Abstract
Context
Severe H2S poisoning leads to death by rapid respiratory and cardiac arrest, the latter can occur within seconds or minutes in severe forms of intoxication.
Objectives
Determine the time course and the nature of H2S induced cardiac arrest and the effects of high dose Hydroxocobalamin administered after the end of sulfide exposure.
Materials and methods
In 16 sedated mechanically ventilated sheep, NaHS was infused to reach concentrations of H2S in the blood we previously found to lead to cardiac arrest within minutes following the cessation of H2S exposure. High dose Hydroxocobalamin (5 g) or saline solution was administered intravenously, one minute after the cessation of NaHS infusion.
Results
All animals were still alive at the cessation of H2S exposure. Three animals (18%) presented a cardiac arrest within 90 seconds and were unable to receive any antidote or vehicle. In the animals that survived long enough to receive either Hydroxocobalamin or saline, 71% (5/7) died in the control group by cardiac arrest within 10 minutes. In all instances, cardiac arrest was the result of a pulseless electrical activity (PEA). In the group that received the antidote, intravenous injection of 5 g Hydroxocobalamin provoked an abrupt increase in blood pressure and blood flow; PEA was prevented in all instances. However, we could not find any evidence for a recovery in oxidative metabolism in the group receiving Hydroxocobalamin, as blood lactate remained elevated and even continued to rise after one hour, despite restored hemodynamics. This, along with an unaltered recovery of H2S kinetics, suggests that Hydroxocobalamin did not act through a mechanism of H2S trapping.
Conclusion
In this sheep model, there was a high risk for cardiac arrest, by PEA, persisting up to 10 minutes after H2S exposure. Very high dose of Hydroxocobalamin (5 g), injected very early after the cessation of H2S exposure, improved cardiac contractility and prevented PEA.
INTRODUCTION
The treatment of Hydrogen sulfide (H2S) intoxication must resolve a difficult challenge: as already mentioned in the early 20th century by Haggard (1), H2S produces its deleterious effects, e.g. coma, apnea, cardiac arrest - within minutes or sometimes seconds (2, 3), while it vanishes, at least in its soluble/diffusible forms, very rapidly as soon as the exposure ceases (4, 5). More specifically, we found that H2S is present in essentially two pools during sulfide exposure: one pool of free/exchangeable sulfide and one larger compartment of sulfide likely combined with proteins or metallo-proteins. When H2S exposure ceases, the free/exchangeable form of sulfide disappears within one minute from the blood and thus from the tissues (1, 5–7). Combined H2S also decreases rapidly in the blood to a few microM, regardless of the initial H2S level, but remains present at very low concentrations for at least one hour (4, 5). The potential benefits of any antidote in patients severely intoxicated is thus limited by the very rapid disappearance of soluble H2S following hydrogen sulfide poisoning and the low and slow accessibility of the pools of combined intracellular H2S. Just like ferric compounds, Cobalt compounds such as Hydroxocobalamin, when used at a very high dose, do combine free H2S and catalyze its oxidation during H2S exposure (4, 8–10). Five grams of Hydroxocobalamin, corresponding to 106 times the recommended daily intake, can drop the concentration of soluble H2S to almost zero during infusion of moderate to severe levels of H2S infusion (4). This observation supports the view that Hydroxocobalamin or other Vitamin B12 analogues (11), could exert a protective effect against H2S toxicity since not only can they diffuse within cells (12) via non-transcobalamin mechanisms (13–15), but they also oppose the effects of Nitric oxide (16–19), recently shown to potentiate the effects of H2S (20, 21). Finally, intravenous infusion of large dose of Hydroxocobalamin is already FDA approved for the treatment of cyanide poisoning (22, 23). There is, however, no evidence that hydroxocobalamin can oppose any of the persisting toxic effects of sulfide related to its combination on cysteine residues or metallo-proteins after an exposure (24) or could enter cells rapidly enough to interact in vivo with the intra-cellular pool of combined sulfide. More specifically, since one of the immediate dangers of H2S toxicity relates to the production of a rapid shock, it remains to be shown that high dose Hydroxocobalamin can counteract the consequences of H2S on circulation. This study intends to determine 1- the natural history of cardiac arrest following H2S exposure in a large mammal, 2- whether hydroxocobalamin can affect the immediate outcome of sulfide poisoning, if administered just after H2S exposure, at a time wherein most soluble sulfide has already vanished. To answer these questions, we have developed a model of H2S exposure in the sheep, which has a high mortality after the cessation of sulfide exposure, using repetitions of short bouts of high levels of NaHS, but allowing enough time after complete cessation of H2S exposure to administer an antidote.
METHODS
Animal preparation
Sixteen sedated, adult Dorset sheep (Ovis aries, ewes, 1–2 years old) weighing 54 ± 13 kg were used in these experiments. All experiments were conducted in accordance with the Guideline for the Care and Use of Laboratory Animals published by the United States National Institutes of Health. The study was approved by the Pennsylvania State University College of Medicine Institutional Care and Use Committee.
The animals were pre-medicated with Ketamine (20 mg/kg) intramuscularly, then anesthesia was induced by an intravenous loading dose of Sodium Thiopental (Pentothal 10 mg/kg). Anesthesia was subsequently maintained by an intravenous solution containing urethane (80 mg/kg) and alphachloralose (15 mg/kg), as previously described (4).
The animals were tracheostomized and an inflatable-cuff tracheal cannula (Shilley #7) was inserted through the tracheostomy. Systemic arterial blood pressure and heart rate were monitored by placing a catheter in one of the carotid arteries, which was also used for sampling arterial blood. Venous catheters (Plastimed 3F) were placed in one jugular and one femoral vein for injection of antidotes and NaHS infusion, respectively. One of the femoral arteries was isolated and a transonic flow probe (Transonic Systems Inc. Ithaca, NY) was placed around the artery for blood flow measurement. Animals were mechanically ventilated throughout the experiments to prevent H2S induced apnea (4, 5).
Measurements
Respiratory flow, along with the pulmonary gas exchange rate, was recorded using a Hans-Rudolph pneumotachograph (Hans-Rudolph, Kansas City, MO) connected to the tracheostomy tube and to a differential pressure transducer. The animals were all ventilated in volume control mode (servo Siemens 900C) with a mandatory ventilation of 8 l/min. FIO2 was maintained at 21% throughout the protocol.
The expiratory valve of the ventilator was connected to a mixing chamber where the mixed expired O2 and CO2 fractions were measured continuously using O2 (Oxystar-100, CWE) and CO2 (model 17630, VacuMed, Ventura, CA) analyzers. H2S expired fractions were also measured continuously from the same mixing chamber (Interscan RM series, Simi Valley, CA; 1–200 ppm).
The gas analyzers were calibrated immediately before use with different gas mixtures containing a known concentration of CO2 and O2 in air and H2S in N2. Arterial blood pressure and respiratory flow signals were digitized at 200 Hz (Power Lab system). The respiratory flow signal was integrated for breath-by-breath calculation of tidal volume and minute ventilation and oxygen consumption. At the end of the experiment, animals were euthanized by lethal injection of barbiturate (Pentothal 200 mg/kg) in the jugular catheter.
The arterial catheter was connected to a pressure transducer (TA-100, CWE Inc., Ardmore, PA), while the transonic flow probe was connected to a perivascular flowmeter (TS420, Transonic Systems Inc., Ithaca, NY). Electrocardiogram was recorded by using a bioelectric amplifier (Veterinary PC-ECG System, VacuMed, Ventura, CA).
Determination of dissolved concentrations of H2S in the arterial blood
Mixed expired H2S (FEH2S) was determined as previously described (5). The partial pressure of expired H2S (PEH2S) was calculated as FEH2S× (PB-PH2O), allowing the determination of the alveolar partial pressure of H2S (PAH2S) as PAH2S=PEH2S× (V̇E/V̇A). V̇E/V̇A was determined from the PECO2/PACO2 ratio assuming that PACO2 equates PETCO2. PaH2S was assimilated to PAH2S and the concentration of gaseous H2S in the blood was calculated as: CgH2S=(0.00012× PaH2S) (see (5) for details and references). Following the assumption that H2S is under the form of H2S gas (30%) and HS− (70%) at a pH of 7 (25, 26).
The time constant of the response of the circuit, included the sulfide analyzer was determined using a 2 liter balloon which was connected to the ventilator (minute ventilation of 10 l/min), with a constant flow of a mixture containing 200 ppm H2S at 1 l/min). FEH2S was monitored from the expiratory chamber connected to the expiratory circuit. When FEH2S reached a steady state, the flow of H2S was stopped and the time constant of the circuit, including gas analyzer and dilution of the gas in the balloon was computed as the time needed to drop H2S concentration by 64 % and was found to average 140 seconds.
Protocol
NaHS infusion and recovery
We needed a model producing an oxygen deficit and increased lactic acidosis, without killing the animal before or immediately after the end of exposure, therefore allowing enough time to inject the antidote. To achieve this goal, we established, based on a series of pilot experiments, a protocol consisting in repetitive bouts of “supra-lethal” iv infusion.
Every animal received NaHS, until the level of expired H2S reached 110 ppm or greater, a level, which we previously found lead to a lethal outcome minutes after the end of infusion. This level of expired H2S was achieved by exposing the animals to bouts of NaHS of 40 seconds at a rate of 4 ml/min NaHS (20 mg/ml in saline) separated by 30 seconds with no infusion. As presented in the results section, 3–4 bouts were necessary to reach a level of expired H2S of 110 ppm. This protocol was compatible with immediate survival while it led to the development of an O2 deficit and lactic acid accumulation with a very high mortality by cardiac arrest occurring within less than 10 minutes after the end of infusion (See result section). NaHS solutions were prepared a few minutes before the infusion and kept in airtight syringes. The solution was infused intravenously in the femoral vein using an infusion pump (Fusion 100; Chemyx Inc; Stafford, TX).
Hydroxocobalamin infusion
One minute after the end of NaHS infusion, a solution of Hydroxocobalamin (Cyanokit 5 g in 200 ml saline, Merck Santé, licensed by Meridian Medical Technologies, Inc.) or 200 ml saline were infused over 3 min in the catheter placed in the jugular vein. Arterial blood was sampled before the antidote infusion and then at 5, 10 and 15, 30 and 60 minutes into recovery for determination of Hydroxocobalamin concentrations, blood gases and blood lactate. For Hydroxocobalamin measurement, blood was centrifuged and 1 ml of supernatant was mixed with 500 μl of PBS. The concentration in Hydroxocobalamin present in the blood was estimated by measuring the plasma absorbance at 525 nm as previously described (27).
Data analysis
Breathing frequency (f) and tidal volume (VT) were determined using peak detection and integration of the respiratory flow signal, respectively, and minute ventilation (VE) was computed as f×VT. Oxygen consumption (VO2) was computed using VE, the inspiratory and mixed expiratory fraction of O2.
All signals were digitized at 200 Hz using an analog-to-digital data acquisition system (Power Lab 16/35, AD Instruments, Colorado Springs, CO). Data were stored for later analysis. Mean arterial blood pressure, femoral blood flow, minute ventilation, expired fraction of H2S, the electrocardiogram (ECG) and VO2 were displayed online for monitoring.
Arterial blood gases partial pressure of O2 (PaO2), CO2 (PaCO2), and lactate concentration were measured using i-STAT1 blood gas analyzer (ABAXIS, Union city, CA).
All data are expressed as mean ± SD. The relationship between CgH2S and the time to death was established for every animal. The number of deaths was compared between the 2 groups using a Fisher’s exact test, while the survival rate as a function of time (post infusion) was analysed using a log-rank (Mantel Cox) test and presented with Kaplan Meier curve. P<0.05 was considered as significant. Second, CgH2S, VO2 and blood pressure were compared within each group, before NaHS infusion then at 1, 2, 3, 5, 10, 15 and 20 minutes after the end of NaHS infusion or up to the moment where the first animal died, using repeated ANOVA, followed by a post-hoc multiple comparison procedure (Turkey’s multiple comparison). A two-way ANOVA was also used to compare animals between the saline and the Hydroxocobalamin groups, but only over the shortest period of survival (see result section for further details).
Blood gases and lactate were compared between and within groups at 5, 10, 15, 30 and 60 minutes using a two-way ANOVA followed by a Turkey’s multiple comparison.
RESULTS
Effects of NaHS infusion in control group (n=8)
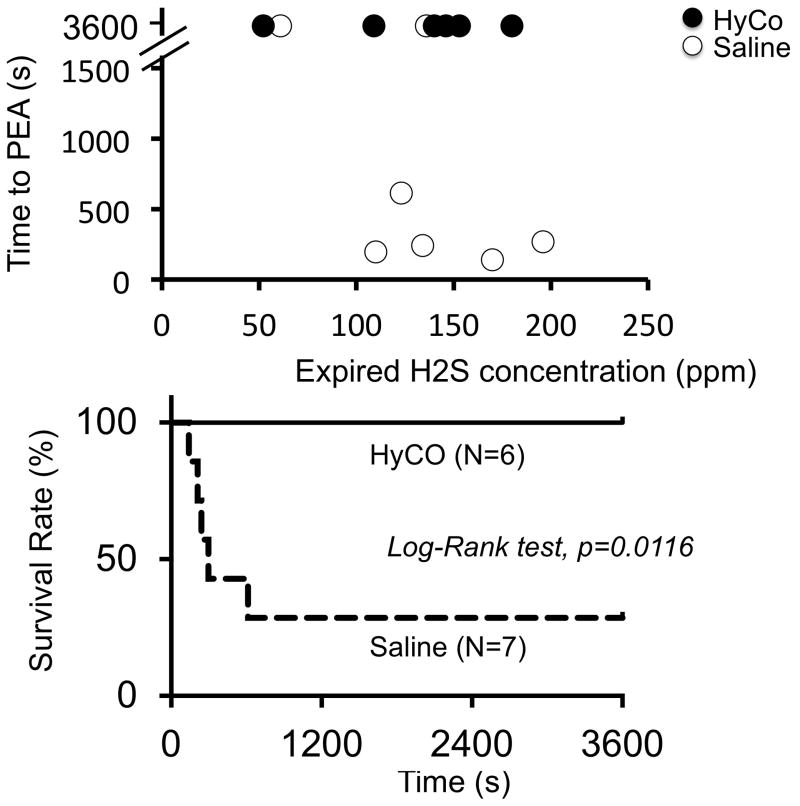
Repetition of 3–4 bouts of NaHS led to a level of expired H2S above 110 ppm in 7 out of 8 control animals. Indeed, in one animal the peak level of mixed expired H2S reached only 61 ppm despite a number of bouts similar to the other sheep. This animal survived the intoxication. In the 7 remaining animals, peak H2S ranged from 122 to 176 ppm (corresponding to a CgH2S between 13 and 22 microM). One of these 7 animals was unable to receive the saline solution in keeping with the protocol, since this sheep survived only 90 seconds after the end of NaHS infusion. This animal was therefore excluded from the comparisons. All of the 6 other animals, along with the animal that reached low levels of expired H2S, received a saline injection. All animals except two (including the animal with the low level of H2S) died by pulseless electrical activity (PEA), as illustrated in figure 1. All of the animals that did not survive displayed a complete disappearance of blood pressure or flow fluctuations between 140 and 613 seconds, while ECG signal was clearly sustained well beyond the period of cardiac arrest (figure 1). Repeated ANOVA was thus performed to analyze data up to 2 minutes following the end of NaHS exposure. Clearly a rapid decrease in blood pressure occurred by the end of NaHS infusion and continued to develop after the cessation of sulfide exposure (drop from 112 ± 8 to 70 ± 32 Torr, 60 seconds after the end of NaHS infusion, p<0.01) along with a severe reduction in femoral blood flow. This was associated to a rapid decrease in VO2 from 203 ± 51 to 148 ± 136 ml/min after one minute (figures 1, 2 and 3) along with a significant rise in blood lactate levels (figure 4). Of note is that in the sheep in which mixed expired H2S reached 61 ppm, no reduction in VO2, blood pressure or increase in lactate levels was observed. In summary, out of the 8 control animals, 7 survived long enough to receive the saline solution. One of these 7 animals did not reach the levels of H2S intended (above 110 ppm) and survived, in the 6 remaining animals, 5 died.
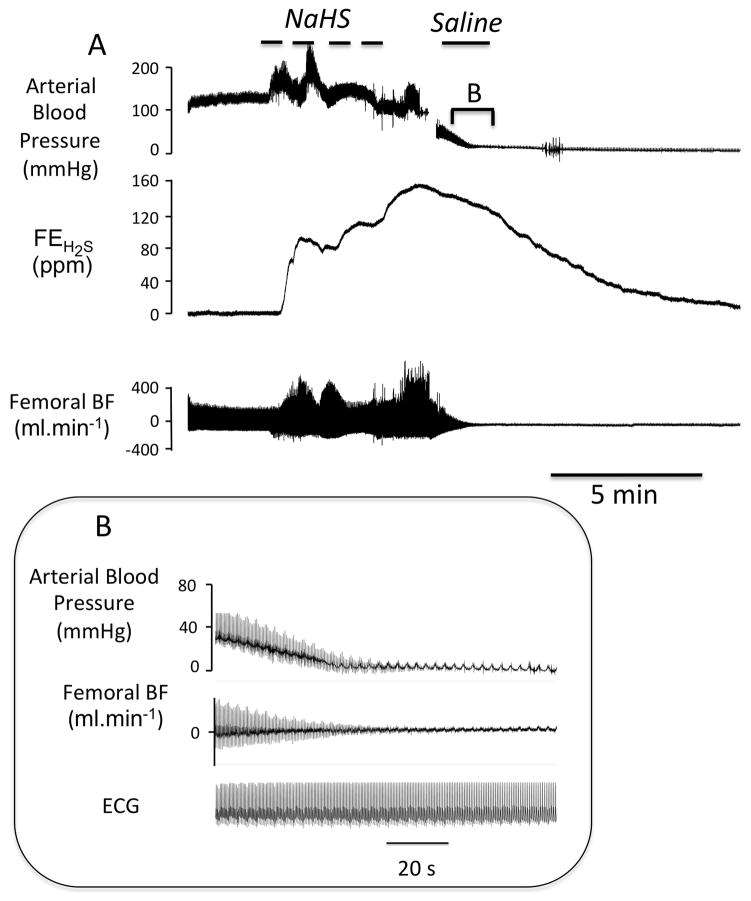
Figure 1.
A Example of a recording obtained in one sheep showing the effects of intravenous infusion of saline administered one minute after the cessation of sodium sulfide (NaHS) infusion. The changes in arterial blood, mixed expired fraction of H2S (FEH2S) and femoral blood flow are shown. In this example, the level of expired H2S reached more than 120 ppm at the end of exposure (see text for corresponding level in the blood). Note that arterial pressure and femoral blood flow decreased very rapidly during the recovery phase from NaHS infusion leading to death by pulseless electrical activity (PEA) within minutes. The inset B is the magnification of period of reduction in blood pressure and cardiac output leading to cardiac arrest, showing the persistence of the normal electrical activity with no arterial pulse pressure defining a PEA. All animals that presented a cardiac arrest kept a persistent electrical activity for several more minutes.
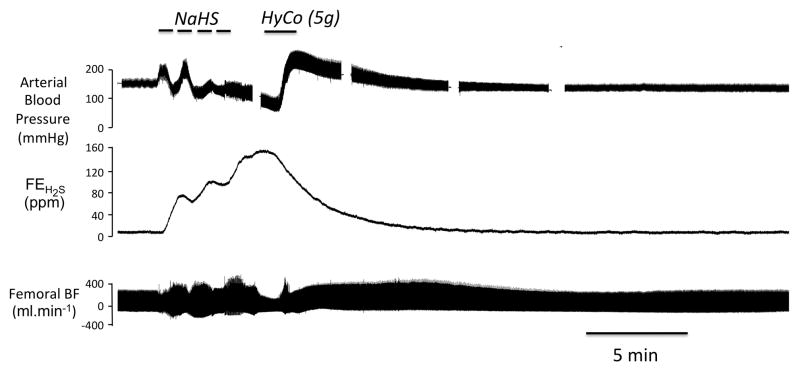
Figure 2.
Example of a recording obtained in one sheep showing the effects of intravenous infusion of 5 g Hydroxocobalamin (HyCo), administered one minute after the cessation of NaHS infusion. The changes in arterial blood, mixed expired fraction of H2S (FEH2S) and femoral blood flow are shown. In this example - like in figure 1- the level of expired H2S also reached more than 120 ppm by the end of period of exposure. In contrast to the animal receiving saline, blood pressure and femoral blood flow, which initially decreased very rapidly during the recovery phase from NaHS infusion, rose during and after Hydroxocobalamin infusion, restoring a normal circulation and preventing death by pulseless electrical activity for the total duration of the protocol.
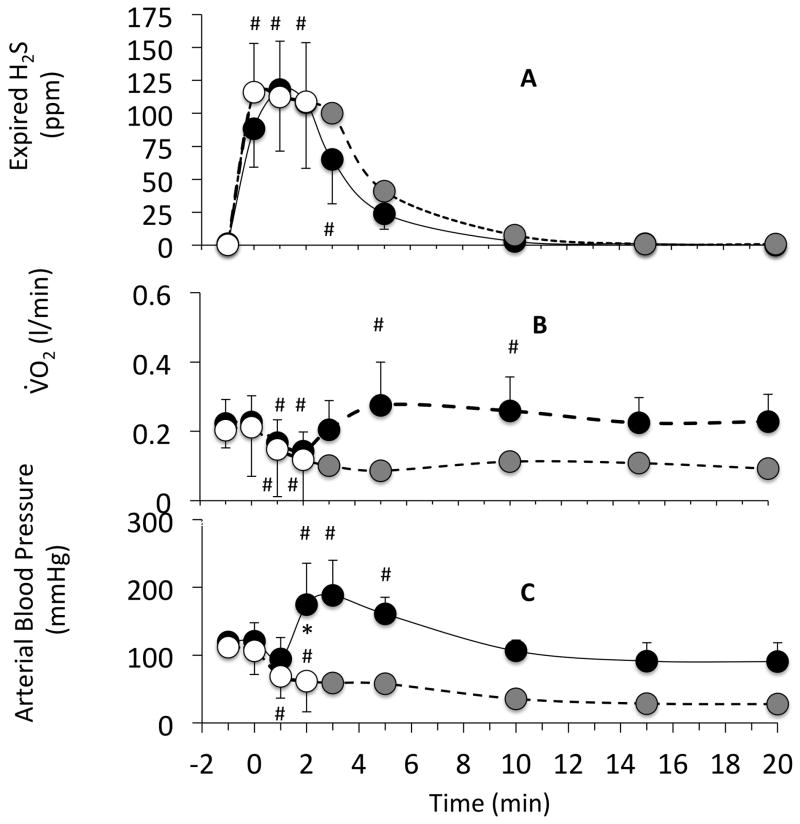
Figure 3.
Time course of expired H2S concentration (panel A), VO2 (panel B) and arterial blood pressure (panel C), following the cessation of NaHS exposure. Data are shown as mean ± SD. The infusion of sulfide was interrupted at time zero. Injections of saline (open circles) or hydroxocobalamin (closed circles) were performed 60 seconds following the end of NaHS infusion. In the hydroxocobalamin group, since all animal survived, averaged data are shown up to 20 minutes and have been compared to baseline data, using a one-way repeated ANOVA. For saline experiments, data points are shown up to 120 seconds as animals started to die thereafter. Averaged data in the saline group are shown using a dotted line after 120 seconds as data were obtained from animals already in cardiac arrest while some were still alive. Data from the saline group were therefore compared to the Hydroxocobalamin group (two-way ANOVA) up to 120 seconds. Expired H2S decreased very rapidly with no difference between the Hydroxocobalamin or saline groups. In contrast both arterial blood pressure and VO2 increased significantly after Hydroxocobalamin, while in the saline group all animals, but 2 displayed a fatal drop in blood pressure. Values are shown as mean ± SD. #significantly from baseline, *significantly different between the 2 groups, p<0.05.
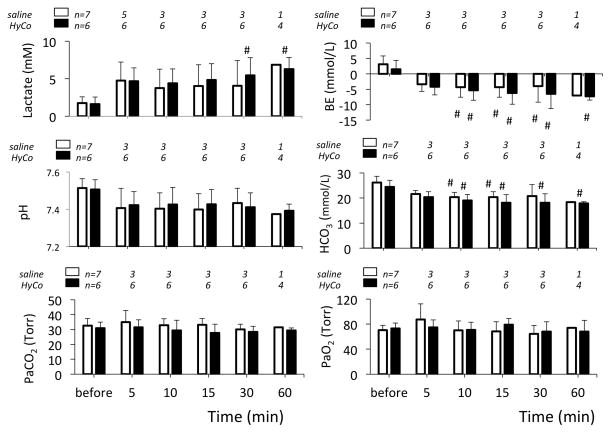
Figure 4.
Arterial lactate, base excess (BE), pH, bicarbonate (HCO3) and blood gases, before NaHS infusion then at 5, 10, 15, 30 and 60 minutes following the cessation of NaHS exposure in the animals receiving saline or Hydroxocobalamin. The number of animals studied is shown. # significantly different from baseline values at P<0.05.
Hydroxocobalamin infusion (n=8)
Following the same protocol as in the control group, two out of the 8 animals presented a PEA 36 and 90 seconds into recovery and could not receive any injection of Hydroxocobalamin, they were therefore not used for comparison and were excluded from the data analysis. One animal reached only 52 ppm (CgH2S = 6 microM) and, just like in the saline group, did not display any drop in blood pressure and VO2, or increase in lactate. In all of the remaining animals, peak levels of H2S reached 110 to 180 ppm at the end of exposure, corresponding to CgH2S ranging from 13 to 22 microM. All of these animals presented a rapid reduction in blood pressure (120 ± 8 to 94 ± 31 Torr) and VO2 (222 ± 69 to 167 ± 66 ml/min) one minute into recovery (figures 2 and 3). There was no difference between this group of animals and the control/saline group in terms of the severity of the circulatory failure, until the infusion of Hydroxocobalamin was performed (figure 2). The 6 animals that received Hydroxocobalamin presented a very rapid and significant increase in blood pressure to 199 ± 48 Torr, 120 seconds after the onset of Hydroxocobalamin injection. Blood pressure and blood flow after this initial overshoot returned towards baseline values within 5 minutes (figure 3). In major contrast to the control group, all of the animals survived (figure 5). Survival was thus significantly different from the control group (Fisher tests and Log Rank test). Comparison with control animals could be performed using repeated ANOVA in all animals up to 120 seconds and is shown in figure 4. Note that since VO2 and circulatory data were recorded up to 20 minutes in all animals, data have been averaged over this entire period in the saline group but data obtained after the first animal died (140 seconds) are shown without standard deviation for visual comparison only.
Figure 5.
Top panel Individual data showing the time to cardiac arrest by pulseless electrical activity (PEA) after the end of NaHS infusion as a function of the peak value of mixed expired H2S. Only the data obtained from the animals that did receive either hydroxocobalamin (HyCo, black symbols) or saline (open symbols) are shown. Lower panel: Survival rate in these 2 groups of animals (survival is significantly different between the 2 groups, log-rank test p=0.0116).
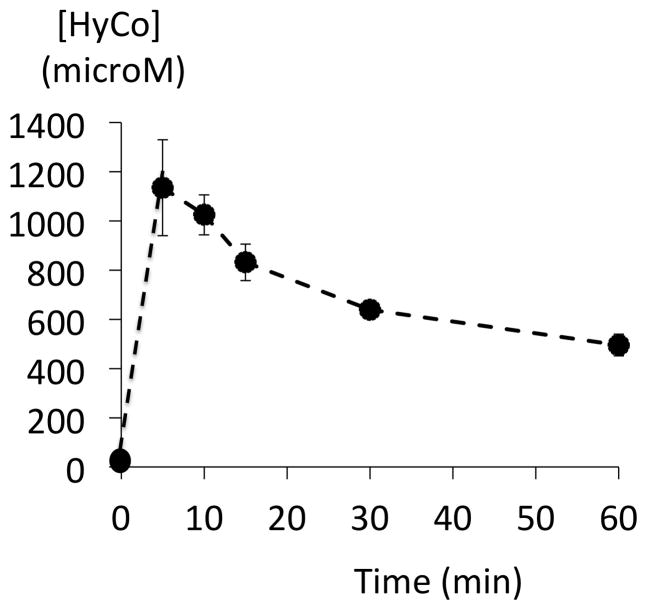
Although VO2 and blood pressure returned to their baseline values (figure 3), lactic acid, which increased up to 5.38 mmol/l one minute into recovery, did not decrease over the entire hour following H2S poisoning as shown on figure 5. This was associated with a progressive increase in base deficit (Figure 4). No hypoxemia was observed over the hour following the cessation of infusion (Figure 4). The concentration of Hydroxocobalamin sampled 2 minutes after the onset of infusion was found to reach levels above 1000 microM before declining progressively. Blood Hydroxocobalamin concentrations remained above 400 microM for at least one hour (figure 6).
Figure 6.
Time course of the concentrations of Hydroxocobalamin (HyCo) in the blood following HyCo infusion. Data are shown as mean ± SD. Blood was collected in the 6 animals that received HyCo. Concentration in HyCo increased within minutes up to 1000 microM and remained above 400 microM up to an hour after the end of infusion.
DISCUSSION
We found that in a large animal model, wherein H2S is rapidly risen to levels able to produce a cardiac arrest, 3 out of 16 adult sheep died within 90 seconds following the end of sulfide exposure and were not able to receive any antidotes. Among the 13 animals that received the antidote or a saline infusion, 71% of the animals died in the saline group by PEA, while all animals survived following Hydroxocobalamin administration, when administered between one and four minutes after the end of H2S exposure.
H2S intoxication
H2S exposure remains an occupational hazard in gas production, well drilling and gas refining industries (28, 29). H2S poisoning has also become a dreadful method of suicide over the last few years (2, 30–32). The incidence of this form of suicide has increased due to a phenomenon of imitation or “copy-cat” following reports in the media (33) and is committed by mixing in a closed environment (usually in a car or in a small room) a source of sulfur and various types of acidic solutions, readily available in most household chemicals. This method of suicide has also created new risks for neighbors and first responders (34, 35). Finally, since it is very easy to manufacture, H2S is also regarded as a possible threat by the Department of Homeland Security (36), and can create a large-scale panic due to its distinguishable smell of rotten egg.
At high levels of exposure - typically around 1000 ppm- hydrogen sulfide has been shown to be rapidly fatal by primary cardiorespiratory arrest. At lower levels, which, for obvious reasons, are not well established in humans, patients can present a reversible coma, often referred as “knock-down”. However, some patients can develop severe post-anoxic/toxic injuries (37). These neurological after-effects, which combine cognitive as well as motor or sensory deficits, result from the combination of the acute inhibition of mitochondrial cytochrome C oxidase in neurons (38, 39) and the depressive effects of sulfide on the cardio-vascular system (40). The latter appears to potentiate the mitochondrial insult produced by H2S on neuron, per se.
H2S induced cardiac toxicity
In the present study, when levels of CgH2S reached 12 to 16 microM, death occurred between 30 seconds and 10 minutes following H2S exposure. A reduction in cardiac function produced by sulfide has been proposed to be one of the main factors dictating the outcome of animals and possibly patients surviving H2S induced coma (40). Cardiac arrest may be precipitated by a terminal apnea induced anoxia, although typically this form of cardiac arrest takes time to develop, or through an acute episode of ischemia-induced ventricular fibrillation. This study revealed that cardiac arrest results from a typical pulseless electrical activity (PEA) phenomenon, which can still occur after the period of exposure. This PEA seems to be the final stage of a very rapid deterioration in cardiac function. Sun et al. and Zhang et al. (41, 42) have established in-vitro that one of the main effects of H2S on cardiomyocytes at toxic concentrations is to inhibit L-Type Calcium channels. This inhibition of L-Type Calcium Currents could be accounted for by the interaction of H2S with free sulfhydric group present on the channels. The redox modulation of L-Type Calcium channel activity (43) could also be affected by the presence of sulfide leading to a rapid inhibition of the mechanical contractions of the cardiomyocytes (41, 42). This inhibition has been studied in vitro and is clearly present at 100 microM of soluble H2S, a figure higher than what was required in-vivo to produce a toxic effect. Interestingly, this makes the effects of H2S intoxication on the heart very similar to those produced by calcium channels blockers poisoning (44). In the 2 animals, where CgH2S only peaked at 6–7 microM, no significant decrease in blood pressure or VO2 was produced. Putting these results in the context of our previous studies (4, 5), concentrations of gaseous H2S around 1 microM are associated with a stimulation in breathing, concentrations between 6–8 microM produces an apnea, and as soon as levels of H2S increase above 10 microM, PEA can occur. Such concentrations of gaseous H2S correspond to a total level of sulfide 10 to 20 times higher (5).
Effects of Hydroxocobalamin
We have recently found that the ferric or cobalt compounds (methemoglobin solution or very high dose of hydroxocobalamin) currently proposed as antidotes against H2S poisoning are very effective in trapping gaseous/free H2S and thus decreasing the amount of H2S diffusing into tissue to exert its toxic effects (4). The impressive effects of these antidotes are, however, somehow misleading, as the pool of free H2S, i.e. gaseous H2S and its sulfhydric anion HS−, is mostly -if not exclusively- present in the blood during H2S exposure (5). The very high ability of the mitochondria from all tissues to oxidize sulfide (45) explains why this pool vanishes within less than one minute after exposure (1, 4). In conditions associated with low cardiac output (or even cardiac arrest), one can imagine that H2S could still be found in the blood, but as soon as circulation is restored, whether by cardio-pulmonary resuscitation or spontaneously, free H2S should disappear very rapidly. As illustrated on figure 3, the time constant of the off-kinetics of arterial H2S was not different from the time constant of the circuit and the response time of the analyzers (140 seconds, see method section). Since the effects of any antidote on H2S poisoning is to be primarily dictated by kinetics of the pools of soluble H2S in the blood and tissues after H2S exposure, i.e. when patients are withdrawn from the source of intoxication and taken care of by paramedic units, one may question the benefit of any treatment based on trapping agents, which includes Hydroxocobalamin (8, 27) and methemoglobinemia (46). In other words, it remains to be explained how Hydroxocobalamin could be effective when no free H2S is present. Indeed, following H2S exposure, VO2 rapidly increased after Hydroxocobalamin administration along with the restoration of blood pressure and flow, markers of the improvement of the cardiac function. These rapid changes in VO2 very likely reflect the increase in cardiac output, rather than an improvement in cellular metabolism since lactate levels remained very high and were no different from control animals. Whether Hydroxocobalamin enters cardiac cells fast enough even via a non-transcobalamin mechanism (13) and can affect the concentrations of H2S already combined on proteins, remain unanswered questions.
Alternative mechanisms unrelated to the ability of Hydroxocobalamin to combine sulfide H2S can be proposed. For instance, the potent anti-NO effects of Hydroxocobalamin (16), as illustrated by the rapid and powerful increase in blood pressure, both in the present study and in the literature (19), could have counteracted some of the toxicity of H2S, which have been shown to be enhanced in the presence of NO (21). The latter can significantly contribute to the reduction in cardiac contractility in various pathological states from septic shock to chronic heart failure (47–50). NO and H2S may thus in concert affect cardiac contractility which could be antagonized by Hydroxocobalamin. Such a mechanism does not require the presence of high level of Hydroxocobalamin in the cells and does not necessarily require the persistence of free H2S. Whether Hydroxocobalamin could counteract the effects of sulfide on Calcium channels (41, 42) is an outstanding question. Finally, in this study, we used a dose of Hydroxocobalamin of about 100 mg/kg, it remains to be determined if lower levels of Hydroxocobalamin can be as protective.
Limitations of the model used
Creating a model of H2S poisoning faithful to a relevant clinical scenario is always challenging. Using a ventilated sedated animal may certainly affect the cardiovascular, metabolic and neuronal responses to the anoxic insult produced by sulfide differently from an un-anesthetized human. This point is, however, to be considered in the light of the use of urethane-chloralose in our model, as this “old” modality of sedation maintains intact a large part of the medullary reflexes (51–54). The initial injections of ketamine and barbiturate may still affect the response to sulfide, though equally in both groups; the doses we used were relatively small and only administered during the initial surgical period. In our view, the major limitation of this study relies on the “artificial” scenario created by our experimental setting in keeping with the specificity of sulfide poisoning. Indeed, a small difference in H2S concentrations can allow survival or can produce death within seconds or minutes. The steepness of the dose-toxicity relationship for H2S along with the fast disappearance of free/soluble forms of H2S from the blood and tissue (within a minute), make the conditions of success of any treatment of sulfide poisoning very difficult to evaluate. To substantiate any potential effect of a Hydroxocobalamin, we had to create specific conditions allowing most animals to survive long enough to be able to receive an antidote, while the model would still be lethal. Any antidote found to be effective one to 4 minutes after the end of exposure may therefore prove to be deceiving in real life conditions, due to this very short window of opportunity. Similarly, scenarios wherein an experimental model would still be exposed to toxic levels of H2S while receiving an antidote do not necessarily correspond to a realistic situation, since the basis for treating H2S poisoning –and reducing its concentration in the blood and tissue- is to allow the very effective sulfide oxidation to take place by preventing new molecules of sulfide to diffuse into blood. The rapid drop of H2S shown in figure 3 and previously reported (4, 5) supports the view that the most effective approach for treating sulfide poisoning is still to remove the subjects from any possible new contamination.
In conclusion we found that 1) H2S can kill by producing PEA even after the cessation of H2S exposure, 2) high dose Hydroxocobalamin (5 g) administered intravenously counteracts this effect, if injected between one to 4 minutes after the cessation of exposure, 3) the effect of large dose of Hydroxocobalamin does not seem to rely on the trapping of H2S.
Acknowledgments
The authors are grateful to Ms. Nicole Tubbs for her skillful technical assistance. This work has been supported in part by the CounterACT Program, National Institutes of Health, Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number 5R21NS080788-02. Dr. Sonobe was supported by a Grant-in-Aid for scientific research (N11J10987) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Haggard HW. The fate of sulfides in the blood. The Journal of biological chemistry. 1921;49:519–29. [Google Scholar]
- 2.Beauchamp RO, Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol. 1984;13(1):25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 3.Guidotti TL. Hydrogen sulfide: advances in understanding human toxicity. International journal of toxicology. 2010 Dec;29(6):569–81. doi: 10.1177/1091581810384882. Epub 2010/11/16. eng. [DOI] [PubMed] [Google Scholar]
- 4.Haouzi P, Sonobe T, Torsell-Tubbs N, Prokopczyk B, Chenuel B, Klingerman CM. In Vivo Interactions Between Cobalt or Ferric Compounds and the Pools of Sulphide in the Blood During and After H2S Poisoning. Toxicological sciences : an official journal of the Society of Toxicology. 2014 Jul 11; doi: 10.1093/toxsci/kfu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klingerman CM, Trushin N, Prokopczyk B, Haouzi P. H2S concentrations in the arterial blood during H2S administration in relation to its toxicity and effects on breathing. American journal of physiology Regulatory, integrative and comparative physiology. 2013 Sep 15;305(6):R630–8. doi: 10.1152/ajpregu.00218.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toombs CF, Insko MA, Wintner EA, Deckwerth TL, Usansky H, Jamil K, et al. Detection of exhaled hydrogen sulphide gas in healthy human volunteers during intravenous administration of sodium sulphide. British journal of clinical pharmacology. 2010 Jun;69(6):626–36. doi: 10.1111/j.1365-2125.2010.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van de Louw A, Haouzi P. Inhibitory effects of hyperoxia and methemoglobinemia on H(2)S induced ventilatory stimulation in the rat. Respiratory physiology & neurobiology. 2012 May 31;181(3):326–34. doi: 10.1016/j.resp.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Truong DH, Mihajlovic A, Gunness P, Hindmarsh W, O’Brien PJ. Prevention of hydrogen sulfide (H2S)-induced mouse lethality and cytotoxicity by hydroxocobalamin (vitamin B(12a)) Toxicology. 2007 Dec 5;242(1–3):16–22. doi: 10.1016/j.tox.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Mihajlovic A. [PhDThesis] Toronto: 1999. Antidotal mechanisms for hydrogen sulfide toxicity. [Google Scholar]
- 10.Van de Louw A, Haouzi P. Ferric iron and cobalt (III) compounds to safely decrease H2S in the body? Antioxidants & redox signaling. 2012 Jan 10; doi: 10.1089/ars.2012.4513. Epub 2012/01/12. Eng. [DOI] [PubMed] [Google Scholar]
- 11.Brenner M, Benavides S, Mahon SB, Lee J, Yoon D, Mukai D, et al. The vitamin B12 analog cobinamide is an effective hydrogen sulfide antidote in a lethal rabbit model. Clinical toxicology. 2014 Apr 9; doi: 10.3109/15563650.2014.904045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Astier A, Baud FJ. Complexation of intracellular cyanide by hydroxocobalamin using a human cellular model. Human & experimental toxicology. 1996 Jan;15(1):19–25. doi: 10.1177/096032719601500104. [DOI] [PubMed] [Google Scholar]
- 13.Hall CA, Begley JA. Atypical cobalamin binding in the serum of congenital deficiency of transcobalamin II. British journal of haematology. 1982 May;51(1):65–71. doi: 10.1111/j.1365-2141.1982.tb07290.x. [DOI] [PubMed] [Google Scholar]
- 14.Hall CA, Begley JA, Green-Colligan PD. The availability of therapeutic hydroxocobalamin to cells. Blood. 1984 Feb;63(2):335–41. Epub 1984/02/01. eng. [PubMed] [Google Scholar]
- 15.Gimpert E, Jakob M, Hitzig WH. Vitamin B12 transport in blood. I. Congenital deficiency of transcobalamin II. Blood. 1975 Jan;45(1):71–82. Epub 1975/01/01. eng. [PubMed] [Google Scholar]
- 16.Sharma VS, Pilz RB, Boss GR, Magde D. Reactions of nitric oxide with vitamin B12 and its precursor, cobinamide. Biochemistry. 2003 Jul 29;42(29):8900–8. doi: 10.1021/bi034469t. [DOI] [PubMed] [Google Scholar]
- 17.Rochelle LG, Morana SJ, Kruszyna H, Russell MA, Wilcox DE, Smith RP. Interactions between hydroxocobalamin and nitric oxide (NO): evidence for a redox reaction between NO and reduced cobalamin and reversible NO binding to oxidized cobalamin. The Journal of pharmacology and experimental therapeutics. 1995 Oct;275(1):48–52. [PubMed] [Google Scholar]
- 18.Brouwer M, Chamulitrat W, Ferruzzi G, Sauls DL, Weinberg JB. Nitric oxide interactions with cobalamins: biochemical and functional consequences. Blood. 1996 Sep 1;88(5):1857–64. [PubMed] [Google Scholar]
- 19.Gerth K, Ehring T, Braendle M, Schelling P. Nitric oxide scavenging by hydroxocobalamin may account for its hemodynamic profile. Clinical toxicology. 2006;44( Suppl 1):29–36. doi: 10.1080/15563650600811805. [DOI] [PubMed] [Google Scholar]
- 20.Altaany Z, Yang G, Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. Journal of cellular and molecular medicine. 2013 Jul;17(7):879–88. doi: 10.1111/jcmm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proceedings of the National Academy of Sciences of the United States of America. 2012 Jun 5;109(23):9161–6. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forsyth JC, Mueller PD, Becker CE, Osterloh J, Benowitz NL, Rumack BH, et al. Hydroxocobalamin as a cyanide antidote: safety, efficacy and pharmacokinetics in heavily smoking normal volunteers. J Toxicol Clin Toxicol. 1993;31(2):277–94. doi: 10.3109/15563659309000395. Epub 1993/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd G, Velez LI. Role of hydroxocobalamin in acute cyanide poisoning. Ann Pharmacother. 2008 May;42(5):661–9. doi: 10.1345/aph.1K559. Epub 2008/04/10. eng. [DOI] [PubMed] [Google Scholar]
- 24.Haouzi P, Chenuel B, Sonobe T, Klingerman CM. Are H2S-trapping compounds pertinent to the treatment of sulfide poisoning? Clinical toxicology. 2014 Jun;52(5):566. doi: 10.3109/15563650.2014.923906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almgren T, Dyrssen D, Elgquist B, Johannsson O. Dissociation of hydrogen sulfide in seawater and comparison of pH scales. Marine Chemistry. 1976;4:289–97. [Google Scholar]
- 26.Millero FJ. The thermodynamics and kinetics of hydrogen sulfide system in natural waters. Marine Chemistry. 1986;18:121–47. [Google Scholar]
- 27.Van de Louw A, Haouzi P. Ferric Iron and Cobalt (III) compounds to safely decrease hydrogen sulfide in the body? Antioxidants & redox signaling. 2013 Aug 10;19(5):510–6. doi: 10.1089/ars.2012.4513. [DOI] [PubMed] [Google Scholar]
- 28.Arnold IM, Dufresne RM, Alleyne BC, Stuart PJ. Health implication of occupational exposures to hydrogen sulfide. J Occup Med. 1985 May;27(5):373–6. doi: 10.1097/00043764-198505000-00018. Epub 1985/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 29.EPA. Toxicological Review of Hydrogen Sulfide (CAC No 7783-06-04) Washington DC: United States Environmental Protection Agency; 2003. [Google Scholar]
- 30.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annual review of pharmacology and toxicology. 1992;32:109–34. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 31.Guidotti TL. Hydrogen sulphide. Occup Med (Lond) 1996 Oct;46(5):367–71. doi: 10.1093/occmed/46.5.367. Epub 1996/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 32.Fuller DC, Suruda AJ. Occupationally related hydrogen sulfide deaths in the United States from 1984 to 1994. J Occup Environ Med. 2000 Sep;42(9):939–42. doi: 10.1097/00043764-200009000-00019. Epub 2000/09/22. eng. [DOI] [PubMed] [Google Scholar]
- 33.Hagihara A, Abe T, Omagari M, Motoi M, Nabeshima Y. The impact of newspaper reporting of hydrogen sulfide suicide on imitative suicide attempts in Japan. Social psychiatry and psychiatric epidemiology. 2013 Jul 14; doi: 10.1007/s00127-013-0741-8. [DOI] [PubMed] [Google Scholar]
- 34.Reedy SJ, Schwartz MD, Morgan BW. Suicide fads: frequency and characteristics of hydrogen sulfide suicides in the United States. West J Emerg Med. 2011 Jul;12(3):300–4. Epub 2011/07/07. eng. [PMC free article] [PubMed] [Google Scholar]
- 35.Truscott A. Suicide fad threatens neighbours, rescuers. CMAJ. 2008 Aug 12;179(4):312–3. doi: 10.1503/cmaj.080878. Epub 2008/08/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DHS. Final rule (FR No 07-5585) Department of Homeland Security; 2007. Appendix to Chemical Facility Anti-Terrorism Standards. [Google Scholar]
- 37.Tvedt B, Skyberg K, Aaserud O, Hobbesland A, Mathiesen T. Brain damage caused by hydrogen sulfide: a follow-up study of six patients. American journal of industrial medicine. 1991;20(1):91–101. doi: 10.1002/ajim.4700200109. [DOI] [PubMed] [Google Scholar]
- 38.Dorman DC, Moulin FJ, McManus BE, Mahle KC, James RA, Struve MF. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicological sciences : an official journal of the Society of Toxicology. 2002 Jan;65(1):18–25. doi: 10.1093/toxsci/65.1.18. [DOI] [PubMed] [Google Scholar]
- 39.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. Journal of bioenergetics and biomembranes. 2008 Oct;40(5):533–9. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 40.Baldelli RJ, Green FH, Auer RN. Sulfide toxicity: mechanical ventilation and hypotension determine survival rate and brain necrosis. J Appl Physiol (1985) 1993 Sep;75(3):1348–53. doi: 10.1152/jappl.1993.75.3.1348. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Sun Y, Tsai H, Tang C, Jin H, Du J. Hydrogen sulfide inhibits L-type calcium currents depending upon the protein sulfhydryl state in rat cardiomyocytes. PloS one. 2012;7(5):e37073. doi: 10.1371/journal.pone.0037073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovascular research. 2008 Sep 1;79(4):632–41. doi: 10.1093/cvr/cvn140. [DOI] [PubMed] [Google Scholar]
- 43.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovascular research. 2006 Jul 15;71(2):310–21. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Kerns W., 2nd Management of beta-adrenergic blocker and calcium channel antagonist toxicity. Emergency medicine clinics of North America. 2007 May;25(2):309–31. doi: 10.1016/j.emc.2007.02.001. abstract viii. [DOI] [PubMed] [Google Scholar]
- 45.Bouillaud F, Blachier F. Mitochondria and sulfide: a very old story of poisoning, feeding, and signaling? Antioxidants & redox signaling. 2011 Jul 15;15(2):379–91. doi: 10.1089/ars.2010.3678. [DOI] [PubMed] [Google Scholar]
- 46.Smith RP. Nitrite treatment for hydrogen sulfide poisoning. Ann Intern Med. 1981 Dec;95(6):782. doi: 10.7326/0003-4819-95-6-782_1. [DOI] [PubMed] [Google Scholar]
- 47.Brady AJ, Poole-Wilson PA, Harding SE, Warren JB. Nitric oxide production within cardiac myocytes reduces their contractility in endotoxemia. Am J Physiol. 1992 Dec;263(6 Pt 2):H1963–6. doi: 10.1152/ajpheart.1992.263.6.H1963. [DOI] [PubMed] [Google Scholar]
- 48.Hare JM, Colucci WS. Role of nitric oxide in the regulation of myocardial function. Progress in cardiovascular diseases. 1995 Sep-Oct;38(2):155–66. doi: 10.1016/s0033-0620(05)80004-0. [DOI] [PubMed] [Google Scholar]
- 49.Hare JM, Loh E, Creager MA, Colucci WS. Nitric oxide inhibits the positive inotropic response to beta-adrenergic stimulation in humans with left ventricular dysfunction. Circulation. 1995 Oct 15;92(8):2198–203. doi: 10.1161/01.cir.92.8.2198. [DOI] [PubMed] [Google Scholar]
- 50.Rastaldo R, Pagliaro P, Cappello S, Penna C, Mancardi D, Westerhof N, et al. Nitric oxide and cardiac function. Life sciences. 2007 Aug 16;81(10):779–93. doi: 10.1016/j.lfs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 51.Haouzi P, Bell HJ. Respiratory effects of changing the volume load imposed on the peripheral venous system. Respiratory physiology & neurobiology. 2010 May 31;171(3):175–80. doi: 10.1016/j.resp.2010.04.007. Epub 2010/04/20. eng. [DOI] [PubMed] [Google Scholar]
- 52.Haouzi P, Notet V, Chenuel B, Chalon B, Sponne I, Ogier V, et al. H2S induced hypometabolism in mice is missing in sedated sheep. Respiratory physiology & neurobiology. 2008 Jan 1;160(1):109–15. doi: 10.1016/j.resp.2007.09.001. Epub 2007/11/06. eng. [DOI] [PubMed] [Google Scholar]
- 53.Haouzi P, Beyaert C, Gille JP, Chalon B, Marchal F. Laryngeal reflex apnea is blunted during and after hindlimb muscle contraction in sheep. Am J Physiol. 1997;272(2 Pt 2):R586–92. doi: 10.1152/ajpregu.1997.272.2.R586. [DOI] [PubMed] [Google Scholar]
- 54.Haouzi P, Huszczuk A, Gille JP, Chalon B, Marchal F, Crance JP, et al. Vascular distension in muscles contributes to respiratory control in sheep. Respir Physiol. 1995;99(1):41–50. doi: 10.1016/0034-5687(94)00083-c. [DOI] [PubMed] [Google Scholar]