Abstract
As a consequence of the high potency and short range of alpha-particles, radiopharmaceutical therapy with alpha-particle emitting radionuclides is a promising treatment approach that is under active pre-clinical and clinical investigation. To understand and predict the biological effects of alpha-particle radiopharmaceuticals, dosimetry is required at the micro or multi-cellular scale level. At such a scale, highly non-uniform irradiation of the target volume may be expected and the utility of a single absorbed dose value to predict biological effects comes into question. It is not currently possible to measure the pharmacokinetic input required for micro scale dosimetry in humans. Accordingly, pre-clinical studies are required to provide the pharmacokinetic data for dosimetry calculations. The translation of animal data to the human requires a pharmacokinetic model that links macro- and micro-scale pharmacokinetics thereby enabling the extrapolation of micro-scale kinetics from macroscopic measurements. These considerations along with a discussion of the appropriate physical quantity and related units for alpha-particle radiopharmaceutical therapy are examined in this review.
Keywords: Alpha-particles, dosimetry, modelling, microdosimetry
INTRODUCTION
Dosimetry for alpha-particle emitting radiopharmaceuticals presents a number of challenges. The fundamental dosimetry formalism, described in the Medical Internal Radiation Dose (MIRD) Committee Pamphlet 21 [1] with the guidance provided in MIRD Pamphlet 22 [2] can accommodate the majority of absorbed dose calculations for alphaparticle emitters. These approaches are optimal for circumstances in which single dosimetric parameter values (e.g., mean absorbed dose to a target volume) are expected to adequately reflect biological effect. Even without invoking the stochastic effects of alpha-particles and the corresponding need for microdosimetry [3], situations can arise in which the short range of alpha-particles relative to the typical scale of human organ dimensions and associated critical or target cell populations (i.e., target volumes) can lead to a highly non-uniform irradiation of the target volume. In these cases the distribution of the absorbed dose to the target volume is required to predict the biological effects and of the microlevel distribution of alpha-emitters relative to the range of the alpha-particles should be carefully considered [4]; traditional organ or even voxel-based calculations will fail to provide dosimetry information relevant to understanding or predicting the biological response. Furthermore, human imaging-based pharmacokinetic information cannot be currently obtained at the needed (sub-mm) resolution. Finally, there is no agreed-upon quantity and associated unit with which to express the results of a correctly performed alpha-emitter absorbed dose calculation that is weighted to reflect deterministic biological effects (tumor kill or toxicity). Although this state of affairs may appear discouraging, substantial progress is being made towards solving each of these problems. This chapter describes some of the progress being made in the various areas highlighted above.
PHARMACOKINETIC INPUT
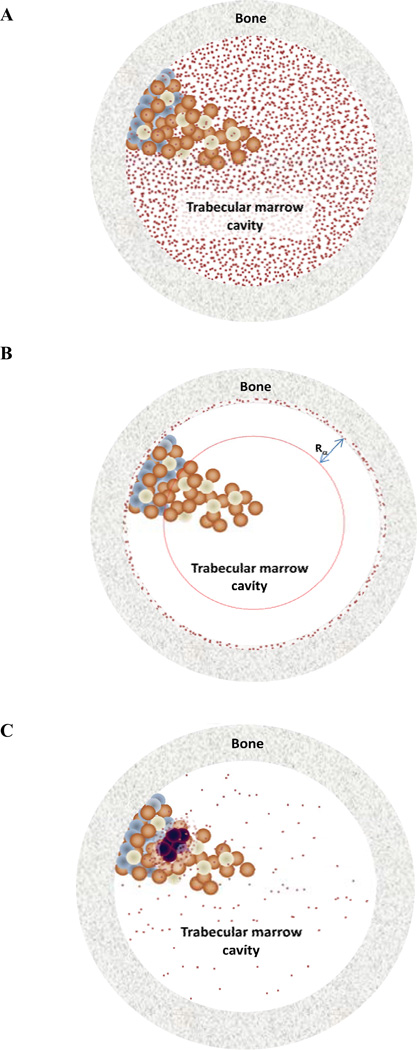
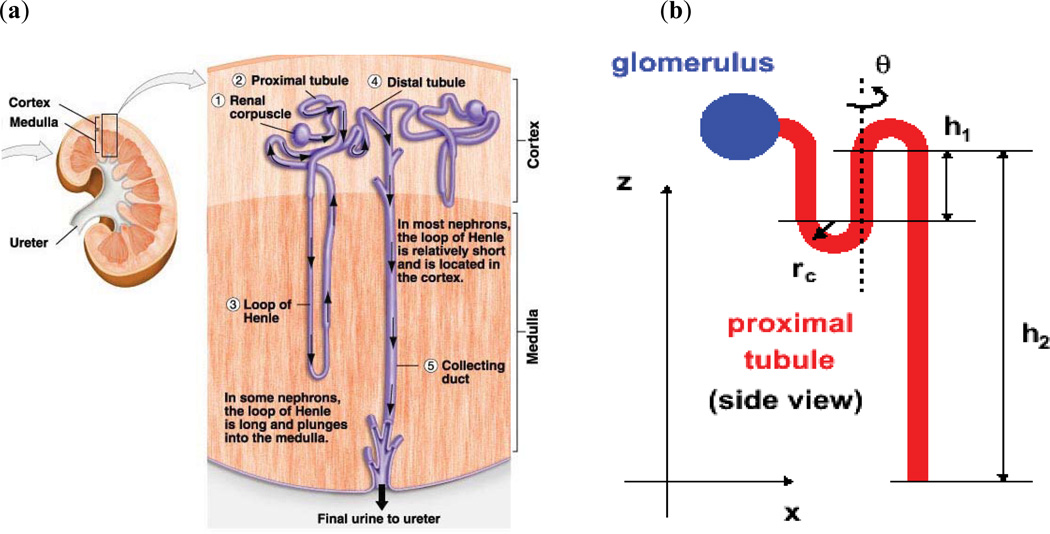
As noted above, human pharmacokinetic data at the resolution required for alpha-emitter dosimetry cannot be obtained from patient imaging. MIRD Pamphlet 22 discusses the essential role that pre-clinical studies must play in measuring the agent’s distribution at the micro-scale level [2]. To satisfy this requirement, alpha-particle emitter specific micro-scale imaging technology, applicable to pre-clinical studies has been recently described [5]. The actual scale required will depend upon: the agent’s localization, the range of the alpha-particles and the extent to which all alpha emissions may be assumed to occur at the targeted site (i.e., extent to which alpha-emitting progeny decay away from the site of parent decay [6,7]). The spatial relation between the source region and target volumes or cell populations within individual organs will also impact the scale of the calculation. For example, in the marrow the scale at which the calculation should be performed will depend upon whether the radiopharmaceutical localizes to the bone surface (e.g, as in calcium analogs) or to the active marrow volume (as in antibody-mediated delivery) or to target cells in the marrow [8–10] (Fig. 1). The average absorbed dose over a marrow cavity volume would be adequate if the relevant target cells were uniformly distributed within this volume (there is evidence, however, that this is not the case [11,12]). On the other hand, if the dose distribution within the cavity were truly uniform, target cell distribution would not impact biological response. If the agent localizes to the bone surface, the scale of the calculation will depend upon the range of the alpha-particles and an average over the marrow cavity volume would not be appropriate. In the kidneys, the scale will depend upon whether the agent localizes in the collecting ducts or the nephrons (Fig. 2). In alpha-emitter therapy, a comprehensive series of studies have been performed to delineate the histopathology of alpha-particle induced kidney damage and physiologic interventions to alleviate it [13–15]. Preclinical dose-response studies have shown that the average alpha-particle absorbed dose to the cortex does not predict toxicity [16]. This observation also highlights the importance of pre-clinical dose-response studies in assessing the adequacy of a particular dosimetry methodology. In the study cited above, mice with aggressive endogenous mammary carcinoma metastases to the lungs survived for over a year following a single IV injection of 225Ac-antibody. At necropsy, however, these otherwise symptom-free mice had shrunken kidneys reflecting severe radiation damage at calculated mean whole-kidney absorbed dose of 2 Gy (not weighted for relative biological effectiveness (RBE)). Studies using a 211At-labeled F(ab’)2 antibody fragment found reductions in glomerular filtration rate (a measure of renal toxicity) and changes in histopathology after 10 Gy to the whole kidneys [17]. External beam and radiopeptide experience has shown that 26 to 27 Gy (low linear energy transfer (LET) radiation) is required for renal damage [18–22]. Renal toxicity in the 225Ac-intact antibody studies is likely due to the differential micro-scale localization of free 213Bi in the kidneys [23]. The discrepancy observed amongst these studies highlights the need for micro-scale dosimetry to better relate absorbed dose to biological effect.
Fig. (1).
Schematic of three potential alpha-emitter distributions (red dots). In (a), the distribution of emitters is uniformly distributed in the trabecular marrow cavity. In panel (b), the agent localizes to the bone surface and only irradiates a portion of the marrow. In (c), the emitters are distributed around tumor cells in the marrow. Each of these configurations will have a different impact on marrow toxicity. Case (a) can be accommodated by single value, absorbed fraction-based calculations. The other two scenarios are best handled by Monte Carlo methods that yield dose-volume histograms to calculate radiobiological parameters that can relate the dose-volume histogram to biological efficacy or toxicity.
Fig. (2).
Schematic representation of the kidney (a) depiction of realistic anatomy (from http://www.uic.edu/classes/bios/bios100/-lecturesf04am/kidney01a.jpg) and (b) idealized model of a nephron that may be used in Monte Carlo calculations once the distribution of emissions is localized to the different micro-scale kidney compartments.
MODELLING
In conjunction with measurements of the alpha-emitting radiopharmaceutical’s micro level distribution, both anatomic and pharmacokinetic modelling is important. Pharmacokinetic modelling, ideally based upon longitudinal autoradiography studies or a priori information regarding the behaviour of the agent (and its decay products) in microscopically defined compartments is needed to assess (a) the time-course of alpha-emitter activity concentration in such compartments and, from this, (b) the spatial distribution of time-integrated activity (i.e., cumulated activity) that can subsequently be used as input for Monte Carlo or point-kernel calculations [24–29] to obtain the absorbed dose or specific energy distribution. Anatomical modelling of the target volume is needed to facilitate the absorbed dose calculation. This will involve an idealized representation of the anatomical region. The modelled region may correspond to an organ sub-region (e.g, the kidney cortex or glomerulus) [30–32], a cluster of cells [33–36], or a sub-cellular compartment [37–39]. Once anatomical models of the source and target regions are established, the calculational methodology must be chosen. As reviewed in MIRD Pamphlet 22, microdosimetry techniques are required when the number of alpha-particles emitted is so low or (equivalently) when the target volume is so small that the energy deposition of a single alpha-particle can significantly influence the absorbed dose to the target. Expressed more formally, microdosimetry techniques are required when the relative deviation of the local dose exceeds 20% [40]. Under such conditions a single dose value is unlikely to reflect the biological outcome and the probability distribution of specific energy must be considered in calculating biologic response (e.g., cell survival, organ toxicity). Microdosimetry methods have been extensively reviewed [3]; additional details may be found in Pamphlet 22 and in Chapter 9 of reference [41]. When microdosimetry techniques are not required, absorbed dose calculations at these dimensions may be performed by previously calculated absorbed fraction values for selected microscopically defined source-target volume combinations [37]. If there is a high level of non-uniformity requiring a large number of voxelized absorbed fractions it will be easier and more tractable to perform such calculations by Monte Carlo or point-kernel convolution calculations. The method selected should provide a histogram of the absorbed dose distribution in the target volume (providing the number of times that a particular absorbed dose value occurs in a target volume -or, depending upon the target dimensions and expected number of events, the microdosimetric specific energy). This information can be used to determine if the mean absorbed dose (or single value microdosimetric parameters [42]) over the target volume will be relevant in determining likely biological effects.
As noted above, the micro-scale measurements of activity distribution require pre-clinical studies. Translation of pre-clinical results to the human requires that the studies be performed in the context of pharmacokinetic modelling so that a pharmacokinetic model that describes kinetics in macroscopic compartments such as the blood and the whole organ be used to fit micro-scale kinetics. Such an approach establishes rate constants and kinetic parameters that describe the relationship of the radiopharmaceutical at the micro-scale to that at the macro-scale. This approach assumes that the macro to micro transfer of the radiopharmaceutical in the pre-clinical model is the same as that in the human. Until it becomes possible to make such micro-scale measurements, in vivo, caution and careful consideration of the chosen pre-clinical model is required in applying this approach.
QUANTITIES AND RELATED UNITS
Dosimetry for internally distributed (unsealed) radionuclides was initially developed for diagnostic imaging in nuclear medicine by the MIRD Committee [43,44] and for radiation protection by the International Commission on Radiologic Protection (ICRP) [45]. The basic equation and concepts were the same but the two organizations chose nomenclatures and symbols tailored to their specific concerns. The MIRD Committee recently published a dosimetry formalism that applies to both realms and that has been also adopted by the ICRP. The dosimetry formalism includes quantities such as equivalent dose and effective dose [1]. Equivalent dose is the absorbed dose weighted by radiation weighting factors and is intended to adjust the absorbed dose for the difference in stochastic risks (e.g., cancer induction) of different particle types. A weighting factor of 20 was chosen by the ICRP for alpha-particles, meaning that a given absorbed dose of alpha-particle radiation is 20 time more likely to lead to cancer and other stochastic effects than the same absorbed dose of electron or photon radiation [46]. The effective dose is a weighted average of individual organ equivalent doses. The weighting factors applied for each organ were also selected by the ICRP to reflect the relative overall detriment that a particular distribution of organ absorbed doses will have to a reference individual, taken to represent an average member of an exposed population. Both equivalent and effective dose values are associated with the sievert (Sv).
None of the dosimetric quantities discussed thus far are relevant to radiopharmaceutical therapy. Although both stochastic (cancer risk) and deterministic (toxicity, tumor kill) effects occur at the administered activities and resulting absorbed doses associated with radiopharmaceutical therapy, the relevant end-points for treatment evaluation (i.e., toxicity and efficacy) are deterministic (the effect-as opposed to the probability that an effect will occur-increases with increasing absorbed dose). For deterministic end-points alpha particle radiation yields 3–7 times more toxicity or efficacy (represented by cell sterilization) per unit absorbed dose than electrons or photons [2]. The factor used to weight the absorbed dose for deterministic end-points is called the relative biological efficacy (RBE). It is analogous to the radiation weighting factors described above except that it is a value obtained by experimental measurements. As noted in MIRD Pamphlets 21 and 22 no dosimetric quantity analogous to the equivalent dose has been defined for deterministic effects. Furthermore, since a dosimetric quantity does not exist for deterministic effects there is also no associated unit for a weighted absorbed dose value that reflects deterministic as opposed to stochastic effects. In short, there is no consensus on what to call an RBE-weighted absorbed dose and also no consensus on what special named unit to assign to the numerical value. These issues have been highlighted by the MIRD Committee and the Committee is working closely with the relevant international regulatory and standards organizations (the International Commission on Radiological Units (ICRU) and the International Bureau of Weights and Measures (Bureau International des Poids et Mesures (BIPM))) towards a resolution of these issues. Towards this objective the MIRD Committee has proposed a special named unit, the barendsen (Bd) for the deterministic dose quantity once it is defined [47]. This will avoid the confusion possible with units such as gray-equivalents or cobalt-gray-equivalents that are in use or have been recommended previously. Until these issues are resolved, absorbed doses for alpha-emitting radionuclides should separately list the absorbed dose (in Gy) of electron+photon emissions (if these are non-negligible) and of alpha-particle emissions [2].
CONCLUSIONS
Dosimetry for alpha-emitter therapy requires a consideration of the microscopic distribution of emitters. As these are generally not measurable in individual patients, pre-clinical studies that characterize the distribution and kinetics of the alpha-emitting radiopharmaceutical at the microscopic level are essential to the dosimetry for these agents. The translation of activity distributions obtained in pre-clinical studies to the human requires micro-scale models of the source-target geometry at human dimensions. Dosimetry reporting for alpha emitters should individually list the contributions of high (alpha-particles) vs. low (electron and photon) LET emissions.
REFERENCES
- 1.Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet No. 21: a generalized schema for radiopharmaceutical dosimetry--standardization of nomenclature. J. Nucl. Med. 2009;50(3):477–484. doi: 10.2967/jnumed.108.056036. [DOI] [PubMed] [Google Scholar]
- 2.Sgouros G, Roeske JC, McDevitt MR, Palm S, Allen BJ, Fisher DR, Brill AB, Song H, Howell RW, Akabani G. MIRD Pamphlet No. 22 (Abridged): Radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J. Nucl. Med. 2010;51(2):311–328. doi: 10.2967/jnumed.108.058651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humm JL, Roeske JC, Fisher DR, Chen GT. Microdosimetric concepts in radioimmunotherapy. Med. Phys. 1993;20(2):535–541. doi: 10.1118/1.597049. [DOI] [PubMed] [Google Scholar]
- 4.Roeske JC, Chen GT, Atcher RW, Pelizzari CA, Rotmensch J, Haraf D, Montag A, Weichselbaum RR. Modeling of dose to tumor and normal tissue from intraperitoneal radioimmunotherapy with alpha and beta emitters. Int. J. Radiat. Oncol. Biol. Phys. 1990;19(6):1539–1548. doi: 10.1016/0360-3016(90)90370-y. [DOI] [PubMed] [Google Scholar]
- 5.Bäck T, Jacobsson L. The alpha-camera: a quantitative digital autoradiography technique using a charge-coupled device for ex vivo high-resolution bioimaging of alpha-particles. J. Nucl. Med. 2010;51(10):1616–1623. doi: 10.2967/jnumed.110.077578. [DOI] [PubMed] [Google Scholar]
- 6.Hamacher KA, Sgouros G. A schema for estimating absorbed dose to organs following the administration of radionuclides with multiple unstable daughters: a matrix approach. Med. Phys. 1999;26(12):2526–2528. doi: 10.1118/1.598788. [DOI] [PubMed] [Google Scholar]
- 7.Hamacher KA, Sgouros G. Theoretical estimation of absorbed dose to organs in radioimmunotherapy using radionuclides with multiple unstable daughters. Med. Phys. 2001;28(9):1857–1874. doi: 10.1118/1.1395026. [DOI] [PubMed] [Google Scholar]
- 8.Akabani G, Zalutsky MR. Microdosimetry of astatine-211 using histological images: application to bone marrow. Radiat. Res. 1997;148(6):599–607. [PubMed] [Google Scholar]
- 9.Charlton DE, Utteridge TD, Allen BJ. Theoretical treatment of human haemopoietic stem cell survival following irradiation by alpha particles. Int. J. Radiat. Biol. 1998;74(1):111–118. doi: 10.1080/095530098141771. [DOI] [PubMed] [Google Scholar]
- 10.Utteridge TD, Charlton DE, Allen BJ. Monte Carlo modeling of the effects of injected short-, medium- and longer-range alpha-particle emitters on human marrow at various ages. Radiat. Res. 2001;156(4):413–418. doi: 10.1667/0033-7587(2001)156[0413:mcmote]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Watchman CJ, Bourke VA, Lyon JR, Knowlton AE, Butler SL, Grier DD, Wingard JR, Braylan RC, Bolch WE. Spatial distribution of blood vessels and CD34+ hematopoietic stem and progenitor cells within the marrow cavities of human cancellous bone. J. Nucl. Med. 2007;48(4):645–654. doi: 10.2967/jnumed.106.035337. [DOI] [PubMed] [Google Scholar]
- 12.Bourke VA, Watchman CJ, Reith JD, Jorgensen ML, Dieudonne A, Bolch WE. Spatial gradients of blood vessels and hematopoietic stem and progenitor cells within the marrow cavities of the human skeleton. Blood. 2009;114(19):4077–4080. doi: 10.1182/blood-2008-12-192922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaggi JS, Kappel BJ, McDevitt MR, Sgouros G, Flombaum CD, Cabassa C, Scheinberg DA. Efforts to control the errant products of a targeted in vivo generator. Cancer Res. 2005;65(11):4888–4895. doi: 10.1158/0008-5472.CAN-04-3096. [DOI] [PubMed] [Google Scholar]
- 14.Jaggi JS, Seshan SV, McDevitt MR, LaPerle K, Sgouros G, Scheinberg DA. Renal tubulointerstitial changes after internal irradiation with _-particle-emitting actinium daughters. J. Am. Soc. Nephrol. 2005;16(9):2677–2689. doi: 10.1681/ASN.2004110945. [DOI] [PubMed] [Google Scholar]
- 15.Jaggi JS, Seshan SV, McDevitt MR, Sgouros G, Hyjek E, Scheinberg DA. Mitigation of radiation nephropathy after internal _-particle irradiation of kidneys. Int. J. Radiat. Biol. 2006;64(5):1503–1512. doi: 10.1016/j.ijrobp.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Song H, Hobbs RF, Vajravelu R, Huso DL, Esaias C, Apostolidis C, Morgenstern A, Sgouros G. Radioimmunotherapy of breast cancer metastases with alpha-particle emitter 225Ac: comparing efficacy with 213Bi and 90Y. Cancer Res. 2009;69(23):8941–8948. doi: 10.1158/0008-5472.CAN-09-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bäck T, Haraldsson B, Hultborn R, Jensen H, Johansson ME, Lindegren S, Jacobsson L. Glomerular filtration rate after alpha-radioimmunotherapy with 211At-MX35-F(ab')2: a long-term study of renal function in nude mice. Cancer Biother. Radiopharm. 2009;24(6):649–658. doi: 10.1089/cbr.2009.0628. [DOI] [PubMed] [Google Scholar]
- 18.Andersson H, Cederkrantz E, Bäck T, Divgi C, Elgqvist J, Himmelman J, Horvath G, Jacobsson L, Jensen H, Lindegren S, Palm S, Hultborn R. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of 211At-MX35 F(ab')2--a phase I study. J. Nucl. Med. 2009;50(7):1153–1160. doi: 10.2967/jnumed.109.062604. [DOI] [PubMed] [Google Scholar]
- 19.Barone R, Borson-Chazot F, Valkema R, Walrand S, Chauvin F, Gogou L, Kvols LK, Krenning EP, Jamar F, Pauwels S. Patient-specific dosimetry in predicting renal toxicity with 90Y-DOTATOC: relevance of kidney volume and dose rate in finding a dose-effect relationship. J. Nucl. Med. 2005;46:99s–106s. [PubMed] [Google Scholar]
- 20.Wessels B. Summary and perspectives on kidney dose-response to radionuclide therapy. Cancer Biother. Radiopharm. 2004;19(3):388–390. doi: 10.1089/1084978041424981. [DOI] [PubMed] [Google Scholar]
- 21.Dawson LA, Kavanagh BD, Paulino AC, Das SK, Miften M, Li XA, Pan C, Ten Haken RK, Schultheiss TE. Radiation-associated kidney injury. Int. J. Radiat. Oncol. Biol. Phys. 2010;76:108s–115s. doi: 10.1016/j.ijrobp.2009.02.089. [DOI] [PubMed] [Google Scholar]
- 22.O'Donoghue J. Relevance of external beam dose-response relationships to kidney toxicity associated with radionuclide therapy. Cancer Biother. Radiopharm. 2004;19(3):378–387. doi: 10.1089/1084978041425025. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz J, Jaggi JS, O'Donoghue JA, Ruan S, McDevitt M, Larson SM, Scheinberg DA, Humm JL. Renal uptake of bismuth-213 and its contribution to kidney radiation dose following administration of actinium-225-labeled antibody. Phys. Med. Biol. 2011;56(3):721–733. doi: 10.1088/0031-9155/56/3/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roeske JC, Humm JL. Microdosimetry of targeted radionuclides. In: Zaidi H, Sgouros G, editors. Therapeutic Applications of Monte Carlo in Nuclear Medicine. Bristol, UK: Institute of Physics Publishing, Ltd; 2003. pp. 204–213. [Google Scholar]
- 25.Incerti S, Gault N, Habchi C, Lefaix JL, Moretto P, Poncy JL, Pouthier T, Seznec H. A comparison of cellular irradiation techniques with alpha particles using the Geant4 Monte Carlo simulation toolkit. Radiat. Prot. Dosimetry. 2006;122(1-4):327–329. doi: 10.1093/rpd/ncl422. [DOI] [PubMed] [Google Scholar]
- 26.Roeske JC, Hoggarth M. Alpha-particle Monte Carlo simulation for microdosimetric calculations using a commercial spreadsheet. Phys. Med. Biol. 2007;52(7):1909–1922. doi: 10.1088/0031-9155/52/7/010. [DOI] [PubMed] [Google Scholar]
- 27.Hunt JG, Watchman CJ, Bolch WE. Calculation of absorbed fractions to human skeletal tissues due to alpha particles using the Monte Carlo and 3-D chord-based transport techniques. Radiat. Prot. Dosimetry. 2007;127(1-4):223–226. doi: 10.1093/rpd/ncm276. [DOI] [PubMed] [Google Scholar]
- 28.Humm JL. A microdosimetric model of astatine-211 labeled antibodies for radioimmunotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1987;13(11):1767–1773. doi: 10.1016/0360-3016(87)90176-3. [DOI] [PubMed] [Google Scholar]
- 29.Humm JL, Chin LM. A model of cell inactivation by alpha-particle internal emitters. Radiat Res. 1993;134(2):143–150. [PubMed] [Google Scholar]
- 30.Barone R, Borson-Chazot FO, Valkerna R, Walrand S, Chauvin F, Gogou L, Kvols LK, Krenning EP, Jamar F, Pauwels S. Patient-specific dosimetry in predicting renal toxicity with Y-90-DOTATOC: relevance of kidney volume and dose rate in finding a dose-effect relationship. J. Nucl. Med. 2005;46:99s–106s. [PubMed] [Google Scholar]
- 31.Bouchet LG, Bolch WE, Blanco HP, Wessels BW, Siegel JA, Rajon DA, Clairand I, Sgouros G. MIRD Pamphlet No 19: absorbed fractions and radionuclide S values for six age-dependent multiregion models of the kidney. J. Nucl. Med. 2003;44(7):1113–1147. [PubMed] [Google Scholar]
- 32.Wessels BW, Konijnenberg MW, Dale RG, Breitz HB, Cremonesi M, Meredith RF, Green AJ, Bouchet LG, Brill AB, Bolch WE, Sgouros G, Thomas SR. MIRD Pamphlet No. 20: The effect of model assumptions on kidney dosimetry and response-Implications for radionuclide therapy. J. Nucl. Med. 2008;49(11):1884–1899. doi: 10.2967/jnumed.108.053173. [DOI] [PubMed] [Google Scholar]
- 33.Ballangrud AM, Yang WH, Charlton DE, McDevitt MR, Hamacher KA, Panageas KS, Ma D, Bander NH, Scheinberg DA, Sgouros G. Response of LNCaP spheroids after treatment with an alpha-particle emitter 213Bi-labeled anti-prostate-specific membrane antigen antibody (J591) Cancer Res. 2001;61(5):2008–2014. [PubMed] [Google Scholar]
- 34.Ballangrud AM, Yang WH, Palm S, Enmon R, Borchardt PE, Pellegrini VA, McDevitt MR, Scheinberg DA, Sgouros G. Alpha-particle emitting atomic generator (Actinium-225)-labeled trastuzumab (herceptin) targeting of breast cancer spheroids: efficacy versus HER2/neu expression. Clin. Cancer Res. 2004;10(13):4489–4497. doi: 10.1158/1078-0432.CCR-03-0800. [DOI] [PubMed] [Google Scholar]
- 35.Charlton DE. Radiation effects in spheroids of cells exposed to alpha emitters. Int. J. Radiat. Biol. 2000;76(11):1555–1564. doi: 10.1080/09553000050176315. [DOI] [PubMed] [Google Scholar]
- 36.Goddu SM, Rao DV, Howell RW. Multicellular dosimetry for micrometastases: dependence of self-dose versus cross-dose to cell nuclei on type and energy of radiation and subcellular distribution of radionuclides. J. Nucl. Med. 1994;35(3):521–530. [PubMed] [Google Scholar]
- 37.Goddu SM, Howell RW, Rao DV. Cellular dosimetry: absorbed fractions for monoenergetic electron and alpha particle sources and S-values for radionuclides uniformly distributed in different cell compartments. J. Nucl. Med. 1994;35(2):303–316. [PubMed] [Google Scholar]
- 38.Goddu SM, Howell RL, Bouchet LG, Bolch WE, Rao DV. MIRD cellular S values. Reston VA: Society of Nuclear Medicine; 1997. [Google Scholar]
- 39.Hamacher KA, Den RB, Den EI, Sgouros G. Cellular dose conversion factors for alpha-particle-emitting radionuclides of interest in radionuclide therapy. J. Nucl. Med. 2001;42(8):1216–1221. [PubMed] [Google Scholar]
- 40.Kellerer AM, Chmelevsky D. Criteria for applicability of LET. Radiat. Res. 1975;63(2):226–234. [PubMed] [Google Scholar]
- 41.Zaidi H, Sgouros G. Therapeutic Applications of Monte Carlo Calculations in Nuclear Medicine. Philadelphia: Institute of Physic Publishing; 2003. [Google Scholar]
- 42.Stinchcomb TG, Roeske JC. Values of "S," <z1> and <(z1)2> for dosimetry using alpha-particle emitters. Med. Phys. 1999;26(9):1960–1971. doi: 10.1118/1.598701. [DOI] [PubMed] [Google Scholar]
- 43.Loevinger R, Berman M. A schema for calculating the absorbed dose from biologically distributed radionuclides. MIRD Pamphlet No. 1. J. Nucl. Med. 1968;9:5. [PubMed] [Google Scholar]
- 44.Loevinger R, Berman M. A revised schema for calculating the absorbed dose from biologically distributed radionuclides. MIRD Pamphlet No. 1 (revised) New York: The Society of Nuclear Medicine; 1976. [Google Scholar]
- 45.Evaluation of radiation doses to body tissues from internal contamination due to occupational exposure. London/New York: Annals of the ICRP (Pergamon Press); 1968. ICRP Publication 10. [Google Scholar]
- 46.Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (WR) London/New York: Annals of the ICRP (Pergamon Press); 2003. ICRP Publication 92. [DOI] [PubMed] [Google Scholar]
- 47.Sgouros G, Howell RW, Bolch WE, Fisher DR. MIRD commentary: proposed name for a dosimetry unit applicable to deterministic biological effects--the barendsen (Bd) J. Nucl. Med. 2009;50(3):485–487. doi: 10.2967/jnumed.108.057398. [DOI] [PubMed] [Google Scholar]