SUMMARY
Background
Natural foods not only contain nutrients, but also non-nutritious and potentially harmful chemicals. Thus, animals need to evaluate food content in order to make adequate feeding decisions.
Results
Here, we investigate the effects of acids on the taste neuron responses and on taste behavior of desirable, nutritious sugars and sugar/bitter compound mixtures in Drosophila melanogaster. Using Ca2+ imaging, we show that acids neither activate sweet nor bitter taste neurons in tarsal taste sensilla. However, they suppress responses to bitter compounds in bitter-sensing neurons. Moreover, acids reverse suppression of bitter compounds exerted on sweet-sensing neurons. Consistent with these observations, behavioral analyses show that bitter compound-mediated inhibition on feeding behavior is alleviated by acids. To investigate the cellular mechanism by which acids modulate these effects, we silenced bitter sensing gustatory neurons. Surprisingly, this intervention had little effect on acid-mediated de-repression of sweet neuron or feeding responses to either sugar/bitter compound mixtures, or sugar/bitter compound/acid mixtures, suggesting two independent pathways by which bitter compounds are sensed.
Conclusions
Our investigations reveal that acids, when presented in dietary relevant concentrations, enhance the perception of sugar/bitter compound mixtures. Drosophila’s natural food sources - fruits and cohabitating yeast - are rich in sugars and acids, but are rapidly colonized by microorganisms, such as fungi, protozoan parasites and bacteria, many of which produce bitter compounds. We propose that acids present in most fruits counteract the inhibitory effects of these bitter compounds during feeding.
Introduction
In addition to nutritious compounds such as sugars, fatty acids, proteins and minerals, most natural foods contain or accumulate many non-nutritious or even harmful chemicals. When these diverse compounds contact the taste sensory system, they activate distinct sets of taste cells, and proper integration of these cellular responses are key events that guide feeding activity and ultimately impact an animal’s metabolism and health. In humans and mice, sugars and amino acids are the main nutritious taste cues, while acids and a range of chemically diverse compounds (alkaloids, phenols, terpenoids), generally referred to as bitter compounds, are the main repulsive cues. These chemicals activate specific receptors expressed in different sets of taste cells in the tongue and elicit sweet and savory, and sour and bitter perception, respectively [1–3]. In Drosophila melanogaster, food chemicals are sensed through approximately 250 taste bristles (sensilla) located on the labial palps and legs [3–6]. Most sensilla harbor four, and some two, gustatory receptor neurons (GRNs), which are tuned to specific taste modalities. Sugars, the major nutritious components of Drosophila’s diet, are sensed by the single sweet neuron found in each taste sensillum [7–12]. Most, but not all, sensilla also contain a bitter/high salt sensing neuron (referred to as bitter neuron), which is activated by bitter compounds found in plants and strongly suppress feeding [8–12]. However, bitter compounds may also accumulate in fruit, along with yeast the main food and egg laying source of Drosophila melanogaster [13, 14], as by-products of microbes and other microorganisms that colonize fruit during ripening and decay [15, 16]. At least two additional types of GRNs are present in most taste sensilla and detect water and low salt solutions, respectively [17, 18]. Surprisingly little is known about the sensing of acids, and their potential role in modulating feeding behavior, even though many carboxylic acids, such as citric, acetic, tartaric and malic acid, are abundantly present in most fruits [19] and therefore integral part of the diet of frugivores, including Drosophila. Although acids were recently reported to activate a subset of bitter sensing neurons in the labial palps and suppress feeding [20], the presence of acids in natural food sources makes it unlikely that these chemicals are perceived as repulsive in the context of nutritious sugars.
Sweet and bitter neurons express distinct members of the gustatory receptor (Gr) protein family. Sugars are detected by proteins of a small, highly conserved subfamily encoded by eight Gr genes, several of which are expressed in sweet neurons [21–25]. Likewise, many members of a larger, but less conserved subfamily, the putative “bitter” receptor proteins, are expressed in partially overlapping fashion in the single bitter neuron present in most taste sensilla and activated by bitter compounds [11, 26–30]. Both, functional bitter and sugar receptors are thought to be composed of two or more Gr subunits [11, 22, 24, 28], providing a combinatorial rationale for the large number of diverse chemicals that can be sensed by the fly.
The close proximity of up to four distinctly tuned GRNs in each sensillum allows for cross-modulation of sensory input at the periphery [4, 12]. Specifically, bitter compounds were shown to suppress the response to sugars in sweet neurons [12, 31]. However, this modulation may or may not involve bitter neurons, as bitter compounds can suppress sugar responses in sweet neurons of sensilla that lack the bitter neuron [12, 31]. Thus, distinct mechanisms may account for repression of sweet neuron responses by bitter compounds.
Despite the abundance of organic acids in fruits, we have a very poor understanding of how these chemicals affect Drosophila feeding. Although attracted by vinegar, flies are repelled by volatile acids, which are sensed by neurons in the olfactory sensilla of antennae [32, 33]. On the other hand, flies choose acid containing food over food lacking acids as an egg-laying substrate [34, 35], a preference that is mediated by GRNs located in the proboscis [34] and by olfactory neurons in the antenna [35]. The authors of one of these studies suggested that acids inhibit growth of bacteria and other harmful microorganisms and thereby provide superior growth conditions for emerging larvae [34]. Soluble carboxylic acids were recently shown to activate a small subset of bitter neurons in the labellum [20]. However, the level of bitter neuron activation at dietary relevant acid concentrations (pH 3 to 6) amounted to only a faction (2 to 10%) of that elicited by bitter compounds [20]. Thus, the role of acids present at dietary relevant concentrations on both cellular and behavioral taste responses remains unknown.
In this paper, we report on the effects of acids on tarsal taste perception using Ca2+ imaging and behavioral assays. Acids activate neither bitter nor sweet neurons, but they modulate the response of both neuron types. Specifically, acids suppress responses in bitter neurons, and they relieve the bitter compound-mediated repression in sweet neurons. Thus, acids appear to modulate bitter and sugar responses in opposite ways, and we propose that these modulations increase acceptance of nutritious, but bitter compound containing foods.
RESULTS
To investigate the effects of bitter compounds and acids on molecularly characterized GRN types, we performed a series of Ca2+ imaging experiments of neurons located in the most distal tarsal segments, the part of the foreleg closest to a food source and hence expected to contribute most to tarsal taste perception. A major advantage of this set-up vs. electrophysiological recordings is the ability to unambiguously visualize the activity of a single cell, which is especially advantageous when ligands from different chemical classes are applied simultaneously. We previously established response profiles for the bitter and sweet neurons located in each of the three pairs of taste sensilla in this segment, the 5D1, 5V1 and 5V2 sensilla [23] (Figure 1A). The 5D1 and 5V2 sensilla contain a neuron expressing Gr64f-Gal4 and a neuron expressing Gr33aGal4, cell markers indicative for sweet and bitter GRNs, respectively. The two 5V1 sensilla harbor a hypersensitive sweet neuron, but lack a bitter sensing neuron based on expression analysis of numerous, broadly expressed, bitter-cell specific Gal4 drivers, such as Gr66a-Gal4, Gr33aGal4 or Gr32a-Gal4 [23] (data not shown).
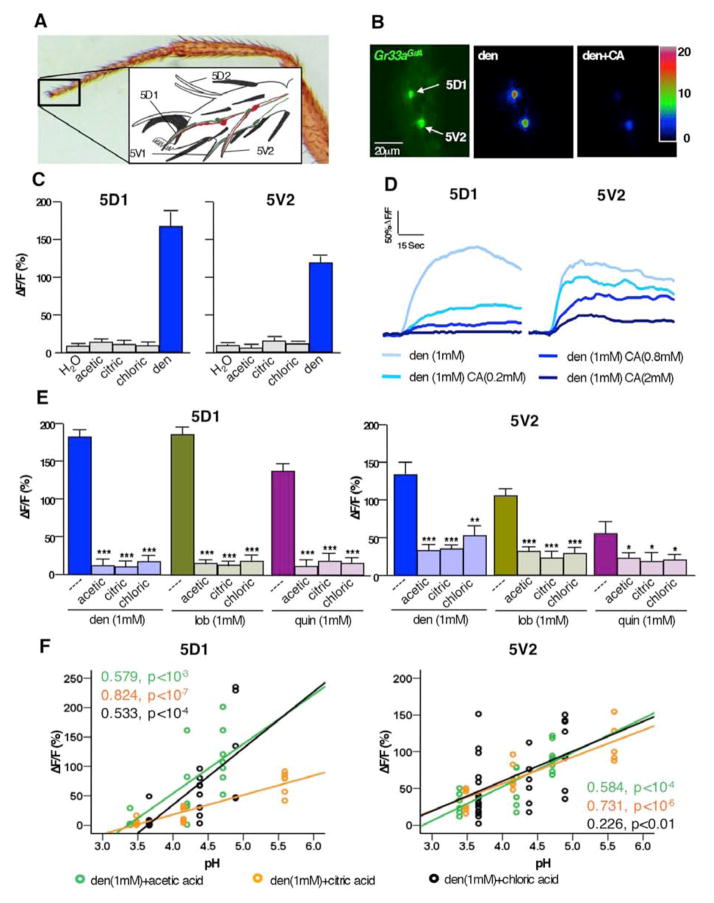
Figure 1. Acids repress bitter neuron response.
A) Photomicrograph of foreleg and schematic drawing (inset) of taste sensilla in the fifth tarsal segment (adapted from [23]). Bitter and sweet neurons in 5D1, 5V1 and 5V2 were used for Ca2+ imaging.
B) Still photos from live Ca2+ imaging recordings of tarsal bitter neurons. Left image shows the bitter neurons of the 5D1 and 5V2 sensilla located in the 5th tarsal segment of flies expressing GCaAMP3.0 under the control of Gr33aGAL4. Maximal fluorescence elicited upon stimulation with 1mM denatonium (middle) or 1mM denatonium-2mM citric acid (right), respectively.
C) Acids do not activate tarsal bitter neurons: Maximum relative fluorescence change (ΔF/F%) of bitter neuron responses in the 5D1 and 5V2 sensilla stimulated with water (---), 500 mM acetic acid (pH ~ 2.5), 100mM citric acid (pH ~ 2.1) or 10 mM HCl (pH ~2), as well as 1mM denatonium.
D) Representative traces of bitter neuron responses in 5D1 and 5V2 sensilla to denatonium- citric acid mixtures. Bitter neuron response decreases as the concentration of acid increases.
E) Suppression of bitter neuron response to denatonium (blue), lobeline (green) and quinine (purple) is suppressed by all three acids tested (acetic acid, 50mM. citric acid, 2mM. chloric acid, 1mM). * represent p<0.05, *** represent p<0.0001, one-way ANOVA with post hoc Bonferroni correction; 5<n<13
F) Acids repress bitter neurons in a pH-dependent manner. Scatter diagram with linear regression indicates that bitter neuron responses in the 5D1 and 5V2 sensilla stimulated with denatonium/acid mixtures are pH dependent. Concentrations: denatonium 1mM; acetic acid (green) 1mM, 5mM, and 50mM; citric acid (orange) 0.2mM, 0.8mM, and 2mM; HCl (black) 0.1mM, 0.4mM and 1mM; 5<n<12. Regression co-efficient (R2) and p values are indicated for each acid.
Abbreviations: den, denatonium; lob, lobeline; quin, quinine. ΔF/F(%), maximum relative fluorescent change before and after tastant application.
Acids suppress the bitter response in bitter neurons in a pH dependent manner
Charlu and colleagues recently reported that high concentration of acids elicited a response in a small subset of bitter neurons in the labellum [20]. In previous studies, we did not observe any bitter neuron responses to 20 mM citric acid (pH 2.5) [23]. To investigate whether low pH can elicit taste neuron responses, we carried out Ca2+ imaging experiments by expressing the Ca2+ indicator GCaMP3.0 in the four bitter neurons associated with the pairs of 5D1 and 5V2 sensilla (Gr33aGAL4 UAS-GCaMP 3.0/+ flies) using five different acids as substrates (Figure 1 and Figure S1). While all neurons responded readily to various bitter compounds tested (Figure 1E), none of them responded to 500 mM acetic, 100 mM citric acid, or 10 mM HCl, concentrations corresponding to a pH range of 2.5 to 2 (Figure 1B). Likewise, no responses were elicited from these neurons by presenting 2 mM tartaric acid (pH ~ 3) or 1 mM sulfuric acid (pH ~ 2.7; Figure S1A).
We next investigated whether acids can modulate the response to bitter compounds in these neurons. Interestingly, we found that each acid suppressed bitter neuron responses, regardless which bitter compound was used for stimulation (Figure 1D and 1E). Suppression appears more closely correlated with pH than acid concentration (Figure 1F, Figure S1B): For example, 5mM acetic acid, 0.8mM citric acid or 0.4mM HCl (pH of ~ 4.3) suppress bitter neuron response to a similar, modest extent, while 50mM acetic acid, 2mM citric acid or 1mM HCl (pH ~ 3.5) caused very strong suppression (Figure 1F). Thus, our data are consistent with the notion that acids can modulate taste neuron activity of tarsal GRNs to increase, rather than decrease, acceptance/feeding behavior.
Opposing modulation of sweet neurons by bitter compounds and acids
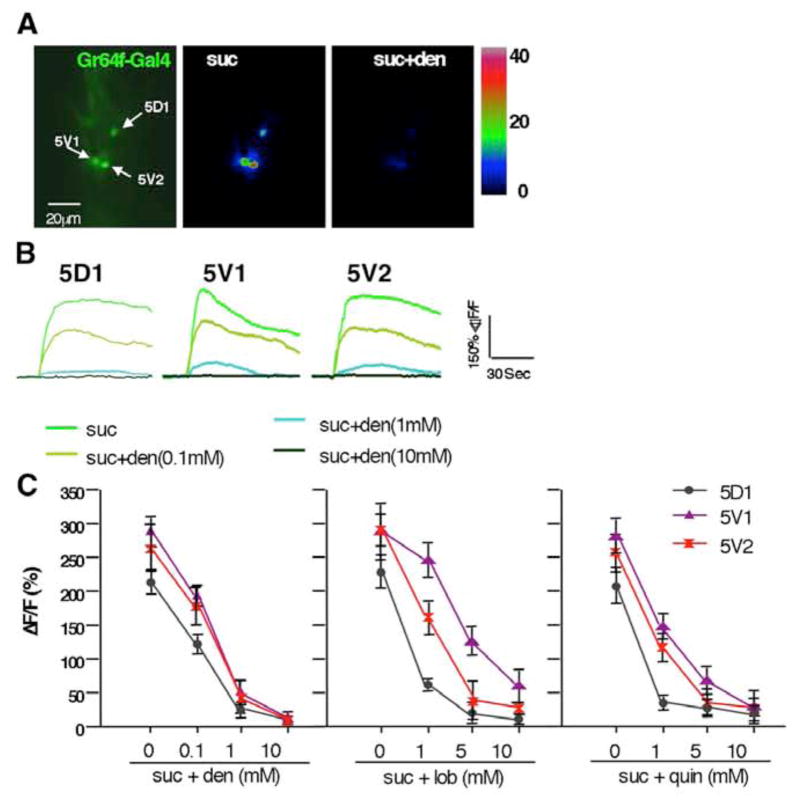
We next asked whether acids and bitter compounds modulate sweet neuron activity in the 5D1, 5V1 and 5V2 sensilla. Suppression of sweet neurons by bitter compounds has been established using electrophysiological recordings of sensilla associated with both major taste organs [12, 31], and we therefore wanted to ascertain that the tarsal sweet neurons behave similarly, using Ca2+ imaging. We first examined the effects of denatonium benzene, lobeline and quinine on the sucrose response of tarsal sweet neurons expressing GCaMP3.0 (Gr64f-Gal4 UAS-GCaMP3.0/+) [11]. Indeed, all three bitter compounds strongly suppress the response in sweet neurons of all three sensilla pairs, including 5V1, which lack a bitter neuron labeled by any known bitter taste receptor in a dosage dependent manner (Figure 2). Moreover, bitter compounds repress the sweet neuron response regardless of the sugar used in the mixtures (Supplemental Figure S2A and S2B). We note that in complementary experiments, sugars had no effect on the bitter neuron response (Figure S2C).
Figure 2. Suppression of sweet neuron responses by bitter compound in Ca2+ imaging.
A) Still photos from live Ca2+ imaging recordings of tarsal sweet neurons. Left image shows the sweet neurons of the 5D1, 5V1, and 5V2 sensilla located in the 5th tarsal segment of flies expressing GCaMP3.0 under the control of Gr64f-GAL4. Maximal fluorescence elicited upon stimulation with 100mM sucrose (middle) or 100mM sucrose/1mM denatonium (right), respectively.
B) Representative traces of sweet neuron responses in 5D1, 5V1, and 5V2 sensilla to sucrose and sucrose-denatonium mixtures. Note that the sweet neuron response decreases as the concentration of denatonium increase.
C) Suppression of sweet neuron response is not bitter compound specific. Maximum relative fluorescence change (ΔF/F%) to sucrose and sucrose/denatonium (left) sucrose/lobeline (middle), and sucrose/quinine (right) shows suppression in a dosage-dependent manner. Responses of sweet neurons of 5D1, 5V1 and 5V2 sensilla are shown in black, purple and red, respectively. 5<n<9.
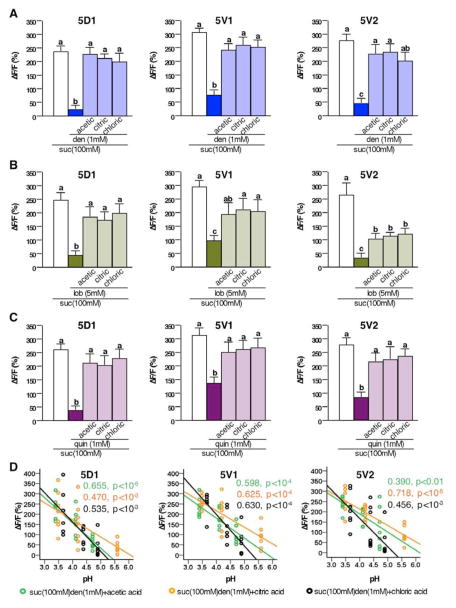
Given that acids are an integral part of Drosophila’s natural diet, we next asked whether they positively affect sweet neuron responses. Acids alone fail to activate sweet neurons, and they neither increase nor decrease sweet neuron responses elicited by sugars (Figure S3A). Thus, sweet neuron responses in tarsal sensilla to pure sugars appear unaffected by low pH. However, acids may exert an effect on sweet neurons indirectly, i.e. by modulating the inhibitory effect of bitter compounds. We therefore conducted Ca2+ imaging experiments of sweet neurons that were challenged with sugar/bitter compound mixtures in the presence and absence of acids (Figure 3). Indeed, sweet neurons suppression caused by any of the three bitter compounds is completely or partially reverted by all acids in the 3 to 4 pH range (Figure 3A to 3C). The extent of recovery was somewhat dependent on the specific sweet neuron, as well as the bitter compound (compare complete recovery for 5D sweet neuron/denatonium to the partial recovery for 5V2 sweet neuron/lobeline; Figure 3A and 3B). Similar to the inhibition of bitter neurons, acid-mediated de-repression of sweet neurons is primarily correlated with pH. For example, sugar/bitter compound mixtures with a pH ~ 4.3 (5mM acetic acid, 0.8mM citric acid or 0.4mM HCl cause robust, but only partial de-repression, while mixtures with a pH ~ 3.5 (50mM acetic acid, 2mM citric acid or 1mM HCl) lead to a complete or almost complete de-repression (Figure 3D). Similar de-repression of sweet neuron responses was also observed with two additional acids, tartaric acid and sulfuric acid (Figure S3C). Lastly, de-repression of bitter compounds by acids appears to be independent of the sugar used for stimulation (Figure S3B). Taken together, our data suggest that acids de-repress the inhibitory effects of bitter compounds to the sugar response of sweet neurons in a pH-dependent manner.
Figure 3. Acids de-repress sweet neuron responses of sugar-bitter compound mixtures in Ca2+ imaging.
A, B and C) Acids de-repress sweet neuron inhibition by denatonium (A), lobeline (B), and quinine (C). Maximum relative fluorescence change (ΔF/F%) of sweet neuron responses stimulated with 100mM sucrose, a sucrose/denatonium mixture (A), sucrose/lobeline mixture (B), and sucrose/quinine mixture (C), and the same mixture complemented with three different acids (acetic acid, 50mM; citric acid, 2mM; HCl, 1mM). Different letters indicate significant differences, p<0.05. One way ANOVA with post hoc Duncan’s test; 5<n<9.
D) Acids de-repress sweet neuron responses of sugar/bitter compound mixtures in a pH-dependent manner. Scatter diagram with linear regression indicates that sweet neuron responses in 5D1, 5V1 and 5V2 sensilla stimulated with sucrose/denatonium mixtures are pH dependent. Concentrations: denatonium 1mM; acetic acid (green) 1mM, 5mM, and 50mM; citric acid (orange) 0.2mM, 0.8mM, and 2mM; HCl (black) 0.1mM, 0.4mM and 1mM; 5<n<9. Regression coefficient (R2) and p values are indicated for each acid.
Abbreviations: den, denatonium; lob, lobeline. quin, quinine; suc, sucrose. (ΔF/F%), maximum relative fluorescent change before and after tastant application.
De-repression of bitter compounds by acids in sweet neurons does not require a functional bitter neuron
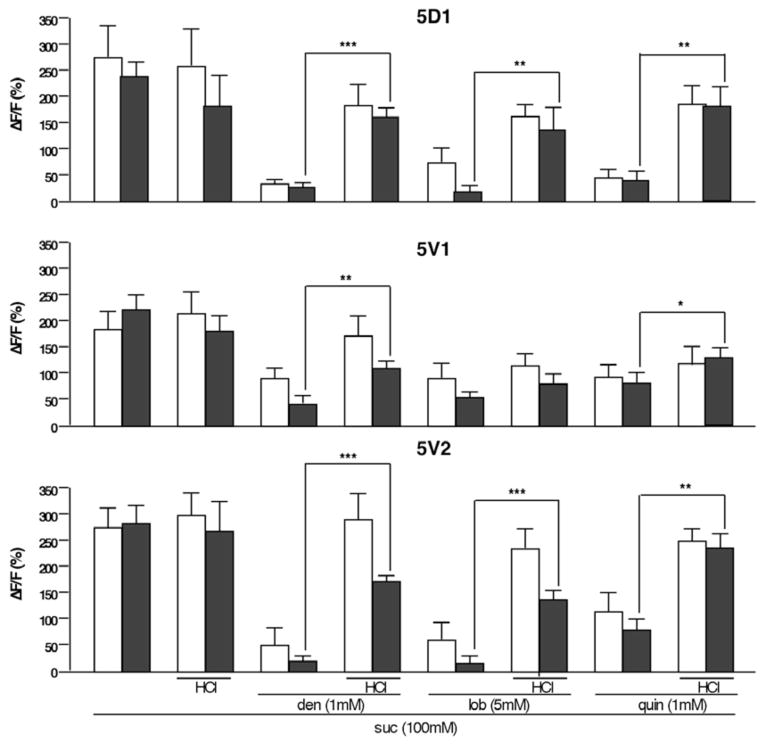
Our analyses have revealed two effects of low pH on taste neurons: acids suppress the activity of bitter neurons (Figure 1), and they release inhibition exerted by bitter compounds on sweet neurons (Figure 3). Given that bitter and sweet neurons are in close proximity within a sensillum, and that mutual inhibition of neurons within structurally similar olfactory neurons has been reported [36], it is possible that the effects of acids on sweet neuron responses are mediated by the bitter neuron. Yet, the observation that the sweet neuron response is also affected in the 5V1 sensilla suggests a more direct role for acids on sweet neuron suppression by bitter compounds. To test whether the bitter neuron plays a critical role in acid mediated de-repression of sweet neuron responses, we silenced it by expressing the rectifying potassium channel Kir2.1 under the control of the Gr33aGAL4 allele and visualized sweet neuron responses of sugar/bitter compound mixtures using a second bimodal expression system (Gr64f-LexA; lexopGCaMP3.0) [37] (Figure 4). Indeed, both the acid and the bitter compound mediated modulation of sweet neuron responses are largely unaffected in the 5D1 and 5V2 sensilla in the absence of a functional bitter neuron: First, sweet neuron responses are still suppressed by bitter compounds in these flies, and second, the acid mediated recovery of that suppression remains intact (Figure 4). We note that sweet neuron responses of the 5V1 sensilla are muted in this genetic background (Gr64f-LexA/lexopGCaMP3.0 UAS-Kir2.1), and that both bitter compound suppression of such responses and their recovery by acids are relatively poor (Figure 4A, middle panel). Regardless, these experiments show that the modulation of the sweet neuron response by bitter compounds can occur in the absence of a functional bitter neuron.
Figure 4. Sweet neuron responses of sugar/bitter compound mixtures are de-repressed by acids in sensilla with silenced bitter neurons.
Maximum relative fluorescence change (ΔF/F%) of sweet neuron responses stimulated by sugar/bitter compound mixtures with or without acid in tarsi of flies expressing the inward-rectifying potassium channel Kir2.1 (Gr64f-LexA, Gr33aGAL4/LexAop-GCaMP3.0,UAS-Kir2.1; black bars). Silencing of the bitter neuron has little or no effect on de-repression; Controls are Gr64f-LexA/LexAop-GCaMP3.0,UAS-Kir2.1 (white bars); den, denatonium. lob, lobeline. quin, quinine. ΔF/F(%), maximum relative fluorescent change before and after tastant application.
Acids render sugar-bitter compound mixtures more acceptable
The effects of pH on the cellular response profile of both sweet and bitter neurons suggest a role for acids in counteracting aversive effects of bitter compounds on feeding behaviors. To test this, we performed two behavioral assays. First, we carried out proboscis extension reflex (PER) experiments to assess whether the observed cellular phenomena are translated into an increase in acceptance response to suboptimal (i.e. bitter compound containing) food. Acids per se have no effect on PER to sugars (Figure 5A). Importantly, and consistent with our Ca2+ imaging experiments (Figure 3), we find that acids counteract the inhibitory effect of bitter compounds on sweet taste, as PER is restored to a large extent (Figure 5B). We note that this effect is also observed in flies with silenced Gr33a bitter-sensing neurons (Figure 5D, left panel).
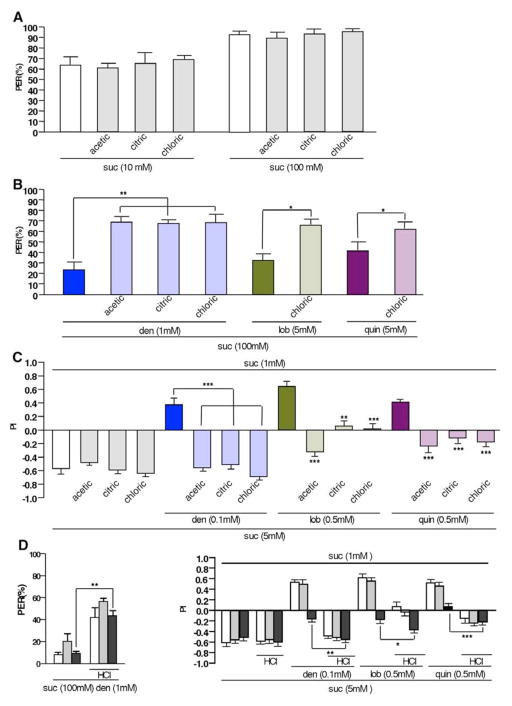
Figure 5. Acids modulate bitter and sweet taste behavior.
A) Acids do not affect sucrose induced proboscis extension reflex (PER) in legs. Sucrose, and sucrose mixed with various acids elicit similar PER when tarsi were stimulated.
B) Acids de-repress bitter compound mediated suppression of PER when legs are stimulated. Recovery of PER by acids is similar, regardless of how strong the bitter compound suppresses PER.
C) Acids shift preference from pure low sugar diet to high sugar/bitter compound mixtures. Flies prefer low but pure sugar at the expense of high sugar-bitter compound mixtures (solid color bars), but shift their preference towards such mixtures in the presence of acids.
D) Feeding modulation by acids is not dependent on functional bitter neurons: Increase of PER response (left panel) by acids occurs in the absence of functional bitter neurons (Gr33aGAL4/UAS-Kir2.1, black bars). Likewise, a modest shift from pure low sugar diet to high sugar/bitter compound mixtures induced by acids is evident in two choice feeding experiments (right panel). Control genotypes were +/UAS-Kir2.1 (white bars) and Gr33aGAL4/+ (grey bars).
* represents p<0.05, ** represents p<0.001, *** represents p<0.0001. For simplicity, only significant differences in experimental flies (but not control flies) feeding on food with and without acids, respectively, are indicated. One-way ANOVA with post hoc Bonferroni correction. 4<n<13 for calcium imaging, 4<n<7 for PER, and 7<n<19 for two-choice feeding assay. Abbreviations: den, denatonium; lob, lobeline; quin, quinine. Acid concentrations: acetic acid 50mM, citric acid 2mM and hydrochloric acid 1mM.
To examine whether tarsi-mediated modulation of PER is indicative of an increase in feeding activity on sugar/bitter compound mixtures, we conducted two choice-feeding assays (Figure 5C and 5D). When given a choice, flies prefer 5 mM sucrose (high sugar) to 1mM sucrose (low sugar), a preference not affected by acids (Figure 5C, left panel). However, when provided with the choice between a pure, but low sugar diet and a high sugar diet containing bitter compounds, they prefer the non-contaminated low sugar diet (Figure 5C, middle and right panels). Intriguingly, flies shift their preference towards the high sugar/bitter compound diet in the presence of acid, a shift that can results in a complete preference switch (Figure 5C; high sugar/denatonium and high sugar/quinine). Again, silencing of bitter neurons does affect the acid-mediated shift in feeding preference (Figure 5D, right panel). Thus, our behavioral analyses are consistent with our Ca2+ imaging experiments, confirming that acids increase the preference of sugar contaminated with bitter compounds.
Discussion
Most fruits contain significant amounts of carboxylic acids. For example, the total carboxylic acid content of apples and grapes is ~ 1%, and that of banana ~ 0.5% [19]. Thus, while not providing caloric value, acids are an integral component of frugivore insects and mammals alike. Our studies revealed that various carboxylic acids found in fruits, as well as chloric and sulfuric acid, fail to activate tarsal bitter or sweet neurons when provided in a pH range found in these foods (pH 3 to 6). Instead, all acids suppress the activity of bitter neurons when stimulated with bitter compounds, and they release the suppression observed in sweet neurons when stimulated with sugar/bitter compound mixtures. Importantly, these cellular effects are translated in corresponding behavioral changes, which show that acids function as acceptance or feeding “enhancer” for food that is suboptimal (i.e. contaminated with bitter compounds), but nutritiously rich. The biological significance of this phenomenon may be related to the fact that almost all frugivore Drosophila, with a few exceptions (i.e. D. suzukii [38]) must feed and lay eggs on “damaged” fruit (i.e. fruit with exposed flesh). Such compromised fruit decomposes faster as it is more likely to be colonized by microorganisms, such as fungi, protozoan parasites and bacteria, many of which produce a wide array of bitter compounds. We suggest that the modulatory properties of acids enables flies to counteract the repulsive effects of bitter compounds, facilitating ingestion of contaminated, sugar rich food. An important future avenue will be to determine the extent of acid induced de-repression, i.e. to determine whether acids fail to revert the repression of the most harmful bitter compounds.
Two mechanisms are likely operational in sweet neuron suppression by bitter compounds. The first mechanism might involve direct interaction between bitter and sweet neuron within the same sensilla. For example, olfactory neurons housed in the same sensilla were shown to repress each other through lateral, non-synaptic inhibition [36]. Furthermore, inhibitory GABA signaling has been shown to modulate output in axonterminals of both the olfactory and gustatory receptor neurons [39, 40]. However, dispensability of bitter neurons in inhibiting sweet neuron responses suggests that bitter chemicals can modulate sweet neurons directly, an observation that is further supported by the fact that acids can reverse the inhibitory effects of bitter compounds in the absence of bitter neurons (Figures 4 and 5D). Yeong and co-workers recently showed that odorant binding proteins (OBPs), which are secreted from support cells associated with taste neurons, form complexes with bitter compounds, and they suggested that these complexes activate bitter and suppress sugar receptors expressed in adjacent neurons within a sensillum [31]. Because low pH has been shown to alter conformation of several OBPs [41], it is intriguing to speculate that such changes inhibit interactions between OBPs and bitter compounds, and hence reduce probability of such complexes as agonist of bitter receptors and antagonist or allosteric inhibitors of sugar receptors.
Are labial palp and tarsi interpreting acid taste differently?
Using electrophysiological recordings of labial palp sensilla, Charlu and colleagues recently identified a small number of bitter neurons that are activated by acids [20]. We note that the effects reported by these investigators were modest in the dietary relevant pH range (pH 3 to 6): In the most sensitive, acid-responsive neurons (i.e. neurons of the S-b sensilla), the response at pH 5.8 amounts to less than 2.5% of the firing rate elicited by 10mM caffeine. At the pH of ~ 3.5 to 4, the responses is typically below 10% of the response elicited by 10 mM caffeine, and even at noxiously high acid levels (pH 1 to 2) does their firing rate approach only ~ 30% of that elicited by caffeine. Thus, the bitter neurons identified by these investigators appear to be tuned to noxious concentration of acids. Charlu and colleagues also observed acid-mediated inhibition of sweet neuron responses, albeit different acids appear to exert different degrees of suppression. Specifically, acetic and citric acid exhibited no apparent inhibitory effect on the sugar response in the dietary relevant pH range, while tartaric and glycolic acid inhibited sugar responses only modestly [20]. Thus, we suggest that the observations by these investigators represent responses to noxiously high levels of acids, commonly not found in the fly’s natural diet, while the enhancement of feeding uncovered in our study reflect acids’ role when flies encounter natural food sources. It is possible that the distribution of bitter neurons responding to different levels of acids is biased in labial palps and tarsi, respectively, i.e. neurons activated by noxious concentration of acids may be predominantly located in the palps, while neurons in which bitter responses are suppressed by acids may be abundant in tarsi. In this context, we note that both bitter and sweet sensing neurons on the fourth tarsal segment show the same response patterns as those in the 5D1, 5V1 and 5V2 sensilla used in this study (data not shown).
Different roles for the labial palps and tarsi in acid sensing is also consistent with our PER analysis. Specifically, we find that the acceptance enhancing effect of acids on sugar/bitter compound mixtures is mediated by the tarsi (Figure 5B), while PER to such mixtures was not affected when the labellum was stimulated (Figure S4). This suggests that the two main organs may differ in their ability to modulate acceptance behavior of suboptimal food. Regardless, the cumulative effect of acids present at dietary relevant concentrations is one of enhancement and not suppression, evident from the increased consumption of bitter/sugar mixtures (Figure 5C and 5D). One other instance where distinct behavioral output is triggered by a single chemical stimulus sensed through two physically separated taste organs has been reported. Lobeline detected by Gr66a expressing bitter neurons in the legs induces repulsion, but it promotes egg laying when sensed by Gr66a bitter neurons located in the internal pharyngeal taste organs [42]. Interestingly, bitter and sugar sensing neurons located in the legs, internal taste organs or labellum project to distinct domains within the subesophageal ganglion and ventral nerve cord [9], suggesting that these different domains are ultimately engaging different motor circuits. Future work will be necessary to elucidate the nature of these differently modulated circuits.
EXPERIMENTAL PROCEDURES
Drosophila Stock
Flies were maintained on standard corn meal food in plastic vials at 25 C0. The LexAop-GCaMP3.0 strain was a gift from Dr. Orie Shafer’s lab [37]. Gr64f-Gal4 and Gr64f-LexA were reported previously [23, 24]. The Gr33aGAL4 [27], UAS-GCaMP3.0 [43], UAS-Kir2.1 [44] strains were received from the Bloomington Drosophila Stock Center. Gr64f-Gal4 UAS-GCaMP3.0/+ and Gr33aGAL4 UAS-GCaMP3.0/+ were used as controls for Ca2+ imaging experiments of sweet and bitter neurons, respectively, while Gr64f-LexA /LexAop-GCaMP3.0 UAS-Kir2.1 was used as control for imaging sweet neurons in sensilla with silenced bitter neurons. For behaviors of “wild type” flies, a w+ strain was used with the genetic background of the w1118 strain: this strain was obtained through repeated back-crossing of hybrid, red-eyed (w+ w1118) females to w1118 males. Gr33aGAL4/UAS-Kir2.1 flies were used to assess the behavioral effects of silenced bitter neurons.
Chemicals
Sucrose, fructose and glucose were purchased from Sigma-Aldrich Co. (St. Louis, MO), with purity >99%. Acetic acid (>99% purity), sulfuric acid (>98% purity) and hydrochloric acid (36.5 to 38%) were purchased from Merck Inc. (Emanuel Merck, Darmstadt, Germany). Citric acid (>99% purity) and tartaric acid (>99% purity) were purchased from Mallinckrodt Pharmaceuticals (St. Louis MO) and Spectrum Chemical MFG Corp. (New Brunswick, NJ). Quinine hydrochloride (#Q1125) and denatonium benzoate (#D5765) were purchased form Sigma-Aldrich and lobeline hydrochloride (LTD #L0096) from Tokyo Chemical Industry Co. (Tokyo, Japan). All bitter chemicals were of > 98 % purity.
Calcium Imaging
Calcium imaging for tarsal GRNs was described previously [23]. Briefly, the foreleg of female flies with 4 to 7 days old was cut with a razor blade between the femur and the tibia and then was placed laterally on double-sided scotch tape, fastened to a glass bottom dish (MatTek Corp). The cut area of the leg was immediately sealed with silicone lubricant (Dow Corning; Midland, MI), and then covered with 1% agarose, except for the fourth and fifth tarsal segments, which were kept free for ligand application. The preparation was immersed in 100μl of water and immediately used for imaging with a Nikon eclipse Ti inverted microscope. Ligand solution was added by pipette and images were acquired every 500 ms, starting 10s before application and ending 50s after application of ligands. Each preparation was tested with 2–4 different compounds. Measurements were taken in the cell bodies and adjacent regions were used to determine background auto fluorescence. Baseline was established by the average of 5 frame measurements during before application of ligand. Max ΔF/F % was calculated by using the max value within 50 seconds after ligand application.
Proboscis Extension Reflex (PER) assays
PER assays were essentially carried out as described by Slone et al [22], with minor modifications. Briefly, 4 to 6 day old flies were starved for 22 to 24 hours in vials with a water-saturated Whatman paper. Flies were chilled on ice and mounted on their backs on a microscope slide using double-sided scotch tape. In PERs elicited from labial palp stimulation, all legs were secured to the tape to avoid accidental contact with tarsal neurons. Flies were allowed to recover for 1 to 2 hours after mounting, and they were allowed to drink water thoroughly prior to testing their response to test solution. A PER was recorded only when a fly fully extended the proboscis after a ligand application. Test solutions were delivered by a 20μl pipette for PER on legs or by wet Kimwipe tissue on the labellum. Each test solution was applied two times per fly, and water saturation was achieved again before the next application. Finally, 100mM sucrose was applied to the flies, only those respond to 100mM sucrose were included in the data. The probability of PER for a single experiment was determined from 16 to 20 test solution applications, using 8 to 10 flies, and at least four independent experiments were included in each data point. Error bars represent +/− the standard error of the mean (SEM), and statistical significance was calculated using one-way ANOVA with post hoc Bonferroni correction.
Two Choice Feeding Assays
Newly eclosed flies were collected and kept on standard food for 4 to 6 days. For each experiment, 35 to 45 flies were starved for 22 to 24 hrs in vials with a water-saturated Whatman paper prior to the assay. 60 well (6 X 10, Falcon) plates were used for the assays. In each plate, 20 wells were filled with 25ul 1% agarose as water source for flies; 20 wells contained 1% agarose plus 1 mM sucrose plus dye1 and 20 wells contained 1% agarose plus test mixture plus dye2. Dye concentrations were 0.125 mg/ml brilliant blue FCF (Wako Chemical Co.; 027-12842) and 0.2 mg/ml sulforhodamine B (Sigma-Aldrich; S9012). Blue and red dyes were exchanged in each experiment to correct for possible dye bias. The flies were chilled on ice and knocked onto the plates. Flies were let to feed for 90 minutes at room temperature in darkness, removed, frozen, grouped according to coloration of abdomen (blue, red, and purple, indicating ingestion of both food sources) and counted. A preference index (PI) was calculated using the following equation: PI = (# of flies w/dye1 abdomen (100 mM sucrose) − # of flies w/dye2 (test mixtures)/(# of flies w/dye1 + # of flies w/dye2 + # of flies w/dye1+dye2).
Supplementary Material
Highlights.
Acids suppress bitter neuron responses
Bitter compounds suppress sweet neuron responses
Acids revert suppression of bitter compounds on sweet neurons
Feeding behaviors reveal that acids enhance perception of contaminated food
Acknowledgments
We thank Dr. Tetsuya Miyamoto for advice on Ca2+ imaging experiments, Dr. Orie Shafer (U. of Michigan) for the lexop-GCaMP3.0 fly strain. Other strains were obtained from the Drosophila stock Center (http://flystocks.bio.indiana.edu). This work was supported by grants from the NIH (RO1-DC009014 and RO1-DC005606) to HA. YC and HA designed the experiments, YC carried out all experiments and HA wrote the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roper SD. Taste buds as peripheral chemosensory processors. Semin Cell Dev Biol. 2013;24:71–79. doi: 10.1016/j.semcdb.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott K. Taste recognition: food for thought. Neuron. 2005;48:455–464. doi: 10.1016/j.neuron.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Liman ER, Zhang YV, Montell C. Peripheral coding of taste. Neuron. 2014;81:984–1000. doi: 10.1016/j.neuron.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amrein H, Thorne N. Gustatory perception and behavior in Drosophila melanogaster. Curr Biol. 2005;15:R673–684. doi: 10.1016/j.cub.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 6.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 7.Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- 8.Hiroi M, Meunier N, Marion-Poll F, Tanimura T. Two antagonistic gustatory receptor neurons responding to sweet-salty and bitter taste in Drosophila. J Neurobiol. 2004;61:333–342. doi: 10.1002/neu.20063. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- 13.Ruebenbauer A, Schlyter F, Hansson BS, Lofstedt C, Larsson MC. Genetic variability and robustness of host odor preference in Drosophila melanogaster. Curr Biol. 2008;18:1438–1443. doi: 10.1016/j.cub.2008.08.062. [DOI] [PubMed] [Google Scholar]
- 14.Beshel J, Zhong Y. Graded encoding of food odor value in the Drosophila brain. J Neurosci. 2013;33:15693–15704. doi: 10.1523/JNEUROSCI.2605-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janzen DH. Why Fruits Rot, Seeds Mold, and Meat Spoils. The American Naturalist. 1977;111:691–713. [Google Scholar]
- 16.Stensmyr MC, Dweck HK, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 17.Zhang YV, Ni J, Montell C. The molecular basis for attractive salt-taste coding in Drosophila. Science. 2013;340:1334–1338. doi: 10.1126/science.1234133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridges M, Mattice M. Over two thousand estimations of the ph of representative foods*. The American Journal of Digestive Diseases. 1939;6:440–449. [Google Scholar]
- 20.Charlu S, Wisotsky Z, Medina A, Dahanukar A. Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nat Commun. 2013;4:2042. doi: 10.1038/ncomms3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr Biol. 2008;18:1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17:1809–1816. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto T, Chen Y, Slone J, Amrein H. Identification of a Drosophila glucose receptor using Ca2+ imaging of single chemosensory neurons. PLoS One. 2013;8:e56304. doi: 10.1371/journal.pone.0056304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci U S A. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon SJ, Kottgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci U S A. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y, Kang MJ, Shim J, Cheong CU, Moon SJ, Montell C. Gustatory receptors required for avoiding the insecticide L-canavanine. J Neurosci. 2012;32:1429–1435. doi: 10.1523/JNEUROSCI.4630-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y, Kim SH, Montell C. Avoiding DEET through insect gustatory receptors. Neuron. 2010;67:555–561. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong YT, Shim J, Oh SR, Yoon HI, Kim CH, Moon SJ, Montell C. An odorant-binding protein required for suppression of sweet taste by bitter chemicals. Neuron. 2013;79:725–737. doi: 10.1016/j.neuron.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ai M, Blais S, Park JY, Min S, Neubert TA, Suh GS. Ionotropic glutamate receptors IR64a and IR8a form a functional odorant receptor complex in vivo in Drosophila. J Neurosci. 2013;33:10741–10749. doi: 10.1523/JNEUROSCI.5419-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, Suh GS. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph RM, Devineni AV, King IF, Heberlein U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc Natl Acad Sci U S A. 2009;106:11352–11357. doi: 10.1073/pnas.0901419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dweck HK, Ebrahim SA, Kromann S, Bown D, Hillbur Y, Sachse S, Hansson BS, Stensmyr MC. Olfactory preference for egg laying on citrus substrates in Drosophila. Curr Biol. 2013;23:2472–2480. doi: 10.1016/j.cub.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 36.Su CY, Menuz K, Reisert J, Carlson JR. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature. 2012;492:66–71. doi: 10.1038/nature11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao Z, Macara AM, Lelito KR, Minosyan TY, Shafer OT. Analysis of functional neuronal connectivity in the Drosophila brain. J Neurophysiol. 2012;108:684–696. doi: 10.1152/jn.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rota-Stabelli O, Blaxter M, Anfora G. Drosophila suzukii. Curr Biol. 2013;23:R8–9. doi: 10.1016/j.cub.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Root CM, Masuyama K, Green DS, Enell LE, Nassel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnie Chu VC, Mann Kevin, Gordon Michael D. Presynaptic gain control drives sweet and bitter taste integration in Drosophila. Curr Biol. 2014 doi: 10.1016/j.cub.2014.07.020. In press. [DOI] [PubMed] [Google Scholar]
- 41.Fan J, Francis F, Liu Y, Chen JL, Cheng DF. An overview of odorant-binding protein functions in insect peripheral olfactory reception. Genet Mol Res. 2011;10:3056–3069. doi: 10.4238/2011.December.8.2. [DOI] [PubMed] [Google Scholar]
- 42.Joseph RM, Heberlein U. Tissue-specific activation of a single gustatory receptor produces opposing behavioral responses in Drosophila. Genetics. 2012;192:521–532. doi: 10.1534/genetics.112.142455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.