Abstract
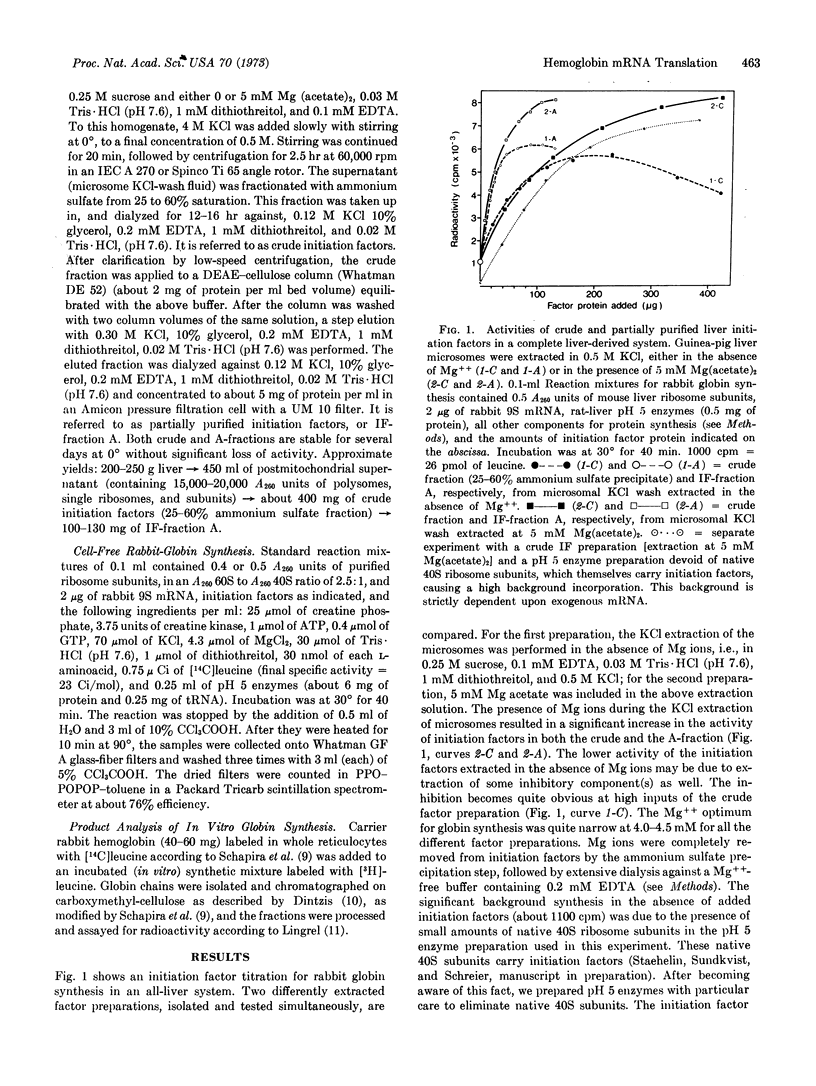
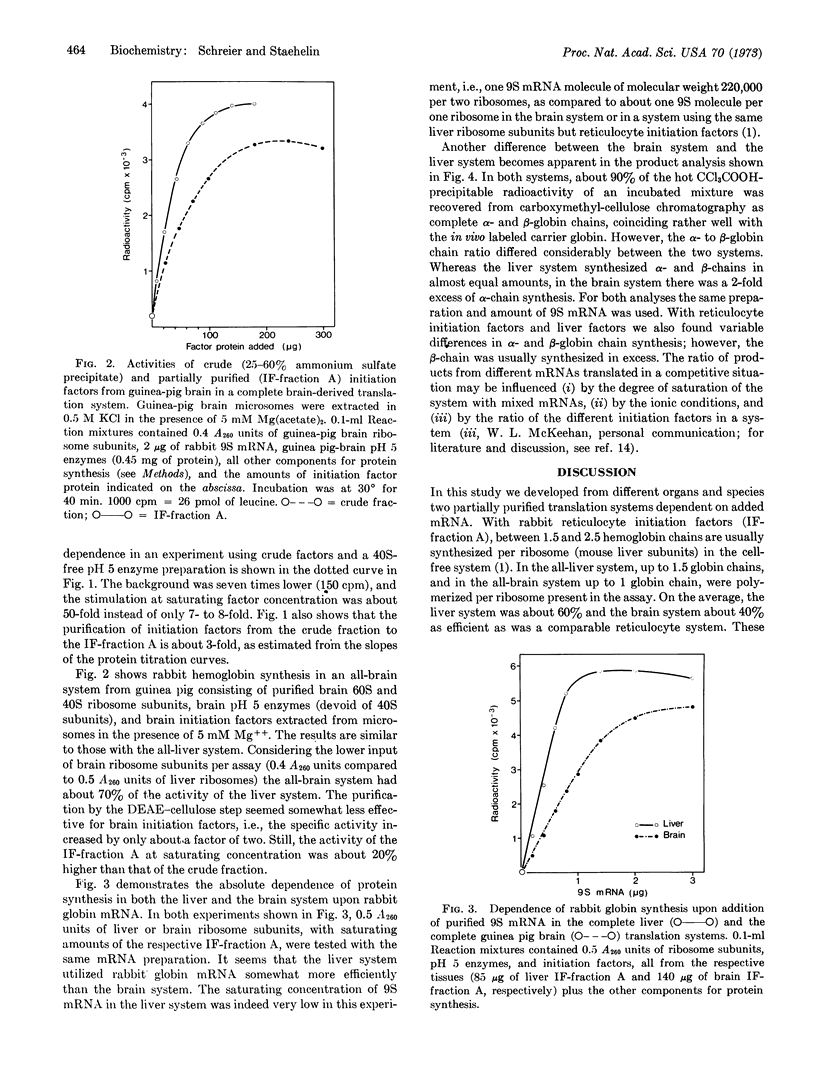
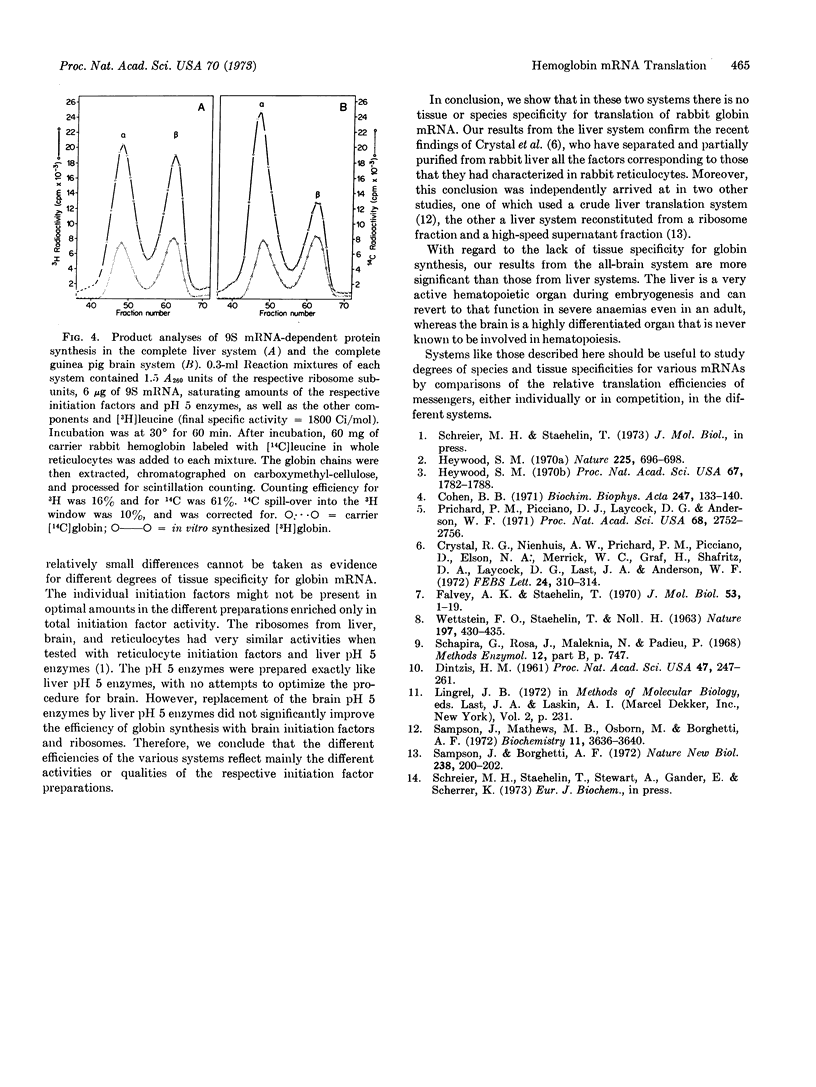
Two mammalian systems have been developed for efficient in vitro translation of exogenous messenger RNA (rabbit globin mRNA). One system is completely derived from guinea pig brain, and the other is from liver of different species. Both systems consist of purified 60S and 40S ribosomal sununits, unseparated initiation factors partially purified by ammonium sulfate precipitation and DEAE-cellulose chromatography, and pH 5 enzyme fractions as sources of elongation and termination factors, aminoacyl-tRNA synthetases, and transfer RNA. Translation depends completely upon exogenous mRNA and initiation factors. Extraction of initiation factors from microsomes or ribosomes has been improved for these tissues by inclusion of Mg++ ions in the 0.5 M KCl extraction solution. Both systems synthesize complete rabbit α- and β-globin chains, but in variable ratios. The overall efficiencies of the two systems are about 60% (liver) and 40% (brain) of a comparable system with rabbit reticulocyte initiation factors.
Keywords: guinea pig, mouse, ribosomal subunits, initiation factors
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen B. B. Cell-free protein synthesis in mixed systems with components from ascites cells and reticulocytes. Biochim Biophys Acta. 1971 Sep 30;247(1):133–140. doi: 10.1016/0005-2787(71)90816-1. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Nienhuis A. W., Prichard P. M., Picciano D., Elson N. A., Merrick W. C., Graf H., Shafritz D. A., Laycock D. G., Last J. A. Initiation of globin synthesis. FEBS Lett. 1972 Aug 15;24(3):310–314. doi: 10.1016/0014-5793(72)80379-x. [DOI] [PubMed] [Google Scholar]
- DINTZIS H. M. Assembly of the peptide chains of hemoglobin. Proc Natl Acad Sci U S A. 1961 Mar 15;47:247–261. doi: 10.1073/pnas.47.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. I. Isolation and characterization of active liver ribosomal subunits. J Mol Biol. 1970 Oct 14;53(1):1–19. doi: 10.1016/0022-2836(70)90042-2. [DOI] [PubMed] [Google Scholar]
- Heywood S. M. Formation of the initiation complex using muscle messenger RNAs. Nature. 1970 Feb 21;225(5234):696–698. doi: 10.1038/225696a0. [DOI] [PubMed] [Google Scholar]
- Heywood S. M. Specificity of mRNA binding factor in eukaryotes. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1782–1788. doi: 10.1073/pnas.67.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard P. M., Picciano D. J., Laycock D. G., Anderson W. F. Translation of exogenous messenger RNA for hemoglobin on reticulocyte and liver ribosomes. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2752–2756. doi: 10.1073/pnas.68.11.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson J., Borghetti A. F. Translation of rabbit globin mRNA. Nat New Biol. 1972 Aug 16;238(85):200–202. doi: 10.1038/newbio238200a0. [DOI] [PubMed] [Google Scholar]
- Sampson J., Mathews M. B., Osborn M., Borghetti A. F. Hemoglobin messenger ribonucleic acid translation in cell-free systems from rat and mouse liver and Landschutz ascites cells. Biochemistry. 1972 Sep 12;11(19):3636–3640. doi: 10.1021/bi00769a022. [DOI] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]


