Atrial fibrillation (AF) affects 2.2 million Americans, making it the most common form of sustained cardiac arrhythmia.1,2 Admissions for inpatient care associated with AF have risen dramatically over the last several years, and are expected to increase further. AF annual hospitalizations in the United States are predicted to rise from 376,000 in 1999 to more than 3.3 million by 2025.3 Furthermore, an estimated 5% of all patients undergoing cardiac surgery have AF, making it an important surgical problem.
AF is associated with significant morbidity and mortality because of its three detrimental sequelae: (1) palpitations, causing patient discomfort and anxiety; (2) loss of synchronous atrioventricular (AV) contraction, compromising cardiac hemodynamics and resulting in ventricular dysfunction; and (3) stasis of blood flow in the left atrium, leading to increased risk for thromboembolism and stroke.4–8 AF confers a three- to fivefold increased risk for stroke, and is responsible for an estimated 15% to 20% of all strokes.1 AF independently increases mortality rates. Using data from the Framingham Heart Study, Benjamin and colleagues9 established the risk factor-adjusted odds ratio for death in men and women with AF as 1.5 and 1.9, respectively. In a separate population-based study, investigators observed a threefold increased risk of death over a relatively short mean follow-up of 3.6 years.10
CLASSIFICATION OF AF
The nomenclature used to classify AF in the medical literature is variable and inconsistent across investigators, but the classification system published jointly by the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society is the most widely used.11 This system defines AF as either paroxysmal or persistent. When a patient has had two or more episodes, AF is considered recurrent. If recurrent AF terminates spontaneously, it is designated as paroxysmal; but if it is sustained beyond 7 days, it is termed persistent. Pharmacologic or electrical cardioversion before expected spontaneous termination does not change the persistent designation. The Heart Rhythm Society recently released a consensus statement to aid in the uniform reporting of trials for the clinical management of AF. The term “permanent” was eliminated and replaced with the term “long-standing AF” when the duration is greater than 1 year.12
ELECTROPHYSIOLOGY OF AF
AF is characterized by irregular activation of the atria. Electrical activity in the atria during AF can exhibit two different patterns. One pattern consists of a stable source, either a focal trigger or a small reentrant circuit, with fibrillatory conduction away from the source. The other pattern is characterized by multiple changing sources or reentrant circuits. These patterns are not mutually exclusive in any particular patient. Data obtained from almost half of the patients who had undergone intraoperative mapping before arrhythmia surgery revealed that the source of AF was not stable and was capable of moving from one atrium to the other.13 As such, any surgical treatment to restore sinus rhythm is not complete without consideration of the substrate for AF in any particular patient.
Four factors contribute to the electrophysiological substrate that determines whether AF is initiated and/or sustained. These are:
A trigger – usually a premature depolarization or runs of focal ectopic depolarizations
Atrial refractory period – both its magnitude and spatial distribution
Conduction velocity – its magnitude and anisotropic spread
Atrial geometry and anatomy – both macroscopic and microscopic.
These four factors interact to create a substrate capable of sustaining AF. A nonreentrant trigger can spread away from a point with differing conduction velocities in differing directions, interacting with an inhomogeneous distribution of refractory periods. Unidirectional block occurs in this setting of anisotropy, leading to the traditional reentrant circuits associated with AF. Any pathology of the heart can affect the atrial myocardium and change its physiology through one or more of these four factors.14 For any given atria, there exists a critical mass as determined by the tissue geometry, the magnitude of refractory periods, and the conduction velocity. This is the amount of tissue required to support a reentrant circuit, and is defined by the equation WL = CV × RP (wavelength = conduction velocity × refractory period). If either CV or RP decreases, the amount of tissue needed to sustain AF decreases and the probability of a patient having AF increases.15 The surgical treatment of AF is directed at altering the geometry and anatomy of the atrium to render the electrophysiological substrate unable to support AF.
There has been a great deal of emphasis placed on the role of the pulmonary veins (PVs) in triggering AF. Haissaguerre found in a clinical study that paroxysmal AF often originates in the PVs.16 Intraoperative mapping studies have shown rapid firing originating from the region of the PVs.14 Although variable between patients, electrically excitable cardiac muscle extends 1 to 4 cm beyond the ostium of the PVs.17 Pacemaker tissue may even be present in the PVs during development.18 Because of their unique physiology, successful cure of AF is achieved in some patients by the isolation of the PVs.16 Furthermore, if triggers of AF were outside the PVs, and other substrates that sustain AF were within the veins, AF would be prevented with PV isolation. Thus, pulmonary vein isolation (PVI) is the mainstay of interventional electrophysiology catheter-based techniques.
Despite these successes, it is important to note that PVI fails to cure AF in many patients, especially those with long-standing AF. More recent studies have shown that up to a third of patients with paroxysmal AF have triggers other than the PVs. This occurs more frequently in women and in patients with large atria.19 In patients with persistent AF, the mechanism of AF is usually more complex and often not dependent on focal triggers to sustain the rhythm.20
Caution should be taken in interpreting interventional studies, whether catheter ablation or surgery, as to whether they imply an underlying mechanism for AF. Most intraoperative and catheter mapping systems do not have the spatial resolution to separate reentrant from non-reentrant mechanisms. Therefore even though focal trigger-based fibrillation may be reported from investigational mapping, the underlying mechanism of AF may be more complex. Claims of cure by PVI alone must be tempered by the knowledge that the PVI intervention often incorporates more than just the PVs. Commonly the PVs, adjacent atrial muscle, and the muscle in the oblique sinus between the veins are ablated during a catheter-based PVI, accounting for over one-third of the left atrium. This large area of ablation substantially reduces the critical mass available to sustain AF and may incorporate other non-PV substrates of AF.
The classifications of paroxysmal, persistent, and long-standing AF do not imply a specific mechanism. Even though clinical results have shown that in paroxysmal AF, PVI is effective 70% to 80% of the time, it is clear that 20% to 30% of the time the PVs are not the only substrate driving AF.13 Furthermore, human mapping data from the authors’ laboratory did not show any significant difference in mechanism between paroxysmal and persistent AF.13
Because AF is a complex arrhythmia, cardiac mapping requires a high density of closely placed electrodes and a sophisticated mapping and signal processing system. Intraoperative mapping has not been useful in providing real-time information during surgery. The traditional surgical algorithm of obtaining preoperative or intraoperative mapping data and using that information to guide surgical technique, as was done with arrhythmias like Wolf-Parkinson-White syndrome, has not been feasible for AF.
Map-guided techniques to treat AF are an area of continued research. Mapping techniques are being developed that may allow interventionalists to customize the incision set to the specific underlying mechanism.21–23 One particularly promising technique is ECG imaging.24 This noninvasive technique involves recording signals from the body surface of awake patients and mathematically fitting these potentials onto the surface of the heart with anatomic data obtained from computed tomography. If performed preoperatively, this would allow for the delineation of the mechanism of AF before the proposed intervention, allowing physicians to triage patients to the most effective procedure.
MEDICAL TREATMENT
Medical therapy for AF in restoring sinus rhythm has had poor results. Antiarrhythmic drugs generally have low therapeutic indices and limited long-term efficacy.25,26 As a consequence, medical therapy is often aimed at rate control rather than rhythm control. Drugs are given to reduce the ventricular response rate, which reduces palpitations and avoids tachycardia-induced cardiomyopathy. The justification for this strategy arises from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study.27 The results of this study showed that management with rhythm control did not have any more survival benefit than a rate control strategy in anticoagulated patients with AF.28,29 Rate control may have potential advantages over rhythm control, such as a lower risk for adverse side effects that are seen with aggressive rhythm control.29
Catheter fulguration of the His bundle is an extreme form of ventricular rate control. First described by Scheinman and colleagues30 in 1982, the ablation of the AV node– His bundle complex effectively controlled the irregular cardiac rhythm in AF and other supraventricular arrhythmias. Unfortunately, ablation of the AV node requires permanent pacemaker insertion. Despite this drawback, AV node ablation is currently a common treatment of medically refractory AF, especially for those patients who cannot tolerate pharmacologic rate control.
Rate control alone does not address all the pathophysiological effects of AF. Although the ventricular response rate can often be controlled pharmacologically or with AV node ablation, the atria are still in fibrillation. The absence of atrial “kick” can result in worsening symptoms of congestive heart failure. More importantly, patients with AF remain at risk for developing thromboembolism, requiring indefinite anticoagulation with warfarin. Warfarin has a significant side effect profile, with a major complication rate of approximately 2% per year.31–33
Although the AFFIRM trial showed no difference in long-term outcome between rhythm versus rate control, there are clinically meaningful advantages of normal sinus rhythm. These advantages include increased exercise tolerance, freedom from anticoagulation medications, decreased palpitations, and prevention of atrial remodeling.27,34 The presence of sinus rhythm was also associated with a significantly decreased risk of death (HR = 0.53, P<.0001) in a post hoc analysis of the AFFIRM trial.35 This beneficial effect on mortality from sinus rhythm is also borne out in the surgical literature.36–39
DEVELOPMENT OF THE COX-MAZE PROCEDURE
In the 1980s, several groups began developing procedures for the surgical treatment of AF. The majority of these have only historical significance now because they were unable to address all 3 detrimental sequelae of AF simultaneously. The left atrial isolation procedure, introduced by Williams and colleagues40 isolated AF to the left atrium, restoring regular rhythm and hemodynamics in patients with left-sided fibrillation. However, patients were still at risk for thromboembolism. In 1985, Guiraudon and colleagues introduced the corridor procedure, which isolated a strip of atrial septum containing the SA and AV nodes. This restored a regular ventricular response, but left both atria fibrillating, leading to hemodynamic inadequacy and a persistent risk for thromboembolism.41 In 1985, Cox described an atrial transection procedure that involved a long incision across both atria and down into the septum. This procedure cured AF in a canine model, but was ineffective in humans.42 Although unsuccessful, these techniques laid the groundwork for the subsequent development of the Cox-Maze procedure.
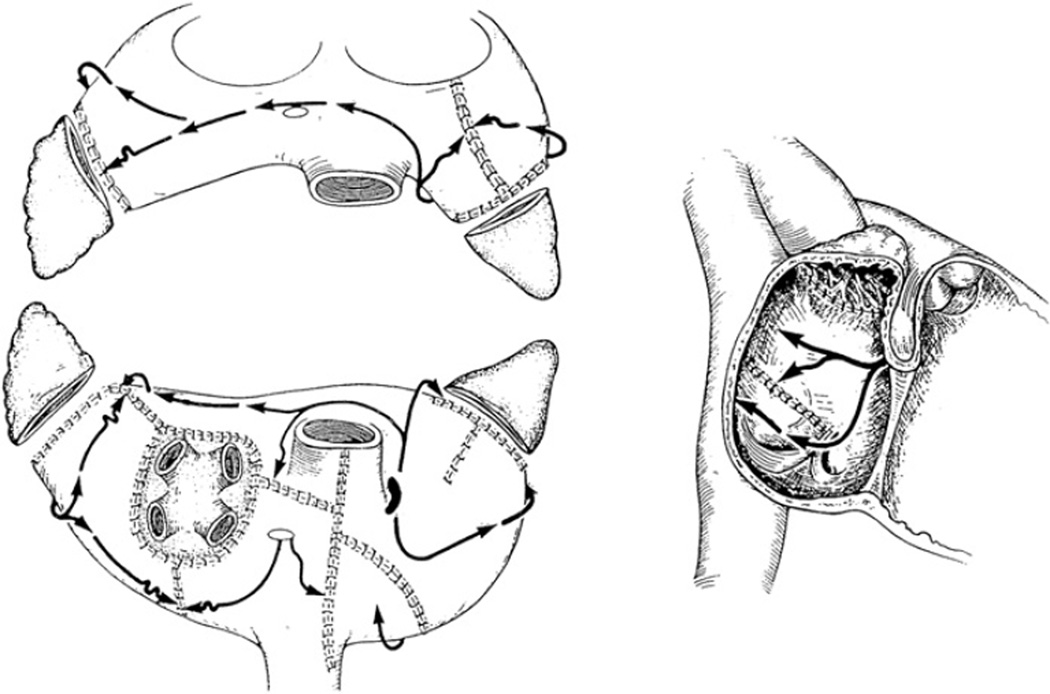
The team led by Dr. James Cox at Washington University in St. Louis developed the Maze procedure in 1987.43–45 The Cox-Maze procedure was an empiric operation designed to interrupt the macro-reentrant circuits thought to be responsible for AF at the time. Unlike earlier procedures, the Maze procedure addressed all three detrimental sequelae of AF, restoring AF synchrony, a regular ventricular response, and decreasing the risk of thromboembolism and stroke.46 The operation involved creating multiple incisions across the right and left atria. In effect, these incisions directed the electrical impulses from the SA node while preventing reentrant circuits from forming. It also allowed all of the atrial myocardium to be activated, preserving atrial transport function in most patients.47 After two iterations addressing late complications and technical difficulty, the Cox-Maze III emerged (Fig. 1).48,49
Fig. 1.
The incisions of the Cox-Maze III procedure with the propagation of the sinus impulse represented by arrows. (From Cox JL. Evolving applications of the Maze procedure for atrial fibrillation [invited editorial]. Ann Thorac Surg 1993;55:578–80. Reprinted with permission from The Society of Thoracic Surgeons.)
The Cox-Maze III procedure has become the gold standard for the surgical treatment of AF. In a long-term study of patients who had the Cox-Maze III procedure, 97% of the patients at late follow-up were free of symptomatic AF.50 Similar results have been reproduced by other institutions around the world.51–53
SURGICAL ABLATION TECHNOLOGY
Although several groups have reported excellent results with the Cox-Maze III, the procedure did not gain widespread acceptance because of its complexity and technical difficulty. During the last 10 years, most groups have replaced the traditional cut-and-sew lesions of the Cox-Maze III with ablations using various energy sources in an effort to make the procedure technically simpler and faster to perform.54 These linear lines of ablation have been created using a variety of energy sources including radio frequency (RF) energy, microwave, cryoablation, laser and high-frequency ultrasound.55–59 The development of ablation technologies has revolutionized the surgical treatment of AF. Although very few patients (<1%) with AF undergoing cardiac surgery before 2000 underwent a Cox-Maze procedure, over 40% of patients with AF undergoing cardiac surgery had a concomitant ablation procedure in 2006.60
The safe and effective use of these new ablation devices requires a thorough understanding of the biophysics of the energy source and their behavior on atrial tissue. Most importantly, a device must reliably produce conduction block to prevent AF. An ablation device must have the capability to reliably make transmural lesions from either the epicardial or endocardial surface. Experimental work has shown that even small gaps in ablation lines can conduct fibrillatory wavefronts.61 Surgeons must also understand the safety profile of ablation devices. This requires a precise definition of dose-response curves to limit excessive or inadequate ablation and knowledge of the effect of the device on surrounding vital cardiac structures, such as coronary arteries and valves. A device should also make AF surgery simpler and require less time to perform than a traditional case. This requires features such as rapidity of lesion formation, simplicity of use, and adequacy of length and flexibility. A device should be amenable to minimally invasive approaches. A brief discussion of cryoablation and RF ablation, the most used ablation technologies, follows. Microwave and laser devices are no longer on the market and are not discussed.
Cryoablation
Cryoablation has been used in arrhythmia surgery for decades. There are currently two commercially available sources of cryothermal energy. The original technology uses nitrous oxide and is manufactured by Atricure (Cincinnati, Ohio). The nitrous oxide devices use rigid reusable probes, although there are flexible probes in development. CryoCath Technologies (Montreal, Quebec, Canada) introduced a device using argon, which is now distributed by ATS (Minneapolis, Minnesota). The original device used a disposable flexible catheter, and newer iterations have included a clamp device. At one atmosphere of pressure, nitrous oxide is capable of achieving a temperature of −89.5°C, whereas argon has a minimum temperature of −185.7°C.
Cryoablation causes tissue injury through the freezing and rewarming process. Acutely, ice crystals disrupt cell membranes, whereas, chronically, microvascular damage leads to local tissue ischemia. Apoptosis plays a clear, but incompletely understood role in lesion formation.62,63 The size and depth of cryolesions are determined by numerous factors, including probe temperature, tissue temperature, probe size, the duration and number of ablations, and the particular liquid used as the cooling agent.64–67
Cryoablation has the distinct advantage of preserving collagen structure.68 Thus, it preserves the fibrous skeleton of the heart, making it safe for use around valvular tissue. Furthermore, cryoablation has a proven track record in the treatment of arrhythmias. Nitrous oxide cryoablation has had extensive clinical use and an excellent safety profile. A European randomized trial comparing mitral valve surgery to mitral valve surgery with concomitant left atrial cryoablation showed increased freedom from AF for the cryoablation group (43% versus 73% at 12 months).69 Ad and Cox developed a less invasive Maze procedure using cryoablation that reduced the number of atriotomies from 12 to 4 using cryoablation with preserved efficacy.70
Despite a proven track record, cryoablation does have some drawbacks. It is largely ineffective on the beating heart. Because of the heat sink provided by circulating endocardial blood, epicardial cryolesions on the beating heart have not been uniformly transmural. In one study, investigators were only able to create transmural lesions 62% of the time around the PVs and only two out of eight (25%) ablations on the left atrial appendage were transmural.71 Experimental studies have shown late intimal hyperplasia of coronary arteries after cryoablation, and these structures should be avoided.63
In summary, cryoablation is unique among the currently available ablation technologies in that it destroys tissue by freezing rather than heating. The important advantage is its ability to preserve tissue architecture. The nitrous oxide technology has a well-defined efficacy and safety profile and is generally safe except around the coronary arteries. The potential disadvantages of cryoablation technology include the relatively long time necessary to create a lesion (1–3 minutes). There is also difficulty in creating lesions on the beating heart because of the heat sink of the circulating blood volume. Furthermore, if blood is frozen during epicardial ablation on the beating heart, it coagulates, creating a potential risk for thromboembolism.
RF Energy
In the electrophysiology laboratory, RF energy has been used for cardiac ablation for many years.72 RF energy uses an alternating current in the range of 100 to 1000 kHz which is high enough to prevent rapid myocardial depolarization and the induction of ventricular fibrillation yet low enough to prevent tissue vaporization and perforation. A lesion is created through thermal injury. As the RF radiation passes through tissue, resistive heating occurs within a narrow rim of tissue in direct contact with the electrode. Passive conduction continues from this interface to create the lesion on deeper tissue.
RF ablation devices can be unipolar or bipolar. With unipolar catheters, the energy is dispersed between the electrode tip and an indifferent electrode, usually the grounding pad applied to the patient. In bipolar clamp devices, alternating current is generated between two closely approximated electrodes, which results in a more focused ablation. The lesion size depends on tissue-electrode contact area, the interface temperature, the current and voltage (power), and the duration of delivery. An obstacle to deeper tissue penetration is char formation at the tissue-electrode interface. Irrigated devices have been developed that reduce charring and allow for deeper penetration of radiation. These irrigated catheters were shown to create larger volume lesions than dry RF devices.73,74
There are numerous unipolar RF devices available. Estech (San Ramon, California) has marketed several Cobra catheters, both dry and irrigated unipolar catheters, which are segmented and flexible. These devices can create variable lesion lengths of 10 mm to 95 mm. The electrodes can be individually selected and temperature controlled. Medtronic has developed a unipolar RF device, the Cardioblate catheter. This is an irrigated unipolar RF catheter used to make point-by-point ablations by dragging it across the tissue to make a linear lesion.
The most widely used devices are bipolar RF clamps. With two electrodes instead of one, the path of energy is more focused. This allows for faster ablation (usually less than 20 s), while limiting destruction to tissue that is in close proximity to the electrodes. With bipolar devices, the electrodes are clamped over the targeted atrial tissue. The first bipolar RF device was introduced by Atricure, Inc. The isolator was a specially designed clamp with 1-mm wide and 5-cm long electrodes embedded in the jaws of the clamp. The device was unique in that it had an algorithm created to detect real-time measurement of lesion transmurality. The conductance between the electrodes was measured during ablation. When the conductance dropped to a stable minimum level, this was well correlated experimentally and clinically to histologically transmural lesions.75–77 More recent iterations have introduced more uniform clamp strength and a unique dual electrode designed to achieve wider and more consistent lesions. The Medtronic bipolar clamp, the Cardioblate BP, has an irrigated, flexible jaw along with an articulating head, with 5-cm long electrodes. This device has an algorithm, like the Atricure device, that predicts transmurality of lesions. This device was shown to be effective in the experimental setting.78,79
Newer RF ablation devices have been released by other manufacturers, and these are all variations of the same themes of earlier devices.80,81 The Cobra Adhere and Cobra Adhere XL (Estech) devices are unipolar devices with suction to aid in minimally invasive applications, such as port access or thoracoscopic approaches. The VisiTrax device (nContact Surgical, Morrisville, North Carolina) is another unipolar device coupled with suction for minimally invasive applications.
Dose-response curves for unipolar RF have been described.82–84 Although these studies show reliable lesion formation in animals with ablation times of 1 to 2 minutes, these devices have not performed well in humans. In one study, after 2-minute endocardial ablations during mitral valve surgery, only 20% of the in vivo lesions were transmural.85 Epicardial ablation was even more difficult. Both animal and human studies have consistently shown that unipolar RF is incapable of creating epicardial transmural lesions on the beating heart.84,85 In contrast, bipolar RF ablation, was capable of creating transmural lesions on the beating heart in animals and humans.55,76,77
Like cryoablation, RF ablation is a well-developed technology, and much is known about its safety profile. A number of clinical complications of unipolar RF devices have been described, including coronary artery injuries, cerebrovascular accidents, and esophageal perforation leading to atrioesophageal fistula.86–89 Use of the bipolar RF devices has eliminated virtually all of the collateral damage seen with the unipolar devices, and there have been no clinical complications reported in the literature. One drawback of the bipolar devices is the requirement for the tissue to be clamped in the jaws of the device. This has limited the potential lesion set, particularly on the beating heart, and requires the use of adjunctive unipolar technology to create a complete Cox-Maze lesion set.
INDICATIONS FOR SURGICAL TREATMENT OF AF
The principal indication for surgery for AF is intolerance of the arrhythmia in patients who have failed medical management. Patients with paroxysmal atrial flutter or fibrillation are often more symptomatic than those with persistent or long-standing AF. Major symptoms include dyspnea on exertion, easy fatigability, lethargy, palpitations, and a general sense of unease.
The Heart Rhythm Society created a task force to evaluate indications for catheter and surgical ablation of AF.12 The recommendations were developed in partnership with the European Heart Rhythm Association, the European Cardiac Arrhythmia Society, the American College of Cardiology, the American Heart Association, and the Society of Thoracic Surgeons. Because the treatment of AF is complex, they recommended that a team-based approach, including electrophysiologists and surgeons, is used to appropriately select patients for stand-alone surgical treatment of AF. The consensus of the Task Force established the following indications for surgical ablation of AF:12
Symptomatic AF patients undergoing other cardiac surgical procedures
Selected asymptomatic AF patients undergoing cardiac surgery in whom the ablation can be performed with minimal risk
Stand-alone AF surgery should be considered for symptomatic AF patients who have failed medical management and prefer a surgical approach, or have failed one or more attempts at catheter ablation, or are not candidates for catheter ablation.
Another important group of patients who should be considered for surgery are those who cannot take warfarin or those who have had a stroke while adequately anticoagulated. The Cox-Maze procedure significantly reduces the risk for stroke in these patients. About 20% of patients who had the original cut-and-sew procedure at the authors’ institution experienced at least one episode of cerebral thromboembolism that resulted in a temporary or permanent neurologic deficit. At a mean follow-up of 5.4 years, less than 1% of patients (1/178) had a late stroke following the Cox-Maze procedure.50
SURGICAL TECHNIQUE: THE COX-MAZE PROCEDURE
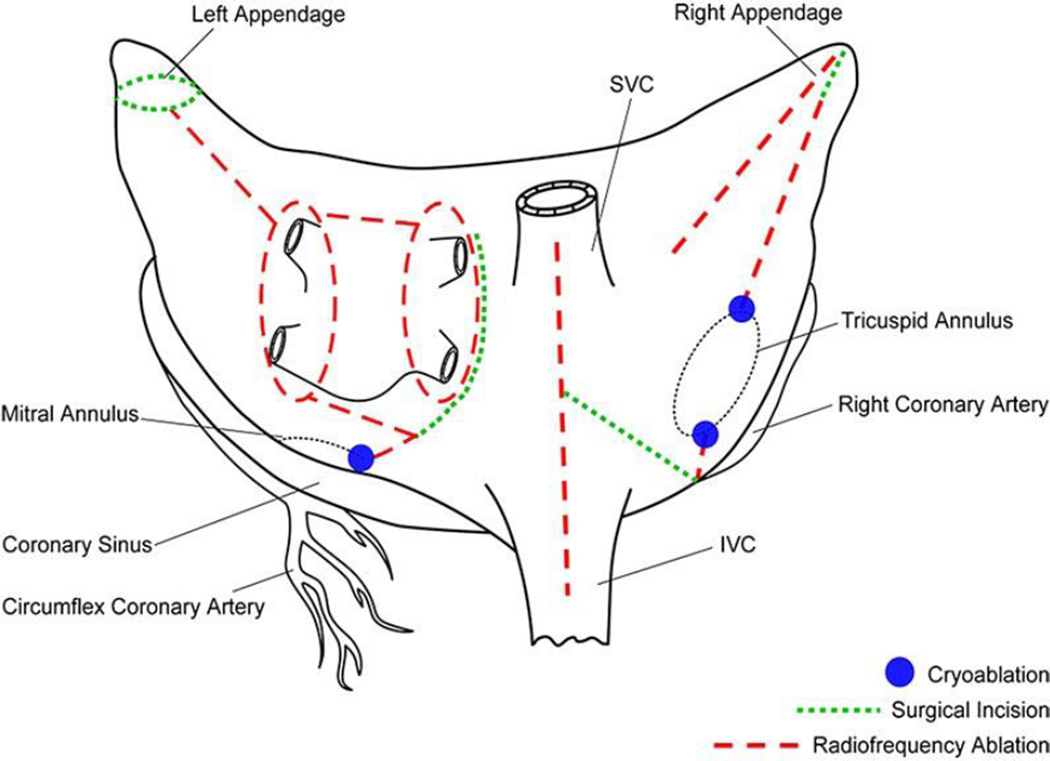
The final version of the standard cut-and-sew technique to cure AF was the Cox-Maze III procedure.43–45 The complexity of the cut-and-sew technique led many surgeons to replace the incisions with linear ablations. Bipolar RF energy has been used successfully by the authors’ group to replace the majority of the surgical incisions of the Cox-Maze III procedure. The authors’ current procedure incorporates most of the lesions of the Cox-Maze III procedure, and has been named the Cox-Maze IV (Fig. 2).55,90 Midterm follow-up has shown that this modification has significantly shortened the operative time without compromising the success rate of the traditional cut-and-sew Cox-Maze III procedure.91
Fig. 2.
Cox-Maze IV procedure lesion set. Most of the incisions of the Cox-Maze III have been replaced with ablation. Modifications included independent isolation of PVs with connecting lesion, and no atrial septal incision (originally used for exposure). Abbreviations: IVC, inferior vena cava; SVC, superior vena cava.
The Cox-Maze IV procedure is performed with the patient on cardiopulmonary bypass either through a median sternotomy or a right mini thoracotomy. The right and left PVs are bluntly dissected to prepare for isolation. If the patient is in AF, an intravenous bolus of amiodarone is given and the patient is cardioverted. This allows for determination of pacing thresholds on both sets of PVs before ablation. The bipolar ablations are then performed on a cuff of atrial tissue surrounding the right and left PVs separately. After isolation, exit block is verified with pacing from all of the PVs.
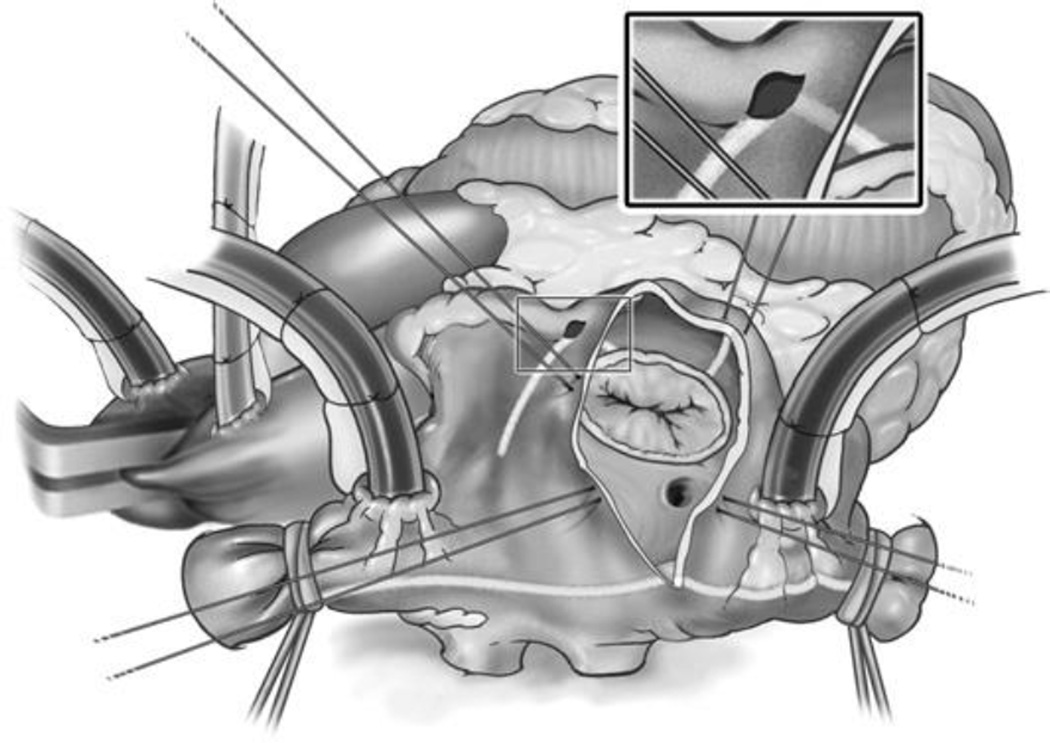
The right atrial lesions are performed with the heart beating through a single vertical atriotomy and a small purse-string suture at the base of the right atrial appendage (Fig. 3). A unipolar energy source (cryoablation, RF, and so forth) is used to complete the ablation lines at the tricuspid valve.
Fig. 3.
The surgeon’s view of the right atrium through the vertical atriotomy used to create the right-sided lesions of the Cox-Maze IV procedure. Note the pale demarcations of the lines of ablation created with the bipolar RF clamps on the atrial wall. Unipolar energy, like cryothermy, is used to finish the ablation lines at the tricuspid annulus.
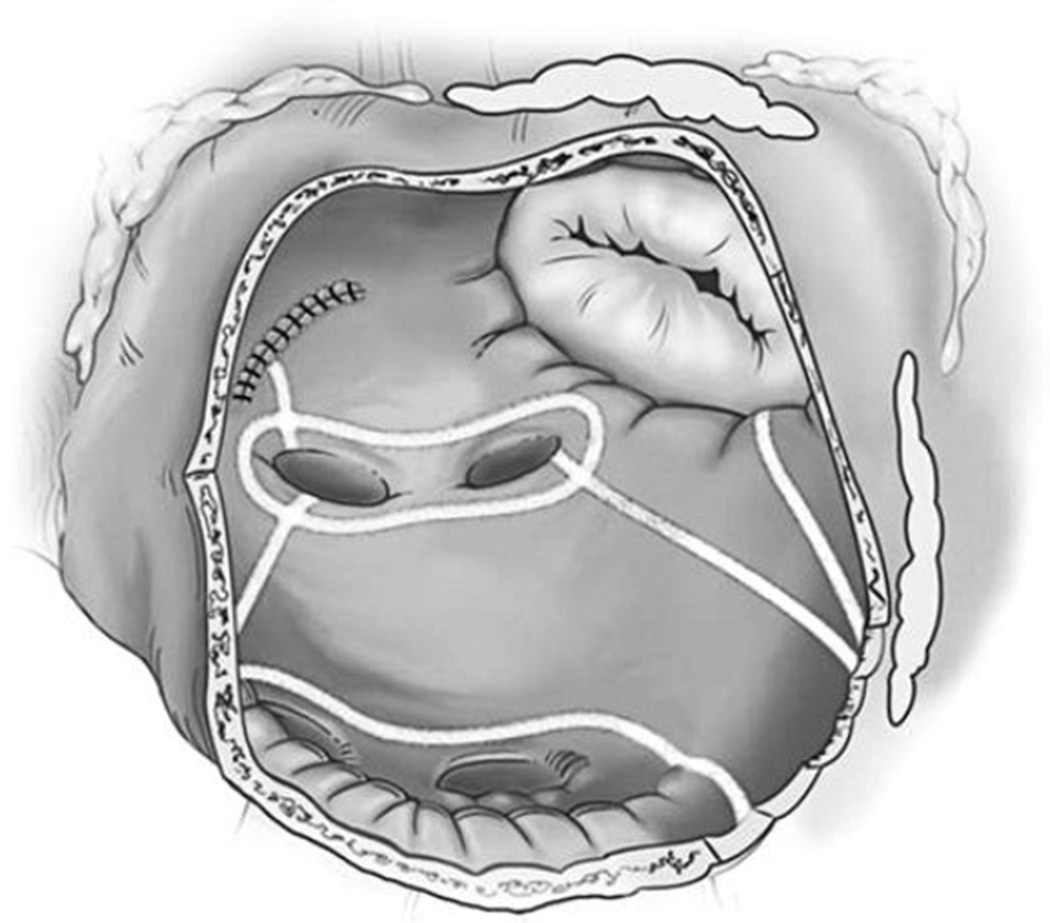
After completion of the right-sided lesions, the left-sided lesions are performed through a standard left atriotomy with the heart arrested. This is illustrated schematically in Fig. 4. The atriotomy is extended inferiorly around the right inferior PV and superiorly onto the dome of the left atrium. Care is taken to connect this incision to the RF ablation line surrounding the right PVs. A lesion is made with the bipolar RF device, connecting the left atrium incision inferiorly to the ablation line encircling the left PVs. Another ablation is performed from the superior aspect of the left atriotomy, across the dome of the left atrium, and into the left superior PV. A bipolar RF lesion is then created up to the mitral valve annulus. This lesion is performed from the inferior aspect of the left atrial incision across the posterior left atrium, AV groove, and the coronary sinus. The ablation is performed in the space between the circumflex and right coronary artery circulation to avoid damage to the coronary arteries.
Fig. 4.
The surgeon’s view of the left atrium through the standard left atriotomy. Note the pale demarcations of the lines of ablation created with the bipolar RF clamps on the atrial wall. On the left atrium, the line toward the mitral valve is also finished with a unipolar energy source, most often cryothermy.
To complete the Cox-Maze, a unipolar energy source (cryoablation, RF, and so forth) is used to connect the last ablation line to the mitral valve annulus. To reduce the risk for systemic thromboembolism, the left atrial appendage is amputated. A final ablation is performed through the amputated left atrial appendage into one of the left PVs. The left atrial appendage is oversewn. In patients undergoing a mitral valve replacement, amputation of the left atrial appendage before performing the other left atrial lesions is recommended to avoid excessive traction.
SURGICAL RESULTS: COX-MAZE PROCEDURE
The long-term results of the Cox-Maze III procedure have been excellent. The experience encompassing 198 patients at Washington University showed that 97% of patients at a mean follow-up of 5.4 years were in sinus rhythm with no difference between patients undergoing lone versus concomitant procedures. The cure rates off antiarrhythmic medications were 80% and 73% in patients undergoing lone and concomitant Cox-Maze procedures, respectively, at late follow-up.50
The results with the ablation-based Cox-Maze IV have been similar. In a prospective, single-center trial from the authors’ institution, 91% of patients at 6-months follow-up were free from AF, and operative mortality was 0%.55 These results were later validated in a multicenter trial.58 Moreover, the adaptation of bipolar RF has shortened the operation considerably, dropping mean cross-clamp times for patients undergoing a lone Cox-Maze III procedure from 92 ± 26 minutes to 44 ± 21 minutes for patients undergoing a Cox-Maze IV.92 A propensity analysis comparing the Cox-Maze IV patients with a historical cohort of patients undergoing the Cox-Maze III showed that the Cox-Maze IV had statistically identical outcomes at 1-year follow-up.91
The Cox-Maze procedure has been effective at decreasing the risk for late stroke in this patient population. Nineteen percent (58/306) of patients undergoing Cox-Maze III at Washington University had experienced a neurologic event before surgery, and there were only two minor strokes during long-term follow-up (mean 3.9 ± 2.7 years). The long-term stroke rate after the Cox-Maze procedure has been 0.1% per year, despite the fact that the majority of patients were able to discontinue anticoagulation medication.46 Other centers have reported similar results. In a series from Japan, patients with chronic AF who had a concomitant Cox-Maze procedure with their mitral valve replacement were 99% stroke-free at a follow-up of 8 years, whereas the group with mitral valve replacement alone were 89% stroke-free.93
OTHER AF PROCEDURES
More limited procedures have been proposed for the surgical treatment of AF. These fall into two broad groups, left atrial lesion sets and operations that isolate the PVs.
PVI
Based on the original reports of Hassaiguerre,16 it has been well documented that the triggers for paroxysmal AF originate from the PVs in most cases. However, over 30% of triggers originate outside the PVs.19 A number of surgeons have published the technique of PVI as a procedure to treat AF.94–97 PVI as a therapeutic strategy is attractive because this procedure can be often done without cardiopulmonary bypass through small incisions, or endoscopically. To increase efficacy, some investigators have added ablation of the ganglionic plexi (GP).98–100
The results of PVI have been variable. In a series by Wolf and colleagues,97 91% of patients undergoing a video-assisted bilateral PVI and left atrial appendage exclusion were free from AF at 3-months follow-up. Edgerton and colleagues101 reported on 57 patients undergoing PVI with GP ablation with more thorough follow-up and found 82% of their patients with paroxysmal AF to be free from AF at 6 months, with 74% off antiarrhythmic drugs. In a study involving 21 patients undergoing PVI with GP ablation, McClelland and colleagues99 reported 88% procedural success in patients with paroxysmal AF, with procedural success defined as freedom from AF at 1 year without antiarrhythmic drugs. A recent multicenter trial reported 87% normal sinus rhythm in a more diverse patient population including patients with long-standing persistent AF, although this subset had only 71% normal sinus rhythm rate.102 Results from the authors’ institution are similar, with 83% freedom from AF and 67% off drugs at a mean follow-up of 24 ± 37 months using data from 24-hour Holter monitoring.
However, the success of PVI is largely dependent on patient selection. Patients with long-standing or persistent AF had very poor results. Fifty-six percent of patients in Edgerton’s group were free from AF at 6 months (35% were off antiarrythmics).103 In patients undergoing concomitant procedures, the results were even worse. Of 23 patients undergoing mitral valve surgery or coronary revascularization with concomitant PVI, only 50% of patients were free from AF at a mean follow-up of 57 ± 37 months.92 In a randomized trial, Gaita and colleagues104 reported similar poor results with 29% freedom from AF in patients undergoing concomitant PVI with valve surgery. In the setting of mitral valve disease Tada and colleagues96 reported 61% freedom from AF and only 17% freedom from antiarrythmic drugs in their series of 66 patients undergoing PVI.
These results of PVI emphasize the importance of understanding the electrophysiological substrate in patients with AF.
Ganglionated Plexus Ablation
The addition of ganglionated plexus ablation by some authors is based on experimental data showing that autonomic ganglia in these plexi play a role in the initiation and maintenance of AF.105,106 However, the authors’ practice is not to ablate the ganglia, as experimental evidence in their laboratory and others have shown recovery of autonomic function as early as 4 weeks after GP ablation.107–109 Furthermore, long-term follow-up regarding the effects of GP ablation is lacking. Thus, GP ablation should be reserved for centers participating in clinical trials.
Left Atrial Lesion Sets
The left atrial lesion set generally involves creating PVI and a lesion to the mitral annulus and removal of the left atrial appendage. A multitude of ablation devices have been used in the formation of these lesions sets, with varying techniques and varying degrees of success.88,104,110–116 A meta-analysis by Barnett and Ad in 2006 showed a 73% success for left atrial lesion sets versus 87% for biatrial lesion sets (P = .05).117 The only randomized control trial comparing the efficacy of left atrial lesion set versus no ablation procedure was reported by Doukas and colleagues.118 For a cohort of patients undergoing mitral valve surgery, 44% had normal sinus rhythm 1 year after undergoing RF ablation of the left atrium versus only 4.5% in the patients undergoing valve surgery alone.
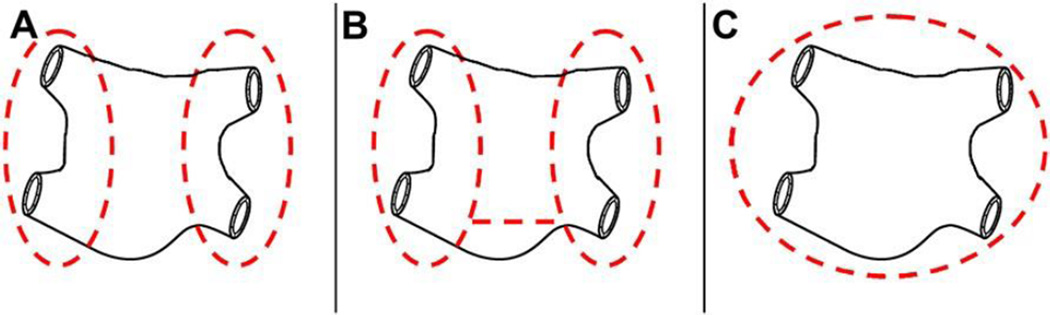
Although the results of Barnett and Ad’s meta-analysis showed that left atrial lesion sets are not as effective as biatrial lesion sets, left atrial lesion sets are better than no treatment at all. There is evidence in the literature that some of the lesions on the left atrium are important to include regardless of surgical approach. One in particular is the isthmus lesion down to the mitral valve annulus (see Fig. 4). In a report on differing left atrial lesion sets by Gillinov and colleagues,119 patients with long-standing persistent AF had a greater chance of recurrent AF if their lesion set did not include a line down to the mitral annulus. Furthermore, data from the authors’ institution show the importance of isolating the entire posterior atrium around the PVs as a single unit (Fig. 5). Freedom from AF, off antiarrhythmic drugs was significantly higher for patients who had a “box” lesion around the PVs than those who had a single ablation connecting the islands of atrial tissue around the left and right PVs. At 6 months, 79% of patients with the “box” lesion were free from AF, off antiarrhythmic drugs versus 54% of the patients without it (P = .011).120
Fig. 5.
Isolation of the pulmonary veins: (A) Isolation of the PVs separately, (B) Isolation of the PVs separately with a bridging ablation. (C) Isolation of the entire posterior left atrium as a unit, including the PVs. The practice of the authors’ institution in performing the Cox-Maze IV is to isolate the entire posterior atrium by connecting the PVs with a superior and inferior ablation, creating a “box” lesion (not shown).
SUMMARY
AF is a complex disease affecting a significant portion of the general population. In the last decade, interventional therapy (catheter and surgical ablation) has emerged to play an ever increasing role for patients with symptomatic, medically refractory arrhythmia. The introduction of ablation devices has revolutionized the surgical treatment of AF making procedures available to a wider patient population.
There have been few randomized trials comparing the different surgical procedures, but conclusions can be drawn from two decades of surgical experience and retrospective series. In patients with lone AF, PVI had good early results for paroxysmal AF.99,101 At the authors’ institution, this procedure is performed in patients who have paroxysmal AF and a left atrial size of 4 cm or less. In patients with large atria, the authors prefer a full Cox-Maze lesion set, because of the high incidence of nonpulmonary vein triggers in this population.19 They also prefer a full lesion set in patients with long-standing, persistent lone AF as a result of the poor results of PVI in this group.
If a patient fails a catheter ablation, the surgeon needs to accurately diagnose the recurrent arrhythmia. A number of these patients will have atypical atrial flutter that cannot be treated with PVI alone and requires either a complete biatrial or left atrial lesion set including the isthmus lesion. Patients may also have atrial tachycardia, which is better treated by catheter ablation or medication. To determine the precise arrhythmia, these patients should be evaluated by an electrophysiologist and a surgeon before an operation. At the authors’ institution, noninvasive ECG imaging is used to help define the mechanism of recurrence.121,122
In patients with AF undergoing concomitant procedures and requiring cardiopulmonary bypass, a Cox-Maze procedure is the procedure of choice. For patients undergoing mitral valve procedures, this adds 10 to 15 minutes of cross-clamp time. In most patients, the authors prefer a biatrial lesion set. The right atrial lesions can be performed with the heart beating (see Fig. 3). In selected patients, with left-sided pathology and a normal size right atrium, a left atrial lesion set alone has been performed with good results. In patients undergoing coronary bypass grafting, the high success rate of the Cox-Maze lesion set has been documented by the authors’ group.123 However, there have been no randomized comparisons of lesion sets in this population.
More limited lesion sets have a more limited role. In patients with paroxysmal AF undergoing off-pump coronary bypass grafting, our policy had been to perform PVI with removal of the left atrial appendage. Poor results in patients with long-standing AF have led the authors to abandon this procedure in those patients.
Acknowledgments
This work was supported in part by National Institutes of Health grants 5R01HL32257, R01HL085113, and T32HL0776.
Financial Disclosures: Dr. Damiano receives consultant fees from AtriCure and Medtronic.
REFERENCES
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 3.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108(6):711–716. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–844. [PubMed] [Google Scholar]
- 5.Cairns JA. Stroke prevention in atrial fibrillation trial. Circulation. 1991;84(2):933–935. doi: 10.1161/01.cir.84.2.933. [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, Halperin JL, Pearce LA, et al. Lessons from the stroke prevention in atrial fibrillation trials. Ann Intern Med. 2003;138(10):831–838. doi: 10.7326/0003-4819-138-10-200305200-00011. [DOI] [PubMed] [Google Scholar]
- 7.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Benjamin EJ, Belanger AJ, et al. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J. 1996;131(4):790–795. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 10.Vidaillet H, Granada JF, Chyou PH, et al. A population-based study of mortality among patients with atrial fibrillation or flutter. Am J Med. 2002;113(5):365–370. doi: 10.1016/s0002-9343(02)01253-6. [DOI] [PubMed] [Google Scholar]
- 11.Fuster V, Ryden LE, Asinger RW, et al. ACC/AHA/ESC Guidelines for the management of patients with atrial fibrillation: executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. Circulation. 2001;104(17):2118–2150. [PubMed] [Google Scholar]
- 12.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4(6):816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Schuessler RB, Kay MW, Melby SJ, et al. Spatial and temporal stability of the dominant frequency of activation in human atrial fibrillation. J Electrocardiol. 2006;39(4) Suppl:S7–S12. doi: 10.1016/j.jelectrocard.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Boineau JP, Schuessler RB, Canavan TE, et al. The human atrial pacemaker complex. J Electrocardiol. 1989;22(Suppl):189–197. doi: 10.1016/s0022-0736(07)80122-1. [DOI] [PubMed] [Google Scholar]
- 15.Byrd GD, Prasad SM, Ripplinger CM, et al. Importance of geometry and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. Circulation. 2005;112(9) Suppl:I7–I13. doi: 10.1161/CIRCULATIONAHA.104.526210. [DOI] [PubMed] [Google Scholar]
- 16.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 17.Spach MS, Barr RC, Jewett PH. Spread of excitation from the atrium into thoracic veins in human beings and dogs. Am J Cardiol. 1972;30(8):844–854. doi: 10.1016/0002-9149(72)90009-4. [DOI] [PubMed] [Google Scholar]
- 18.Blom NA, Gittenberger-de Groot AC, DeRuiter MC, et al. Development of the cardiac conduction tissue in human embryos using HNK-1 antigen expression: possible relevance for understanding of abnormal atrial automaticity. Circulation. 1999;99(6):800–806. doi: 10.1161/01.cir.99.6.800. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Tai CT, Hsieh MH, et al. Predictors of non-pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation: implication for catheter ablation. J Am Coll Cardiol. 2005;46(6):1054–1059. doi: 10.1016/j.jacc.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415(6868):219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 21.Nitta T, Ishii Y, Miyagi Y, et al. Concurrent multiple left atrial focal activations with fibrillatory conduction and right atrial focal or reentrant activation as the mechanism in atrial fibrillation. J Thorac Cardiovasc Surg. 2004;127(3):770–778. doi: 10.1016/j.jtcvs.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Nitta T, Ohmori H, Sakamoto S, et al. Map-guided surgery for atrial fibrillation. J Thorac Cardiovasc Surg. 2005;129(2):291–299. doi: 10.1016/j.jtcvs.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Schuessler RB. Do we need a map to get through the maze? J Thorac Cardiovasc Surg. 2004;127(3):627–628. doi: 10.1016/j.jtcvs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh S, Avari JN, Rhee EK, et al. Hypertrophic cardiomyopathy with preexcitation: insights from noninvasive electrocardiographic imaging (ECGI) and catheter mapping. J Cardiovasc Electrophysiol. 2008;19(11):1215–1217. doi: 10.1111/j.1540-8167.2008.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuster V, Ryden LE, Asinger RW, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. 2001;38(4):1231–1266. doi: 10.1016/s0735-1097(01)01587-x. [DOI] [PubMed] [Google Scholar]
- 26.Miller MR, McNamara RL, Segal JB, et al. Efficacy of agents for pharmacologic conversion of atrial fibrillation and subsequent maintenance of sinus rhythm: a meta-analysis of clinical trials. J Fam Pract. 2000;49(11):1033–1046. [PubMed] [Google Scholar]
- 27.Waldo AL. Management of atrial fibrillation: the need for AFFIRMative action. AFFIRM investigators. Atrial Fibrillation Follow-up Investigation of Rhythm Management. Am J Cardiol. 1999;84(6):698–700. doi: 10.1016/s0002-9149(99)00419-1. [DOI] [PubMed] [Google Scholar]
- 28.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347(23):1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 29.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 30.Scheinman MM, Morady F, Hess DS, et al. Catheter-induced ablation of the atrioventricular junction to control refractory supraventricular arrhythmias. JAMA. 1982;248(7):851–855. [PubMed] [Google Scholar]
- 31.Copland M, Walker ID, Tait RC. Oral anticoagulation and hemorrhagic complications in an elderly population with atrial fibrillation. Arch Intern Med. 2001;161(17):2125–2128. doi: 10.1001/archinte.161.17.2125. [DOI] [PubMed] [Google Scholar]
- 32.DiMarco JP, Flaker G, Waldo AL, et al. Factors affecting bleeding risk during anticoagulant therapy in patients with atrial fibrillation: observations from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149(4):650–656. doi: 10.1016/j.ahj.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Levine MN, Raskob G, Landefeld S, et al. Hemorrhagic complications of anticoagulant treatment. Chest. 2001;119(1)(Suppl):108S–121S. doi: 10.1378/chest.119.1_suppl.108s. [DOI] [PubMed] [Google Scholar]
- 34.Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. J Am Coll Cardiol. 2003;42(1):20–29. doi: 10.1016/s0735-1097(03)00559-x. [DOI] [PubMed] [Google Scholar]
- 35.Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 36.Bando K, Kasegawa H, Okada Y, et al. Impact of preoperative and postoperative atrial fibrillation on outcome after mitral valvuloplasty for nonischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2005;129(5):1032–1040. doi: 10.1016/j.jtcvs.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 37.Bando K, Kobayashi J, Kosakai Y, et al. Impact of Cox maze procedure on outcome in patients with atrial fibrillation and mitral valve disease. J Thorac Cardiovasc Surg. 2002;124(3):575–583. doi: 10.1067/mtc.2002.124392. [DOI] [PubMed] [Google Scholar]
- 38.Itoh A, Kobayashi J, Bando K, et al. The impact of mitral valve surgery combined with maze procedure. Eur J Cardiothorac Surg. 2006;29(6):1030–1035. doi: 10.1016/j.ejcts.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 39.Melo J, Santiago T, Aguiar C, et al. Surgery for atrial fibrillation in patients with mitral valve disease: results at five years from the International Registry of Atrial Fibrillation Surgery. J Thorac Cardiovasc Surg. 2008;135(4):863–869. doi: 10.1016/j.jtcvs.2007.08.069. [DOI] [PubMed] [Google Scholar]
- 40.Williams JM, Ungerleider RM, Lofland GK, et al. Left atrial isolation: new technique for the treatment of supraventricular arrhythmias. J Thorac Cardiovasc Surg. 1980;80(3):373–380. [PubMed] [Google Scholar]
- 41.Leitch JW, Klein G, Yee R, et al. Sinus node-atrioventricular node isolation: long-term results with the “corridor” operation for atrial fibrillation. J Am Coll Cardiol. 1991;17(4):970–975. doi: 10.1016/0735-1097(91)90881-9. [DOI] [PubMed] [Google Scholar]
- 42.Smith PK, Holman WL, Cox JL. Surgical treatment of supraventricular tachyar-rhythmias. Surg Clin North Am. 1985;65(3):553–570. doi: 10.1016/s0039-6109(16)43637-6. [DOI] [PubMed] [Google Scholar]
- 43.Cox JL. The surgical treatment of atrial fibrillation. IV. Surgical technique. J Thorac Cardiovasc Surg. 1991;101(4):584–592. [PubMed] [Google Scholar]
- 44.Cox JL, Canavan TE, Schuessler RB, et al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101(3):406–426. [PubMed] [Google Scholar]
- 45.Cox JL, Schuessler RB, D’Agostino HJ, Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101(4):569–583. [PubMed] [Google Scholar]
- 46.Cox JL, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg. 1999;118(5):833–840. doi: 10.1016/s0022-5223(99)70052-8. [DOI] [PubMed] [Google Scholar]
- 47.Feinberg MS, Waggoner AD, Kater KM, et al. Restoration of atrial function after the maze procedure for patients with atrial fibrillation. Assessment by Doppler echocardiography. Circulation. 1994;90(5 Pt 2):II285–II292. [PubMed] [Google Scholar]
- 48.Cox JL. The minimally invasive Maze-III procedure. Oper Tech Thorac Cardiovasc Surg. 2000;5:79–92. [Google Scholar]
- 49.Cox JL, Boineau JP, Schuessler RB, et al. Modification of the maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results. J Thorac Cardiovasc Surg. 1995;110(2):473–484. doi: 10.1016/S0022-5223(95)70244-X. [DOI] [PubMed] [Google Scholar]
- 50.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126(6):1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy PM, Gillinov AM, Castle L, et al. The Cox-Maze procedure: the Cleveland Clinic experience. Semin Thorac Cardiovasc Surg. 2000;12(1):25–29. doi: 10.1016/s1043-0679(00)70013-x. [DOI] [PubMed] [Google Scholar]
- 52.Raanani E, Albage A, David TE, et al. The efficacy of the Cox/maze procedure combined with mitral valve surgery: a matched control study. Eur J Cardiothorac Surg. 2001;19(4):438–442. doi: 10.1016/s1010-7940(01)00576-0. [DOI] [PubMed] [Google Scholar]
- 53.Schaff HV, Dearani JA, Daly RC, et al. Cox-Maze procedure for atrial fibrillation: Mayo Clinic experience. Semin Thorac Cardiovasc Surg. 2000;12(1):30–37. doi: 10.1016/s1043-0679(00)70014-1. [DOI] [PubMed] [Google Scholar]
- 54.Khargi K, Hutten BA, Lemke B, et al. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg. 2005;27(2):258–265. doi: 10.1016/j.ejcts.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128(4):535–542. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 56.Gillinov AM, Smedira NG, Cosgrove DM., 3rd Microwave ablation of atrial fibrillation during mitral valve operations. Ann Thorac Surg. 2002;74(4):1259–1261. doi: 10.1016/s0003-4975(02)03760-8. [DOI] [PubMed] [Google Scholar]
- 57.Lee JW, Choo SJ, Kim KI, et al. Atrial fibrillation surgery simplified with cryoablation to improve left atrial function. Ann Thorac Surg. 2001;72(5):1479–1483. doi: 10.1016/s0003-4975(01)03176-9. [DOI] [PubMed] [Google Scholar]
- 58.Mokadam NA, McCarthy PM, Gillinov AM, et al. A prospective multicenter trial of bipolar radiofrequency ablation for atrial fibrillation: early results. Ann Thorac Surg. 2004;78(5):1665–1670. doi: 10.1016/j.athoracsur.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 59.Reddy VY, Houghtaling C, Fallon J, et al. Use of a diode laser balloon ablation catheter to generate circumferential pulmonary venous lesions in an open-thoracotomy caprine model. Pacing Clin Electrophysiol. 2004;27(1):52–57. doi: 10.1111/j.1540-8159.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 60.Gammie JS, Haddad M, Milford-Beland S, et al. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Data-base. Ann Thorac Surg. 2008;85(3):909–914. doi: 10.1016/j.athoracsur.2007.10.097. [DOI] [PubMed] [Google Scholar]
- 61.Melby SJ, Lee AM, Zierer A, et al. Atrial fibrillation propagates through gaps in ablation lines: implications for ablative treatment of atrial fibrillation. Heart Rhythm. 2008;5(9):1296–1301. doi: 10.1016/j.hrthm.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 63.Mikat EM, Hackel DB, Harrison L, et al. Reaction of the myocardium and coronary arteries to cryosurgery. Lab Invest. 1977;37(6):632–641. [PubMed] [Google Scholar]
- 64.Ghalili K, Roth JA, Kwan SK, et al. Comparison of left ventricular cryolesions created by liquid nitrogen and nitrous oxide. J Am Coll Cardiol. 1992;20(6):1425–1429. doi: 10.1016/0735-1097(92)90258-o. [DOI] [PubMed] [Google Scholar]
- 65.Holman WL, Ikeshita M, Douglas JM, Jr, et al. Cardiac cryosurgery: effects of myocardial temperature on cryolesion size. Surgery. 1983;93(2):268–272. [PubMed] [Google Scholar]
- 66.Hunt GB, Chard RB, Johnson DC, et al. Comparison of early and late dimensions and arrhythmogenicity of cryolesions in the normothermic canine heart. J Thorac Cardiovasc Surg. 1989;97(2):313–318. [PubMed] [Google Scholar]
- 67.Markovitz LJ, Frame LH, Josephson ME, et al. Cardiac cryolesions: factors affecting their size and a means of monitoring their formation. Ann Thorac Surg. 1988;46(5):531–535. doi: 10.1016/s0003-4975(10)64691-7. [DOI] [PubMed] [Google Scholar]
- 68.Gage AM, Montes M, Gage AA. Freezing the canine thoracic aorta in situ. J Surg Res. 1979;27(5):331–340. doi: 10.1016/0022-4804(79)90149-5. [DOI] [PubMed] [Google Scholar]
- 69.Blomstrom-Lundqvist C, Johansson B, Berglin E, et al. A randomized double-blind study of epicardial left atrial cryoablation for permanent atrial fibrillation in patients undergoing mitral valve surgery: the SWEDish Multicentre Atrial Fibrillation study (SWEDMAF) Eur Heart J. 2007;28(23):2902–2908. doi: 10.1093/eurheartj/ehm378. [DOI] [PubMed] [Google Scholar]
- 70.Ad N, Cox JL. The Maze procedure for the treatment of atrial fibrillation: a minimally invasive approach. J Card Surg. 2004;19(3):196–200. doi: 10.1111/j.0886-0440.2004.4036_1.x. [DOI] [PubMed] [Google Scholar]
- 71.Doll N, Kornherr P, Aupperle H, et al. Epicardial treatment of atrial fibrillation using cryoablation in an acute off-pump sheep model. Thorac Cardiovasc Surg. 2003;51(5):267–273. doi: 10.1055/s-2003-43086. [DOI] [PubMed] [Google Scholar]
- 72.Viola N, Williams MR, Oz MC, et al. The technology in use for the surgical ablation of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2002;14(3):198–205. doi: 10.1053/stcs.2002.35292. [DOI] [PubMed] [Google Scholar]
- 73.Khargi K, Deneke T, Haardt H, et al. Saline-irrigated, cooled-tip radiofrequency ablation is an effective technique to perform the maze procedure. Ann Thorac Surg. 2001;72(3):S1090–S1095. doi: 10.1016/s0003-4975(01)02940-x. [DOI] [PubMed] [Google Scholar]
- 74.Nakagawa H, Wittkampf FH, Yamanashi WS, et al. Inverse relationship between electrode size and lesion size during radiofrequency ablation with active electrode cooling. Circulation. 1998;98(5):458–465. doi: 10.1161/01.cir.98.5.458. [DOI] [PubMed] [Google Scholar]
- 75.Gaynor SL, Ishii Y, Diodato MD, et al. Successful performance of Cox-Maze procedure on beating heart using bipolar radiofrequency ablation: a feasibility study in animals. Ann Thorac Surg. 2004;78(5):1671–1677. doi: 10.1016/j.athoracsur.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 76.Prasad SM, Maniar HS, Diodato MD, et al. Physiological consequences of bipolar radiofrequency energy on the atria and pulmonary veins: a chronic animal study. Ann Thorac Surg. 2003;76(3):836–841. doi: 10.1016/s0003-4975(03)00716-1. [discussion 841-2] [DOI] [PubMed] [Google Scholar]
- 77.Prasad SM, Maniar HS, Schuessler RB, et al. Chronic transmural atrial ablation by using bipolar radiofrequency energy on the beating heart. J Thorac Cardiovasc Surg. 2002;124(4):708–713. doi: 10.1067/mtc.2002.125057. [DOI] [PubMed] [Google Scholar]
- 78.Hamner CE, Potter DD, Jr, Cho KR, et al. Irrigated radiofrequency ablation with transmurality feedback reliably produces Cox maze lesions in vivo. Ann Thorac Surg. 2005;80(6):2263–2270. doi: 10.1016/j.athoracsur.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 79.Melby SJ, Gaynor SL, Lubahn JG, et al. Efficacy and safety of right and left atrial ablations on the beating heart with irrigated bipolar radiofrequency energy: a long-term animal study. J Thorac Cardiovasc Surg. 2006;132(4):853–860. doi: 10.1016/j.jtcvs.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 80.Demazumder D, Mirotznik MS, Schwartzman D. Biophysics of radiofrequency ablation using an irrigated electrode. J Interv Card Electrophysiol. 2001;5(4):377–389. doi: 10.1023/a:1013224110550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruchat P, Schlaepfer J, Delabays A, et al. Left atrial radiofrequency compartmentalization for chronic atrial fibrillation during heart surgery. Thorac Cardiovasc Surg. 2002;50(3):155–159. doi: 10.1055/s-2002-32411. [DOI] [PubMed] [Google Scholar]
- 82.Kress DC, Krum D, Chekanov V, et al. Validation of a left atrial lesion pattern for intraoperative ablation of atrial fibrillation. Ann Thorac Surg. 2002;73(4):1160–1168. doi: 10.1016/s0003-4975(01)03586-x. [DOI] [PubMed] [Google Scholar]
- 83.Santiago T, Melo JQ, Gouveia RH, et al. Intra-atrial temperatures in radiofrequency endocardial ablation: histologic evaluation of lesions. Ann Thorac Surg. 2003;75(5):1495–1501. doi: 10.1016/s0003-4975(02)04990-1. [DOI] [PubMed] [Google Scholar]
- 84.Thomas SP, Guy DJ, Boyd AC, et al. Comparison of epicardial and endocardial linear ablation using handheld probes. Ann Thorac Surg. 2003;75(2):543–548. doi: 10.1016/s0003-4975(02)04314-x. [DOI] [PubMed] [Google Scholar]
- 85.Santiago T, Melo J, Gouveia RH, et al. Epicardial radiofrequency applications: in vitro and in vivo studies on human atrial myocardium. Eur J Cardiothorac Surg. 2003;24(4):481–486. doi: 10.1016/s1010-7940(03)00344-0. [discussion 486] [DOI] [PubMed] [Google Scholar]
- 86.Demaria RG, Page P, Leung TK, et al. Surgical radiofrequency ablation induces coronary endothelial dysfunction in porcine coronary arteries. Eur J Cardiothorac Surg. 2003;23(3):277–282. doi: 10.1016/s1010-7940(02)00810-2. [DOI] [PubMed] [Google Scholar]
- 87.Gillinov AM, Pettersson G, Rice TW. Esophageal injury during radiofrequency ablation for atrial fibrillation. J Thorac Cardiovasc Surg. 2001;122(6):1239–1240. doi: 10.1067/mtc.2001.118041. [DOI] [PubMed] [Google Scholar]
- 88.Kottkamp H, Hindricks G, Autschbach R, et al. Specific linear left atrial lesions in atrial fibrillation: intraoperative radiofrequency ablation using minimally invasive surgical techniques. J Am Coll Cardiol. 2002;40(3):475–480. doi: 10.1016/s0735-1097(02)01993-9. [DOI] [PubMed] [Google Scholar]
- 89.Laczkovics A, Khargi K, Deneke T. Esophageal perforation during left atrial radiofrequency ablation. J Thorac Cardiovasc Surg. 2003;126(6):2119–2120. doi: 10.1016/j.jtcvs.2003.08.007. [author reply 2120] [DOI] [PubMed] [Google Scholar]
- 90.Damiano RJ, Jr, Gaynor SL. Atrial fibrillation ablation during mitral valve surgery using the Atricure device. Oper Tech Thorac Cardiovasc Surg. 2004;9(1):24–33. [Google Scholar]
- 91.Lall SC, Melby SJ, Voeller RK, et al. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg. 2007;133(2):389–396. doi: 10.1016/j.jtcvs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 92.Melby SJ, Zierer A, Bailey MS, et al. A new era in the surgical treatment of atrial fibrillation: the impact of ablation technology and lesion set on procedural efficacy. Ann Surg. 2006;244(4):583–592. doi: 10.1097/01.sla.0000237654.00841.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bando K, Kobayashi J, Hirata M, et al. Early and late stroke after mitral valve replacement with a mechanical prosthesis: risk factor analysis of a 24-year experience. J Thorac Cardiovasc Surg. 2003;126(2):358–364. doi: 10.1016/s0022-5223(03)00550-6. [DOI] [PubMed] [Google Scholar]
- 94.Geidel S, Lass M, Boczor S, et al. Monopolar and bipolar radiofrequency ablation surgery: 3-year experience in 90 patients with permanent atrial fibrillation. Heart Surg Forum. 2004;7(5):E398–E402. doi: 10.1532/HSF98.20041054. [DOI] [PubMed] [Google Scholar]
- 95.Salenger R, Lahey SJ, Saltman AE. The completely endoscopic treatment of atrial fibrillation: report on the first 14 patients with early results. Heart Surg Forum. 2004;7(6):E555–E558. doi: 10.1532/HSF98.20041111. [DOI] [PubMed] [Google Scholar]
- 96.Tada H, Ito S, Naito S, et al. Long-term results of cryoablation with a new cryoprobe to eliminate chronic atrial fibrillation associated with mitral valve disease. Pacing Clin Electrophysiol. 2005;28(Suppl 1):S73–S77. doi: 10.1111/j.1540-8159.2005.00011.x. [DOI] [PubMed] [Google Scholar]
- 97.Wolf RK, Schneeberger EW, Osterday R, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg. 2005;130(3):797–802. doi: 10.1016/j.jtcvs.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 98.Doll N, Pritzwald-Stegmann P, Czesla M, et al. Ablation of ganglionic plexi during combined surgery for atrial fibrillation. Ann Thorac Surg. 2008;86(5):1659–1663. doi: 10.1016/j.athoracsur.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 99.McClelland JH, Duke D, Reddy R. Preliminary results of a limited thoracotomy: new approach to treat atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18(12):1289–1295. doi: 10.1111/j.1540-8167.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- 100.Mehall JR, Kohut RM, Jr, Schneeberger EW, et al. Intraoperative epicardial electrophysiologic mapping and isolation of autonomic ganglionic plexi. Ann Thorac Surg. 2007;83(2):538–541. doi: 10.1016/j.athoracsur.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 101.Edgerton JR, Jackman WM, Mack MJ. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. J Interv Card Electrophysiol. 2007;20(3):89–93. doi: 10.1007/s10840-007-9177-y. [DOI] [PubMed] [Google Scholar]
- 102.Beyer E, Lee R, Lam BK. Point: minimally invasive bipolar radiofrequency ablation of lone atrial fibrillation: early multicenter results. J Thorac Cardiovasc Surg. 2009;137(3):521–526. doi: 10.1016/j.jtcvs.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 103.Edgerton JR, Edgerton ZJ, Weaver T, et al. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. Ann Thorac Surg. 2008;86(1):35–38. doi: 10.1016/j.athoracsur.2008.03.071. [discussion 39] [DOI] [PubMed] [Google Scholar]
- 104.Gaita F, Riccardi R, Caponi D, et al. Linear cryoablation of the left atrium versus pulmonary vein cryoisolation in patients with permanent atrial fibrillation and valvular heart disease: correlation of electroanatomic mapping and long-term clinical results. Circulation. 2005;111(2):136–142. doi: 10.1161/01.CIR.0000151310.00337.FA. [DOI] [PubMed] [Google Scholar]
- 105.Po SS, Scherlag BJ, Yamanashi WS, et al. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm. 2006;3(2):201–208. doi: 10.1016/j.hrthm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 106.Scherlag BJ, Nakagawa H, Jackman WM, et al. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13(Suppl 1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 107.Mounsey JP. Recovery from vagal denervation and atrial fibrillation inducibility: effects are complex and not always predictable. Heart Rhythm. 2006;3(6):709–710. doi: 10.1016/j.hrthm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 108.Oh S, Zhang Y, Bibevski S, et al. Vagal denervation and atrial fibrillation inducibility: epicardial fat pad ablation does not have long-term effects. Heart Rhythm. 2006;3(6):701–708. doi: 10.1016/j.hrthm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 109.Sakamoto S, Schuessler RB, Lee AM, et al. Focal ablation of epicardial ganglionated plexus: electrophysiological modification and regeneration of vagal tone in canine atria. Innov Technol Tech Cardiothorac Vasc Surg. 2008;3(3):67. [Google Scholar]
- 110.Benussi S, Nascimbene S, Agricola E, et al. Surgical ablation of atrial fibrillation using the epicardial radiofrequency approach: mid-term results and risk analysis. Ann Thorac Surg. 2002;74(4):1050–1056. doi: 10.1016/s0003-4975(02)03850-x. [discussion 1057] [DOI] [PubMed] [Google Scholar]
- 111.Fasol R, Meinhart J, Binder T. A modified and simplified radiofrequency ablation in patients with mitral valve disease. J Thorac Cardiovasc Surg. 2005;129(1):215–217. doi: 10.1016/j.jtcvs.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 112.Imai K, Sueda T, Orihashi K, et al. Clinical analysis of results of a simple left atrial procedure for chronic atrial fibrillation. Ann Thorac Surg. 2001;71(2):577–581. doi: 10.1016/s0003-4975(00)02254-2. [DOI] [PubMed] [Google Scholar]
- 113.Knaut M, Spitzer SG, Karolyi L, et al. Intraoperative microwave ablation for curative treatment of atrial fibrillation in open heart surgery–the MICRO-STAF and MICRO-PASS pilot trial. MICROwave application in surgical treatment of atrial fibrillation. MICROwave application for the treatment of atrial fibrillation in bypass-surgery. Thorac Cardiovasc Surg. 1999;47(Suppl 3):379–384. doi: 10.1055/s-2007-1013205. [DOI] [PubMed] [Google Scholar]
- 114.Kondo N, Takahashi K, Minakawa M, et al. Left atrial maze procedure: a useful addition to other corrective operations. Ann Thorac Surg. 2003;75(5):1490–1494. doi: 10.1016/s0003-4975(02)04900-7. [DOI] [PubMed] [Google Scholar]
- 115.Schuetz A, Schulze CJ, Sarvanakis KK, et al. Surgical treatment of permanent atrial fibrillation using microwave energy ablation: a prospective randomized clinical trial. Eur J Cardiothorac Surg. 2003;24(4):475–480. doi: 10.1016/s1010-7940(03)00377-4. [discussion 480] [DOI] [PubMed] [Google Scholar]
- 116.Sie HT, Beukema WP, Misier AR, et al. Radiofrequency modified maze in patients with atrial fibrillation undergoing concomitant cardiac surgery. J Thorac Cardiovasc Surg. 2001;122(2):249–256. doi: 10.1067/mtc.2001.114633. [DOI] [PubMed] [Google Scholar]
- 117.Barnett SD, Ad N. Surgical ablation as treatment for the elimination of atrial fibrillation: a meta-analysis. J Thorac Cardiovasc Surg. 2006;131(5):1029–1035. doi: 10.1016/j.jtcvs.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 118.Doukas G, Samani NJ, Alexiou C, et al. Left atrial radiofrequency ablation during mitral valve surgery for continuous atrial fibrillation: a randomized controlled trial. JAMA. 2005;294(18):2323–2329. doi: 10.1001/jama.294.18.2323. [DOI] [PubMed] [Google Scholar]
- 119.Gillinov AM, McCarthy PM, Blackstone EH, et al. Surgical ablation of atrial fibrillation with bipolar radiofrequency as the primary modality. J Thorac Cardiovasc Surg. 2005;129(6):1322–1329. doi: 10.1016/j.jtcvs.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 120.Voeller RK, Bailey MS, Zierer A, et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg. 2008;135(4):870–877. doi: 10.1016/j.jtcvs.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Schuessler RB, Damiano RJ, et al. Noninvasive electrocardiographic imaging (ECGI) of scar-related atypical atrial flutter. Heart Rhythm. 2007;4(12):1565–1567. doi: 10.1016/j.hrthm.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y, Cuculich PS, Woodard PK, et al. Focal atrial tachycardia after pulmonary vein isolation: noninvasive mapping with electrocardiographic imaging (ECGI) Heart Rhythm. 2007;4(8):1081–1084. doi: 10.1016/j.hrthm.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Damiano RJ, Jr, Gaynor SL, Bailey M, et al. The long-term outcome of patients with coronary disease and atrial fibrillation undergoing the Cox maze procedure. J Thorac Cardiovasc Surg. 2003;126(6):2016–2021. doi: 10.1016/j.jtcvs.2003.07.006. [DOI] [PubMed] [Google Scholar]