Abstract
Purpose
To our knowledge the reasons for the high rates of prostate cancer in black American men are unknown. Genetic and lifestyle factors have been implicated. Better understanding of prostate cancer rates in West African men would help clarify why black American men have such high rates since the groups share genetic ancestry and yet have different lifestyles and screening practices. To estimate the prostate cancer burden in West African men we performed a population based screening study with biopsy confirmation in Ghana.
Materials and Methods
We randomly selected 1,037 healthy men 50 to 74 years old from Accra, Ghana for prostate cancer screening with prostate specific antigen testing and digital rectal examination. Men with a positive screen result (positive digital rectal examination or prostate specific antigen greater than 2.5 ng/ml) underwent transrectal ultrasound guided biopsies.
Results
Of the 1,037 men 154 (14.9%) had a positive digital rectal examination and 272 (26.2%) had prostate specific antigen greater than 2.5 ng/ml, including 166 with prostate specific antigen greater than 4.0 ng/ml. A total of 352 men (33.9%) had a positive screen by prostate specific antigen or digital rectal examination and 307 (87%) underwent biopsy. Of these men 73 were confirmed to have prostate cancer, yielding a 7.0% screen detected prostate cancer prevalence (65 patients), including 5.8% with prostate specific antigen greater than 4.0 ng/ml.
Conclusions
In this relatively unscreened population in Africa the screen detected prostate cancer prevalence is high, suggesting a possible role of genetics in prostate cancer etiology and the disparity in prostate cancer risk between black and white American men. Further studies are needed to confirm the high prostate cancer burden in African men and the role of genetics in prostate cancer etiology.
Keywords: prostatic neoplasms, prostate-specific antigen, mass screening, African Americans, Africa
Prostate cancer is the most commonly diagnosed nonmelanoma cancer in men in most Western countries.1 Despite the high morbidity and mortality of prostate cancer (it is the second leading cause of cancer death in men in the United States) until recently the only established risk factors were advancing age, race and a family history of prostate cancer.1 Of the well established risk factors race is the most dramatic with incidence and mortality rates in black American men almost twice those of white American men and 5 times higher than those of Asian men living in Asia.2,3 Genetics, environmental and lifestyle factors have been implicated to explain the large racial difference in prostate cancer risk, including differences in 5α-reductase activity in the prostate.4,5 More recently genome-wide association studies implicated several areas of the genome as risk factors.6–12 It is currently unclear how much of the racial difference in prostate cancer risk can be attributable to these risk loci.
Reports of population based incidence rate of prostate cancer in African men are limited because there are few population based cancer registries in this continent.13 Using the limited reported incidence we recently observed that the age adjusted incidence of prostate cancer in Africa is increasing and the prostate cancer incidence varies widely on the continent.14,15 More comprehensive population based data on the prostate cancer burden in West Africa are needed for cancer prevention and control efforts.
Better understanding of prostate cancer rates in West African men would provide insight into prostate cancer etiology and reasons for the large racial disparity since West African and black American men share similar genetic ancestry. To help determine the burden of prostate cancer in West African men, we performed a population based prostate cancer screening with diagnostic and therapeutic followup in a probability sample of healthy men between ages 50 and 74 in Greater Accra, Ghana.
MATERIALS AND METHODS
Study Subjects
This study was approved by the NCI (National Cancer Institute) and University of Ghana institutional review boards. Details of this study population were described previously.16,17 Briefly, to enroll a population based probability sample of men from Accra for screening, we collaborated with the Ghana Census Bureau and used 2000 Ghana Population and Housing Census data to construct a sampling frame for enrolling about 1,000 men 50 to 74 years old in the Greater Accra Region (population about 3 million). Based on census data we used a 3-stage design to select probability samples. 1) The primary sampling unit was the enumeration unit, which is the smallest well-defined geographic unit in Greater Accra. 2) The secondary sampling unit was the household in the enumeration areas. 3) The ultimate sampling unit was men between ages 50 and 74 years who resided in the selected households.
We first selected 300 enumeration areas randomly with probability proportional to the number of households in each enumeration area. We estimated that we would need to sample 7,500 households to identify about 1,000 men eligible for study. Thus, we selected 25 households randomly from each enumeration area, resulting in 7,500 households from Greater Accra. Door-to-door visits were made to enumerate all members of the selected household and identify men eligible for study. We selected the oldest eligible man in each household as the potential study participant. Based on these mechanisms we identified 1,049 men who were eligible for study, of whom 3 were too ill to be screened and 9 refused to participate, yielding a 98.8% response.
Interview and Blood Collection
Consenting participants were brought to the Korle-Bu Hospital for an interview in person and a health examination. Trained interviewers used a structured questionnaire to elicit epidemiological information, including ethnicity, education, smoking, alcohol use, medical history, screening history, family history of cancer and medical care system utilization. Height, weight, and waist and hip circumferences were measured at the interview. Overnight fasting blood was collected from each participant before DRE. Collected blood was processed at a central laboratory in Korle-Bu Hospital within 4 hours of collection and stored at −70C.
Prostate Cancer Screening
We used DRE and serum PSA for prostate cancer screening in study participants between September 2004 and September 2006. DRE was performed by experienced Ghanaian urologists. Total PSA and fPSA were measured in duplicate at UCLA. Before January 2004 the Hybritech Tandem®-R PSA and fPSA assays were used. Because these assays were not available after 2004, we changed to the Access® 2 Hybritech PSA and fPSA assays. Parallel evaluation data between these 2 generations of assays were within 10%. Samples with PSA greater than 100 ng/ml were retested. The percent ratio of fPSA to total PSA was calculated using the formula, (fPSA/PSA) × 100.
TRUS Guided Biopsy
Men with total PSA 2.5 ng/ml or greater and/or positive DRE were offered TRUS guided biopsy at Korle-Bu Hospital. TRUS was also used to estimate prostate volume. PSAD was calculated by the formula, PSA/prostate volume. At 24 hours before and 5 days after biopsy the men received ciprofloxacin (500 mg) or Zinnat™ (cefuroxime 250 mg) twice daily to prevent infection. A short medical history was taken before biopsy to assess contraindications to the procedure. A total of 12, 17 to 19 mm biopsy cores were collected with 2 cores from each of 6 designated areas of the prostate. Any visible lesions were also taken. Each core was preserved in 10% formalin buffer solution for preparation of pathology slides.
Pathology Review
Diagnoses were made by a Ghanaian pathologist (YT) as the preliminary clinical decision. Hematoxylin and eosin stained biopsy sections (1 slide per biopsy core) were subsequently reviewed by Johns Hopkins University pathologists (AMDM and GJN) to confirm the diagnosis. Slides of patients identified as having benign prostatic hyperplasia by Ghanaian pathologists were also reviewed. Sections were examined for pathological features, including acute or chronic inflammation, basal cell hyperplasia, prostatic intraepithelial neoplasia, foci of glandular atypia and adenocarcinoma. Immunohistochemistry staining was done for high molecular weight cytokeratin in 2 cases to help establish the diagnosis. In each case a consensus diagnosis had to be reached by all 3 pathologists on all biopsies to render the final study diagnosis. Gleason scores were determined for each section with a diagnosis of carcinoma. When present, perineural invasion was noted. The Johns Hopkins University diagnosis was used as the gold standard and for analysis in this study.
Men confirmed to have prostate cancer or severe benign prostatic hyperplasia received treatment at Korle-Bu Hospital based on the standard clinical care protocol in Ghana. Posttreatment followup included PSA testing, physical examination, imaging and management. All patients with cancer will be followed for life.
Statistical Analysis
We used SUDAAN18 to calculate age specific prevalence rates per 100 screened men by adjusting sampling weights according to the survey design. Age adjusted prevalence rates were calculated as a weighted average of age specific prevalence rates. Weights were the proportion of subjects in the corresponding age groups of the IARC (International Agency for Research on Cancer) world standard population.19
RESULTS
Table 1 shows select characteristics of the 1,037 men in the study. The largest ethnic groups were Akan and Ga/Adangbe (72% combined). About 85% of the men were younger than 70 years, 77% had at least a middle school education, 66% had ever used alcohol and 43% had ever smoked cigarettes. Only 25 men (2.4%) reported ever having had a PSA test and 54 (5.2%) reported ever having had a DRE. Only 4.3% of the men had health insurance.
Table 1.
Select characteristics of 1,037 healthy men in Accra, Ghana
| No. Age (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. Pts (%) | 50–59 | 60–69 | 70–74 | |||||
| Overall | 1,037 | (100.0) | 507 | (100.0) | 374 | (100.0) | 156 | (100.0) |
| Age: | – | – | – | |||||
| 50–59 | 507 | (48.9) | ||||||
| 60–69 | 374 | (36.1) | ||||||
| 70–74 | 156 | (15.0) | ||||||
| Ethnic group: | ||||||||
| Akan | 347 | (33.5) | 206 | (40.6) | 101 | (27.0) | 40 | (25.6) |
| Ga/Adangbe | 399 | (38.5) | 164 | (32.3) | 164 | (43.9) | 71 | (45.5) |
| Ewe | 186 | (17.9) | 88 | (17.4) | 73 | (19.5) | 25 | (16.0) |
| Mole-Dagbani | 15 | (1.4) | 8 | (1.6) | 5 | (1.3) | 2 | (1.3) |
| Grussi | 8 | (0.8) | 4 | (0.8) | 3 | (0.8) | 1 | (0.6) |
| Hausa | 26 | (2.5) | 11 | (2.2) | 9 | (2.4) | 6 | (3.8) |
| Other | 55 | (5.3) | 25 | (4.9) | 19 | (5.1) | 11 | (7.1) |
| Education: | ||||||||
| None/primary | 186 | (17.9) | 54 | (10.7) | 82 | (21.9) | 50 | (32.1) |
| Junior/middle | 472 | (45.5) | 240 | (47.3) | 163 | (43.6) | 69 | (44.2) |
| Senior/secondary | 186 | (17.9) | 103 | (20.3) | 65 | (17.4) | 18 | (11.5) |
| Higher | 142 | (13.7) | 83 | (16.4) | 44 | (11.8) | 15 | (9.6) |
| Other | 40 | (3.9) | 20 | (3.9) | 16 | (4.3) | 4 | (2.6) |
| Alcohol use* | 688 | (66.3) | 337 | (66.5) | 254 | (67.9) | 97 | (62.2) |
| Smoking† | 445 | (42.9) | 208 | (41.0) | 174 | (46.5) | 63 | (40.4) |
| PSA screening history | 25 | (2.4) | 6 | (1.2) | 11 | (2.9) | 8 | (5.1) |
| DRE history | 54 | (5.2) | 15 | (3.0) | 28 | (7.5) | 11 | (7.1) |
| Sought medical care‡ | 834 | (80.4) | 395 | (77.9) | 300 | (80.2) | 139 | (89.1) |
| Has medical insurance | 44 | (4.3) | 20 | (4.0) | 15 | (4.1) | 9 | (5.9) |
Ever drank alcohol at least once per week for 6 months or longer.
Ever smoked at least 1 cigarette per day for 6 months or longer.
Sought medical care in last 5 years.
Table 2 shows the results of screening and biopsy. Of the 1,037 screened men 154 (14.9%) had positive DRE and 272 (26.2%) had PSA 2.5 ng/ml or greater, including 166 with PSA 4.0 gm/ml or greater. A total of 352 men (33.9%) had a positive screen result by PSA (2.5 ng/ml or greater) or DRE and were recommended for biopsy, of whom 307 (87%) returned for biopsy. Reasons why the other 45 men did not return for biopsy included death (7), too ill (9), moved out of Accra (7) and refusal (22). Of the 307 men who underwent biopsy 73 were confirmed to have prostate cancer, yielding a 7.0% screen detected prevalence of prostate cancer (6.6% after age adjustment to the world population). When PSA 4.0 ng/ml or greater was used as the cutoff for screening, the yield was 6.3% or 65 cases (age adjusted 5.8%). As expected, the prevalence of abnormal DRE or PSA increased with advancing age, as did the prevalence of screen detected prostate cancer. In men older than 70 years the prevalence reached 17%.
Table 2.
Screening and biopsy results in 1,037 healthy men in Accra, Ghana
| Age Group | ||||
|---|---|---|---|---|
| Overall | 50–59 | 60–69 | 70–74 | |
| No. pos DRE | 154 | 53 | 56 | 45 |
| No. PSA 2.5 ng/ml or greater group: | 272 | 72 | 123 | 77 |
| Pos DRE with/without PSA 2.5 ng/ml or greater | 352 | 112 | 149 | 91 |
| Biopsy | 307 | 98 | 132 | 77 |
| Prostate Ca/total No. screened (%) | 73/1,037 (7.03)* | 19/507 (3.72) | 27/374 (7.22) | 27/156 (17.26) |
| No. PSA 4.0 ng/ml or greater group: | 166 | 42 | 75 | 49 |
| Pos DRE with/without PSA 4.0 ng/ml or greater | 264 | 85 | 109 | 70 |
| Biopsy | 237 | 75 | 101 | 61 |
| Prostate Ca/total No. screened (%) | 65/1,037 (6.26)† | 16/507 (3.12) | 23/374 (6.15) | 26/156 (16.66) |
Crude and age adjusted prevalence 7.0% and 6.6%, respectively.
Crude and age adjusted prevalence 6.3% and 5.8%, respectively.
Table 3 shows the clinical characteristics of the 65 confirmed prostate cancer cases (PSA greater than 4.0 ng/ml or positive DRE). Of these men 61.5% had positive DRE at screening. PSA in cases ranged from 0.66 to 8,423 ng/ml (mean 202.6, median 12.1). In 60% of cases clinical stage was T2 or greater and in 65% Gleason score was 7 or greater. The prevalence of basal hyperplasia, acute inflammation, chronic inflammation and high grade prostatic intraepithelial neoplasia was 6.8%, 17.8%, 60.3% and 15.1%, respectively.
Table 3.
Select clinical characteristics of 65 patients with prostate cancer with PSA 4.0 ng/ml or greater and/or positive DRE in Accra, Ghana
| No. age (%): | |
| 50–59 | 16 (24.6) |
| 60–69 | 23 (35.4) |
| 70–74 | 26 (40.0) |
| No. pos DRE (%) | 40 (61.5) |
| No. ng/ml PSA (%): | |
| Less than 2.5 | 5 (7.7) |
| 2.5–3.9 | 3 (4.6) |
| 4.0–9.9 | 24 (36.9) |
| 10.0 or Greater | 33 (50.8) |
| Mean/median PSA (ng/ml) | 202.6/12.1 |
| Mean/median free PSA (ng/ml) | 14.4/1.7 |
| No. clinical stage (%): | |
| T1 | 13 (20.0) |
| T2 | 40 (61.5) |
| T2, T3 | 3 (4.6) |
| T3 | 5 (7.7) |
| T3, T4 | 3 (4.6) |
| T4 | 1 (1.5) |
| Gleason score (%): | |
| 6 | 23 (35.4) |
| 7 | 18 (27.7) |
| 8 | 11 (16.9) |
| 9 | 11 (16.9) |
| 10 | 2 (3.1) |
Table 4 shows mean prostate volume and PSAD by 3 levels of PSA in the 236 men who underwent prostate biopsy based on the screening criteria of PSA 4.0 ng/ml or greater and/or positive DRE. Men with higher PSA had a higher mean prostate volume and PSAD. Of men with PSA greater than 10 ng/ml mean PSAD was much higher in those with than without cancer.
Table 4.
Prostate volume and PSAD in men who underwent prostate biopsies based on screening criteria of PSA 4.0 ng/ml or greater and/or positive DRE in Accra, Ghana
| PSA (ng/ml) | Ca | No Ca |
|---|---|---|
| No. pts: | 65 | 171 |
| Less than 4 | 8 | 78 |
| 4–10 | 24 | 61 |
| Greater than 10 | 33 | 32 |
| Mean ± SD TRUS prostate vol (cc): | ||
| Less than 4 | 29.7 ± 12.9 | 26.5 ± 16.9 |
| 4–10 | 35.3 ± 17.3 | 44.6 ± 22.9 |
| Greater than 10 | 50.3 ± 27.1 | 59.3 ± 35 |
| Mean ± SD PSA density (ng/ml/cc): | ||
| Less than 4 | 0.07 ± 0.03 | 0.06 ± 0.04 |
| 4–10 | 0.23 ± 0.1 | 0.17 ± 0.09 |
| Greater than 10 | 6.38 ± 20.6 | 0.42 ± 0.35 |
DISCUSSION
In this population based study of more than 1,000 Ghanaian men randomly selected from Accra we found that the age adjusted, screen detected prevalence of prostate cancer was 6.6% using a PSA cutoff of 2.5 ng/ml and 5.8% using a 4.0 ng/ml cutoff. In the 65 confirmed cases with PSA 4.0 ng/ml or greater the mean PSA level was 202 ng/ml and greater than 60% of the cases showed clinically significant high grade tumors (clinical stage T2 or greater and Gleason 7 or greater). The findings further support the conclusion that these men were from a relatively unscreened population.
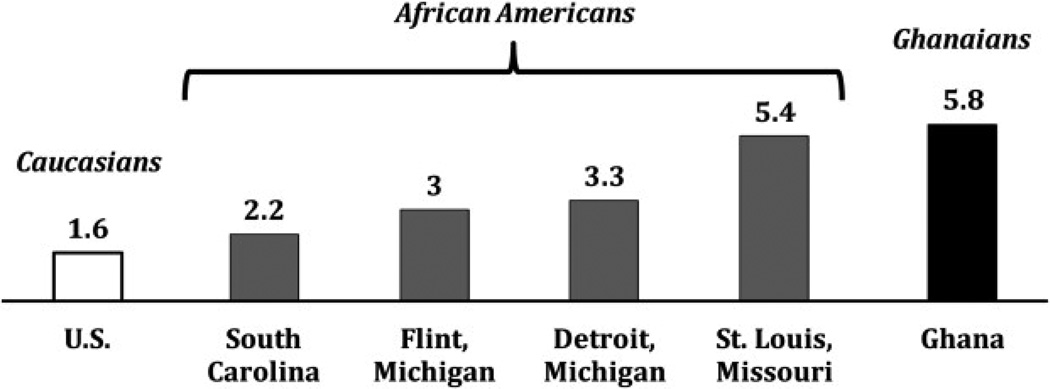
With the widespread use of PSA screening in the United States it is difficult to find data on black American men without a history of prostate cancer screening for comparison. Therefore, we compared our findings to data from several studies (see figure). Studies of black men, including populations in South Carolina and Michigan, showed an age adjusted screen detected prevalence of between 2.2% and 3.3%.20–23 In a study of a slightly older population in Missouri the reported prevalence was 5.4%.24 The prevalence observed in Ghanaian men was higher than in white American men of all age groups.25
Figure.
Screening detected prevalence of prostate cancer in white American men 50 years old or older,25 black American men 40 to 79 years old in 4 studies20–23 and 1,037 men 50 to 74 years old in Ghana. Screening criteria for all studies were PSA 4.0 ng/ml or greater and/or positive DRE.
Several strengths of the current study lend credibility to this attempt to estimate the true population prevalence of prostate cancer in Ghanaian men. These strengths include the probability sample of the population, the high participation rate in all study components, and the high quality PSA testing and pathology review. However, several factors have a substantial impact on prostate cancer detection and, thus, on the prevalence and reported incidence of prostate cancer. Most notably a history of PSA testing has the most profound impact on the reported prostate cancer prevalence. Of studies mentioning a history of prior testing the one with the lowest screen detected prostate cancer prevalence in black American men (2%) showed a relatively high prevalence of such a history (24%) (see figure).20 The low prevalence of screen identified cancer in this population in the United States results from the fact that some cancers in the population may have been identified by prior screening.
In Ghana the lack of screening is likely to explain much of the observation of a higher screen detected prevalence since PSA screening is uncommon in this country (2.4% in our study). Access to medical care also has an important role in cancer disparity among populations. To minimize the impact of unequal access to care we used a population based screening approach that provided equal medical care to all study participants. However, if screening or access to medical care in African men were similar to that in black or white American men, we would have seen a higher reported incidence than what was previously reported, including in an IARC monograph.14,15
The number of biopsies and the anatomical locations from which biopsies are taken also impact the prostate cancer detection rate since the more thorough the prostate examination, the higher the probability of detecting prostate cancer.26 To minimize this impact we used procedures similar to those used in the United States, that is TRUS guided biopsy from 6 areas of the prostate. Notably detecting the true prostate cancer prevalence in the population requires collecting the whole prostate as well as serial step sections of the prostate, which are usually only performed in autopsy studies and are not feasible in studies such as ours.
Although direct comparison of rates between populations is challenging due to the mentioned factors in our study, it is evident that prostate cancer is a much more common disease in West Africa than implied by reported registry incidence data14,15 and likely at a level quite comparable to that in black American men. In the last few years prostate cancer has become one of the more common cancers in men in Africa based on clinical data. It is likely that prostate cancer incidence rates in Africa are increasing and will be much higher if the level of medical care, prostate cancer screening and westernization is similar to that in Western countries.
Because West African and black American men 1) are at high risk for prostate cancer, 2) have different lifestyle factors and 3) share a similar genetic ancestry,27 our findings support the hypothesis that genetics may have an important role in prostate cancer etiology and in the disparity in prostate cancer risk between black and white American men. This is also consistent with the observation that a family history of prostate cancer is an important risk factor. Perhaps most notable in this regard is the exceptionally high heritability estimate for prostate cancer in studies of twins in which men who were twins of patients with prostate cancer were at 45% greater risk for prostate cancer than for any other common tumor.28
More recently genome-wide association studies identified more than 75 genomic locations containing common polymorphisms associated with prostate cancer risk.6–12 Notably a number of these newly identified genetic variants are more common in black than in white American men and several were first identified in an underpowered scan in black men.29 Currently to our knowledge the degree to which these genetic variants contribute to prostate cancer in African men and the amount of disease for which they may be responsible in African and black American men are unknown and warrant further research.
CONCLUSIONS
Our population based screening study shows that the prostate cancer prevalence is relatively high in West African men and likely comparable to that in black American men, suggesting a role of genetics in prostate cancer etiology and the disparity in prostate cancer risk between black and white American men. Future studies in West African and black men incorporating genetic and lifestyle data are needed to clarify further the role of these factors and their interplay in prostate cancer risk.
ACKNOWLEDGMENTS
Drs. William Catalona and Isaac Powell provided data. Evelyn Tay and Vicky Okyne coordinated the study. Violet Devairakkam, Norma Kim and John Heinrich, Research Triangle Institute, managed the study. Ann Truelove, Westat, provided study support and data management. Dr. B.J. Stone provided editorial help with the manuscript.
Supported by the National Institutes of Health Intramural Research Program (National Cancer Institute), National Center on Minority Health and Center to Reduce Cancer Disparities (National Cancer Institute).
Abbreviations and Acronyms
- DRE
digital rectal examination
- fPSA
free PSA
- PSA
prostate specific antigen
- PSAD
PSA density
- TRUS
transrectal ultrasound
Footnotes
Study received institutional review board approval.
Financial interest and/or other relationship with Gilead Sciences.
Financial interest and/or other relationship with Westat.
REFERENCES
- 1.Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 2.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Hsing AW, Devesa SS. Trends and patterns of prostate cancer: what do they suggest? Epidemiol Rev. 2001;23:3. doi: 10.1093/oxfordjournals.epirev.a000792. [DOI] [PubMed] [Google Scholar]
- 4.Ross R, Schottenfeld D. Prostate cancer. In: Schottenfeld D, Fraumeni J Jr, editors. Cancer Epidemiology and Prevention. 2nd ed. Oxford, United Kingdom: Oxford University Press; 1996. pp. 1180–1206. [Google Scholar]
- 5.Chu LW, Reichardt JK, Hsing AW. Androgens and the molecular epidemiology of prostate cancer. Curr Opin Endocrinol Diabetes Obes. 2008;15:261. doi: 10.1097/MED.0b013e3282febcf6. [DOI] [PubMed] [Google Scholar]
- 6.Amundadottir LT, Sulem P, Gudmundsson J, et al. A common variant associated with prostate cancer in European and African populations. Nat Genet. 2006;38:652. doi: 10.1038/ng1808. [DOI] [PubMed] [Google Scholar]
- 7.Yeager M, Orr N, Hayes RB, et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39:645. doi: 10.1038/ng2022. [DOI] [PubMed] [Google Scholar]
- 8.Yeager M, Chatterjee N, Ciampa J, et al. Identification of a new prostate cancer susceptibility locus on chromosome 8q24. Nat Genet. 2009;41:1055. doi: 10.1038/ng.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eeles RA, Kote-Jarai Z, Al Olama AA, et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet. 2009;41:1116. doi: 10.1038/ng.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haiman CA, Chen GK, Blot WJ, et al. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat Genet. 2011;43:570. doi: 10.1038/ng.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Mo Z, Ye D, et al. Genome-wide association study in Chinese men identifies two new prostate cancer risk loci at 9q31.2 and 19q13.4. Nat Genet. 2012;44:1231. doi: 10.1038/ng.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eeles RA, Olama AA, Benlloch S, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45 doi: 10.1038/ng.2560. 385, 391e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkin DM, Sitas F, Chirenje M, et al. Part I: cancer in indigenous Africans–burden, distribution, and trends. Lancet Oncol. 2008;9:683. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- 14.Chu LW, Ritchey J, Devesa SS, et al. Prostate cancer incidence rates in Africa. Prostate Cancer. 2011;2011:947870. doi: 10.1155/2011/947870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebbeck TR, Devesa SS, Chang B-L, et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of African descent. Prostate Cancer. 2013;2013:12. doi: 10.1155/2013/560857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chokkalingam AP, Yeboah ED, Demarzo A, et al. Prevalence of BPH and lower urinary tract symptoms in West Africans. Prostate Cancer Prostatic Dis. 2012;15:170. doi: 10.1038/pcan.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook M, Wang Z, Yeboah E, et al. A genome-wide association study of prostate cancer in West African men. Hum Genet. 2013;2013:1. [Google Scholar]
- 18.SUDAAN Language Manual, Release 9.0. Research Triangle Park: Research Triangle Institute; 2004. [Google Scholar]
- 19.Parkin DM, Whelan SL, Ferlay J, et al. Cancer Incidence in Five Continents. VIII. Lyon: International Agency for Research on Cancer Press; 2002. [Google Scholar]
- 20.Weinrich SP, Boyd MD, Bradford D, et al. Recruitment of African Americans into prostate cancer screening. Cancer Pract. 1998;6:23. doi: 10.1046/j.1523-5394.1998.1998006023.x. [DOI] [PubMed] [Google Scholar]
- 21.Cooney KA, Strawderman MS, Wojno KJ, et al. Age-specific distribution of serum prostate-specific antigen in a community-based study of African-American men. Urology. 2001;57:91. doi: 10.1016/s0090-4295(00)00873-6. [DOI] [PubMed] [Google Scholar]
- 22.Andriole GL, Levin DL, Crawford ED, et al. Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial: findings from the initial screening round of a randomized trial. J Natl Cancer Inst. 2005;97:433. doi: 10.1093/jnci/dji065. [DOI] [PubMed] [Google Scholar]
- 23.Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444. doi: 10.1016/j.juro.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Smith DS, Bullock AD, Catalona WJ, et al. Racial differences in a prostate cancer screening study. J Urol. 1996;156:1366. [PubMed] [Google Scholar]
- 25.Richie JP, Catalona WJ, Ahmann FR, et al. Effect of patient age on early detection of prostate cancer with serum prostate-specific antigen and digital rectal examination. Urology. 1993;42:365. doi: 10.1016/0090-4295(93)90359-i. [DOI] [PubMed] [Google Scholar]
- 26.Haas GP, Delongchamps NB, Jones RF, et al. Needle biopsies on autopsy prostates: sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst. 2007;99:1484. doi: 10.1093/jnci/djm153. [DOI] [PubMed] [Google Scholar]
- 27.Hellenthal G, Busby GBJ, Band G, et al. A genetic atlas of human admixture history. Science. 2014;343:747. doi: 10.1126/science.1243518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 29.Freedman ML, Haiman CA, Patterson N, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A. 2006;103:14068. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]