(hydroxy)-pentadecanoyl BAC | 258, hydroxy pentadecanoic acid | |||
| 854 | C50H79NO10 | 3-Deoxy-8-O-propionyl BAC | 284, stearic acid | |||
|
| ||||||
| 602 | C32H43NO10 | 1,16-O-Didemethyl-3-deoxy AC (M1) | NA | 16-O-Demethyl-3-deoxy AC (C33H45NO10, 616) from AC; human; intestinal bacteria; anaerobic incubation, in vitro. |
IT, FT-ICR | [30] |
| 600 | C33H45NO9 | 16-O-Demethyl-3-deoxy-deoxy AC (M2) | NA | |||
| 574 | C31H43NO9 | 16-O-Demethyl-3-deoxy BAC (M3) | NA | |||
| 470 | C24H39NO8 | 16-O-Demethyl-3-deoxy aconine (M4) | NA | |||
| 630 | C34H47NO10 | 16-O-Demethyl-3-deoxy-8-O-propionyl BAC | 74, propionic acid | |||
| 644 | C35H49NO10 | 16-O-Demethyl-3-deoxy-8-O-butyryl BAC | 88, butyric acid | |||
| 696 | C39H53NO10 | 16-O-Demethyl-3-deoxy-8-O-octadienoyl BAC | 140, octadienoic acid | |||
| 700 | C39H57NO10 | 16-O-Demethyl-3-deoxy-8-O-octanoyl BAC | 144, octanoic acid | |||
| 702 | C38H55NO11 | 16-O-Demethyl-3-deoxy-8-O-(hydroxy)-heptanoyl BAC | 146, hydroxy heptanoic acid | |||
| 730 | C40H59NO11 | 16-O-Demethyl-3-deoxy-8-O-(hydroxy)-nonanoyl BAC | 174, hydroxy nonanoic acid | |||
| 746 | C43H55NO10 | 16-O-Demethyl-3-deoxy-8-O-dodecapentaenoyl BAC | 190, dodecapentaenoic acid | |||
| 762 | C44H59NO10 | 16-O-Demethyl-3-deoxy-8-O-tridecatetraenoyl BAC | 206, tridecatetraenoic acid | |||
| 778 | C45H63NO10 | 16-O-Demethyl-3-deoxy-8-O-tetradecatrienoyl BAC | 222, tetradecatrienoic acid | |||
aNot available.
bDeoxy may also be referred to as dehydroxy in the literature.
Ester exchange metabolites are classified as lipoalkaloids or lipoaconitines with an acetyl group at the C-8 position of DDAs replaced by other fatty acid acyl groups [24, 31]. Presumably, the short chain fatty acids (such as propionic, butyric, hexanoic, phenylacetic, and phenylpropionic acids) for ester exchange are generated from xenobiotics, such as food decomposed by intestinal bacteria, while certain long chain fatty acids (such as palmitic, oleic, and stearic acids) are generated from bacterial cell walls [24]. DDA toxicity is reduced after ester exchange. For example, the LD50 of 8-O-butyryl- (from short chain fatty acid) benzoylmesaconine is 15.78 mg/kg, which is 5.5-fold greater than MA (8-O-acetyl-benzoylmesaconine) [22]. The LD50 for mice with lipomesaconitines (from long chain fatty acids) are from 10 to 40 mg/kg, which are 20-fold greater than MA [32].
2.3. Metabolism in the Liver
The liver is an important organ for drug metabolism, and it expresses many drug-metabolising enzymes. After oral administration, drugs are typically subjected to hepatic metabolism, including CEs that catalyse ester hydrolysis [18], phase I drug metabolic enzymes that catalyse oxidation, and phase II metabolic enzymes that catalyse conjugation [21]. The metabolites are hydrophilic and are more rapidly excreted from the body than parent drugs. Cytochrome P450 enzymes (CYP450s) and uridine 5′-diphosphate (UDP)-glucuronosyltransferases (UGTs) are the most common phase I and phase II metabolic enzymes, respectively [33].
The hepatic metabolism studies reviewed herein were mainly performed in vitro through incubation with liver microsomes. CYP450- or UGT-catalysed metabolism in microsomes can be selectively performed in different reaction systems with auxiliary enzymes and exclusive substrates [34, 35].
The DDA and MDA phase I metabolic pathways are similar and include hydroxylation, deoxylation, demethylation, didemethylation/deethylation, dehydrogenation, and demethylation with dehydrogenation (Table 5). The individual CYP450s responsible for specific metabolites were further determined via individual inhibitors or recombinant isoenzymes. CYP3A4 and CYP3A5 are the most common isoenzymes that catalyse both DDAs and MDAs. In addition, CYP2D6, CYP1A1/2, CYP2C9, CYP2C8, CYP2C19, and CYP2E1 also partially catalyse DDAs.
Table 5.
Metabolites of DDAs and MDAs converted in the liver.
| Alkaloids | m/z (ESI+) | Formula | Identification | Involved CYP450s | Metabolic procedure | MS detection | References |
|---|---|---|---|---|---|---|---|
| AC | 662 | C34H47NO12 | Hydroxy AC | CYP3A5, CYP2D6 | Human; liver microsomes and recombinant CYP450s; incubation, in vitro. | Q-TOF | [35] (M6) |
| NAa | Rats; liver microsome S9 fraction; incubation, in vitro. | IT | [36] (M5) | ||||
| Guinea pigs and mice; liver microsomes; incubation, in vitro. | HRMS, MS2 | [37] (M6) | |||||
| 644 | C34H45NO11 | 3-Dehydrogen AC | CYP3A4, CYP3A5 | Human; liver microsomes and recombinant CYP450s; incubation, in vitro. | Q-TOF | [35] (M5) | |
| NA | Guinea pigs and mice; liver microsomes; incubation, in vitro. | HRMS, MS2 | [37] (M5) | ||||
| Dehydrogen AC | CYP3A, CYP1A1/2 | Rats; liver microsomes; incubation, in vitro. | IT | [4] (M6) | |||
| NA | Rats; liver microsome S9 fraction; incubation, in vitro. | IT | [36] (M7) | ||||
| 632 | C33H45NO11 | 16-O-Demethyl AC | CYP3A, CYP1A1/2 | Rats; liver microsomes; incubation, in vitro. | IT | [4] (M2) | |
| CYP3A4, CYP3A5, CYP2D6, CYP2C9 | Human; liver microsomes and recombinant CYP450s; incubation, in vitro. | Q-TOF | [35] (M2) | ||||
| NA | Rats; liver microsome S9 fraction; incubation, in vitro. | IT | [36] (M6) | ||||
| NA | Guinea pigs and mice; liver microsomes; incubation, in vitro. | HRMS, MS2 | [37] (M2) | ||||
| O-Demethyl AC | CYP3A, CYP1A1/2 | Rats; liver microsomes; incubation, in vitro. | IT | [4] (M1) | |||
| CYP3A4, CYP3A5, CYP2C8, CYP2D6 | Human; liver microsomes and recombinant CYP450s; incubation, in vitro. | Q-TOF | [35] (M1) | ||||
| NA | Guinea pigs and mice; liver microsomes; incubation, in vitro. | HRMS, MS2 | [37] (M1) | ||||
| 630 | C34H47NO10 | Deoxyaconitine (3-deoxy AC) | NA | Guinea pigs and mice; liver microsomes; incubation, in vitro. | HRMS, MS2 | [37] (M7) | |
| Deoxy AC | NA | Rats; liver microsome S9 fraction; incubation, in vitro. | IT | [36] (M8) | |||
| 618 | C32H43NO11 | O-Didemethyl AC | CYP3A, CYP1A1/2 | Rats; liver microsomes; incubation, in vitro. | IT | [4] (M3) | |
| CYP2D6, CYP3A5 | Human; liver microsomes and recombinant CYP450s; incubation, in vitro. | Q-TOF | [35] (M4) | ||||
| NA | Rats; liver microsome S9 fraction; incubation, in vitro. | IT | [36] (M4) | ||||
| NA | Guinea pigs and mice; liver microsomes; incubation, in vitro. | HRMS, MS2 | [37] (M3) | ||||
| N-Deethyl AC | CYP3A, CYP1A1/2 | Rats; liver microsomes; incubation, in vitro. | IT | [4] (M4) | |||
| CYP3A4, CYP3A5, CYP2D6, CYP2C9 | Human; liver microsomes and recombinant CYP450s; incubation, in vitro. | Q-TOF | [35] (M3) | ||||
| NA | Rats; liver microsomes; incubation, in vitro. | Q-TOF | [38] (M4) | ||||
| NA | Rats; liver microsome S9 fraction; incubation, in vitro. | IT | [36] (M2) | ||||
| NA | Guinea pigs and mice; liver microsomes; incubation, in vitro. | HRMS, MS2 | [37] (M4) | ||||
| 604 | C32H45NO10 | BAC | CYP3A, CYP1A1/2 | Rats; liver microsomes; incubation, in vitro. | IT | [4] (M5) | |
| NA | Rats; liver microsome and S9 fraction; incubation, in vitro. | Q-Trap | [39] | ||||
| NA | Rats; liver microsomes; incubation, in vitro. | Q-TOF | [38] (M2) | ||||
| NA | Rats; liver microsome S9 fraction; incubation, in vitro. | IT | [36] (M1) | ||||
| NA | Guinea pigs and mice; liver microsomes; incubation, in vitro. | HRMS, MS2 | [37] (M8) | ||||
| 586 | C32H43NO9 | Deacetoxy ACb | NA | Rats; liver microsome S9 fraction; incubation, in vitro. | IT | [36] (M3) | |
| 482 | C25H39NO8 | Dehydrated aconine | NA | Rabbits; liver; ig, in vivo. | IT | [40] | |
|
| |||||||
| MA | 648 | C33H45NO12 | Hydroxy MA | CYP3A4, CYP3A5 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [41] (M5) |
| 2-Hydroxy MA | NA | Rats; liver microsomes; incubation, in vitro. | Q-TOF, QQQ | [38] (M5) | |||
| CYP3A, CYP2C, CYP2D | Rats; liver microsomes; incubation, in vitro. | QQQ; IM | [42] (M5) | ||||
| 630 | C33H43NO11 | Dehydrogen MA | CYP3A4, CYP3A5 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [41] (M4) | |
| NA | Rats; liver microsomes; incubation, in vitro. | Q-TOF, QQQ | [38] (M6) | ||||
| 3-Dehydrogen MA | CYP3A, CYP2D | Rats; liver microsomes; incubation, in vitro. | QQQ; IM | [42] (M2) | |||
| 618 | C32H43NO11 | 16-O-Demethyl MA | CYP2C8, CYP3A4, CYP3A5 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [41] (M2) | |
| CYP3A | Rats; liver microsomes; incubation, in vitro. | QQQ; IM | [42] (M4) | ||||
| 1-O-Demethyl MA | CYP3A, CYP2C | Rats; liver microsomes; incubation, in vitro. | QQQ; IM | [42] (M3) | |||
| 18-O-Demethyl MA | CYP3A, CYP2C | Rats; liver microsomes; incubation, in vitro. | QQQ; IM | [42] (M6) | |||
| Demethyl MA | CYP2C8, CYP2D6, CYP3A5 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [41] (M1) | |||
| Demethyl MA | CYP3A4, CYP3A5 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [41] (M3) | |||
| 616 | C32H41NO11 | Demethyl-dehydrogen MA | CYP3A4, CYP3A5 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [41] (M6) | |
| Demethyl-dehydrogen MA | CYP2C8, CYP3A4, CYP3A5 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [41] (M7, M8) | |||
| Demethyl-dehydrogen MA | CYP2C8, CYP2C9, CYP2D6, CYP3A4, CYP3A5 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [41] (M9) | |||
| 590 | C31H44NO10 | BMA | NA | Rats; liver microsome and S9 fraction; incubation, in vitro. | Q-Trap | [39] | |
| NA | Rats; liver microsomes; incubation, in vitro. | Q-TOF, QQQ | [38] (M1) | ||||
|
| |||||||
| HA | 632 | C33H45NO11 | MA | CYP3A4, CYP3A5, CYP2C19, CYP2D6, CYP2E1 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [43] (M8) |
| CYP3A, CYP2D, CYP2C, CYP2E1 | Rats; liver microsomes; incubation, in vitro. | QQQ | [44] (M6) | ||||
| 2-Hydroxy HA | CYP3A, CYP2C, CYP2D, CYP1A2 | Rats; liver microsomes; incubation, in vitro. | QQQ | [44] (M4) | |||
| Hydroxy HA | CYP3A4, CYP3A5, CYP2C19, CYP2D6, CYP2E1 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [43] (M7) | |||
| 614 | C33H43NO10 | 15-Dehydrogen HA | CYP3A, CYP2D, CYP2E1 | Rats; liver microsomes; incubation, in vitro. | QQQ | [44] (M2) | |
| 602 | C32H43NO10 | 16-O-Demethyl HA | CYP3A4, CYP3A5, CYP2C19, CYP2D6, CYP2E1 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [43] (M2) | |
| 1-O-Demethyl HA | CYP3A, CYP2D, CYP2C | Rats; liver microsomes; incubation, in vitro. | QQQ | [44] (M5) | |||
| 18-O-Demethyl HA | CYP3A, CYP2C | Rats; liver microsomes; incubation, in vitro. | QQQ | [44] (M7) | |||
| Demethyl HA | CYP3A4, CYP3A5, CYP2C8, CYP2C19, CYP2D6, CYP2E1 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [43] (M1) | |||
| Demethyl HA | CYP3A4, CYP3A5, CYP1A2, CYP2C8, CYP2C19, CYP2D6, CYP2E1 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [43] (M3) | |||
| 600 | C32H41NO10 | Demethyl-dehydrogen HA | CYP3A4, CYP3A5, CYP2C19, CYP2D6, CYP2E1 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [43] (M4–M6) | |
| 590 | C31H43NO10 | 2-Hydroxy BHA | CYP3A, CYP2C | Rats; liver microsomes; incubation, in vitro. | QQQ | [44] (M1) | |
| 588 | C31H41NO10 | Didemethyl HA | CYP3A4, CYP3A5, CYP2C19, CYP2D6, CYP2E1 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [43] (M9, M10) |
|
| Didemethyl HA | CYP3A4, CYP3A5, CYP2C19 | Human (male); liver microsomes and recombinant CYP450s; incubation, in vitro. |
Q-TOF | [43] (M11) | |||
| 574 | C31H43NO9 | BHA | CYP3A, CYP2D | Rats; liver microsomes; incubation, in vitro. | QQQ | [44] (M3) | |
| NA | Rats; liver microsomes; incubation, in vitro. | Q-TOF, QQQ | [38] (M3) | ||||
| NA | Rats; liver microsome and S9 fraction; incubation, in vitro. | Q-Trap | [39] | ||||
|
| |||||||
| BAC | 602 | C32H43NO10 | Dehydrogen BAC (M1, M2) | CYP3A4, CYP3A5 | Human; liver microsomes; incubation, in vitro. |
Q-TOF | [45] |
| 590 | C31H43NO10 | Demethyl BAC (M5) | CYP3A4, CYP3A5, CYP2D6 | ||||
| Demethyl BAC (M6) | CYP3A4, CYP3A5 | ||||||
| 588 | C31H41NO10 | Demethyl-dehydrogen BAC (M3) | CYP3A4, CYP3A5 | ||||
| 576 | C30H41NO10 | Deethyl BAC or didemethyl BAC (M7) |
CYP3A4, CYP3A5 | ||||
| 574 | C30H39NO10 | Didemethyl-dehydrogen BAC or deethyl-dehydrogen BAC (M4) |
CYP3A4, CYP3A5 | ||||
|
| |||||||
| BMA | 606 | C31H43NO11 | Hydroxy BMA (M8) | CYP3A4, CYP3A5 | Human; liver microsomes; incubation, in vitro. |
Q-TOF | [45] |
| 588 | C31H41NO10 | Dehydrogen BMA (M1, M2) | CYP3A4, CYP3A5 | ||||
| 576 | C30H41NO10 | Demethyl BMA (M5) | CYP3A4, CYP3A5, CYP2D6, CYP2C8 | ||||
| Demethyl BMA (M6, M7) | CYP3A4, CYP3A5 | ||||||
| 574 | C30H39NO10 | Demethyl-dehydrogen BMA (M3, M4) | CYP3A4, CYP3A5 | ||||
|
| |||||||
| BHA | 590 | C31H43NO10 | Hydroxy BHA (M7) | CYP3A4, CYP3A5 | Human; liver microsomes; incubation, in vitro. |
Q-TOF | [45] |
| BMA (M8) | CYP3A4, CYP3A5 | ||||||
| 572 | C31H41NO9 | Dehydrogen BHA (M1, M2) | CYP3A4, CYP3A5 | ||||
| 560 | C30H41NO9 | Demethyl BHA (M5) | CYP3A4 | ||||
| Demethyl BHA (M4, M6) | CYP3A4, CYP3A5 | ||||||
| 558 | C30H39NO9 | Demethyl-dehydrogen BHA (M3) | CYP3A4, CYP3A5 | ||||
| 556 | C30H37NO9 | Demethyl-didehydrogen BHA (M9) | CYP3A4, CYP3A5 | ||||
aNot available.
bDeacetoxy aconitine may also be referred to as pyroaconitine in the literature.
Hydrophobic drug biotransformation commonly occurs first through phase I metabolism in which functional groups, such as hydroxy, sulfhydryl, carboxyl, and amino group, are formed and provide reaction sites for the subsequent phase II conjugation [46, 47]. For lipophilic DDAs and MDAs, hydroxy groups are initially present and are formed after hydroxylation during the phase I metabolism. However, phase II metabolites of either DDAs or MDAs were not detected in hepatic metabolism in vitro and in vivo, which demonstrates that phase II metabolism is not dominant compared with phase I metabolism in the liver. DDA ester hydrolysis should be catalysed by CEs. However, CYP3A, CYP1A1, and CYP1A2 are also involved in ester hydrolysis of AC, which reflects the complexity of metabolism.
2.4. A Comparison of DDA and MDA Metabolism in the Gastrointestinal Tract and Liver
The metabolites generated in the stomach, intestine, and liver are compared in Table 6. The polarity of most metabolites increased after DDA gastrointestinal and hepatic metabolism, except lipoalkaloids. Metabolites of AC from dehydrogenation and demethylation with dehydrogenation were only observed in the liver. The AC metabolites from demethylation with deoxylation observed from intestinal bacteria incubation [24] were also detected in the urine after oral AC administration in rabbits. However, these metabolites were not found in the urine after intravenous injection [48]. This observation suggests that the gastrointestinal tract may participate in biotransformation. The characteristic metabolites in the gastrointestinal tract were lipoalkaloids, which might be converted by enzymes that are only produced by intestinal bacteria. In addition, more lipoalkaloid varieties were detected in the intestine than in the stomach, which is consistent with abundant bacterial distribution in the gastrointestinal tract [16]. More studies have focused on DDAs than MDAs. However, it is speculated that MDAs may share similar metabolic pathways (except for ester hydrolysis at the C-8 position) with DDAs in the gastrointestinal tract based on the similarity in their hepatic metabolism and chemical structures.
Table 6.
A comparison of DDA and MDA metabolites in different metabolic procedures.
| Alkaloids | Stomach | Intestine | Liver (CYP450s, phase I metabolism) |
|---|---|---|---|
| DDAs | Ester hydrolysis | Ester hydrolysis commonly occurs at C-8 | Ester hydrolysis commonly occurs at C-8 |
| Hydroxylation at 2′/3′/4′ of the benzoyl group | Hydroxylation at C-10 | Hydroxylation at C-2 | |
| Deoxylation at C-3/15 | Deoxylation at C-3/15 | Deoxylation at C-3/15 | |
| Demethylation at the methoxy group | Demethylation at the methoxy group, often at C-1/6/16 or the N-methyl group |
Demethylation at the methoxy group, often at C-1/6/16 or the N-methyl group |
|
| Didemethylation at the methoxy group or deethylation at the N-ethyl group | NAa | Didemethylation at the methoxy group or deethylation at the N-ethyl group | |
| NA | Deacetoxylation (pyrolysis) | Deacetoxylation (pyrolysis) | |
| NA | NA | Dehydrogenation at C-3/15 | |
| NA | NA | Demethylation at C-1/6/16 or the N-methyl group with dehydrogenation at C-3/15; demethylation with dehydrogenation at the same methoxyl group, O remained as a carbonyl group. |
|
| NA | Demethylation and deoxylation | NA | |
| Lipoalkaloids via ester exchange at C-8 with long chain fatty acids. | Lipoalkaloids via ester exchange at C-8 with short/long chain fatty acids. | NA | |
|
| |||
| MDAs | NA | NA | Hydroxylation |
| Demethylation | |||
| Didemethylation or deethylation | |||
| Dehydrogenation | |||
| Demethylation and (di)dehydrogenation | |||
aNot available.
Interestingly, phase I metabolites of hydroxylation, deoxylation, demethylation, and didemethylation/deethylation were detected not only in the liver but also in the gastrointestinal tract. As mentioned above in Section 2.2, intestinal bacteria participate in metabolism, such as through deoxylation, reduction, and deacetylation. However, it has also been reported that human small intestinal epithelial cells express a range of P450s, which include CYP3A, the isoenzyme that dominates in the liver [49]. Intestinal metabolism was performed in vitro through anaerobic incubation in a feces suspension, despite the symbiotic intestinal bacteria, which should also contain apoptosis-undergoing intestinal epithelial cells that release phase I and phase II metabolic enzymes into the suspension. Thus, intestinal metabolites are likely converted by both bacteria and phase I metabolic enzymes.
Metabolic isoenzyme expression is not identical among different species [50] that lead to metabolic differences in different species. Based on references in this review, we find that DDAs were ester hydrolysed to MDAs in rat intestine and liver, but not in humans. On the other hand, the same metabolites converted in different species have been reported. For example, 16-O-demethyl BAC, the ester hydrolysed products from 16-O-demethyl AC in intestinal metabolism, was detected not only in rats but also in humans. Hydroxy aconitine from AC was detected through incubation in liver microsomes or S9 from humans, rats, guinea pigs, and mice. It is notable that the AC demethylation at the C-16 position is catalysed by CYP3A and CYP1A1/2 in rats while it is catalysed by CYP3A, CYP2D6, and CYP2C9 in humans. However, no studies have specifically compared metabolites from DDAs or MDAs among humans and different experimental animals. Briefly, the metabolic differences in different species yield certain risks in predicting human drug metabolism based on data from experimental animals.
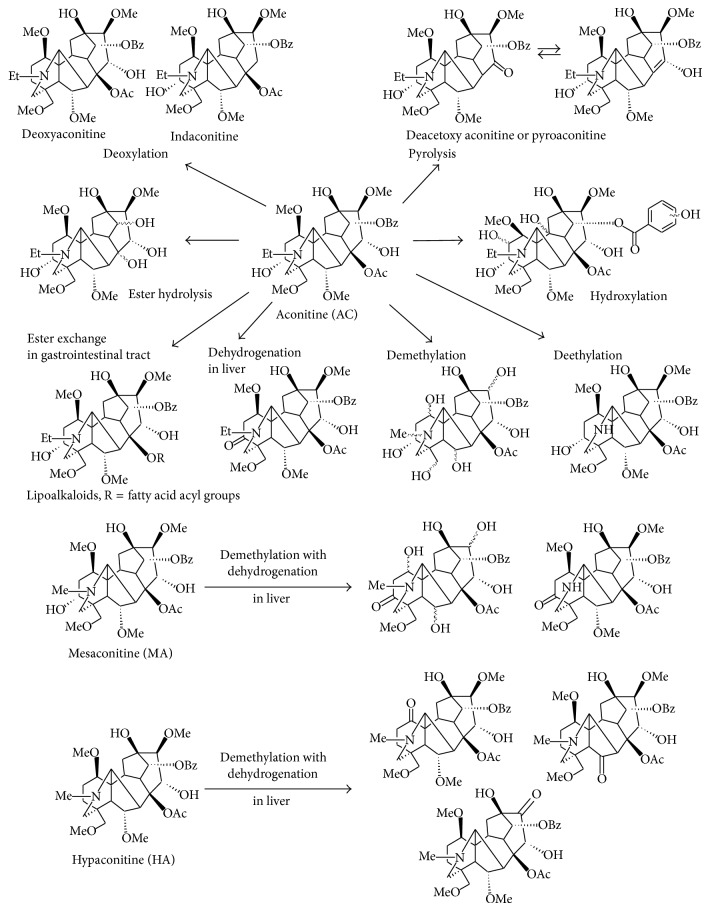
The metabolic pathways proposed for DDAs are generalized in Figure 1.
Figure 1.
Proposed DDA metabolic pathways. The organ/tissue metabolic processes are partially indicated. The wavy bonds indicate the potential metabolic positions. Me, Et, Ac, and Bz indicate methyl, ethyl, acetyl, and benzoyl groups, respectively.
The organ/tissue metabolic processes are partially indicated. The wavy bonds indicate the potential metabolic positions. Me, Et, Ac, and Bz indicate methyl, ethyl, acetyl, and benzoyl groups, respectively.
3. Metabolites Detected in the Blood
MDAs and alcohol amines are the main DDA metabolites in the blood (Table 7). It has been suggested that AC and related alkaloids can be rapidly absorbed by the upper gastrointestinal tract for the short latent period between the ingestion of aconite roots and the onset of poisoning features [3]. Therefore, the absorbed DDAs may be partially and gradually ester hydrolysed to less toxic MDAs and nontoxic alcohol amines by CEs distributed in the blood. Furthermore, the blood provides a suitable pH environment for ester hydrolysis. This hypothesis is supported by an analysis of rat plasma after DDA administration via a tail vein, wherein MDAs and alcohol amines were detected [39].
Table 7.
DDA metabolites detected in the plasma.
| DDAs | m/z (ESI+) | Formula | Identification | Metabolic procedure | MS detection | References |
|---|---|---|---|---|---|---|
| AC | 604 | C32H45NO10 | BAC | Mouse; plasma; ig, in vivo. | GC/MS | [51] |
| Rabbit; plasma; ig, in vivo. | IT | [52] (M2) | ||||
| 590 | C31H43NO10 | 16-O-Demethyl BAC | Rabbit; plasma; ig, in vivo. | IT | [52] (M3) | |
| 500 | C25H41NO9 | Aconine | Rats; plasma; iv, in vivo.a | Q-Trap | [39] | |
| Mouse; plasma; ig, in vivo. | GC/MS | [51] | ||||
| Rabbit; plasma; ig, in vivo. | IT | [52] (M4) | ||||
|
| ||||||
| MA | 590 | C31H43NO10 | BMA | Rats; plasma; iv, in vivo.a | Q-Trap | [39] |
| 486 | C24H40NO9 | Mesaconine | ||||
|
| ||||||
| HA | 574 | C31H44NO9 | BHA | Rats; plasma; iv, in vivo.a | Q-Trap | [39] |
aA mixture of AC, MA, and HA was administered via the tail vein.
MDAs and alcohol amines are commonly considered markers in forensic and clinical evaluations of aconitine poisoning because their half-lives are longer than DDAs [19], which might lead to the neglect of other metabolites in the blood. Additionally, many efflux/influx transporters, such as P-glycoprotein (P-gp), multidrug resistance-associated protein 2 (MRP2), and MRP3 expressed in intestinal epithelial and hepatic cells, are involved in drug absorption [53]. It is difficult to determine whether the various metabolites produced in the gastrointestinal tract and liver are transported into the blood from the few studies on their transport mechanism.
4. Metabolites Detected in the Urine
The metabolites found in the urine are shown in Table 8. Compared with intestinal and hepatic metabolites, most metabolites from hydroxylation, deoxylation, demethylation, deethylation/didemethylation, dehydrogenation, ester hydrolysis, deacetoxylation (pyrolysis), and demethylation with deoxylation have been found in the urine. Further, a few phase II metabolites as glucuronide and sulfate conjugates have been found in the urine but have not been reported in hepatic or intestinal metabolism in vitro. Glucuronidation catalysed by UGTs occurs in human and rat kidneys [63, 64]; glucuronidation might be responsible for phase II biotransformation processes in addition to hepatic and intestinal metabolism.
Table 8.
Metabolites of AC, MA, and HA (DDAs) detected in the urine.
| DDAs | m/z (ESI+) | Formula | Identification | Metabolic procedure | MS detection | References |
|
| ||||||
| AC | 780 | C38H53NO16 | BAC glucuronide conjugate | Rats; ig, in vivo. | IT | [54] |
| 726 | C34H47NO14S | AC sulfate conjugate | ||||
| 662 | C34H47NO12 | 10-Hydroxy AC | Rats; ig, in vivo. | IT | [54] | |
| Rats; ig, in vivo. | IT | [36] (M5) | ||||
| 644 | C34H45NO11 | 3-Dehydrogen AC | Rats; ig, in vivo. | IT | [36] (M7) | |
| 632 | C33H45NO11 | 16-O-Demethyl AC | Rats; ig, in vivo. | IT | [54] | |
| Rats; ig, in vivo. | IT | [55] (M2) | ||||
| Rabbits; ig, in vivo. | IT | [56] (M1) | ||||
| Rabbits; iv and ig, in vivo. | IT | [48] (M1, found in both iv and ig) | ||||
| Rabbits (male and female); ig, in vivo. | IT | [57] (M5) | ||||
| Human (female); po, in vivo.a | IT | [58] (M4) | ||||
| Rats; ig, in vivo. | IT | [36] (M6) | ||||
| Rabbits; ig, in vivo. | IT | [59] (M1) | ||||
| Human (female); po, in vivo.b | IT | [60] (M7) | ||||
| 1-O-Demethyl AC | Rats; ig, in vivo. | IT | [54] | |||
| 6-O-Demethyl AC | ||||||
| MA | Rats; ig, in vivo. | IT | [55] (M1) | |||
| 630 | C34H47NO10 | Deoxy AC | Rats; ig, in vivo. | IT | [54] | |
| Rats; ig, in vivo. | IT | [36] (M8) | ||||
| 618 | C32H43NO11 | 16-O-Demethyl MA | Rats; ig, in vivo. | IT | [55] (M3) | |
| 8-Methoxy BAC | Rats; ig, in vivo. | IT | [54] | |||
| 1-O-Demethyl MA | Rats; ig, in vivo. | IT | [54] | |||
| N-Deethyl AC (M2) | Rats; ig, in vivo. | IT | [36] | |||
| O-Didemethyl AC (M4) | ||||||
| 616 | C33H45NO10 | 1-O-Demethyl-13-deoxy AC | Rats; ig, in vivo. | IT | [54] | |
| Demethyl-deoxy AC | Rabbits; iv and ig, in vivo. | IT | [48] (M2, found in ig only) | |||
| 606 | C31H43NO11 | 10-Hydroxy BMA | Rats; ig, in vivo. | IT | [54] | |
| 604 | C32H45NO10 | BAC | Rabbits; ig, in vivo. | IT | [56] (M2) | |
| Rats; ig, in vivo. | IT | [55] (M4) | ||||
| Rabbits (male and female); ig, in vivo. | IT | [57] (M2) | ||||
| Rabbits; ig, in vivo. | IT | [59] (M2) | ||||
| Rats; ig, in vivo. | IT | [54] | ||||
| Human (female); po, in vivo.a | IT | [58] (M1) | ||||
| Human (female); po, in vivo.b | IT | [60] (M4) | ||||
| Rats; ig, in vivo. | IT | [36] (M1) | ||||
| 590 | C31H43NO10 | 16-O-Demethyl BAC | Rabbits; ig, in vivo. | IT | [56] (M3) | |
| Rabbits (male and female); ig, in vivo. | IT | [57] (M3) | ||||
| Rabbits; ig, in vivo. | IT | [59] (M3) | ||||
| 588 | C32H45NO9 | 3-Deoxy BAC | Rats; ig, in vivo. | IT | [54] | |
| 586 | C32H43NO9 | Pyroaconitine (deacetoxy AC) | Rabbits (male and female); ig, in vivo. | IT | [57] (M6, found in male only) | |
| Rats; ig, in vivo. | IT | [54] | ||||
| Rats; ig, in vivo. | IT | [36] (M3) | ||||
| 500 | C25H41NO9 | Aconine | Rabbits; ig, in vivo. | IT | [56] (M4) | |
| Rabbits (male and female); ig, in vivo. | IT | [57] (M4) | ||||
| Rabbits; ig, in vivo. | IT | [59] (M4) | ||||
| Rats; ig, in vivo. | IT | [54] | ||||
| 482 | C25H39NO8 | Dehydrated aconine | Human; po, in vivo.c | IT | [40] | |
|
| ||||||
| Alkaloids | m/z (ESI+) | Formula | Identification | Metabolic procedure | MS detection | References |
|
| ||||||
| MA | 766 | C37H51NO16 | BMA glucuronide conjugate | Rats; ig, in vivo. | IT | [61] (M1) |
| 648 | C33H45NO12 | 10-Hydroxy MA | Rats; ig, in vivo. | IT | [61] (M2) | |
| 618 | C32H43NO11 | 1-O-Demethyl MA | Rats; ig, in vivo. | IT | [61] (M3) | |
| Demethyl MA | Rats; ig, in vivo.d | TOF | [62] (M10) | |||
| 616 | C33H45NO10 | Deoxy MA | Rats; ig, in vivo. | IT | [61] (M4) | |
| 590 | C31H43NO10 | BMA | Rats; ig, in vivo. | IT | [61] (M5) | |
| Human (female); po, in vivo.a | IT | [58] (M2) | ||||
| Human (female); po, in vivo.b | IT | [60] (M5) | ||||
| 468 | C24H37NO8 | Dehydrated mesaconine | Human; po, in vivo.c | IT | [40] | |
|
| ||||||
| HA | 602 | C32H43NO10 | 16-O-Demethyl HA | Human (female); po, in vivo.a | IT | [58] (M5) |
| Human (female); po, in vivo.b | IT | [60] (M8) | ||||
| 574 | C31H43NO9 | BHA | Human (female); po, in vivo.a | IT | [58] (M3) | |
| Human (female); po, in vivo.b | IT | [60] (M6) | ||||
a,bDDA was produced through decoction containing Aconiti and Aconiti Kusnezoffii Radix.
It is not clear whether these compounds were directly metabolized from DDAs or originally ingested.
cDDA was produced from a medical liquor containing Aconiti Kusnezoffii Radix.
It is not clear whether these compounds were directly metabolized from DDAs or originally ingested.
dDDA was produced from a liquid of crude aconite root decoction via ethanol precipitation.
It is not clear whether these compounds were directly metabolized from DDAs or originally ingested.
Additionally, mRNA for CYP3A4 and CYP3A5, which are the major isoforms that catalyse DDA metabolism, is also expressed in human kidneys, but the expression levels are much lower than in the liver and intestine [65]. Based on the data in Section 3, metabolites from DDAs in the blood are fewer than in the urine. Further, the urine is converted from the blood in the kidney. Perhaps, the various metabolites in the urine are converted from DDAs and their ester hydrolysed products in the blood by metabolic enzymes expressed at low levels in the kidney. Is it possible that various metabolites from DDAs produced in the intestine and liver are absorbed in the blood and excreted in the urine? However, as noted in Section 3, the data on metabolites in the blood is insufficient.
No studies have reported on metabolites of lipoalkaloids in the urine, which are the metabolites characteristically produced in the gastrointestinal tract. DDA lipophilicity may be reasonably increased through ester exchange with long chain fatty acids at the C-8 position, which results in easier absorption of lipoalkaloids into the blood. Are the ester groups then hydrolysed by CEs in the blood and liver, producing MDAs and alcohol amines, or are they directly excreted through the feces? Such conjecture requires further investigation.
5. Original Compound Stability
All of the in vivo and in vitro metabolism reactions occur in fluid. Therefore, the stability of DDAs and MDAs in different pH aqueous solutions should be considered. One study reported that AC and MA were decomposed dramatically after incubation in water for 24 h at 25°C (degrees Celsius), and the products of AC were BAC, aconine, deacetoxy AC, and deoxy AC. In addition, almost half of the AC and MA were depleted in phosphate buffer at pH 2.0 and 6.8 over 12 h at 25°C (degrees Celsius); these pH values are similar to gastric acid and intestinal juice, respectively [66]. These results imply that metabolites, such as BAC and aconine, may be partially converted from DDAs in body fluid without enzyme catalysis. On the other hand, the rate of MDA formation from DDAs was much higher in phosphate buffer (pH 7.4) with hepatic microsomes than in the negative control without hepatic microsomes [39]. The facts imply that the enzymes did affect bioconversion of instable DDAs.
6. Metabolite Detection and Identification
Metabolites are typically varied at trace levels with endogenous interference from biological matrices, such as tissue, the blood, or urine. Liquid chromatography multiple-stage tandem mass spectrum (LC/MSn) has been widely applied for drug metabolite detection due to its high sensitivity and selectively.
For DDAs and MDAs, positive electrospray ionization (ESI+) is suitable for alkaloid ionization. Quadrupole time of flight (Q-TOF) and Fourier transform ion cyclotron resonance (FT-ICR) MS techniques are applied to metabolite identification due to their high resolution of pseudomolecular ions. Fragment ions are obtained step-by-step through ion trap (IT) MS, which is helpful for deducing the chemical structures. The acyl groups from fatty acids are confirmed by GC-MS, and neutral fatty acid losses are observed in LC-MS [24].
The fragmentation pathways of different types of Aconitum alkaloids include diagnostic ions. For the AC-type of alkaloid, the diagnostic ions are [M+H-18 (water)]+, [M+H-60 (acetate from C-8 and C-15)]+, [M+H-60-32 (methanol)-28 (carbonyl group)]+, and [M+H-60-32-28-122 (benzoic acid at C-14)]+[14, 22]. For the BAC-type, the diagnostic ions are [M+H-50 (methanol and water)]+, [M+H-50-32]+, and [M+H-50-32-18]+ [60]. For lipoaconitine, the diagnostic ions are 586 ([Mass of AC+H-60]+) with neutral fatty acid losses that correspond to acyl groups at the C-8 position [24].
However, MSn analyses only provide a possible fragmentation pattern based on the mass difference between pseudomolecular and fragment ions, and the metabolite confirmations are not necessarily accurate. Considering HA, the demethylation reaction position is ambiguous due to the five methyl groups at the C-1, C-6, C-16, C-18, and nitro positions. Demethylation with dehydrogenation was inferred to occur at the methoxy and hydroxy groups that attach to different skeleton carbons in MA [41] (see Figure 1), while it occurs at the same methoxy group in HA, forming a carbonyl group [43] (see Figure 1). However, detailed structure determination for these two types of metabolites was not provided.
7. Conclusions
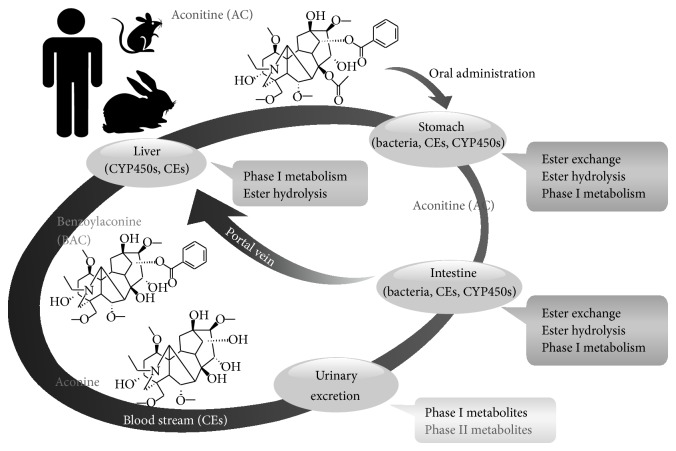
In this review, we classify and summarize metabolites of highly toxic DDAs and less toxic MDAs from the gastric and intestinal content, intestinal bacterial juice, hepatic microsomes, blood, and urine from different animal species and humans in vivo and in vitro. For example, considering AC, which is the most researched toxic DDA, we generalize a process of toxicity reduction in body after oral AC administration for the first time (Figure 2).
Figure 2.
The proposed process of toxicity reduction after oral AC administration in humans and experimental animals. The metabolites from ester exchange are lipo-alkaloids. Ester hydrolysis occurs at the C-8 or/and C-14 position, producing benzoylaconine (BAC) and aconine. Phase I metabolism refers to hydroxylation, deoxylation, dehydrogenation, demethylation, and didemethylation/deethylation. A few phase II metabolites were detected in the urine, including BAC glucuronide and AC sulfate conjugates. Cytochrome P450 enzymes (CYP450s), carboxylesterases (CEs), and enzymes produced by intestinal bacteria are involved in gastrointestinal and hepatic metabolism of aconitine (AC).
The metabolites from ester exchange are lipoalkaloids. Ester hydrolysis occurs at the C-8 or/and C-14 position, producing benzoylaconine (BAC) and aconine. Phase I metabolism refers to hydroxylation, deoxylation, dehydrogenation, demethylation, and didemethylation/deethylation. A few phase II metabolites were detected in the urine, including BAC glucuronide and AC sulfate conjugates. Cytochrome P450 enzymes (CYP450s), carboxylesterases (CEs), and enzymes produced by intestinal bacteria are involved in gastrointestinal and hepatic metabolism of aconitine (AC).
In conclusion, CYP450s, CEs, and enzymes produced by intestinal bacteria are mainly involved in DDA metabolism in both the gastrointestinal tract and liver after oral administration, including hydroxylation, deoxylation, demethylation, dehydrogen, pyrolysis, ester hydrolysis, and ester exchange. Phase II conjugation of DDAs is not the dominant metabolic process and only a few conjugated DDAs are found in the urine. DDA metabolites in the blood are not as various as those in the urine.
Thus far, reports of less toxic MDA metabolism have only been related to hepatic metabolism. Nevertheless, MDAs may share similar metabolic pathways (except ester hydrolysis at the C-8 position) with DDAs in the gastrointestinal tract based on the same DDA and MDA diterpenoid skeletons and similar hepatic metabolism between DDAs and MDAs.
As summarized above, toxic DDAs and MDAs are converted to metabolites that are less toxic or easier to excrete in the gastrointestinal tract and liver after oral administration. However, for drug excretion, few phase II metabolism conjugations are formed, which are the most hydrosoluble metabolites. Further, this detoxification effect is likely restricted due to rapid DDA absorption by the upper gastrointestinal tract.
Although the many available studies on metabolism and toxicity of DDAs and MDAs are helpful, they are insufficient for safe clinical administration of Aconitum herbs. Several issues must be further studied and verified. More attention should be paid to metabolism of MDAs because they are not sufficiently safe for clinical use. Due to metabolic interspecific differences, it is more reasonable to apply human recombinant metabolic isozymes or humanized animal models [67] to a human metabolism study. Studies have not confirmed whether the various metabolites detected in the urine are from gastrointestinal and hepatic metabolism via absorption into the blood or from biotransformation in the kidney. Because the metabolites are detected at trace levels, it is difficult to accumulate such metabolites for identification, bioassays, or toxicity studies. However, the changes in bioactivity or toxicity after metabolism are unambiguous.
Based on our conclusions, it is worthwhile to perform an in-depth investigation of the Aconitum herbs compatible with other medicines, such as prescription licorice, which is featured in and crucial to clinical application of Aconitum herbs in traditional Chinese medicine. To a certain extent, drug-drug interactions are the essence of a drug-drug combination, in which drug metabolism and/or absorption is changed by affecting (inducing or inhibiting) another with respect to metabolic enzymes or/and transporters; thus, drug pharmacological activity or toxicity is consequently affected [12, 13, 67].
Acknowledgment
This work was mainly supported by the National Natural Science Foundation of China (no. 81274062).
Abbreviations
- AC:
Aconitine
- BAC:
14-Benzoylaconine or 8-O-deacetyl aconitine
- BHA:
14-Benzoylhypaconine or 8-O-deacetyl hypaconitine
- BMA:
14-Benzoylmesaconine or 8-O-deacetyl mesaconitine
- CEs:
Carboxylesterases
- CYP450s:
Cytochrome P450 enzymes
- DDAs:
Diester diterpenoid alkaloids
- FT-ICR:
Fourier transform ion cyclotron resonance
- HA:
Hypaconitine
- HLM:
Human liver microsomes
- IM:
Ion mobility
- IT:
Ion trap
- LD50:
Half-maximally lethal dose
- MA:
Mesaconitine
- MDAs:
Monoester diterpenoid alkaloids
- MRP:
Multidrug resistance-associated protein
- MS:
Mass spectrometry
- NA:
Not available
- P-gp:
P-glycoprotein
- QQQ:
Triple quadrupole
- Q-trap:
Quadrupole trap
- Q-TOF:
Quadrupole time of flight
- UGTs:
Uridine 5-diphosphate- (UDP-) glucuronosyltransferases.
Conflict of Interests
There is no financial conflict of interests with the authors of this review.
References
- 1.Chan T. Y. K. Aconite poisoning presenting as hypotension and bradycardia. Human and Experimental Toxicology. 2009;28(12):795–797. doi: 10.1177/0960327109353056. [DOI] [PubMed] [Google Scholar]
- 2.Singhuber J., Zhu M., Prinz S., Kopp B. Aconitum in traditional Chinese medicine—a valuable drug or an unpredictable risk? Journal of Ethnopharmacology. 2009;126(1):18–30. doi: 10.1016/j.jep.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Chan T. Y. K. Aconite poisoning. Clinical Toxicology. 2009;47(4):279–285. doi: 10.1080/15563650902904407. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y.-G., Wang S.-Q., Liu Y.-X., Yan L.-P., Dou G.-F., Gao Y. Characterization of metabolites and cytochrome P450 isoforms involved in the microsomal metabolism of aconitine. Journal of Chromatography B. 2006;844(2):292–300. doi: 10.1016/j.jchromb.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 5.Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia, Part I. Beijing, China: China Medical Science Press; 2010. Aconiti Radix; p. p. 36. [Google Scholar]
- 6.Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia Part I. Beijing, China: China Medical Science Press; 2010. Aconiti Kusnezoffii Radix Cocta; p. p. 220. [Google Scholar]
- 7.Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia. 2010. part 1. Beijing, China: China Medical Science Press; 2010. Aconiti lateralis radix praeparata; p. p. 177. [Google Scholar]
- 8.Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia, Part I. Beijing, China: China Medical Science Press; 2010. Aconiti Radix Cocta; p. p. 37. [Google Scholar]
- 9.Huang Q.-A., Zhang Y.-M., He Y., Lu J., Lin R.-C. Studies on hydrolysis of aconitine. China Journal of Chinese Materia Medica. 2007;32(20):2143–2145. [PubMed] [Google Scholar]
- 10.Zheng Q., Lu H.-W., Hao W.-W., Liu J.-Y., Wang S.-J., Yang M. Study on hydrolysis of Aconitum alkaloids and quantitative analysis method of their hydrolysates. Chinese Pharmaceutical Journal. 2011;46(9):652–656. [Google Scholar]
- 11.Zhou Y.-P., Liu W.-H., Zeng G.-Y., Chen D.-H., Li H.-Y., Song W.-L. The toxicity of aconitine and its analogs and their effects on cardiac contractile function. Acta Pharmaceutica Sinica. 1984;19(9):641–646. [PubMed] [Google Scholar]
- 12.Rahimi R., Abdollahi M. An update on the ability of St. John's wort to affect the metabolism of other drugs. Expert Opinion on Drug Metabolism & Toxicology. 2012;8(6):691–708. doi: 10.1517/17425255.2012.680886. [DOI] [PubMed] [Google Scholar]
- 13.König J., Müller F., Fromm M. F. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacological Reviews. 2013;65(3):944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- 14.Sui Z.-G., Li N., Liu Z.-Q., Yan J., Liu Z.-Y. Metabolite profile analysis of aconitine in rabbit stomach after oral administration by liquid chromatography/electrospray ionization/multiple-stage tandem mass spectrometry. Xenobiotica. 2013;43(7):628–635. doi: 10.3109/00498254.2012.753490. [DOI] [PubMed] [Google Scholar]
- 15.Enayetallah A. E., French R. A., Thibodeau M. S., Grant D. F. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. Journal of Histochemistry and Cytochemistry. 2004;52(4):447–454. doi: 10.1177/002215540405200403. [DOI] [PubMed] [Google Scholar]
- 16.DiBaise J. K., Zhang H., Crowell M. D., Krajmalnik-Brown R., Decker G. A., Rittmann B. E. Gut microbiota and its possible relationship with obesity. Mayo Clinic Proceedings. 2008;83(4):460–469. doi: 10.4065/83.4.460. [DOI] [PubMed] [Google Scholar]
- 17.Fukuhara A., Imai T., Otagiri M. Stereoselective disposition of flurbinprofen from a mutual prodrug with a histamine H-2-antagonist to reduce gasrointestinal lesions in the rat. Chirality. 1996;8:494–502. doi: 10.1002/(SICI)1520-636X(1996)8:7<494::AID-CHIR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Satoh T., Hosokawa M. The mammalian carboxylesterases: from molecules to functions. Annual Review of Pharmacology and Toxicology. 1998;38:257–288. doi: 10.1146/annurev.pharmtox.38.1.257. [DOI] [PubMed] [Google Scholar]
- 19.Usui K., Hayashizaki Y., Hashiyada M., Nakano A., Funayama M. Simultaneous determination of 11 aconitum alkaloids in human serum and urine using liquid chromatography-tandem mass spectrometry. Legal Medicine. 2012;14(3):126–133. doi: 10.1016/j.legalmed.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Ley R. E., Peterson D. A., Gordon J. I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Sousa T., Paterson R., Moore V., Carlsson A., Abrahamsson B., Basit A.-W. The gastrointestinal microbiota as a site for the biotransformation of drugs. International Journal of Pharmaceutics. 2008;363(1-2):1–25. doi: 10.1016/j.ijpharm.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Wang X.-Y., Pi Z.-F., Liu W.-L., Zhao Y.-F., Liu S.-Y. Effect of pH on the metabolism of aconitine under rat intestinal bacteria and analysis of metabolites using HPLC/MS-MSn technique. Chinese Journal of Chemistry. 2010;28(12):2494–2500. doi: 10.1002/cjoc.201190028. [DOI] [Google Scholar]
- 23.Sui Z.-G., Jiang Y.-Q., Liu Z.-Q., Liang F., Yan J., Liu Z.-Y. Study on metabolites of aconitine in rabbit intestines by using LC/ESI-MSn. Acta Chimica Sinica. 2009;67(21):2439–2444. [Google Scholar]
- 24.Zhao Y.-F., Song F.-R., Guo X.-H., Liu S.-Y. Studies on the biotransformation of aconitine in human intestinal bacteria using soft-ionization mass spectrometry. Chemical Journal of Chinese Universities. 2008;29(1):55–59. [Google Scholar]
- 25.Wang X.-Y., Pi Z.-F., Liu W.-L., Song F.-R., Liu Z.-Q., Liu S.-Y. Biotransformation of aconitum alkaloids before and after the combination of Radix Aconiti Preparata by rat intestinal flora using semiquantitative analysis method of electrospray ionization mass spectrometry. Chemical Journal of Chinese Universities. 2011;32(7):1526–1531. [Google Scholar]
- 26.Wang X.-Y., Pi Z.-F., Song F.-R., Liu Z.-Q., Liu S.-Y. Studies on the biotransformation of Licorice and Aconite accessory root decoction and Atractylodes Macrocephala and Aconite accessory root decoction under rat intestinal bacteria. Acta Chimica Sinica. 2011;69(11):1368–1374. [Google Scholar]
- 27.Xin Y., Pi Z.-F., Song F.-R., Liu Z.-Q., Liu S.-Y. Study on the metabolic characteristics of aconite alkaloids in the extract of Radix aconiti under intestinal bacteria of rat by UPLC/MSn technique. Chinese Journal of Chemistry. 2012;30(3):656–664. doi: 10.1002/cjoc.201100228. [DOI] [Google Scholar]
- 28.Zhao Y.-F., Song F.-R., Wang X.-Y., Guo X.-H., Liu Z.-Q., Liu S.-Y. Studies on the biotransformation of 16-O-demethylaconitine and electrospray ionization tandem mass spectrometry. Acta Chimica Sinica. 2008;66(5):525–530. [Google Scholar]
- 29.Zhao Y.-F., Song F.-R., Yue H., et al. Biotransformation of deoxyaconitine of metabolite of aconitine by human intestinal bacteria and electrospray ionization tandem mass spectrometry. Chemical Journal of Chinese Universities. 2007;11:2051–2055. [Google Scholar]
- 30.Zhao Y.-F., Song F.-R., Yue H., et al. Studies on the biotransformation of 16-O-edmethyldeoxyaconitine of the metabolite of aconitine in human intestinal bacteria. Chinese Journal of Analytical Chemistry. 2007;35(12):1711–1715. [Google Scholar]
- 31.Liu W.-L., Liu Z.-Q., Song F.-R., Liu S.-Y. Specific conversion of diester-diterpenoid aconitum alkaloids components into hydrolysis monoester-diterpenoid alkaloids components and lipo-alkaloids components. Chemical Journal of Chinese Universities. 2011;32(3):717–720. [Google Scholar]
- 32.Qi M.-F. Another explanation of processing mechanism of Aconiti radix and Aconiti lateralis radix praeparata. Journal of Chinese Medicinal Materials. 1986;(6):37–38. [Google Scholar]
- 33.Bock K. W. Functions and transcriptional regulation of adult human hepatic UDP-glucuronosyl-transferases (UGTs): mechanisms responsible for interindividual variation of UGT levels. Biochemical Pharmacology. 2010;80(6):771–777. doi: 10.1016/j.bcp.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 34.Zhu L., Ge G., Liu Y., et al. Characterization of UDP-glucuronosyltransferases involved in glucuronidation of diethylstilbestrol in human liver and intestine. Chemical Research in Toxicology. 2012;25(12):2663–2669. doi: 10.1021/tx300310k. [DOI] [PubMed] [Google Scholar]
- 35.Tang L., Ye L., Lv C., Zheng Z.-J., Gong Y., Liu Z.-Q. Involvement of CYP3A4/5 and CYP2D6 in the metabolism of aconitine using human liver microsomes and recombinant CYP450 enzymes. Toxicology Letters. 2011;202(1):47–54. doi: 10.1016/j.toxlet.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Chen X.-G., Lai Y.-Q., Cai Z.-W. Simultaneous analysis of aconitine and its metabolites by liquid chromatography-electrospray ion trap mass spectrometry. Journal of Chinese Mass Spectrometry Society. 2012;33:65–73. doi: 10.1093/jat/bks004. [DOI] [PubMed] [Google Scholar]
- 37.Xie L.-X., Lv C., Ye L., Tang L. Study on metabolism of aconitine in liver microsomes of guinea pig and mice. China Pharmacy. 2012;23:590–593. [Google Scholar]
- 38.Bi Y.-F., Liu S., Li X., Liu Z.-Q., Song F.-R. Metabolic fingerprint and effects of aconite alkaloid components on the activities of CYP450 isozymes in rat liver microsomes. Chemical Journal of Chinese Universities. 2013;34(9):2084–2089. doi: 10.7503/cjcu20130492. [DOI] [Google Scholar]
- 39.Ye L., Gao S., Feng Q., et al. Development and validation of a highly sensitive UPLC-MS/MS method for simultaneous determination of aconitine, mesaconitine, hypaconitine, and five of their metabolites in rat blood and its application to a pharmacokinetics study of aconitine, mesaconitine, and hypaconitine. Xenobiotica. 2012;42(6):518–525. doi: 10.3109/00498254.2011.641608. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y.-G., Sun M.-Q., Zhang H.-G. Studies on the metabolic pathway of aconitine in rabbit and human using electrospray ionization-mass spectrometry. Journal of Liquid Chromatography and Related Technologies. 2013;36(12):1686–1696. doi: 10.1080/10826076.2012.695315. [DOI] [Google Scholar]
- 41.Ye L., Tang L., Gong Y., et al. Characterization of metabolites and human P450 isoforms involved in the microsomal metabolism of mesaconitine. Xenobiotica. 2011;41(1):46–58. doi: 10.3109/00498254.2010.524950. [DOI] [PubMed] [Google Scholar]
- 42.Bi Y.-F., Liu S., Zhang R.-X., Song F.-R., Liu Z.-Q. Metabolites and metabolic pathways of mesaconitine in rat liver microsomal investigated by using UPLC-MS/MS method in vitro. Yaoxue Xuebao. 2013;48(12):1823–1828. [PubMed] [Google Scholar]
- 43.Ye L., Wang T., Yang C.-H., et al. Microsomal cytochrome P450-mediated metabolism of hypaconitine, an active and highly toxic constituent derived from Aconitum species. Toxicology Letters. 2011;204(1):81–91. doi: 10.1016/j.toxlet.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 44.Bi Y.-F., Li X., Pi Z.-F., Song F.-R., Liu Z.-Q. Analysis of hypaconitine's metabolites and related metabolic CYP isoforms in rat liver microsomal by UPLC-MS/MS. Journal of Chinese Mass Spectrometry Society. 2013;34(6):330–337. doi: 10.7538/zpxb.2013.34.06.0330. [DOI] [Google Scholar]
- 45.Ye L., Yang X.-S., Lu L.-L., et al. Monoester-diterpene Aconitum alkaloid metabolism in human liver microsomes: predominant role of CYP3A4 and CYP3A5. Evidence-Based Complementary and Alternative Medicine. 2013;2013:24. doi: 10.1155/2013/941093.941093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., Yu Y.-N. Advance in research of drug phase II metabolisms and conjugatio enzymes. Chinese Journal of Clinical Pharmacology. 2000;16:458–465. [Google Scholar]
- 47.Silva M. J., Barr D. B., Reidy J. A., et al. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Archives of Toxicology. 2003;77:561–567. doi: 10.1007/s00204-003-0486-3. [DOI] [PubMed] [Google Scholar]
- 48.Liang F., Sui Z.-G., Yan J., Liu Z.-Y. Comparison of metabolites of aconitine in rabbit urine under different routes of administration. Journal of Jilin University. 2010;36(3):443–445. [Google Scholar]
- 49.Galetin A., Gertz M., Houston J.-B. Potential role of intestinal first-pass metabolism in the prediction of drug-drug interactions. Expert Opinion on Drug Metabolism and Toxicology. 2008;4(7):909–922. doi: 10.1517/17425255.4.7.909. [DOI] [PubMed] [Google Scholar]
- 50.Komura H., Iwaki M. In vitro and in vivo small intestinal metabolism of CYP3A and UGT substrates in preclinical animals species and humans: species differences. Drug Metabolism Reviews. 2011;43(4):476–498. doi: 10.3109/03602532.2011.597401. [DOI] [PubMed] [Google Scholar]
- 51.Wada K., Nihira M., Hayakawa H., Tomita Y., Hayashida M., Ohno Y. Effects of long-term administrations of aconitine on electrocardiogram and tissue concentrations of aconitine and its metabolites in mice. Forensic Science International. 2005;148(1):21–29. doi: 10.1016/j.forsciint.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H.-G., Shi X.-G., Sun Y., Zhong D.-F., Zhang H.-Q. Study on the metabolites of aconitine in rabbit blood. Journal of Jilin University (Science edition) 2006;44:284–286. [Google Scholar]
- 53.Zamek-Gliszczynski M. J., Chu X.-Y., Polli J. W., Paine M. F., Galetin A. Understanding the transport properties of metabolites: case studies and considerations for drug development. Drug Metabolism and Disposition. 2014;42:650–664. doi: 10.1124/dmd.113.055558. [DOI] [PubMed] [Google Scholar]
- 54.Ye X.-L., He X.-W., Song Q.-Q., et al. Analysis on the metabolites of aconitine in Sini decoction (SND) in the rat urine by liquid chromatography and electrospray ionization mass spectrometry. Proceedings of the Annual Conference Symposium of Innovation and Development Forum on Chinese Medicine Preparations; 2011; Kunming, China. pp. 556–562. [Google Scholar]
- 55.Wang C.-H., Wen J., Chen Y.-H., He Y. Study on determination of metabolites of aconitine in rat urine by HPLC/MS. Chinese Journal of Forensic Medicine. 2006;21(2):88–90. [Google Scholar]
- 56.Sun Y., Zhang H.-G., Shi X.-G., Duan M.-Y., Zhong D.-F. Study on metabolites on aconitine in rabbit urine. Acta pharmaceutica Sinica. 2002;37(10):781–783. [PubMed] [Google Scholar]
- 57.Sun Y., Zhang Q.-S., Dong L.-D., Chen Y.-J. Metabolites of major alkaloids of Aconitum Chinese herbal medicine in different gender rabbit urine. Journal of Jilin University (Science edition) 2007;45:1032–1034. [Google Scholar]
- 58.Ai L., Sun Y., Zhang H.-G. Metabolites of aconitum alkaloids from compound formula of Chinese medicine in human body. Journal of Beijing University of Traditional Chinese Medicine. 2007;30:417–422. [Google Scholar]
- 59.Zhang H.-G., Shi X.-G., Sun Y., Duan M.-Y., Zhong D.-F. New metabolites of aconitine in rabbit urine. Chinese Chemical Letters. 2002;13(8):758–760. [Google Scholar]
- 60.Zhang H.-G., Sun Y., Duan M.-Y., Chen Y.-J., Zhong D.-F., Zhang H.-Q. Separation and identification of Aconitum alkaloids and their metabolites in human urine. Toxicon. 2005;46(5):500–506. doi: 10.1016/j.toxicon.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 61.Chen P.-P., Zhao N., Xu X.-L., Ruan Y.-P., Wei Y.-H., Li F.-Z. Analysis on the metabolites of mesaconitine in the rat urine by liquid chromatography and electrospray ionization mass spectrometry. Acta Pharmaceutica Sinica. 2010;45(8):1043–1047. [PubMed] [Google Scholar]
- 62.Tan G.-G., Lou Z.-Y., Jing J., et al. Screening and analysis of aconitum alkaloids and their metabolites in rat urine after oral administration of aconite roots extract using LC-TOFMS-based metabolomics. Biomedical Chromatography. 2011;25(12):1343–1351. doi: 10.1002/bmc.1607. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura A., Nakajima M., Yamanaka H., Fujiwara R., Yokoi T. Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metabolism and Disposition. 2008;36(8):1461–1464. doi: 10.1124/dmd.108.021428. [DOI] [PubMed] [Google Scholar]
- 64.Shelby M. K., Cherrington N. J., Vansell N. R., Klaassen C. D. Tissue mRNA expression of the rat UDP-glucuronosyltransferase gene family. Drug Metabolism and Disposition. 2003;31(3):326–333. doi: 10.1124/dmd.31.3.326. [DOI] [PubMed] [Google Scholar]
- 65.Nishimura M., Yaguti H., Yoshitsugu H., Naito S., Satoh T. Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi. 2003;123(5):369–375. doi: 10.1248/yakushi.123.369. [DOI] [PubMed] [Google Scholar]
- 66.Yue H., Pi Z.-F., Li H.-L., Song F.-R., Liu Z.-Q., Liu S.-Y. Studies on the stability of diester-diterpenoid alkaloids from the genus Aconitum L.-by high performance liquid chromatography combined with electrospray ionisation tandem mass spectrometry (HPLC/ESI/MSn) Phytochemical Analysis. 2008;19(2):141–147. doi: 10.1002/pca.1027. [DOI] [PubMed] [Google Scholar]
- 67.Scheer N., Wolf C. R. Genetically humanized mouse models of drug metabolizing enzymes and transporters and their applications. Xenobiotica. 2014;44(2):96–108. doi: 10.3109/00498254.2013.815831. [DOI] [PubMed] [Google Scholar]