Abstract
Objective
Assessing and treating pain in nonverbal children with developmental disabilities are a clinical challenge. Current assessment approaches rely on clinical impression and behavioral rating scales completed by proxy report. Given the growing health relevance of the salivary metabolome, we undertook a translational-oriented feasibility study using proton nuclear magnetic resonance (NMR) spectroscopy and neuropeptide/cytokine/hormone detection to compare a set of salivary biomarkers relevant to nociception.
Design
Within-group observational design.
Setting
Tertiary pediatric rehabilitation hospital.
Subjects
Ten nonverbal pediatric patients with cerebral palsy with and without pain.
Methods
Unstimulated (passively collected) saliva was collected using oral swabs followed by perchloric acid extraction and analyzed on a Bruker Avance 700 MHz NMR spectrometer. We also measured salivary levels of several cytokines, chemokines, hormones, and neuropeptides.
Results
Partial least squares discriminant analysis showed separation of those children with/without pain for a number of different biomarkers. The majority of the salivary metabolite, neuropeptide, cytokine, and hormone levels were higher in children with pain vs no pain.
Conclusions
The ease of collection and noninvasive manner in which the samples were collected and analyzed support the possibility of the regular predictive use of this novel biomarker-monitoring method in clinical practice.
Keywords: Saliva, Metabolite, Pain, Developmental Disability, Nuclear Magnetic Resonance (NMR), Partial Least Squares Discriminant Analysis (PLS-DA)
Introduction
Pain is a universal phenomenon causing tremendous human suffering and compromising the quality of life for countless individuals. Pain is the number one reason people seek medical appointments and costs the United States in excess of $100 billion annually in health care and lost productivity [1]. Individuals with intellectual disability (e.g., mental retardation) associated with neurodevelopmental disabilities (e.g., cerebral palsy [CP]), however, are often assumed to be insensitive or indifferent to pain [2]. Expression of pain by individuals with neurodevelopmental disorders is frequently ambiguous and its recognition by caregivers can be highly subjective [3]. This presents a tremendous challenge for clinicians and researchers alike. There is no reason, however, to believe that pain is any less frequent in someone with a developmental disability, or that such an individual would be insensitive or indifferent to pain. Numerous functional limitations as well as the underlying neurologic condition itself frequently confound the presentation of pain. Regardless of the degree of the disability, however, pain or conditions associated with it are often a part of daily life for individuals with neurodevelopmental disability [4].
The problem is significant as children with developmental disabilities experience markedly higher rates of reported pain or pain-related procedures (30–50%) than their typically developing peers with enormous associated costs (extended hospital stays, emotional cost of suffering, reduced adaptive function, pain “memory” associated with hospital settings) [5]. Assessment approaches that are not language dependent and a measurement framework that can account for and predict pain trajectory/course over time are needed. A major advance in pain assessment and treatment in this context could be made by translating basic research evaluating associations between pain etiologies and biomarker profiles to complement clinical assessments, particularly for vulnerable populations (cognitive impairment associated with a range of clinical conditions).
Current diagnostic and therapeutic approaches to manage chronic or recurring pain experienced by individuals with neurodevelopmental disabilities are limited by our narrow understanding of the underlying biological mechanisms contributing to pain of various etiologies (i.e., congenital abnormalities, spasticity, neural tube defects, muscle strain) and the lack of biomarkers predictive of therapeutic outcome. Although imaging methods (i.e., magnetic resonance imaging, computed tomography scan) can be used successfully to assist diagnosis in some cases, imaging findings by themselves frequently have a low correlation to pain and disability levels found upon physical evaluation [6]. This may be because biochemical factors, which are undetectable by imaging, are contributing to a chronic pain state. Because of problems related to self-report and cognitive, motor, and sensory impairments, adequate pain management often eludes individuals with neurodevelopmental disabilities [7]. Therapeutic decisions are frequently based on trial and error, creating a situation frustrating to physicians and nurses as well as individual patients and their families.
A growing literature documents the value of salivary biomarkers in general and the health relevance of the salivary metabolome in particular [8]. Considering the vulnerable clinical population for the current project, saliva is regarded as very attractive because it is relatively easy to collect and noninvasive. As a first step to understanding salivary metabolites, hormones, and peptides in relation to clinical pain in a vulnerable patient population, we undertook a preliminary feasibility study using proton nuclear magnetic resonance (NMR) and immunoassays to identify and compare a set of nociceptive-relevant salivary biomarkers from a clinical sample of pediatric patients with CP with and without ongoing pain.
Methods
Participants
Following approval of the University of Minnesota and Gillette Children’s Specialty Healthcare Institutional Review Boards, informed consent was obtained initially from parents for 12 (8 male) children with CP, but 2 patients were omitted from the final analysis because of anomalous glucose/maltose levels (1 patient) or incomplete cytokine data/insufficient saliva volume (1 patient), leaving a final sample of 10 nonverbal pediatric patients with CP (mean age = 9.2 years, standard deviation [SD] = 5.3 years; mean Gross Motor Function Classification Score = 2.9, SD = 1.5). Our recruitment source was specific to nonverbal Gillette Children’s Specialty Health-care pediatric patients with CP who were scheduled for initial intrathecal baclofen (ITB) pump implantation to manage chronic spasticity. Our reasoning for recruiting from a presurgical group was because it permitted some degree of control in a field-based clinical setting (i.e., patients had not eaten for a minimum of 8 hours, no liquids for 4 hours, and specimen collections were in a narrow early morning time-band prior to surgery). Specific inclusion criteria were 1) diagnosis of CP; 2) between 3 and 18 years of age; and 3) scheduled for initial ITB pump implant surgery. Individuals were excluded if 1) their parent(s)/guardian(s) did not consent to the study; or 2) they had an existing cerebral shunt.
Behavioral Measurement
Pain intensity and duration were characterized by the Dalhousie Pain Interview (DPI) using parental report based on a 1-week recall. The DPI consists of 10 items designed to measure the frequency and intensity of pain and was designed explicitly as an interview/survey script; the DPI takes approximately 10 minutes to complete. Specific items are anchored to whether there has been pain in the past week, its general description, possible cause, duration, and intensity. The DPI has been used successfully in a prior pain study with children with CP receiving botulinum toxin (“Botox”) injections for spasticity management [9]. In the current sample, four individuals (100% male, mean age = 9.6 years, SD = 3.5 years) were reported to have had significant pain (at least 1 episode, >4 intensity [0–10], duration >5 minutes) during the 1 week prior to saliva collection (all three criteria [frequency, intensity, duration] had to be met). The distinction is referred to throughout the remainder of the manuscript as “pain” vs “no pain.”
Sample Collection and Preparation
Briefly, approximately 3 mL of unstimulated (i.e., passively collected) saliva was procured at approximately the same time for each patient (7:00–9:00 AM) using toothette oral swabs. Because of motor and communicative impairments, saliva was collected by swabbing the participant’s mouth with the oral swabs and draining sponges into salivette plastic vials. Samples were centrifuged immediately at 900 × g for 5 minutes then aliquoted (500 μL) into cryovials and frozen at −80°C.
Chemicals
The following reagents were purchased from the indicated sources: phosphate-buffered saline, perchloric acid, potassium hydroxide (KOH), NaOD, DCl, and D2O (Sigma-Aldrich, St. Louis, MO, USA), cortisol (Alpco Diagnostics, Salem, NH, USA), dynorphin A (Phoenix Pharmaceuticals, Burlingame, CA, USA), neuropeptide Y (RayBiotech, Norcross, GA, USA), somatostatin (BACHEM—Peninsula Labs, San Carlos, CA, USA), and nerve growth factor (NGF; Promega, Madison, WI, USA).
Salivary Assays
Simultaneous profiling of multiple cytokines in addition to a set of brain-derived proteins (endocrine, neuropeptide) was performed in saliva samples in the cytokine reference laboratory located in the University of Minnesota Department of Pediatrics using a commercially available 22-plex Human Cytokine Array Panel (LUH000, R&D Systems, Minneapolis, MN, USA). Salivary levels of cytokines/chemokines, agouti-related peptide (AgRP), and prolactin were determined by multiplex method on the Luminex platform (Austin, TX, USA) with Bioplex software (BioRad, Hercules, CA, USA) using human-specific bead sets from Millipore (Billerica, MA, USA). Enzyme-linked immunosorbent assays (ELISAs) were used to determine levels of cortisol (Alpco Diagnostics), dynorphin A (Phoenix Pharmaceuticals), neuropeptide Y (RayBiotech), somatostatin (BACHEM—Peninsula Labs), and NGF (Promega). Values were interpolated from standard curves of the relevant recombinant human proteins set up on each plate. Dilution series and standard curves were run for all samples; all assays were performed in duplicate.
Perchloric Acid Cell Extraction
Saliva samples were first centrifuged at 5,000× g for 5 minutes at 4°C. The supernatant was transferred followed by addition of ice-cold perchloric acid to reach 12% (v:v). The samples were then sonicated on ice twice for 30 seconds each (Branson sonicator). The sonicated lysates were centrifuged at 5,000× g for 20 minutes at 4°C. The supernatants were neutralized with ice-cold 2 M KOH followed by centrifugation at 3,000× g for 20 minutes at 4°C. The supernatants were collected, lyophilized, and stored at −80°C.
1H NMR Analysis
Lyophilized extracts were reconstituted in 50 mM phosphate buffer pH 7.4 made in D2O (Sigma-Aldrich). Trimethylsilyl-tetradeuterosodium propionate (TSP) was added as an internal standard for metabolite concentrations and as a chemical shift reference. The pH was adjusted with either DCl (35%) or NaOD (30%) (Sigma-Aldrich). NMR experiments were performed at 25°C on a Bruker Avance 700 MHz NMR spectrometer (Bruker Bio Spin Corporation, Billerica, MA, USA). Spectra were acquired using a 30° pulse every 6 seconds with 63,022 data points and acquisition time of 3 seconds. The residual water peak was suppressed using a presaturation pulse. Collection of 2,048 scans was performed on each sample. Resonance assignments were done using Chenomx software (Edmonton, Canada). Spectra were uploaded into the software and then Fourier-transformed with line broadening of 0.5 Hz. Metabolite assignment was done according to the chemical shifts and pattern of coupling constants and searched against the Chenomx library. Metabolite concentrations were determined after a baseline correction, using TSP as an internal standard.
Statistical Analysis
Partial least squares discriminant analysis (PLS-DA) was conducted using MetaboAnalyst, a metabolomics-oriented web interface to the R statistical package, with the application of mean centering and unit variance scaling. PLS-DA was used because of its ability to utilize pain classification information in conjunction with the collected metabolite and cytokine data to help identify those metabolites/cytokines that are important for discriminating between the pain/no pain states. Peptides (cytokines, chemokines, growth factors, etc.), hormones (cortisol), and metabolite concentrations were log10-transformed as necessary prior to analysis and subjected to parametric (t-tests) or nonparametric (e.g., Wilcoxon-rank sum tests) tests as appropriate. Significance was set at α = 0.05 for all tests.
Results
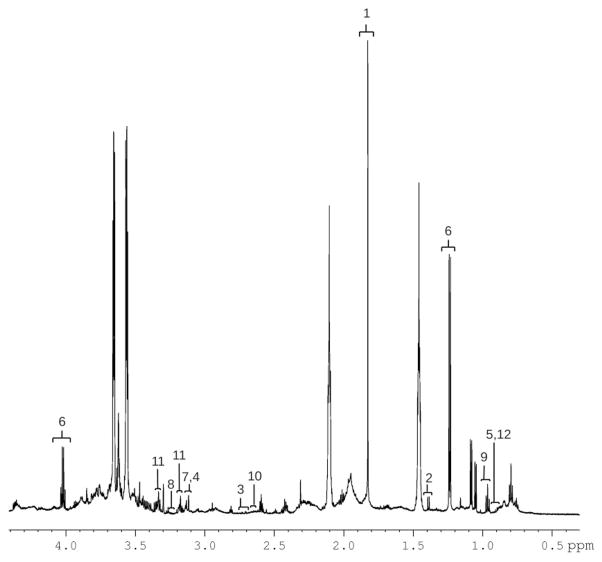
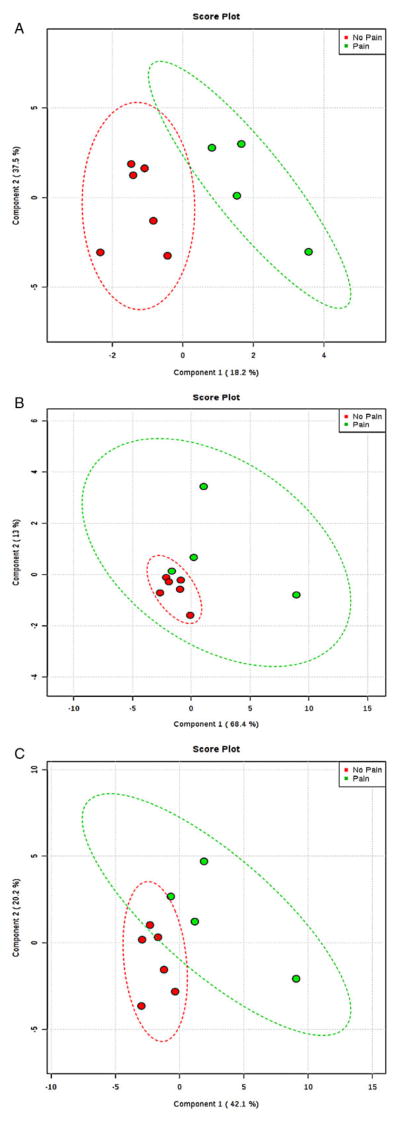
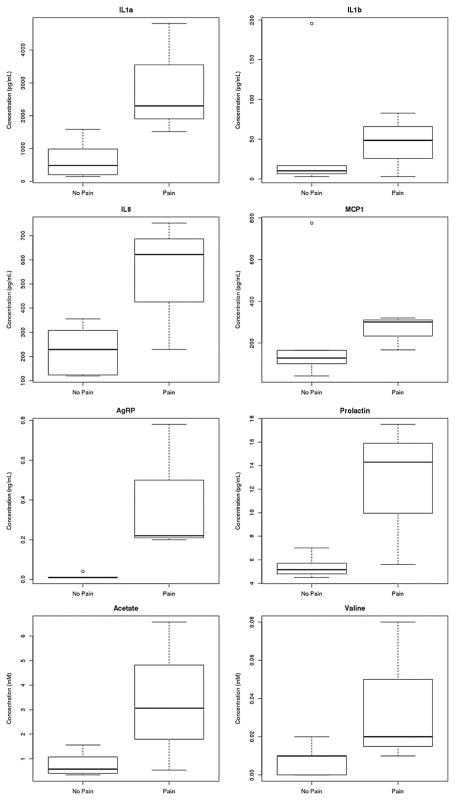
A representative sample 1H NMR spectrum of unstimulated saliva from a male participant manifesting behavior consistent with pain within the past week shows several metabolite resonances identified (Figure 1). Resonance assignments were made using the database associated with the Chenomx software. To determine differences among participants and to optimize class separation, PLS-DAs were performed. Gender, CP severity, and prematurity status were examined using PLS-DA but did not reliably separate. More reliable separations were obtained using pain status (“pain” vs “no pain”) as a classifier, particularly for the neuropeptides, cytokines, and hormones (Figure 2A), but much less so for the metabolites (Figure 2B). Among the most important neuropeptides, cytokines, and hormones for distinguishing between the “pain” and “no pain” group were interleukin (IL)1a, IL8, AgRP, cortisol, monocyte chemotactic protein-1 (MCP1), dynorphin A, and prolactin. There was much less clear separation for the metabolites, but based on variable importance for the DA, among the most important metabolites for partially distinguishing the “pain” from “no pain” group were valine, proline, hypoxanthine, propionate, formate, and acetate. We combined the metabolites and neuropeptides, cytokines, and hormones, which resulted in partial group separation relative to metabolites only (Figure 2C). Nonparametric statistical analysis of individual analytes and metabolites revealed significant differences (P < 0.05) in median concentration values for several identified molecules including gender differences for IFNalpha2 and IL12p70 (female > male) and pain differences for AgRP (pain > no pain), with marginally (P < 0.10) significant differences between pain and no pain groups for the metabolite propionate and the cytokine IL1alpha. Notable descriptive differences (box and whisker plots) were found for several molecules relevant to inflammation and neuroendocrine function (Figure 3).
Figure 1.
A representative 700 MHz 1H NMR spectrum of unstimulated saliva from a male pain participant manifesting behavior consistent with pain within the past week. 1) Acetate, 2) alanine, 3) aspartate, 4) choline, 5) isoleucine, 6) lactate, 7) o-phosphocholine, 8) proline, 9) propionate, 10) sarcosine, 11) taurine, and 12) valine. NMR = nuclear magnetic resonance.
Figure 2.
(A) Neuro-hormones only: Component 1 top loadings: IL1a, IL8, AgRP; Component 2 top loadings: cortisol, MCP1, DynA. Green = pain, red = no pain. Ellipses are 95% confidence intervals. (B) Metabolites only: Component 1 top loadings: valine, proline, hypoxanthine; Component 2 top loadings: propionate, formate, acetate. Green = pain, red = no pain. Ellipses are 95% confidence intervals. (C) Metabolites + neuro-hormones: metabolites (Component 1 loadings: valine, proline, hypoxanthine), hormones/peptides (Component 2 top loadings: cortisol, MCP1, prolactin). Green = pain, red = no pain. Ellipses are 95% confidence intervals. AgRP = agouti-related peptide; DynA = dynorphin A; IL = interleukin; MCP1 = monocyte chemotactic protein-1.
Figure 3.
Box and whisker plots of select molecules in saliva relevant to inflammation and neuroendocrine function; in all instances, concentration values were elevated in patients with cerebral palsy (CP) and pain in the prior week. AgRP = agouti-related peptide; IL = interleukin; MCP1 = monocyte chemotactic protein-1.
Discussion
Translational pain research in vulnerable populations has been hampered by the unreliable nature of instruments based on self-report. In specialized and vulnerable populations with communicative impairments, the instrumentation relies almost exclusively on third-party proxy reports. The establishment of objective, affordable, and reliable biomarkers and measurements would advance our understanding of pain mechanisms, provide a basis for improved clinical management of pain, help assess an individual’s risk for analgesic failure, and establish much needed objective measures of treatment success or failure. In this preliminary feasibility study, the ease with which the samples were collected and analyzed would lend support to the possibility of further testing the predictive use of this novel neuropeptide/hormone and metabolite monitoring method into clinical practice for pain management for specialized populations. There were no specimen collection issues with regard to the children; the children easily tolerated the technique and there were no difficulties in obtaining the specimen samples. Parents were supportive of the approach. Overall, it seemed that salivary collection in this population was feasible and given that ELISA analysis are readily available, it seems like it may be a reasonable approach to pursue further. But it should be acknowledged that sample preparation, analysis, and interpretation on the NMR spectrometer are much more time-consuming and costly. Given that the NMR results were much less clear, it may be a less clinically favorable route. Overall, we demonstrated the noninvasive potential possibility for discrimination of pain vs non-pain in nonverbal children with CP. However, if this application is to truly translate into the clinical setting, a more rapid assessment of the patient will likely be needed. Point-of-care salivary diagnostics are rapidly emerging in other contexts and patient populations such as children with CP and related developmental disabilities may benefit from this technology.
Beyond the feasibility aim of the study, it is worth pointing out that the pathogenesis of chronic pain associated with CP is not well understood. Given the nature of the condition, it would seem reasonable to focus on factors related to musculoskeletal dysfunction and immune-related inflammatory molecules. Most of the research to date has been descriptive with limited attempts to characterize underlying mechanisms. There would be good reason to adopt an approach based on metabolic pathways to help clarify the pathophysiology of CP and muscle pain. Prolonged muscle contraction produces several metabolites (lactic acid, adenosine triphospate [ATP]) that in turn activate sensory nociceptive afferents innervating muscle [10]. In our analysis of saliva, there were several metabolites of interest based on the primary polylactic acid (PLA) component loadings, including valine, proline, hypoxanthine, propionate, formate, and acetate. Acetate is of interest given some evidence that it may interact with transient receptor potential V1 (capsaicin receptor, sensitized by low pH) in such a way as to sensitize nociceptors to painful stimuli [11], but it is not clear whether it would function as a marker for ongoing pain. Although there is no clear relation among the individual metabolites and known nociceptive pathways, it may well be that their involvement reflects more general metabolic changes associated with a recurring or chronic pain state. This idea is speculative, however, and would need to be more fully evaluated.
The neuropeptides, hormones, and cytokines of interest based on the primary PLA component loadings included IL1alpha, IL8, AgRP, cortisol, MCP1, dynorphin A, and prolactin. The interleukins (IL1alpha, IL8) indicate the probability of an ongoing immune system upregulation among the individuals with ongoing pain [12]. In diseases with acute or chronic inflammation, cytokines can be recognized by neurons and used to trigger numerous cellular responses influencing immune cells activity (proliferation, survival), as well as the production and activity of other cytokines. There has been limited work specific to CP and immune modulation of inflammation, but the emerging work regarding inflammation and CP is beginning to point out the possible importance for immune-mediated inflammatory factors in CP [13,14]. The role of cortisol and dynorphin A are well established as integral components of stress-induced analgesia (in part through the hypothalamic-pituitary-adrenocortical [HPA] axis) as well as neurohormone molecules implicated in nociceptive signaling and regulation. However, the relationships can be complex; under some circumstances, for example, it appears that dynorphin may have pronociceptive functions [15,16]. AgRP is a paracrine-signaling molecule regulated by inflammatory signals that is capable of modulating the HPA axis, resulting in increased adrenocorticotropic hormone (ACTH), cortisol, and prolactin production [17,18]. Prolactin was also a major contributor to the component loadings in our salivary samples. As an endocrine hormone, prolactin can be increased by opioids and appears to act as a signaling molecule capable of modulating immune system response among chronic pain patients with autoimmune disorders [19,20]. MCP1 (also chemokine [C-C motif] ligand 2) is a small chemokine molecule implicated in a variety of neuro-immune and inflammatory regulatory functions. Of particular note, it may have a specific role as an endogenous trigger for blood-spinal cord barrier leakage leading to inflammation [21]. Observations of its presence in individuals with CP with pain may have prognostic relevance given the increasing likelihood that neuroinflammatory mechanisms play an important role in the pathogenesis of some forms of CP [14].
Clearly, our results are not confirmatory of any of the pathways just described, and the interpretations above are necessarily speculative. Our goal of the preliminary investigation was first to evaluate feasibility of the approach (collecting the saliva, conducting the assays, determining appropriate analytic models). But given the almost complete absence of biomarker-oriented research in this area, it would seem worth speculating to generate testable hypotheses about putative nociceptive and inflammatory mediators in nonverbal children with neurodevelopmental disability associated with CP.
There are some specific and general limitations to this preliminary study that are worth noting because they delimit what can reasonably be concluded from the findings and also because they point up more general issues that need to be addressed to further our scientific understanding of pain in communicatively impaired populations. The sample was created by clinical convenience and there were no controls, so the results should be understood as specific to the individuals reported on and not as representative of individuals with CP. With regard to the analysis model, PLS is a variant of structural equation modeling but focuses on the variance explained in the dependent variables rather than reproducing the empirical covariance matrix [22]. It is based, in part, on the assumption that all variance measured in the variables are useful variance to be explained in the model. There are no assumptions about the population or measurement scale. Although PLS appears robust with respect to problems such as skewness or multicollinearity among indicators, to paraphrase Fornell and Cha [23] (as cited in Hanlein and Kaplan [22]), it is prone to consistency problems because the case values for the latent variables in PLS are aggregates of manifest variables involving measurement error, such that they must be considered as inconsistent. The solution to the “problem of consistency” is increasing both the number of cases and the number of indicators per latent variable. More generally, as we have mentioned, this was a small sample and much more work with larger samples will be needed to generate more consistent estimates.
From a more general perspective, the approach itself—searching for biomarkers for a complex construct like pain in a communicatively impaired population—may be problematic. It is unlikely that a complex construct like pain would be related in a statistically straightforward way with any single biologically pain-relevant molecule. To complicate matters, pain in the current sample was measured via proxy report. Some part of the variance in the pain measure is therefore likely attributable to the proxy (an issue not widely acknowledged), not the individual. Alternatively, absent strong theory, there is always the danger of finding statistically significant relations because of the empiric nature of preliminary investigative work (i.e., “fishing”). The statistical relations, however, may not always be clinically relevant. The former issue relates to false negatives while the latter to false positives. Taken together, the two issues suggest caution about what to expect from a single measurement time point and that much more work with larger samples will be required to discover meaningful functional biobehavioral pain relationships in vulnerable patient populations.
The value of the approach, however, is not likely in the notion of finding a single “pain biomarker” in any diagnostic sense. Rather, the value may have more to do with looking for evidence relevant to nociceptive and inflammatory system activity to possibly point the way forward for reasonable consideration of pharmaceutical treatment targets. It is worth noting that in this particular sample, there were no significant loadings with CP type or severity, only with pain as measured. Perhaps more work that ties in with natural observation by clinician and/or primary caregiver that anchors pain expression to an event and building biomarker models accordingly may be a relevant way to move forward. Surely, we owe it to such a vulnerable group of patients to be as innovative as possible to improve practice; current best practice for pain management remains empiric (trial and error).
In sum, the preliminary findings and the approach that generated them may help improve our understanding of the clinical and biochemical manifestations of the underlying pathophysiologic processes associated with pain and CP. Further work seems warranted, investigating the scientific and ultimately the clinical value of measuring salivary biomarkers relevant to nociception and inflammation in relation to chronic pain among children with developmental disabilities and severe communicative impairments. Using the bench to explore and expose putative molecular mechanisms of pain may be a useful translational strategy in clarifying a difficult, ambiguous clinical presentation and making a difference in the lives of children with developmental disabilities suffering needlessly in pain.
Acknowledgments
We are grateful for the families and our participants and their willingness to take part in research; our sincere thanks also to Jody Evenson and Gillette Children’s Specialty Healthcare Research Administration for assisting in the coordination of this study and Mike Ehrhardt for the cytokine laboratory work. The work was supported, in part, by a Minnesota Futures Research Grant and NIH Grant Nos. 47201 and 69985.
Footnotes
Disclosure: The authors have no disclosures/conflicts of interest to report.
References
- 1.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Symons FJ, Shinde SK, Gilles E. Perspectives on pain and intellectual disability. J Intellect Disabil Res. 2008;52:275–86. doi: 10.1111/j.1365-2788.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 3.Oberlander TF, Craig KD. Pain and children with developmental disabilities. In: Schechter NL, Berde CB, Yasters M, editors. Pain in Infants, Children, and Adolescents. 2. New York: Lippincott Williams & Williams; 2003. pp. 599–619. [Google Scholar]
- 4.Bottos S, Chambers CT. The epidemiology of pain in developmental disabilities. In: Oberlander TF, Symons FJ, editors. Pain in Children & Adults with Developmental Disabilities. Baltimore, Maryland: Paul H. Brookes Publishing Co; 2006. pp. 67–87. [Google Scholar]
- 5.Abu-Saad HH. Challenge of pain in the cognitively impaired. Lancet. 2000;356:1867–8. doi: 10.1016/S0140-6736(00)03253-0. [DOI] [PubMed] [Google Scholar]
- 6.Kupers R, Kehlet H. Brain imaging of clinical pain states: A critical review and strategies for future studies. Lancet Neurol. 2006;5:1033–44. doi: 10.1016/S1474-4422(06)70624-X. [DOI] [PubMed] [Google Scholar]
- 7.Symons FJ, Rivard PF, Nugent AC, Tervo RC. Parent evaluation of spasticity treatment using botulinum toxin type A in cerebral palsy. Arch Phys Med Rehabil. 2006;87:1658–60. doi: 10.1016/j.apmr.2006.08.343. [DOI] [PubMed] [Google Scholar]
- 8.Takeda I, Stretch C, Barnaby P, et al. Understanding the human salivary metabolome. NMR Biomed. 2009;22:577–84. doi: 10.1002/nbm.1369. [DOI] [PubMed] [Google Scholar]
- 9.Rivard PF, Nugent A, Symons FJ. Parent-rated pain pre- and post-botulinum toxin type A treatment for spasticity in children with cerebral palsy. Clin J Pain. 2009;25:413–7. doi: 10.1097/AJP.0b013e31819a6d07. [DOI] [PubMed] [Google Scholar]
- 10.Light AR, Hughen RW, Zhang J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nascimento FP, Figueredo SM, Marcon R, et al. Inosine reduces pain-related behavior in mice: Involvement of adenosine A1 and A2a receptor subtypes and protein kinase C pathways. J Pharmacol Exp Ther. 2010;334:590–8. doi: 10.1124/jpet.110.166058. [DOI] [PubMed] [Google Scholar]
- 12.Shubayev V, Kato K, Meyers RR. Cyotokines in pain. In: Kruger L, Light AR, editors. Translational Research from Mouse to Man. New York: CRC Press, Taylor & Francis Group; 2010. pp. 187–214. [Google Scholar]
- 13.Lin CY, Chang YC, Wang ST, et al. Altered inflammatory responses in preterm children with cerebral palsy. Ann Neurol. 2010;68:204–12. doi: 10.1002/ana.22049. [DOI] [PubMed] [Google Scholar]
- 14.Kannan S, Dai H, Navath RS, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4:130–46. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci U S A. 1979;76(12):6666–70. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31:98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarlett JM, Zhu X, Enriori PJ, et al. Regulation of agouti-related protein messenger ribonucleic acid transcription and peptide secretion by acute and chronic inflammation. Endocrinology. 2008;149:4837–45. doi: 10.1210/en.2007-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao E, Xia-Zhang L, Vulliémoz NR, Ferin M, Wardlaw SL. Agouti-related protein stimulates the hypothalamic-pituitary-adrenal (HPA) axis and enhances the HPA response to interleukin-1 in the primate. Endocrinology. 2003;144(5):1736–41. doi: 10.1210/en.2002-220013. [DOI] [PubMed] [Google Scholar]
- 19.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–68. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 20.Jara LJ, Lavalle C, Fraga A, et al. Prolactin, immunoregulation, and autoimmune diseases. Semin Arthritis Rheum. 1991;20:273–84. doi: 10.1016/0049-0172(91)90028-x. [DOI] [PubMed] [Google Scholar]
- 21.Echeverry S, Shi XQ, Rivest S, Zhang J. Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J Neurosci. 2011;31:10819–28. doi: 10.1523/JNEUROSCI.1642-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanlein M, Kaplan AM. A beginner’s guide to partial least squares analysis. Underst Stat. 2004;3(4):283–97. [Google Scholar]
- 23.Fornell C, Cha J. Partial least squares. In: Bagozzi RP, editor. Advanced Methods of Marketing Research. Cambridge, England: Blackwell; 1994. pp. 52–78. [Google Scholar]