Abstract
We developed an automatic occlusion device for remote control of tumor tissue ischemia. The device consists of a flexible cannula encasing a shape memory alloy wire with its distal end connected to surgical suture. Regional tissue occlusion was tested on both the benchtop and the animal models. In the benchtop test, the occlusion device introduced quantitative and reproducible changes of blood flow in a tissue simulating phantom embedding a vessel simulator. In the animal test, the device generated a cyclic pattern of reversible ischemia in the right hinder leg tissue of a black male C57BL/6 mouse. We also developed a multimodal detector that integrates near infrared spectroscopy and electron paramagnetic resonance spectroscopy for continuous monitoring of tumor tissue oxygenation, blood content, and oxygen tension changes. The multimodal detector was tested on a cancer xenograft nude mouse undergoing reversible tumor ischemia. The automatic occlusion device and the multi-modal detector can be potentially integrated for closed-loop feedback control of tumor tissue ischemia. Such an integrated occlusion device may be used in multiple clinical applications such as regional hypoperfusion control in tumor resection surgeries and thermal ablation processes. In addition, the proposed occlusion device can also be used as a research tool to understand tumor oxygen transport and hemodynamic characteristics.
Keywords: Occlusion, Reperfusion, Ischemia, Tumor, Oxygenation, Blood flow, Oxygen tension, Near infrared spectroscopy, Electron paramagnetic resonance spectroscopy, Shape memory alloy wire
Introduction
Adequate blood supply provides necessary oxygen and nutrition to maintain biological tissue viability and normal metabolic processes. Ischemia represents the restriction of blood supply that may cause tissue hypoxia, metabolic waste build-up, system dysfunction, organ failure, and even patient death (1). Sudden restoration of blood circulation after a certain period of ischemia may result in acute inflammatory reaction, oxidative damage, and irreversible reperfusion injury (2).
Introduction of tissue ischemia is sometimes inevitable in oncological surgeries. For example, warm renal ischemia is used in partial nephrectomy to reduce blood loss and to provide a bloodless field for tumor excision (3). Warm renal ischemia is typically achieved by clamping the renal vessels to cut off the blood supply for global hypoperfusion. However, global hypoperfusion will also affect the viability of the whole kidney. Therefore, the tumor resection process has to be completed in less than 30 minutes in order to prevent irreversible damage of normal kidney tissue (4). Various regional hypoperfusion techniques, such as cable tie, tourniquet, and endoloop (5-7), were explored for the extended surgical time and the minimal ischemic damage of normal tissue. However, many of these techniques are only applicable to polar lesions. In addition, controlling these devices for a designated level of occlusion is very difficult. Inappropriate occlusion may cause either tissue hemorrhage or mechanical damage.
Adequate control of tumor tissue ischemia is also very important in cancer ablation therapies. Thermal ablation destroys tumor tissue by localized deposition or extraction of thermal energy that causes protein denaturation and coagulation necrosis (8, 9). Commonly used thermal ablation processes include laser ablation, radiofrequency ablation, microwave ablation, and focused ultrasound ablation. Previous in vivo and ex vivo ablation studies have demonstrated that vascular perfusion is among the most important factors contributing to the size and the shape of tissue necrosis (10-14). Therefore, appropriate control of the vascular perfusion has clinical significance. In the past, balloon catheters were used for temporary arterial occlusion during a thermal ablation process (10, 14, 15). A hydraulic occluder consisting of analog electronics and mechanical components was developed for repetitive coronary occlusion and myocardial ischemia (16). An inflatable silastic occluder driven by a motor syringe pump was used for acute and chronic regulation of renal perfusion (17). These methods are able to change the global vascular perfusion. However, regional control of tumor tissue ischemia can not be achieved with accuracy and reproducibility. Therefore, we are motivated to develop a low cost automatic occlusion device for remote control of tumor tissue ischemia.
Materials and Methods
Occlusion System Prototype
The automatic occlusion device consists of a flexible cannula encasing a Flexinol 100LT shape memory alloy wire (also called “muscle wire”, Images SI, Staten Island, New York) and a control box encasing driver circuits and electronics, as shown in Figure 1. A rotary potentiometer on the control box sets five different occlusion levels. At each occlusion level, the driver circuit applies a designated voltage to the shape memory alloy wire that consequently tightens or releases the surgical suture tied at the distal end of the muscle wire. The muscle wire is 50 cm long and is able to shrink by up to 1.5cm (3%) upon 12 volt DC power.
Figure 1.
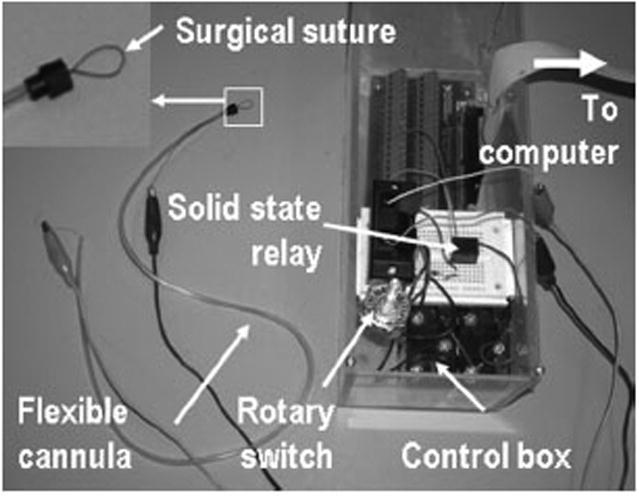
The prototype of the automatic occlusion device. The device comprises a flexible cannula encasing a shape memory alloy wire and a control box encasing a rotary switch, a solid state relay, and a driver circuit board connected to a computer. The distal end of the automatic occlusion device is connected to a surgical suture.
Figure 2 sketches the driver circuit for the occlusion device. A potentiometer switch sets five resistance levels for the shrinkage control of the muscle wire. A laptop computer with a DAQ-700 data acquisition card (National Instruments, Austin, Texas) was used to trigger a solid state relay (OUAZ-SS-105D 900, Tyco Electronics, Wilmington, Delaware) and activate the occlusion. A Labview program (National Instruments, Austin, Texas) was developed to synchronize occlusion with other data acquisition tasks.
Figure 2.
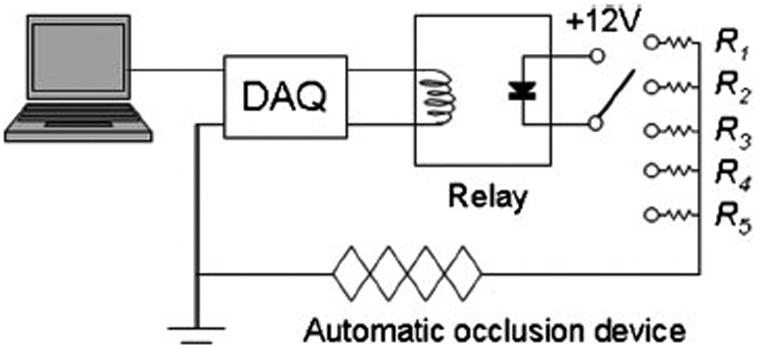
Schematic of the control circuit for the automatic occlusion device. The computer is used to control the digital output of a data acquisition card to activate the solid state relay. The rotary switch allows for the selection of five resistors corresponding to different occlusion voltage levels.
Benchtop Validation
The automatic occlusion device was quantitatively validated on a benchtop setup as shown in Figure 3. A tissue simulating phantom was made from VytaFlex 60 urethane rubber (Smooth-On, Easton, Pennsylvania). A soft silicon tube was embedded in the phantom to simulate a blood vessel. Tissue ischemia was simulated by using a surgical suture to ligate a portion of the tissue simulating phantom around the embedded vessel. The ends of the surgical suture were connected to the automatic occlusion device. Diluted porcine whole blood (1:40) was circulated by a peristaltic pump and the flow rate was monitored by a MoorLAB laser Doppler flow meter (Moor Instruments, Wilmington, Delaware). A pressure release bypass in the blood circuit prevents the pressure accumulation and vessel rupture during ligation. Once a stable blood circulation was established in the tissue simulating block, the rotary potentiometer was switched to set different ligation voltages. The corresponding blood flow changes were recorded by the laser Doppler flow meter.
Figure 3.
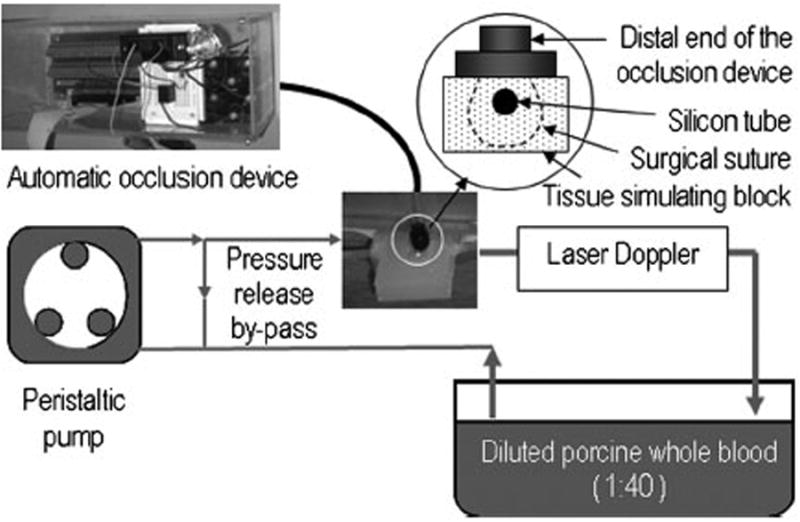
Experimental setup for benchtop validation of the automatic occlusion device. The enlarged sketch shows how the silicon tube and the surrounding tissue simulating material are ligated by the surgical suture that is connected to the distal end of the occlusion device.
Animal Validation
The experimental setup for animal validation tests is shown in Figure 4. The femoral artery, the femoral vein, and the surrounding muscle tissue of the right leg of a male C57BL/6 mouse were cyclically ligated by the automatic occlusion device. A fiber optical sensor head continously monitored the leg tissue oxygen saturation ([StO2]) during the cyclic ligation. Optical fibers from the sensor head were connected to an OxiplexTS tissue spectrophotometer (ISS, Champaign, Illinois). Due to viscoelastic relaxation and vascular regulation of in vivo biological tissue, it is difficult to establish a stable level of tissue ischemia at the designated occlusion voltage. Therefore, a fixed occlusion voltage (12V) was used in this in vivo experiment.
Figure 4.
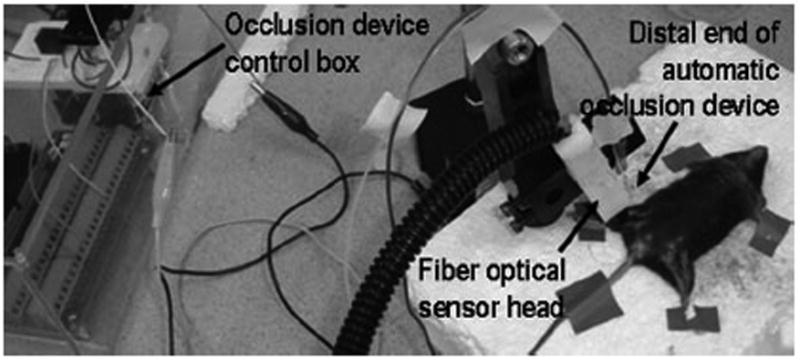
Experimental setup for femoral vessel ligation on a black male C57BL/6 mouse.
Noninvasive detection of tumor ischemia and reperfusion
Maintaining a consistent level of ischemia is difficult in an in vivo tissue system because of various physical and physiologic changes introduced by mechanical preconditioning, viscoelastic relaxation, and vascular regulation. Therefore, continuous monitoring and feedback control of tissue ischemia is necessary. We developed a multimodal detector for continuous detection of multiple tissue parameters, such as [StO2], blood flow, and oxygen tension (pO2), as shown in Figure 5. The sensor head comprises multiple optical fibers connected to a home-made tissue oximeter for [StO2] measurement, an optical fiber connected to a MoorLAB laser Doppler blood flow meter for blood flow measurement, and an electron paramagnetic resonance spectroscopic (EPRS) surface coil resonator for pO2 measurement. The multimodal detector was tested using a female athymic nude mouse with xenograft pancreatic cancer implanted on the right leg. Reversible tumor ischemia was achieved by ligating the right leg of the mouse for over 20 minutes and then releasing the ligation. Tumor tissue [StO2], blood flow, and pO2 were measured by the home-made tissue oximeter (sampling rate every 2 seconds), the laser Doppler meter (sampling rate 10Hz), and the EPRS system (sampling rate every 30 seconds) respectively after tumor ischemia and reperfusion. The achievable measurement depths for tissue [StO2], blood flow, and pO2 vary with the detection systems. In our design, the home-made tissue oximeter has a measurement depth from several millimeters to a couple of centimeters, depending on the source-detector separation distance. The laser Doppler blood flow meter has a measurement depth of a couple of millimeters (an ultrasound Doppler system may be used for the greater measurement depth). The EPRS system has a measurement depth ranging from millimeters to centimeters, depends on the location of the injected oxygen-sensitive lithium phthalocyanine probe.
Figure 5.
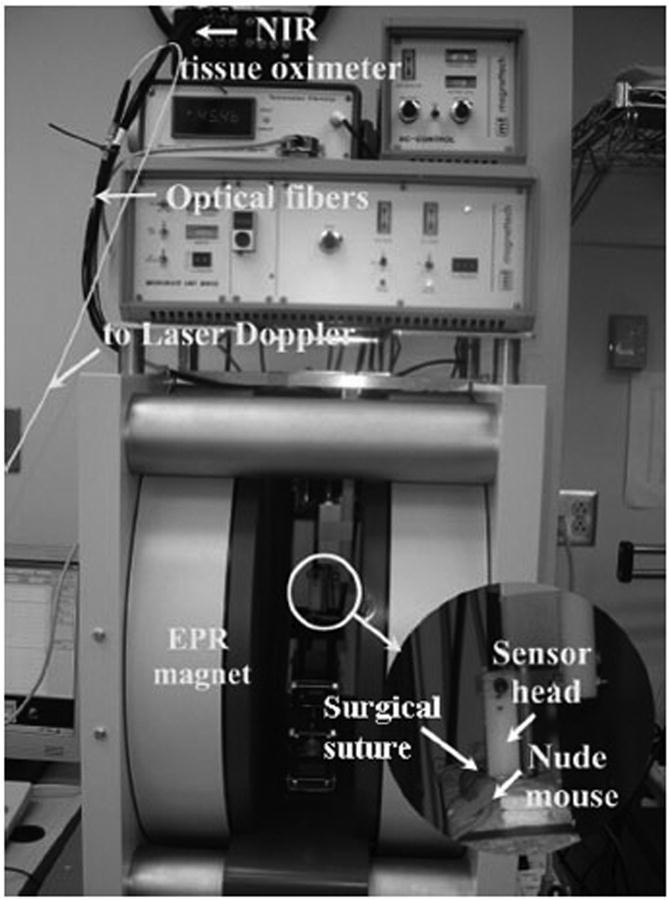
Simultaneous monitoring of tumor oxygen and blood flow dynamics during reversible tumor ischemia on a xenograft nude mouse.
The home-made tissue oximeter is a continuous wave (CW) near infrared (NIR) tissue oximeter using two laser sources (each combines two wavelengths of 690nm and 830nm) and four photodiode detectors for tissue [StO2] measurement (18). The tissue oximeter was designed following a pre-commercial oximeter prototype we developed several years ago (19). The pre-commercial oximeter prototype was validated on both blood-lipid phantoms and isolated canine limbs within a full range of [StO2] levels (19). Linear correlation was observed between the tissue oximeter measurements and the invasive gas analyzer measurements of blood oxygenation (19). Compared with time domain and frequency domain tissue oximeters, a CW tissue oximeter has advantages of low cost and simplicity. However, a CW tissue oximeter can not determine the absolute tissue [StO2] levels since it can not measure tissue scattering characteristics. In this paper, we used a CW tissue oximeter to monitor relative changes of tissue [StO2] during ischemia and reperfusion.
Results and Discussion
Figure 6 plots the measured flow rate versus the occlusion voltage for the benchtop experiment as shown in Figure 3. The occlusion voltage was set by selecting different resistance levels of the rotary potentiometer switch. The resultant flow rate is linearly correlated with the occlusion voltage (correlation coefficient R2 = 0.996) and the occlusion performance is reproducible. The benchtop results indicate that the proposed automatic occlusion device is able to control the flow rate of a vessel simulator reliably.
Figure 6.
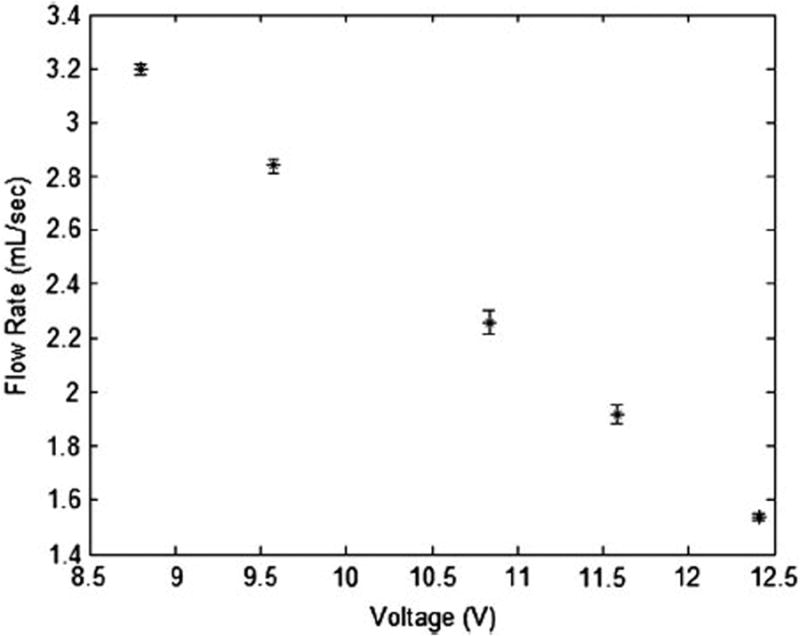
Correlation between the occlusion voltage applied to the automatic occlusion device and the resulting blood flow rate in a vessel simulator.
In the animal validation test as shown in Figure 4, reversible tissue ischemia was achieved by cyclic ligation at a duration of 120 second and a duty cycle of 50% (Figure 7). The cyclic ligation introduced up to 10% changes in tissue [StO2]. After the ligation was removed, tissue [StO2] resumed the base-line level, indicating no permanent effect on tissue viability. Since it is difficult to maintain a stable [StO2] level at different ischemic plateaus on an in vivo tissue system, the ligation test was carried out at a fixed occlusion voltage only. The animal validation results indicate that the proposed automatic occlusion device is able to introduce cyclically reproducible ischemia in an in vivo tissue system.
Figure 7.
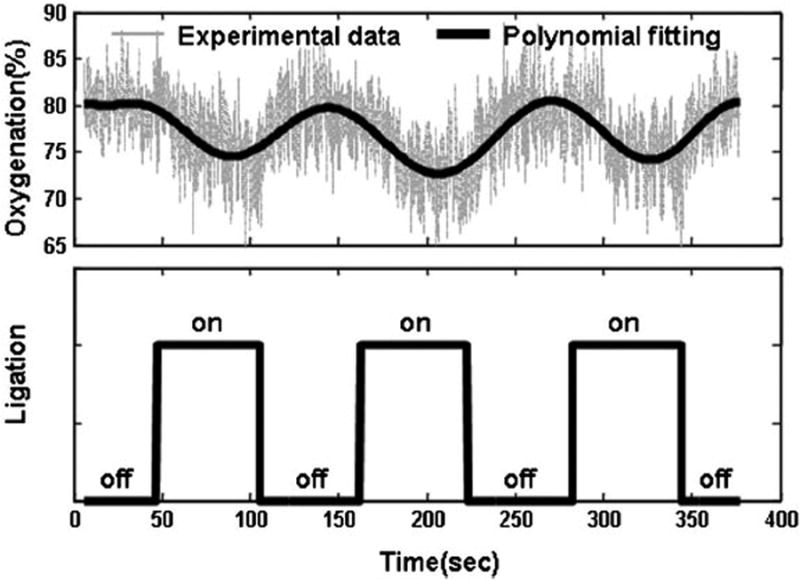
Tissue oxygenation measured on the right leg of a black male C57BL/6 mouse during the successive femoral vessel ligation. The top figure shows the tissue oxygenation history during repetitive ligation. The grey data points in the background are actual oxygenation measurements and the thick solid line in the foreground is the 12th order least square polynomial fitting of the experimental data. The bottom figure shows the ligation status. “On” represents the status that the occlusion voltage was applied. “Off” represents no ligation. At the “on” status, the vessel ligation caused tissue ischemia and the reduction of tissue oxygenation. At the “off” status, tissue oxygenation resumes back to its original level.
Figure 8 plots tumor tissue [StO2], blood flow, and pO2 levels as well as their deviations during ischemia and reperfusion. The ischemic data were continuously acquired for 3 minutes after ligating the tumor for 20 minutes. The reperfusion data were continuously acquired for 3 minutes after releasing the ligation for 20 minutes. Descriptive statistics were used to calculate the averaged tumor tissue [StO2], blood flow, and pO2 levels as well as their deviations during the 3-minute ischemia and the 3-minute reperfusion processes. According to the figure, the ischemic tissue had an averaged [StO2] level of 34.0 ± 6.8% and an averaged pO2 level of 3.8 ± 0.7 mmHg. After reperfusion, the [StO2] level increased to 99.0 ± 1.0% and the pO2 level increased to 11.3 ± 0.9 mmHg. The blood flow level also increased from 0.117 ± 0.07 (a.u.) to 0.153 ± 0.019 (a.u.) after reperfusion. The paired Student's t-test showed that tissue [StO2], blood flow, and PO2 changes during ischemia and reperfusion were statistically significant (P value < 0.001). Since the changes of tumor tissue [StO2], blood flow, and pO2 levels were only measured on one cancer xenograft nude mouse, they may not represent the typical ranges of ligation-induced tumor tissue property changes. Nevertheless, Figure 8 suggests that the multi-modal detector described in this paper may provide effective measurements of tumor tissue oxygen and perfusion during reversible tissue ischemia. Extensive animal studies are required for better understanding of ligation-induced tumor tissue changes.
Figure 8.
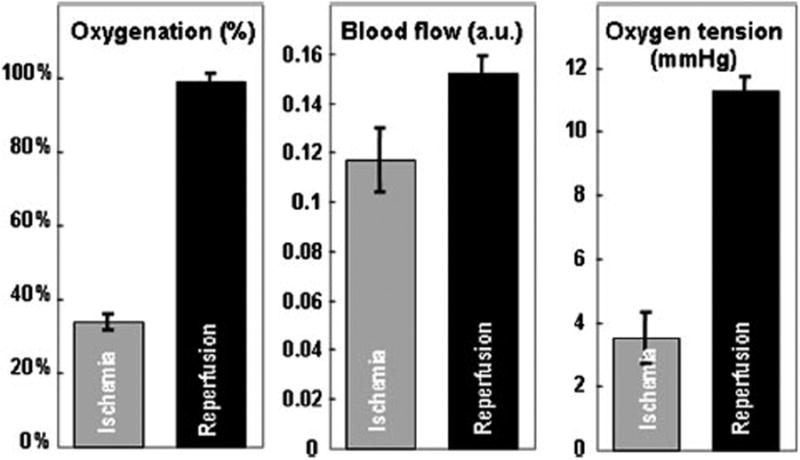
Relative changes of tumor tissue oxygenation, blood flow, and oxygen tension during ischemia and reperfusion on the xenograft nude mouse. The Student's paired t-Test shows that ligation significantly reduced tumor tissue oxygenation, blood flow, and oxygen tension (P <0.001).
In summary, we have developed an automatic occlusion device for remote control of reversible tissue ischemia. The automatic occlusion device was tested on both benchtop and animal models. In the benchtop test, the occlusion device introduced quantitative and reproducible changes of blood flow in a tissue simulating phantom embedding a vessel simulator. In the animal test, the occlusion device generated a cyclic pattern of reversible ischemia in the right leg of a black male C57BL/6 mouse. We also developed a multimodal detector for continuous monitoring of tumor tissue [StO2], blood flow, and pO2 changes. The multimodal detector was tested on a cancer xenograft nude mouse. The automatic occlusion device and the multimodal detector we developed have the potential to be integrated into one system for closed-loop feedback control of tumor tissue ischemia.
The work described in this report demonstrated the technical potential of using the shape memory alloy material for remote quantitative control of reversible tissue ischemia. Further developmental works are required before the proposed automatic occlusion device can be practically used in cancer ablation procedures or oncological surgeries for quantitative control of tumor tissue ischemia. First of all, since consistent tissue ischemia can not be maintained at a fixed occlusion voltage in an in vivo tissue system, it is necessary to control the ligation condition in real-time based on the multimodal feedback measurements of tissue ischemia. The closed-loop feedback control of the tissue ligation condition will also help to prevent the collateral injury to the ligated tissue. Second, the surgical suture tied at the end of the occlusion device may be replaced by an endoloop or a laparoscopic tool for better clinical usability. Finally, the occlusion device needs to be encased in an appropriate cannula to protect the surrounding tissue from possible resistance heating of the shape memory alloy wire and to provide maximal mechanical flexibility.
In addition to therapeutic applications, the proposed automatic occlusion device may also be used as a research tool to understand tumor oxygen transport and hemodynamic characteristics. Many solid tumors are hypoxic and angiogenic (20-22). Previous clinical studies have suggested that malignant tumors may be associated with greater vascularization and lower [StO2] (23-25). However, clinical reliability of these criteria is still debatable, partially due to the intratumoral heterogeneity in oxygen distribution and vascularization (26), and is further complicated by tissue structural heterogeneity, inter-patient differences, and hormone changes (27, 28). To minimize the effect of these variations, we proposed a dynamic schema for noninvasive characterization of relative changes of tumor tissue functional properties in response to external stimuli (29, 30). This dynamic schema is based on the hypothesis that tumor tissue can be differentiated by their hemodynamic characteristics associated with vascular leakiness, interstitial hypertension, and increased flow resistance (21, 31). In the past, we used a stepped compression load as an external stimulation to study tumor dynamic responses (32). The mechanical compression introduces simultaneous tissue deformation and blood redistribution secondary to tissue physiologic responses. Consequently, accurate interpretation of pressure-induced tissue dynamics presents a major technical challenge (33). In this regard, an automatic occlusion device may provide a clean stimulation that uncouples tissue metabolic dynamics from tissue mechanical deformation. By introducing reversible ischemia to the tumor tissue and monitoring tissue [StO2], blood flow, and pO2 responses simultaneously, it is possible to develop mathematical models for quantitative understanding of tumor tissue metabolic reactions and transport mechanisms during stepped ischemia and reperfusion processes (34).
Acknowledgments
The authors are grateful to Dr. Jay Zweier of Davis Heart and Lung Research Institute at The Ohio State University for his technical guidance and support.
References
- 1.Leach RM, Treacher DF. Oxygen transport-2. Tissue hypoxia. BMJ. 1998;317:1370–1373. doi: 10.1136/bmj.317.7169.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–2989. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- 3.Weise ES, Winfield HN. Laparoscopic partial nephrectomy. J Endourol. 2005;19:634–642. doi: 10.1089/end.2005.19.634. [DOI] [PubMed] [Google Scholar]
- 4.Novick AC. Renal hypothermia: in vivo and ex vivo. Urol Clin North Am. 1983;10:637–644. [PubMed] [Google Scholar]
- 5.Cadeddu JA, Corwin TS, Traxer O, Collick C, Saboorian HH, Pearle MS. Hemostatic laparoscopic partial nephrectomy: cable-tie compression. Urology. 2001;57:562–566. doi: 10.1016/s0090-4295(00)01009-8. [DOI] [PubMed] [Google Scholar]
- 6.Gill IS, Munch LC, Clayman RV, McRoberts JW, Nickless B, Roemer FD. A new renal tourniquet for open and laparoscopic partial nephrectomy. J Urol. 1995;154:1113–1116. [PubMed] [Google Scholar]
- 7.Beck SD, Lifshitz DA, Cheng L, Lingeman JE, Shalhav AL. Endoloop-assisted laparoscopic partial nephrectomy. J Endourol. 2002;16:175–177. doi: 10.1089/089277902753716151. [DOI] [PubMed] [Google Scholar]
- 8.VanSonnenberg E, McMullen WN, Solbiati L, Livraghi T, Mueller PR, Silverman SG. Tumor ablation : principles and practice. Springer Science+Business Media; New York: 2005. [Google Scholar]
- 9.Le Pivert PJ, Morrison DR, Haddad RS, Renard M, Aller A, Titus K, Doulat J. Percutaneous tumor ablation: microencapsulated echo-guided interstitial chemotherapy combined with cryosurgery increases necrosis in prostate cancer. Technol Cancer Res Treat. 2009;8:207–216. doi: 10.1177/153303460900800305. [DOI] [PubMed] [Google Scholar]
- 10.Aschoff AJ, Sulman A, Martinez M, Duerk JL, Resnick MI, MacLennan GT, Lewin JS. Perfusion-modulated MR imaging-guided radiofrequency ablation of the kidney in a porcine model. AJR Am J Roentgenol. 2001;177:151–158. doi: 10.2214/ajr.177.1.1770151. [DOI] [PubMed] [Google Scholar]
- 11.Bitsch RG, Dux M, Helmberger T, Lubienski A. Effects of vascular perfusion on coagulation size in radiofrequency ablation of ex vivo perfused bovine livers. Invest Radiol. 2006;41:422–427. doi: 10.1097/01.rli.0000201231.60420.a2. [DOI] [PubMed] [Google Scholar]
- 12.Chang I, Mikityansky I, Wray-Cahen D, Pritchard WF, Karanian JW, Wood BJ. Effects of perfusion on radiofrequency ablation in swine kidneys. Radiology. 2004;231:500–505. doi: 10.1148/radiol.2312021248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denys AL, De Baere T, Mahe C, Sabourin JC, Sa Cunha A, Germain S, Roche A. Radio-frequency tissue ablation of the liver: effects of vascular occlusion on lesion diameter and biliary and portal damages in a pig model. Eur Radiol. 2001;11:2102–2108. doi: 10.1007/s003300100973. [DOI] [PubMed] [Google Scholar]
- 14.Kariya Z, Yamakado K, Nakatuka A, Onoda M, Kobayasi S, Takeda K. Radiofrequency ablation with and without balloon occlusion of the renal artery: an experimental study in porcine kidneys. J Vasc Interv Radiol. 2003;14:241–245. doi: 10.1097/01.rvi.0000058327.82956.f1. [DOI] [PubMed] [Google Scholar]
- 15.Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchiano A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V, Blum HE, Bartolozzi C. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 16.Rys R, LaDisa JF, Jr, Tessmer JP, Gu W, Kersten JR, Warltier DC, Pagel PS. An automated coronary artery occlusion device for stimulating collateral development in vivo. J Pharmacol Toxicol Methods. 2002;48:111–118. doi: 10.1016/S1056-8719(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 17.Hester RL, Granger JP, Williams J, Hall JE. Acute and chronic servo-control of renal perfusion pressure. Am J Physiol. 1983;244:F455–460. doi: 10.1152/ajprenal.1983.244.4.F455. [DOI] [PubMed] [Google Scholar]
- 18.Xu RX, Povoski SP. Diffuse optical imaging and spectroscopy for cancer. Expert Rev Med Devices. 2007;4:83–95. doi: 10.1586/17434440.4.1.83. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Zhu W, Padival V, Xia M, Cheng X, Bush R, Christenson L, Chan T, Doherty T, Iatridis A. Validation of NIRS In Measuring Tissue Hemoglobin Concentration And Oxygen Saturation Using Benchtop and Isolated Limb Model. Proc SPIE. 2003;4955:369–378. [Google Scholar]
- 20.Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9 Suppl 5:4–9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- 21.Boucher Y, Leunig M, Jain RK. Tumor angiogenesis and interstitial hypertension. Cancer Res. 1996;56:4264–4266. [PubMed] [Google Scholar]
- 22.Myhr G. Multimodal cancer treatment: real time monitoring, optimization, and synergistic effects. Technol Cancer Res Treat. 2008;7:409–414. doi: 10.1177/153303460800700510. [DOI] [PubMed] [Google Scholar]
- 23.Chance B, Nioka S, Zhang J, Conant EF, Hwang E, Briest S, Orel SG, Schnall MD, Czerniecki BJ. Breast cancer detection based on incremental biochemical and physiological properties of breast cancers: a six-year, two-site study. Acad Radiol. 2005;12:925–933. doi: 10.1016/j.acra.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Ntziachristos V, Yodh AG, Schnall MD, Chance B. MRI-guided diffuse optical spectroscopy of malignant and benign breast lesions. Neoplasia. 2002;4:347–354. doi: 10.1038/sj.neo.7900244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munaron L, Tomatis C, Pla AF. The secret marriage between calcium and tumor angiogenesis. Technol Cancer Res Treat. 2008;7:335–339. doi: 10.1177/153303460800700408. [DOI] [PubMed] [Google Scholar]
- 26.Wilson DF, Evans SM, Jenkins WT, Vinogradov SA, Ong E, Dewhirst MW. Oxygen distributions within R3230Ac tumors growing in dorsal flap window chambers in rats. Adv Exp Med Biol. 1998;454:603–609. doi: 10.1007/978-1-4615-4863-8_71. [DOI] [PubMed] [Google Scholar]
- 27.Shah N, Cerussi AE, Jakubowski D, Hsiang D, Butler J, Tromberg BJ. Spatial variations in optical and physiological properties of healthy breast tissue. J Biomed Opt. 2004;9:534–540. doi: 10.1117/1.1695560. [DOI] [PubMed] [Google Scholar]
- 28.Pogue BW, Jiang S, Dehghani H, Kogel C, Soho S, Srinivasan S, Song X, Tosteson TD, Poplack SP, Paulsen KD. Characterization of hemoglobin, water, and NIR scattering in breast tissue: analysis of intersubject variability and menstrual cycle changes. J Biomed Opt. 2004;9:541–552. doi: 10.1117/1.1691028. [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Povoski SP, Cao X, Sun D, Xu RX. Dynamic schema for near infrared detection of pressure-induced changes in solid tumors. Appl Opt. 2008;47:3053–3063. doi: 10.1364/ao.47.003053. [DOI] [PubMed] [Google Scholar]
- 30.Xu RX, Young DC, Mao JJ, Povoski SP. A prospective pilot clinical trial evaluating the utility of a dynamic near-infrared imaging device for characterizing suspicious breast lesions. Breast Cancer Res. 2007;9:R88. doi: 10.1186/bcr1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002;62:5381–5385. [PubMed] [Google Scholar]
- 32.Xu RX, Ewing J, El-Dahdah H, Wang B, Povoski SP. Design and benchtop validation of a handheld integrated dynamic breast imaging system for noninvasive characterization of suspicious breast lesions. Technol Cancer Res Treat. 2008;7:471–482. doi: 10.1177/153303460800700609. [DOI] [PubMed] [Google Scholar]
- 33.Wang B, Povoski S, Cao X, Sun D, Xu R. Dynamic schema for near infrared detection of pressure-induced changes in solid tumors. Applied Optics. 2008;47:3053–3063. doi: 10.1364/ao.47.003053. [DOI] [PubMed] [Google Scholar]
- 34.Saidel GM, DiBella JA, Cabrera ME. Metabolic system dynamics: lumped and distributed models. In: Arnez ZM, editor. Simulations in Biomedicine. WIT Press; 2003. pp. 100–110. [Google Scholar]


