Abstract
We examined the effect of therapeutic anticoagulation on overall survival in men with metastatic castration-resistant prostate cancer (mCRPC) receiving first-line docetaxel chemotherapy. Anticoagulant use (low-molecular-weight heparin [LMWH] or warfarin) was retrospectively ascertained from a large single-institution database; all patients who were prescribed anticoagulants had a clinical indication for anticoagulation (ie, deep vein thrombosis or pulmonary embolism, or both). Our study found that anticoagulant use was an independent predictor of improved survival in men with mCRPC receiving docetaxel.
Background
Anticoagulants have been postulated to possess antitumor activity, although clinical data supporting this claim are conflicting. No definitive data exist on the clinical impact of anticoagulation therapy in patients with prostate cancer. The aim of this study was to investigate the association between therapeutic anticoagulant use and survival in men with metastatic castration-resistant prostate cancer (mCRPC) receiving docetaxel chemotherapy.
Patients and Methods
We retrospectively reviewed the records of 247 consecutive patients with mCRPC who received first-line docetaxel chemotherapy between 1998 and 2010 at a single institution. Among them, 29 patients (11.7 %) received therapeutic anticoagulation (low-molecular-weight heparin [LMWH] or warfarin) for the treatment of venous thromboembolism. Univariate and multivariable Cox proportional hazards regression models were used to investigate the effect of anticoagulant use on overall survival.
Results
In univariate analysis, anticoagulant use was associated with improved survival (hazard ratio [HR], 0.61; P = .024). Median survival was 20.9 months in the anticoagulation group versus 17.1 months in the control group (P = .024). In multivariable analysis, anticoagulant use remained a significant predictor of survival after adjusting for other baseline prognostic factors (HR, 0.49; P = .023). When each anticoagulant was considered separately in the multivariable model, LMWH remained significantly prognostic for survival (HR, 0.48; P = .035), whereas warfarin use did not.
Conclusions
Anticoagulant use (LMWH in particular) is an independent predictor of improved survival in men with mCRPC receiving docetaxel. These data provide the impetus to further explore the antitumor properties of anticoagulants in patients with prostate cancer and warrant validation in prospective studies.
Keywords: Anticoagulation, Docetaxel, Metastatic castration-resistant prostate cancer, Overall survival
Introduction
Tumor-mediated activation of the coagulation cascade leads to dysregulation of hemostasis and could result in venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism in patients with cancer.1 VTE is a major cause of cancer-related morbidity and mortality and has been validated as an independent risk factor for mortality.2,3 In addition, various tumor-associated coagulation factors such as tissue factor, fibrin, thrombin, and plasmin are implicated in mechanisms of tumor cell survival and progression, including tumor stroma formation,4 tumor cell migration and adhesion,5-7 antitumor immune evasion,8,9 induction of oncogenes,10 and angiogenesis.11-13 Furthermore, several anticoagulant agents have been found to possess various antitumor properties in addition to their antithrombotic effects.14
A number of studies have evaluated the association between the use of anticoagulants and clinical outcomes in patients with cancer. Low-molecular-weight heparin (LMWH) and unfractionated heparin have both been associated with enhanced tumor responses to chemotherapy and improved progression-free survival (PFS) when combined with various chemotherapy regimens in a number of cancer types.15-18 In patients with VTE, LMWH has consistently demonstrated superior survival benefits over unfractionated heparin or warfarin, without a significant difference in VTE recurrence.19-22 However, in patients who have no therapeutic or prophylactic indication for anticoagulation, anticoagulant use has produced conflicting survival outcomes in randomized controlled trials.23-26 However, the majority of these trials included heterogeneous patient populations with various tumor types (and different disease stages) and included only a small number of patients with prostate cancer.
To date, no definitive data exist on the impact of anticoagulation therapy on overall survival in patients with advanced prostate cancer. Hence, we conducted a single-institution retrospective analysis to evaluate the impact of therapeutic anticoagulation on survival in patients with metastatic castration-resistant prostate cancer (mCRPC) receiving first-line docetaxel chemotherapy.
Patients and Methods
Patient Selection and Treatment
Data were retrospectively collected on consecutive patients diagnosed with mCRPC and treated with first-line docetaxel chemotherapy between 1998 and 2010 at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland. All patients had pathologically confirmed adenocarcinoma of the prostate with evidence of progressive metastatic disease either by prostate-specific antigen (PSA) or clinical/radiographic criteria. All patients received first-line docetaxel-containing chemotherapy at the US Food and Drug Administration—approved dose and schedule or as participants in clinical trials incorporating first-line docetaxel at the same dose and schedule (ie, docetaxel 75 mg/m2 administered intravenously every 21 days together with prednisone 5 mg twice daily).
Data Collection and Analysis
Demographic data, type of primary treatment (surgery vs. radiation), Gleason score, Eastern Cooperative Oncology Group (ECOG) performance status, baseline PSA level, number and location of metastatic lesions, hematologic and metabolic laboratory parameters, previous treatment history, and number of chemotherapy cycles were determined from patient records. Data were also collected on anticoagulant use, type of anticoagulant, indication for anticoagulation therapy, and duration of anticoagulant use. Treatment response and disease progression characteristics were captured according to the recommendations of the Prostate Cancer Clinical Trials Working Group.27
Patients were divided into 2 groups: those who received therapeutic anticoagulation and those who did not. The distribution of baseline characteristics was compared between the groups (for descriptive purposes only) using a χ2 test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Overall survival (the primary outcome measure of interest) was defined as the time from the first chemotherapy treatment with docetaxel to the date of death from any cause. Patients who had not died at last follow-up were censored at that time point. Secondary outcome measures included PSA response rates, objective response rates, and PFS estimates. Survival analysis was performed using the Kaplan-Meier method, and differences between curves were sought using the log-rank test. Univariate and multivariable analyses were performed using Cox proportional hazards analysis to determine if anticoagulant use was an independent prognostic factor for survival. The specific anticoagulants used (LMWH or warfarin) were also studied with respect to their effect on survival.
All statistical tests were 2-sided, and a P value of < .05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics, version 20.0 (SPSS Inc, Chicago, IL).
Results
Patient Characteristics
Between 1998 and 2010, a total of 247 consecutive patients with mCRPC who had received docetaxel as first-line chemotherapy were identified. Among them, 29 patients (11.7 %) were receiving therapeutic anticoagulation for a concomitant VTE during the time of their chemotherapy treatment. The median age was 68 years (range, 44-84 years), and the majority of patients were white (76%). Baseline characteristics including ECOG status, PSA level, Gleason score, previous treatment history, location and number of metastatic lesions, and baseline laboratory values were well balanced between the anticoagulated and nonanticoagulated groups (Table 1).
Table 1.
Baseline Characteristics
| Variable | Anticoagulation (n = 29) |
No Anticoagulation (n = 218) |
P Value |
|---|---|---|---|
| Clinical Characteristics | |||
| Race, n (%) | .184 | ||
| White | 20 (71.4) | 167 (77) | |
| Nonwhite | 9 (28.6) | 52 (23) | |
| Median Age, (range) years | 65.7 (44.6-84.5) | 68.5 (41.8-84.1) | .694 |
| ECOG Performance Score, n (%) | .647 | ||
| 0 | 5 (31.2) | 53 (37.1) | |
| ≥1 | 11 (68.8) | 90 (62.9) | |
| Gleason Score, n (%) | .353 | ||
| ≤6 | 3 (13) | 10 (5.6) | |
| 7 | 7 (30.4) | 68 (37.8) | |
| ≥8 | 13 (56.5) | 102 (56.7) | |
| Treatment History | |||
| Primary Treatment, n (%) | .564 | ||
| Surgery | 8 (29.6) | 42 (20.7) | |
| Radiation | 8 (29.6) | 77 (37.9) | |
| Both | 4 (14.8) | 42 (20.7) | |
| None | 7 (25.9) | 42 (20.7) | |
| Median Number of Previous Hormonal Therapies (Range) | 2 (1-4) | 2 (0-5) | .872 |
| Bisphosphonate Use, n (%) | 6 (21.4) | 69 (34.8) | .158 |
| Median number Of Chemotherapy Cycles (range) | 6 (2-18) | 6 (1-19) | .869 |
| Biochemical Values, median (range) | |||
| Hemoglobin, g/dL | 13 (8-15) | 12 (7-16) | .280 |
| AST (U/L) | 26 (11-115) | 25 (10-207) | .639 |
| ALT (U/L) | 21 (10-112) | 20 (7-125) | .108 |
| Albumin (mg/dL) | 4.1 (3.2 -4.9) | 4.1 (3-5) | .981 |
| Alkaline phosphatase (U/L) | 126 (54-1949) | 134.5 (12-2986) | .805 |
| Baseline PSA (ng/mL) | 128 (0-1377) | 91 (0-5326) | .363 |
| Metastatic Lesions, n (%) | |||
| Presence of Bone Metastases | 26 (92.9) | 193 (89.8) | .606 |
| ≤10 lesions | 3 (17.9) | 31 (24.7) | .562 |
| >10 lesions | 23 (82.1) | 162 (75.3) | |
| Presence of Lymph Node Metastases | 18 (64.3) | 106 (49.3) | .136 |
| ≤5 nodes | 5 (27.8) | 22 (20.8) | .504 |
| >5 nodes | 13 (72.2) | 84 (79.2) | |
| Presence of Visceral Metastases a | 3 (10.3) | 34 (15.5) | .462 |
| Presence of Measurable Disease b | 12 (42.9) | 85 (39.4) | .721 |
| Anticoagulation Therapy Details | |||
| Indication for Anticoagulation, n (%) | |||
| DVT | 15 (51.7) | NA | |
| PE | 9 (31.0) | NA | |
| Both DVT and PE | 5 (17.2) | NA | |
| Type of Anticoagulant, n (%) | |||
| Warfarin | 12 (41.4) | NA | |
| LMWH | 17 (58.6) | NA | |
| Duration of anticoagulation, mo, median (range) | 5.7 (0.56-35.29) | NA |
Abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; DVT = deep vein thrombosis; LMWH = low-molecular-weight heparin; NA = not available; PE = pulmonary embolism; PSA = prostate-specific antigen.
Visceral metastases consists of combined lung and liver metastases.
Presence of visceral or lymph node lesions, or both.
At the time of docetaxel initiation, 39.3% of all men (97 of 247) had measurable soft tissue disease and were included in tumor response rate analysis according to Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 criteria.28 Median PSA level was numerically higher in the anticoagulated group than in the nonanticoagulated group (128 ng/mL vs. 91 ng/mL, respectively). Other disease-related characteristics are summarized in Table 1.
All patients receiving anticoagulation therapy had a concurrent or previous diagnosis of VTE. The indications for anticoagulation were DVT in 15 patients (51.7%), PE in 9 patients (31.0%), and both DVT and PE in 5 patients (17.2%). Seventeen patients (58.6%) received LMWH, and 12 patients (41.4%) received warfarin. The median duration of anticoagulation therapy was 5.7 months (range, 0.6-35.3 months) (Table 1).
Survival and Other Treatment Outcomes
In univariate analysis (Table 2), use of any anticoagulant (ie, either LMWH or warfarin) was associated with improved survival compared with no anticoagulant (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.40-0.94; P = .024) (Figure 1A). Median survival was 20.9 months in the group receiving anticoagulation versus 17.1 months in the group not receiving anticoagulation (log-rank P = .024). Furthermore, patients receiving > 6 months of anticoagulation had even superior survival compared with those receiving < 6 months of anticoagulation (HR, 0.47; 95% CI, 0.26-0.88; P = .018). When overall survival was analyzed for each anticoagulant separately, a significant improvement in survival was observed with LMWH use (HR, 0.58; 95% CI, 0.34-0.99; P = .048) but not with warfarin use (HR, 0.82; 95% CI, 0.55-1.28; P = .23) (Figure 1, B and C).
Table 2.
Univariate Analysis of Overall Survival
| Variable | Categories | HR (95% CI) | P Value |
|---|---|---|---|
|
Anticoagulation
use |
Yes versus no | 0.61 (0.40-0.94) | .024 |
| LMWH | Yes versus no anticoagulation |
0.58 (0.34-0.99) | .048 |
| Warfarin | Yes versus no anticoagulation |
0.82 (0.55-1.28) | .230 |
|
Length of
anticoagulation, mo |
>6 versus ≤6 | 0.47 (0.26-0.88) | .018 |
| Age, years | ≤65 versus >65 | 0.87 (0.65-1.17) | .354 |
|
Primary
treatment |
Radiation alone versus surgery ± radiation |
1.19 (0.88-1.59) | .260 |
|
No. of
chemotherapy cycles |
≥6 versus <6 cycles |
0.62 (0.46-0.84) | .002 |
| Gleason score | 7 versus ≤6 | 1.37 (0.71-2.62) | .347 |
| 8-10 versus ≤6 | 1.60 (0.85-3.00) | .144 | |
|
No. of hormonal
therapies |
Continuous | 0.94 (0.78-1.13) | .510 |
|
Bisphosphonate
use |
Yes versus no | 0.86 (0.63-1.17) | .340 |
| ECOG score | ≥1 versus 0 | 1.60 (1.10-2.35) | .013 |
|
Hemoglobin
(g/dL) |
<12.0 versus ≥12.1 |
2.18 (1.63-2.94) | <.001 |
| Albumin (U/L) | <4 versus ≥4 | 2.64 (1.66-4.22) | <.001 |
|
Alkaline
phosphatase (U/L) |
≥140 versus <140 |
1.72 (1.30-2.28) | <.001 |
|
AST or ALT
elevation, or both (>40 U/L) |
Yes versus no | 2.45 (1.66-3.62) | <.001 |
|
Lymph node
metastases |
Yes versus no | 0.96 (0.73-1.27) | .786 |
|
Visceral
metastases |
Yes versus no | 1.98 (1.36-2.87) | <.001 |
|
Baseline (log)
PSA |
Continuous | 1.11 (1.02-1.21) | .017 |
Bold values indicate statistically significant results.
Abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; HR = hazard ratio; LMWH = low-molecular-weight heparin; PSA = prostate-specific antigen.
Figure 1.
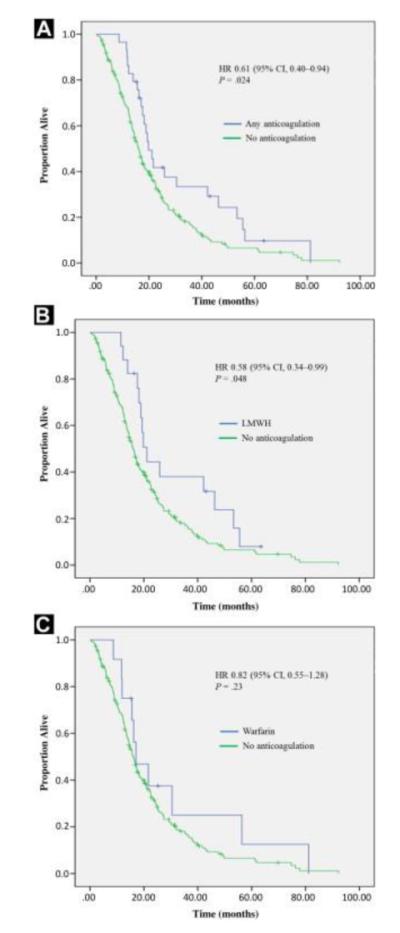
Kaplan-Meier Curves Depicting the Effect of (A) any Anticoagulation, (B) Low-Molecular-Weight Heparin (LMWH), and (C) Warfarin on Overall Survival
Other clinical factors that showed statistically significant correlations with survival in univariate analyses included number of chemotherapy cycles received, ECOG performance status, presence of anemia, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation, low serum albumin level, elevated alkaline phosphatase level, baseline PSA level, and presence of visceral (ie, liver or lung) metastases (Table 2).
Anticoagulant use remained a significant predictor of overall survival in multivariable Cox regression analysis (HR, 0.49; 95% CI, 0.26-0.90; P = .023) (Table 3). In addition, LMWH use was also significantly associated with survival (HR, 0.48; 95% CI, 0.24-0.95; P = .035), whereas warfarin use was not (HR, 0.53; 95% CI, 0.13-2.16; P = .374). Number of chemotherapy cycles, AST/ALT elevation, ECOG score, and anemia also showed significant association with survival in multivariable analysis (Table 3).
Table 3.
Multivariable Analysis of Overall Survival
| Variable | HR (95% CI) | P Value |
|---|---|---|
|
Any anticoagulant versus no
anticoagulation |
0.487 (0.26-0.90) | .023 |
| LMWH versus no anticoagulation | 0.480 (0.24-0.95) | .035 |
| Warfarin versus no anticoagulation | 0.527 (0.13-2.16) | .374 |
|
Alkaline phosphatase: ≥140
versus <140 U/L |
1.400 (0.94-2.08) | .097 |
|
AST or ALT elevation (>40 U/L),
or both, present versus absent |
2.638 (1.55-4.50) | <.001 |
| Albumin: <4 versus ≥4 mg/dL | 1.832 (0.95-3.53) | .103 |
|
Number of chemotherapy cycles:
≥6 versus <6 cycles |
0.490 (0.33-0.72) | <.001 |
| Hemoglobin: <12 versus ≥12 g/dL | 1.511 (0.99-2.28) | .002 |
| ECOG score: ≥1 versus 0 | 1.531 (1.03-2.29) | .037 |
Bold values indicate statistically significant results.
Abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; HR = hazard ratio; LMWH = low-molecular-weight heparin.
Additional treatment outcomes, including objective tumor responses (in patients with measurable disease), best PSA response, and PFS, did not show statistically significant differences between the anticoagulation and control groups (Table 4)
Table 4.
Treatment Response and Progression-Free Survival
| Variable | Anticoagulation (n = 29) | No Anticoagulation (n = 219) | P Value |
|---|---|---|---|
| Objective tumor response, n (%) | 5 of 12 (41.7) | 35 of 80 (43.5) | .496 |
| Best PSA response, % reduction, median (range) | 57.1 (0-98.2) | 57.2 (0-99.7) | .613 |
| Progression-free survival, mo, median (range) | 7.5 (1-36) | 7.0 (0-79) | .535 |
Abbreviation: PSA = prostate-specific antigen.
Discussion
Tumor-associated dysregulation of the coagulation system has long been speculated to be associated with proliferation and spread of cancer cells.14 Thus the potential antitumor effects of anticoagulants have been explored, and several mechanisms besides their anticoagulating properties have been identified. For example, LMWH exerts an antiangiogenic effect through fibrin structure modification, inhibition of the endothelial/tissue factor pathway, and direct inhibition of ligand binding to the VEGF receptor.12,13,29 Heparin has also been shown to inhibit tumor metastasis through inhibition of vascular cell adhesion receptors P- and L-selectin,30 modulation of chemokines,31 and inhibition of heparinase activity.32 Notably, Drago et al demonstrated that heparin significantly reduced metastasis in a murine CRPC model.33
The antitumor properties of anticoagulants have been further supported by demonstration of improvements in clinical outcomes in patients with cancer in several prospective trials.23 Prandoni et al first demonstrated the survival benefit of LMWH compared with unfractionated heparin in patients with cancer and VTE (without differences in recurrence rates of VTE).34 Survival benefits of LMWH in patients with VTE have been further validated by subsequent meta-analyses,35,36 which has led to several randomized controlled trials to specifically assess the survival benefit of LMWH in patients without VTE. FAMOUS (Fragmin Advanced Malignancy Outcome Study), which randomized 385 patients with various advanced cancers to receive either dalteparin or placebo for 12 months, showed a nonsignificant trend toward a survival advantage in the group treated with dalteparin.23 Furthermore, the MALT (Malignancy and Low Molecular Weight Heparin Therapy) trial showed that a 6-week course of nadroparin in 302 patients with advanced malignancy without VTE resulted in significant improvements in survival compared with placebo (HR, 0.75; 95% CI, 0.59-0.96).24 However, a subsequent similar randomized study by Sideras et al evaluating dalteparin in patients with advanced cancers failed to confirm the survival benefit.25 Both studies included only a small number of patients with prostate cancer (18 patients in each study). A more recent open-label randomized trial by van Doormaal et al included a significant proportion of patients with CRPC (n = 197) and showed a 21% relative reduction in risk of death among patients with prostate cancer receiving nadroparin versus no anticoagulation (median survival, 20.0 vs. 17.5 months), although this difference did not reach statistical significance.37
Our retrospective analysis of 247 consecutive patients with mCRPC starting docetaxel-based chemotherapy showed that men who received therapeutic anticoagulation (all of whom also had a documented VTE) demonstrated improved survival compared with the control group that did not receive anticoagulation. This finding is particularly interesting because the control group had comparable baseline prognostic characteristics but had no VTE (which is an indicator of poor survival in patients with cancer).2,3 This finding lends further support to the notion that anticoagulants may possess antitumor activity in men with mCRPC.
In our analysis, the survival benefit was more robust with LMWH use than with warfarin use (the survival trend with warfarin was not statistically significant). This observation is consistent with previous reports.38-40 Despite preclinical evidence of anti-metastatic41,42 and antiangiogenic43 effects of warfarin, a survival benefit with warfarin has not been demonstrated in patients with cancer. The first randomized prospective study, the Veterans Administration Cooperative Study No. 75 (evaluating the effect of warfarin on survival in lung, colon, head and neck, and prostate cancers) showed significant improvements in survival only in patients with small-cell lung cancer.26 However, subsequent randomized controlled trials failed to confirm the survival benefit of warfarin in patients with small-cell lung cancer.44,45 In addition, several randomized trials and meta-analyses comparing LMWH versus warfarin consistently showed a superior survival benefit of LMWH over warfarin in various solid tumor types.38-40
Despite the lack of specific recommendations for optimal duration of anticoagulation therapy in patients with cancer and established VTE, the majority of these patients receive anticoagulation therapy for 6 months or longer. Median duration of anticoagulation therapy in our study was 6 months, and the observed survival benefit was more pronounced in patients receiving anticoagulation for > 6 months. Previous prospective trials assessing the survival benefit of anticoagulants used various durations of anticoagulation (from 4 weeks to lifelong).46,47 This wide variation in duration of anticoagulation therapy for the purposes of cancer control precludes determination of appropriate anticoagulation duration and needs to be addressed in future studies.
Several prospective studies suggest that anticoagulation may also enhance the tumor response to cytotoxic chemotherapy. A phase II trial of single-agent docetaxel in combination with enoxaparin in patients with metastatic non—small-cell lung cancer showed a tumor response rate of 53% and a PFS duration of 5 months, which was longer than historical data in this setting, suggesting a synergistic effect of LMWH and docetaxel chemotherapy.17 An improvement in chemotherapy response as well as a survival benefit with the addition of LMWH has also been seen with various other chemotherapy regimens.15,16,18 A recent preclinical study also suggested that LMWH may prevent development of chemoresistance to cisplatin.48 However, our data did not show improved tumor response rates or prolonged PFS with anticoagulation therapy, despite the observed improvements in overall survival. Therefore, it is not clear if the survival benefit demonstrated in our study is related to direct antitumor effects of anticoagulants or their ability to prevent further clinical thromboembolic events or micro-thrombotic phenomena that may potentially accelerate death in these patients.
Our study has the inherent limitations of a retrospective analysis and a relatively small sample size. However, to our knowledge, this is the first report of a significant survival benefit from therapeutic anticoagulation in patients with mCRPC receiving docetaxel chemotherapy. Our findings provide the rationale for further evaluation of anticoagulants (especially LMWH) in patients with mCRPC in randomized prospective studies. In addition, evaluation of the safety and antineoplastic activity of next-generation oral anticoagulants (such as direct factor X inhibitors or thrombin inhibitors) may be warranted because increasing use of these novel agents in patients with cancer is anticipated.
Conclusion
Our retrospective analysis of 247 patients with mCRPC receiving docetaxel-based chemotherapy showed that patients who received therapeutic anticoagulation had improved survival compared with those not receiving anticoagulation therapy. The survival benefit was limited to LMWH use, whereas warfarin therapy did not appear to confer a survival benefit. Our findings are particularly interesting because the anticoagulated group also had concurrent VTE, which is a poor prognostic factor for survival in patients with cancer, further supporting the antitumor properties of LMWH in patients with mCRPC. Despite the inherent limitations of this retrospective analysis and a relatively small sample size, this study provides the impetus for further prospective evaluation of LMWH in patients with mCRPC.
Clinical Practice Points.
Tumor-associated dysregulation of the coagulation system is associated with survival and spread of cancer cells.
Various anticoagulants have been examined for potential antitumor effects, but no definitive data exist on the impact of anticoagulation therapy on survival in patients with advanced prostate cancer.
Anticoagulation therapy resulted in improved survival in patients with mCRPC who received first-line docetaxel chemotherapy.
The survival benefit appeared to be limited to LMWH use and not warfarin use.
A larger effect on survival was observed in men receiving anticoagulation therapy for > 6 months.
Acknowledgments
ESA is partially funded by grant P30 CA006973.
Footnotes
Disclosure The authors have stated that they have no conflicts of interest.
References
- 1.Prandoni P, Piccioli A, Girolami A. Cancer and venous thromboembolism:an overview. Haematologica. 1999;84:437–45. [PubMed] [Google Scholar]
- 2.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–50. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 3.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–64. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 4.Dvorak HF, Nagy JA, Berse B, et al. Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann NY Acad Sci. 1992;667:101–11. doi: 10.1111/j.1749-6632.1992.tb51603.x. [DOI] [PubMed] [Google Scholar]
- 5.Chew EC, Wallace AC. Demonstration of fibrin in early stages of experimental metastases. Cancer Res. 1976;36:1904–9. [PubMed] [Google Scholar]
- 6.Antachopoulos CT, Iliopoulos DC, Gagos S, et al. In vitro effects of heparin on SW480 tumor cell-matrix interaction. Anticancer Res. 1995;15:1411–6. [PubMed] [Google Scholar]
- 7.Lersch C, Gericke D, Classen M. Efficacy of low-molecular-weight heparin and unfractionated heparin to prevent adhesion of human prostate and bladder carcinoma and melanoma cells to bovine endothelial monolayers. An in vitro study and review of the literature. Urol Int. 1996;56:230–3. doi: 10.1159/000282848. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak HF, Senger DR, Dvorak AM. Fibrin as a component of the tumor stroma: origins and biological significance. Cancer Metastasis Rev. 1983;2:41–73. doi: 10.1007/BF00046905. [DOI] [PubMed] [Google Scholar]
- 9.Sylvester DM, Liu SY, Meadows GG. Augmentation of antimetastatic activity of interferon and tumor necrosis factor by heparin. Immunopharmacol Immunotoxicol. 1990;12:161–80. doi: 10.3109/08923979009019667. [DOI] [PubMed] [Google Scholar]
- 10.Martin CB, Mahon GM, Klinger MB, et al. The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene. 2001;20:1953–63. doi: 10.1038/sj.onc.1204281. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak HF, Harvey VS, Estrella P, Brown LF, McDonagh J, Dvorak AM. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest. 1987;57:673–86. [PubMed] [Google Scholar]
- 12.Norrby K. Heparin and angiogenesis: a low-molecular-weight fraction inhibits and a high-molecular-weight fraction stimulates angiogenesis systemically. Haemostasis. 1993;23(suppl 1):141–9. doi: 10.1159/000216923. [DOI] [PubMed] [Google Scholar]
- 13.Collen A, Smorenburg SM, Peters E, et al. Unfractionated and low molecular weight heparin affect fibrin structure and angiogenesis in vitro. Cancer Res. 2000;60:6196–200. [PubMed] [Google Scholar]
- 14.Bobek V, Kovarik J. Antitumor and antimetastatic effect of warfarin and heparins. Biomed Pharmacother. 2004;58:213–9. doi: 10.1016/j.biopha.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Lebeau B, Chastang C, Brechot JM, et al. Subcutaneous heparin treatment increases survival in small cell lung cancer. “Petites Cellules” Group. Cancer. 1994;74:38–45. doi: 10.1002/1097-0142(19940701)74:1<38::aid-cncr2820740108>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemostas. 2004;2:1266–71. doi: 10.1111/j.1538-7836.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- 17.Robert F, Busby E, Marques MB, Reynolds RE, Carey DE. Phase II study of docetaxel plus enoxaparin in chemotherapy-naive patients with metastatic nonsmall cell lung cancer: preliminary results. Lung Cancer. 2003;42:237–45. doi: 10.1016/s0169-5002(03)00354-4. [DOI] [PubMed] [Google Scholar]
- 18.Icli F, Akbulut H, Utkan G, et al. Low molecular weight heparin (LMWH) increases the efficacy of cisplatinum plus gemcitabine combination in advanced pancreatic cancer. J Surg Oncol. 2007;95:507–12. doi: 10.1002/jso.20728. [DOI] [PubMed] [Google Scholar]
- 19.Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011:CD006649. doi: 10.1002/14651858.CD006649.pub4. [DOI] [PubMed] [Google Scholar]
- 20.Hettiarachchi RJ, Smorenburg SM, Ginsberg J, Levine M, Prins MH, Buller HR. Do heparins do more than just treat thrombosis? The influence of heparins on cancer spread. Thromb Haemostas. 1999;82:947–52. [PubMed] [Google Scholar]
- 21.Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann Intern Med. 1999;130:800–9. doi: 10.7326/0003-4819-130-10-199905180-00003. [DOI] [PubMed] [Google Scholar]
- 22.van Dongen CJ, van den Belt AG, Prins MH, Lensing AW. Fixed dose subcutaneous low molecular weight heparins vs. adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2004:CD001100. doi: 10.1002/14651858.CD001100.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Kakkar AK, Levine MN, Kadziola Z, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS) J Clin Oncol. 2004;22:1944–8. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130–5. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 25.Sideras K, Schaefer PL, Okuno SH, et al. Low-molecular-weight heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clin Proc. 2006;81:758–67. doi: 10.4065/81.6.758. [DOI] [PubMed] [Google Scholar]
- 26.Zacharski LR, Henderson WG, Rickles FR, et al. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate. Final report of VA Cooperative Study #75. Cancer. 1984;53:2046–52. doi: 10.1002/1097-0142(19840515)53:10<2046::aid-cncr2820531007>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 27.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29.Mousa SA, Mohamed S. Anti-angiogenic mechanisms and efficacy of the low molecular weight heparin, tinzaparin: anti-cancer efficacy. Oncol Rep. 2004;12:683–8. [PubMed] [Google Scholar]
- 30.Stevenson JL, Varki A, Borsig L. Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb Res. 2007;120(suppl 2):S107–11. doi: 10.1016/S0049-3848(07)70138-X. [DOI] [PubMed] [Google Scholar]
- 31.Simka M. Anti-metastatic activity of heparin is probably associated with modulation of SDF-1-CXCR4 axis. Med Hypotheses. 2007;69:709. doi: 10.1016/j.mehy.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Li JP. Heparin, heparan sulfate and heparanase in cancer: remedy for metastasis? Anticancer Agents Med Chem. 2008;8:64–76. doi: 10.2174/187152008783330824. [DOI] [PubMed] [Google Scholar]
- 33.Drago JR, Weed P, Fralisch A. The evaluation of heparin in control of metastasis of Nb rat androgen-insensitive prostate carcinoma. Anticancer Res. 1984;4:171–2. [PubMed] [Google Scholar]
- 34.Prandoni P, Lensing AW, Buller HR, et al. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deepvein thrombosis. Lancet. 1992;339:441–5. doi: 10.1016/0140-6736(92)91054-c. [DOI] [PubMed] [Google Scholar]
- 35.Hettiarachchi RJ, Prins MH, Lensing AW, Buller HR. Low molecular weight heparin versus unfractionated heparin in the initial treatment of venous thromboembolism. Curr Opin Pulm Med. 1998;4:220–5. doi: 10.1097/00063198-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Siragusa S, Cosmi B, Piovella F, Hirsh J, Ginsberg JS. Low-molecular-weight heparins and unfractionated heparin in the treatment of patients with acute venous thromboembolism: results of a meta-analysis. Am J Med. 1996;100:269–77. doi: 10.1016/S0002-9343(97)89484-3. [DOI] [PubMed] [Google Scholar]
- 37.van Doormaal FF, Di Nisio M, Otten HM, Richel DJ, Prins M, Buller HR. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J Clin Oncol. 2011;29:2071–6. doi: 10.1200/JCO.2010.31.9293. [DOI] [PubMed] [Google Scholar]
- 38.Akl EA, Kamath G, Kim SY, et al. Oral anticoagulation may prolong survival of a subgroup of patients with cancer: a cochrane systematic review. J Exp Clin Cancer Res. 2007;26:175–84. [PubMed] [Google Scholar]
- 39.Kuderer NM, Khorana AA, Lyman GH, Francis CW. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: impact on survival and bleeding complications. Cancer. 2007;110:1149–61. doi: 10.1002/cncr.22892. [DOI] [PubMed] [Google Scholar]
- 40.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin vs. a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–53. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 41.Maat B. Selective macrophage inhibition abolishes warfarin-induced reduction of metastasis. Br J Cancer. 1980;41:313–6. doi: 10.1038/bjc.1980.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCulloch P, George WD. Warfarin inhibits metastasis of Mtln3 rat mammary carcinoma without affecting primary tumour growth. Br J Cancer. 1989;59:179–83. doi: 10.1038/bjc.1989.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoji M, Hancock WW, Abe K, et al. Activation of coagulation and angiogenesis in cancer: immunohistochemical localization in situ of clotting proteins and vascular endothelial growth factor in human cancer. Am J Pathol. 1998;152:399–411. [PMC free article] [PubMed] [Google Scholar]
- 44.Chahinian AP, Propert KJ, Ware JH, et al. A randomized trial of anticoagulation with warfarin and of alternating chemotherapy in extensive small-cell lung cancer by the Cancer and Leukemia Group B. J Clin Oncol. 1989;7:993–1002. doi: 10.1200/JCO.1989.7.8.993. [DOI] [PubMed] [Google Scholar]
- 45.Maurer LH, Herndon JE, 2nd, Hollis DR, et al. Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: a Cancer and Leukemia Group B study. J Clin Oncol. 1997;15:3378–87. doi: 10.1200/JCO.1997.15.11.3378. [DOI] [PubMed] [Google Scholar]
- 46.Akl EA, Vasireddi SR, Gunukula S, et al. Oral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev. 2011:CD006466. doi: 10.1002/14651858.CD006466.pub3. [DOI] [PubMed] [Google Scholar]
- 47.Akl EA, Gunukula S, Barba M, et al. Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev. 2011:CD006652. doi: 10.1002/14651858.CD006652.pub3. [DOI] [PubMed] [Google Scholar]
- 48.Niu Q, Wang W, Li Y, et al. Low molecular weight heparin ablates lung cancer cisplatin-resistance by inducing proteasome-mediated ABCG2 protein degradation. PloS One. 2012;7:e41035. doi: 10.1371/journal.pone.0041035. [DOI] [PMC free article] [PubMed] [Google Scholar]


