Abstract
OBJECTIVE
To assess the utility of an extensive restaging examination performed after the completion of neoadjuvant chemotherapy (NAC) but before radical cystectomy (RC) in the management of patients with advanced bladder cancer.
METHODS
We studied 62 patients who underwent NAC with the intent of proceeding to consolidative RC. A restaging examination, including endoscopic and bimanual examination, as well as cross-sectional imaging of the abdomen and pelvis, was performed after chemotherapy. The impact of restaging on clinical management was determined. In patients proceeding to RC, the degree of correlation between clinical stage (at diagnosis vs on restaging) and pathologic stage was determined.
RESULTS
Restaging altered the treatment course in 6 patients (9.7%) in whom RC was not performed because of restaging findings. An additional 56 patients (90.3%) proceeded to RC. In these patients, compared with clinical stage at diagnosis, the postchemotherapy clinical stage correlated more strongly with pathologic stage (κ = 0.02 vs 0.17). On multivariate analysis, diagnostic clinical stage was not associated with pathologic stage (P = .85), whereas postchemotherapy clinical stage was strongly predictive of pathologic stage (P <.01).
CONCLUSION
An extensive restaging examination altered treatment strategy in a small, but clinically significant subset of patients treated with NAC for bladder cancer. Furthermore, restaging allowed for more accurate prediction of pathologic stage after RC, thereby improving assessment of patient prognosis. Consideration should be given to incorporating a restaging evaluation into the standard management paradigm for bladder cancer.
Level-1 evidence supports the use of neoadjuvant chemotherapy (NAC) in the management of muscle-invasive bladder cancer.1,2 In recent years, these findings have resulted in the increased use of NAC before radical cystectomy (RC) in the United States.3 The enrollment criteria for the 2 largest randomized trials of NAC in bladder cancer2,4 required a clinical diagnosis of muscle-invasive disease (clinical stage T2-T4). After the completion of NAC, patients proceeded directly to cystectomy without a repeat staging examination. Based on these study protocols, this management strategy has become the standard of care for muscle-invasive bladder cancer.
At our institution, we routinely perform a restaging evaluation after the completion of NAC but before proceeding to RC. This evaluation involves a chest radiography or chest computed tomography scan (CT), cross-sectional imaging of the abdomen and pelvis (either CT or magnetic resonance imaging), endoscopic examination of the bladder under anesthesia, and a bimanual examination. This extensive restaging evaluation, including endoscopic and bimanual examination, is not routinely performed by all urologists, and its clinical utility is unclear.
Furthermore, in patients managed with RC alone, there are often discrepancies between the clinical stage assigned at the time of diagnosis and the pathologic stage determined by analysis of the RC specimen.5–7 The lack of correlation between clinical and pathologic stage compromises accurate assessment of patient prognosis at the time of diagnosis.8 In patients receiving NAC, there is likely to be an even greater discrepancy between clinical and pathologic stage, as NAC leads to pathologic downstaging in a significant percentage of patients.2 It is unknown whether a restaging evaluation performed after the completion of NAC allows for the more accurate prediction of pathologic stage after RC.
The purpose of the present study was thus 2-fold. First, we aimed to assess the clinical utility of an extensive restaging evaluation after NAC and determine how often the results of this restaging evaluation change patient management. Second, we investigated the correlation between clinical stage on postchemotherapy restaging evaluation and pathologic stage after RC, to determine whether restaging improves the assessment of patient prognosis.
MATERIALS AND METHODS
We performed a retrospective analysis on our institutional bladder cancer database. We identified patients with bladder cancer who elected to receive NAC with a plan to proceed to consolidative cystectomy, treated between January 2011 and July 2012. It is our institutional policy to recommend NAC to all patients with clinical T2-T4 tumors before proceeding to RC. Patients who received chemotherapy as part of a definitive bladder-sparing chemotherapy and radiation protocol were excluded, as were patients who had previously received chemotherapy or radiation for bladder cancer and subsequently developed recurrence.
All pathology slides were reviewed by a urologic pathologist at our institution. The NAC regimen was left to the discretion of the medical oncologists. Patients receiving gemcitabine and cisplatin or carboplatin received a minimum of 3 cycles of chemotherapy. Patients treated with methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy received 3 cycles of chemotherapy. Patients who did not complete the prescribed course of chemotherapy were excluded.
All patients underwent a restaging examination after completing chemotherapy and before proceeding to RC. The restaging examination included chest x-ray or chest CT, cross-sectional imaging of the abdomen and pelvis (either CT or magnetic resonance imaging), endoscopic examination of the bladder under anesthesia, and a bimanual examination. On restaging examination, patients with evidence of metastatic disease, lymph-adenopathy meeting response evaluation criteria in solid tumors (RECIST) criteria,9 or unresectable tumors were not considered candidates for RC. Patients without these findings proceeded to RC. A standard-template pelvic lymphadenectomy was performed at the beginning of the procedure. At the time of lymphadenectomy, lymph nodes that were concerning for metastatic disease were sent for frozen-section pathologic analysis intraoperatively. In patients with lymph node metastases confirmed by frozen section, the procedure was aborted and RC was not performed. In the remainder of patients, RC was completed and lower urinary tract reconstruction or diversion was performed.
Clinical stage of the bladder tumor was assigned at the time of diagnosis according to the American Joint Committee on Cancer clinical staging manual.10 After NAC, a second clinical stage was assigned based on results of the restaging evaluation. At the time of restaging, biopsy or transurethral resection of visible abnormalities in the bladder was performed when the operating surgeon judged that the results of biopsy may change the recommendation to proceed with RC. Patients with no evidence of residual tumor on restaging evaluation were deemed cT0. For patients with residual tumor, postchemotherapy stage was based on results of restaging biopsy or on a constellation of endoscopic findings at the time of restaging, imaging studies, and pathology results from the initial resection. Clinical stage was determined by consensus of the 2 attending physicians (M.J.S. and T.J.B.) and the primary author of the study (A.C.R.).
For each patient who proceeded to RC, the clinical stages at the time of diagnosis and on postchemotherapy restaging evaluation were compared with the pathologic stage after RC. Rates of upstaging and downstaging from clinical stage to pathologic stage were determined. Upstaging was defined as a more advanced pathologic stage than clinical stage, whereas downstaging was defined as a less advanced pathologic stage than clinical stage. The Cohen kappa statistic was used to quantify the degree of correlation of the initial diagnostic clinical stage and the post-chemotherapy stage with the pathologic stage after RC.
Two ordered logistic regression models were then performed to identify clinical factors associated with advanced pathologic stage after RC. Covariates in the first model included patient sex, age, smoking history, and initial diagnostic clinical stage. The second model contained the same covariates, with the exception of substituting postchemotherapy clinical stage for diagnostic clinical stage.
RESULTS
Between January 2011 and July 2012, 62 patients with bladder cancer underwent NAC with a plan to proceed to consolidative RC. The mean patient age was 64.4 years. Additional demographic data for the study population, data on tumor histology as determined by the initial diagnostic transurethral resection, and NAC regimen administered are shown in Table 1. More than 80% of patients were male and approximately 75% had a smoking history. More than 90% of tumors were urothelial carcinomas, all of which were high-grade tumors. Gemcitabine and cisplatin served as the NAC regimen in 76% of patients.
Table 1.
Demographic, tumor histology, and neoadjuvant chemotherapy data
| Variable | N (%) |
|---|---|
| Sex | |
| Male | 50 (81) |
| Female | 12 (19) |
| Smoking history | |
| Yes | 47 (76) |
| No | 14 (22) |
| Unknown | 1 (2) |
| Tumor histology | |
| Urothelial carcinoma | 57 (91) |
| Poorly differentiated carcinoma | 2 (3) |
| Adenocarcinoma | 1 (2) |
| Squamous cell carcinoma | 1 (2) |
| Small cell carcinoma | 1 (2) |
| Chemotherapy regimen | |
| Gemcitabine/cisplatin | 47 (76) |
| Gemcitabine/carboplatin | 3 (5) |
| Cisplatin/etoposide | 2 (3) |
| MVAC | 2 (3) |
| Pacitaxel/carboplatin | 2 (3) |
| Pacitaxel/gemcitabine | 1 (2) |
| Unknown | 5 (8) |
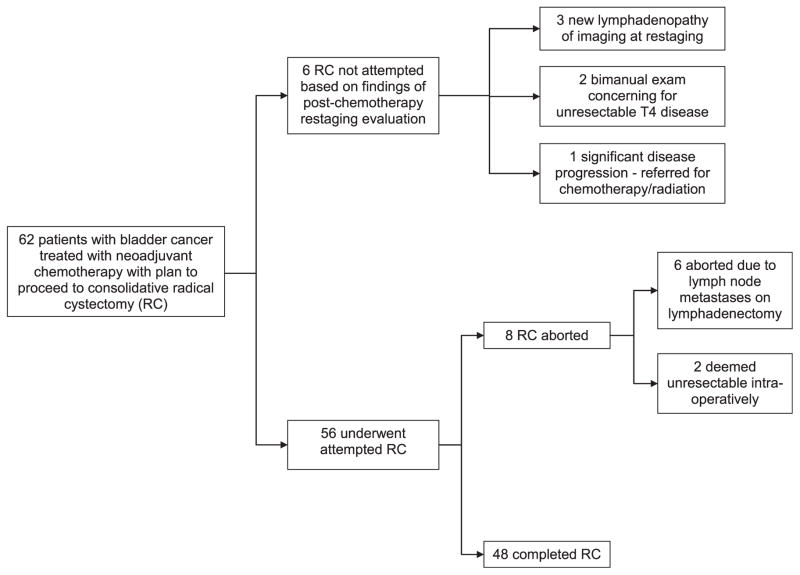
The course of treatment for the study patients is illustrated in Figure 1. Of the 62 patients, 48 (77.4%) ultimately completed RC after NAC. In 8 patients (12.9%), RC was attempted but aborted. Of the aborted cases, 6 (75%) were aborted because of the intraoperative finding of lymph node metastases, and 2 cases (25%) were deemed unresectable. In 6 patients (9.7%), RC was not attempted because of findings of the postchemotherapy restaging evaluation. Of these 6 patients, 3 (50%) had developed new pelvic lymphadenopathy on cross-sectional imaging, 2 (33%) were suspected of having unresectable T4 disease based on bimanual examination, and 1 (16.7%) had significant disease progression on endoscopic examination and biopsy and was referred for chemotherapy and radiation. At the time of restaging, no patients were found to have pulmonary metastases on chest imaging. No patients were unable to undergo cystectomy because of chemotherapy toxicity. No patients were denied cystectomy because of a complete response to chemotherapy.
Figure 1.
Course of treatment for study patients. RC, radical cystectomy.
At the time of restaging, 14 patients underwent biopsy or transurethral resection of visible abnormalities in the bladder. Management course was altered in only 1 of these 14 patients (7%). This patient was noted to have a significantly increased volume of small cell carcinoma and was referred for additional chemotherapy and radiation. The remainder of the patients who underwent biopsy proceeded to RC as planned.
The mean and median times from diagnosis to post-chemotherapy restaging examination were 163 days (standard deviation, 66 days) and 162 days (range, 50–558 days), respectively. Mean and median times from restaging examination to cystectomy were 35 days (standard deviation, 25 days) and 32 days (range, 4–109 days), respectively.
Table 2 shows distribution of clinical stage at the time of diagnosis, clinical stage on postchemotherapy restaging evaluation, and pathologic stage after RC. Compared with the clinical stage at the time of diagnosis, there was a significant shift toward lower stages on the restaging evaluation and on pathologic examination after RC.
Table 2.
Distribution of clinical and pathological stages
| Stage | Clinical Stage at Diagnosis, N (%) | Clinical Stage on Restaging Evaluation After Chemotherapy, N (%) | Pathologic Stage After RC, N (%) | Clinical Stage on Restaging in Patients who Completed RC, N (%) |
|---|---|---|---|---|
| T0 | 0 (0) | 22 (35) | 12 (25) | 21 (44) |
| TIS | 0 (0) | 1 (2) | 7 (15) | 0 (0) |
| Ta | 0 (0) | 0 (0) | 3 (6) | 0 (0) |
| T1 | 3 (5) | 5 (8) | 5 (10) | 5 (10) |
| T2 | 36 (58) | 20 (32) | 10 (21) | 13 (27) |
| T3 | 21 (34) | 9 (15) | 5 (10) | 7 (15) |
| T4 | 2 (3) | 5 (8) | 6 (13) | 2 (4) |
| Total | 62 | 62 | 48 | 48 |
RC, radical cystectomy.
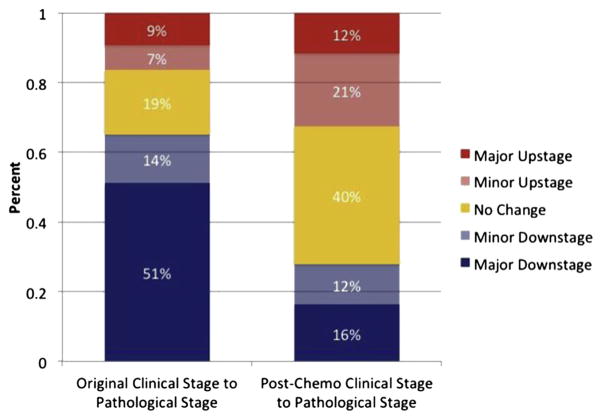
Figure 2 illustrates how clinical stage at diagnosis, and clinical stage on postchemotherapy restaging evaluation correlate with pathologic stage after RC. Initial diagnostic stage was poorly correlated with pathologic stage (κ =0.02), whereas the stage assigned on postchemotherapy restaging evaluation was more strongly correlated with pathologic stage (κ = 0.17). When comparing the clinical stage at the time of diagnosis to pathologic stage after RC, 30 patients (62.4%) were downstaged, 9 patients (18.8%) were upstaged, and the 2 stages were similar in 9 patients (18.8%). When comparing the postchemotherapy clinical stage with the pathologic stage after RC, 12 patients (25.0%) were downstaged, 17 patients (35.4%) were upstaged, and the 2 stages were similar in 19 patients (39.6%).
Figure 2.
Correlation between clinical and pathologic stages. Groups defined as follows: major downstage, pathologic stage less than clinical stage by >1 stage interval (eg, clinical stage T4, pathologic stage T2). Minor downstage: pathologic stage less than clinical stage by 1 stage interval (eg, clinical stage T4, pathologic stage T3). Major upstage: pathologic stage greater than clinical stage by >1 stage interval (eg, clinical stage T1, pathologic stage T3). Minor upstage: pathologic stage greater than clinical stage by 1 stage interval (eg, clinical stage T1, pathologic stage T2). (Color version available online.)
Of the 48 patients who completed RC, 12 patients (25.0%) had no residual tumor in the final pathology specimen (pT0). Of these 12 patients, 8 (66.7%) were clinical stage T0 on postchemotherapy restaging evaluation, 3 (25.0%) were cT2, and 1 (8.3%) was cT3. The stage at diagnosis in these 12 patients was cT2 in 8 (66.7%), cT3 in 3 (25.0%), and cT4 in 1 (8.3%).
On postchemotherapy staging evaluation, 21 patients were staged as cT0 and proceeded to RC. Of these, 8 patients (38.1%) were pT0 and an additional 6 (28.6%) had non—muscle-invasive tumors at RC.
Downstaging from diagnostic clinical stage to post-chemotherapy clinical stage was strongly associated with ultimate downstaging after RC. When compared with the initial clinical stage, 43 patients were either downstaged or had no stage change on postchemotherapy evaluation. Only 6 of these patients (14.0%) were ultimately upstaged from their initial clinical stage to pathologic stage after RC. In comparison, 4 of the 5 patients (80%) who were upstaged on postchemotherapy evaluation were ultimately upstaged after RC. There was no association between chemotherapeutic regimen received and pathologic stage after RC (P = .31).
Two ordered logistic regression models were run to identify associations between preoperative variables and pathologic stage after RC. Both models contained patient age, sex, and smoking history as covariates in the model, whereas the first model contained initial diagnostic clinical stage and the second model contained clinical stage on postchemotherapy staging evaluation. In both models, neither age and sex nor smoking history was significantly associated with pathologic stage (all P values >.05). Likewise, in the first model, no significant association was seen between clinical stage at diagnosis and pathologic stage after RC (P = .85). However, in the second model, a significant association was seen between postchemotherapy clinical stage and advanced pathologic stage (P <.01).
COMMENT
The present study evaluates the utility of an extensive restaging examination performed before RC for patients undergoing NAC for bladder cancer. We found that this restaging examination changes management in a small but significant number of patients and enables more accurate prediction of pathologic stage in those patients who proceed to RC.
Of the 62 patients in the present study, 6 (9.7%) were spared a potentially unnecessary RC by a restaging evaluation. This represents a relatively small percentage of the study population, and thus the merits of completing a restaging evaluation in all patients are debatable. We feel, however, that the potential benefit to patients who do not undergo RC is significant. RC is an invasive procedure, with a significant complication rate and the potential for peri-operative mortality.11–13 After NAC, patients with residual muscle-invasive or node-positive disease are thought to potentially harbor chemoresistant tumors and clearly have significantly worse survival than those patients with a favorable response to chemotherapy.14 In these non-responders, the significant morbidity of RC may outweigh any potential survival benefit. Restaging allows for the identification of many of these nonresponders, sparing them a potentially unnecessary operation. Furthermore, the morbidity of a restaging evaluation is minimal. Thus, even if the restaging evaluation only impacts a small number of patients, the significant morbidity it spares these patients may justify the routine restaging of all patients before RC.
Furthermore, the additional costs associated with restaging appear to be minimal. Charge data from our institution suggest that a full restaging examination costs approximately $4600. In this study, restaging 62 patients prevented a potentially unnecessary cystectomy in 6 patients, at a mean cost of $37,100 per cystectomy (inclusive of charges associated with procedure and postoperative stay). Restaging thus results in an additional cost of approximately $1010 per patient. Notably, the costs of potential complications requiring readmission in the 6 patients spared cystectomy were not included in this analysis. A recent report of prospectively collected complication data suggests that 58% of patients experience a complication within 90 days after discharge after RC.8 Thus, avoiding complications in the 6 patients spared cystectomy by a restaging examination may result in cost-neutrality or perhaps even cost saving.
Although restaging potentially spared 9.7% of the study population from RC, it failed to identify another 8 patients (12.9%) who likely proceeded to RC unnecessarily. This includes 2 patients with unresectable tumors that were not identified by bimanual examination, and 6 patients with lymph node involvement that was not seen on preoperative imaging. This underscores the need for improved imaging modalities and biomarkers to better identify patients who have not responded to chemotherapy before surgery.
An additional benefit of the restaging evaluation was that restaging allowed for more accurate prediction of pathologic stage in patients proceeding to RC. An individual patient’s response to NAC is highly variable. Depending on the chemotherapy regimen used, pathologic complete-response rates have been reported to vary from 7% to 38%.2,4,15 Furthermore, we are currently unable to accurately differentiate those patients likely to respond to chemotherapy from those likely to progress.16 In the present study, this variable response to chemotherapy resulted in significant discrepancy between the clinical stage at diagnosis and the pathologic stage after RC. In comparison, there was a significantly stronger correlation between the stage assigned on the post-chemotherapy evaluation and the pathologic stage.
The stronger correlation between postchemotherapy clinical stage and pathologic stage allows for an improved assessment of patient prognosis before RC. It appears that the benefit of NAC may extend only to those patients whose disease does not progress through chemotherapy.14 Our findings suggest that chemotherapy nonresponders can be identified on a restaging evaluation after the completion of chemotherapy. In fact, 80% of patients with evidence of disease progression on restaging evaluation were ultimately upstaged after RC, compared with a 14.0% rate of upstaging in patients with no evidence of progression on restaging. Perhaps nonresponders could be identified on a restaging evaluation midway through a course of NAC, and these patients could be taken straight to RC, avoiding potential morbidity associated with additional chemotherapy cycles and avoiding further delay in definitive treatment of their tumors.
We acknowledge that our practice of foregoing or abandoning cystectomy in patients with persistent or progressive lymph node metastases after chemotherapy can be debated. Certainly, cystectomy may result in disease palliation or improved cancer control in a subset of these patients. Particularly, medically fit patients with minimal evidence of disease progression may derive benefit from cystectomy in this setting. However, we feel there is insufficient evidence that surgery significantly improves cancer-specific survival in this setting. Thus, for most patients, we feel the potential morbidity of cystectomy outweighs the potential benefits of surgery. Furthermore, the recovery from an invasive surgical procedure will likely delay the administration of second-line chemotherapy regimens, which may be of some benefit in patients with progressive locoregional disease.
The present study is not without limitations. There was some variability in the method used to assign clinical stage at the time of postchemotherapy restaging, as a biopsy was performed in some, but not all, patients. This practice likely led to an overestimation of the number of patients staged cT0 at the time of restaging evaluation. Moreover, interindividual variation in clinical stage assignment may bias our findings, although all staging data were re-reviewed to ensure uniformity when assigning clinical stage. Additionally, bimanual examination was used at the time of restaging to identify unresectable tumors. However, others have reported variability in the accuracy of the bimanual examinations,17 and thus, some tumors thought to be locally invasive may in fact have been resectable had these patients proceeded to RC. Finally, the retrospective nature of the study design may bias our findings, and additional prospective studies are needed to confirm the findings of this manuscript.
CONCLUSION
These data suggest that restaging after NAC alters treatment course in a small, but clinically significant, percentage of patients with advanced bladder cancer. Furthermore, the restaging evaluation improves prediction of pathologic stage, and thus allows for a more accurate assessment of patient prognosis before RC. These benefits, combined with the minimal morbidity of the restaging examination, argue that restaging could be routinely incorporated into the treatment paradigm for muscle-invasive bladder cancer to reduce unnecessary application of RC and to provide surgeons and patients with more accurate staging information on which to base important clinical decisions.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Griffiths G, Hall R, Sylvester R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–2177. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 3.Fedeli U, Fedewa SA, Ward EM. Treatment of muscle invasive bladder cancer: evidence from the National Cancer Database, 2003 to 2007. J Urol. 2011;185:72–78. doi: 10.1016/j.juro.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet. 1999;354:533–540. [PubMed] [Google Scholar]
- 5.McLaughlin S, Shephard J, Wallen E, et al. Comparison of the clinical and pathologic staging in patients undergoing radical cystectomy for bladder cancer. Int Braz J Urol. 2007;33:25–31. doi: 10.1590/s1677-55382007000100005. [DOI] [PubMed] [Google Scholar]
- 6.Shariat SF, Palapattu GS, Karakiewicz PI, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol. 2007;51:137–149. doi: 10.1016/j.eururo.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Svatek RS, Shariat SF, Novara G, et al. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. 2011;107:898–904. doi: 10.1111/j.1464-410X.2010.09628.x. [DOI] [PubMed] [Google Scholar]
- 8.Canter D, Long C, Kutikov A, et al. Clinicopathological outcomes after radical cystectomy for clinical T2 urothelial carcinoma: further evidence to support the use of neoadjuvant chemotherapy. BJU Int. 2011;107:58–62. doi: 10.1111/j.1464-410X.2010.09442.x. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Greene FL. AJCC cancer Staging Manual. New York: Springer; 2010. American Joint Committee on Cancer and American Cancer Society. [Google Scholar]
- 11.Novara G, De Marco V, Aragona M, et al. Complications and mortality after radical cystectomy for bladder transitional cell cancer. J Urol. 2009;182:914–921. doi: 10.1016/j.juro.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–174. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Yuh BE, Nazmy M, Ruel NH, et al. Standardized analysis of frequency and severity of complications after robot-assisted radical cystectomy. Eur Urol. 2012;62:806–813. doi: 10.1016/j.eururo.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115:4104–4109. doi: 10.1002/cncr.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weight CJ, Garcia JA, Hansel DE, et al. Lack of pathologic down-staging with neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma of the bladder: a contemporary series. Cancer. 2009;115:792–799. doi: 10.1002/cncr.24106. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neo-adjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61:1229–1238. doi: 10.1016/j.eururo.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Ploeg M, Kiemeney LA, Smits GA, et al. Discrepancy between clinical staging through bimanual palpation and pathological staging after cystectomy. Urol Oncol. 2012;30:247–251. doi: 10.1016/j.urolonc.2009.12.020. [DOI] [PubMed] [Google Scholar]