Abstract
Purpose
Sex steroid hormone concentrations and insulin-like growth factor (IGF) proteins have been independently associated with risk of cancer, chronic diseases, and mortality. However, studies that evaluated the inter-relation between the sex hormones and IGF pathways have provided mixed results. We examined the association between endogenous sex hormones and sex hormone-binding globulin (SHBG) with IGF-1 and IGF-binding protein 3 (IGFBP-3) in a population-based sample of US men.
Methods
Data from 1,135 men aged 20 years or older participating in the third National Health and Nutrition Examination Survey (NHANES III) were analyzed. Weighted linear regression was used to estimate geometric means and 95 % confidence intervals for IGF-1 and IGFBP-3 concentrations by sex steroid hormones and SHBG after adjusting for age, race/ethnicity, body mass index, waist circumference, alcohol consumption, cigarette smoking, physical activity, diabetes, and mutually adjusting for other sex hormones and SHBG.
Results
No significant association was observed between sex steroid hormones, SHBG, and IGF-1 concentrations. Total estradiol (% difference in Q5 − Q1 geometric means −9.7 %; P-trend 0.05) and SHBG (% difference −7.3 %; P-trend 0.02) were modestly inversely associated with IGFBP-3. Total testosterone was modestly inversely associated with IGFBP-3 (% difference −6.2 %; P-trend 0.01), but this association disappeared after adjustment for total estradiol and SHBG (% difference 2.6 %; P-trend 0.23). Androstanediol glucuronide was not associated with IG-FBP-3.
Conclusions
These findings suggest that there may be inter-relationships between circulating total estradiol, SHBG, and IGFBP-3 concentrations. Future research may consider these inter-relationships when evaluating potential joint effects of the sex hormones and IGF pathways.
Keywords: Testosterone, Estradiol, Sex hormone-binding globulin (SHBG), Insulin growth factor-1 (IGF-1), Insulin growth factor-binding protein 3 (IGFBP-3)
Introduction
Endogenous sex steroid hormones and insulin-like growth factor 1 (IGF-1) are anabolic hormones implicated in the aging process with a declining concentration with increasing age [1, 2]. Testosterone, which is the principal male sex hormone, plays an important role in complex mechanisms that determine muscle mass, fertility, glucose metabolism, and visceral adiposity [3]. Low testosterone concentrations have been associated with increased risks of metabolic syndrome and type II diabetes [4], hypertension, and atherosclerosis [5]. In addition, it has been recently demonstrated that low concentrations of total testosterone are associated with increased risk of all-cause and cardiovascular mortality [6]. The association between serum androgens and prostate cancer risk has been generally null, and a pooled analysis of the worldwide data showed no association [7].
IGF-1 is the main mediator of the growth hormone actions that involve cell replication and proliferation, lipid metabolism, and protein synthesis [8]. Low concentrations of IGF-1 have been associated with a wide range of morbidities including osteoporosis, metabolic syndrome [8], diabetes, and cardiovascular disease [9], whereas both high and low levels have been associated with all-cause mortality forming a U-shaped association [10]. A pooled analysis of prospective studies reported a moderately increased risk of prostate cancer with higher circulating IGF-1 concentrations [11]. Associations between serum concentration of insulin-like growth factor-binding protein 3 (IGFBP-3) and the risk of prostate [12] and other common cancers [13] are inconsistent. Moreover, a prospective study showed that multiple deficiencies in anabolic hormones such as IGF-1 and total testosterone may have a higher impact on mortality than a deficiency in a single hormone alone [14].
Independent quantification of the sex steroid hormones or the IGF system components may not provide complete information regarding their contribution to cancer, chronic disease incidence, and mortality. Few studies have addressed the inter-relationship between sex hormones and IGF-1 or IGFBP-3 concentrations in men, which produced inconsistent results [15–18]. In the present cross-sectional analysis, we explored the inter-relationships between sex steroid hormones (total testosterone, free testosterone, total estradiol, free estradiol, and androstanediol glucuronide) and sex hormone-binding globulin (SHBG) with IGF-1 and IGFBP-3 in a large nationally representative sample of US men after adjusting for important potential confounders.
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a periodic survey conducted by the National Center for Health Statistics (NCHS) of the United States (US) Centers for Disease Control and Prevention (CDC) to assess the health and nutritional status of the civilian non-institutionalized US population aged 2 months and older [19]. NHANES III is based on a multistage, stratified, clustered probability sample collected from 1988 to 1994 in two phases (1988–1991 and 1991–1994). Within each phase, participants were randomly assigned to either the morning or afternoon/evening examination session. NHANES over-sampled specific groups within the study population (persons over 60 years, African Americans and Hispanics). This survey included an interview, a physical examination, and a blood collection, and investigators are allowed to access surplus sera for approved studies.
More than 33,000 subjects participated in NHANES III. Of these, 1,998 men at least 20 years of age participated in the morning session of phase I. Morning sample participants were chosen to minimize extraneous variation due to diurnal production of hormones. For the purposes of our study, serum samples for hormone measurements were still available for 1,190 of these men. Additionally, seven men had missing values for sex hormones, and 16 men were excluded for having extreme hormone measurements. The following cut-off points were used to determine extreme hormone measurements and were based on identification of outliers from a graphical representation of the distribution of each hormone: testosterone >12 ng/mL (n = 3), estradiol >100 pg/mL (n = 4), androstanediol glucuronide >100 ng/mL (n = 6), SHBG > 150 nmol/L (n = 1), IGF-1 > 700 ng/mL (n = 1), and IGFBP-3 > 8,000 ng/mL (n = 1). Finally, 32 men were excluded because they had missing waist circumference, leaving 1,135 men for the statistical analysis.
Informed consent was obtained from all participants. The Institutional Review Board of the NCHS at CDC approved the NHANES program. The measurement of sex steroid hormones was approved by the Institutional Review Boards at the Johns Hopkins Bloomberg School of Public Health and at the NCHS at CDC.
Measurement of sex steroid hormones, SHBG, IGF-1, and IGFBP-3
Blood was collected after overnight fasting for participants in the morning sample during an examination at home or at a medical center and was stored at the NCHS’ main repository in Atlanta, GA. The serum samples were transferred on dry ice directly from the repository to the assay laboratory. Details on the blood draw, process, storage, and shipping methods are provided elsewhere [19].
The laboratory of Dr. Nader Rifai at Children’s Hospital in Boston, MA, performed all the measurements for serum sex hormone concentrations. Competitive electrochemiluminescence immunoassays on the 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN) were used to quantify serum testosterone, estradiol, and SHBG. Androstanediol glucuronide was measured by an enzyme immunoassay (Diagnostic Systems Laboratories, Webster, TX). The participant samples were tested in random order, and the laboratory technicians were blinded to the identities, age, and race/ethnicity of the participants. The lowest limits of detection were 0.02 ng/mL for testosterone, 5.00 pg/mL for estradiol, 0.33 ng/mL for androstanediol glucuronide, and 3.00 nmol/L for SHBG, but there were no values below those limits in our sample. The coefficients of variation for the quality control samples were 5.9 and 5.8 % for testosterone at 2.5 and 5.5 ng/mL, 6.5 and 6.7 % for estradiol at 102.7 and 474.1 pg/mL, 9.5 and 5.0 % for androstanediol glucuronide at 2.9 and 10.1 ng/mL, and 5.3 and 5.9 % for SHBG at 5.3 and 16.6 nmol/L. Additionally, quality control samples were separately run with a mean estradiol concentration of 39.4 pg/mL, which is in the range of typical male concentrations; the intra-assay CV % was 5.2 %, and the inter-assay CV % was 2.5 %. Free testosterone [20] and free estradiol [21] concentrations were estimated from published formulas including values for total testosterone, total estradiol, SHBG, and albumin.
IGF-1 and IGFBP-3 were measured by Diagnostic Systems Laboratories (Webster, TX). IGF-1 concentrations were quantified by an ELISA (DSL 10-5600), which is an enzymatically amplified “one-step” sandwich-type immunoassay. This procedure includes an extraction step during which IGF-I is separated form its binding protein in serum. IGFBP-3 concentrations were measured with an immunoradiometric assay (IRMA; DSL 6600). This is a non-competitive assay in which the analyte to be measured is “sandwiched” between two antibodies. The first antibody is placed to the inside walls of the tubes, and the other is radiolabeled for detection. A single technician performed all procedures. Throughout the study, samples were reanalyzed if the coefficient of variation for replicate samples from a single vial was greater than 15 %. Coefficients of variation varied significantly among individual QC subjects ranging from 12.3 to 17.6 % for IGF-1 and 8.9 to 12.8 % for IGFBP-3 [22].
Assessment of covariates
Demographic characteristics including age, race/ethnicity, cigarette smoking, and physical activity were self-reported during the NHANES interviews. The consumption of alcoholic beverages (beer, wine, liquor) was assessed using a food frequency questionnaire. Waist circumference at the iliac crest, height, and weight were measured during the examination. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. The definition of diabetes was based on self-reported information of diabetes diagnosis and blood concentration of glucose [no diabetes (no diagnosis and glucose <100 mg/dL), pre-diabetes (no diagnosis and glucose ≥100 to ≤125 mg/dL), and diabetes [diagnosis or glucose >125 mg/dL)]. Glucose was measured in NHANES using the glucose hexokinase method with a Hitachi Model 704 multichannel analyzer (Boehringer Manheim Diagnostics, Indianapolis, IN).
Statistical analysis
We calculated the pairwise Spearman rank correlation coefficients for all the hormones examined. We used weighted linear regression models to take into account the unequal probabilities of selection, oversampling, and non-response and thus correctly estimate the geometric means and 95 % confidence intervals of IGF-1 and IGFBP-3 concentrations according to each of the sex hormones. IGF-1 and IGFBP-3 were transformed using the natural logarithm because their distributions were right skewed. We used quintiles of the sex hormone concentrations, and when we tested the models using tertiles or deciles, the inferences were similar. Lowess plots between endogenous sex hormones and IGF-1 or IGFBP-3 were used to capture the best modeling approach, but no violation of a linear association was identified. Restricted cubic splines [23] were also used to further investigate the relationship between sex hormones or SHBG with IGF-1 or IGFBP-3, where knot positions were specified according to the quintiles of the sex hormone concentrations. The slope of the change in the natural logarithm of IGF-1 and IGFBP-3 with increasing quintiles of sex steroid hormones and SHBG concentrations was estimated by entering into the models an ordinal variable with its values corresponding to the quintiles of each hormone measure (1, 2, 3, 4, 5), the coefficients of which were evaluated by the Wald test. We also calculated the percentage difference in the geometric means of the hormones between the fifth and the first quintile by subtracting the geometric mean of the first quintile from the geometric mean of the fifth quintile divided by the geometric mean of the first quintile ((Q5 − Q1)/Q1). Negative values mean a decrease in the hormone concentrations with increasing quintiles, while positive values mean an increase.
Three multivariable linear regression models were built for each of the IGF-1, IGFBP-3, and sex hormone pair including non-endogenous factors that are known to influence hormone levels. Model 1 was adjusted for age (as a continuous variable) and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic including Mexican American, and other). Model 2 included also BMI (<25, 25–30 and ≥30 kg/m2), waist circumference (as a continuous variable), cigarette smoking status (never, former, current smoker), type 2 diabetes (no diabetes, pre-diabetes, diabetes), alcohol consumption (never drinker, ≤1 drink per week, two or three drinks per week, four to six drinks per week, and daily or more), and moderate or vigorous physical activity (no physical activity, ≤3 times per week, 3–6 times per week, >6 times per week). Model 3 further included mutual adjustments for testosterone, estradiol, and SHBG because these hormones compete for binding to SHBG. We further adjusted for IGF-1, IGFBP-3, and percentage body fat [24] in respective models, but inferences did not change.
Based on evidence from previous studies, age [25], race [26, 27], obesity [28], and diabetes [29] are factors that were significantly associated with both sex steroid hormones and the IGF system proteins; thus, we performed stratified analyses by age (at the median, ≤43 vs. >43 years), race/ethnicity, obesity (not obese by both BMI and waist circumference definitions [BMI < 30 kg/m2 and waist circumference <102 cm], obese by only one of the two definitions, obese by both definitions [BMI ≥ 30 kg/m2 and waist circumference ≥102 cm]), and type 2 diabetes (no, pre-diabetes, diabetes). In all interaction models, we also adjusted for percentage body fat to avoid possible residual confounding due to the extent of body fatness, but the results remained unchanged, and therefore, it was not included in our final models. We tested for possible interactions using the ordinal sex steroid hormone and SHBG variables, categorical variables for the potentially modifying factors, and their product terms. The statistical significance of the interaction terms was evaluated by the Wald test.
All tests were two-sided. P values <0.05 were considered statistically significant, and all analyses were performed using survey data analysis commands in STATA version 10 (College Station, TX). The p values from the interaction tests were not corrected for multiple hypothesis testing, but they were interpreted in view of the 48 (IGF-1 and IGFBP-3 × 6 steroid hormones × 4 modifying variables) comparisons made.
Results
Baseline characteristics of the 1,135 adult men included in the analysis are presented in Table 1. The mean age was 41 years, and the majority of the participants were white (78 %). The mean BMI was 26.4 kg/m2, and the prevalence of obesity based on BMI (≥30 kg/m2) was 21 %, whereas it was 26 % based on waist circumference (≥102 cm). Thirty-two percent of the participants were current smokers, 29 % never consumed alcohol, and 44 % performed moderate or vigorous physical activity more than six times per week. Six percent of the participants were diabetic, and 37 % were in an undiagnosed pre-diabetic state. The geometric mean values of IGF-1, IGFBP-3, sex steroid hormones, and SHBG were within the normal range.
Table 1.
Selected characteristics of the U.S. male population of 20 years or older, NHANES III 1988–1991
| Characteristics | Unweighted sample size | Mean or percentage (SE)† |
|---|---|---|
| Age (years) | 1,135 | 41.2 (0.7) |
| Race/ethnicity (%) | ||
| Non-Hispanic white | 537 | 78.3 (3.1) |
| Non-Hispanic black | 277 | 9.3 (1.3) |
| Hispanic | 273 | 5.0 (1.0) |
| Other | 48 | 7.5 (2.2) |
| Body mass index (kg/m2) | 1,135 | 26.4 (0.3) |
| Waist circumference (cm) | 1,135 | 94.4 (0.7) |
| Cigarette smoking (%) | ||
| Never | 408 | 36.3 (2.2) |
| Former | 368 | 31.5 (2.9) |
| Current | 359 | 32.2 (1.9) |
| Diabetes (%) | ||
| No | 609 | 57.7 (1.7) |
| Pre-diabetes | 421 | 36.7 (2.0) |
| Yes | 105 | 5.6 (0.8) |
| Alcohol consumption (%) | ||
| Never | 395 | 28.8 (2.8) |
| ≤1 per week | 205 | 17.6 (1.6) |
| 2 or 3 per week | 174 | 18.5 (1.7) |
| 4–6 per week | 171 | 17.5 (1.9) |
| Daily or more | 190 | 17.6 (2.8) |
| Moderate or vigorous physical activity (%) | ||
| No | 146 | 7.6 (1.3) |
| ≤3 times per week | 356 | 30.7 (2.2) |
| 3–6 times per week | 157 | 17.4 (1.6) |
| >6 times per week | 476 | 44.3 (2.9) |
| IGF-1 ng/mL‡ | 1,135 | 273 (262–285) |
| IGFBP-3 ng/mL‡ | 1,135 | 4,347 (4,231–4,467) |
| Total testosterone (ng/mL)‡ | 1,135 | 5.0 (4.8–5.3) |
| Total estradiol (pg/mL)‡ | 1,135 | 35.8 (34.2–37.5) |
| Sex hormone-binding globulin (nmol/L)‡ | 1,135 | 33.9 (32.4–35.4) |
| Androstanediol glucuronide (ng/mL)‡ | 1,135 | 11.7 (11.0–12.5) |
| Free testosterone (ng/mL)‡ | 1,135 | 0.10 (0.1–0.11) |
| Free estradiol (pg/mL)‡ | 1,135 | 0.92 (0.87–0.97) |
NHANES National Health and Nutrition Examination Survey, SE standard error
Sampling weights were applied
Geometric mean (95 % CI)
Table 2 shows the pairwise Spearman rank correlation coefficients for all hormones examined. There were several statistically significant correlations with the strongest being the positive association between free and total estradiol (r = 0.89) and the strongest negative association observed between SHBG and free estradiol (r = −0.34). Geometric means and 95 % CIs of IGF-1 concentrations by quintiles of sex steroid hormones from the weighted linear regression analysis are shown in Table 3. We found no statistically significant association between the concentrations of total (P-trend 0.13) and free testosterone (P-trend 0.44), total (P-trend 0.28) and free estradiol (P-trend 0.52), SHBG (P-trend 0.39), or androstanediol glucuronide (P-trend 0.96) and IGF-1 in any of the multivariable models. When we ran the analyses again after further adjustment for IGFBP-3, the results remained not statistically significant.
Table 2.
Spearman rank correlation coefficients (univariate) for sex hormones and IGF-1/IGFBP-3
| IGF-1 (ng/mL) |
IGFBP-3 (ng/mL) |
Total testosterone (ng/mL) |
Free testosterone (ng/mL) |
Total estradiol (pg/mL) |
Free estradiol (pg/mL) |
SHBG (nmol/L) |
Androstanediol glucuronide (ng/mL) |
|
|---|---|---|---|---|---|---|---|---|
| IGF-1 | 1.0 | |||||||
| IGFBP-3 | 0.63* | 1.0 | ||||||
| Total testosterone | 0.19* | 0.02 | 1.0 | |||||
| Free testosterone | 0.35* | 0.22* | 0.79* | 1.0 | ||||
| Total estradiol | 0.04 | −0.05 | 0.45* | 0.44* | 1.0 | |||
| Free estradiol | 0.11* | 0.05 | 0.26* | 0.49* | 0.89* | 1.0 | ||
| SHBG | −0.26* | −0.32* | 0.30* | −0.28* | 0.04 | −0.34* | 1.0 | |
| Androstanediol glucuronide | 0.17* | 0.14* | 0.23* | 0.31* | 0.11* | 0.14* | −0.13* | 1.0 |
SHBG sex hormone-binding globulin
Correlation coefficients with a star (*) are significant at the 0.05 level
Table 3.
Geometric means and 95 % confidence intervals of IGF-1 (ng/mL) concentrations by quintiles of sex steroid hormones in the U.S. male population of 20 years or older, NHANES III 1988–1991
| Sex steroid hormones | Model 1†
|
Model 2†
|
Model 3§
|
|||
|---|---|---|---|---|---|---|
| Mean | 95 % CI | Mean | 95 % CI | Mean | 95 % CI | |
| Total testosterone (ng/mL) | ||||||
| ≤3.67 | 276 | 259–293 | 282 | 270–294 | 271 | 256–289 |
| 3.68–4.64 | 248 | 226–271 | 252 | 227–279 | 245 | 222–270 |
| 4.65–5.62 | 283 | 260–307 | 281 | 259–306 | 279 | 258–303 |
| 5.62–6.91 | 276 | 262–291 | 274 | 259–289 | 278 | 262–294 |
| >6.91 | 283 | 258–310 | 278 | 257–300 | 291 | 267–317 |
| Slope, P-trend* | 0.02 | 0.22 | 0.01 | 0.70 | 0.02 | 0.13 |
| Free testosterone (ng/mL) | ||||||
| ≤0.069 | 277 | 253–304 | 278 | 258–300 | 273 | 252–295 |
| 0.070–0.092 | 270 | 248–293 | 272 | 245–301 | 269 | 242–298 |
| 0.093–0.113 | 261 | 244–279 | 263 | 247–280 | 262 | 245–279 |
| 0.113–0.14 | 280 | 262–298 | 277 | 260–296 | 278 | 261–297 |
| >0.14 | 279 | 252–309 | 277 | 254–303 | 283 | 260–309 |
| Slope, P-trend* | 0.01 | 0.66 | 0.01 | 0.80 | 0.01 | 0.44 |
| Total estradiol (pg/mL) | ||||||
| ≤28.56 | 292 | 278–306 | 288 | 273–302 | 290 | 274–306 |
| 28.57–33.29 | 269 | 258–281 | 265 | 252–277 | 265 | 252–278 |
| 33.30–38.66 | 265 | 249–282 | 269 | 252–288 | 269 | 252–288 |
| 38.67–46.2 | 287 | 257–320 | 287 | 258–318 | 286 | 257–317 |
| >46.2 | 257 | 237–279 | 261 | 242–281 | 259 | 241–279 |
| Slope, P-trend* | −0.02 | 0.17 | −0.01 | 0.38 | −0.01 | 0.28 |
| Free estradiol (pg/mL) | ||||||
| ≤0.70 | 280 | 269–292 | 272 | 260–285 | 276 | 262–291 |
| 0.71–0.85 | 282 | 268–296 | 276 | 263–290 | 278 | 265–292 |
| 0.86–1.0 | 265 | 244–288 | 266 | 246–288 | 267 | 246–289 |
| 1.1–1.2 | 281 | 268–295 | 285 | 271–301 | 284 | 270–298 |
| >1.2 | 259 | 232–290 | 266 | 240–294 | 262 | 238–288 |
| Slope, P-trend* | −0.02 | 0.26 | −0.002 | 0.89 | −0.01 | 0.52 |
| SHBG (nmol/L) | ||||||
| ≤24.0 | 272 | 250–296 | 278 | 255–303 | 282 | 255–311 |
| 24.1–31.6 | 270 | 253–289 | 274 | 257–292 | 275 | 256–296 |
| 31.7–40.52 | 270 | 256–284 | 271 | 258–285 | 271 | 258–284 |
| 40.53–53.5 | 283 | 267–301 | 277 | 263–293 | 275 | 261–290 |
| >53.5 | 271 | 252–293 | 262 | 242–285 | 260 | 237–286 |
| Slope, P-trend* | 0.005 | 0.67 | −0.01 | 0.51 | −0.02 | 0.39 |
| Androstanediol glucuronide (ng/mL) | ||||||
| ≤6.72 | 275 | 262–289 | 274 | 261–287 | Not applicable | |
| 6.73–9.68 | 283 | 267–301 | 284 | 268–302 | ||
| 9.69–12.77 | 256 | 237–277 | 258 | 235–283 | ||
| 12.78–17.82 | 268 | 252–286 | 270 | 253–287 | ||
| >17.82 | 283 | 264–303 | 280 | 262–299 | ||
| Slope, P-trend* | 0.002 | 0.88 | 0.001 | 0.96 | ||
SHBG sex hormone-binding globulin
From a weighted linear regression model of sex steroid hormones on IGF-1 adjusted for age and race/ethnicity
Same as model 1 plus adjustment for body mass index, waist circumference, diabetes, alcohol consumption, cigarette smoking, and physical activity
Same as model 2 plus testosterone, estradiol, and SHBG mutually adjusted, and free testosterone and free estradiol mutually adjusted
Change in IGF-1 concentration in ng/mL per one quintile change in sex steroid hormone concentration
Table 4 presents the associations between sex steroid hormones and IGFBP-3. Total testosterone concentration was modestly inversely associated with IGFBP-3 in models 1 (% difference Q5 − Q1 geometric means −5.7 %; slope per quintile increase in concentration −0.01; P-trend 0.06) and 2 (% difference −6.2 %; slope −0.01; P-trend 0.01), but this association became nonsignificant after additional mutual adjustment for total estradiol and SHBG (% difference 2.6 %; slope 0.01; P-trend 0.23). There was no association between free testosterone and IGFBP-3 (% difference 3 %; slope 0.01; P-trend 0.36). Total estradiol concentration was modestly inversely associated with IGFBP-3 in model 1 (% difference −10.2 %; slope −0.02; P-trend 0.01), model 2 (% difference −10.3 %; slope −0.02; P-trend 0.01), and model 3 (% difference −9.7 %; slope −0.02; P-trend 0.05) (Fig. 1). In model 3, the geometric mean of IGFBP-3 in the estradiol lowest quintile was 4,574 ng/mL, while in the highest quintile it was 4,130 ng/mL. However, free estradiol was not associated with IGFBP-3 (% difference −4.7 %; slope −0.01; P-trend 0.17). SHBG concentrations were also modestly inversely associated with IGFBP-3 in all models (% difference −7.3 %; slope −0.02; P-trend 0.02) (Fig. 2). In model 3, the geometric mean of IGFBP-3 in the SHBG first quintile was 4,501 ng/mL, whereas in the fifth it was 4,171 ng/mL. Androstanediol glucuronide concentration was not associated with IGFBP-3 (P-trend 0.40). When we further adjusted for IGF-1 concentrations, all the associations remained unchanged.
Table 4.
Geometric means and 95 % confidence intervals of IGFBP-3 (ng/mL) concentrations by quintiles of sex steroid hormones of the U.S. male population of 20 years or older, NHANES III 1988–1991
| Sex steroid hormones | Model 1†
|
Model 2†
|
Model 3§
|
|||
|---|---|---|---|---|---|---|
| Mean | 95 % CI | Mean | 95 % CI | Mean | 95 % CI | |
| Total testosterone (ng/mL) | ||||||
| ≤3.67 | 4,477 | 4,331–4,627 | 4,493 | 4,348–4,642 | 4,313 | 4,162–4,470 |
| 3.68–4.64 | 4,303 | 4,118–4,496 | 4,316 | 4,110–4,531 | 4,184 | 3,968–4,411 |
| 4.65–5.62 | 4,471 | 4,244–4,710 | 4,476 | 4,264–4,700 | 4,444 | 4,238–4,660 |
| 5.62–6.91 | 4,293 | 4,143–4,448 | 4,272 | 4,133–4,416 | 4,341 | 4,200–4,487 |
| >6.91 | 4,222 | 4,032–4,422 | 4,215 | 4,077–4,357 | 4,428 | 4,309–4,551 |
| Slope, P-trend* | −0.01 | 0.06 | −0.01 | 0.01 | 0.01 | 0.23 |
| Free testosterone (ng/mL) | ||||||
| ≤0.069 | 4,318 | 4,099–4,549 | 4,329 | 4,134–4,534 | 4,234 | 4,032–4,447 |
| 0.070–0.092 | 4,347 | 4,128–4,577 | 4,345 | 4,091–4,613 | 4,290 | 4,026–4,571 |
| 0.093–0.113 | 4,390 | 4,207–4,581 | 4,401 | 4,226–4,585 | 4,375 | 4,204–4,554 |
| 0.113–0.14 | 4,414 | 4,224–4,612 | 4,405 | 4,222–4,595 | 4,421 | 4,247–4,601 |
| >0.14 | 4,258 | 4,036–4,492 | 4,253 | 4,054–4,462 | 4,359 | 4,156–4,572 |
| Slope, P-trend* | −0.003 | 0.71 | −0.003 | 0.65 | 0.01 | 0.36 |
| Total estradiol (pg/mL) | ||||||
| ≤28.56 | 4,588 | 4,449–4,732 | 4,593 | 4,445–4,745 | 4,574 | 4,392–4,765 |
| 28.57–33.29 | 4,359 | 4,160–4,567 | 4,371 | 4,173–4,578 | 4,356 | 4,134–4,591 |
| 33.30–38.66 | 4,311 | 4,141–4,487 | 4,308 | 4,151–4,471 | 4,310 | 4,152–4,474 |
| 38.67–46.2 | 4,381 | 4,159–4,615 | 4,370 | 4,162–4,588 | 4,388 | 4,168–4,619 |
| >46.2 | 4,121 | 3,936–4,316 | 4,120 | 3,950–4,297 | 4,130 | 3,950–4,320 |
| Slope, P-trend* | −0.02 | 0.01 | −0.02 | 0.01 | −0.02 | 0.05** |
| Free estradiol (pg/mL) | ||||||
| ≤0.70 | 4,415 | 4,265–4,751 | 4,426 | 4,273–4,586 | 4,423 | 4,239–4,615 |
| 0.71–0.85 | 4,448 | 4,301–4,600 | 4,457 | 4,320–4,597 | 4,455 | 4,308–4,607 |
| 0.86–1.0 | 4,346 | 4,161–4,539 | 4,335 | 4,138–4,542 | 4,334 | 4,139–4,538 |
| 1.1–1.20 | 4,313 | 4,152–4,481 | 4,317 | 4,181–4,459 | 4,319 | 4,186–4,456 |
| >1.20 | 4,225 | 4,013–4,448 | 4,213 | 4,014–4,422 | 4,217 | 3,998–4,449 |
| Slope, P-trend* | −0.01 | 0.11 | −0.01 | 0.08 | −0.01 | 0.17 |
| SHBG (nmol/L) | ||||||
| ≤24.0 | 4,500 | 4,326–4,681 | 4,501 | 4,343–4,665 | 4,501 | 4,316–4,694 |
| 24.1–31.62 | 4,481 | 4,292–4,679 | 4,483 | 4,296–4,678 | 4,472 | 4,279–4,675 |
| 31.63–40.52 | 4,202 | 4,040–4,369 | 4,201 | 4,053–4,353 | 4,195 | 4,051–4,344 |
| 40.53–53.50 | 4,335 | 4,191–4,484 | 4,347 | 4,208–4,490 | 4,353 | 4,203–4,508 |
| >53.50 | 4,174 | 3,965–4,395 | 4,157 | 3,957–4,367 | 4,171 | 3,963–4,391 |
| Slope, P-trend* | −0.02 | 0.01 | −0.02 | 0.005 | −0.02 | 0.02** |
| Androstanediol glucuronide (ng/mL) | ||||||
| ≤6.72 | 4,310 | 4,123–4,504 | 4,333 | 4,143–4,532 | Not applicable | |
| 6.73–9.68 | 4,279 | 4,098–4,469 | 4,281 | 4,107–4,462 | ||
| 9.69–12.77 | 4,332 | 4,176–4,494 | 4,332 | 4,173–4,497 | ||
| 12.78–17.82 | 4,476 | 4,313–4,644 | 4,466 | 4,297–4,642 | ||
| >17.82 | 4,321 | 4,155–4,494 | 4,313 | 4,161–4,470 | ||
| Slope, P-trend* | 0.005 | 0.35 | 0.003 | 0.4 | ||
SHBG sex hormone-binding globulin
From a linear regression model of sex steroid hormones on IGFBP-3 adjusted for age and race/ethnicity
Same as model 1 plus adjustment for body mass index, waist circumference, diabetes, cigarette smoking, alcohol consumption, and physical activity
Same as model 2 plus testosterone, estradiol, and SHBG mutually adjusted, and free testosterone and free estradiol mutually adjusted
Change in IGFBP-3 concentration in ng/mL per one quintile change in sex steroid hormone concentration
The −0.02 slope for total estradiol and SHBG was based on the logarithmic distribution of IGFBP-3 and corresponded to a 82 ng/mL decrease in IGFBP-3 per quintile change of estradiol and 74 ng/mL decrease in IGFBP-3 per quintile change of SHBG in the natural scale
Fig. 1.
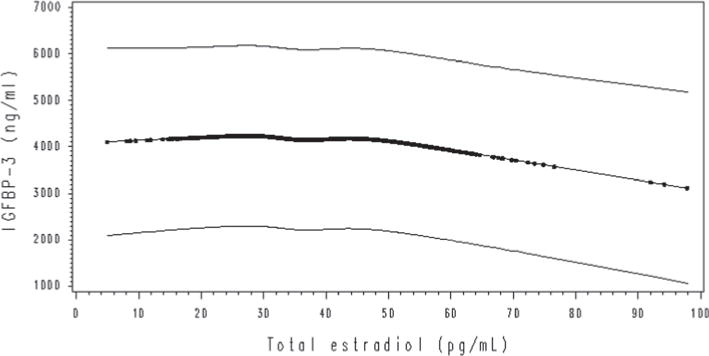
Change in concentrations of IGFBP-3 with total estradiol (unadjusted quadratic spline model estimation with 95 % CI). The results were similar after adjustment for confounders
Fig. 2.
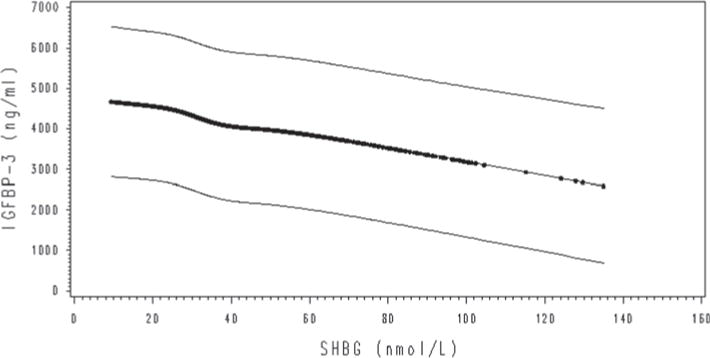
Change in concentrations of IGFBP-3 with SHBG (unadjusted quadratic spline model estimation with 95 % CI). The results were similar after adjustment for confounders
The results of the stratified analysis for the fully adjusted models are shown in Online Resources 1 and 2. The strongest interaction based on the P value was observed for the association between SHBG and IGFBP-3 by obesity (P-interaction 0.002) where SHBG was inversely associated with IGFBP-3 in obese men (slope −0.09; P-trend <0.001), but no association was observed in men who were not obese (slope −0.002; P-trend 0.79). Significant interactions were also observed for the associations between SHBG and IGFBP-3 by age (P-interaction 0.01), where there was an inverse association in men older than 43 years (slope −0.05; P-trend 0.001), but no association was observed in young men (slope −0.02; P-trend 0.06). Other significant interactions were seen for the associations between SHBG and IGFBP-3 by race/ethnicity (P-interaction 0.007), between androstanediol glucuronide and IGFBP-3 by age (P-interaction 0.03), and between free testosterone and IGFBP-3 by obesity (P-interaction 0.02). In the associations between IGF-1 and sex steroid hormones, significant interactions were observed in the association between SHBG and IGF-1 by race/ethnicity (P-interaction 0.04) and between total testosterone and IGF-1 by obesity (P-interaction 0.03).
Discussion
In the present analysis of cross-sectional data from NHANES III, we found no statistically significant associations between sex steroid hormones and IGF-1 concentrations. Total estradiol concentration was modestly inversely associated with IGFBP-3 concentration, although a significant association was not observed for free estradiol. SHBG was also modestly inversely associated with IG-FBP-3. An association between serum androgens and IG-FBP-3 was not observed.
Our results did not support an association between serum androgens and IGF-1. Few studies have examined the inter-relation between sex steroid hormones and IGF-1, and they produced mixed results. In a large population-based sample of 3,057 men participating in the European Male Aging Study (EMAS), free testosterone concentrations were positively associated with IGF-1 [15], but these results were not adjusted for other sex hormones, physical activity, alcohol consumption, and smoking. Total and free androgens were not associated with IGF-1 in other smaller studies [16–18]. In vitro studies have shown that IGF-1 enhances sex steroid synthesis in the testis [30]. Nevertheless, these results may apply only at the cellular level where the local associations between IGF-1 and androgens may be different than those observed in the plasma levels that our measurements represent.
We found a statistically significant inverse association between total testosterone and IGFBP-3 that disappeared after mutual adjustment for total estradiol and SHBG. This inverse association was observed also in a previous study that adjusted only for age and BMI [17]. Free testosterone was not associated with IGFBP-3 in any of our multivariable models. Earlier studies have reported mixed results for the association between free testosterone and IGFBP-3 [15–17].
We did not find a significant association between serum estrogens and IGF-1, and there are limited and conflicting published data on this association in men. Free estradiol was not associated with IGF-1 in the EMAS study [15], but another cross-sectional analysis among 103 healthy elderly men showed a positive association between free estradiol and IGF-1 after adjusting for age [16]. We observed a significant inverse association between total estradiol and IGFBP-3, which was not observed in previous studies [16, 17]. It has been proposed that estrogens attenuate growth hormone (GH) actions through the increase in the GH-binding protein [31], and this may be an explanation for the decrease in IGFBP-3, as it is partly regulated by GH.
Sex hormone-binding globulin (SHBG) was not associated with IGF-1 in our analysis, and this is in contrast to the results from other studies that reported a negative association [15, 16, 18, 20, 32]. These discrepancies could be due to differences in sample size, different population characteristics, and inadequate adjustment for confounders. SHBG concentration was inversely associated with IGFBP-3 in our study, which is consistent with other studies [15– 17, 20, 32]. The biological mechanism that explains this inverse association may involve regulatory pathways mediated by insulin. Reduced serum SHBG concentrations have been described in insulin resistance states coupled with hyperinsulinemia, as in polycystic ovary syndrome [33] or obesity [34], and also insulin has been shown to induce the expression of the IGFBP-3 gene [35] and thus increase the production of IGFBP-3 directly or indirectly through IGF-1 increase.
We reported some conventionally significant interactions for the association between sex steroid hormones and IGFBP-3 by age, race/ethnicity, obesity, and diabetes. The strongest suggestion for an interaction was the inverse association between SHBG and IGFBP-3 by obesity; there was an inverse association in obese participants, whereas the association was null in non-obese individuals. One of the metabolic effects of obesity includes a rise in insulin concentrations that leads to a progressive reduction in SHBG, and this might be the reason for the observed association in the obese, and not in the normal-weight individuals who have a normal insulin balance. This is the first study to our knowledge that examined potential effect modification by a number of contributing factors, and more data are needed to further explore these associations.
The strengths of this study include the large sample of adult men, who are representative of the general US population and the adjustment for demographic characteristics, lifestyle and health factors, and other sex steroid hormones, which were not consistently adjusted for in prior studies. The measurement method of estradiol may not be optimal for the range of values described in men [36, 37]; therefore, the results should be interpreted with caution. On the other hand, serum testosterone concentrations have high correlation with mass spectrometry-based methods. Our results are based on cross-sectional data, and we cannot make inferences about temporality. We cannot be certain on whether sex steroid hormones influence the concentration of IGF-1 and IGFBP-3 or the opposite. In addition, the methodologies that were used to measure circulating IGF-1 and IGFBP-3 concentrations have changed over time. Especially for IGFBP-3, the correlation between the older radioimmunoassay (RIA) method and the newer ELISAs was moderate (r = 0.58; 95 % CI 0.49–0.66) [38], so this could partly explain the differences between our results and results from other studies. One single measurement of a biomarker may not represent the average lifetime concentration, and it certainly does not incorporate the instability of the concentrations throughout the day or from day to day. However, there is evidence that, for all these hormones, a single fasting serum measurement can fairly reliably reflect the hormonal milieu over periods of months [39].
In conclusion, our results suggest that there is no association between circulating sex steroid hormones and IGF-1 concentrations, but total estradiol and SHBG concentrations may be inversely associated with IGFBP-3. Researchers should examine in the future the potential joint effect of these two pathways on chronic disease etiology, including cancer and cardiovascular disease.
Supplementary Material
Acknowledgments
This is the 27th study from the Hormone Demonstration Program, which is supported by the Maryland Cigarette Restitution Fund at Johns Hopkins. We thank Dr. David Berrigan for the useful comments he provided on this manuscript.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10552-013-0336-4) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare no conflict of interest.
Contributor Information
Stefania I. Papatheodorou, Cyprus International Institute for Environmental and Public Health, Cyprus University of Technology, Limassol, Cyprus
Sabine Rohrmann, Division of Cancer Epidemiology and Prevention, Institute of Social and Preventive Medicine, University of Zurich, Zurich, Switzerland.
David S. Lopez, Division of Epidemiology, University of Texas School of Public Health, Houston, TX, USA
Gary Bradwin, Department of Laboratory Medicine, Harvard Medical School and Children’s Hospital, Boston, MA, USA.
Corinne E. Joshu, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Norma Kanarek, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Medical Institutions, Baltimore, MD, USA; Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
William G. Nelson, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Medical Institutions, Baltimore, MD, USA Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA; Department of Oncology, Pathology, Pharmacology and Molecular Sciences, Radiation Oncology and Molecular Radiation Sciences, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Nader Rifai, Department of Laboratory Medicine, Harvard Medical School and Children’s Hospital, Boston, MA, USA; King Abdulaziz University, Jeddah, Saudi Arabia.
Elizabeth A. Platz, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Medical Institutions, Baltimore, MD, USA; James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA; Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Konstantinos K. Tsilidis, Email: kostas.tsilidis@ceu.ox.ac.uk, ktsilidi@cc.uoi.gr, Department of Hygiene and Epidemiology, University of Ioannina School of Medicine, Stavros Niarchos Av., 45110 Ioannina, Greece.
References
- 1.Anversa P. Aging and longevity: the IGF-1 enigma. Circ Res. 2005;97(5):411–414. doi: 10.1161/01.RES.0000182212.09147.56. [DOI] [PubMed] [Google Scholar]
- 2.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore longitudinal study of A. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 3.Phillips GB, Jing T, Heymsfield SB. Relationships in men of sex hormones, insulin, adiposity, and risk factors for myocardial infarction. Metabolism. 2003;52(6):784–790. doi: 10.1016/s0026-0495(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 4.Corona G, Monami M, Rastrelli G, Aversa A, Tishova Y, Saad F, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8(1):272–283. doi: 10.1111/j.1743-6109.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 5.Nettleship JE, Jones RD, Channer KS, Jones TH. Testosterone and coronary artery disease. Front Horm Res. 2009;37:91–107. doi: 10.1159/000176047. [DOI] [PubMed] [Google Scholar]
- 6.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(10):3007–3019. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endogenous Hormones and Prostate Cancer Collaborative Group. Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100(3):170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13(4):113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 9.Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet. 2002;359(9319):1740–1745. doi: 10.1016/S0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- 10.Burgers AM, Biermasz NR, Schoones JW, Pereira AM, Renehan AG, Zwahlen M, et al. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab. 2011;96(9):2912–2920. doi: 10.1210/jc.2011-1377. [DOI] [PubMed] [Google Scholar]
- 11.Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB, et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med. 2008;149(7):461–71. W83–8. doi: 10.7326/0003-4819-149-7-200810070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. Int J Cancer. 2009;124(10):2416–2429. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 14.Friedrich N, Schneider HJ, Haring R, Nauck M, Volzke H, Kroemer HK, et al. Improved prediction of all-cause mortality by a combination of serum total testosterone and insulin-like growth factor I in adult men. Steroids. 2012;77(1–2):52–58. doi: 10.1016/j.steroids.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Pye SR, Almusalam B, Boonen S, Vanderschueren D, Borghs H, Gielen E, et al. Influence of insulin-like growth factor binding protein (IGFBP)-1 and IGFBP-3 on bone health: results from the European Male Ageing Study. Calcif Tissue Int. 2011;88(6):503–510. doi: 10.1007/s00223-011-9484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen JA, Stolk RP, Pols HA, Grobbee DE, de Jong FH, Lamberts SW. Serum free IGF-I, total IGF-I, IGFBP-1 and IGFBP-3 levels in an elderly population: relation to age and sex steroid levels. Clin Endocrinol (Oxf) 1998;48(4):471–478. doi: 10.1046/j.1365-2265.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- 17.Pfeilschifter J, Scheidt-Nave C, Leidig-Bruckner G, Woitge HW, Blum WF, Wuster C, et al. Relationship between circulating insulin-like growth factor components and sex hormones in a population-based sample of 50- to 80-year-old men and women. J Clin Endocrinol Metab. 1996;81(7):2534–2540. doi: 10.1210/jcem.81.7.8675573. [DOI] [PubMed] [Google Scholar]
- 18.Erfurth EM, Hagmar LE, Saaf M, Hall K. Serum levels of insulin-like growth factor I and insulin-like growth factor-binding protein 1 correlate with serum free testosterone and sex hormone binding globulin levels in healthy young and middle-aged men. Clin Endocrinol (Oxf) 1996;44(6):659–664. doi: 10.1046/j.1365-2265.1996.731552.x. [DOI] [PubMed] [Google Scholar]
- 19.Plan and operation of the Third National Health and Nutrition Examination Survey. Series 1: programs and collection procedures. Vital Health Stat 1. 1988–94;1994(32):1–407. [PubMed] [Google Scholar]
- 20.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 21.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 22.Berrigan D, Potischman N, Dodd KW, Nicar M, McQuillan G, Lavigne JA, et al. Serum levels of insulin-like growth factor-I and insulin-like growth factor-I binding protein-3: quality control for studies of stored serum. Cancer Epidemiol Biomarkers Prev. 2007;16(5):1017–1022. doi: 10.1158/1055-9965.EPI-07-0044. [DOI] [PubMed] [Google Scholar]
- 23.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6(4):356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Rohrmann S, Shiels MS, Lopez DS, Rifai N, Nelson WG, Kanarek N, et al. Body fatness and sex steroid hormone concentrations in US men: results from NHANES III. Cancer Causes Control. 2011;22(8):1141–1151. doi: 10.1007/s10552-011-9790-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohrmann S, Platz EA, Selvin E, Shiels MS, Joshu CE, Menke A, et al. The prevalence of low sex steroid hormone concentrations in men in the Third National Health and Nutrition Examination Survey (NHANES III) Clin Endocrinol (Oxf) 2011;75(2):232–239. doi: 10.1111/j.1365-2265.2011.04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92(7):2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 27.Berrigan D, Potischman N, Dodd KW, Hursting SD, Lavigne J, Barrett JC, et al. Race/ethnic variation in serum levels of IGF-I and IGFBP-3 in US adults. Growth Horm IGF Res. 2009;19(2):146–155. doi: 10.1016/j.ghir.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faupel-Badger JM, Berrigan D, Ballard-Barbash R, Potischman N. Anthropometric correlates of insulin-like growth factor 1 (IGF-1) and IGF binding protein-3 (IGFBP-3) levels by race/ethnicity and gender. Ann Epidemiol. 2009;19(12):841–849. doi: 10.1016/j.annepidem.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, et al. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30(2):234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 30.Spiteri-Grech J, Nieschlag E. The role of growth hormone and insulin-like growth factor I in the regulation of male reproductive function. Horm Res. 1992;38(Suppl 1):22–27. doi: 10.1159/000182566. [DOI] [PubMed] [Google Scholar]
- 31.Rajkovic IA, Valiontis E, Ho KK. Direct quantitation of growth hormone binding protein in human serum by a ligand immunofunctional assay: comparison with immunoprecipitation and chromatographic methods. J Clin Endocrinol Metab. 1994;78(3):772–777. doi: 10.1210/jcem.78.3.8126156. [DOI] [PubMed] [Google Scholar]
- 32.Gomez JM, Maravall FJ, Gomez N, Navarro MA, Soler J. Determinants of sex hormone-binding globulin concentrations in a cross-sectional study of healthy men randomly selected. J Nutr Health Aging. 2007;11(1):60–64. [PubMed] [Google Scholar]
- 33.Jayagopal V, Kilpatrick ES, Jennings PE, Hepburn DA, Atkin SL. The biological variation of testosterone and sex hormone-binding globulin (SHBG) in polycystic ovarian syndrome: implications for SHBG as a surrogate marker of insulin resistance. J Clin Endocrinol Metab. 2003;88(4):1528–1533. doi: 10.1210/jc.2002-020557. [DOI] [PubMed] [Google Scholar]
- 34.Onat A, Hergenc G, Karabulut A, Albayrak S, Can G, Kaya Z. Serum sex hormone-binding globulin, a determinant of cardiometabolic disorders independent of abdominal obesity and insulin resistance in elderly men and women. Metabolism. 2007;56(10):1356–1362. doi: 10.1016/j.metabol.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Paquette J, Bessette B, Ledru E, Deal C. Identification of upstream stimulatory factor binding sites in the human IGFBP3 promoter and potential implication of adjacent single-nucleotide polymorphisms and responsiveness to insulin. Endocrinology. 2007;148(12):6007–6018. doi: 10.1210/en.2006-1729. [DOI] [PubMed] [Google Scholar]
- 36.Huhtaniemi IT, Tajar A, Lee DM, O’Neill TW, Finn JD, Bartfai G, et al. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur J Endocrinol. 2012;166(6):983–991. doi: 10.1530/EJE-11-1051. [DOI] [PubMed] [Google Scholar]
- 37.Ohlsson C, Nilsson ME, Tivesten A, Ryberg H, Mellstrom D, Karlsson MK, et al. Comparisons of immunoassay and mass spectrometry measurements of serum estradiol levels and their influence on clinical association studies in men. J Clin Endocrinol Metab. 2013;98(6):E1097–E1102. doi: 10.1210/jc.2012-3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rinaldi S, Kaaks R, Zeleniuch-Jacquotte A, Arslan AA, Shore RE, Koenig KL, et al. Insulin-like growth factor-I, IGF binding protein-3, and breast cancer in young women: a comparison of risk estimates using different peptide assays. Cancer Epidemiol Biomarkers Prev. 2005;14(1):48–52. [PubMed] [Google Scholar]
- 39.Missmer SA, Spiegelman D, Bertone-Johnson ER, Barbieri RL, Pollak MN, Hankinson SE. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers Prev. 2006;15(5):972–978. doi: 10.1158/1055-9965.EPI-05-0848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


