Abstract
Background
Our earlier work suggested that the cognitive performance impairment in individuals with schizophrenia relative to healthy control subjects was generalized, cutting across narrower cognitive ability dimensions. Current analyses sought to extend these findings.
Methods
Seventeen neuropsychological variables, available for 148 schizophrenia subjects and 157 control subjects, were subjected to structural equation modeling. Analyses incorporated a hierarchical model, grouping the variables into six familiar cognitive domains and linking these to a higher-order, general cognitive ability factor. We added diagnosis to the model as a grouping factor and estimated loadings from diagnosis to the general cognitive factor and, separately, to the domain factors.
Results
The fit of the final model was good (e.g., Non-Normed Fit Index [NNFI] = .988). Approximately 63.6% of the diagnosis-related variance in cognitive performance was mediated through the general factor, with smaller direct effects on verbal memory (13.8%) and processing speed (9.1%).
Conclusions
The schizophrenia cognitive deficit is largely generalized across performance domains, with small, direct effects of diagnostic group confined to selected domains. This generalized deficit sometimes has been seen as a function of the psychometric limitations of traditional cognitive test batteries. Alternatively, it may be a fundamental manifestation of schizophrenia, with similarly general neurobiological underpinnings.
Keywords: Cognitive deficit, neuropsychological testing, processing speed, schizophrenia, structural equation modeling, verbal memory
A large literature supports a multifactor structure of neuropsychological test performance among individuals with schizophrenia. Deficits in episodic memory, sustained attention, working memory, processing speed, and reasoning/problem solving have been reported frequently (1). However, these separable dimensions are strongly correlated, reflecting their shared relationship with a common cognitive ability factor (2,3), referred to as “g” (4). Most of the schizophrenia factor analytic literature has addressed the “within-group” structure of cognition. The structure of “between-groups” cognitive deficit evident in comparisons of schizophrenia patients and healthy control subjects has been less studied. Using structural equation modeling (SEM), we found that most of this deficit was generalized across cognitive dimensions—in essence, a “deficit g”—and that domain-specific effects of diagnosis were selective and small in magnitude (5).
Generalized deficit findings are a challenge to domain-specific interpretations of neuropsychological findings in clinical trials and studies of schizophrenia genetics, and may raise fundamental questions about the neural underpinnings of cognitive dysfunction in this illness. However, the 2004 analysis used a simplified model of cognitive structure and was limited to measures drawn from the Wechsler intelligence and memory scales. To confirm and extend these findings, we analyzed data from a comprehensive neuropsychological battery administered to large schizophrenia and control samples (6). We examined whether the between-groups performance deficit is better understood as a single, generalized deficit; a number of discrete domain-specific deficits; or some combination of generalized and specific effects. Based on our earlier work, we hypothesized that the deficit would be mediated mainly through a common cognitive ability factor, with smaller direct effects on selected cognitive domains.
Methods and Materials
Participants
Patients and control subjects were assessed and followed by the Schizophrenia Research Center at the University of Pennsylvania. Data for the present analyses were collected from January 1993 to May 1998, as described previously (6). Patients had DSM-III-R diagnoses of schizophrenia established by clinical examination and structured clinical interview but were free of other psychiatric conditions. Control subjects were free of psychiatric disorders in themselves and their first-degree relatives. Patients and control subjects were between the ages of 18 and 45 and had no medical conditions or head injury history that might affect cognitive functioning. All schizophrenia participants were stable outpatients. Forty-two schizophrenia participants were in their first episode of illness with little medication history at the time of testing, 77 were on stable doses of antipsychotic medication, and 29 were missing medication information. One-way analyses of variance (ANOVAs) showed no significant differences among these patient subgroups on any of the cognitive test variables (all ps > .05, all but one p > .25).
Statistical Analysis
Data from 148 schizophrenia patients and 157 healthy control subjects were analyzed. These samples were nonoverlapping with those analyzed for the 2004 report. LISREL 8.8 (7), with maximum likelihood estimation, was used to estimate parameters and evaluate model fit. Our primary analyses incorporated a hierarchical six-factor model of cognitive performance for patients and control subjects validated in our earlier analyses of within-group cognitive structure in these same samples (6). Central premises of the hierarchical model are that cross-domain associations between individual cognitive test variables actually reflect the interrelationships among a smaller number of latent cognitive domain factors (e.g., processing speed, verbal memory) and that these interrelationships are caused, in turn, by the shared relationship of the domain factors to a higher-order latent factor representing general cognitive ability or g (8). Consistent with these ideas, each of the 17 cognitive variables was assigned (by consensus of the authors) to load exclusively on the factor indicated in Table 1, and these factors were assigned to load on the common factor.
Table 1.
Grouping of Individual Neuropsychological Variables into Cognitive Domains
| Cognitive Domain | Individual Cognitive Tests/Variablesa |
|---|---|
| Executive/Working Memory | WCST Categories, WCST Perseverative Errors, Trail Making Test B, WAIS Digit Span |
| Verbal Ability | WAIS Vocabulary, MAE Naming |
| Spatial Ability | WAIS Block Design, Benton Judgment of Line Orientation |
| Verbal Learning and Memory | WMS Logical Memory I, WMS Logical Memory II, CVLT Trials 1–5, CVLT Delayed Free Recall |
| Visual Learning and Memory | WMS Visual Reproduction I, WMS Visual Reproduction II |
| Processing Speed | Trail Making Test A, Symbol Cancellation, Category Fluency |
CVLT, California Verbal Learning Test; MAE, Multilingual Aphasia Examination; WAIS, Wechsler Adult Intelligence Scale; WCST, Wisconsin Card Sorting Test; WMS, Wechsler Memory Scale.
Specific citations for the neuropsychological measures referred to in this article are available in standard neuropsychological reference works (9).
To examine the structure of the between-groups deficit, we added diagnostic group to the hierarchical model (Figure 1, bracket b). We first estimated a common factor model with no independent paths from diagnosis to individual cognitive domain factors. Then, we added direct paths (e.g., the curved arrow in Figure 1), one at a time, and re-estimated. The model was considered final when additional direct diagnosis factor paths no longer contributed significantly to model fit. The goodness-of-fit index (GFI) (10), non-normed fit index (NNFI) (11), and root mean square error of approximation (RMSEA) (12) were used to assess overall goodness of fit of the estimated model variance-covariance matrix to the observed matrix. Interpretation of these indices is described in our earlier report (6).
Figure 1.
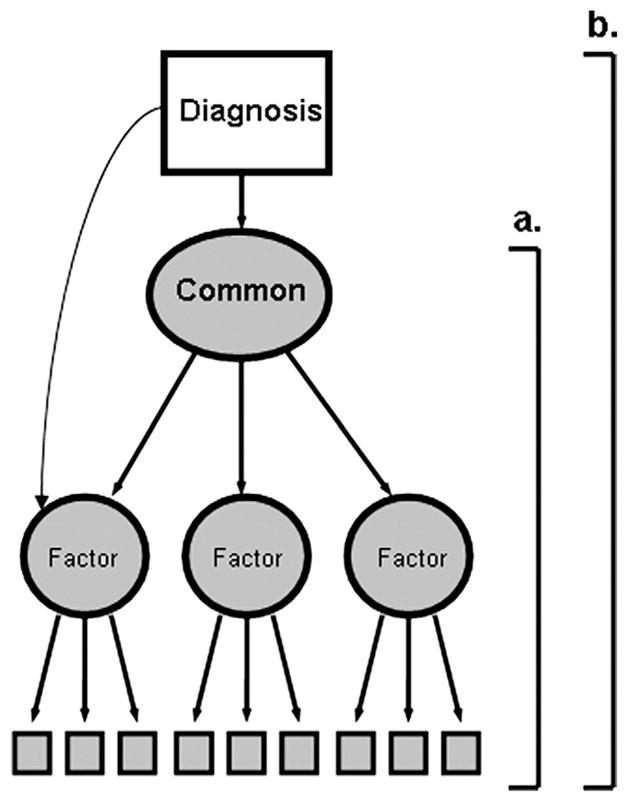
Illustration of the hypothesized model and structural equation modeling methodology. The part of the figure indicated by the bracket labeled “a” illustrates the hierarchical measurement model. Performance on observed variables (boxes) is assumed to be driven by domain-specific latent factors. These factors are determined, in turn, by the common cognitive ability factor. The whole figure (indicated by bracket “b”) illustrates the overall structural model for current analyses. These analyses test whether the effects of diagnostic grouping (i.e., schizophrenia vs. healthy control status; represented by the unshaded box marked “Diagnosis”) on the observed cognitive variables is mediated through the common factor or whether diagnosis affects observed variables through direct effects on domain-specific factors.
Results
Sample Characteristics
Patients were significantly older than control subjects, more likely to be male, and less likely to be Caucasian. Patients also had fewer years of education, although parental education was statistically equivalent between groups (Table 2). To reduce the impact of demographic differences, all analyses covaried age, sex, race/ethnicity, and mother’s/father’s education.
Table 2.
Demographic Characteristics and Intellectual Performance of Schizophrenia Patients and Healthy Control Subjects
| SZ (n = 148) | HC (n = 157) | |
|---|---|---|
| Age at Testinga | 33.5 (7.5) | 30.2 (6.2)f |
| Educationb | 13.2 (2.2) | 15.4 (2.1)f |
| Mother’s Educationc | 13.2 (2.8) | 12.4 (3.3) |
| Father’s Educationd | 13.7 (3.6) | 12.8 (3.8) |
| Age at Onsete | 22.5 (5.6) | |
| % Malea | 65.5 | 45.9f |
| Race (% W-AA-O)a | 52-46-2 | 69-22-9f |
| Vocabulary SS | 8.8 (3.0) | 11.9 (2.3)f |
| Digit Span SS | 8.5 (2.6) | 10.8 (2.6)f |
HC, healthy control subject; SS, standard score; SZ, schizophrenia patients; W-AA-O, White, African-American, Other.
Based on 150 HC, 121 SZ.
Based on 149 HC, 119 SZ.
Based on 147 HC, 100 SZ.
Based on 143 HC, 91 SZ.
Based on 112 SZ.
Group differences significant at p < .001. Other group differences not statistically significant.
The schizophrenia deficit was significant for every neuropsychological variable (all ps < .01). Wechsler Adult Intelligence Scale (WAIS) Vocabulary and Digit Span standard scores indicated that the control subjects were above average in intellectual performance, while the schizophrenia group was below average. The performance discrepancy between groups on these intellectual measures was comparable with other schizophrenia/control group comparisons on similar measures (13,14).
Modeling Results
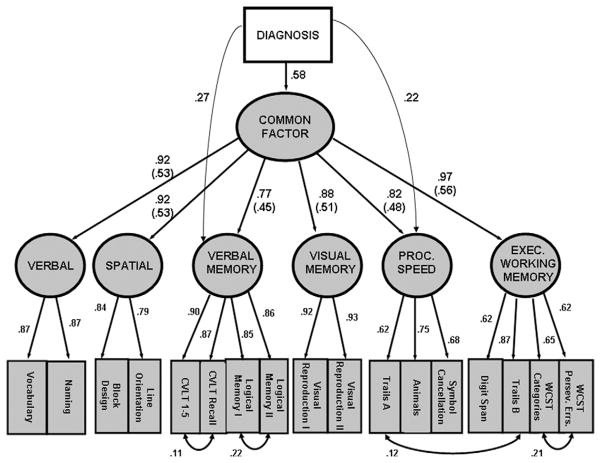
A model with all diagnosis effects mediated through the common factor fit the data marginally (Table 3, Model 1). Model fit improved significantly with the addition of a path representing the direct effects of diagnosis on verbal memory (Table 3, Model 2) and improved further with the addition of a direct processing speed path (Table 3, Model 3). No other direct paths from diagnosis to cognitive factors significantly improved model fit. Figure 2 shows the final model and statistics (Table 3, Model 3) indicate good model fit (i.e., GFI near .90 [10]; NNFI above .95 [11]; and RMSEA near .05 [12]). Multivariate analysis of variance (with the same covariates) indicated that, across all neuropsychological measures, diagnosis was associated with 53% of the overall variance in cognitive performance [F(16,283) =19.83; partial eta-squared =.529]. Squaring the relevant diagnosis loadings (Figure 2), the common factor accounted for approximately 63.6% of this diagnosis-related variance; the direct diagnosis-verbal memory and diagnosis-processing speed associations accounted for 13.8% and 9.1%, respectively.
Table 3.
Structural Model Fit Statistics
| Model | Chi-Square (df) | GFI | NNFI | RMSEA | Chi-Square Difference |
|---|---|---|---|---|---|
| 1. Diagnosis effects through common factor only | 545 (221) | .866 | .975 | .0714 | — |
| 2. Diagnosis effects through common factor and verbal memory | 485 (214) | .883 | .978 | .0643 | 60a |
| 3. Diagnosis effects through common factor, verbal memory and processing speed | 460 (207) | .888 | .979 | .0632 | 25a |
GFI, goodness-of-fit index; NNFI, non-normed fit index; RMSEA, root mean square error of approximation.
Chi-square difference significant at p < .001.
Figure 2.
The final model with standardized estimates for all significant parameters. Parenthesized values are the common factor-domain factor coefficients multiplied by the diagnosis-common factor coefficient (i.e., .58). The parenthesized values are thus scaled to be directly comparable with the direct diagnosis coefficients for verbal memory and processing speed. CVLT, California Verbal Learning Test; WCST, Wisconsin Card Sorting Test.
Discussion
Results indicate that the deficit in schizophrenia neuropsychological performance relative to healthy control subjects is largely mediated through a common ability factor. Using a hierarchical model of cognitive structure, large samples of patients and control subjects, and a comprehensive neuropsychological battery, this factor accounted for 63% of the diagnosis-related variance in overall cognitive performance, consistent with our earlier report (5). Analyses revealed direct diagnosis effects on verbal memory and processing speed. Against a backdrop of generalized poor performance, these domains consistently show disproportionate deficits in schizophrenia (13,14) and their emergence here again suggests that they could be more specifically implicated in schizophrenia than other cognitive domains. Still, the specific effects were small compared with the common factor effect and were confined to two of six domains. The absence of direct diagnosis effects on executive and working memory, visual memory, language, or spatial factors strongly suggests that poor patient performance reflected a generalized effect of illness, not multiple, domain-specific effects.
A growing body of work suggests that the latent structure of cognitive performance in schizophrenia, within and between groups, is more unitary than has been assumed (2,3,6,15). Thus, many popular neuropsychological instruments largely measure general cognitive deficits rather than domain-specific cognition. One prominent response to this problem is a psychometric critique that generalized deficit findings reflect the multifactorial character of the neuropsychological measures used to assess cognitive function, not something fundamental about the structure of cognition (16). In this regard, it does seem likely in coming years that advances in measurement science will enable researchers to link genetic and molecular markers to focused behavioral paradigms with greater precision (17,18). It is unclear how findings from such measurement strategies will relate to the common ability factor identified in the current work. The present verbal memory and processing speed results, converging with other research, may highlight these domains as targets for genetics studies or clinical trials.
At the same time, the drive toward ever more precise biology-to-behavior mappings raises concerns about reductionism. A systems-level approach is essential to explain the illness phenomena that are ultimately of greatest interest to schizophrenia researchers. In part, traditional neuropsychological measures may have shown longevity in this field precisely because they index behavior at a molar level. Moreover, it is not obvious that the generalized performance deficit on such measures can be dismissed as a mere psychometric phenomenon. Indeed, there are many possible links to the neurobiological literature. A relatively specific lesion (e.g., in dopamine-mediated frontal-striatal loops [19]) could create a bottleneck (e.g., poor response programming) broadly affecting performance on neuropsychological measures. Alternatively, evidence that the neurobiology underlying generalized cognitive dysfunction in schizophrenia is likewise general includes: 1) widespread reductions in gray matter and neuronal arborization (20); 2) diminished myelin density and white matter coherence (21,22); 3) poor signal integration at the level of the neuron and neural network (23); and 4) abnormalities associated with excitatory and inhibitory neurotransmitters, glutamate, and gamma-aminobutyric acid (GABA) (24). Related evidence in schizophrenia relatives and healthy groups also is emerging (23,25–27). In sum, investigation of associations at more integrated levels of cognition and neurobiology seems fundamental to unraveling schizophrenia and should be pursued in balance with narrowly targeted approaches.
Acknowledgments
This work was supported by the University of Pennsylvania Schizophrenia Research Center; Research Career Development funding from the Department of Veteran’s Affairs, Rehabilitation Research and Development Service (DD); and National Institute of Mental Health (NIMH) Grants MH62103, MH67764, MH42191, MH00586, and MH43880.
Footnotes
Drs. Dickinson and Ragland report no biomedical financial interests or potential conflicts of interest. Dr. Gold receives royalty payments from the Brief Assessment of Cognition in Schizophrenia test battery and has served as a consultant for Pfizer, Solvay, and AstraZeneca. Dr. Gur serves as consultant, and his laboratory performs studies for Merck. He has investigator-initiated grants from AstraZeneca, and he is consultant to Brain Resource Center and Current Designs, Inc. (manufacturers of a fiber optic response pad used in functional magnetic resonance imaging [fMRI] studies).
References
- 1.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson D, Gold JM. Less unique variance than meets the eye: Overlap among traditional neuropsychological dimensions in schizophrenia. Schizophr Bull. 2008;34:423–434. doi: 10.1093/schbul/sbm092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keefe RS, Bilder RM, Harvey PD, Davis SM, Palmer BW, Gold JM, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31:2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- 4.Jensen AR. Psychometric g: Definition and substantiation. In: Sternberg RJ, Grigorenko EL, editors. The General Factor of Intelligence. Mahwah, NJ: Lawrence Earlbaum; 2002. pp. 39–54. [Google Scholar]
- 5.Dickinson D, Iannone VN, Wilk CM, Gold JM. General and specific cognitive deficits in schizophrenia. Biol Psychiatry. 2004;55:826–833. doi: 10.1016/j.biopsych.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson D, Ragland JD, Calkins ME, Gold JM, Gur RC. A comparison of cognitive structure in schizophrenia patients and healthy controls using confirmatory factor analysis. Schizophr Res. 2006;85:20–29. doi: 10.1016/j.schres.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joreskog KG, Sorbom D. LISREL 8.72. Lincolnwood, Illinois: Scientific Software International, Inc; 2005. [Google Scholar]
- 8.Carroll JB. Human Cognitive Abilities: A Survey of Factor-Analytic Studies. New York: Cambridge University Press; 1993. [Google Scholar]
- 9.Lezak MD. Neuropsychological Assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 10.Joreskog KG. Testing structural equation models. In: Bollen KA, Lang JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage Publications; 1993. pp. 294–316. [Google Scholar]
- 11.Bentler PM, Bonnett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88:588–606. [Google Scholar]
- 12.Browne MW, Cudek R. Alternative ways of assessing model fit. In: Bollen KA, Lang JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage Publications; 1993. pp. 136–159. [Google Scholar]
- 13.Dickinson D, Ramsey M, Gold JM. Overlooking the obvious: A meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 14.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 15.Harvey PD, Green MF, Bowie C, Loebel A. The dimensions of clinical and cognitive change in schizophrenia: Evidence for independence of improvements. Psychopharmacology (Berl) 2006;187:356–363. doi: 10.1007/s00213-006-0432-1. [DOI] [PubMed] [Google Scholar]
- 16.Jonides J, Nee DE. Assessing dysfunction using refined cognitive methods. Schizophr Bull. 2005;31:823–829. doi: 10.1093/schbul/sbi053. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald AW, 3rd, Carter CS, Flory JD, Ferrell RE, Manuck SB. COMT val158Met and executive control: A test of the benefit of specific deficits to translational research. J Abnorm Psychol. 2007;116:306–312. doi: 10.1037/0021-843X.116.2.306. [DOI] [PubMed] [Google Scholar]
- 18.Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, et al. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007;27:13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbins TW. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16:391–402. doi: 10.1093/schbul/16.3.391. [DOI] [PubMed] [Google Scholar]
- 20.Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: A selective review. Schizophr Res. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr Bull. 2008;34:72–92. doi: 10.1093/schbul/sbm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, et al. DTI and MTR abnormalities in schizophrenia: Analysis of white matter integrity. Neuroimage. 2005;26:1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, et al. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004;161:490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- 24.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: Convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 25.Boos HB, Aleman A, Cahn W, Pol HH, Kahn RS. Brain volumes in relatives of patients with schizophrenia: A meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 26.Fan Y, Gur RE, Gur RC, Wu X, Shen D, Calkins ME, et al. Unaffected family members and schizophrenia patients share brain structure patterns: A high-dimensional pattern classification study. Biol Psychiatry. 2008;63:118–124. doi: 10.1016/j.biopsych.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIntosh AM, Moorhead TW, Job D, Lymer GK, Munoz Maniega S, McKirdy J, et al. The effects of a neuregulin 1 variant on white matter density and integrity [published online ahead of print October 9] Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]