Abstract
OBJECTIVE
The purpose of this study was to establish the predictive value of 18F-FDG parameters for overall survival in biopsy-proven recurrent head and neck squamous cell cancer (HNSCC) patients after definitive chemoradiotherapy.
MATERIALS AND METHODS
We conducted a retrospective study including 34 patients with HNSCC who had biopsy-proven recurrence between April 2004 and March 2012 and underwent FDG PET/CT at our institution at the time of recurrence. Maximum standardized uptake value (SUVmax), peak SUV (SUVpeak), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) were measured. The primary outcome measure was overall survival. ROC analysis, univariate and multivariate Cox regression models, and Kaplan-Meir survival curves were performed.
RESULTS
In univariate analyses, human papillomavirus (HPV) status (p = 0.04), primary site recurrence of MTV (p = 0.03), metastasis of MTV (p = 0.02), metastasis of TLG (p = 0.02), total MTV (p = 0.002), and total TLG (p = 0.04) were significantly associated with overall survival outcome. Total MTV remained as significant independent prognostic factor when adjusted for all other covariates except for primary site recurrence SUVmax and SUVpeak and lymph node SUVmax and SUVpeak. There was a significant difference in time to survival between patients with total MTV above and below the 50th percentile (Mantel-Cox log-rank test, p = 0.05 and Gehan-Breslow-Wilcoxon test, p = 0.03) and the optimum threshold of 16.8 mL (Mantel-Cox log-rank test, p = 0.01 and Gehan-Breslow-Wilcoxon test, p = 0.01; hazard ratio [HR], 0.25).
CONCLUSION
FDG PET/CT–based total MTV and clinical HPV status may be significant prognostic markers for overall survival of patients with recurrent HNSCC after definitive chemoradiotherapy.
Keywords: head and neck squamous cell carcinoma recurrence, human papillomavirus, metabolic tumor volume
Head and neck cancer is the sixth most common type of cancer, representing about 6% of all cases and accounting for an estimated 650,000 new cancer cases and 350,000 cancer deaths worldwide each year [1]. Up to 50% of head and neck squamous cell carcinoma (HNSCC) patients will experience recurrence or residual disease even after therapy with curative intent [2]. Locoregional recurrence remains the most common cause of failure in head and neck cancer and accounts for approximately 40–60% of head and neck cancer related deaths [3–5]. The majority of recurrences occur within 3 years of completion of treatment [2, 6, 7]. Recurrence in the neck offers a distinctly higher risk of mortality as opposed to local failure [8]. Historically, patients with recurrence had a poor survival of 4–7 months [9, 10]. However, recent studies suggest that there is improvement in survival for these patients with new treatment options [11].
Over the past decade, 18F-FDG PET/CT has evolved as a valuable imaging modality for staging, treatment response evaluation, and surveillance for recurrence in patients with many human solid tumors [12–20]. Studies have shown that FDG PET/CT is highly sensitive and specific [21–23] in the posttreatment setting. Various FDG parameters have been tested in the pre- and postradiotherapy settings as potential prognostic factors for outcome in HNSCC. Maximum standardized up-take value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) are the common FDG quantitative parameters that have been studied along with qualitative assessments. The purpose of our study was to establish the prognostic value of the volumetric FDG PET parameters of MTV and TLG in recurrent head and neck cancer after definitive chemoradiotherapy.
Materials and Methods
Patient Characteristics
This is a retrospective study approved by the Johns Hopkins institutional review board (IRB). HIPAA guidelines were followed, and an informed consent waiver was obtained from the IRB. Inclusion criteria included biopsy-proven or follow-up imaging confirmation of recurrence of head and neck squamous cell carcinoma (HNSCC) after definitive chemoradiotherapy. We excluded patients with previously treated recurrences or distant metastases at initial presentation or treated primarily with surgery. The study group was composed of 34 patients (13 women and 21 men; mean age [± SD], 57 ± 14 years) who presented with recurrence between April 2004 and March 2012 and underwent FDG PET/CT at our institution.
All patients received radiation therapy with or without chemotherapy. The radiation therapy regimen included a total dosage of 70 Gy that was given in 35 fractions at a dose of 200 cGy per fraction over a course of 50 days. Most patients were treated with an intensity modulated radiation therapy approach. The chemotherapy regimen was variable and included either concurrent chemotherapy alone (n = 24) or induction chemotherapy followed by concurrent chemotherapy (n = 6). Patients receiving induction chemotherapy were treated with 2–3 cycles of cisplatin, taxotere, and 5-fluorouracil or cisplatin and cetuximab. Patients treated with concurrent chemotherapy were treated either with cisplatin or with cetuximab started within 3–7 days of radiation therapy commencement. Carboplatin was used instead of cisplatin in patients with an elevated creatinine or those who developed side effects precluding continuation of cisplatin during treatment.
Outcome Endpoints
Data collection included patient age, sex, and overall stage at presentation; risk factors; treatment received; and mortality data at last follow-up. Overall mortality was determined from the Social Security database or hospital electronic medical records. Time to overall survival was measured from the date of the original positive PET/CT to diagnosis of recurrence or to the last follow-up in patients who were still alive or to the date of death for patients who had died. For all patients, including those who died, the mean length of follow-up was 27.03 ± 25.58 months and the median length of follow-up was 17.5 months (interquartile range [IQR], 8.0–37.5).
PET/CT Protocol
All PET/CT studies were performed using either a Discovery LS (2D) or a Discovery RX VCT (3D) scanner (both scanners, GE Healthcare). All patients were scanned using a dedicated head and neck protocol as part of the standard clinical protocol at our institution. Patients were scanned from skull vertex to mid thigh in two separate acquisitions starting from mid thigh to chin and then from carina to skull vertex. Head and neck images were acquired with the arms down and body images were obtained with the arms up. The average patient blood glucose level was 91 mg/dL (range, 70–114 mg/dL). Mean height and weight of the study population were 171 ± 12 cm and 67 ± 17 kg, respectively. Patients were injected with an average of 15.02 ± 3.58 mCi of FDG and the mean uptake time was 67 ± 10 minutes.
The ordered-subsets expectation maximization algorithm was used to reconstruct all PET images. The 2D implementation on the Discovery LS scanner used two iterations, 28 subsets, a 5.5-mm postreconstruction gaussian filter, and 3.9-mm pixels. The fully 3D implementation on the Discovery RX VCT used two iterations, 21 subsets, a 3.0-mm reconstruction gaussian filter, and 4.7-mm pixels. All PET data were reconstructed with and without CT-based attenuation correction. Helical CT images were obtained with a matrix of 512 × 512. Beam collimation was 10 mm with a pitch of 0.984. Table speed was 9.84 mm/rotation and the slice thickness was 0.625 mm; 120 kV and 440 mAs were used.
PET/CT Image Analysis
All PET/CT studies were electronically retrieved and reviewed on a Mobile MIM workstation (MIM Software) by a board-certified nuclear medicine fellow. The reader was blinded to the clinical outcome. PET, CT, and fused PET/CT images were displayed in axial, coronal, and sagittal planes. For this study, the relevant imaging parameters were the maximum SUV (SUVmax), peak SUV (SUVpeak), MTV, and TLG. All parameters were measured for each primary site recurrence (SUVmax, SUVpeak, MTV, and TLG), lymph nodal recurrence (SUVmax, SUVpeak, MTV, and TLG), and distant metastasis (SUVmax, SUVpeak, MTV, and TLG). We only included positive lymph nodes with SUVmax more than 2.0. MTV was defined as the tumor volume with FDG uptake segmented by a gradient-based method. The MIM-vista software analysis suite, version 5.2 (MIM Software) includes a contouring PET/CT suite. Once the ROI was segmented, SUVmax and MTV were semi-automatically calculated by the MIMvista software. The gradient segmentation methods of volume measurement available in MIMvista software rely on an operator-defined starting point near the center of the lesion. As the operator drags the cursor out from the center of the lesion, six axes extend, providing visual feedback for the starting point of gradient segmentation. Spatial gradients are calculated along each axis interactively, and the length of an axis is restricted when a large gradient is detected along that axis. The six axes define an ellipsoid that is then used as an initial bounding region for gradient detection. The MTV, TLG, SUVpeak, and SUVmax with-in the bounding region are automatically calculated [24]. We further calculated the total tumor burden by combining the activity at the primary site recurrence, lymph node recurrence, and distant metastasis and measured the following metabolic parameters: total MTV and total TLG. We used gradient-based segmentation because our previous studies have suggested that MTV measured by gradient-based segmentation may be a superior prognostic marker [24].
Statistical Analysis
We present central tendencies as mean ± SD or as median (IQR) when data were skewed or as frequency and percentage for categoric variables. ROC analysis was performed for identifying the optimum thresholds. Survival is presented as Kaplan-Meir survival curves with analysis performed using the Mantel-Cox log-rank test and Gehan-Breslow-Wilcoxon Test. Cox multivariate analyses were performed to adjust for important prognostic factors. Multicollinearity was established using Pearson correlation coefficients (r > 0.70). A Mann-Whitney U test was performed to compare distribution of parameters between those who survived and those who died. We used the Prism 5 (GraphPad Software) and SPSS 20 (IBM) statistical packages for all analyses, and all hypothesis tests are two-sided with a significance level of 0.05.
Results
The mean age (± SD) of our study population was 57 ± 14 years. Twenty-one (62%) patients were men and 13 (38%) were women. Twenty-seven (79%) patients had American Joint Commission on Cancer stage IV cancer at the time of initial diagnosis. Human papillomavirus (HPV) status for 13 (38.2%) patients was available, and among those, eight of 13 (61.5%) were positive for HPV and five of 13 (38.5%) were negative. Eighteen (53%) patients had died and 16 (47%) were alive at the end of follow-up. The median length of follow-up for the study population was 17.5 months (IQR, 8.0–37.5 months).
Among the 34 patients included in this study, all the recurrences occurred within 3 years of completion of treatment. Of these recurrences, 21 of 34 (61.8%) were noted in the first year, 10 of 34 (29.4%) in the second year, and three of 34 (8.8%) in the third year after completion of treatment. Tumor recurred at the primary site in 23 of 34 (67.6%) patients, lymph nodes in 16 of 34 (47.1%) patients, and distant sites in 15 of 34 (44.1%) patients. There was a significant difference in median total MTV (p = 0.025) and total TLG (p = 0.004) between those patients who were alive and those who were dead at the end of follow-up (Table 1). There was also a significant difference in the overall survival time of those who were HPV positive (mean rank, 8.75) versus those who were HPV negative (mean rank, 4.2) (p = 0.04).
TABLE 1.
FDG Parameters of Local Recurrence, Nodal Recurrence, Distant Metastasis, and Total Tumor Burden
| Parameter | Dead | Alive | p | ||
|---|---|---|---|---|---|
|
| |||||
| Median | Interquartile Range | Median | Interquartile Range | ||
|
| |||||
| Total MTV (mL) | 11.25 | 5.07–18.75 | 3.84 | 2.25–9.34 | 0.03 |
| Total TLG (g) | 59.64 | 11.93–141.50 | 8.86 | 5.70–31.27 | 0.00 |
| Primary SUVmax | 7.17 | 4.56–9.73 | 4.49 | 3.20–6.71 | 0.10 |
| Primary SUVpeak | 4.80 | 3.09–8.66 | 3.20 | 2.40–4.10 | 0.10 |
| Primary MTV (mL) | 6.17 | 1.79–11.23 | 2.80 | 2.23–5.40 | 0.24 |
| Primary TLG (g) | 24.51 | 4.49–75.66 | 8.86 | 5.47–49.69 | 0.15 |
| Lymph node SUVmax | 7.41 | 3.86–14.02 | 5.80 | 4.12–6.21 | 0.52 |
| Lymph node SUVpeak | 6.70 | 2.80–9.40 | 3.95 | 2.75–4.48 | 0.57 |
| Lymph node MTV (mL) | 4.06 | 2.21–7.85 | 4.45 | 2.21–16.71 | 0.77 |
| Lymph node TLG (g) | 19.88 | 6.08–36.84 | 13.53 | 7.50–64.12 | 0.77 |
| Metastasis SUVmax | 6.72 | 6.33–10.04 | 8.79 | 4.81–11.29 | 0.78 |
| Metastasis SUVpeak | 5.00 | 3.35–7.35 | 4.75 | 1.83–7.9 | 1.00 |
| Metastasis MTV (mL) | 14.69 | 3.06–43.41 | 2.62 | 1.74–5.17 | 0.46 |
| Metastasis TLG (g) | 90.82 | 7.36–181.50 | 13.23 | 4.81–19.66 | 0.46 |
Note—MTV = metabolic tumor volume, TLG = total lesion glycolysis, SUVmax = maximum standardized uptake value (SUV), SUVpeak = peak SUV.
Univariate Cox Regression Model
Age, sex, stage, site of recurrence, HPV status, FDG metabolic parameters of primary site recurrence (primary SUVmax, primary SUVpeak, primary MTV, and primary TLG), lymph nodal recurrence (nodal SUVmax, nodal SUVpeak, nodal MTV, and nodal TLG), distant metastasis (metastasis SUVmax, metastasis SUVpeak, metastasis MTV, and metastasis TLG), and total tumor (total MTV and total TLG) were included in the univariate and multivariate Cox regression models for overall survival outcome. In univariate analyses, when stratified by overall survival status at the end of follow-up, HPV status, primary site recurrence MTV, metastasis MTV, metastasis TLG, total MTV, and total TLG were significantly associated with overall survival outcome (Table 2).
TABLE 2.
Cox Univariate Analysis for Clinical and Imaging Parameters and Overall Mortality
| Parameters | Overall Mortality | p | |
|---|---|---|---|
|
| |||
| Hazard Ratio | 95% CI | ||
|
| |||
| Age | 1.03 | 0.99–1.07 | 0.14 |
| Sex | 1.19 | 0.47–3.02 | 0.72 |
| Stage | 2.23 | 0.78–6.39 | 0.14 |
| Site | 0.62 | 0.24–1.60 | 0.31 |
| HPV status | 6.21 | 1.08–35.84 | 0.04 |
| Primary SUVmax | 1.06 | 0.97–1.15 | 0.20 |
| Primary SUVpeak | 1.08 | 0.97–1.21 | 0.17 |
| Primary MTV (mL) | 1.05 | 1.01–1.10 | 0.03 |
| Primary TLG (g) | 1.00 | 1.00–1.01 | 0.11 |
| Lymph node SUVmax | 1.05 | 0.93–1.19 | 0.39 |
| Lymph node SUVpeak | 1.13 | 0.94–1.36 | 0.20 |
| Lymph node MTV (mL) | 1.01 | 1.00–1.02 | 0.21 |
| Lymph node TLG (g) | 1.00 | 1.00–1.00 | 0.14 |
| Metastasis SUVmax | 0.97 | 0.79–1.19 | 0.78 |
| Metastasis SUVpeak | 1.04 | 0.80–1.34 | 0.77 |
| Metastasis MTV (mL) | 1.01 | 1.00–1.02 | 0.02 |
| Metastasis TLG (g) | 1.00 | 1.00–1.00 | 0.02 |
| Total MTV (mL) | 1.01 | 1.00–1.01 | 0.00 |
| Total TLG (g) | 1.00 | 1.00–1.00 | 0.00 |
Note—Human papillomavirus (HPV) status, primary tumor metabolic tumor volume (MTV), metastasis MTV, metastasis total lesion glycolysis (TLG), maximum standardized uptake value (SUVmax), peak SUV (SUVpeak), total MTV, and total TLG were significantly associated with overall survival outcome.
Multivariate Cox Regression Model
Given the strong correlation between MTV and TLG (0.98, p < 0.001), we further studied only total MTV and primary site recurrence MTV with exploratory multivariate models to identify whether the univariate results were an artifact due to confounding variables. We tested the association between total recurrence MTV and outcome (Table 3) while controlling for each covariate individually. Total MTV remained a significantly independent prognostic factor associated with overall survival when adjusted for age, sex, stage and site of recurrence, HPV status, metastasis SUVmax, and SUVpeak but not when adjusted for primary site recurrence SUVmax, SUVpeak, lymph node SUVmax, and SUVpeak.
TABLE 3.
Cox Multivariate Analysis Model of Total Metabolic Tumor Volume (MTV) of Recurrence as Predictor of Overall Survival Adjusted for Other Covariates
| Parameters | Hazard Ratio | 95% CI | p |
|---|---|---|---|
|
| |||
| Total MTV | 1.01 | 1.00–1.01 | 0.00 |
| Age (per y) | 1.02 | 0.99–1.06 | 0.23 |
| Total MTV | 1.01 | 1.00–1.01 | 0.00 |
| Male | 1.06 | 0.40–2.79 | 0.91 |
| Total MTV | 1.01 | 1.00–1.01 | 0.00 |
| Stage (stage III vs stage IV) | 2.15 | 0.74–6.22 | 0.16 |
| Total MTV | 1.01 | 1.00–1.01 | 0.01 |
| Site (locoregional recurrence vs distant metastasis) | 0.82 | 0.30–2.23 | 0.70 |
| Total MTV | 1.02 | 1.00–1.04 | 0.04 |
| HPV (positive vs negative) | 9.03 | 1.25–65.25 | 0.03 |
| Total MTV | 1.014 | 0.99–1.04 | 0.21 |
| Primary SUVmax | 1.031 | 0.93–1.15 | 0.57 |
| Total MTV | 1.02 | 0.99–1.04 | 0.21 |
| Primary SUVpeak | 1.05 | 0.91–1.20 | 0.52 |
| Total MTV | 1.00 | 1.00–1.01 | 0.15 |
| Lymph node SUVmax | 1.00 | 0.86–1.16 | 0.98 |
| Total MTV | 1.00 | 1.00–1.01 | 0.32 |
| Lymph node SUVpeak | 1.03 | 0.80–1.34 | 0.80 |
| Total MTV | 1.01 | 1.00–1.01 | 0.01 |
| Metastasis SUVmax | 0.89 | 0.70–1.12 | 0.30 |
| Total MTV | 1.00 | 1.00–1.01 | 0.02 |
| Metastasis SUVpeak | 0.88 | 0.65–1.19 | 0.41 |
Note—Total MTV remained as a significant independent prognostic factor when adjusted for all other covariates individually except for primary site recurrence maximum standardized uptake value (SUVmax) and lymph node recurrence SUVmax and peak SUV (SUVpeak). HPV = human papillomavirus.
ROC Analysis
ROC curve analysis was performed to determine the accuracy of total MTV to differentiate those living at 24 months from those who died (AUC, 0.66; 95% CI, 0.48–0.85; p< 0.09). The median cut point of 9.69 mL of total MTV had sensitivity, specificity, and likelihood ratio (LR) of 67.7%, 63.2%, and 1.81, respectively. An optimum threshold of 27.8 mL had 46.7% sensitivity, 84.2% specificity, and LR of 2.96 in identifying patients who died within 2 years. ROC analysis was also performed for overall survival. The AUC for total MTV was 0.72 (0.55–0.90, p = 0.03) for differentiating those who had died at the end of follow-up from those still alive. The median cut point of 9.69 mL had sensitivity, specificity, and LR of 66.7%, 68.8%, and 2.13, respectively. For an optimum cut point of 16.8 mL, the sensitivity, specificity, and LR were 55.6%, 87.5%, and 4.44, respectively.
Kaplan-Meier Survival Curves
Overall survival curves were compared using the Mantel-Cox log-rank test and Gehan-Breslow-Wilcoxon test. A significant difference in time to survival was observed between patients with total metabolic tumor volume above and below the optimum threshold of 16.8 mL (Mantel-Cox log-rank test, p = 0.01 and Gehan-Breslow-Wilcoxon test, p = 0.01; HR, 0.25; 0.09–0.72). A significant difference in time to survival was also observed between patients with total metabolic tumor volume above and below the median threshold total metabolic volume of 9.69 mL (Mantel-Cox log-rank test, p = 0.05 and Gehan-Breslow-Wilcoxon test, p = 0.03) (Fig. 1). Illustrative cases are provided in Figures 2 and 3.
Fig. 1.

Kaplan Meir survival curves.
A and B, Kaplan-Meier plots for total metabolic tumor volume were generated using 50th percentile (9.69 mL) (A) and optimum (16.8 mL) (B) cutoff thresholds. Significant difference in time to survival was observed between patients with total metabolic tumor volume above and below median threshold (Mantel-Cox log-rank test, p = 0.05 and Gehan-Breslow-Wilcoxon test, p = 0.03) as well as optimum threshold (Mantel-Cox log-rank test, p = 0.01 and Gehan-Breslow-Wilcoxon test, p = 0.01).
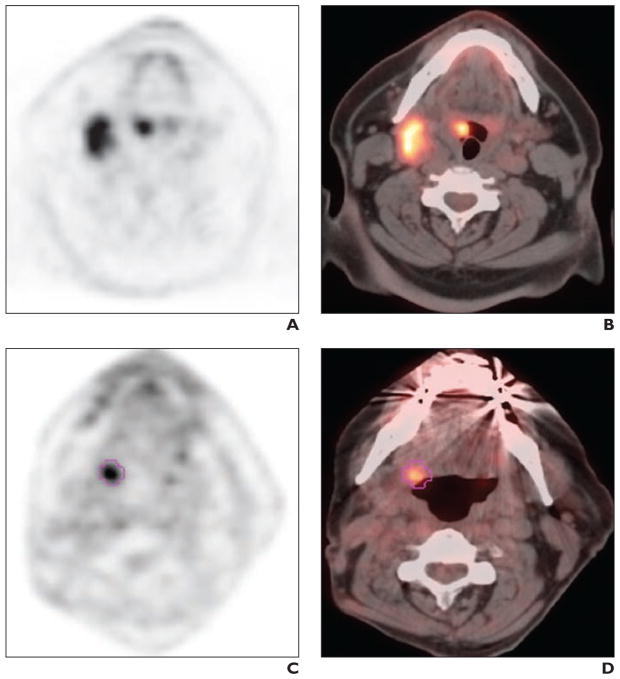
Fig. 2.
59-year-old man with history of human papillomavirus–associated T2N2M0 squamous cell carcinoma of right tonsil.
A and B, Initial staging PET/CT images show intense 18F-FDG uptake in right tonsil and right level IIA nodes. Patient was treated with cisplatin and erlotinib with 70 Gy radiation over 6 weeks followed by neck dissection and remained disease free for 3 years. C and D, Restaging PET/CT images at 3 years after completion of treatment show intense FDG uptake in right side of tongue base (maximum standardized uptake value [SUV], 4.7; peak SUV, 3.1; total metabolic tumor volume, 1.77 mL; total lesion glycolysis, 5.14 g). There was no evidence of regional nodal or distant metastatic disease. Patient was treated with transoral robotic surgery for recurrence and was alive at 33 months after PET/CT at time of last follow-up.
Fig. 3.
32-year-old man with history of human papillomavirus–negative T3N1M0 squamous cell carcinoma of right tonsil.
A and B, Initial staging PET/CT images show intense 18F-FDG uptake in right tonsil. Patient was treated with cetuximab and 70-Gy radiotherapy over 6 weeks. Cisplatin was stopped after 5 days because of fever and cytopenia. Patient remained disease-free for 2 years. C and D, Restaging PET/CT images at 2 years after completion of treatment show intense FDG uptake in right side of tongue base (maximum standardized uptake value [SUV], 25.5; peak SUV, 19.4; total metabolic tumor volume, 37.97 mL; total lesion glycolysis, 379.9 g). There was no regional or distant metastatic disease. Patient died 19 months after PET/CT.
Discussion
Our study showed that the total metabolic tumor volume is a significant prognostic marker for patients with recurrent HNSCC after definitive chemoradiotherapy. We found that patients with large total MTV above the 50th percentile at the time of diagnosis of recurrence had a significantly worse overall survival even when adjusted for all the clinical covariates in the Cox regression analysis.
A review of the literature shows that 30–50% of HNSCC patients treated with curative intent will develop residual disease or recurrence [2]. Recurrent head and neck cancers are particularly challenging to physicians because we have to consider resectability, prior treatment, age, performance status, and patient wishes in the overall management strategy. Moreover, the cost of treatment may need to be considered because most therapies may fail to provide overall survival benefit [25]. However, in the past few years there have been many emerging alternative treatment options for patients with recurrent HNSCC. These treatment options have shown promising results with improved survival [26–28]. The value of PET/CT in detecting recurrence is already established [23], including identifying the distant metastasis before salvage surgery [29]. Risk stratifying patients at the time of recurrence may allow modifying treatment strategies to individualize patient management using quantitative imaging parameters, such as MTV or TLG of the total tumor burden.
Several studies have shown that baseline SUVmax [30], MTV [31, 32], and TLG [33] are significant independent prognostic factors for survival in HNSCC. Studies have evaluated posttreatment SUVmax [34–36] and posttreatment MTV [37] for prediction of disease-free survival and overall survival. Wong et al. [34] suggested that for every one unit increase in posttreatment SUVmax, the relative risk of death increases by 14%. Similarly, Murphy et al. [37] suggested that an increase in posttreatment MTV of 21 cm3 measured by the threshold segmentation method was associated with an increased risk of death (HR = 2.0, p = 0.003). In our study we focused on those with biopsy-proven recurrence. We included patients who were stage III and stage IV at initial staging, treated with definitive chemoradiotherapy, and developed a recurrence, and we investigated the FDG volumetric parameters as well as SUVmax and SUVpeak. Our study suggests total MTV is a marker for prognosis in patients with recurrent HNSCC after treatment with chemoradiation. Our study results are consistent with recent reports supporting the emerging value of metabolic tumor volume as a prognostic marker [24, 38–41].
HPV status was a significant factor associated with overall survival both in univariate and multivariate analyses adjusted for imaging variables in our study. HPV-positive HN-SCC patients have improved survival in comparison with HPV-negative patients [42, 43]. A recent study by O’Sullivan et al. [44] reported that HPV-positive patients have high local and regional control but similar distant control in comparison with HPV-negative patients. Our preliminary results suggest that HPV status is associated with the prognosis of patients with recurrence of HNSCC.
This study has several limitations, including its retrospective design and relatively small sample size of 34 patients. The findings are therefore only hypothesis-generating and will need to be validated in a larger prospective study. Because of the small numbers within each stage, we did not stratify within each stage, but we performed Cox regression analysis adjusting the clinical parameters, including stage. Because of the small number of patients with HPV-positive status, we could not perform stratification analyses to establish the value of total MTV among these patients. We used segmentation from only one commercial vendor’s software workstation for analysis and need to investigate multiple vendor-provided segmentation algorithms.
In conclusion, our study suggests that total metabolic tumor volume (gradient-based segmentation) and clinical HPV status are potential markers for overall survival in patients with recurrent advanced HNSCC treated with chemoradiotherapy. Total MTV and HPV status may be valuable in risk-stratifying patients to individualize therapy.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Beswick DM, Gooding WE, Johnson JT, Branstetter BF., 4th Temporal patterns of head and neck squamous cell carcinoma recurrence with positron-emission tomography/computed tomography monitoring. Laryngoscope. 2012;122:1512–1517. doi: 10.1002/lary.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coatesworth AP, Tsikoudas A, MacLennan K. The cause of death in patients with head and neck squamous cell carcinoma. J Laryngol Otol. 2002;116:269–271. doi: 10.1258/0022215021910726. [DOI] [PubMed] [Google Scholar]
- 4.Vargo JA, Heron DE, Ferris RL, et al. Prospective evaluation of patient-reported quality-of-life outcomes following SBRT ± cetuximab for locally-recurrent, previously-irradiated head and neck cancer. Radiother Oncol. 2012;104:91–95. doi: 10.1016/j.radonc.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin WJ., Jr Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000;110:1–18. doi: 10.1097/00005537-200003001-00001. [DOI] [PubMed] [Google Scholar]
- 6.Boysen M, Lovdal O, Tausjo J, Winther F. The value of follow-up in patients treated for squamous cell carcinoma of the head and neck. Eur J Cancer. 1992;28:426–430. doi: 10.1016/s0959-8049(05)80068-1. [DOI] [PubMed] [Google Scholar]
- 7.Ritoe SC, Krabbe PF, Kaanders JH, van den Hoogen FJ, Verbeek AL, Marres HA. Value of routine follow-up for patients cured of laryngeal carcinoma. Cancer. 2004;101:1382–1389. doi: 10.1002/cncr.20536. [DOI] [PubMed] [Google Scholar]
- 8.Deschamps DR, Spencer HJ, Kokoska MS, Spring PM, Vural EA, Stack BC., Jr Implications of head and neck cancer treatment failure in the neck. Otolaryngol Head Neck Surg. 2010;142:722–727. doi: 10.1016/j.otohns.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Kowalski LP, Carvalho AL. Natural history of untreated head and neck cancer. Eur J Cancer. 2000;36:1032–1037. doi: 10.1016/s0959-8049(00)00054-x. [DOI] [PubMed] [Google Scholar]
- 10.Choe KS, Haraf DJ, Solanki A, et al. Prior chemo-radiotherapy adversely impacts outcomes of recurrent and second primary head and neck cancer treated with concurrent chemotherapy and reirradiation. Cancer. 2011;117:4671–4678. doi: 10.1002/cncr.26084. [DOI] [PubMed] [Google Scholar]
- 11.Wong SJ, Bourhis J, Langer CJ. Retreatment of recurrent head and neck cancer in a previously irradiated field. Semin Radiat Oncol. 2012;22:214–219. doi: 10.1016/j.semradonc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Kao J, Vu HL, Genden EM, et al. The diagnostic and prognostic utility of positron emission tomography/computed tomography-based follow-up after radiotherapy for head and neck cancer. Cancer. 2009;115:4586–4594. doi: 10.1002/cncr.24493. [DOI] [PubMed] [Google Scholar]
- 13.Davison JM, Ozonoff A, Imsande HM, Grillone GA, Subramaniam RM. Squamous cell carcinoma of the palatine tonsils: FDG standardized uptake value ratio as a biomarker to differentiate tonsillar carcinoma from physiologic uptake. Radiology. 2010;255:578–585. doi: 10.1148/radiol.10091479. [DOI] [PubMed] [Google Scholar]
- 14.Kubicek GJ, Champ C, Fogh S, et al. FDG-PET staging and importance of lymph node SUV in head and neck cancer. Head Neck Oncol. 2010;2:19. doi: 10.1186/1758-3284-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lonneux M, Hamoir M, Reychler H, et al. Positron emission tomography with [18F]fluorodeoxy-glucose improves staging and patient management in patients with head and neck squamous cell carcinoma: a multicenter prospective study. J Clin Oncol. 2010;28:1190–1195. doi: 10.1200/JCO.2009.24.6298. [DOI] [PubMed] [Google Scholar]
- 16.Subramaniam RM, Clayton AC, Karantanis D, Collins DA. Hibernoma: 18F FDG PET/CT imaging. J Thorac Oncol. 2007;2:569–570. doi: 10.1097/JTO.0b013e31805fe2a5. [DOI] [PubMed] [Google Scholar]
- 17.Subramaniam RM, Wilcox B, Aubry MC, Jett J, Peller PJ. 18F-fluoro-2-deoxy-D-glucose positron emission tomography and positron emission tomography/computed tomography imaging of malignant pleural mesothelioma. J Med Imaging Radiat Oncol. 2009;53:160–169. doi: 10.1111/j.1754-9485.2009.02058.x. quiz, 170. [DOI] [PubMed] [Google Scholar]
- 18.Dibble EH, Lara Alvarez AC, Truong MT, Mercier G, Cook EF, Subramaniam RM. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med. 2012;53:709–715. doi: 10.2967/jnumed.111.099531. [DOI] [PubMed] [Google Scholar]
- 19.Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of primary hepatobiliary tumors: part 2. AJR. 2011;197:W260–W265. doi: 10.2214/AJR.11.6995. [web] [DOI] [PubMed] [Google Scholar]
- 20.Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of liver metastases: part 1. AJR. 2011;197:W256–W259. doi: 10.2214/AJR.10.6331. [web] [DOI] [PubMed] [Google Scholar]
- 21.Wong RJ. Current status of FDG-PET for head and neck cancer. J Surg Oncol. 2008;97:649–652. doi: 10.1002/jso.21018. [DOI] [PubMed] [Google Scholar]
- 22.Zundel MT, Michel MA, Schultz CJ, et al. Comparison of physical examination and fluorodeoxy-glucose positron emission tomography/computed tomography 4–6 months after radiotherapy to assess residual head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:e825–e832. doi: 10.1016/j.ijrobp.2010.11.072. [DOI] [PubMed] [Google Scholar]
- 23.Abgral R, Querellou S, Potard G, et al. Does 18F-FDG PET/CT improve the detection of posttreatment recurrence of head and neck squamous cell carcinoma in patients negative for disease on clinical follow-up? J Nucl Med. 2009;50:24–29. doi: 10.2967/jnumed.108.055806. [DOI] [PubMed] [Google Scholar]
- 24.Davison J, Mercier G, Russo G, Subramaniam RM. PET-based primary tumor volumetric parameters and survival of patients with non-small cell lung carcinoma. AJR. 2013;200:635–640. doi: 10.2214/AJR.12.9138. [DOI] [PubMed] [Google Scholar]
- 25.Kurzweg T, Mockelmann N, Laban S, Knecht R. Current treatment options for recurrent/metastatic head and neck cancer: a post-ASCO 2011 update and review of last year’s literature. Eur Arch Otorhinolaryngol. 2012;269:2157–2167. doi: 10.1007/s00405-012-1998-3. [DOI] [PubMed] [Google Scholar]
- 26.Jiménez B, Trigo JM, Pajares BI, et al. Efficacy and safety of weekly paclitaxel combined with cetuximab in the treatment of pretreated recurrent/metastatic head and neck cancer patients. Oral Oncol. 2013;49:182–185. doi: 10.1016/j.oraloncology.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Cohen MH, Chen H, Shord S, et al. Approval summary: cetuximab in combination with cisplatin or carboplatin and 5-fluorouracil for the first-line treatment of patients with recurrent locoregional or metastatic squamous cell head and neck cancer. Oncologist. 2013;18:460–466. doi: 10.1634/theoncologist.2012-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argiris A, Ghebremichael M, Gilbert J, et al. Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an Eastern Cooperative Oncology Group Trial. J Clin Oncol. 2013;31:1405–1414. doi: 10.1200/JCO.2012.45.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi JS, Kim JS, Lee JH, et al. 18F-FDG PET/CT for detecting distant metastases in patients with recurrent head and neck squamous cell carcinoma. J Surg Oncol. 2012;106:708–712. doi: 10.1002/jso.23185. [DOI] [PubMed] [Google Scholar]
- 30.Xie P, Li M, Zhao H, Sun X, Fu Z, Yu J. 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol. 2011;137:1085–1093. doi: 10.1007/s00432-010-0972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romesser PB, Qureshi MM, Shah BA, et al. Superior prognostic utility of gross and metabolic tumor volume compared to standardized uptake value using PET/CT in head and neck squamous cell carcinoma patients treated with intensity-modulated radiotherapy. Ann Nucl Med. 2012;26:527–534. doi: 10.1007/s12149-012-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang C, Murphy JD, Khong B, et al. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;83:1514–1520. doi: 10.1016/j.ijrobp.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang KP, Tsang NM, Liao CT, et al. Prognostic significance of 18F-FDG PET parameters and plasma Epstein-Barr virus DNA load in patients with nasopharyngeal carcinoma. J Nucl Med. 2012;53:21–28. doi: 10.2967/jnumed.111.090696. [DOI] [PubMed] [Google Scholar]
- 34.Wong RJ, Lin DT, Schoder H, et al. Diagnostic and prognostic value of [18F]Fluorodeoxyglucose positron emission tomography for recurrent head and neck squamous cell carcinoma. J Clin Oncol. 2002;20:4199–4208. doi: 10.1200/JCO.2002.02.590. [DOI] [PubMed] [Google Scholar]
- 35.Kim G, Kim YS, Han EJ, et al. FDG-PET/CT as prognostic factor and surveillance tool for postoperative radiation recurrence in locally advanced head and neck cancer. Radiat Oncol J. 2011;29:243–251. doi: 10.3857/roj.2011.29.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherriff JM, Ogunremi B, Colley S, Sanghera P, Hartley A. The role of positron emission tomography/CT imaging in head and neck cancer patients after radical chemoradiotherapy. Br J Radiol. 2012;85:e1120–e1126. doi: 10.1259/bjr/20976707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy JD, La TH, Chu K, et al. Postradiation metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;80:514–521. doi: 10.1016/j.ijrobp.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bazan JG, Koong AC, Kapp DS, et al. Metabolic tumor volume predicts disease progression and survival in patients with squamous cell carcinoma of the anal canal. J Nucl Med. 2013;54:27–32. doi: 10.2967/jnumed.112.109470. [DOI] [PubMed] [Google Scholar]
- 39.Fonti R, Larobina M, Del Vecchio S, et al. Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med. 2012;53:1829–1835. doi: 10.2967/jnumed.112.106500. [DOI] [PubMed] [Google Scholar]
- 40.Park GC, Kim JS, Roh JL, Choi SH, Nam SY, Kim SY. Prognostic value of metabolic tumor volume measured by 18F-FDG PET/CT in advanced-stage squamous cell carcinoma of the larynx and hypopharynx. Ann Oncol. 2013;24:208–214. doi: 10.1093/annonc/mds247. [DOI] [PubMed] [Google Scholar]
- 41.Lim R, Eaton A, Lee NY, et al. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med. 2012;53:1506–1513. doi: 10.2967/jnumed.111.101402. [DOI] [PubMed] [Google Scholar]
- 42.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48:1191–1201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 44.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]