Abstract
Background
Incidental pancreatic cysts are common, a small number of which are premalignant or malignant. Multidisciplinary care has been shown to alter management and improve outcomes in many types of cancers, but its role has not been examined in patients with pancreatic cysts. We assessed the effect of a multidisciplinary pancreatic cyst clinic (MPCC) on the diagnosis and management of patients with pancreatic cysts.
Methods
The referring institution and MPCC diagnosis and management plan were recorded. Patient were placed into one of five categories—no, low, intermediate, or high risk of malignancy within the cyst, and malignant cyst—on the basis of their diagnosis. Patients were assigned one of four management options: surveillance, surgical resection, further evaluation, or discharge with no further follow-up required. The MPCC was deemed to have altered patient care if the patient was assigned a different risk or management category after the MPCC review.
Results
Referring institution records were available for 262 patients (198 women; mean age 62.7 years), with data on risk category available in 138 patients and management category in 225. The most common diagnosis was branch duct intraductal papillary mucinous neoplasm. MPCC review altered the risk category in 11 (8.0%) of 138 patients. The management category was altered in 68 (30.2%) of 225 patients. Management was increased in 52 patients, including 22 patients who were recommended surgical resection. Management was decreased in 16 patients, including 10 who had their recommendation changed from surgery to surveillance.
Conclusions
MPCC is helpful and alters the management over 30% of patients.
Incidental pancreatic cysts are common diagnoses, with asymptomatic cysts identified in 2.6 % of individuals undergoing abdominal computed tomographic (CT) scan.1 Pancreatic cysts represent a spectrum of disease ranging from benign to malignant lesions, which include both inflammatory and neoplastic processes. Although the majority of pancreatic cysts are benign, certain types are either precursors to malignancy or occur in association with a malignancy.
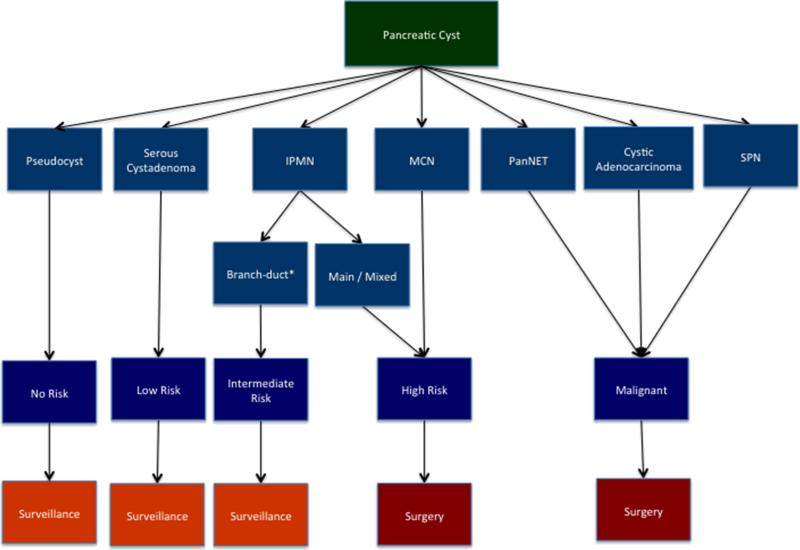
Each type of cyst is associated with unique biology and a different risk of malignancy (Fig. 1). Inflammatory cysts, or pseudocysts, are the sequelae of acute pancreatitis and have no malignant potential. In contrast, the risk of malignancy in cystic neoplasms varies greatly. Serous cystadenoma (SCA) have essentially no malignant potential, while mucin-producing neoplasms are precursors to invasive ductal adenocarcinoma, and their risk of malignancy depends on certain features. The risk is considered intermediate for most branch duct intraductal papillary mucinous neoplasms (IPMN) and high for main duct IPMN, branch duct IPMN with a solid component, mixed type IPMN, and mucinous cystic neoplasms (MCN). Finally, some pancreatic neoplasms can present as cysts, such as solid pseudopapillary neoplasm and cystic pancreatic neuroendocrine neoplasm. Patients with pancreatic cysts of a low or intermediate risk of malignant transformation are suitable for surveillance, whereas those with high-risk lesions or those with malignant cysts (cystic degeneration of an adenocarcinoma, invasive IPMN, pancreatic neuroendocrine tumors, or solid pseudopapillary tumors) should undergo surgical resection.2,3
FIG. 1.
Management of pancreatic cysts. Risk of malignant transformation of cysts depends on type of cyst. Management of pancreatic cysts is based on determining risk of malignant transformation. Those with no, low, or intermediate risk can be followed with surveillance, while surgery should be considered in cysts that have either high risk of malignant transformation or that appear malignant. IPMN intraductal papillary mucinous neoplasm, MCN mucinous cystic neoplasm, PanNET pancreatic neuroendocrine tumor, SPN solid pseudopapillary neoplasm
The management of patients with a pancreatic cyst greatly relies on determining the type of the cyst. The determination of cyst type is made on the basis of clinical information, imaging characteristics, and cyst fluid analysis. The accuracy of making this determination is limited by the lack of definitive markers of each cyst type, and a wrong diagnosis is made in a significant number of patients. This is evidenced by the fact the over 20 % of resected pancreatic cysts are found to be benign.4 The management of patients with cystic neoplasms is complex and has the potential to benefit from input by multiple disciplines.
Multidisciplinary care has been shown to alter management and improve outcomes in many types of cancers.5–7 Both the Commission on Cancer and the American College of Surgeons require multidisciplinary conferences for the accreditation of cancer centers delivering multidisciplinary care. In patients with pancreatic cancer, a multidisciplinary clinic has been observed to alter management in over 20 % of patients.8 However, to our knowledge, there are no studies reporting the effect of a multidisciplinary clinic on the management of patients with pancreatic cysts.
A multidisciplinary outpatient clinic dedicated exclusively to patients with pancreatic cysts was established at our institution in November 2010. The purpose of the clinic is to provide a comprehensive multispecialty evaluation for patients with pancreatic cysts. The aim of this study was to assess the effect of this multidisciplinary clinic on the diagnosis and management of patients with pancreatic cysts.
METHODS
The study was approved by the institutional review board for human research and complied with Health Insurance Portability and Accountability Act regulations.
Multidisciplinary Pancreatic Cyst Clinic
The multidisciplinary pancreatic cyst clinic (MPCC) is conducted on a weekly basis. The MPCC sees both new and return patients with pancreatic cysts. Patients are first assessed by a gastroenterologist and pancreatic surgeon, with the physicians and trained pancreatic cyst clinic advanced practitioners (physician assistant and nurse practitioner) providing education on the various types of pancreatic cysts and their management. Each case is then presented at a multidisciplinary conference that consists of five pancreatic surgeons, CT and magnetic resonance imaging (MRI) radiologists, pancreatic pathologists, cytopathologist, and a gastroenterologist who is an expert pancreatic endosonographer. The patients’ clinical history, radiological imaging, endoscopic ultrasound, cytology, and cyst fluid analysis are reviewed. The CT and MRI images are reviewed by a pancreatic CT and MRI radiologist, while a cytopathologist presents any cases in which there is marked atypia or a suspicion of malignancy. In addition, the patient's goals and preferences are presented to the group. A consensus diagnosis is made on the basis of review of the imaging features, cyst fluid analysis, and cytology, and treatment recommendations are provided.
The management of pancreatic cysts is one of the following: no further follow-up, surveillance, or surgical resection based on the estimated risk of malignant transformation. The management recommendations usually follow the International Consensus Criteria for the management of pancreatic cysts.2,3 These guidelines recommend consideration of surgical resection in patients with main duct IPMN, patients with an enhancing solid component within the cyst, or those presenting with obstructive jaundice resulting from a pancreatic cyst.2,3 In addition, patients who have a definite mural nodule, a main pancreatic duct measuring 5 to 9 mm but with features suspicious for main duct involvement, or those in which the cyst fluid cytology is suspicious or positive for malignancy should be considered for surgical resection.2 In the original guidelines, surgical resection was recommended for all branch duct IPMN that measured over 3 cm3; however, according to the 2012 guidelines, surveil-lance can be considered, particularly if the patient is older or is a poor surgical candidate.2 The most recent guidelines and surveillance intervals are shown in Supplementary Fig. 1.
Patients
We included in our analysis all new referrals to the MPCC. These included patients with a known pancreatic cyst diagnosed at another institution but newly referred to the MPCC as well as patients with a new diagnosis of a pancreatic cyst made at our institution. The referring clinic and MPCC records of consecutive patients assessed at the MPCC from November 2010 to December 2011 were reviewed. Patients who did not undergo surgical resection were followed until November 2013. Patients with no referring records were excluded from the study.
Effect of MPCC Review on Patient Diagnosis and Risk of Malignant Transformation
The referring and MPCC diagnoses were recorded. Referring diagnoses were placed in one of five different categories on the basis of the risk of malignancy or progression to malignancy of the pancreatic cyst (Fig. 1). Cysts such as pseudocysts and patients referred with cystic lesions on outside imaging that on review were judged to be noncystic pancreatic lesions such as a focal fat deposition were considered to harbor no risk of malignancy. Cysts considered to be SCA were considered to be at low risk of malignant progression. Branch duct IPMN with no worrisome features was defined as intermediate risk. 2,3 Mixed and main duct IPMN, branch duct IPMN with suspicious features, and MCN were considered high risk for malignant transformation. The final category consisted of malignant cysts and included cystic degeneration of an adenocarcinoma, invasive adenocarcinoma arising in an IPMN, pancreatic neuroendocrine tumors, and solid pseudopapillary neoplasms. Patients were determined to have an unclear diagnosis if multiple different diagnoses were given that crossed risk categories. A significant change was deemed to have been made if the risk category assigned to the cyst changed after MPCC review. Patients were excluded from this analysis if they were referred with an unknown or unclear diagnosis because it would not be possible to determine what effect the MPCC review had on their risk category.
Effect of MPCC Review on Management
The management recommendations the patients received at the MPCC and at the referring institution were documented. Patients who did not have a management plan documented were excluded from this analysis. Patients were assigned one of four management options: surveillance, surgical resection, further evaluation, or discharge with no further follow-up. The MPCC was determined to have altered the management of a patient if the MPCC consensus recommendation changed the management category.
Statistical Analysis
Demographics, changes in diagnosis, risk of malignant transformation, and management were compared by the chi-square test.
RESULTS
The MPCC assessed 305 new patients during the study period. Clinical records from the referring institution were not available for 43 patients, who were excluded from this study, thus leaving 262 patients (198 women; mean age 62.7 years, range 19–87 years) available for analysis.
Referring Institution versus MPCC Diagnosis
The referring institution and the MPCC diagnosis for all 262 patients are shown in Table 1. The most common diagnosis in both groups was branch duct IPMN (43.1 and 67.2 %, respectively). The etiology of the cyst was unclear in 46.6 % in the cases referred from referring institutions, while only 14.5 % of the cysts were classified as having an unclear etiology after review by the MPCC.
TABLE 1.
Referring institution versus MPCC diagnosis
| Risk | Diagnosis | Referring institution (n = 262) | MPCC (n = 262) |
|---|---|---|---|
| No risk | No cyst | 3 (1.1 %) | 7 (2.7 %) |
| Pseudocyst | 3 (1.1 %) | 3 (1.1 %) | |
| Low | Serous cystadenoma | 7 (2.7 %) | 15 (5.7 %) |
| Intermediate | Branch duct IPMN | 113 (43.1 %) | 176 (67.2 %) |
| High | Main duct IPMN | 3 (1.1 %) | 2 (0.8 %) |
| Mixed IPMN | 2 (0.8 %) | 3 (1.1 %) | |
| Mucinous cystic neoplasm | 4 (1.5 %) | 3 (1.1 %) | |
| Malignant | Adenocarcinoma/adenocarcinoma arising in IPMN | 5 (1.9 %) | 8 (3.1 %) |
| Pancreatic neuroendocrine tumor | 0 (0 %) | 6 (2.3 %) | |
| Solid pseudopapillary neoplasm | 0 (0 %) | 1 (0.4 %) | |
| Unknown | Unclear type | 122 (46.6 %) | 38 (14.5 %) |
MPCC multidisciplinary pancreatic cyst clinic, IPMN intraductal papillary mucinous neoplasm
Change in Risk Category after MPCC Review
The change in risk category after MPCC review is shown in Table 2. Of the 262 patients, 124 were excluded from this analysis because there was either an unclear or no outside diagnosis, leaving 138 patients who had a diagnostic classification at the time of referral. The MPCC review altered the risk category in 11 (8 %) of 138 patients. Seven patients (5.1 %) had their risk category decreased (Supplementary Table 1). Five cases were reclassified as branch duct IPMN that had been previously classified as main/mixed IPMN (n = 1), MCN (n = 2), and suspicious for neoplasm (n = 2). One patient whose cyst was considered suspicious for cancer before attending the MPCC was found to have a microcystic cyst and was reclassified as a SCA. Another patient whose cyst was classified as a branch duct IPMN before our assessment was reclassified as having a pseudocyst. Four patients (2.9 %) had their risk category increased (Supplementary Table 2). One patient initially classified as having a pseudocyst was reclassified as having a branch duct IPMN; another patient initially classified as having a SCA was reclassified as having a branch duct IPMN; and two patients initially classified as having branch duct IPMN were reclassified as having mixed IPMN (Supplementary Table 2). There was no change in risk category in 109 patients (79 %). In 18 patients (13.0 %), the change in risk could not be determined because the etiology of the cyst was unclear even after MPCC review.
TABLE 2.
Change in risk category after MPCC review
| Risk category | Before MPCC review (n = 138) | Change in risk category after MPCC review (n = 138) |
|||
|---|---|---|---|---|---|
| No change (n = 109, 79 %) | Increase (n = 4, 2.9 %) | Decrease (n = 7, 5.1 %) | Unknown (n = 18) | ||
| No risk | 6 | 3 (1.5 %) | 1 (0.7 %) | NA | 2 (1.4 %) |
| Low risk | 7 | 4 (3.7 %) | 1 (0.7 %) | 0 | 2 (1.4 %) |
| Intermediate risk | 113 | 96 (69.6 %) | 2 (1.4 %) | 1 (0.7 %) | 14 (10.1 %) |
| High risk | 7 | 4 (2.9 %) | 0 | 3 (1.5 %) | 0 |
| Malignant | 5 | 2 (1.4 %) | NA | 3 (1.5 %) | 0 |
MPCC multidisciplinary pancreatic cyst clinic, NA not applicable
Effect of MPCC Review on Patient Treatment
The referring institution and MPCC management recommendations are presented in Table 3. Of the 262 patients in the study, 37 (14.1 %) had no management plan from the referring institution and were excluded from this analysis. Of the 225 patients with a management plan from the referring institution, the MPCC review altered the management of 68 patients (30.2 %) (Table 4). There was an escalation in management in 52 patients (23.1 %). This included 22 patients whose management was changed to surgical resection. For patients in whom the management was changed to surgery, the pathologic diagnosis correlated with the MPCC diagnosis in 18 cases (82 %) (Supplementary Table 3). In contrast, 16 patients (7.1 %) had a decrease in their management plan, including 10 (4.4 %) in whom the recommendation was changed from surgery to surveillance after MPCC review (Supplementary Table 4). Further evaluation was recommended in 40 patients (15.3 %) by the referring institution, compared with 1 patient (0.4 %) after MPCC review. Six patients (2.7 %) were discharged from the MPCC with no further follow-up required.
TABLE 3.
Referring institution and MPCC management recommendations
| Management | Referring institution (n = 262) | MPCC (n = 262) |
|---|---|---|
| No recommendations | 37 (14.1 %) | 0 |
| Further evaluation | 40 (15.3 %) | 1 (0.4 %) |
| No further follow-up | 1 (0.4 %) | 8 (3.1 %) |
| Surveillance | 163 (62.2 %) | 211 (80.5 %) |
| Surgery | 21 (8.0 %) | 42 (16.0 %) |
MPCC multidisciplinary pancreatic cyst clinic
TABLE 4.
Effect of MPCC on management
| Management | Referring institution management (n = 225) | Management recommendations after MPCC review |
||
|---|---|---|---|---|
| No change (n = 157, 69.8 %) | Increase in level of management (n = 52, 23.1 %) | Decrease in level of management (n = 16, 7.1 %) | ||
| No follow-up | 1 | 0 | 1 (0.4 %) | NA |
| Further assessment | 40 | 0 | 9 (4.0 %) surgery, 29 (12.9 %) surveillance | 2 (0.9 %) no follow-up |
| Surveillance | 163 | 146 (64.9 %) | 13 (5.8 %) surgery | 4 (1.8 %) no follow-up |
| Surgery | 21 | 11 (4.9 %) | NA | 10 (4.4 %) surveillance |
MPCC multidisciplinary pancreatic cyst clinic, NA not applicable
Patients who Underwent Surgical Resection
Overall, 36 patients underwent surgical resection in whom surgical pathology was available for review (Supplementary Table 3). The MPCC and surgical pathology risk category correlated in 28 patients (78 %), as follows: benign (n = 2), low risk (n = 3), intermediate risk (n = 8), high risk (n = 5), and malignant (n = 10). The MPCC diagnosis was incorrect in 8 patients (22 %). In 2 cases, the incorrect diagnosis was found to be branch duct IPMN at final pathology but were misclassified as mixed IPMN at the MPCC. One case was originally thought to be an MCN at the MPCC as a result of the lack of communication of the main pancreatic duct on imaging but was found to be a branch duct IPMN on pathology. Two cases proven to be SCA were misclassified at the MPCC. The first had a solid appearance and was mistaken for a MCN with a solid component; the second was a macrocystic SCA that appeared to be communicating with the main pancreatic duct and was incorrectly classified as a branch duct IPMN. A lesion proven to be a retention cyst pathologically, which was associated with a cyst fluid carcinoembryonic antigen (CEA) of >4,000 ng/ml, atypical cytology, and a thickened wall, was incorrectly identified as a branch duct IPMN. One patient, in addition to the cystic lesion in the body that was diagnosed as a branch duct IPMN, had a mass in the tail of the pancreas suspicious for a pancreatic adenocarcinoma on imaging, with cytology revealing focal atypia, which was found to be autoimmune pancreatitis on surgical pathology. In this case, the solid mass observed on cross-sectional imaging was the indication for surgery. The patients’ serum IgG4 was normal, and she had no extrapancreatic manifestations of autoimmune pancreatitis.
Patients whose Recommendation Was Changed from Surgery to Surveillance
Fourteen patients were recommended to undergo surgical resection by the referring institution but had this recommendation changed by the MPCC. Three were discharged because they had lesions with no malignant potential. Of the 11 remaining patients, 2 chose to be followed by their local physician. None of the remaining 9 patients had evidence of malignant transformation during surveillance with a median of 14 months (range 3–50 months) of follow-up (Supplementary Table 4).
DISCUSSION
Over 18 million abdominal CT scans are performed per year in the United States.9 It is estimated that almost 500,000 patients a year in the United States have pancreatic cysts detected incidentally on CT scans.1 Identifying the type of pancreatic cyst and its risk of malignant transformation, as well as instituting appropriate management are essential for the appropriate care of these patients. International consensus guidelines exist to guide the management of these patients.2 However, many physicians are unaware of these guidelines.10 To our knowledge, ours is the first study to review the effect of a MPCC on the diagnosis and management of patients with pancreatic cysts.
The first step when evaluating a patient with a pancreatic cyst is to determine the type of cyst. This study highlights the difficulties that referring physicians have when caring for these patients. Almost 50 % of patients referred to the MPCC either had no diagnosis or multiple diagnoses, which ranged from benign, premalignant, and occasionally malignant differential diagnoses in the same patient. In contrast, multidisciplinary input appears to be helpful: less than 15 % of patients had an unclear diagnosis after MPCC review.
The second step in evaluating a patient with a pancreatic cyst is to determine the risk of malignant transformation of the cyst. This step is critical and is the key to determining whether patients can be discharged with no further follow-up, whether they will require surveillance, or whether they should be considered for surgical resection. In our study, the majority of patients referred for the evaluation of an intermediate risk of malignant transformation remained unchanged after MPCC review. However, in cases categorized as either high risk or malignant by the referring physician, MPCC review was important and altered the risk category in 50 %. Likewise, patients who were labeled as having no risk benefited from MPCC review, with 15 % assigned to a higher risk category. It appears that a multidisciplinary review is helpful in those referred with an unclear diagnosis, in patients with a diagnosis of a high-risk or malignant cyst, and in patients in whom the cyst is thought to be benign. In these groups of patients, review by a MPCC may alter their risk category, which in turn will affect their management.
The final step for a patient with a pancreatic cyst is determining which cysts require surveillance and which are at high risk of malignant transformation and should undergo surgical resection. These are important decisions: pancreatic surgery is associated with morbidity in more than a third of patients, with a mortality of between 0.5 and 5 % for a pancreaticoduodenectomy even in high-volume centers.4,11,12 The MPCC altered the management of a third of patients assessed in the clinic. In the majority of cases, surveillance was recommended, with surgery recommended in just under 10 % of all patients, although no further follow-up was required in just under 2 % of patients who had benign disease. None of the patients in whom the recommendation was changed from surgery to surveillance developed evidence of malignancy during follow-up.
Despite a multidisciplinary review, 8 patients who underwent surgical resection were incorrectly classified. This highlights one of the key problems facing physicians caring for patients with pancreatic cysts: the inadequacy of the tests currently available to correctly identify the type of pancreatic cysts. The accuracy of cross-sectional imaging for classifying pancreatic cysts is reported to be 39.5–46 %.13,14 In a large prospective multicenter study, endoscopic ultrasonography fared little better, with an accuracy of just over 50 % for differentiating mucinous from nonmucinous cysts.15 Although cytology has a specificity reported to be >80 %, it has a low sensitivity of only 34.5 %.15 Cyst fluid CEA is currently the best marker available to identify IPMN and MCN. One multicenter prospective trial reported a specificity and sensitivity of elevated cyst fluid CEA of 84 and 79 %, respectively.15 However, more recent studies have reported much lower accuracies.16,17 Molecular marker testing is available commercially; however, a recent prospective trial reported an accuracy of 56 %.18 More recently discovered genetic markers, such as GNAS and RNF43 gene mutations, hold real promise in classifying cyst type.19,20 Ideally, combining clinical assessments, imaging studies, CEA analysis, cytology, and molecular markers together, either as a nomogram or a model, may provide the optimal approach.21
This study has limitations that should be considered when interpreting the results. It is a single-center retrospective study. There may be a bias in the patients who are referred, with complex cases or those in whom the diagnosis is unclear being more likely to be referred for tertiary opinion. The diagnoses and management recommendations of the MPCC for patients who did not have surgery may not have been correct. However, of the 36 patients who underwent surgery, the MPCC risk category correlated with the surgical pathology in 78 % of cases. Furthermore, none of the patients in whom surveillance was recommended developed evidence of progression of disease. Long-term follow-up will be needed to determine whether an MPCC translates into improved outcomes and to confirm that the patients who were recommended surveillance do not harbor malignant cysts. Despite these limitations, to our knowledge, this is the first study to describe the effect of a multidisciplinary approach on the management of patients with pancreatic cysts.
In conclusion, multidisciplinary input associated with a MPCC is helpful in the management of patients with pancreatic cysts and alters the management of up to 30 % of patients assessed.
Supplementary Material
ACKNOWLEDGMENT
Supported by Cancer Center Grant P30 CA006973.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1245/s10434-014-3739-x) contains supplementary material, which is available to authorized users.
DISCLOSURE Dr. Lennon is a consultant for Boston Scientific. The other authors declare no conflict of interest.
REFERENCES
- 1.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–7. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka M, Fernandez-Del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 4.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152(3 Suppl 1):S4–12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Back MF, Ang EL, Ng WH, et al. Improvements in quality of care resulting from a formal multidisciplinary tumour clinic in the management of high-grade glioma. Ann Acad Med Singapore. 2007;36:347–51. [PubMed] [Google Scholar]
- 6.Gabel M, Hilton NE, Nathanson SD. Multidisciplinary breast cancer clinics. Do they work? Cancer. 1997;79:2380–4. [PubMed] [Google Scholar]
- 7.Petty JK, Vetto JT. Beyond doughnuts: tumor board recommendations influence patient care. J Cancer Educ. 2002;17:97–100. doi: 10.1080/08858190209528807. [DOI] [PubMed] [Google Scholar]
- 8.Pawlik TM, Laheru D, Hruban RH, et al. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081–8. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buscaglia JM, Shin EJ, Giday SA, et al. Awareness of guidelines and trends in the management of suspected pancreatic cystic neoplasms: survey results among general gastroenterologists and EUS specialists. Gastrointest Endosc. 2009;69:813–20. doi: 10.1016/j.gie.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 11.de Wilde RF, Besselink MG, van der Tweel I, et al. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg. 2012;99:404–10. doi: 10.1002/bjs.8664. [DOI] [PubMed] [Google Scholar]
- 12.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Sainani NI, Saokar A, Deshpande V, Fernandez-del Castillo C, Hahn P, Sahani DV. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR Am J Roentgenol. 2009;193:722–31. doi: 10.2214/AJR.08.1253. [DOI] [PubMed] [Google Scholar]
- 14.Visser BC, Yeh BM, Qayyum A, Way LW, McCulloch CE, Coakley FV. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR. Am J Roentgenol. 2007;189:648–56. doi: 10.2214/AJR.07.2365. [DOI] [PubMed] [Google Scholar]
- 15.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Park WG, Mascarenhas R, Palaez-Luna M, et al. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas. 2011;40:42–5. doi: 10.1097/MPA.0b013e3181f69f36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maire F, Voitot H, Aubert A, et al. Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol. 2008;103:2871–7. doi: 10.1111/j.1572-0241.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 18.Al-Haddad M, Dewitt J, Sherman S, et al. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc. 2014;79:79–87. doi: 10.1016/j.gie.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188–93. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correa-Gallego C, Do R, Lafemina J, et al. Predicting dysplasia and invasive carcinoma in intraductal papillary mucinous neoplasms of the pancreas: development of a preoperative nomogram. Ann Surg Oncol. 2013;20:4348–55. doi: 10.1245/s10434-013-3207-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.