Abstract
Objectives
The aim of this article was to review the current role of brain PET in the diagnosis of Alzheimer dementia. The characteristic patterns of glucose metabolism on brain FDG-PET can help in differentiating Alzheimer’s disease from other causes of dementia such as frontotemporal dementia and dementia of Lewy body. Amyloid brain PET may exclude significant amyloid deposition and thus Alzheimer’s disease in appropriate clinical setting.
Conclusions
FDG-PET and amyloid PET imaging are valuable in the assessment of patients with Alzheimer’s disease.
Keywords: FDG brain PET, amyloid brain PET, dementia, Alzheimer’s disease
LEARNING OBJECTIVES
After completing this journal-based CME activity, participants will be able to:
Identify patterns of FDG spatial distribution in brain PET of patients with MCI, AD, frontotemporal dementia, and dementia of Lewy Body.
Identify the value and learn the appropriateness criteria for amyloid brain PET.
Dementia is a collective group of neurodegenerative disorders characterized by memory impairment and cognitive decline, leading to difficulty in performing activities of daily living that has been present for a minimum period of 6 months.1 Depending on the underlying pathology and clinical manifestation of the disease, dementia can be further grouped into Alzheimer’s disease (AD), Lewy body dementia (DLB), frontotemporal dementia (FTD),2 and vascular dementia. Alzheimer’s disease accounts for 60% to 80% of dementia cases, and the incidence increases with the age of the patients, especially older than 65 years. It is the sixth leading cause of mortality in the United States and causes a considerable financial burden to the country. In 2012, the estimated overall care expenses accounted to US $200 billion.3
The diagnosis of AD was mainly based on the clinical manifestation of symptoms. However, advances in genetics, the development of biomarkers of neurodegeneration, and neuroimaging has lead to the incorporation of these modalities in the diagnosis of AD. It is believed that the pathophysiology of AD starts years ahead of the manifestation of clinical signs, and this discovery mandates the use of methods to detect AD earlier than conventional diagnostic tools.4 Most available treatment modalities aim at slowing the progression of disease and controlling symptoms, and this further stresses the importance of early diagnosis of the disease.5 18F-FDG-PET/CT has been not only a valuable tool in tumor imaging6–11 but also a very promising neuroimaging tool in the diagnosis of AD because it reflects resting state cerebral metabolic rates of glucose, which is an indicator of neuronal activity, and several studies have shown that cerebral metabolic alterations precede the clinical manifestation of AD symptoms. The distinct patterns of cerebral glucose metabolism also help in differentiating AD from other causes of dementia.12 Recently, PET imaging tracers, which correlate β-amyloid deposition in the brain, have been approved by the Food and Drug Administration (FDA). The amyloid deposition in the brain can be detected years before the onset of clinical symptoms. The PET imaging tracers help in differentiating dementia syndromes, which do not have overlap of the underlying pathological process.13 The purpose of this article was to review the role of brain PET imaging in the diagnosis of AD.
CLINICAL FEATURES, DIAGNOSIS, AND TREATMENT OF AD
The clinical manifestation of AD demonstrates a spectrum of presentations, depending on its severity. The National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association have worked together to establish criteria to assist the clinical diagnosis of AD. The first manifestation of AD symptoms, also referred to as the predementia phase of AD, is termed mild cognitive impairment (MCI). This entity is characterized by lower performance in 1 or more cognitive domains for the age and educational background of the patient in the absence of dementia and preservation of independence in functional abilities.14 The National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association classifies AD broadly as “probable AD dementia” or “possible AD.” Probable dementia is diagnosed when the patient, in addition to the criteria for all-cause dementia, has insidious onset of symptoms, clear history of cognitive worsening by report or observation, and the cognitive deficits present with either amnestic or nonamnestic presentations, in the absence of evidence of all other causes of dementia. Possible dementia of AD is considered in patients who present with an atypical course of cognitive impairment or have a mixed presentation, such as concomitant cerebrovascular disease, features of DLB or evidence of another neurological disease or nonneurological medical comorbidity, or medication use that could affect cognition.
Available treatment options for AD aim at slowing the progression of the disease and controlling symptoms. Drugs under development are aimed at targeting the pathological processes leading to AD. The approved groups of drugs for the treatment of AD include the acetylcholinesterase inhibitors, which aim at increasing the levels of acetylcholine at the sites of neurotransmission and memantine, a noncompetitive N-methyl-aspartate receptor antagonist. The common adverse effects of acetylcholinesterase inhibitors are nausea, vomiting, diarrhea, sleep disturbances, muscle cramps, weakness, bradycardia, and urinary incontinence. The adverse effects encountered with memantine include dizziness, confusion, headache, and incontinence and is used with caution in patients with renal failure or epilepsy.5
PATHOPHYSIOLOGY OF AD
Alzheimer’s disease is characterized by the accumulation of the β-amyloid peptide (Aβ) within the brain along with neurofibrillary tangles of hyperphosphorylated tau protein.15 Amyloid deposits has been described to be caused by the deposition of Aβ, a cleavage product of the amyloid precursor protein that originates from degenerating mitochondria in dystrophic neurons, and the deposition causes further disruption of axons and further deposition of amyloid.16,17 Neurofibrillary tangles caused by hyperphosphorylation of tau protein have also been identified as an important pathophysiological step in the development of AD. Studies have shown significant correlation between the concentration of phosphorylated tau protein and neurofibrillary tangles in patients with AD.18 Based on these pathophysiological processes, the measurement of certain biomarkers such as β-amyloid peptide (Aβ42), total tau, and phosphorylated tau, reflect the pathological features of AD.19 Studies have shown that these markers may be used to monitor patients with AD overtime and can also serve as a surrogate marker for treatment efficacy.20 Another biomarker of importance is the apolipoprotein E (ApoE). It has been implicated that ApoE isoforms influence clearance and deposition of Aβ. The ApoE is coded by the APOE gene, which has 3 major alleles; ε2, ε3, and ε4. Apolipoprotein E-ε4 (ApoE4) has been recognized as the strongest risk factor for sporadic AD.21
FDG BRAIN PET IMAGING
Normal Cerebral Glucose Metabolism With Aging
Cerebral glucose metabolism patterns are similar among individuals who are age matched. The mean cerebral glucose metabolism has been found to gradually decrease with age. A study by Kuhl et al22 evaluating the effects of aging on the cerebral glucose metabolism of 40 normal subjects showed that the average cerebral glucose metabolic rate at age 78 years was 26% less than at age 18 years. Within the brain, the anatomical regions that show the greatest decrease in FDG uptake with aging are the bilateral superior medial frontal, motor, anterior, and middle cingulate and bilateral parietal cortices. The superior temporal pole was found to be particularly affected. The regions that show the least changes in glucose metabolism with aging are the bilateral medial temporal lobes, putamen, pallidum, lateral thalamic nuclei, right posterior cingulate cortex, precuneus, and both sides of the occipitotemporal cortex.23
Value of FDG-PET in the Evaluation of AD
FDG-PET has been proven to be a promising modality for detecting functional brain changes in AD, identifying changes in early AD, and helping to differentiate AD from other causes of dementia. Many studies have been published evaluating the value of FDG-PET in AD for the last 3 decades. A meta-analysis including 27 studies evaluating FDG-PET in the diagnosis of AD resulted in a pooled sensitivity (SN) of 91% (95% confidence interval [CI], 86%–94%] and specificity (SP) of 86% (95% CI, 79%–91%). The analysis included 119 studies evaluating the role of different modalities in the diagnosis of AD. The results from the meta-analysis showed that FDG-PET has superior diagnostic accuracy in comparison with the other available diagnostic methods such as clinical guidelines, MRI, CT, SPECT, and biomarkers.24 Studies have also shown that FDG-PET has the potential to differentiate patients with AD from normal subjects and patients with other causes of dementia. A study by Mosconi et al25 has shown that FDG-PET can differentiate patients with AD from normal subjects with an SN and SP of 99% and 98%, respectively, from patients with DLB with an SN and SP of 99% and 71%, respectively, and from patients with FTD with an SN and SP of 99% and 65%, respectively.
FDG-PET in MCI
In patients with MCI, FDG-PET has been found to be better than other imaging modalities in diagnosing and predicting conversion of MCI to dementia.26 This has a significant impact on the management strategy and quality of life of affected patients because all available treatment options aim at slowing the progression of the disease and also help patients plan ahead and make important life decisions.
Studies have been published evaluating the pattern of glucose metabolism in patients with AD and MCI. Del Sole et al25 evaluated FDG-PET scans performed in 16 patients with MCI and in 14 patients with AD showing that the areas of decreased glucose metabolism involved the posterior cingulate cortex (PCC), precuneus, inferior parietal lobule, and middle temporal gyrus in patients with AD. In patients with MCI, the authors found decreased glucose metabolism only in the PCC. The areas of decreased glucose metabolismin the PCC was found to be wider in patients with AD, compared with those with MCI and extended to the precuneus. Decreased glucose metabolism in the lateral parietal cortex was found only in AD. The study has also shown that 86%, 71%, 64%, and 35% of patients with AD demonstrated decrease in the cerebral glucose metabolic rate in the PCC, temporal cortex, parietal cortex, and frontal cortex, respectively. The corresponding percentages for patients with MCI were 56%, 44%, 18%, and 0%, respectively.25 Other studies have shown changes in glucose metabolism in similar regions of the brain27,28 (Fig. 1).
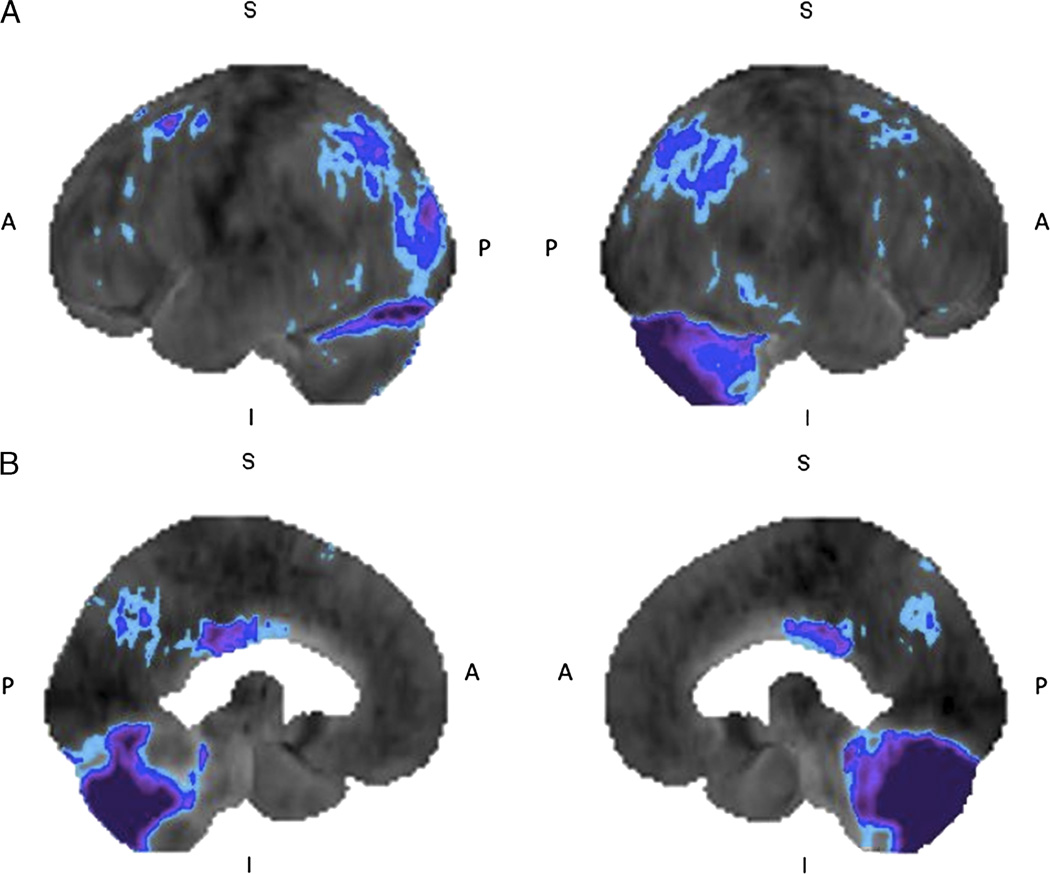
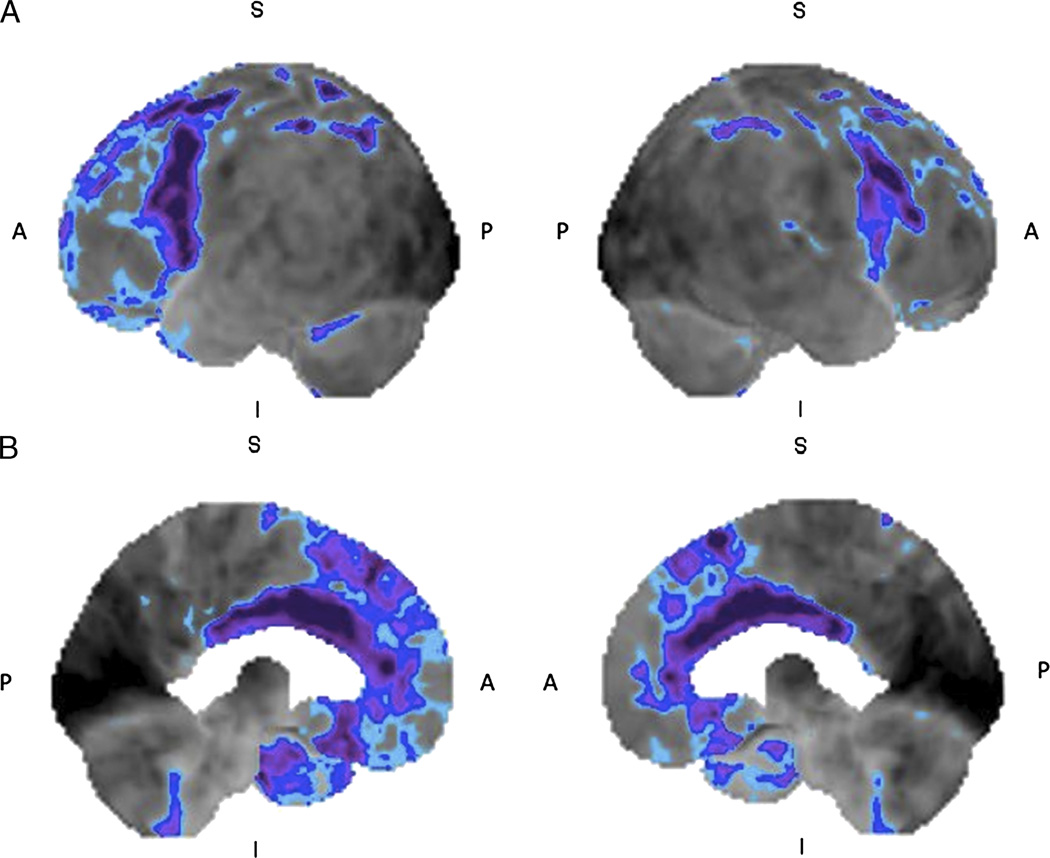
FIGURE 1.
Mild cognitive impairment: 18F-FDG brain PET/CT study of a 61-year-old woman who presented with history of progressive confusion, disorientation, and urinary incontinence. She has a history of surgery for a benign cerebellar tumor at 5 years of age. The left and right lateral (A) and left and right medial (B) 3-dimensional stereotactic surface projection images of FDG hypometabolism demonstrate mild glucose hypometabolism in the parietal and posterior cingulate cortices (compared with a normal brain database, Mim software 6.2, Cleveland, OH. Light blue, −1 SD; dark blue, −2 SDs; purple, −3 SDs), features consistent with MCI. Postsurgical changes appreciated in the cerebellum.
Another important application of FDG-PET in the evaluation of patients with MCI is its ability to predict progression to dementia. In a meta-analysis by Yuan et al,29 of 24 studies involving 1112 patients, the pooled SN, SP, positive likelihood ratio, negative likelihood ratio, and odds ratio of FDG-PET to predict conversion of MCI to AD were 88.8%, 84.9%, 4.61, 0.15, and 40.15, respectively. Regions of decreased cerebral glucose metabolic rate have been observed in the temporoparietal, inferior parietal, medial temporal, and posterior cingulate cortices in patients with MCI who developed AD within a year, in comparison with normal subjects and patients with stable MCI. The progression of the disease is accompanied by a continued decrease in the glucose uptake in the regions and emergence of a new affected region involving the lateral prefrontal cortex.30–32 Mosconi et al31 evaluated 37 patients with MCI, followed them up for a year, and found that all patients who progressed to dementia showed reduced cerebral glucose metabolic rates in the inferior parietal cortex as compared with those patients who did not. Anchisi et al32 evaluated 67 patients with MCI and found that patients who converted to AD showed bilateral hypometabolism in the inferior parietal, posterior cingulate and medial temporal cortices, whereas those with stable MCI had hypometabolism in the dorsolateral frontal cortex.
FDG-PET in AD
As mentioned earlier, in patients with early AD, the areas of glucose hypometabolism have been commonly observed in the parietotemporal association cortices, posterior cingulate cortex, and the precuneus (Figs. 2 and 3). As the disease progresses, the affected regions spread to involve the frontal cortices, whereas the metabolism in the striatum, thalamus, primary sensorimotor cortices, visual cortices, and cerebellumare relatively preserved.33,34 Silverman et al35 evaluated 146 patients undergoing evaluation for dementia, of whom 97 patients were histopathologically confirmed to have AD and observed focal hypometabolism in the parietal, temporal, and/or frontal cortices or global hypometabolism. Hoffman et al36 evaluated 22 patients with dementia, of whom 16 were found to have a pathological diagnosis of AD, and observed that bilateral temporoparietal hypometabolism had relatively high SN (93%), positive predictive value (81%), and negative predictive value (83%) as well as lower SP (63%) for the diagnosis of AD. This study proves that the finding of bilateral temporoparietal hypometabolism highly correlates with the pathological diagnosis of AD. Minoshima et al37 evaluated the glucose metabolism patterns in patients with autopsy-confirmed AD and found that the patients had significant metabolic reductions in the lateral parietal, temporal, frontal, and posterior cingulate cortices. They also observed either mild or insignificant reduction in the glucose metabolism in the primary visual, sensorimotor cortices and subcortical structures. Ossenkoppele et al38 compared the SUV ratio (SUVr) obtained with the cerebellar grey matter as the reference tissue and observed that in patients with AD at baseline, there was a decrease in SUVr in the parietal, posterior cingulate and temporal cortices. At a mean interval follow-up of 2.5 years, the authors observed that there was a decrease in SUVr from baseline in the frontal, parietal, and lateral temporal lobes over time in patients with AD.
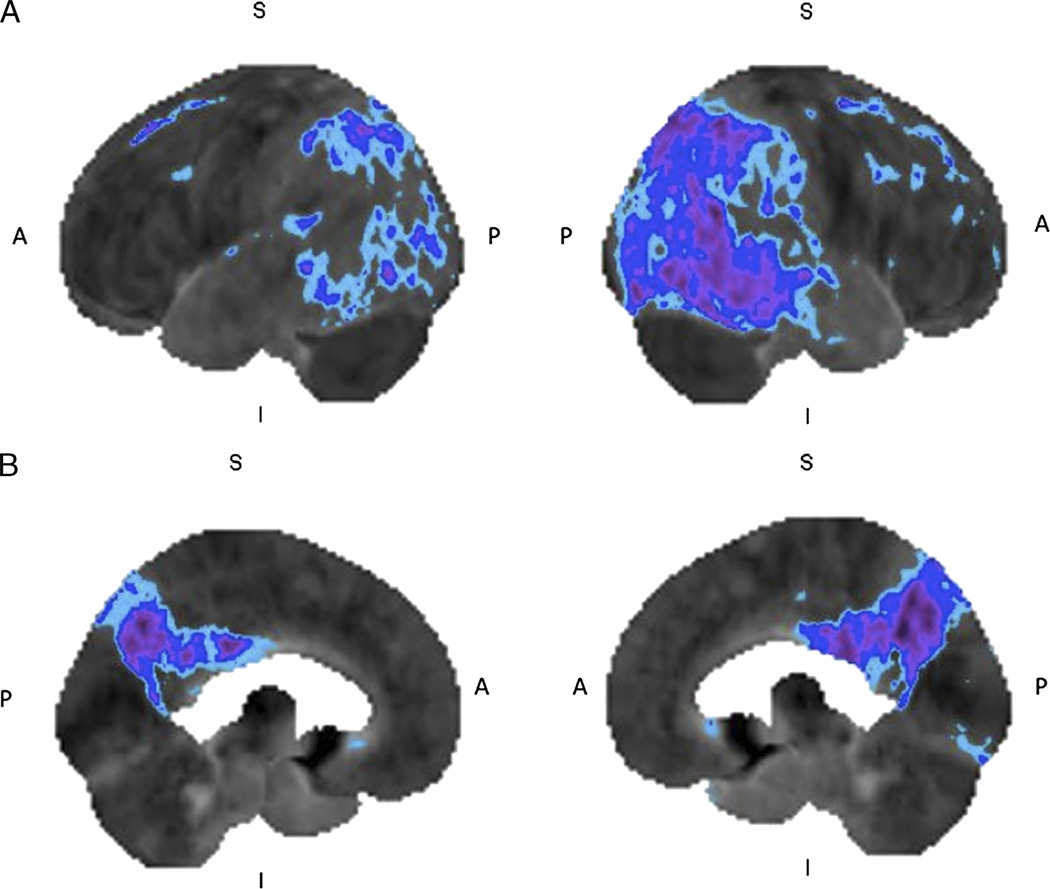
FIGURE 2.
Alzheimer’s disease: 18F-FDG brain PET/CT study of a 50-year-old woman with progressive amnesia and dysgraphia for 18 months. The left and right lateral (A) and left and right medial (B) 3-dimensional stereotactic surface projection images of FDG hypometabolism demonstrate glucose hypometabolism involving the posterior cingulate gyrus and bilateral parietal cortices extending to the temporal region (compared with a normal brain database, Mim software 6.2. Light blue, −1 SD; dark blue, −2 SDs; purple, −3 SDs), features consistent with early AD.
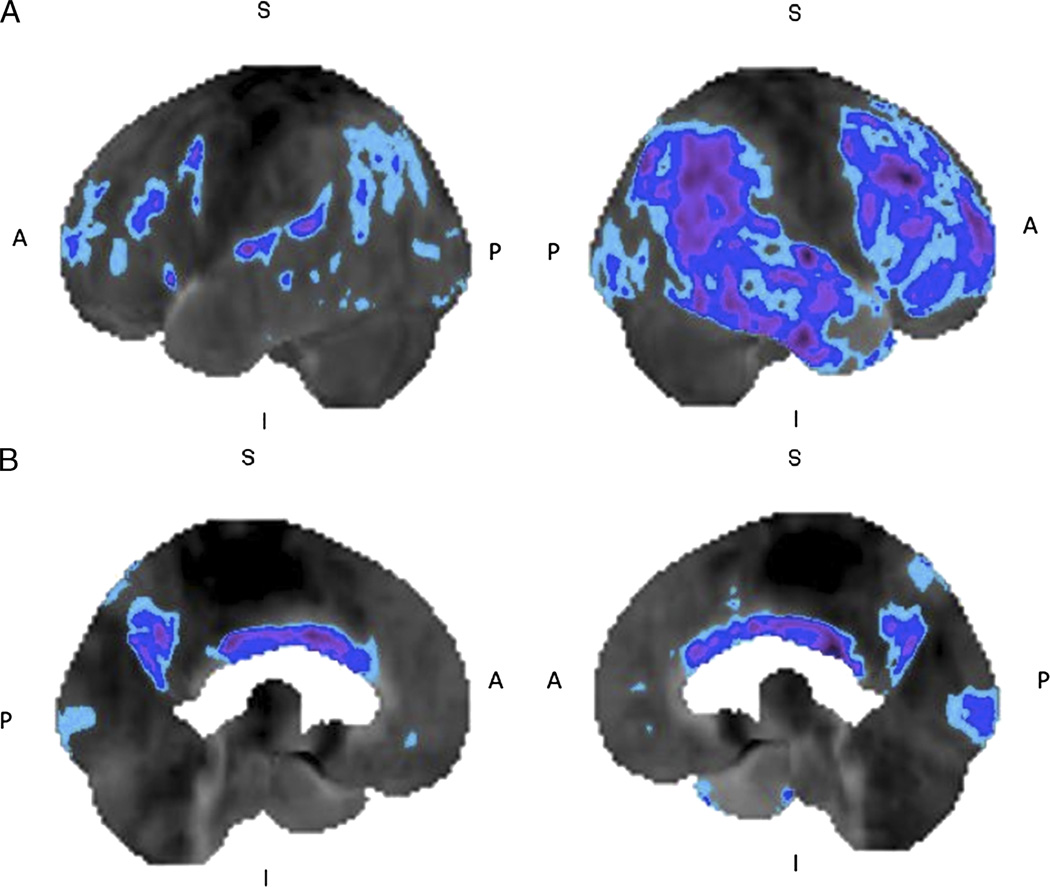
FIGURE 3.
Alzheimer’s disease: 18F-FDG brain PET/CT study of a 81-year-old man with cognitive decline during the past few months. The left and right lateral (A) and left and right medial (B) 3-dimensional stereotactic surface projection images of FDG hypometabolism demonstrate glucose hypometabolism involving the bilateral frontal, temporal, and parietal cortices (compared with a normal brain database, Mim software 6.2. Light blue, −1 SD; dark blue, −2 SDs; purple, −3SDs), with sparing of the sensorimotor and occipital cortices, features consistent with AD.
FDG-PET in Differentiating AD From Other Types of Dementias
An important application of FDG-PET in clinical practice in the context of dementia is its ability to differentiate AD from other causes of dementia. Many studies have been performed to help clinicians differentiate between the types of dementias. Gilman et al39 evaluated 25 patients with AD, 20 with DLB, and 19 normal control subjects and found that the FDG-PET studies showed significantly lower cerebral glucose metabolic rates in the visual cortex (Brodmann areas 17, 18, and 19) in patients with DLB compared with patients with AD. They also observed no significant difference in the glucose metabolism in the PCC, superior parietal lobe, lateral temporal lobe, and prefrontal region between the 2 groups. However, a study by Lim et al40 evaluating 14 patients with DLB and 10 patients with AD has shown that hypometabolism in the lateral occipital cortex had the highest SN (88%) and the relative preservation of the mid or posterior cingulate gyrus had the highest SP (100%) in differentiating DLB from AD. Minoshima et al37 have shown that significant cerebral glucose metabolic reduction in the occipital cortex, especially in the primary visual cortex, distinguished DLB from AD with an SN and SP of 90% and 80%, respectively (Fig. 4). Clinically, it is important to differentiate patients with DLB from patients with AD because patients with DLB can be severely dopaminergic deficient and can adversely react severely to neuroleptic treatment.
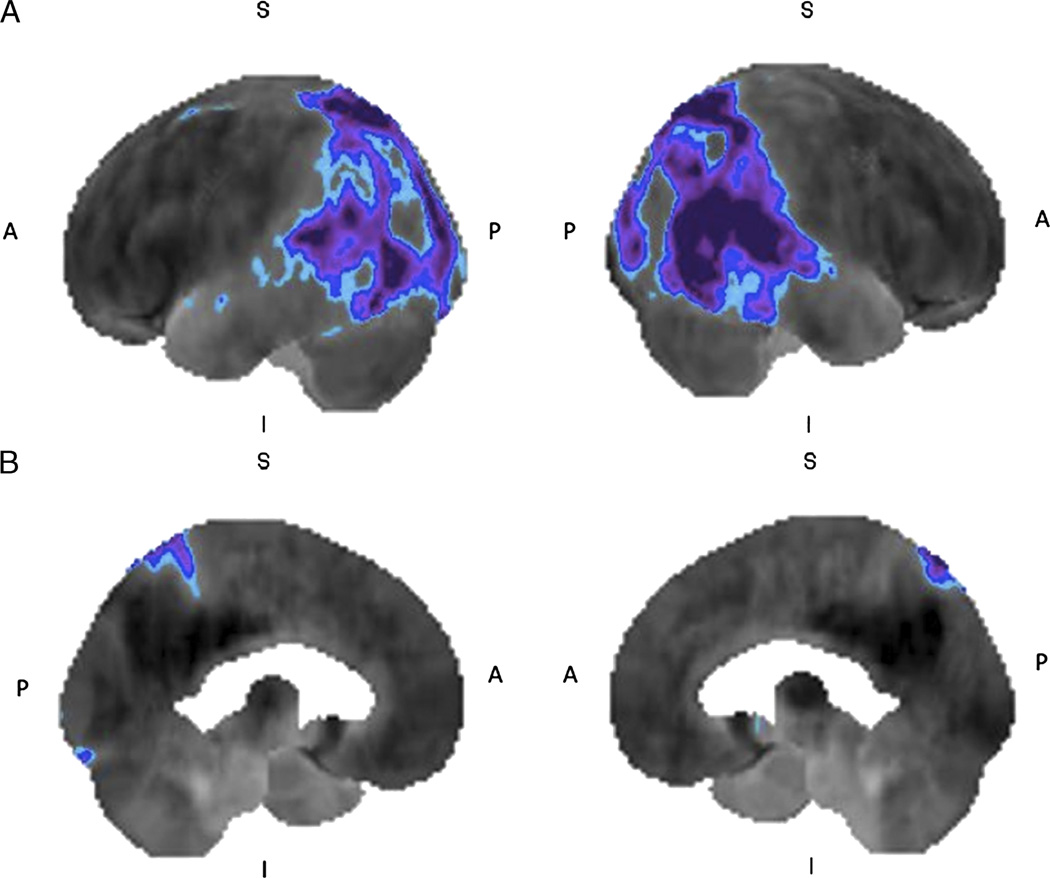
FIGURE 4.
Lewy body dementia: 18F-FDG brain PET/CT study of a 43-year-old woman with memory loss and visuospatial dysfunction. The left and right lateral (A) and left and right medial (B) 3-dimensional stereotactic surface projection images of FDG hypometabolism demonstrate glucose hypometabolism involving the bilateral occipital and parietal cortices, extending to the temporal cortex (compared with a normal brain database, Mim software 6.2. Blue, −2 SDs; purple, −3 SDs), features suggestive of Lewy body dementia.
Foster et al41 evaluated the glucose metabolism patterns in patients with pathologically confirmed AD from those with FTD. The authors found glucose hypometabolism in the frontal, anterior cingulate and anterior temporal regions more frequently in patients with FTD, whereas patients with AD demonstrated hypometabolism in the temporoparietal and posterior cingulate regions, more commonly. The authors found a mean diagnostic accuracy of 84.8% by transaxial FDG-PET imaging and 89.2% by stereotactic surface projection of the FDG-PET images (Fig. 5). Clinically, it is important to differentiate patients with FTD from patients with AD because patients with FTD can have serious adverse effects if treated with anticholinesterase inhibitors.
FIGURE 5.
Frontotemporal dementia: 18F-FDG brain PET/CT study of a 72-year-old woman with anomia, progressive memory impairment, cognitive decline, and gradual paucity of speech. The left and right lateral (A) and left and right medial (B) 3-dimensional stereotactic surface projection images of FDG hypometabolism demonstrate glucose hypometabolism involving the bilateral frontal and temporal cortices (compared with a normal brain database, Mim software 6.2. Blue, −2 SDs; purple, −3 SDs), compatible with a diagnosis of frontotemporal dementia.
AMYLOID BRAIN PET IMAGING
Although the etiology of AD has not been fully established, there is evidence to suggest that the amyloid-β peptide plays an important role in AD pathogenesis.42 Accumulation of Aβ fibrils in the form of amyloid plaques is a neuropathological hallmark for autopsy-based diagnosis confirmation of dementia caused by AD,43 and more importantly, Aβ deposition is thought to precede cognitive symptoms in AD and is therefore a potential preclinical marker of disease.44 There have been different approaches to noninvasively visualize amyloid deposition in human brains with amyloid PET radiotracers.
PET Amyloid Radiopharmaceuticals
Typically, amyloid imaging agents bind to insoluble fibrillar forms of Aβ 40 and Aβ 42 deposits, which are a major component of compact neuritic plaques and vascular deposits. PET amyloid-β imaging agent could facilitate the clinical evaluation of late-life cognitive impairment by providing an objective measure for AD pathology. PET imaging probes of Aβ plaques have been extensively developed during the last decade.
11C-Pittsburgh Compound B (PiB) was the first amyloid imaging PET agent used in humans subjects in 2002.45 In April 2012, the US FDA approved the first Aβ imaging PET probe, 18F-florbetapir46 (Amyvid, Eli Lilly and Company), to identify Aβ plaque accumulation in patients with suspected AD. Subsequently, another amyloid PET radiopharmaceutical derived 11C-PiB, flutemetamol (Vizamyl, GE Healthcare)47 and more recently a third amyloid imaging probe, 18F-florbetaben injection48 (Neuraceq, Piramal Imaging) had been approved by the FDA. Many other amyloid ligands are available for research use, such as the benzofuranes 11C-AZD-2184 and 18F-AZD-4694,49 benzoxazole 11C-BF-227,50 as well as stilbene compounds 11C-SB,51 and naphthol 18F-FDDNP.52 The following discussion will focus on FDA-approved amyloid PET radiotracers.
11C-PiB [N-methyl-[11C]2-(4-methylaminophenyl)-6-hydroxybenzothiazole]
11C-PiB is a derivative of a fluorescent amyloid dye, thioflavin T, and has been found to possess high affinity and high SP for fibrillar Aβ.45,53 It was developed by Chet Mathis and William Klunk at the University of Pittsburgh and was first used in human research studies in 2002 in collaboration with Uppsala University, Sweden. The compound was named Pittsburgh Compound B (11C-PiB). The initial human study of 11C-PiB was expanded to include 16 subjects with AD and 9 cognitively healthy controls, which was published in 2004.53 Patients with AD were found to show significantly higher 11C-PiB retention in the frontal cortex (1.94-fold), parietal cortex (1.71-fold), corpus striatum (1.76-fold), temporal cortex (1.52-fold), and occipital cortex (1.52-fold). Since that study, 11C-PiB imaging has been rapidly spread to academic centers worldwide and widely used in many research studies. PiB compound is labeled with 11C, with short half-life of only 20 minutes, limiting its use to PET centers equipped with an on-site cyclotron and 11C radiochemistry expertise. To overcome these limitations, 18F-labeled Aβ tracers, with longer half-life of 110 minutes, have been developed and found to successfully correlate with 11C-PiB results, providing a reliable assessment of brain amyloid with a single scan of 15- to 20-minute duration.
18F-Florbetapir (18F-AV-45; Amyvid)
18F-Florbetapir was the first amyloid imaging agent approved by the FDA in April 2012.54 It is a stilbene derivative, first synthesized by Kung et al at the University of Pennsylvania. It has rapid reversible binding characteristics, allowing scanning to commence 45 to 50 minutes after injection, similar to 11C-PiB. There are a number of studies evaluating the efficacy of florbetapir in detecting Aβ pathology. In a multicenter study of 59 patients with different levels of cognitive function, florbetapir showed an SN and SP of 92% and 100%, respectively, in detecting amyloid plaques.44,46,48,54–56 The accumulation of amyloid detected by florbetapir PET imaging significantly correlated with postmortem amyloid assessment.56
18F-florbetaben Injection, Also Known as AV1 or BAY04-9172) (Neuraceq, Piramal Imaging)
18F-florbetaben was also synthesized by Kung et al. It was the first 18F-labeled amyloid imaging agent used in humans and has been recently approved by the FDA in March 2014. Approximately 50% to 70% of its binding is reached in approximately 90 minutes after injection in subjects with AD. Florbetaben had shown an SN of 100% and an SP of 90% in detecting AD by visual interpretation.46,48,54,56,57 Rowe et al48 also reported florbetaben binding in areas matching the postmortem Aβ plaques distribution. Brain retention of 18F-florbetaben is also highly correlated with 11C-PiB (r = 0.97).
18F-flutemetamol (also known as GE067) (Vizamyl, GE Healthcare)
Flutemetamol is another 18F-labeled amyloid imaging approved by the FDA in 2013. 18F-flutemetamol has demonstrated dosimetry comparable with other 18F-labeled radiopharmaceuticals and performed similarly to 11C-PiB with high correlation (r = 0.91).46,54,56–58 Studies have provided additional data supporting the concordance between in vivo 18F-flutemetamol imaging and histopathology.59
AMYLOID PET IMAGING IN AD
Studies suggest that the visual inspection of PET imaging amyloid scan shows a typical regional brain distribution in patients with AD, which seems to replicate the sequence of Aβ deposition found at autopsy,46,54,56,59,60 with initial deposition in the precuneus, orbitofrontal cortex, and inferior temporal, posterior cingulate gyrus, followed in time by the remaining prefrontal cortex as well as lateral temporal and parietal cortices. There is relative sparing of the sensorimotor, occipital, and medial temporal regions.
A negative amyloid PET scan result (Fig. 6) indicates a reduced likelihood that cognitive impairment is caused by AD, but a positive scan result (Fig. 7) does not establish a diagnosis of AD. However, amyloid PET imaging must be performed in a setting of a clinically suspected AD because there is a relative low SP. Klunk et al conducted a review of 15 studies with a total of 341 patients with AD and 651 subjects with normal cognitive performance, concluding that 96% of the patients with clinically suspected AD had amyloid imaging positive result, with an SP of 76%.46,54,56,60,61
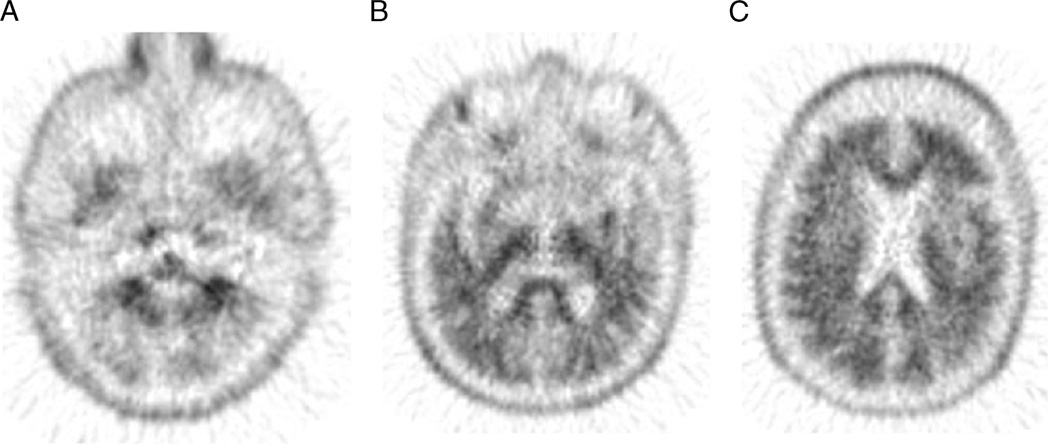
FIGURE 6.
Negative amyloid brain PET result: 18F-florbetapir brain PET/CT study of a 75-year-old man with history of cerebral amyloid angiopathy with gradual decline in cognitive function over 3 years. Axial PET images demonstrate normal grey-white differentiation in the cerebellum (A) and in the cerebrum (B and C) consistent with sparce amyloid neuritic plaques density, inconsistent with the neuropathology of AD.
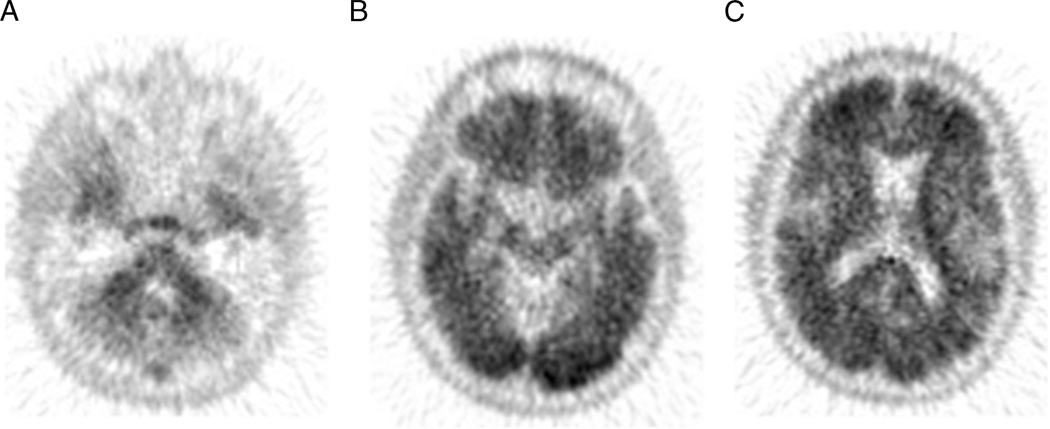
FIGURE 7.
Positive amyloid brain PET result: 18F-florbetapir brain PET/CT study of a 71-year-old woman with history of short-term memory loss for 2 years. The axial PET images of the cerebellum (A) demonstrate the normal grey-white differentiation, but there is loss of grey-white differentiation in occipital and frontal lobes (B and C), suggesitve of moderate-to-severe amyloid neuritic plaques density. This can be observed in cognitively normal individuals as well as those with AD.
The degree of cortical binding of amyloid agent in patients with AD is highly variable and does not correlate with clinical measures of cognitive impairment severity. This has been shown in longitudinal studies assessing for disease progression demonstrating stable PiB retention after 2 years of follow-up in a whole spectrum of cognitive stages, from cognitively unimpaired individuals to patients with AD dementia.46,54,56,61,62 Aβ accumulation is a slow process, and the evidence suggests that it remains constant in the preclinical and prodromal stages of the disease.
There have been efforts to evaluate the impact of amyloid PET in clinical management of patients with AD. Grundman et al62 assessed the impact of florbetapir imaging in 229 patients, reporting change in the diagnosis 54.6% of patients (CI, 48.1%–60.9%) after amyloid imaging scan with 18F-florbetapir, and the diagnostic confidence was increased by an average of 21.6% (CI, 18.3%–24.8%). Thus, the incorporation of amyloid PET into the management of patients with AD may be valuable, offering the possibility of monitoring and assessing the effectiveness of pharmaceutical therapeutic agents directed against Aβ levels. Rinne et al63 conducted a phase II study (n = 19 patients), where PiB PET imaging was used to investigate whether bapineuzumab, a monoclonal antiamyloid antibody, would reduce cortical fibrillar Aβ load in patients with AD. The difference in mean PiB retention ratio changes between the bapineuzumab and the placebo group was −0.24 (P < 0.003). The differences in the individual regions between the 2 groups were similar. Ostrowitzki et al64 studied the effectiveness of gantenerumab treatment using PiB PET imaging (n = 16 patients) and found that using 60 mg of gantenerumab decreased the Aβ deposition by 15.6% (95% CI, −42.7 to 11.6%), whereas using 200 mg of gantenerumab decreased the deposition by 35.7% (95% CI, −63.5% to −7.9%). These studies illustrate the potential value of amyloid imaging in assessing the effectiveness of new treatment options against Aβ in clinical trials as well as monitoring the effect of treatment in clinical practice.
Amyloid PET Imaging in Mild Cognitive Disorder
Approximately 25% to 35% of elderly subjects with adequate performance in cognitive tests demonstrate high cortical radiotracer amyloid imaging retention in the prefrontal, posterior cingulate and precuneus regions.46,54,56,64,65 These findings are concordant with postmortem reports, which show that approximately 25% of nondemented subjects older than 75 years have Aβ plaques.46,54–56,65,66 In nondemented subjects, the presence of Aβ deposition might reflect a preclinical stage of AD.
Unlike AD, there is correlation between amyloid imaging binding, using 11C-PiB, and the degree of memory impairment in nondemented subjects, and approximately 50% to 60% of indivuals with MCI will progress to AD during 3 to 5 years of follow-up.46,54,56,66,67 There is evidence that a positive amyloid PET scan result in patients with MCI will help in predicting conversion to AD and, thus, can potentially help to identify patients who will benefit from specific therapies. This was assessed by Jack et al in 218 patients who concluded that amyloid positive scan results were associated with a significantly higher chance to progress to AD, with a hazard ratio of 3.2 (P = 0.004).46,54,56,67,68 By contrast, only less than 10% of patients with 11C-PiB–negative MCI progress to AD, whereas approximately 20% of subjects with of 11C-PiB–negative MCI progress to other types of dementia, such as Lewy bodies dementia or frontotemporal dementia.46,54,56,68,69
Appropriate Use Criteria for Brain Amyloid Imaging with PET in AD
Criteria for appropriate clinical use are needed to avoid potential patient harm if the amyloid imaging scans are performed in inappropriate circumstances or are poorly read or if the significance of the results is not correctly applied to the clinical context.
The Society of Nuclear Medicine and Molecular Imaging and the Alzheimer’s Association Amyloid Imaging Taskforce have jointly developed consensus recommendations for the appropriate use of brain amyloid imaging to aid in the diagnosis of people with suspected AD.44 The Amyloid Imaging Taskforce concluded that amyloid imaging could potentially be helpful in the diagnosis of cognitive impairment when considered along with other clinical information and when performed according to standardized protocols by trained staff. The appropriate use of amyloid PET requires a fully comprehensive evaluation of patients undertaken by a clinician with expertise in evaluating cognitive neurodegenerative disorders.
Appropriate candidates for amyloid PET imaging evaluation would include (1) patients complaining of persistent or progressive unexplained MCI; (2) patients meeting tests for possible AD but who have unclear clinical presentation, either atypical clinical course or etiologically mixed presentations; and (3) patients with progressive dementia and atypically early age of onset (defined as before the age of 65 years). Inappropriate candidates for amyloid PET imaging include (1) patients who are 65 years or older and meet standard clinical criteria for probable AD; (2) those whose severity of disease has to be determined; (3) those undergoing imaging solely based on a family history of dementia or presence of other risk factors for AD, such as the ApoE-ε4 gene; (4) patients with a cognitive complaint but no clinical confirmation of impairment; (5) those undergoing imaging in lieu of genotyping for suspected autosomal mutation carriers; (6) asymptomatic individuals; (7) those undergoing imaging for nonmedical use, such as insurance coverage and legal or employment screening.
Although the accumulation of β-amyloid plaques is one of the defining pathological features of AD, many elderly people, with normal cognition, have elevated levels of Aβ plaques. Population-based studies have reported age-specific positivity rates for amyloid PET of less than 5% in those 50 to 60 years old, 10% in those 60 to 70 years old, 25% in those 70 to 80 years old, and greater than 50% in those 80 to 90 years old.46,54,56,69,70 Therefore, the potential clinical use of amyloid PET requires careful consideration in the proper clinical setting.
Another limitation is that a positive amyloid scan result can also be seen not only in AD but also in other medical conditions, such as dementia with Lewy bodies,46,54,56,70,71 or cerebral amyloid angiopathy.46,54,55,71,72 Thus, it is important to emphasize that amyloid positivity does not establish differential diagnosis between AD and Aβ disorders. Furthermore, amyloid PET would not add any useful information in differentiating disorders that are not associated with Aβ, such as frontotemporal dementia syndromes, or in detecting rare forms of AD in which ligand binding is greatly reduced because of unusual forms of Aβ.46,54,56,72,73
CONCLUSIONS
FDG-PET and amyloid PET imaging are valuable in the assessment of patients with dementia. The characteristic spatial distribution of glucose metabolism on brain FDG-PET can help in differentiating AD from other causes of dementia such as frontotemporal dementia and dementia of Lewy body. A negative amyloid brain PET result is useful in excluding significant amyloid deposition and thus AD in appropriate clinical setting.
Acknowledgments
Dr Subramaniamwas a speaker for Eli Lilly in 2013 in brain amyloid PET and received speaker fees.
Footnotes
The remaining authors and staff in a position to control the content of this CME activity and their spouses/life partners (if any) have disclosed that they have no other financial relationships with, or financial interests in, any commercial organizations pertaining to this educational activity.
Lippincott CME Institute has identified and resolved all conflicts of interest concerning this educational activity.
REFERENCES
- 1.Eschweiler GW, Leyhe T, Kloppel S, et al. New developments in the diagnosis of dementia. Dtsch Arztebl Int. 2010;107:677–683. doi: 10.3238/arztebl.2010.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3.2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Bagnoli S, Piaceri I, Sorbi S, et al. Advances in imaging-genetic relationships for Alzheimer’s disease: clinical implications. Neurodegener Dis Manag. 2014;4:73–81. doi: 10.2217/nmt.13.68. [DOI] [PubMed] [Google Scholar]
- 5.Ghezzi L, Scarpini E, Galimberti D. Disease-modifying drugs in Alzheimer’s disease. Drug Des Devel Ther. 2013;7:1471–1478. doi: 10.2147/DDDT.S41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramaniam RM, Wilcox B, Aubry MC, et al. 18F-fluoro-2-deoxy-D-glucose positron emission tomography and positron emission tomography/computed tomography imaging of malignant pleural mesothelioma. J Med Imaging Radiat Oncol. 2009;53:160–169. doi: 10.1111/j.1754-9485.2009.02058.x. quiz 170. [DOI] [PubMed] [Google Scholar]
- 7.Dibble EH, Karantanis D, Mercier G, et al. PET/CT of cancer patients: part 1, pancreatic neoplasms. AJR Am J Roentgenol. 2012;199:952–967. doi: 10.2214/AJR.11.8182. [DOI] [PubMed] [Google Scholar]
- 8.Romesser PB, Qureshi MM, Shah BA, et al. Superior prognostic utility of gross and metabolic tumor volume compared to standardized uptake value using PET/CT in head and neck squamous cell carcinoma patients treated with intensity-modulated radiotherapy. Ann Nucl Med. 2012;26:527–534. doi: 10.1007/s12149-012-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davison J, Mercier G, Russo G, et al. PET-based primary tumor volumetric parameters and survival of patients with non-small cell lung carcinoma. AJR Am J Roentgenol. 2013;200:635–640. doi: 10.2214/AJR.12.9138. [DOI] [PubMed] [Google Scholar]
- 10.Davison JM, Subramaniam RM, Surasi DS, et al. FDG PET/CT in patients with HIV. AJR Am J Roentgenol. 2011;197:284–294. doi: 10.2214/AJR.10.6332. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal A, Chirindel A, Shah BA, et al. Evolving role of FDG PET/CT in multiple myeloma imaging and management. AJR Am J Roentgenol. 2013;200:884–890. doi: 10.2214/AJR.12.9653. [DOI] [PubMed] [Google Scholar]
- 12.Mosconi L. Glucose metabolism in normal aging and Alzheimer’s disease: methodological and physiological considerations for PET studies. Clin Transl Imaging. 2013;1:217–233. doi: 10.1007/s40336-013-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantarci K. Molecular imaging of Alzheimer’s disease pathology. AJNR Am J Neuroradiol. 2014;35(6 suppl):S12–S17. doi: 10.3174/ajnr.A3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sery O, Povova J, Misek I, et al. Molecular mechanisms of neuropathological changes in Alzheimer’s disease: a review. Folia Neuropathol. 2013;51:1–9. doi: 10.5114/fn.2013.34190. [DOI] [PubMed] [Google Scholar]
- 16.Fiala JC. Mechanisms of amyloid plaque pathogenesis. Acta Neuropathol. 2007;114:551–571. doi: 10.1007/s00401-007-0284-8. [DOI] [PubMed] [Google Scholar]
- 17.Esler WP, Stimson ER, Ghilardi JR, et al. In vitro growth of Alzheimer’s disease beta-amyloid plaques displays first-order kinetics. Biochemistry. 1996;35:749–757. doi: 10.1021/bi951685w. [DOI] [PubMed] [Google Scholar]
- 18.Buerger K, Ewers M, Pirttila T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 19.de Souza LC, Sarazin M, Teixeira-Junior AL, et al. Biological markers of Alzheimer’s disease. Arq Neuropsiquiatr. 2014;72:227–231. doi: 10.1590/0004-282x20130233. [DOI] [PubMed] [Google Scholar]
- 20.Buchhave P, Blennow K, Zetterberg H, et al. Longitudinal study of CSF biomarkers in patients with Alzheimer’s disease. PLoS One. 2009;4:e6294. doi: 10.1371/journal.pone.0006294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Morillo E, Hansson O, Atagi Y, et al. Total apolipoprotein E levels and specific isoform composition in cerebrospinal fluid and plasma from Alzheimer’s disease patients and controls. Acta Neuropathol. 2014;127:633–643. doi: 10.1007/s00401-014-1266-2. [DOI] [PubMed] [Google Scholar]
- 22.Kuhl DE, Metter EJ, Riege WH, et al. Effects of human aging on patterns of local cerebral glucose utilization determined by the [18F]fluorodeoxyglucose method. J Cereb Blood Flow Metab. 1982;2:163–171. doi: 10.1038/jcbfm.1982.15. [DOI] [PubMed] [Google Scholar]
- 23.Kalpouzos G, Chetelat G, Baron JC, et al. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2009;30:112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Bloudek LM, Spackman DE, Blankenburg M, et al. Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J Alzheimers Dis. 2011;26:627–645. doi: 10.3233/JAD-2011-110458. [DOI] [PubMed] [Google Scholar]
- 25.Mosconi L, Tsui WH, Herholz K, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med. 2008;49:390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davison CM, O’Brien JT. A comparison of FDG-PET and blood flow SPECT in the diagnosis of neurodegenerative dementias: a systematic review. Int J Geriatr Psychiatry. 2014;29:551–561. doi: 10.1002/gps.4036. [DOI] [PubMed] [Google Scholar]
- 27.Mosconi L, Andrews RD, Matthews DC. Comparing brain amyloid deposition, glucose metabolism, and atrophy in mild cognitive impairment with and without a family history of dementia. J Alzheimers Dis. 2013;35:509–524. doi: 10.3233/JAD-121867. [DOI] [PubMed] [Google Scholar]
- 28.Murayama N, Iseki E, Fujishiro H, et al. Detection of early amnestic mild cognitive impairment without significantly objective memory impairment: a case-controlled study. Psychogeriatrics. 2010;10:62–68. doi: 10.1111/j.1479-8301.2010.00316.x. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Y, Gu ZX, Wei WS. Fluorodeoxyglucose-positron-emission tomography, single-photon emission tomography, and structural MR imaging for prediction of rapid conversion to Alzheimer’s disease in patients with mild cognitive impairment: a meta-analysis. AJNR Am J Neuroradiol. 2009;30:404–410. doi: 10.3174/ajnr.A1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 31.Mosconi L, Perani D, Sorbi S, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 32.Anchisi D, Borroni B, Franceschi M, et al. Heterogeneity of brain glucose metabolism in mild cognitive impairment and clinical progression to Alzheimer’s disease. Arch Neurol. 2005;62:1728–1733. doi: 10.1001/archneur.62.11.1728. [DOI] [PubMed] [Google Scholar]
- 33.Ishii K. PET approaches for diagnosis of dementia. AJNR Am J Neuroradiol. 2013 doi: 10.3174/ajnr.A3695. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 35.Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman JM, Welsh-Bohmer KA, Hanson M, et al. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med. 2000;41:1920–1928. [PubMed] [Google Scholar]
- 37.Minoshima S, Foster NL, Sima AA, et al. Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol. 2001;50:358–365. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]
- 38.Ossenkoppele R, Tolboom N, Foster-Dingley JC, et al. Longitudinal imaging of Alzheimer pathology using [11C]PIB, [18F]FDDNP and [18F]FDG PET. Eur J Nucl Med Mol Imaging. 2012;39:990–1000. doi: 10.1007/s00259-012-2102-3. [DOI] [PubMed] [Google Scholar]
- 39.Gilman S, Koeppe RA, Little R, et al. Differentiation of Alzheimer’s disease from dementia with Lewy bodies utilizing positron emission tomography with [18F]fluorodeoxyglucose and neuropsychological testing. Exp Neurol. 2005;191(Suppl 1):S95–S103. doi: 10.1016/j.expneurol.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Lim SM, Katsifis A, Villemagne VL, et al. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50:1638–1645. doi: 10.2967/jnumed.109.065870. [DOI] [PubMed] [Google Scholar]
- 41.Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130:2616–2635. doi: 10.1093/brain/awm177. [DOI] [PubMed] [Google Scholar]
- 42.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer’s disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 44.Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9:e106–e109. doi: 10.1016/j.jalz.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Mathis CA, Bacskai BJ, Kajdasz ST, et al. A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg Med Chem Lett. 2002;12:295–298. doi: 10.1016/s0960-894x(01)00734-x. [DOI] [PubMed] [Google Scholar]
- 46.Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer’s disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18) J Nucl Med. 2010;51:913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rinne JO, Wong DF, Wolk DA, et al. [(18)F]Flutemetamol PET imaging and cortical biopsy histopathology for fibrillar amyloid beta detection in living subjects with normal pressure hydrocephalus: pooled analysis of four studies. Acta Neuropathol. 2012;124:833–845. doi: 10.1007/s00401-012-1051-z. [DOI] [PubMed] [Google Scholar]
- 48.Rowe CC, Ackerman U, Browne W, et al. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7:129–135. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- 49.Cselenyi Z, Jonhagen ME, Forsberg A, et al. Clinical validation of 18F-AZD4694, an amyloid-beta-specific PET radioligand. J Nucl Med. 2012;53:415–424. doi: 10.2967/jnumed.111.094029. [DOI] [PubMed] [Google Scholar]
- 50.Kudo Y, Okamura N, Furumoto S, et al. 2-(2-[2-Dimethylaminothiazol-5-yl]ethenyl)-6- (2-[fluoro]ethoxy)benzoxazole: a novel PET agent for in vivo detection of dense amyloid plaques in Alzheimer’s disease patients. J Nucl Med. 2007;48:553–561. doi: 10.2967/jnumed.106.037556. [DOI] [PubMed] [Google Scholar]
- 51.Verhoeff NP, Wilson AA, Takeshita S, et al. In-vivo imaging of Alzheimer’s disease beta-amyloid with [11C]SB-13 PET. Am J Geriatr Psychiatry. 2004;12:584–595. doi: 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- 52.Agdeppa ED, Kepe V, Liu J, et al. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer’s disease. J Neurosci. 2001;21:RC189. doi: 10.1523/JNEUROSCI.21-24-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 54.Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, Oya S, Kung MP, et al. F-18 Polyethyleneglycol stilbenes as PET imaging agents targeting Abeta aggregates in the brain. Nucl Med Biol. 2005;32:799–809. doi: 10.1016/j.nucmedbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 57.Villemagne VL, Mulligan RS, Pejoska S, et al. Comparison of 11C-PiB and 18F-florbetaben for Aβ imaging in ageing and Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2012;39:983–989. doi: 10.1007/s00259-012-2088-x. [DOI] [PubMed] [Google Scholar]
- 58.Hatashita S, Yamasaki H, Suzuki Y, et al. [18F]Flutemetamol amyloid-beta PET imaging compared with [11C]PIB across the spectrum of Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2014;41:290–300. doi: 10.1007/s00259-013-2564-y. [DOI] [PubMed] [Google Scholar]
- 59.Wolk DA, Grachev ID, Buckley C, et al. Association between in vivo fluorine 18-labeled flutemetamol amyloid positron emission tomography imaging and in vivo cerebral cortical histopathology. Arch Neurol. 2011;68:1398–1403. doi: 10.1001/archneurol.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engler H, Forsberg A, Almkvist O, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129:2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 62.Grundman M, Pontecorvo MJ, Salloway SP, et al. Potential impact of amyloid imaging on diagnosis and intended management in patients with progressive cognitive decline. Alzheimer Dis Assoc Disord. 2013;27:4–15. doi: 10.1097/WAD.0b013e318279d02a. [DOI] [PubMed] [Google Scholar]
- 63.Rinne JO, Brooks DJ, Rossor MN, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 64.Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer’s disease treated with gantenerumab. Arch Neurol. 2012;69:198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 65.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer’s disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 66.Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 67.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 68.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Villemagne VL, Pike KE, Chetelat G, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer’s disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomperts SN, Locascio JJ, Marquie M, et al. Brain amyloid and cognition in Lewy body diseases. Mov Disord. 27:965–973. doi: 10.1002/mds.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 73.Tomiyama T, Nagata T, Shimada H, et al. A new amyloid beta variant favoring oligomerization in Alzheimer’s-type dementia. Ann Neurol. 2008;63:377–387. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]