Abstract
Genetically modified bacterial flagellin (Fla), a Toll-like receptor-5 (TLR5) ligand, was evaluated as a fusion partner for human papillomavirus (HPV) L2-based immunogens in two animal challenge models; either cutaneous inoculation of rabbits with HPV ‘quasivirions’ containing cottontail rabbit papillomavirus (CRPV) genomes that induce warts, or intra-vaginal inoculation of mice with HPV ‘pseudovirions’ encapsidating a luciferase reporter plasmid and measurement of bioluminescence to determine infectivity. An Escherichia coli production system was developed for flagellin-L2 (Fla-L2) fusions containing either monomeric HPV-16 L2 a.a. 11(× 11–200) or oligomeric L2 comprising a fusion of the a.a. 11–88 peptides of five (Fla~5 × 11–88) or eight (Fla~8 × 11–88) genital HPV types. Immunogenicity and bioactivity of Fla-L2 constructs were assessed using an in vitro neutralization and cell-based TLR-5 binding assay, respectively. Efficacy was evaluated following active immunization of rabbits or mice administered 3 intramuscular doses of Fla-L2 recombinants without exogenous adjuvant, followed by challenge. In addition, passive immunization studies of naïve rabbits with serial dilutions of pooled immune sera were used to determine End-Point Protection Titers (EPPT) for each formulation against a broader spectrum of HPV quasivirions. Efficacy was assessed for up to 10 weeks on the basis of wart volume induced following challenge and results compared to licensed L1-VLP vaccines (Gardasil and Cervarix). Following active immunization at doses as low as 1 μg, Fla-L2 fusions afforded complete protection against infection (mice) and disease (rabbits) following either homologous or heterologous HPV challenge. Passive immunization with anti-L2 immune sera discriminated between the different vaccine candidates under evaluation, demonstrated the protective role of antibody and suggested the superiority of this oligomeric L2-TLR5 agonist fusion approach compared to L1-based vaccines in its ability to cross-protect against non-vaccine HPV types.
Keywords: Human papillomavirus, Vaginal challenge, Cutaneous challenge, L2, HPV, Prophylactic vaccine, Neutralizing antibody, Protective efficacy
1. Introduction
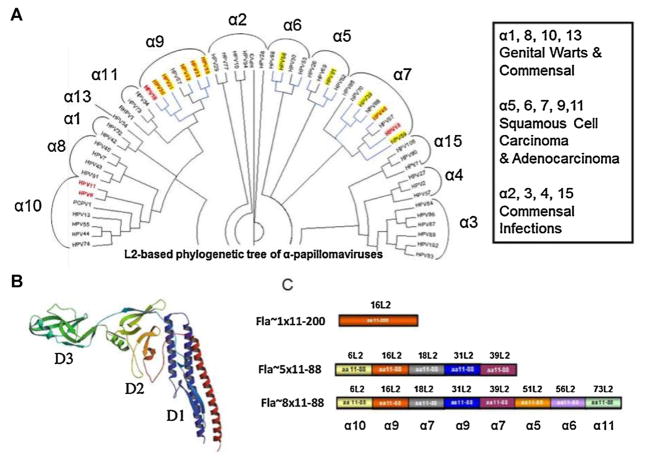
Over 120 identified HPV types have been classified into five genera: Alpha-, Beta-, Gamma-, Mu- and Nu-papillomaviruses [1]. Most alpha HPV types infect the genital tract and are sexually transmitted. Within the alpha papillomaviruses, fifteen genotypes [2–8] are classified as “high-risk” (HR-HPV) and are considered the causal agents of cervical cancer [9] (Fig. 1A). Cervical cancer represents 9% of cases of female cancer and is the third leading cause of cancer in women worldwide, with more than 529,000 new cases and 275,000 deaths per year [10], and 99% of cases contain HPV DNA [11,12].
Fig. 1.
Phylogenetic tree of alphapapillomavirus and design of Fla-L2 vaccines. (A) Phylogenetic tree of alpha papillomavirus based on L2 amino acid sequences was built using the neighbor-joining Jukes-Cantor method [1]. The final tree is a consensus made from bootstrap resampling of 100 replicates. The circular representation was generated using Dendroscope [2]. Bold red font indicates HPV types represented in nona (9)-valent Merck vaccine (NCT00543543); blue lines indicate HPV types associated with cancers; pink background indicate HPV types against which licensed vaccines are ~100% protective; yellow background indicates HPV types reported to be weakly cross-protected by licensed vaccines. Alpha clade numbering is designated over the brackets. (A) (inset) – General characteristics of alpha clades (cited from Schiffman et al. [3]). (B) 3D models of Flagellin modified from [4]. Domain D1, D2, D3 domains are depicted by color. (C) A diagram showing the composition of L2 inserts for individual Fla-L2 vaccine candidates. Individual subunits were derived from different HPV species. Specific α-clades for each type represented in vaccine is depicted below Fla~8 × 11–88 L2 diagram. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Both HPV vaccines, Gardasil (Merck & Co. Inc.) and Cervarix (GlaxoSmithKline), are based upon virus-like particles (VLPs) derived from major capsid protein L1 and are licensed for protection against the two HPV types (HPV-16 and HPV-18) that cause 70% of cervical cancers, 80% of anal cancers, 60% of vaginal cancers, and 40% of vulvar cancers. Gardasil also targets the two HPV types that cause 90% of benign genital warts, HPV-6 and HPV-11. Although there is accumulating evidence suggesting that HPV vaccination also confers some cross-protection against types most closely related to those used to produce the VLPs, efficacy against non-vaccine non-alpha-7/9 HPV types is significantly lower [13,14], and this cross-protection may potentially wane faster than immunity to the vaccinal types HPV16 and HPV18 [15]. In addition, the non-vaccine HR-HPV types tend to induce cervical intraepithelial neoplasia grade 3+ (CIN3+) more slowly than the vaccine-targeted types(9), which implies that CIN3+ caused by slow progressor HPV types may account for a higher percentage of CIN3+ over time [16]. Therefore a new generation of broader spectrum HPV vaccines is being developed, including a 9-valent L1-VLP vaccine including 7 HR-HPV types 16, 18, 31, 33, 45, 52 and 58, and the low risk types HPV6 and 11 (Merck & Co. Inc., NCT00543543). A potential benefit to the development of the 9-valent vaccine is to lower the continuing need to screen after vaccination.
The potential impact of widespread HPV vaccination in developing countries, wherein 80% of cervical cancer cases occur, is enormous, but the current cost of HPV vaccination remains a barrier to their introduction [17]. Reduced dosing schedules for the L1-based vaccines may be sufficient and reduce cost [18]. HPV vaccines are licensed in many low- and middle-income countries, although few have established national immunization programs. Although tiered pricing and 2-dose regimens have potential to greatly expand access, an affordable HPV vaccine covering the majority of HR-HPV incident infections is still urgently needed [19].
A cost-effective HPV vaccine based on a single antigen Escherichia coli- produced minor capsid protein L2 might address this problem. Vaccination with the N-terminus of the L2 protein protects animals from experimental challenge with either animal papillomaviruses [19–21] or HPV pseudovirions that carry a reporter plasmid [2,20]. The N-terminus of L2 does not assemble into a VLP but does effectively present its linear protective epitopes when fused in tandem with the same region of several HPV types [22]. Indeed immunization with such concatemers/multimers of L2 derived from several high risk HPV types, induces neutralizing antibodies that protect mice from vaginal HPV challenge by diverse genotypes [22] despite eliciting neutralization titers significantly lower than L1 VLP vaccines [23].
Engagement of TLRs by their cognate agonists and the subsequent signaling within antigen presenting cells (APC) leads to enhanced processing and presentation of antigens that are co-delivered to those APC [24,25,26]. A TLR-2 agonist was required to adjuvant a short L2 epitope (HPV16; AA 17–36) linked to a universal T-helper epitope and provided mice protection against heterologous HPV challenge [2]. Further, use of an adjuvant with L2 multimer vaccination is an important factor in obtaining effective protection [22], and inclusion of a TLR agonist, such as monophosphoryl lipid A (MPL) or CpG, with 1 μg L2 multimer formulated in alum can provide dose sparing [27].
The principle of utilizing flagellin as a carrier/adjuvant is well described [28–31]. The adjuvant property of flagellin is mediated by TLR5, linking innate and adaptive immunity via MYD88 and TRAF6, leading to NF-κB activation, cytokine secretion and an inflammatory response [28,29,32,33]. Epitope based vaccines delivered via fusion with flagellin are efficacious against a number of viral [34–36] and bacterial [37,38] targets. The safety and ability to induce protective levels of serum antibody have been demonstrated in preclinical [4,5,34–36,39–41] as well as in recent clinical studies [42,43] of flagellin-based candidate influenza vaccines. Therefore fusion with flagellin, which offers a combination of TLR activity and T-helper epitopes, was examined as a self-adjuvanting carrier for L2.
2. Materials and methods
Detailed descriptions of all methods are shown in Supplemental materials.
2.1. “L2-based” in vitro neutralization method
Flat bottom 96-well cell culture plates were coated with Extra Cellular Matrix prepared from MCF10 cells [23], covered with neutralization medium (DMEM without phenol red, 10% FBS, 1% non-essential amino acids, 1% GlutaMax) and incubated the plate at 37 °C, 5% CO2 culture incubator for 4 h. Plates were washed three times with PBS and 80 μL of the diluted PsV prepared in Delta Furin CHO conditional Medium were added to each well. Plates were incubated in a 37 °C culture incubator overnight, carefully washed three times with PBS and 80 μL of the neutralization medium was added to each well. 20 μL of serially diluted antiserum (in neutralization medium) was added to each well and the plate was incubated at 37 °C culture incubator overnight. Upon completion of incubation, 100 μL of medium containing 104 pgsA-745 cells (ATCC, VA) were added to each well. Cells were incubated at 37 °C for 72 h, and cell supernatants were analyzed for luminescence using New England BioLab’s BioLux Gaussia Luciferase Assay Kit (NEB#E3300L, NewEngland Biolabs, MA), using 15 μL supernatant and 50 μL of GLuc assay solution provided in the kit.
2.2. Vaginal HPV challenge (mice)
Mice received 3 mg of medroxyprogesterone (Depoprovera; Pfizer) diluted in 100 μL of sterile PBS in a subcutaneous injection 4 days prior to HPV pseudovirus challenge. The pseudovirus inoculum was a 20 μL dose composed of purified HPV pseudovirus carrying the luciferase reporter gene mixed in 2% carboxymethyl cellulose (CMC) (Sigma C5013). The virus was delivered in two doses. Half was deposited into mouse vagina by using an M50 positive-displacement pipette (Gilson). A cytobrush cell collector was inserted in the vagina and twirled clockwise and counterclockwise 10 times, and the remaining 10 μL was introduced. Three days later, the mice were anesthetized to effect with isoflurane (Baxter), luciferin (20 μL at concentration of 7.8 mg/ml) was deposited intravaginally, and their images were acquired for 10 min using Xenogen IVIS 200, as previously described [45]. The average radiance within the region of interest was determined.
Statistical analysis
One-way ANOVA and Bonferroni’s multiple comparison tests were performed with GraphPad 4.0 (GraphPad Software, San Diego, CA).
3. Results
In our initial immunization studies in rabbits using HPV16 L2 (a.a. 11–200) fused to full length flagellin, the antibody response predominantly targeted the immunodominant domain D-3 of Flagellin (Fig. 1B), whereas minimal L2-specific antibody was detected by ELISA, albeit still sufficient for complete protection against challenge with HPV16 and HPV18 (data not shown). Since the D3 domain is not required for TLR5 activation, a flagellin D3 domain deletion (Fla~; Fig. 1B) was instead fused to HPV16 L2 a.a. 11–200 (Fig. 1C). This construct (Fla~ 1 × 11–200) retained its ability to activate TLR5 signaling (Supplementary Fig. 1D) and afforded a significant shift of immune response toward L2 (data not shown), and therefore this Fla~ backbone was selected for further studies.
Two versions of concatenated L2 representing either alpha-9 and -7 species (Fla~5 × 11–88) or all five oncogenic alpha species (Fla~8 × 11–88) were selected (Fig. 1A and C). Both of these oligomeric fusions also contain an HPV6 L2 sequence from the alpha-10 species, and the most common type found in genital warts (Fig. 1). Both multimeric recombinants were fused to the C terminus of FlaΔD3. A FlaΔD3 alone construct (Fla~) was used as a control.
Each Fla~L2 fusion and Fla~ was purified to homogeneity (Supplementary Fig. 1A–C), and tested for TLR5-dependent signaling in a cell-based assay (Supplementary Fig. 1D). As it is evident from Supplementary Fig. 1D, all Fla-L2 fusions activated TLR5 signaling. However recombinants with longer L2-fusions triggered TLR5 activation at slightly higher doses than Fla~, suggesting some steric hindrance in ligand binding by L2 concatamers.
For in vivo efficacy assessments, the schedule of immunization is shown in Supplementary Fig. 2. Groups of ten mice were immunized on days 0, 14 and 28 with 25 μg of Fla~L2 immunogen alone, or one-tenth the human dose per animal of each commercial L1-based vaccine. Groups of five rabbits were immunized on days 0, 21 and 42 with 125 μg of Fla~L2 protein alone, or a full human dose per animal of Gardasil or Cervarix. Both species were bled before challenges (mice at day 42, and rabbits at day 77), and the sera were utilized to measure humoral immune responses.
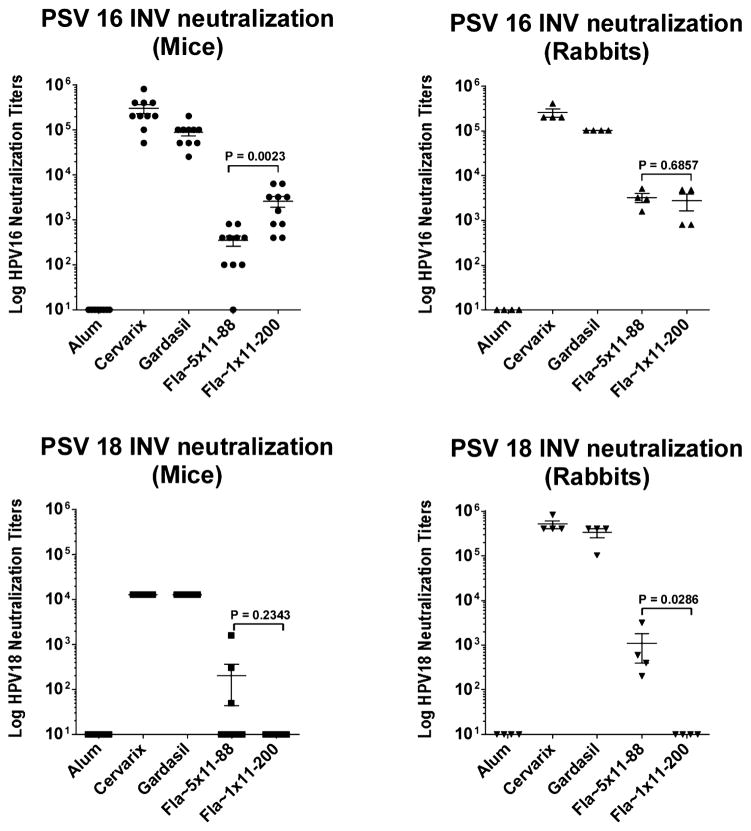
Serum antibody responses were first assayed for in vitro neutralization (IVN) titer using pseudovirions (PsV) of HPV16 and HPV18 (Fig. 2). In both rabbits and mice, Gardasil and Cervarix demonstrated similarly robust PsV16 and PsV18 neutralization titers, two orders of magnitude higher than those elicited by L2-based constructs. In mice Fla~1 × 11–200 elicited higher PsV16 neutralizing titers than Fla~5 × 11–88 (P = 0.0023), but in rabbits there was no difference (P = 0.6857). A significant difference between mono-and oligomeric-L2-vaccines was observed in rabbits for PsV18 neutralization titer (P = 0.0286). While PsV18 neutralization titers induced by Fla~5 × 11–88 were higher than for Fla~1 × 11–200, the results in mice were lower and variable. Immunization with Fla~1 × 11–200 did not result in a detectable PsV18 neutralizing response in either species. However, recent studies have suggested that the conventional neutralization assay is insensitive for L2-specific neutralizing antibodies, and a modified “L2-based” assay has been developed to improve sensitivity [23] by utilizing spatiotemporal separation of HPV binding phases to primary (HSPG) and secondary (unknown) receptors, which is thought to better reflect the infectious process in vivo [23]. This approach allowed detection of neutralizing titers for anti-Fla~1 × 11–200 sera across all HPV types tested, albeit at lower levels than for Fla~5 × 11–88 antisera (Table 1).
Fig. 2.
HPV16 and HPV18 neutralizing serum antibody response elicited by Fla~1 × 11–200 and Fla~5 × 11–88 in mice and rabbits (conventional in vitro neutralization (IVN) method [44]). Neutralizing potency of individual pre-challenge anti-Fla~1 × 11–200, Fla~5 × 11–88, Gardasil™ and Cervarix® serum samples from mice (left panels) and rabbits (right panels) were compared via conventional IVN method [44] against pseudovirions (PsV) of HPV16 and 18 (see Section 2). Neutralization titers for both L2 vaccines against HPV16 (upper panels) were similar, while against PsV18 were dramatically different: Fla~5 × 11–88 induced significant anti-PsV18 response, while Fla~1 × 11–200 immunization did not result in detectable PsV18 neutralization titers (lower panels). L1-VLP vaccines elicited circa two order of magnitude higher neutralizing responses to both pseudiovirions than Fla-L2 fusions.
Table 1.
Neutralization titers determined using the “L2-based” in vitro neutralization assay. Representative in vitro neutralization titers of rabbit antisera to Fla~1 × 11–200 and Fla~5 × 11–88 vaccine candidates measured using the “L2-based assay” performed according to Day et al. [20] with spatiotemporal separation of L2 epitope exposure on the basement membrane (HSPG) and binding to the secondary receptor (unknown) on the HSPG-free epithelial cell surface.
| Fla~1 × 11–200 | Fla~5 × 11–88 | |
|---|---|---|
| HPV6 | 563 | 64,708 |
| HPV16 | 37,275 | 231,538 |
| HPV18 | 544 | 29,831 |
| HPV31 | 1253 | 27,923 |
| HPV45 | 467 | 40,439 |
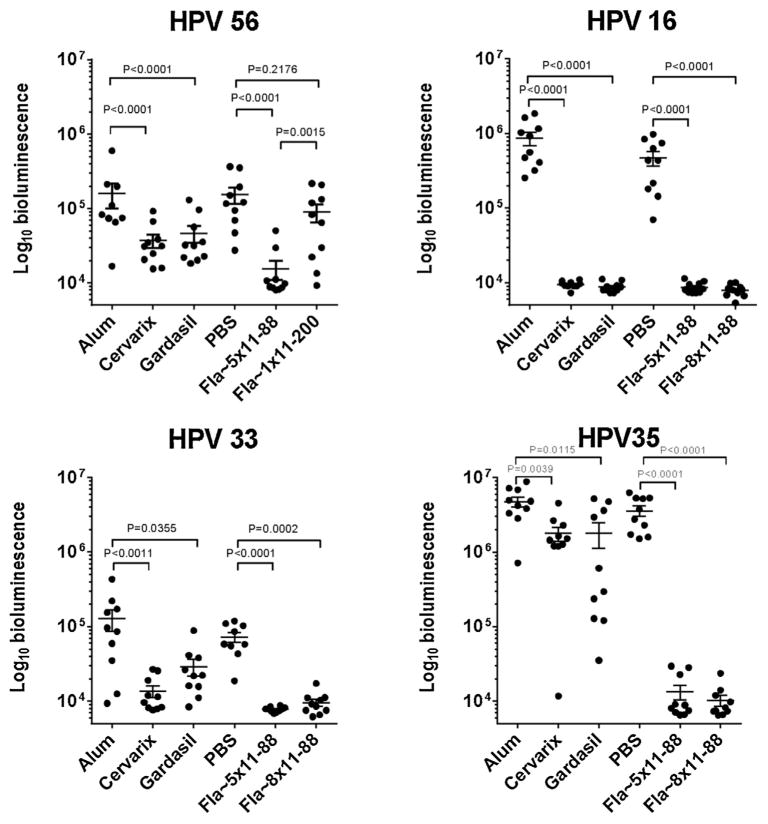
While greater neutralization afforded by Fla~5 × 11–88 against HPV18 challenge compared with Fla~1 × 11–200 is likely associated with incorporation of homologous L2 sequence, the fact that HPV45 PsV was neutralized to a similar extent yet HPV45 L2 was not incorporated into Fla~5 × 11–88 might be attributed to expansion of B-cell clones recognizing the most conserved features of the five L2 types, which are also represented in L2 HPV45. To examine the breadth of cross-protection afforded by mono- and oligomeric-Fla-L2 fusions, mice were challenged intra-vaginally at 4 months post vaccination with luciferase-expressing PsV56, derived from an alpha-6 papillomavirus type that is not directly targeted by either vaccine (Fig. 3A). Monomeric Fla~1 × 11–200 as well as L1-VLP vaccines showed only partial efficacy (41 and 76% reduction of bioluminescence (RoB), respectively), while the oligomeric L2 fusion (Fla~5 × 11–88) produced more dramatic reduction in bioluminescence (98% RoB), the quantitative marker of infection (Fig. 3A).
Fig. 3.
Protective efficacies of Fla~1 × 11–200, Fla~5 × 11–88, Fla~8 × 11–88 against various HPV type vaginal challenges in mice. (A) Vaginal challenge with pseudovirion of HPV56. Mice (10 per group) were immunized biweekly with 3 doses of either of Fla~1 × 11–200, Fla~5 × 11–88, Gardasil™ or Cervarix® and challenged with heterologous pseudovirion HPV56 at 164 days post immunization (see Section 2 and Supplementary Fig. 1). (B–D) Vaginal challenge with pseudovirion of HPV16, 35, 33. Mice (10 per group) were immunized biweekly with 3 doses of either of Fla~5 × 11–88, Fla~8 × 11–88, Gardasil™ or Cervarix® and challenged with heterologous pseudovirion of either HPV16, 36 or 33 at 45 days post immunization (see Section 2 and Supplementary Fig. 1). Individual animal bioluminescence in the genital area was detected by Xenogen IVIS 200 3 days after PsV challenge (see Section 2). The background luminescence signal is ~104 RLU.
After confirming the limits of protection of the monovalent Fla~1 × 11–200 immunogen, the remaining murine studies were focused on assessing breadth of protection using multimeric Fla-L2 constructs. The Fla~5 × 11–88 and Fla~8 × 11–88 immunogens (Fig. 3B) were tested for protective efficacy against vaginal challenge with a homologous (HPV16) or heterologous (HPV33 and HPV35) PsVs (Fig. 3C and D). These immunogens provided similarly robust protection against challenges with HPV16 (~98% RoB), HPV33 (~87% RoB) and HPV35 (~99% RoB). Conversely, L1-vaccine protection was robust for the homologous type HPV16 (99% RoB), and differentiated against the heterologous type HPV33 (87% and 77% RoB for Cervarix and Gardasil, respectively), HPV35 (~62% RoB for both vaccines). In contrast to HPV16 and 56, both HPV 33 and 56 efficacy windows were small reducing the precision of estimates, reflecting intrinsically different infectivity of PsV types rather than the use of less challenge virus as determined by L1. Although the lack of Fla~ only controls in the initial mouse studies is to be noted, it is unlikely that generalized immune activation via TLR5 played a role in protection since immunization of rabbits with this antigen did not elicit protection.
Surprisingly, when rabbits were immunized, the commercial and Fla-L2 vaccines were similarly protective against experimental cutaneous challenge with all six quasivirus (QV) types tested (HPV types 6, 16, 18, 31, 45, 58) at 1 month (Supplementary Fig. 3A–D), 6 months (Supplementary Fig. 4A–D) and 12 months (Supplementary Fig. 5A–D) post immunization. This cross-protection may reflect the use of a high dose (the same as that for humans) of the licensed vaccines and challenge at peak immunity. Notably, the Fla-L2 vaccines provided significant protection against CRPV challenge, but the L1 vaccines did not.
To investigate further the role of antibodies in protection, a passive transfer of serially diluted pooled serum samples was administered via the intravenous route into naive rabbits followed by a challenge with the same set of HPV types as in the active immunization phase. Efficacy was quantified as EPPT determined as the highest serum dilution sufficient for complete protection, e.g. the EPPT for Fla~5 × 11–88 antiserum against HPV45 QV challenge is determined as 100 because the first warts appeared on animals immunized with serum dilution of 1/500, and therefore the EPPT is considered as the prior dilution (Supplementary Fig. 6A and B).
Passive transfer of immune sera into naïve animals better allowed discrimination between HPV vaccines. The EPPT of L2-based immunogens (Fla~1 × 11–200 and Fla~5 × 11–88) showed broader protection compared to both licensed L1-based vaccines (Table 2). Indeed, immunization with Fla~1 × 11–200 resulted in EPPTs higher than 20 against all types except for HPV45 and CRPV, while L1 vaccines provided strong (EPPT = 2500), but mostly type-specific protection. Anti-Fla~5 × 11–88 sera exhibited robust EPPTs ranging from 100 to 2500 across all challenge types tested.
Table 2.
End-point protection titers of L1 and L2 vaccines in rabbits.
| Fla~1 × 11–200 | Fla~5 × 11–88 | Cervarix | Gardasil | |
|---|---|---|---|---|
| HPV6 | 20 | 500 | <20 | 2500 |
| HPV16 | 2500 | 500 | 2500 | 2500 |
| HPV18 | 100 | 500 | 2500 | 2500 |
| HPV31 | 20 | 500 | 100 | <20 |
| HPV45 | <20 | 100 | <20 | <20 |
| HPV58 | 100 | 2500 | <20 | <20 |
| CRPV | <20 | 100 | <20 | <20 |
A study designed to determine the minimum protective dose for a flagellin fusion was then conducted. A dose escalation study in rabbits with Fla~5 × 11–88 was evaluated; strong protection against all HPV types tested, as well as partial protection against CRPV, was demonstrated at doses of this Fla-fusion as low as 1 μg (Fig. 4). This is consistent with the recent human data for flagellin fusions displaying the influenza protective determinants (M2e and HA), which were shown to be efficacious at doses of 1–8 μg [42,43]. Importantly, vaccination at the highest dose (125 μg) with the Fla~ alone immunogen alone did not confer protection from challenge by any type, demonstrating that immunity is L2-dependent rather than a consequence of a broad innate activation via TLR5 signaling.
Fig. 4.
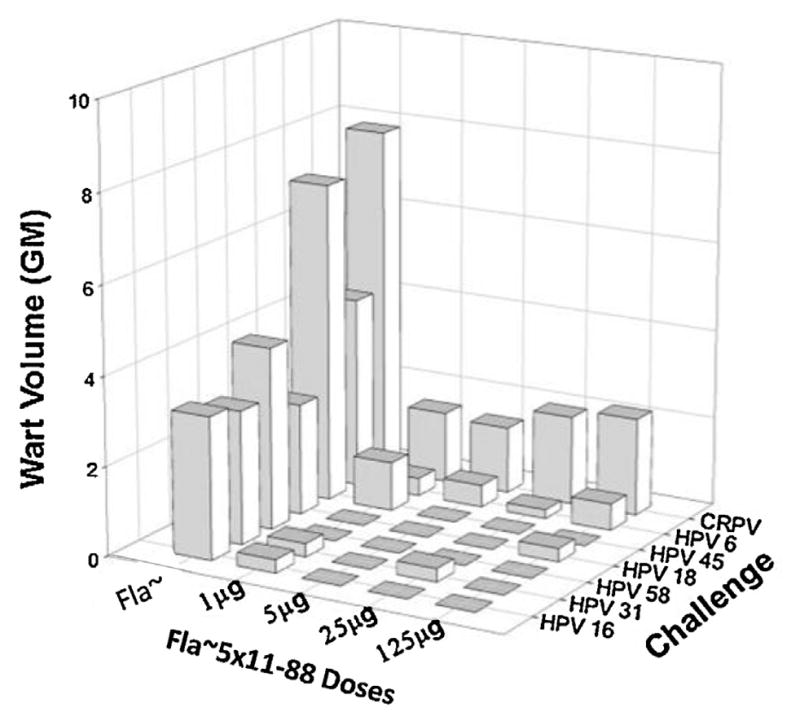
Dose-sparing potential of Fla~5 × 11–88. Groups of four female rabbits were immunized 3 times i.m. with Fla~5 × 11–88 (1, 5, 35 and 125 μg) or a 125 μg dose of Fla~ (flagellin backbone) which was used as a control. Immunized animals were challenged with QV6, 16, 45, 18, 31, 58 and CRPV.
4. Discussion
The commercial L1-VLPs vaccines are known to provide robust protection against HPV16 and HPV18, and this was borne out in our rabbit model. In addition, clinical data supports significant activity against HPV types most highly related to HPV16 and HPV18; for HPV31 77.1% (95% CI 67.2–84.4) for Cervarix vs. 46.2% (15.3–66.4) for Gardasil vaccine; P = 0.003), for HPV 45 (79.0% (61.3–89.4) vs. 7.8% (−67.0 to 49.3); P = 0.0003), but there is little evidence for activity against HPV58 with either vaccine [46]. Vaccination of rabbits with either vaccine was protective against HPV31 and Cervarix was protective against HPV45, as might be expected from the clinical data. However, both vaccines were protective against HPV58 in the rabbit model, and Gardasil was also protective against HPV45. Gardasil is also highly protective against HPV6 in both humans and the rabbit model because it contains HPV6 L1 VLPs, whereas Cervarix does not. Unexpectedly, vaccination of rabbits with Cervarix elicited cross-neutralization and protection against HPV6 challenge. However, although the early trials of Cervarix did not test for efficacy against HPV6, a population in the United Kingdom vaccinated with Cervarix showed significant reductions in genital warts [47] that are predominantly associated with HPV6 infections, and a 35% (95% CI 9–54%) protection against HPV6 was detected in a post hoc analysis of a Cervarix trial [48]. Although a higher efficacy was observed in the rabbit study, this may reflect the use of a full human dose of Cervarix in the rabbits and that the animals were challenged at 1 month after the third dose. One-month post third vaccination likely reflects the peak immune response, whereas the clinical data reflects challenges that occurred over an extended period after vaccination where we speculate that weak cross-protective immunity might wane. Indeed, this level cross-protection by vaccination with the licensed HPV vaccines was not seen in the mouse model (herein and [49], probably because the vaccination dose was lower (1/10th of human dose) and the neutralization response was weaker in mice, but it may also reflect the delivery of a luciferase-expressing vector rather than the CRPV genome by PsV used in the rabbit model.
Passive immunization of naïve rabbits with Gardasil antiserum provided robust protection against HPV6, 16 and 18 challenge with EPPT of 2500, further supporting a central role for type-specific neutralizing antibodies in mediating protection. However, passive immunization with Gardasil antiserum provided no cross-protection, and analogous results were obtained with Cervarix antiserum except for weak cross-protection against HPV31 (EPPT of 100), and these findings are more in line with clinical observations of their efficacy.
The differences observed in cross-protective efficacy between the active and passive immunization models, suggests either a possible role of L1-specific cell-mediated immunity in protection elicited in rabbits by active vaccination with the commercial vaccines or that insufficient sera (maximally a 1:20 final dilution in the host) was transferred. Since neither of the L1 VLP-based vaccines protected against CRPV virion challenge (which deliver the same CRPV genome as is packaged by the HPV PsV), T cell-mediated protection would require recognition and killing of the keratinocytes as the HPV PsV enter the cells rather than as a consequence of CRPV L1 expression, an unlikely scenario.
The more likely explanation for the unexpectedly broad cross-protection by the commercial vaccines in the active immunization rabbit model is that protection is mediated by low levels of cross-neutralizing antibodies. There is good evidence for the induction of low level cross-neutralizing antibodies against HPV31 and HPV45 by the commercial vaccines, although not for HPV58. Furthermore, in vivo protection in the mouse cervicovaginal challenge assay has been shown to be a more sensitive measure of protective VLP antibodies than in vitro neutralization assays [17] and the rabbit challenge model may be similarly sensitive.
The passive immunization method reported here provides a powerful tool for assessing protective thresholds for different vaccine formulations. It is noteworthy that both EPPT profiles of the antisera to the commercial vaccines in the rabbit challenge model (Table 2) and the data from active vaccination studies in the mouse model are generally consistent with clinical trial results [8,14], in that neither offer complete protection for most non-vaccinal types.
In contrast to the L1 vaccines, Fla-L2 demonstrated significant protection not only against all HPV PsV challenges but also against CRPV virion challenge, which can be attributed to cross-neutralizing antibodies rather than a cell-mediated response since the cross-protection was passively transferred in serum. Indeed HPV16 L2 11–200 vaccination of rabbits has been reported previously to be efficacious against CRPV virion, but not CRPV naked DNA challenges, and to afford no therapeutic benefit [21].
Although vaccination with HPV16 L2 11–200 fused to Fla~ elicited significant cross-protection against CRPV virion and HPV PsV challenges in rabbits, it is clear that the multimeric construct provided significantly broader immunity upon both active and passive immunization. Further, in the mouse vaginal challenge model, the multimer constructs were more broadly protective against diverse types of HPV PsV than Fla~1 × 11–200, similar to data obtained without the Fla~ fusion [27]. In contrast there was no significant difference in the cross-protection elicited by Fla~5 × 11–88 and Fla~8 × 11–88, as recently described for similar constructs without the Fla~ fusion [27]. Since neither immunogen contained an HPV56 L2 sequence (Fig. 1A), the superior efficacy against PsV56 of Fla~5 × 11–88 over Fla~1 × 11–200 further supports the concept that multitype L2 immunization may preferentially expand cross-reactive B cells [22].
In summary, we show that vaccination with oligomeric Fla~L2 induces cross-protection against divergent HPV types from different oncogenic clades after challenge at both cutaneous and mucosal sites in relevant animal models. Following active immunization, oligomeric Fla-L2 fusions provided complete protection against infection and disease induced by both homologous and heterologous HPV challenge. The longevity of the cross-protective immune response was demonstrated for Fla~L2 vaccines for up to 1 year. Through the determination of the EPPT, passive transfer studies showed broader cross-protection afforded by L2 delivered as a flagellin fusion than by L1 VLPs. These findings suggest the merit of an oliogomeric L2-TLR5 agonist fusion approach and its potential superiority to L1-based vaccines for cross-protection against non-vaccinal HPV types. Dose ranging studies indicate the dose-sparing potential of the flagellin approach with significant cross-protection observed at doses as low as 1 μg.
Supplementary Material
Acknowledgments
Authors are grateful for technical assistance provided by Susana Pang (NIH), Karla Balogh (PennState University) as well as to Raymond Oomen, Mark Parrington, and Michael Watson (Sanofi Pasteur) for valuable discussions. We are also thankful to Yves Gried-Chambaz (Sanofi Pasteur) for building of L2-based HPV phylogenetic tree.
Funding: This study was funded by Sanofi Pasteur.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2014.04.032.
Footnotes
Ethical statement: Mouse studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal studies were performed with the prior approval of the Animal Care and Use Committee of Johns Hopkins University (protocol MO08M19). Rabbit studies were performed according to the quality service and ethical treatment policy of Covance Inc. All Covance programs are AAALAC International accredited, and meet or exceed USDA Research License requirements, as well as those of the “Guide for Care and Use of Laboratory Animals”. All animal studies are performed with the prior approval of the Institutional Animal Care and Use Committee.
Conflict of interests: SJ and RBSR are co-inventors on L2 patents licensed to Shantha Biotechnics Ltd., GlaxoSmithKline, PaxVax Inc. and Acambis Inc., and have received grant support from Sanofi Pasteur. The terms of these arrangements are managed by Johns Hopkins University in accordance with its conflict of interest policies. JTS is a co-inventor on U.S. Government owed patents on papillomavirus L1 vaccines that are licensed to Merck & Co. and GlaxoSmithKline and L2 vaccines that are licensed to Shantha Biotechnics Ltd., GlaxoSmithKline, PaxVax Inc., Acambis Inc. and Sanofi Pasteur.
References
- 1.Bernard HU, Burk RD, Chen Z, van DK, Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401(1):70–9. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alphs HH, Gambhira R, Karanam B, Roberts JN, Jagu S, Schiller JT, et al. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci U S A. 2008;105(15):5850–5. doi: 10.1073/pnas.0800868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newton SM, Kotb M, Poirier TP, Stocker BA, Beachey EH. Expression and immunogenicity of a streptococcal M protein epitope inserted in Salmonella flagellin. Infect Immun. 1991;59(6):2158–65. doi: 10.1128/iai.59.6.2158-2165.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong SH, Byun YH, Nguyen CT, Kim SY, Seong BL, Park S, et al. Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection. Vaccine. 2012;30(2):466–74. doi: 10.1016/j.vaccine.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 5.Skountzou I, Martin MD, Wang B, Koutsonanos D, Weldon W, Jacob J, et al. Salmonella flagellins are potent adjuvants for intranasally administered whole inactivated influenza vaccine. Vaccine. 2009;28(24):4103–12. doi: 10.1016/j.vaccine.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mejia AF, Culp TD, Cladel NM, Balogh KK, Budgeon LR, Buck CB, et al. Preclinical model to test human papillomavirus virus (HPV) capsid vaccines in vivo using infectious HPV/cottontail rabbit papillomavirus chimeric papillomavirus particles. J Virol. 2006;80(24):12393–7. doi: 10.1128/JVI.01583-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson KM, Kines RC, Roberts JN, Lowy DR, Schiller JT, Day PM. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J Virol. 2009;83(5):2067–74. doi: 10.1128/JVI.02190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199(7):926–35. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 9.Munoz A, Barcelo JR, Lopez-Vivanco G. Chemotherapy for bladder cancer. N Engl J Med. 2003;349(23):2272–3. doi: 10.1056/NEJM200312043492323. [DOI] [PubMed] [Google Scholar]
- 10.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 11.Walboomers JM, Melchers WJ, Mullink H, Meijer CJ, Struyk A, Quint WG, et al. Sensitivity of in situ detection with biotinylated probes of human papilloma virus type 16 DNA in frozen tissue sections of squamous cell carcinomas of the cervix. Am J Pathol. 1988;131(3):587–94. [PMC free article] [PubMed] [Google Scholar]
- 12.Walboomers JM, Fokke HE, Polak M, Volkers H, Houthoff HJ, Barents J, et al. In situ localization of human papilloma virus type 16 DNA in a metastasis of an endocervical adenocarcinoma. Intervirology. 1987;27(2):81–5. doi: 10.1159/000149723. [DOI] [PubMed] [Google Scholar]
- 13.Herrero R. Human papillomavirus (HPV) vaccines: limited cross-protection against additional HPV types. J Infect Dis. 2009;199(7):919–22. doi: 10.1086/597308. [DOI] [PubMed] [Google Scholar]
- 14.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 15.Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6. 4 years. Lancet. 2009;374(9706):1975–85. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Lowy DR, Smith-McCune KK, Melief CJM. The value of HPV vaccination. Nat Med. 2012;18(1):28–9. doi: 10.1038/nm0112-28. [DOI] [PubMed] [Google Scholar]
- 17.Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011;85(24):13253–9. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013;6(11):1242–50. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, et al. Cross-neutralization of cutaneous and mucosal papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337(2):365–72. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, et al. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol. 2007;81(24):13927–31. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J Virol. 2007;81(21):11585–92. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, et al. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst. 2009;101(11):782–92. doi: 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day PM, Pang YY, Kines RC, Thompson CD, Lowy DR, Schiller JT. A human papillomavirus (HPV) in vitro neutralization assay that recapitulates the in vitro process of infection provides a sensitive measure of HPV L2 infection-inhibiting antibodies. Clin Vaccine Immunol. 2012;19(7):1075–82. doi: 10.1128/CVI.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37(11):3040–53. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from Toll-like receptors. Science. 2004;304(5673):1014–8. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 26.Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol. 2006;7(10):1029–35. doi: 10.1038/ni1006-1029. [DOI] [PubMed] [Google Scholar]
- 27.Jagu S, Kwak K, Schiller JT, Lowy DR, Kleanthous H, Kalnin K, et al. Phylogenetic considerations in designing a broadly protective multimeric L2 vaccine. J Virol. 2013;87(11):6127–36. doi: 10.1128/JVI.03218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J, Sun PD. The structure of the TLR5-flagellin complex: a new mode of pathogen detection, conserved receptor dimerization for signaling. Sci Signal. 2012;5(216):e11. doi: 10.1126/scisignal.2002963. [DOI] [PubMed] [Google Scholar]
- 29.Yoon SI, Kurnasov O, Natarajan V, Hong M, Gudkov AV, Osterman AL, et al. Structural basis of TLR5-flagellin recognition and signaling. Science. 2012;335(6070):859–64. doi: 10.1126/science.1215584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Applequist SE, Rollman E, Wareing MD, Liden M, Rozell B, Hinkula J, et al. Activation of innate immunity, inflammation, and potentiation of DNA vaccination through mammalian expression of the TLR5 agonist flagellin. J Immunol. 2005;175(6):3882–91. doi: 10.4049/jimmunol.175.6.3882. [DOI] [PubMed] [Google Scholar]
- 31.Agrawal S, Agrawal A, Doughty B, Liden M, Rozell B, Hinkula J, et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171(10):4984–9. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 32.Aderem A, Hayashi F, Smith KD, Underhill DM, Ozinsky A, inventors. US20030044429A1. Toll-like receptor 5 ligand and method of use. 2003
- 33.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410(6832):1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 34.Song L, Zhang Y, Yun NE, Poussard AL, Smith JN, Smith JK, et al. Superior efficacy of a recombinant flagellin:H5N1 HA globular head vaccine is determined by the placement of the globular head within flagellin. Vaccine. 2009;27(42):5875–84. doi: 10.1016/j.vaccine.2009.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adar Y, Singer Y, Levi R, Tzehoval E, Perk S, Banet-Noach C, et al. A universal epitope-based influenza vaccine and its efficacy against H5N1. Vaccine. 2009;27(15):2099–107. doi: 10.1016/j.vaccine.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Song L, Nakaar V, Kavita U, Price A, Huleatt J, Tang J, et al. Efficacious recombinant influenza vaccines produced by high yield bacterial expression: a solution to global pandemic and seasonal needs. PLoS ONE. 2008;3(5):e2257. doi: 10.1371/journal.pone.0002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizel SB, Graff AH, Sriranganathan N, Ervin S, Lees CJ, Lively MO, et al. Flagellin-F1-V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clin Vaccine Immunol. 2009;16(1):21–8. doi: 10.1128/CVI.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton SM, Jacob CO, Stocker BA. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989;244(4900):70–2. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- 39.Leng J, Stout-Delgado HW, Kavita U, Jacobs A, Tang J, Du W, et al. Efficacy of a vaccine that links viral epitopes to flagellin in protecting aged mice from influenza viral infection. Vaccine. 2011;29(45):8147–55. doi: 10.1016/j.vaccine.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G, Tarbet B, Song L, Reiserova L, Weaver B, Chen Y, et al. Immunogenicity and efficacy of flagellin-fused vaccine candidates targeting 2009 pandemic H1N1 influenza in mice. PLoS ONE. 2011;6(6):e20928. doi: 10.1371/journal.pone.0020928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huleatt JW, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, et al. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine. 2008;26(2):201–14. doi: 10.1016/j.vaccine.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 42.Taylor DN, Treanor JJ, Strout C, Johnson C, Fitzgerald T, Kavita U, et al. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2. HA1 SI) Vaccine. 2011;29(31):4897–902. doi: 10.1016/j.vaccine.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2. 4xM2e) in healthy adults. Vaccine. 2011;29(32):5145–52. doi: 10.1016/j.vaccine.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 44.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. In: Davy C, Doorbar J, editors. Methods in molecular medicine. Vol. 119. Totowa, NJ: 2014. pp. 445–61. [DOI] [PubMed] [Google Scholar]
- 45.Johnson KM, Kenes RC, Roberts JN, Lowy DR, Schiller JT, Day PM. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J Virol. 2009;83(5):2067–74. doi: 10.1128/JVI.02190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(10):781–9. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 47.Howell-Jones R, Soldan K, Wetten S, Mesher D, Williams T, Gill ON, et al. Declining genital Warts in young women in England associated with HPV 16/18 vaccination: an ecological study. J Infect Dis. 2013;208(9):1397–403. doi: 10.1093/infdis/jit361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szarewski A, Skinner SR, Garland SM, Romanowski B, Schwarz TF, Apter D, et al. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low-risk HPV types (PATRICIA randomized trial): an unexpected observation. J Infect Dis. 2013;208(9):1391–6. doi: 10.1093/infdis/jit360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jagu S, Kwak K, Karanam B, Huh WK, Damotharan V, Chivukula SV, et al. Optimization of multimeric human papillomavirus L2 vaccines. PLoS ONE. 2013;8(1):e55538. doi: 10.1371/journal.pone.0055538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.