Abstract
A new study reveals that the majority of macrophages in established atherosclerotic lesions are derived from local proliferation, rather than from the influx of blood-borne monocytes. While the factors driving proliferation remain to be understood, the findings suggest that targeting macrophage proliferation could represent a new therapeutic opportunity in established atherosclerosis.
In recent years macrophages have garnered the lion's share of attention as the key cell populating and inciting atherosclerotic lesions. The prevalent view has been that blood monocytes are attracted to susceptible regions of arterial endothelium, migrate into the subendothelial space, take up modified lipoproteins and become macrophage foam cells; in this model one monocyte gives rise to one lesional macrophage. A few older studies provided hints that macrophages could proliferate in lesions 1-5; however, for the most part macrophage proliferation has not been considered to make an important contribution to macrophage burden in atherosclerotic lesions. A recent study from Swirski's group has provided compelling evidence that local proliferation is a major source of macrophages present in advanced atherosclerotic lesions in Apoe-/- and likely Ldlr-/- mice. While the significance of these observations for human lesions is still uncertain, these novel findings are potentially paradigm changing and have important implications for understanding the mechanisms of atherosclerotic lesion development and complications6.
In a tour de force of clever experimentation and innovative use of technology Robbins et al. establish the role of macrophage proliferation in advanced lesions by multiple approaches. They first show >90% BrdU labeling of macrophages in 4 month old Apoe-/- mice fed a western type diet (WTD) WTD for 4-8 weeks, indicating a high turnover rate of macrophages in established lesions. Earlier studies had shown incorporation of BrdU into macrophages in lesions6, but did not distinguish the point at which BrdU was incorporated as this could have occurred in bone marrow progenitors or in monocytes. Surprisingly, Robbins et al. found that monocyte depletion by clodronate liposome treatment had no effect either on the macrophage content of advanced lesions or on BrdU incorporation into macrophages6. Another interesting observation was that while the atherosclerotic lesion continued to grow, the area of the lesion made up of macrophages remained the same, suggesting that a lot of the macrophages must have died or perhaps migrated out of the lesion.
The most compelling evidence supporting a major role of macrophage proliferation in established lesions came from experiments where pairs of WTD fed Apoe-/- mice were joined by parabiosis; in this coupling one mouse was CD45.1+, while its partner was CD45.2+. Analysis of the CD45.1+ parabiont revealed the expected high level of chimerism (i.e. mixing of CD45.2+ with CD45.1+ cells) for blood, spleen and aortic monocytes, but amazingly, less than 5% chimerism of aortic macrophages6. This approach was then combined with BrdU labeling and flow cytometry to show that 87% of lesional macrophages that had incorporated BrdU were derived from the CD45.1 (host) parabiont, indicating that local proliferation of macrophages was largely responsible for accumulating macrophages in the advanced lesions. A comparable experiment in younger parabiotic mice fed a WTD for 4 weeks showed that only 30% of macrophages in earlier lesions were derived from local proliferation, while the rest were derived by monocyte recruitment. Corroborating evidence for these conclusions was obtained by showing that CD45.1+ macrophages were proliferating locally in advanced lesions based on Ki67 antigen positivity and flow cytometry combined with single cell fluorescence imaging to show that there were increased numbers of G2M phase macrophages and increased cells undergoing mitosis. Studies in advanced human atherosclerotic lesions have shown only a very low % of PCNA positive proliferating macrophages (<1 % of cells)1. However, this represents a snapshot and does not provide any definite information on the quantitative importance of macrophage proliferation in human lesions.
Somewhat surprisingly, an alternative approach of bone marrow transplantation of CD45.1+ Apoe-/- bone marrow into CD45.2+ Apoe-/- mice with established lesions, showed that after 3 or 5 months of further diet feeding 74% or 97% respectively of lesional cells were donor derived. These results contrast with the shorter term parabiosis experiments and suggest that macrophage proliferation does not continue indefinitely and that the macrophage pool of established lesions is ultimately derived from a circulating precursor. Moreover, in Apoe-/- mice net monocyte recruitment continues to increase as lesions progress throughout the aorta up to 50 weeks of age, underlining the ongoing importance of monocyte recruitment in lesion formation and progression7. Randolph8 has attempted to reconcile these seemingly paradoxical results by suggesting that most monocyte-derived macrophages may undergo rapid cell death, while a small pool continue to proliferate and become the dominant population in established lesions. However, this proliferating pool also appears to die after a month or two. Overall, the various studies suggest important roles for monocyte recruitment, local macrophage proliferation and macrophage death in established lesions6, 9, 10 (Figure 1).
Figure 1.
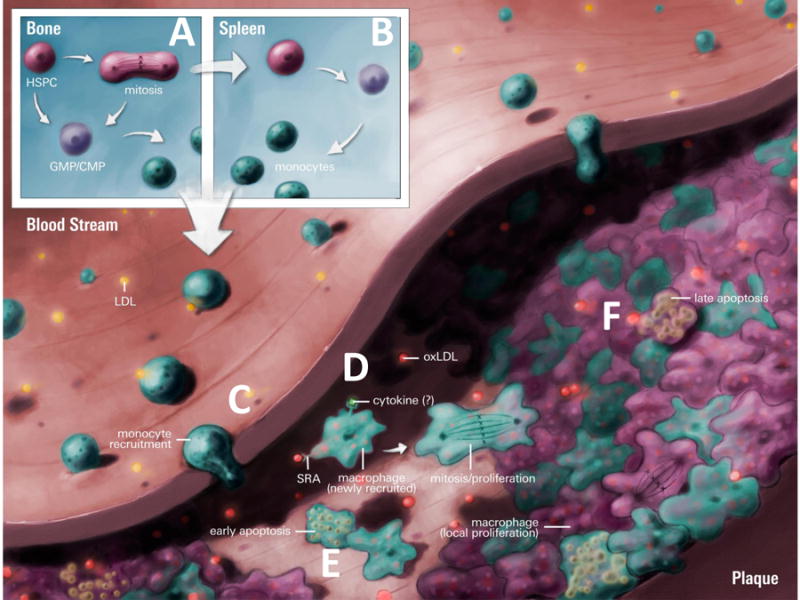
Monocyte production, recruitment and local macrophage proliferation in atherosclerosis.
A) Defective cholesterol homeostasis results in BM hematopoietic stem and multipotential progenitor cells (HSPCs), common myeloid, and granulocyte-macrophage progenitors (CMPs & GMPs) expansion and enhanced monocyte production. B) BM HSPCs also mobilize to the spleen, initiating extramedullary hematopoiesis and further increasing monocyte production. C) In early atherosclerosis, BM and splenic derived monocytes are recruited to the plaque and differentiate into macrophages. D) As the lesion develops macrophages via SR-A sense modified LDL (e.g.oxLDL) and this, along with local cytokines, induces proliferation and sustains the lesional macrophage population. E) While there is still recruitment of monocytes to the lesion some of these cells undergo early apoptosis. F) Macrophages may also undergo late apoptosis or senescence after an unknown number of divisions. Artwork by Dr. Derek Ng.
These studies add to a growing body of literature indicating that macrophages can proliferate locally in tissues; for example following IL-4 treatment or helminth exposure very high numbers of M2 macrophages in tissues appear to be derived by local proliferation11. The underlying mechanisms responsible for macrophage proliferation in advanced lesions are not well understood. Robbins et al ruled out a role for GM-CSF6, a cytokine that would have been a leading candidate as a proliferative stimulus, based on previous studies showing it to be responsible for dendritic cell proliferation in the hypercholesterolemic aortic intima12. They also showed using a parabiosis approach that a WT mouse joined to a WTD fed Apoe-/- mice did not experience local macrophage proliferation in aorta of the WT mouse, suggesting a role of the microenvironment of the lesion. However, in this experiment ApoE expression in the WT mouse could have lowered plasma cholesterol13-15, so an alternative interpretation would be that ongoing hypercholesterolemia was required as a stimulant to macrophage proliferation. This is also plausible in view of mounting evidence that cellular cholesterol accumulation enhances hematopoietic cell proliferation in response to a variety of growth factors16-18. A mixed chimera model employing 45.2+Msr1-/- and CD45.1+ bone marrow cells transplanted into irradiated Ldlr-/- mice, showed a role of the scavenger receptor A in lesional macrophage proliferation. Earlier work showed a key role of the scavenger receptor A in macrophage proliferation stimulated by oxidized LDL19. Thus the continued entry of atherogenic lipoproteins into lesions with uptake of modified particles via SRA may provide an ongoing stimulus for macrophage proliferation, perhaps involving growth factors such as M-CSF (Figure 1).
These studies further illustrate the complex life cycle of monocyte/macrophages and their progenitors in mouse models of atherosclerosis. Recent work has demonstrated that hypercholesterolemia and defective cholesterol efflux pathways result in excessive proliferation of hematopoietic stem and progenitor cells in the bone marrow, hematopoietic stem cell mobilization to the spleen, myeloid progenitor and monocyte proliferation in the spleen, all of which fuel monocytosis and increased entry of inflammatory monocytes into atherosclerotic lesions17, 18, 20, 21. Macrophage proliferation may be viewed as the final amplification step in this overall process of multi-stage inflammatory cell expansion6, 20. Robbins et al., show there may be new therapeutic opportunities aimed specifically at reducing macrophage proliferation in advanced plaques, as shown for 5-FU or potentially other cell cycle regulators. Improvements in cholesterol homeostasis for example via LDL lowering, LXR activation or rHDL infusion could also be anti-proliferative18. One potential downside to directly targeting lesional macrophage proliferation may be the yin and yang between cell proliferation and cell death, with potential adverse effects on plaque stability. Overall, this elegant study provides a surprising new view of the multifaceted lesional macrophage and suggests potential novel approaches for the treatment of established atherosclerosis.
Acknowledgments
We thank Dr. Derek Ng, University of Toronto for the figure. AJM was supported by a grant from the Viertel Foundation, managed by ANZ Trustees and administered by the Diabetes Australia Research Trust. AT is supported in part by NIH grant HL107653.
References
- 1.Gordon D, Reidy MA, Benditt EP, Schwartz SM. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990;87:4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamharzi N, Renard CB, Kramer F, Pennathur S, Heinecke JW, Chait A, Bornfeldt KE. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: Potential role of glucose-oxidized ldl. Diabetes. 2004;53:3217–3225. doi: 10.2337/diabetes.53.12.3217. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld ME, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of whhl and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990;10:680–687. doi: 10.1161/01.atv.10.5.680. [DOI] [PubMed] [Google Scholar]
- 4.Lessner SM, Prado HL, Waller EK, Galis ZS. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol. 2002;160:2145–2155. doi: 10.1016/S0002-9440(10)61163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diez-Juan A, Perez P, Aracil M, Sancho D, Bernad A, Sanchez-Madrid F, Andres V. Selective inactivation of p27(kip1) in hematopoietic progenitor cells increases neointimal macrophage proliferation and accelerates atherosclerosis. Blood. 2004;103:158–161. doi: 10.1182/blood-2003-07-2319. [DOI] [PubMed] [Google Scholar]
- 6.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randolph GJ. Proliferating macrophages prevail in atherosclerosis. Nat Med. 2013;19:1094–1095. doi: 10.1038/nm.3316. [DOI] [PubMed] [Google Scholar]
- 9.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ ccr2, ccr5, and cx3cr1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of th2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. Gm-csf regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein e-deficient mice by bone marrow transplantation. Science. 1995;267:1034–1037. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- 14.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of apoe-/- mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gough PJ, Raines EW. Gene therapy of apolipoprotein e-deficient mice using a novel macrophage-specific retroviral vector. Blood. 2003;101:485–491. doi: 10.1182/blood-2002-07-2131. [DOI] [PubMed] [Google Scholar]
- 16.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. Lxr signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. Apoe regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai M, Miyazaki A, Hakamata H, Kodama T, Suzuki H, Kobori S, Shichiri M, Horiuchi S. The scavenger receptor serves as a route for internalization of lysophosphatidylcholine in oxidized low density lipoprotein-induced macrophage proliferation. J Biol Chem. 1996;271:27346–27352. doi: 10.1074/jbc.271.44.27346. [DOI] [PubMed] [Google Scholar]
- 20.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates ly-6c(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


