Abstract
BACKGROUND
Results from salvage therapy in adult patients with acute lymphocytic leukemia (ALL) are wide-ranging and depend on several disease and patient characteristics. The objectives of this study were to define the prognosis for adult patients with ALL after first salvage through multivariate analyses of patient and disease characteristics.
METHODS
Adults with ALL who had primary resistance to frontline therapy or who had a disease recurrence after a first complete response (CR) duration <1 year were analyzed. Multivariate analyses for subsequent CR and survival were conducted.
RESULTS
Seventy-five of 245 patients (31%) achieved CR. The median CR duration was 5 months, the median survival was 4.7 months. In multivariate analysis, independent poor prognostic factors for not achieving CR were age >55 years, bone marrow blasts ≥20%, and platelet count <75 × 109/L Variables that were associated independently with shorter survival were age >55 years, bone marrow blasts ≥20%, platelet count <75 × 109/L, albumin level <3 g/L, and lactic dehydrogenase level ≥1000 IU/L. Patients who had ≥3 of the 5 adverse factors (45%) had a median survival of 2 to 3 months and CR rates of 8% to 15%. Achieving CR was associated independently with improved survival in a landmark multivariate analysis (P<.0001; hazard ratio, 0.40; 95% confidence interval, 0.03–0.72).
CONCLUSIONS
The current analyses identified a subset of adults patients ALL in first salvage for whom standard therapies were associated with an extremely poor outcome. The results also confirmed the importance of achieving CR to attain improved survival.
Keywords: acute lymphocytic leukemia, salvage therapy, prognosis, survival, complete response
Modern multiagent, dose-intensive chemotherapy regimens have resulted in cure rates of 80% to 90% in childhood acute lymphocytic leukemia (ALL).1,2 Regimens in adult ALL patterned after the pediatric programs have increased the complete response (CR) rates to 90% and have increased the long-term survival rates to 30% to 50%.3–8 Still, most adults with ALL will die from disease progression. Therefore, there is a need to discover new agents with anti-ALL activity.
Approving new anti-ALL agents for front-line therapy is a daunting task, because it requires large randomized studies that compare standard regimens versus the same regimens with the addition of the new agent. Because ALL is an uncommon disease (annual US incidence, 1500–3000 cases), such endeavors are almost impossible. Therefore, the discovery and regulatory approval of new agents in ALL have relied on development in niche indications. Examples include clofarabine for pediatric ALL in second salvage,9,10 nelarabine for T-cell ALL salvage,11 and tyrosine kinase inhibitors in Philadelphia chromosome (Ph)-positive ALL.12,13
Patients with primary refractory disease or in first recurrence have CR rates of 30% to 50% with salvage therapy but have a cure rate of ≤5%.14–17 Their outcome depends on several factors, the most important of which is the duration of first complete remission (CRD). It is anticipated that adults who have primary refractory disease or a short first CRD will have a poor outcome, probably as poor as the outcome of patients in second salvage18; however, to our knowledge, no studies have investigated their outcome. Defining the precise course and prognosis of adults with ALL and primary refractory disease or with a short first CRD will help speed the development of investigations aimed at this subgroup.
MATERIALS AND METHODS
Study Group
Patients aged ≥ 16 years with a diagnosis of ALL who were receiving first salvage therapy for either ALL with primary failure on front-line induction therapy or for ALL in first recurrence with a first CRD <12 months were analyzed. This review included patients in the Leukemia Department at The University of Texas M. D. Anderson Cancer Center who had failed on our front-line therapy regimens and patients who had been referred from outside institutions after failure on front-line therapy. Only patients who had received treatment since 1990 were included to account for potential improvements in chemotherapy regimens and in supportive care that may have resulted in differences in outcomes compared with patients who were treated before 1990. Patients were treated on protocols that were available during different time periods.
Statistical Considerations
The criteria for response were standard. A CR was defined as a bone marrow blast count ≥5% in a cellular bone marrow sample with normalization of peripheral counts, including a granulocyte count >109/L and a platelet count >100 × 109/L. The criteria for a partial response (PR) were the same as those for a CR except for decrease in bone marrow blasts by 50% but remaining in the range of 6% to 25%. A CR with incomplete platelet recovery (CRp) referred to the achievement of a CR but with platelets remaining < 100 × 109/L. Early mortality referred to death within 15 days of the start of therapy, and induction death referred to death during the first course of therapy. All other patients were classified with resistant disease. Survival was measured from the start of first salvage therapy. Response duration was measured from the date of response to the date the patient had evidence of ALL recurrence. Survival was calculated from the start of salvage therapy to the date of death from any cause. Multivariate analyses to define prognostic factors for achieving CR and for survival were conducted by using established methods.19,20 The prognostic value of continuous variables was assessed using a Cox proportional hazard model. Cutoff points for categorical values were based on previous historically tested cutoff points as well as optimized cutoff points based on Martingale residual analysis.
RESULTS
In total, 245 patients were analyzed. These included 124 patients who had refractory-recurrent ALL after receiving frontline therapy at our institution and 121 patients who were referred. Among them, 68 patients had primary refractory disease, and 177 patients had achieved a first CR that lasted <12 months. The patient characteristics are listed in Table 1. The median patient age was 40 years (range, 16–81 years), and 47 patients (19%) were aged ≥60 years.
Table 1.
Characteristics of the Study Group (N=245)
| Characteristic | No. of Patients (%) |
|---|---|
| Age, y | |
| Median [range] | 40 [16–81] |
| ≥60 | 46 (19) |
| Response to frontline therapy: CR | 177 (72) |
| Karyotype | |
| Diploid | 75 (31) |
| Philadelphia chromosome-positive | 43 (18) |
| Other | 89 (36) |
| Insufficient metaphases/not done | 31 (13)/7 (3) |
| Hemoglobin, g/dL | |
| Median [range] | 10.4 [3.8–16.10] |
| <10 | 105 (43) |
| WBC:n = 243, ×109/L | |
| Median [range] | 8 [0.2–292.5] |
| 5–20 | 73 (30) |
| >20 | 77 (32) |
| Bone marrow blasts, % | |
| Median [range] | 68 [0–99] |
| 20–50 | 43 (18) |
| >50 | 155 (64) |
| Peripheral blasts, % | |
| Median [range] | 8 [0–98] |
| 1–10 | 41 (17) |
| >10 | 115 (47) |
| Platelets, ×109/L | |
| Median [range] | 62 [4–418] |
| <25 | 56 (23) |
| 25–50 | 47 (19) |
| >50 | 140 (58) |
| Albumin, g/dL | |
| Median [range] | 3.6 [1.7–5.0] |
| <3.0 | 35 (15) |
| Year of study | |
| 1990–1999 | 149 (61) |
| ≥2000 | 96 (39) |
| LDH, IU/L | |
| Median [range] | 970.5 [195–12,100] |
| ≥970 | 119 (50) |
CR indicates complete response; WBC, white blood cells; LDH, lactate dehydrogenase.
Overall, 75 patients (31%) achieved a CR with first salvage therapy (Table 2). Response by treatment regimen is shown in Table 3. Nine patients received salvage therapy with single-agent clofarabine (n = 6) or with clofarabine combinations (n = 3), and 2 CRs were observed.
Table 2.
Response (n=245)
| Response | No. of Patients (%) |
|---|---|
| CR | 75 (31) |
| PR | 2 (1) |
| CRp | 3 (1) |
| Early death <2 wk | 9 (4) |
| Induction death | 19 (8) |
| Resistant disease | 132 (54) |
| Unknown response | 5 (2) |
CR indicates complete response; PR, partial response; CRp, complete response with incomplete platelet recovery.
Table 3.
Response by Therapy
| Therapy | No. of Patients | No. of CR+CRp (%) |
|---|---|---|
| VAD/Hyper-CVAD | 103 | 48 (47) |
| Cytarabine combinations | 32 | 8 (25) |
| Methotrexate-asparaginase combinations | 15 | 5 (33) |
| Other combinations | 29 | 6 (21) |
| Single agents | 54 | 7 (13) |
| Allogeneic stem cell transplantation | 12 | 4 (33) |
CR indicates complete response; CRp, complete response with incomplete platelet recovery; VAD, vincristine, doxorubicin, and dexamethasone; Hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone.
Response by pretreatment characteristics is shown in Table 4. Prognostic factors that were associated with CR rates were age, karyotype, hemoglobin level, white blood cell count, platelet count, percentage blasts, lactic dehydrogenase (LDH) and albumin levels, and year of salvage. In multivariate analysis, age >55 years, platelet count <75 × 109/L, and bone marrow blasts ≥20% remained as independent poor prognostic factors for response (P< .05). Response according to the presence of zero, 1, 2, or 3 adverse factors is shown in Table 5.
Table 4.
Prognostic Factors for Response and Survival
| Characteristic | No. | No. of CRs (%) | P | 1-Year Survival, % | P |
|---|---|---|---|---|---|
| Age, y | .003 | <.001 | |||
| <50 | 169 | 62 (37) | 19 | ||
| ≥50 | 76 | 13 (17) | 13 | ||
| Response to frontline therapy | .25 | .07 | |||
| CR | 177 | 50 (28) | 26 | ||
| No CR | 68 | 25 (37) | 15 | ||
| Karyotype | .04 | .02 | |||
| Diploid | 75 | 31 (41) | 22 | ||
| Philadelphia chromosome-positive | 43 | 12 (16) | 21 | ||
| Other | 89 | 19 (25) | 10 | ||
| Insufficient metaphases/not done | 31/7 | 9 (12)/4 (5) | 26/0 | ||
| Hemoglobin, g/dL | .02 | .004 | |||
| <10 | 105 | 23 (22) | 11 | ||
| ≥10 | 138 | 51 (37) | 22 | ||
| WBC, ×109/L | .12 | .03 | |||
| <5 | 93 | 34 (37) | 23 | ||
| 5–20 | 73 | 23 (32) | 18 | ||
| >20 | 77 | 17 (22) | 9 | ||
| Bone marrow blasts, % | .0002 | <.0001 | |||
| <20 | 45 | 25 (56) | 35 | ||
| 20–50 | 43 | 12 (28) | 14 | ||
| >50 | 155 | 37 (24) | 13 | ||
| Peripheral blasts, % | .03 | .001 | |||
| 0 | 86 | 35 (41) | 30 | ||
| 1–10 | 41 | 10 (24) | 12 | ||
| >10 | 115 | 28 (24) | 10 | ||
| Platelets, ×109/L | <.0001 | <.0001 | |||
| <25 | 56 | 5 (9) | 5 | ||
| 25–99 | 100 | 27 (27) | 12 | ||
| 100–140 | 22 | 8 (36) | 32 | ||
| >140 | 65 | 34 (52) | 29 | ||
| Albumin, g/dL | .01 | <.0001 | |||
| <3.6 | 108 | 24 (22) | 13 | ||
| ≥3.6 | 129 | 49 (38) | 20 | ||
| LDH, IU/L | .005 | <.0001 | |||
| <970 | 119 | 47 (40) | 25 | ||
| ≥970 | 119 | 26 (22) | 8 | ||
| Year of study | .04 | .36 | |||
| 1990–1994 | 85 | 24 (28) | 21 | ||
| 1995–1999 | 64 | 13 (20) | 11 | ||
| 1999–2004 | 47 | 16 (34) | 21 | ||
| 2004–2009 | 49 | 22 (45) | 15 | ||
| Salvage therapy | .001 | .0002 | |||
| VAD/Hyper-CVAD | 103 | 47 (46) | 22 | ||
| Cytarabine combinations | 32 | 8 (25) | 9 | ||
| Allogeneic transplantation | 12 | 3 (25) | 17 | ||
| Methotrexate-asparaginase combinations | 15 | 4 (27) | 40 | ||
| Other | 29 | 6 (21) | 14 | ||
| Single agent | 54 | 7 (13) | 9 |
CR indicates complete response; WBC, white blood cells; LDH, lactate dehydrogenase; VAD, vincristine, doxorubicin, and dexamethasone; Hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone.
Table 5.
Response and Survival According to the Number of Independent Adverse Factors Present
| No. of Adverse Factors | No. of Patients | No. of CRs (%) | Median Survival, mo | 1-Year Survival, % |
|---|---|---|---|---|
| Independent adverse factors for responsea | ||||
| 0 | 24 | 16 (67) | 9 | 41 |
| 1 | 78 | 36 (46) | 7 | 25 |
| 2 | 107 | 21 (20) | 4 | 13 |
| 3 | 36 | 2 (6) | 2 | 0 |
| Independent adverse factors for survivalb | ||||
| 0–1 | 71 | 36 (51) | 9 | 35 |
| 2 | 63 | 25 (40) | 6 | 23 |
| 3 | 74 | 11 (15) | 5 | |
| 4–5 | 37 | 3 (8) | 2 | 0 |
The median remission duration was 5 months (95% confidence interval, 4–8 months) (Fig. 1). The median survival was 4.7 months (95% confidence interval, 3.8–6.0 months) (Fig. 1).
Figure 1.
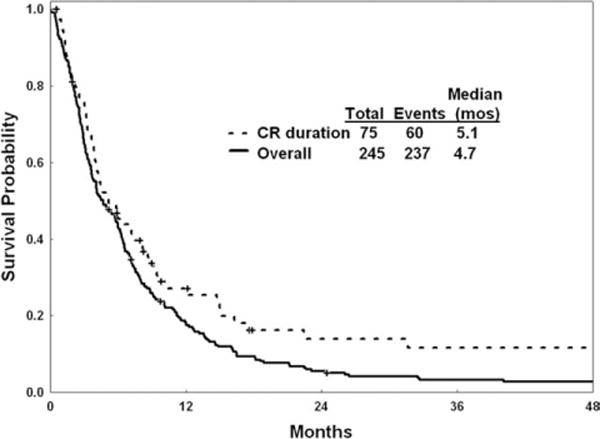
Remission duration and survival are illustrated. CR indicates complete response.
Prognostic factors (excluding first salvage treatment effect) that were associated significantly with survival were age, karyotype, hemoglobin level, percentage bone marrow blasts, platelet count, LDH, and albumin levels. Multivariate analysis selected the following independent poor prognostic factors for survival: age >55 years (P= .001), bone marrow blasts ≥20% (P = .002), platelet count <75 × 109/L (P< .001), albumin level <3 g/L (P< .001), and LDH level ≥1000 IU/L (P< .001). Survival according to the presence of zero or 1, 2, 3, and 4 or 5 adverse factors is shown in Table 5 and Figure 2.
Figure 2.
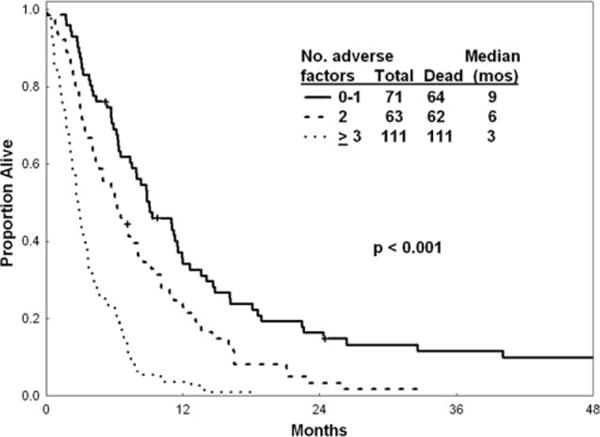
Survival is illustrated according to the number of adverse factors derived from multivariate analysis.
Table 5 also shows the prediction of survival based on the risk model for CR and prediction of the CR rate based on the risk model for survival. This suggests that, for simplicity, each group of predictive factors still can be used to predict both the CR rate and survival.
Twelve patients underwent allogeneic stem cell transplantation as first salvage for active disease with preparative regimens that included total body irradiation (TBI) in 2 patients and non-TBI regimens in 10 patients. The donor was a related matched sibling in 11 patients and a matched unrelated donor in 1 patient. Overall, 4 patients (33%; 3 CRs and 1 CRp) achieved a CR with allogeneic SCT (Table 3). Their 1-year survival rate was 17%, which was not different from the outcome of other patients (17%; P = .7). Of the 75 patients who achieved a CR, 20 underwent allogeneic SCT in second CR. Five of those patients remained alive without disease for a median of ≥52 months (range, from ≥7 months to ≥190 months). The median survival of patients after allogeneic SCT in second CR was 14 months (95% confidence interval, 10–26 months).
Effect of First Salvage Therapy on Prognosis
The previous model included pretreatment patient and disease characteristics. First salvage regimens may have different efficacies (Table 3) and significant interactions with pretreatment characteristics. To account for the treatment-related factors, we repeated the multivariate analyses and included first salvage therapy (vincristine, doxorubicin, and dexamethasone-hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; cytarabine combinations; and allogeneic SCT vs others). The multivariate analysis for CR still identified a higher percentage bone marrow blasts (P = .0004), older age (P = .04), and thrombocytopenia (P < .0001) as independently prognostic and also identified the “ineffective regimens” (defined as single-agent therapy regimens) as independently prognostic (P = .001). Similarly, the multivariate survival analysis selected some previously identified significant factors (percentage bone marrow blasts [P = .02], platelet count [P = .002], albumin level [P = .001], age [P = .003], and LDH [P < .0001]) and also identified type of salvage therapy as an independent prognostic factors.
Achievement of CR and Prognosis
To assess the benefit of achieving a CR, we repeated the multivariate survival analysis using a 6-week landmark that excluded patients who died within 6 weeks. The multivariate analysis included 213 patients and selected independent factors associated with survival similar to those described above (ie, age, percentage bone marrow blasts, LDH, and albumin) but did not select platelet count. Adding response (CR vs no CR) to the multivariate analysis indicated that a CR added significantly to the survival benefit (P < .0001; hazard ratio, 0.40; 95% confidence interval, 0.03–0.72).
DISCUSSION
In the current analysis, we attempted to identify a subgroup of adult patients with ALL who have an extremely poor prognosis when receiving salvage therapy. It is known from previous studies that patients with a long first CRD (>12 months) still may have a favorable early outcome and can achieve reasonable CR rates of 40% to 70% with combination salvage regimens. They also may have reasonable long-term expectations with estimated 3-year survival rates of 10% to 20%. The objective of this study was to identify patients in first salvage for whom the prognosis with standard salvage combination regimens is poor. Such patients may benefit from novel investigational therapies that potentially might be approved for these niche orphan indications, as in the case with clofarabine, nelarabine, and the Bcr-Abl tyrosine kinase inhibitors.9–13 Therefore, we focused on patients in first salvage who had failed primary front-line therapy or who had a first CRD <12 months. In this analysis of 245 patients, the CR rate was only 31%, the median response duration was 5 months, and the median survival for the entire study cohort was 4.7 months (Fig. 1). A multivariate analysis of prognostic factors independently associated with worse survival identified older age, increased bone marrow blasts, lower platelet count, lower albumin level, and higher LDH level as adverse features (Table 5). Patients who had ≥3 or of these 5 factors (45% of patients) had a median survival of 2 months to 3 months. Achieving a CR improved survival significantly (P< .0001; hazard ratio, 0.40).
In the current analysis, we identified a subgroup of patients with ALL in first salvage who have a very poor prognosis. In such patients, novel single-agent or combination strategies are justified. Such approaches that have demonstrated promise include monoclonal antibodies targeted against CD19 (which is expressed universally in all patients with ALL), against CD20 (which is expressed in 30%–50% of adults with ALL), against CD22 (which is expressed in ≥80% of adults with ALL), and against dual monoclonal antibodies (eg, against CD19 and CD22) either as native antibodies or attached to toxins (eg, diphtheria toxin, calicheamicin).
Although several studies have reported experience with ALL in first salvage,9–13 to our knowledge, no studies have detailed the outcome of patients who had a poor prognosis after first salvage, ie, those with primary refractory disease or with short first CRD. Clofarabine has been approved for the treatment of pediatric ALL in second salvage,9,10 nelarabine has been approved for the treatment of T-cell ALL salvage,11 and the Bcr-Abl tyrosine kinase inhibitors imatinib and dasatinib have been approved for the treatment of Ph-positive ALL.12,13 Most patients in the current study were either treated prior to availability of these agents or were not candidates for these approved therapeutic options and, thus, are in need of alternative new agents with anti-ALL activity. In summary, our experience in adults with ALL who had a poor prognosis after first salvage emphasizes the need to develop novel strategies in this setting and establishes baseline expectations against which new approaches can be compared.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Pui CH, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998;339:605–615. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]
- 2.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 4.Faderl S, Jeha S, Kantarjian HM. The biology and therapy of adult acute lymphoblastic leukemia. Cancer. 2003;98:1337–1354. doi: 10.1002/cncr.11664. [DOI] [PubMed] [Google Scholar]
- 5.Larson RA. Recent clinical trials in acute lymphocytic leukemia by the Cancer and Leukemia Group B. Hematol Oncol Clin North Am. 2000;14:1367–1379. doi: 10.1016/s0889-8588(05)70191-x. [DOI] [PubMed] [Google Scholar]
- 6.Durrant IJ, Richards SM, Prentice HG, Goldstone AH. The Medical Research Council trials in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2000;14:1327–1352. doi: 10.1016/s0889-8588(05)70189-1. [DOI] [PubMed] [Google Scholar]
- 7.Gokbuget N, Hoelzer D, Arnold R, et al. Treatment of adult ALL according to protocols of the German Multicen-ter Study Group for Adult ALL (GMALL) Hematol Oncol Clin North Am. 2000;14:1307–1325. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 8.Thiebaut A, Vernant JP, Degos L, et al. Adult acute lymphocytic leukemia study testing chemotherapy and autologous and allogeneic transplantation. A follow-up report of the French protocol LALA 87. Hematol Oncol Clin North Am. 2000;14:1353–1366. doi: 10.1016/s0889-8588(05)70190-8. [DOI] [PubMed] [Google Scholar]
- 9.Jeha S, Kantarjian H. Clofarabine for the treatment of acute lymphoblastic leukemia. Expert Rev Anticancer Ther. 2007;7:113–118. doi: 10.1586/14737140.7.2.113. [DOI] [PubMed] [Google Scholar]
- 10.Jeha S, Gandhi V, Chan KW, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–789. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 11.DeAngelo DJ, Yu D, Johnson JL, et al. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801. Blood. 2007;109:5136–5142. doi: 10.1182/blood-2006-11-056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ottmann OG, Druker BJ, Sawyers CL, et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood. 2002;100:1965–1971. doi: 10.1182/blood-2001-12-0181. [DOI] [PubMed] [Google Scholar]
- 13.Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110:2309–2315. doi: 10.1182/blood-2007-02-073528. [DOI] [PubMed] [Google Scholar]
- 14.Fielding AK, Richards SM, Chopra R, et al. Medical Research Council of the United Kingdom Adult ALL Working Party; Eastern Cooperative Oncology Group Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL): an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 15.Tavernier E, Boiron JM, Huguet F, et al. GET-LALA Group; Swiss Group for Clinical Cancer Research SAKK; Australasian Leukaemia and Lymphoma Group Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21:1907–1914. doi: 10.1038/sj.leu.2404824. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Manero G, Thomas DA. Salvage therapy for refractory or relapsed acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2001;15:163–205. doi: 10.1016/s0889-8588(05)70204-5. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86:1216–1230. doi: 10.1002/(sici)1097-0142(19991001)86:7<1216::aid-cncr17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.George SL, Ochs JJ, Mauer AM, Simone JV. The importance of an isolated central nervous system relapse in children with acute lymphoblastic leukemia. J Clin Oncol. 1985;3:776–781. doi: 10.1200/JCO.1985.3.6.776. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113:3186–3191. doi: 10.1002/cncr.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life tables. J Stat Soc (B) 1972;34:187–220. [Google Scholar]


