Abstract
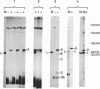
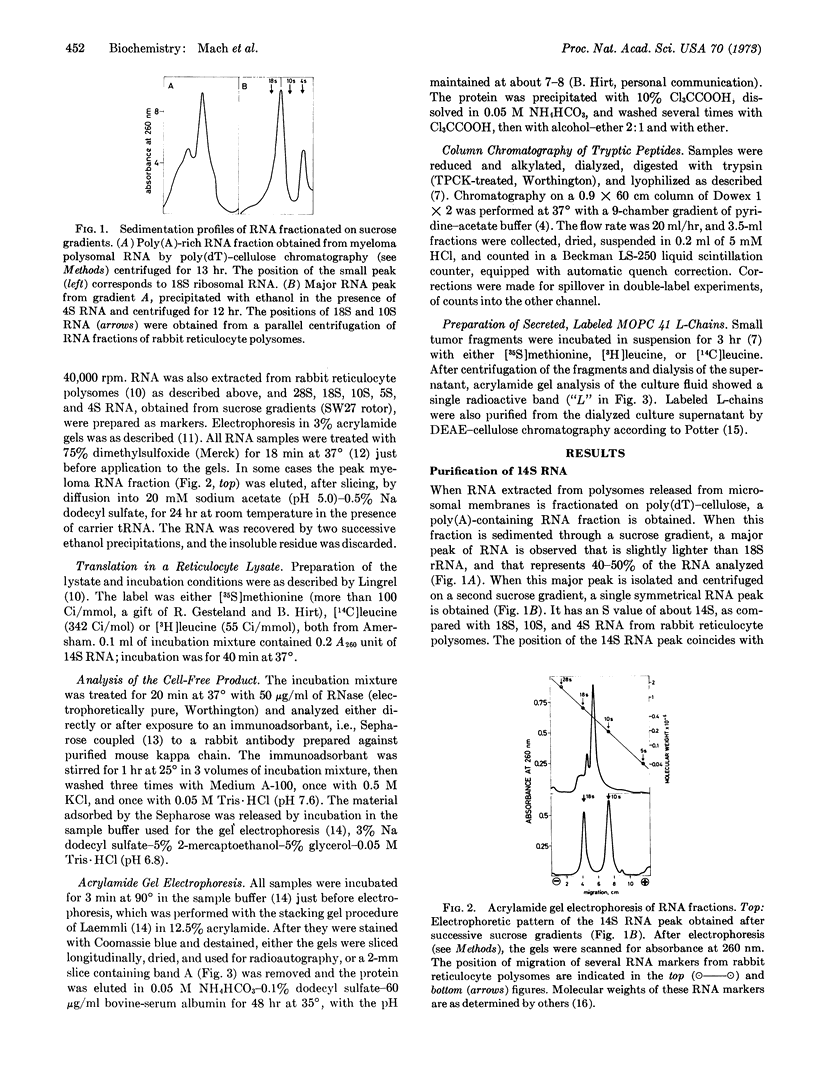
Polysomes released from microsomes of MOPC 41 mouse myeloma were used to prepare a poly(A)-containing fraction of RNA by chromatography on poly-(dT)-cellulose. From that fraction, a 14S RNA species was purified to a single peak by successive sucrose gradient centrifugations, followed by acrylamide gel electrophoresis. The RNA has an apparent molecular weight of 380,000 (1100 nucleotides), as estimated from the electrophoretic analyses. In a reticulocyte lysate this RNA directs the synthesis of a protein that migrates more slowly in sodium dodecylsulfate-acrylamide gels than does the light chain secreted by the same tumor. This difference in migration corresponds to a size difference appropriate for polypeptide chain about 20 amino acids longer than the light chain. The tryptic peptides of this protein correspond to those of the secreted light chain, except for the presence of two additional peptides from the product synthesized in vitro and for the absence of one light-chain peptide. The purified RNA is, therefore, the mRNA of the light chain, and it seems to code for a precursor protein slightly larger than the light chain. From the estimated size of the 14S mRNA, it appears that only 65% of the RNA is translated.
Keywords: poly(dT) chromatography, mRNA molecular weight, mRNA translation, mouse myeloma
Full text
PDF
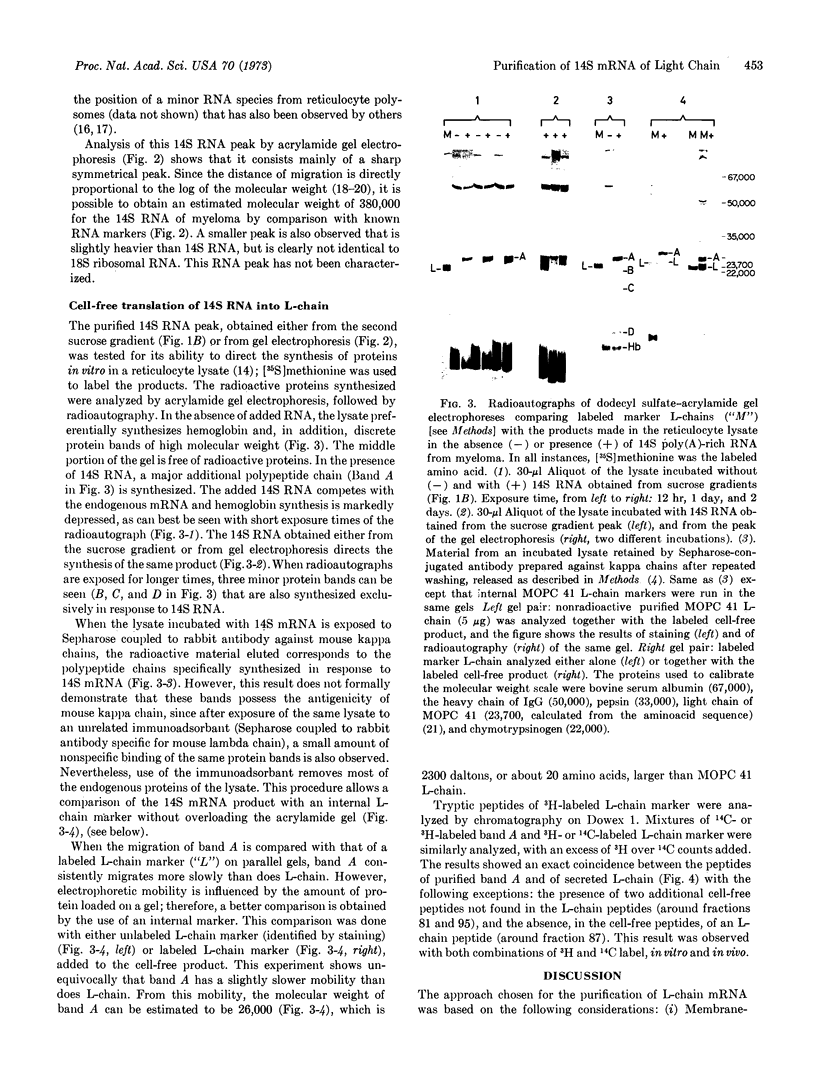

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
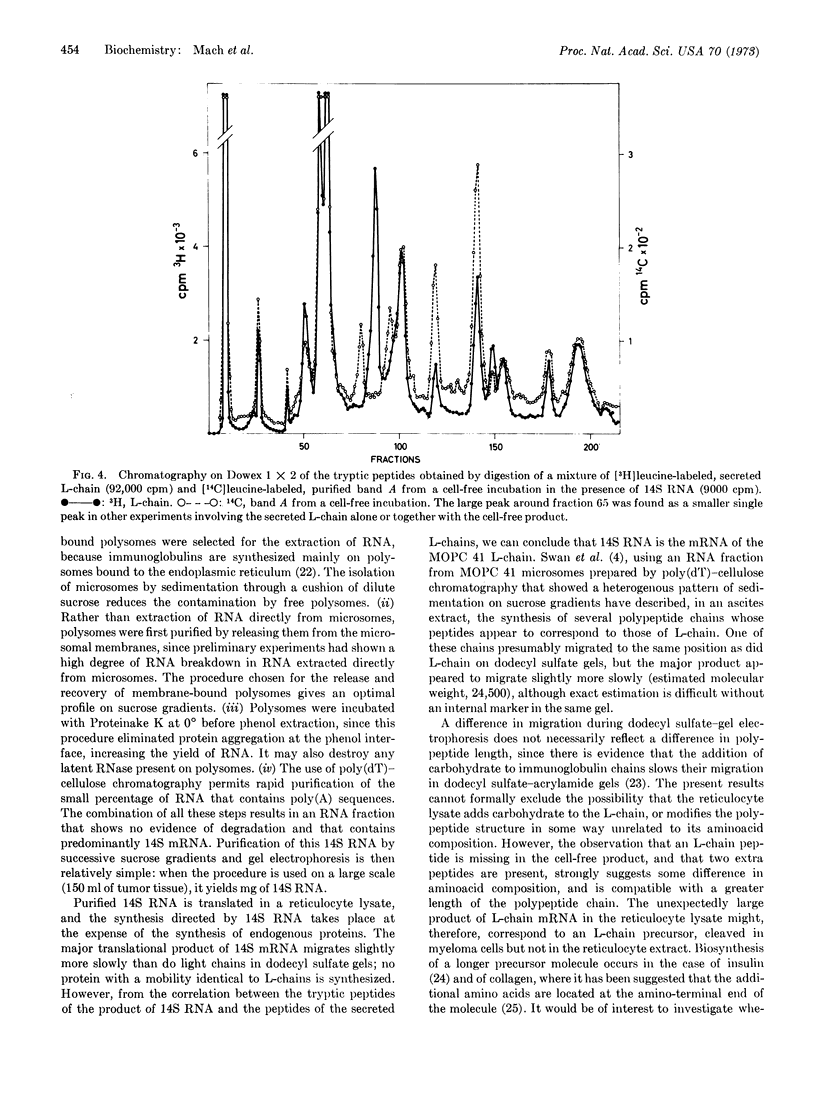
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson D. S. The regulation of plasma lipoprotein concentrations as affected in human mutants. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1138–1146. doi: 10.1073/pnas.64.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill P., Kabat D. Unexpectedly large size of globin messenger ribonucleic acid. Proc Natl Acad Sci U S A. 1971 Jan;68(1):72–75. doi: 10.1073/pnas.68.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W. R., Dreyer W. J., Hood L. Mechanism of antibody synthesis: size differences between mouse kappa chains. Science. 1967 Jan 27;155(3761):465–467. doi: 10.1126/science.155.3761.465. [DOI] [PubMed] [Google Scholar]
- Katz L., Penman S. The solvent denaturation of double-stranded RNA from poliovirus infected HeLa cells. Biochem Biophys Res Commun. 1966 May 25;23(4):557–560. doi: 10.1016/0006-291x(66)90765-0. [DOI] [PubMed] [Google Scholar]
- Labrie F. Isolation of an RNA with the properties of haemoglobin messenger. Nature. 1969 Mar 29;221(5187):1217–1222. doi: 10.1038/2211217a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowska-Bernstein B., Lamm M. E., Vassalli P. Synthesis of immunoglobulin heavy and light chains by the free ribosomes of a mouse plasma cell tumor. Proc Natl Acad Sci U S A. 1970 Jun;66(2):425–432. doi: 10.1073/pnas.66.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Mach B., Koblet H., Gros D. Chemical identification of specific immunoglobulins as the product of a cell-free system from plasmocytoma tumors. Proc Natl Acad Sci U S A. 1968 Feb;59(2):445–452. doi: 10.1073/pnas.59.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechler B., Mach B. Preparation and properties of ribosomal subunits from mouse plasmocytoma tumors. Eur J Biochem. 1971 Aug 25;21(4):552–564. doi: 10.1111/j.1432-1033.1971.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Scherrer K. Isolation of preribosomes from HeLa cells and their characterization by electrophoresis on uniform and exponential-gradient-polyacrylamide gels. Eur J Biochem. 1971 Nov 11;23(2):372–386. doi: 10.1111/j.1432-1033.1971.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Pemberton R. E., Housman D., Lodish H. F., Baglioni C. Isolation of duck haemoglobin messenger RNA and its translation by rabbit reticulocyte cell free system. Nat New Biol. 1972 Jan 26;235(56):99–102. doi: 10.1038/newbio235099a0. [DOI] [PubMed] [Google Scholar]
- Schubert D. Immunoglobulin biosynthesis. IV. Carbohydrate attachment to immunoglobulin subunits. J Mol Biol. 1970 Jul 28;51(2):287–301. doi: 10.1016/0022-2836(70)90143-9. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Huang R. C. Synthesis of a mouse immunoglobulin light chain in a rabbit reticulocyte cell-free system. Nat New Biol. 1971 Apr 7;230(14):172–176. doi: 10.1038/newbio230172a0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Specific IgG mRNA molecules from myeloma cells in heterogeneous nuclear and cytoplasmic RNA containing poly-A. Nature. 1972 Sep 15;239(5368):143–146. doi: 10.1038/239143a0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Brown D. D. Isolation and identification of the messenger RNA for silk fibroin from Bombyx mori. J Mol Biol. 1972 Feb 14;63(3):409–429. doi: 10.1016/0022-2836(72)90437-8. [DOI] [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]