Abstract
Recent studies have proposed activation of brown adipose tissue (BAT) thermogenesis as a new strategy to combat obesity. Currently, there is no effective noninvasive imaging agent to directly detect unstimulated BAT and quantify the core mechanism of mitochondrial thermogenesis. We investigated an approach to detect BAT depots and monitor thermogenesis using the mitochondria-targeting voltage sensor radiolabeled fluorobenzyltriphenyl phosphonium (FBnTP).
Methods
18F-FBnTP, 14C-FBnTP, 18F-FDG, and 99mTc-sestamibi uptake in BAT at room temperature (n = 8) and cold-treated (n = 8) Lewis rats was assayed. The effect of the cold condition on 18F-FBnTP retention in BAT was assessed in 8 treated and 16 control rats. The effect of the noradrenergic inhibitor propranolol on 14C-FBnTP response to cold stimulation was investigated in an additional 8 treated and 8 control mice.
Results
At room temperature, 18F-FBnTP accumulated in BAT to an extent similar to that in the heart, second only to the kidney and twice as much as 99mTc-sestamibi. Prior exposure to cold (4 °C) for 4 h resulted in an 82% decrease of 14C-FBnTP uptake and an 813% increase of 18F-FDG uptake in BAT. 99mTc-sestamibi uptake was not affected by cold. Administration of 18F-FBnTP at room temperature 60 min before 120 and 240 min of exposure to cold resulted in marked washout of the tracer from BAT. Propranolol significantly diminished the effect of cold on 14C-FBnTP and 18F-FDG uptake into BAT.
Conclusion
The intense uptake of 18F-FBnTP into BAT at room temperature and the response to cold stimulation suggest the unique potential advantage of 18F-FBnTP not only in detecting unstimulated BAT at high contrast but also in quantifying the mitochondrial thermogenic activity. 18F-FBnTP PET may serve as a useful technique to assess BAT volume and function.
Keywords: PET, 18F-FBnTP, 18F-FDG, brown adipose tissue, thermogenesis
The incidence of obesity has reached epidemic proportions, with 1.6 billion people worldwide being overweight, of whom 400 million are obese (body mass index > 30) (1). Complications related to obesity, such as diabetes and heart failure, contribute substantially to health care costs. Despite the prevalence and burden of obesity, there are no consistently effective means to control weight gain (2,3).
Obesity can be defined as an imbalance between food intake and energy expenditure. Thermogenic brown adipose tissue (BAT) has been implicated in the modulation of energy balance. Several studies have proposed BAT as a target for antiobesity therapy, focusing on increased energy expenditure·(4,5). BAT is known to generate heat in response to a cold environment and a high-fat diet (6,7).
Previously, we have shown that 18F-FDG accumulates extensively in BAT in rats (also termed uptake in supraclavicular area fat [USA-Fat] in humans) (8,9) and that this uptake is markedly augmented by cold stimulation in a noradrenergic-dependent manner (10). Until recently, the common belief was that BAT is restricted to hibernating animals and to young children. Recent clinical studies using 99mTc-sestamibi SPECT and 18F-FDG PET/CT revealed that a fraction of adults retains metabolically active depots of BAT mainly in the neck, supraclavicular, and paravertebral regions (11–14). The PET finding triggered extensive investigation of the potential use of BAT thermogenesis as a tool to transform caloric intake into heat rather than white fat depots. However, partly because of the lack of appropriate noninvasive tools, most of our knowledge of BAT stems from animal studies. Relatively little is known about BAT in humans. The incidence and contribution to overall energy expenditure in adult humans, the distribution among geographic regions or ethnic groups, and the modes of metabolic activation are still unknown.
A recent study has shown increased uptake of 99mTc-sestamibi in unstimulated BAT in a small fraction of patients (11). Presently, 18F-FDG PET/CT is the technology commonly used to detect metabolically active BAT depots in humans (12–15). However, clinical studies have shown that 18F-FDG uptake in BAT is highly variable and nonreproducible. Conflicting reports suggest a prevalence of subjects with positive 18F-FDG PET scans showing BAT ranging from a low percentage to 80% (12,13,15,16). These inconsistent results may be attributed to endogenous and exogenous factors, such as environmental temperature, body mass index, blood glucose level, and diet. Yet, even within the same subjects (33 breast cancer patients) undergoing repeated PET scans (n = 5) under similar basal conditions (e.g., room temperature, body weight, blood glucose level, and fasting conditions), 18F-FDG BAT uptake was nonreproducible in 32 of 33 patients (17). A well-controlled PET study in healthy adults found no quantitative correlation between cold-induced thermogenesis and the extent of 18F-FDG BAT uptake (14).
The increase of 18F-FDG uptake in stimulated BAT is most likely due to enhanced glycolysis taking place during thermogenesis (7). Mitochondria are the generator of heat, which is produced due to the uncoupling of oxidative phosphorylation from adenosine triphosphate (ATP) synthesis (18–20). Enhanced glycolysis was suggested as a compensatory mechanism to address the need for ATP (7). This is supported by the marked increase of lactate efflux to the bloodstream, measured in rats exposed to cold (21). In other words, 18F-FDG targets a metabolic process secondary to thermogenesis, and this may explain the reported high variability and poor reproducibility of 18F-FDG PET/CT. These data highlight the need for a noninvasive and direct approach that could allow detection and localization of BAT in the intact body in a more reliable and accurate manner than that afforded by 18F-FDG.
In this study, we tested the hypothesis that targeting the mitochondria in a membrane potential–dependent manner using the PET agent 4-18F-fluorobenzyltriphenyl phosphonium (18F-FBnTP) may afford an effective in vivo approach to detect metabolically active BAT depots. Brown adipocytes have abundant mitochondria—the organelles responsible for the heat production during thermogenesis—and 18F-FBnTP is expected to accumulate extensively in BAT. We assayed 18F-FBnTP and 14C-FBnTP in rats under thermoneutral and cold conditions and compared the results to those for 18F-FDG and 99mTc-sestamibi. The mechanistic link between 14C-FBnTP and thermogenesis was studied using the noradrenergic inhibitor propranolol.
MATERIALS AND METHODS
Species and Materials
Male Lewis rats (~200 g in body weight) were purchased from Charles River. 18F-FBnTP was prepared in our radiochemistry laboratory with a specific activity ranging from 111 to 185 GBq (3,000–5,000 mCi)/mmol, as described elsewhere (22). 14C-FBnTP was prepared by International Isotopes Clearing House, Inc. 18F-FDG was purchased from PETNET Solutions. 99mTc-sestamibi was purchased from Mallinckrodt. Propranolol was purchased from Sigma.
Uptake Assays
Effect of Cold Stimulation on 14C-FBnTP, 18F-FDG, and 99mTc-Sestamibi Uptake in BAT
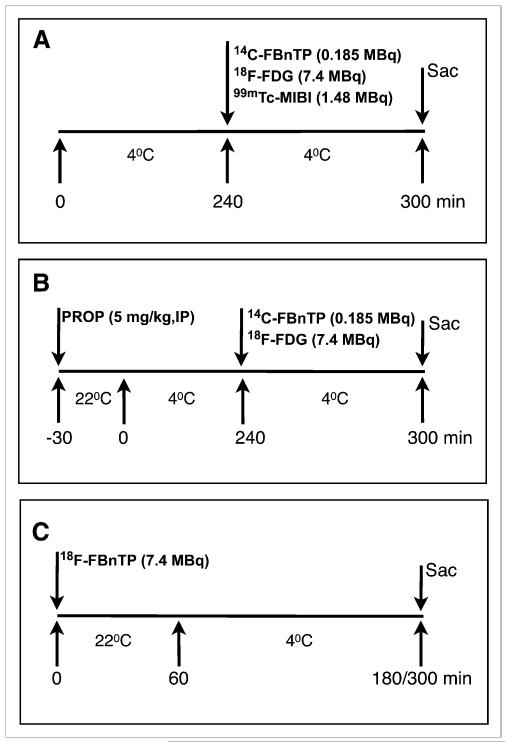
Studies were performed according to protocol A, which is schematically outlined in Figure 1A. Rats were kept in the cold (4 °C, n = 8) and at room temperature (22 °C, n = 8) for 4 h before a tail vein injection of a mixture of 14C-FBnTP (185 kBq [5 μCi]), 18F-FDG (7,400 kBq [200 μCi]), and 99mTc-sestamibi (1,480 kBq [40 μCi]) in 0.2 mL of saline. After a 60-min uptake period in the cold, interscapular BAT and selected organs were harvested. 18F-FDG radioactivity was counted in a γ-counter. 99Tc-sestamibi was counted after 24 h in a γ-counter. The tissue samples were then incubated in 1 mL of tissue dissolver and placed on a hot plate (40 °C) for 72 h. H2O2 (0.4 mL) was added; after 60 min, 10 mL of scintillation liquid were added, and 14C-FBnTP was counted in a β-counter. γ and β tissue radioactivity was counted along with standards (1:200). Tissue radioactivity was expressed as the percentage of injected dose per gram of tissue per kilogram of body weight.
FIGURE 1.
Study protocols. (A) Effect of prior exposure to cold. Rats were placed in cold (4 °C) environment for 4 h before tracer administration. Rats were replaced in cold for additional 60 min before sacrifice. Control rats underwent similar treatment, but without exposure to cold. (B) Effect of propranolol. Propranolol (5 mg/kg) was administered intraperitoneally to rats. After 30 min at room temperature (22 °C), rats were placed in cold environment for 4 h. Radioactive tracers were then administered intravenously, and rats were put back in cold for 60 min before sacrifice. Control rats underwent same cold treatment, but without propranolol administration. (C) Effect of cold on 18F-FBnTP retention in BAT. 18F-FBnTP was administered at room temperature, and after 60 min, rats were placed in cold environment for either 120 or 240 min before sacrifice. Control rats were kept at room temperature and sacrificed at 60, 180, and 240 min after tracer administration. IP = intraperitoneal; MIBI = sestamibi; PROP = propranolol; Sac = sacrifice.
Effect of the Noradrenergic Antagonist Propranolol
For protocol B, outlined in Figure 1B, propranolol (5 mg/kg, intraperitoneally) was administered 30 min before a 4-h exposure to cold. The isotope mixture 14C-FBnTP and 18F-FDG was administered via a tail vein, and tissue was harvested 60 min thereafter. Tissue radioactivity was measured as described for protocol A.
Effect of Cold Stimulation on 18F-FBnTP Retention in BAT
For protocol C, schematically outlined in Figure 1C, rats were administered 18F-FBnTP (7,400 kBq [200 μCi]) via a tail vein. After a 60-min uptake period at room temperature, rats were placed in a cold environment for 120 min. In a separate study, rats were placed in the cold environment for 240 min before tissue harvesting. In each of the studies, tissue radioactivity in the cold group was compared with 2 baseline groups, one maintained at room temperature for 60 min and the other for a duration equal to that of the treated group (180 or 300 min). Tissue radioactivity was measured as indicated for protocol A.
RESULTS
The Differential Effect of Cold on 14C-FBnTP, 18F-FDG, and 99mTc-Sestamibi Uptake in BAT
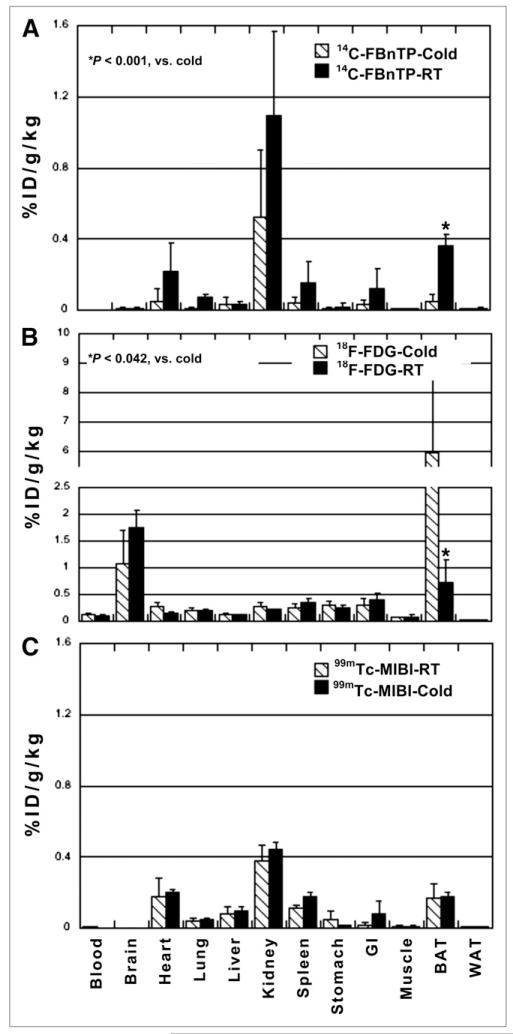
Figure 2 depicts 14C-FBnTP, 18F-FDG, and 99mTc-sestamibi biodistribution in cold-treated rats and control rats maintained at room temperature. At room temperature, 14C-FBnTP demonstrated intense uptake in BAT, to an extent similar to that in the heart and second only to the kidney (Fig. 2A). 14C-FBnTP BAT uptake was nearly twice that of 99mTc-sestamibi (Fig. 2C).
FIGURE 2.
Effect of prior exposure to cold. Compared with rats kept at room temperature for entire study, cold induced significant increase of 18F-FDG (A) and decrease of 14C-FBnTP (B) uptake in BAT, whereas 99mTc-sestamibi uptake was not affected (C). There was also a change in 14C-FBnTP activity in heart and kidneys. GI = gastrointestinal; ID = injected dose; RT = room temperature; WAT = white adipose tissue.
Exposure for 4 h to a cold environment resulted in a decrease of 14C-FBnTP and an increase of 18F-FDG uptake in BAT (Fig. 2B). 14C-FBnTP BAT uptake declined by more than 80% (from 0.34 ± 0.07 to 0.049 ± 0.045, P < 0.01), whereas 18F-FDG BAT uptake increased by more than 8 times (from 0.73 ± 0.51 to 5.94 ± 1.30, P < 0.042), compared with control. Cold stimulation did not affect 99mTc-sestamibi uptake in BAT (room temperature, 0.18 ± 0.079, vs. cold, 0.2 ± 0.018, P > 0.72).
Interestingly, exposure to cold reduced 14C-FBnTP uptake and increased 18F-FDG uptake in organs other than BAT, including the heart and kidney. However, in this small cohort of animals, these changes were not statistically significant (P < 0.17). 14C-FBnTP uptake in the liver was the least affected by cold stimulation.
Noradrenergic Mediation of Cold Effect on 14C-FBnTP and 18F-FDG Uptake in BAT
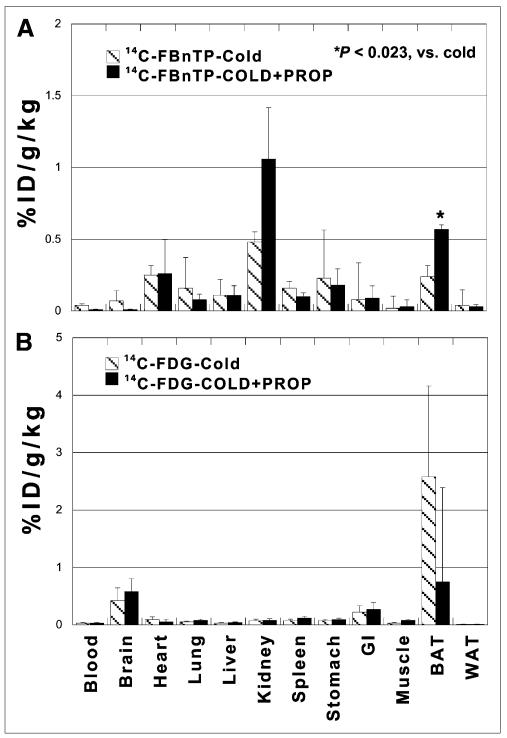
The mechanism responsible for the differential effect of cold on 14C-FBnTP and 18F-FDG was investigated using the nonselective noradrenergic antagonist propranolol, according to protocol B. Prior administration of propranolol significantly decreased the effect of cold stimulation on both 14C-FBnTP and 18F-FDG (Fig. 3). 14C-FBnTP uptake increased by more than 100% (from 0.24 ± 0.073 to 0.57 ± 0.022, P < 0.023) (Fig. 3A), compared with nontreated rats exposed to cold, whereas 18F-FDG BAT uptake declined to levels observed at room temperature (from 2.58 ± 1.58 to 0.75 ± 0.3, P < 0.043) (Fig. 3B).
FIGURE 3.
Effect of propranolol. Prior administration of propranolol augmented 14C-FBnTP BAT uptake (A) and diminished 18F-FDG BAT uptake (B) in rats exposed to cold, compared with nontreated rats. GI = gastrointestinal; ID = injected dose; PROP = propranolol; WAT = white adipose tissue.
Effect of Exposure to Cold on 18F-FBnTP Retention in BAT
The effect of cold treatment on 18F-FBnTP retention in BAT was investigated according to protocol C, which entailed administration of 18F-FBnTP at room temperature and 60 min later exposing the animals to either 120 or 240 min of cold. Results obtained in each cold-treated group were compared with the control group, which was maintained at room temperature before sacrifice for the same duration as the treated group (180 and 300 min). 18F-FBnTP retention in BAT at room temperature in the 180- and 300-min control group was compared with rats that were kept at room temperature for 60 min after 18F-FBnTP administration.
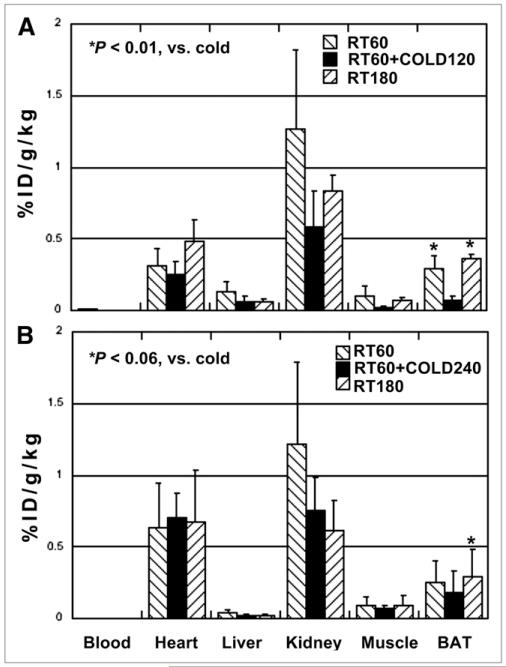
Exposure to cold for 120 min resulted in a marked decrease (81%) of 18F-FBnTP uptake in BAT, compared with rats maintained at room temperature for a similar post-administration duration (180 min). 18F-FBnTP decreased from 0.36 ± 0.03 in the room temperature control group to 0.07 ± 0.03 in the cold-treated group (Fig. 3A). Increasing the duration of cold stimulation to 240 min did not elicit an additive effect on 18F-FBnTP washout from BAT (Fig. 3B). Similar uptakes of 18F-FBnTP into BAT were found in the room temperature 180-min and 60-min groups (Fig. 4A; P > 0.34) and in the room temperature 300-min and 60-min rats (Fig. 4B, P > 0.21). This finding indicates that at room temperature, 18F-FBnTP reached a plateau in BAT within less than 60 min and that 18F-FBnTP maintained a steady-state concentration in BAT for at least 300 min.
FIGURE 4.
Effect of cold treatment on 18F-FBnTP retention in BAT. (A) Exposure for 120 min in cold (RT60+COLD120) resulted in marked decrease of 18F-FBnTP uptake into BAT, compared with 60-min (RT60) and 180-min (RT180) control groups maintained at room temperature. (B) Prolongation of cold treatment to 240 min (RT60+COLD240) did not elicit additive effect. Similar uptake is seen in room temperature control groups at 60-, 180-, and 300-min post-treatment duration. ID = injected dose; RT = room temperature.
DISCUSSION
The major findings of the current study are that under thermoneutral conditions 18F-FBnTP accumulates extensively in unstimulated BAT depots, whereas cold stimulation suppresses the uptake and retention of 18F-FBnTP. The effect of cold is mediated by the noradrenergic system. The extensive uptake and response to cold stimulation suggest that 18F-FBnTP could be an effective biomarker for detecting and localizing BAT and could be a tool for monitoring the thermogenic activity of BAT.
18F-FBnTP demonstrated extensive uptake in BAT—to an extent similar to that observed in the heart. The heart is a major target organ of 18F-FBnTP, second only to the kidney, as found in small and large animals (23,24). The strong accumulation of 18F-FBnTP in BAT is most likely due to the highly dense population of mitochondria in brown adipocytes. In fact, the brown color of the tissue is due to the abundance of mitochondria. In mitochondria-rich myocytes isolated from dog heart, 18F-FBnTP concentration was more than 100 times greater than in an equal volume of the extracellular medium (23). 18F-FBnTP is a potentiometric probe; it accumulates in cells as a function of the membrane potential. The electrical gradient across the mitochondrial membrane (ΔΨm) is much greater than that of the plasma membrane (~180–240 mV vs. ~30–60 mV, respectively) (25). According to the Nernst equation, every 60-mV difference results in a 10-fold increase of the potentiometric probe uptake, which may lead to a more than 1,000 times greater concentration in mitochondria than in the cytosol (26). This possibility is in line with previous observations that selective dissipation of ΔΨm resulted in a more than 80% decrease in 18F-FBnTP cellular uptake (24). Because the mitochondria are only a fraction of the total cellular volume, 18F-FBnTP concentration is manyfold greater in mitochondria than in the cytosol. It is not surprising that 18F-FBnTP accumulated extensively in BAT, where there is an abundance of mitochondria. This finding suggests the suitability of 18F-FBnTP as a PET biomarker to detect and localize, at high-resolution, unstimulated BAT depots in the body.
Three sets of studies were performed to explore the relationship between 18F-FBnTP and BAT thermogenesis. First, the effect of cold stimulation was investigated. Prior exposure to cold resulted in a marked decrease (~80%) of 14C-FBnTP uptake in BAT. The mitochondria are the heat generator in BAT, and the mechanism of heat production was studied in detail (18,20,29). The uncoupler protein 1 (UCP1), a constituent of the mitochondrial inner membrane, is the key mechanism of heat production in BAT (19,20).
Under thermoneutral conditions, the energy produced by the mitochondrial respiratory chain’s electron transport is stored in the form of the electrochemical proton gradient and used for the enzymatic activity of ATP synthase. Under cold conditions, most of the energy produced by the mitochondrial respiratory chain is not used for ATP synthesis but is dissipated as heat. The translation to heat is obtained by the fast translocation of protons back to the mitochondrial matrix via UCP1 (18–20,27). ΔΨm is the voltage analog of UCP1 activity, and the proton leak mediated by UCP1 results in a proportional decrease of ΔΨm (18,26,27).
The intimate nexus between ΔΨm and UCP1 provides the mechanism underlying the decline in 18F-FBnTP cellular uptake induced by cold stimulation. 18F-FBnTP is a voltage sensor whose cellular uptake is driven mainly by ΔΨm. Uptake assays in a malignant cell line have shown that stepwise depolarization of membrane potential (by increasing extracellular potassium concentration) resulted in a linear decrease of 18F-FBnTP cellular uptake with a slope (a measure of the probe sensitivity) and coefficient of variance (a measure of the probe accuracy) nearly identical to that of 3H-tetraphenylphosphonium (24), which is a common and accurate method for the measurement of ΔΨm in vitro (26). Moreover, selective dissipation of ΔΨm induced by the protonophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP) resulted in a dose-dependent decrease of 18F-FBnTP cellular uptake (24). CCCP collapses ΔΨm by activation of UCP1 in a manner similar to that induced by cold stimulation (20). Incubation of isolated brown adipocytes with the uncoupler carbonyl cyanide p-(trifluoromethoxy)-phenylhydrazone (FCCP) resulted in a significant increase of oxygen use, as compared with the coupled state (20). Increased oxygen use is typical of thermogenesis and is due to uncoupling of oxidative phosphorylation and ATP synthesis (7). These data highlight the unique advantage of 18F-FBnTP as a tool not only to detect unstimulated BAT at high contrast but also to quantify the thermogenic activity, by direct targeting of the core mechanism: UCP1.
Second, the effect of cold stimulation on 18F-FBnTP retention in BAT was investigated. In this set of studies, 18F-FBnTP was introduced at room temperature, which eliminated any possible intervention of cold-induced effects on blood flow and drug delivery (30). Exposure to 120 min of cold resulted in a marked washout of 18F-FBnTP. This finding indicates that 18F-FBnTP cellular uptake in BAT is not only driven but also retained by ΔΨm. This finding is supported by the prolonged retention of 18F-FBnTP in BAT observed in the control groups at room temperature. Similar 18F-FBnTP BAT uptake was measured at 60, 180, and 300 min after administration. At room temperature, BAT is in coupled state and ΔΨCm is intact. Under this condition, 18F-FBnTP was retained in BAT for a prolonged period (300 min). In contrast, cold stimulation activates UCP1-mediated proton leakage, which leads to a decrease of 18F-FBnTP retention force: ΔΨm. This explains 18F-FBnTP washout from BAT on cold stimulation, as observed in the present study.
Importantly, targeting ΔΨm affords the unique potential advantage of realizing the dual capacity of 18F-FBnTP to detect and localize BAT depots, as well as quantify the thermogenic function, using a single dynamic PET scan. PET tracers that provide a positive signal on BAT stimulation, such as increased uptake of 18F-FDG, require separate PET scans at baseline and stimulated phases because of clearance from the vascular system. In contrast, 18F-FBnTP is taken up extensively and retained for a prolonged period in unstimulated BAT. Therefore, 18F-FBnTP BAT uptake at room temperature can serve as a reliable baseline for detecting the effect of a change in environmental conditions taking place during the same PET scan.
Comparison of duration of exposure to cold on 18F-FBnTP retention in BAT did not find an additive effect of 240 min of cold, compared with 120 min of cold. This result may suggest that a 120-min cold duration elicited maximal stimulation of BAT thermogenesis. This assumption is in line with our previous observation that mitochondrial uncoupling using varying doses of the protonophore CCCP resulted in a maximal decrease of 80% in 18F-FBnTP cellular uptake (24). Recent dynamic PET studies in isolated perfused heart have found maximal washout of 75% of 18F-FBnTP distribution in the left ventricular wall as induced by the uncoupler FCCP (Madar Igal, Ting Liu, Brian O’Rourke, unpublished data, 2010). In the experimental protocol adopted in the present study, a 120-min cold stimulation induced a washout of 80%, compared with control, which is similar to the labile fraction of 18F-FBnTP available to efflux when maximal activation of UCP1 is achieved. As we showed before, the remaining 18F-FBnTP cell-bound activity either is stored in the cytosol (15%) or is nonspecific (5%) (24).
Third, prior administration of the adrenergic antagonist propranolol significantly reduced the effect of cold stimulation on 14C-FBnTP. BAT is densely innervated by nor-adrenergic nerve endings (20). β3-adrenergic agonists are potent inducers of BAT thermogenesis and were shown to activate the proton carrier UCP1 (18,20,27). In the present study, the noradrenergic inhibitor significantly decreased the suppressive effect of cold on 14C-FBnTP. This finding further validates that changes in 14C-FBnTP uptake into stimulated BAT are coupled to the thermogenic function.
Taken together, these data demonstrate the potentially unique role of 18F-FBnTP as a noninvasive biomarker for the detection and localization of BAT depots, as well as for quantifying UCP1 activity. UCP1 is the pivotal player in thermogenic function (27,28). UCP1 is expressed exclusively in brown adipocytes (7). The centrality of UCP1 may be best exemplified by the finding that no thermogenesis can be induced by norepinephrine in brown adipocytes isolated from UCP1-ablated mice (19). The expression of UCP1 genes in brown adipocytes is upregulated by cold stimulation (18,28,29). Mice lacking BAT or UCP1 are more susceptible to diet-induced obesity (31,32). Therefore, the capacity of 18F-FBnTP to directly target the core mechanism of heat production equipped this imaging agent with the unique capacity to monitor noninvasively the dynamics of thermogenic activity.
Recent studies have suggested that BAT is present and can be activated in a fraction of adult humans and that total BAT activity is inversely associated with adiposity (11–14). Thus, increasing BAT mass or activity may be a target for pharmacologic and nutritional interventions that modulate energy expenditure to treat obesity (33–36). In humans, it has been estimated that as little as 50 g of BAT could use up to 20% of basal caloric needs if maximally stimulated (37). It is tempting to assume that induction of thermogenesis may afford a successful strategy to shift the balance in obese subjects from storing energy intake in the form of white fat depots into energy expenditure in the form of heat. Extensive efforts for biogenesis and stimulation of BAT depots are under way, and novel approaches are currently under investigation, including inducers of brown adipogenesis (e.g., bone morphogenetic protein 7, fibroblast growth factor 21, and growth differentiation factor 3) (38–40).
The dual capacity of 18F-FBnTP to detect and localize unstimulated BAT at high contrast and to report the kinetics of thermogenic function can provide information critical for developing and assessing the efficacy of therapeutic approaches to antiobesity drugs. BAT is an elastic structure that waxes and wanes in response to environmental temperature (7). Drugs designed to increase BAT mass and mitochondrial population are expected to result in a proportional increase in uptake of the mitochondria-targeting 18F-FBnTP. An alternative therapeutic approach is drugs designed to improve and facilitate the thermogenic capacity of BAT by elevating the expression of UCP1. The efficacy of such drugs can be quantified by the rate of washout of 18F-FBnTP from BAT, which can be documented by dynamic PET.
Changes in 18F-FBnTP uptake in non-BAT organs, such as the heart and kidney, were observed in some of the experiments of the present research (Fig. 1) but not in others (Fig. 4). At this early stage of study, it may be premature to draw conclusions on the effect of cold stimulation on mitochondrial uncoupling in organs other than BAT. Whole-body 18F-FBnTP PET studies in larger groups of animals are required to further elucidate this important aspect.
CONCLUSION
The intense uptake of 18F-FBnTP into BAT at thermoneutral conditions and the response to cold stimulation suggest the unique potential advantage of 18F-FBnTP not only for detecting unstimulated BAT at high contrast but also for quantifying mitochondrial thermogenic activity. 18F-FBnTP PET may serve as a useful technique to assess BAT volume and function.
ACKNOWLEDGMENTS
We thank Caroline Esaias for participating in some of the uptake assays. This study was supported in part by NIDDK R21 DK090780-01.
REFERENCES
- 1.Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 3.Bray GA. Drug insight: appetite suppressants. Nat Clin Pract Gastroenterol Hepatol. 2005;2:89–95. doi: 10.1038/ncpgasthep0092. [DOI] [PubMed] [Google Scholar]
- 4.Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ. Safety of drug therapies used for weight loss and treatment of obesity. Drug Saf. 2006;29:277–302. doi: 10.2165/00002018-200629040-00001. [DOI] [PubMed] [Google Scholar]
- 5.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol. 1979;57:257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 7.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 8.Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: prevalence is related to ambient outdoor temperature—evaluation with 18F-FDG PET/CT. J Nucl Med. 2003;44:1267–1270. [PubMed] [Google Scholar]
- 9.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET CT. J Nucl Med. 2003;44:170–176. [PubMed] [Google Scholar]
- 10.Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense 18F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med. 2004;45:1189–1193. [PubMed] [Google Scholar]
- 11.Goetze S, Lavely WC, Ziessman HA, Wahl RL. Visualization of brown adipose tissue with 99mTc methoxyisobutylisonitrile on SPECT/CT. J Nucl Med. 2008;49:752–756. doi: 10.2967/jnumed.107.048074. [DOI] [PubMed] [Google Scholar]
- 12.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 13.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 15.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 17.Rousseau C, Bourbouloux E, Campion L, et al. Brown fat in breast cancer patients: analysis of serial 18F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging. 2006;33:785–791. doi: 10.1007/s00259-006-0066-x. [DOI] [PubMed] [Google Scholar]
- 18.Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504:82–106. doi: 10.1016/s0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- 20.Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent: UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty acid-induced thermogenesis. J Biol Chem. 2000;275:25073–25081. doi: 10.1074/jbc.M000547200. [DOI] [PubMed] [Google Scholar]
- 21.López-Soriano FJ, Fernández-López JA, Mampel T, Villarroya F, Iglesias R, Alemany M. Amino acid and glucose uptake by rat brown adipose tissue: effect of cold-exposure and acclimation. Biochem J. 1988;252:843–849. doi: 10.1042/bj2520843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravert HT, Madar I, Dannals RF. Radiosynthesis of 3-(18F)-fluoropropyl and 4-(18F)-fluorobenzyl triarylphosphonium ions. J Labelled Comp Radiopharm. 2004;47:469–476. [Google Scholar]
- 23.Madar I, Ravert RT, Du Y, et al. Characterization of uptake of the new PET imaging compound 18F-fluorobenzyl triphenyl phosphonium in dog myocardium. J Nucl Med. 2006;47:1359–1366. [PubMed] [Google Scholar]
- 24.Madar I, Ravert H, Nelkin B, et al. Characterization of membrane potential-dependent uptake of the novel PET tracer 18F-fluorobenzyl triphenylphosphonium cation. Eur J Nucl Med Mol Imaging. 2007;34:2057–2065. doi: 10.1007/s00259-007-0500-8. [DOI] [PubMed] [Google Scholar]
- 25.Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev. 2000;41:235–250. doi: 10.1016/s0169-409x(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 26.Nicholls DG. The effective proton conductance of the inner membrane of mitochondria from brown adipose tissue: dependency on proton electrochemical potential gradient. Eur J Biochem. 1977;77:349–356. doi: 10.1111/j.1432-1033.1977.tb11674.x. [DOI] [PubMed] [Google Scholar]
- 27.Bouillaud F, Ricquier D, Mory G, Thibault J. Increased level of mRNA for the uncoupling protein in brown adipose tissue of rats during thermogenesis induced by cold exposure or norepinephrine infusion. J Biol Chem. 1984;259:11583–11511. [PubMed] [Google Scholar]
- 28.Beattie JH, Black DJ, Wood AM, Trayhurn P. Cold-induced expression of the metallothionein-1 gene in brown adipose-tissue of rats. Am J Physiol. 1996;270:R971–R977. doi: 10.1152/ajpregu.1996.270.5.R971. [DOI] [PubMed] [Google Scholar]
- 29.Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med. 2004;4:149–177. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- 30.Madar I, Ravert H, Dipaula A, Du Y, Dannals RF, Becker L. Assessment of severity of coronary artery stenosis in a canine model using the PET agent 18F-fluorobenzyl triphenyl phosphonium: comparison with 99mTc-tetrofosmin. J Nucl Med. 2007;48:1021–1030. doi: 10.2967/jnumed.106.038778. [DOI] [PubMed] [Google Scholar]
- 31.Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:143–149. doi: 10.1097/MED.0b013e328337a81f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kontani Y, Wang Y, Kimura K. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005;4:147–155. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 33.Argyropoulos G, Harper ME. Uncoupling proteins and thermoregulation. J Appl Physiol. 2002;92:2187–2198. doi: 10.1152/japplphysiol.00994.2001. [DOI] [PubMed] [Google Scholar]
- 34.Frühbeck G, Becerril S, Sáinz N, Garrastachu P, García Velloso MJ. BAT: a new target for human obesity? Trends Pharmacol Sci. 2009;30:387–396. doi: 10.1016/j.tips.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Ghorbani M, Claus TH, Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem Pharmacol. 1997;54:121–131. doi: 10.1016/s0006-2952(97)00162-7. [DOI] [PubMed] [Google Scholar]
- 36.Guerra C, Koza RA, Yamashita H, et al. Emergence of brown adipocytes in white fat in mice is under genetic control: effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowell BB, Susulic V, Hamann A, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 38.Melnikova I, Wages D. Antiobesity therapies. Nat Rev Drug Discov. 2006;5:369–370. doi: 10.1038/nrd2037. [DOI] [PubMed] [Google Scholar]
- 39.Rothwell NJ, Stock MJ. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci (Lond) 1983;64:19–23. doi: 10.1042/cs0640019. [DOI] [PubMed] [Google Scholar]
- 40.Wangsness M. Pharmacological treatment of obesity: past, present, and future. Minn Med. 2000;83:21–26. [PubMed] [Google Scholar]